Abstract
INTRODUCTION:
Polycystic ovary syndrome (PCOS) is one of the most common infertility factor for which women are enrolled in in vitro fertilization (IVF) technique. In the recent years, gonadotropin releasing hormone antagonist protocol has emerged as the protocol of choice for controlled ovarian hyperstimulation in these patients.
OBJECTIVES:
The objective of the present study is to compare conventional long agonist protocol with fixed antagonist protocol in PCOS patients undergoing IVF cycle.
MATERIALS AND METHODS:
Retrospective analysis of 4 years data of a single center from northern India. Totally 81 patients who had long agonist protocol were compared with 36 patients with similar baseline characteristics who had antagonist protocol.
RESULT:
Total dose of gonadotropin required was significantly lower (P - 0.004) in the antagonist group. There was no significant difference in pregnancy rate or incidence of ovarian hyperstimulation syndrome between two groups. Cycle cancellation due to arrest of follicular growth was significantly higher in the antagonist group (P - 0.027).
CONCLUSION:
More randomized control trials and meta-analysis are required before replacing conventional long agonist protocol with antagonist protocol in patients with polycystic ovary syndrome.
KEY WORDS: Gonadotropin-releasing hormone agonist, gonadotropin-releasing hormone antagonist, in vitro fertilization, polycystic ovarian syndrome
INTRODUCTION
First described by Irving Fstain and Michael Leventhal in 1935, polycystic ovary syndrome (PCOS) is one of the most common endocrinopathy that affects 5-7% of reproductive age group females.[1] Common clinical features include irregular menstruation, hirsutism, acne and infertility. Anovulation/oligo-ovulation is responsible for 40% of female infertility and PCOS accounts for 80% of these cases.[2,3] in vitro fertilization (IVF)/intracytoplasmic sperm injection (ICSI) is the final step of treatment for PCOS patients with infertility.[4] However, controlled ovarian stimulation (COS) in these patients remains a challenge till date because of risk of potentially lethal complication like ovarian hyperstimulation syndrome (OHSS).[5] Different stimulation protocols have been suggested, but still there is no consensus as to which protocol is best for patients with PCOS.
Gonadotropin-releasing hormone (GnRH) antagonist is being increasingly used in COS for IVF from late 1990s. GnRH antagonists do not require long desensitization as in agonist protocol and induce rapid reduction in the level of follicle stimulating hormone (FSH) and luteinizing hormone (LH) without initial flair up thus ensuring a short and simple IVF cycle and better patient compliance. Although there was initial reports that antagonist cycles were associated with lower ongoing pregnancy rate when compared to long agonist cycles,[6,7] recent randomized control trials (RCT) show that there is no significant difference in fertilization rate and pregnancy outcome.[8,9,10] Three recent meta-analysis pointed out certain advantages of antagonist cycles like shorter period of gonadotropin stimulation, smaller dose of gonadotropin required and reduced incidence of OHSS.[11,12]
There are only limited number of studies in literature comparing GnRH agonist and antagonist protocol in PCOS patients[13,14,15] and we are yet to reach a final conclusion regarding the best IVF protocol in PCOS population. Hence the aim of the present study was to compare GnRH agonist and antagonist protocols in PCOS patients by retrospective analysis of data of 4 years from a single IVF center of Northern India.
MATERIALS AND METHODS
A retrospective analysis of records of PCOS patients who entered assisted reproductive technology program in All India Institute of Medical Sciences, New Delhi, India, from January 2007 to December 2012 (6 years) was performed. The diagnosis of PCOS was based on Rotterdam criteria.[16] Even though it was a retrospective study, we tried to omit the confounding factors by setting an inclusion criteria which include age between 20 and 35, body mass index (BMI) between 20 and 30, day 2 FSH level below 10 IU/L, no past history of genital tuberculosis, first IVF cycle, no documented evidence of hypothyroidism or hyper-prolactinemia and no other associated infertility factors except for tubal factor. Couples with male factor infertility were barred however those who underwent donor semen IVF were included into the analysis. Out of 944 patients who underwent IVF cycles in this time period, 153 had PCOS out of which 117 met the inclusion criteria and complete case record of these patients were reviewed thoroughly. Among 117 patients of PCOS, 81 patients had conventional long agonist protocol and 36 had fixed antagonist protocol.
In agonist group, patients were given 1 mg injection of leuprolide acetate (Injection Lupride, Sun Pharmaceutical Industries Ltd., Mumbai) starting from day 21 of menstruation for 14 days. Down regulation was confirmed by biochemical markers (LH <5 IU/ml, E2 <50 pg/ml and progesterone <1 ngm/ml) and trans vaginal ultrasound (TVS) assessment of endometrial thickness (ET) and ovarian status (ET <3 mm, no ovarian cyst >2 cm). After down-regulation, dose of leuprolide was reduced to 0.5 mg/day and patients were started on recombinant FSH (Injection Gonal-f, Merck Serono Specialties Pvt. Ltd., Italy). The starting dose was between 150 IU/day to 225 IU/day depending upon patient's characteristics. In antagonist group, patients were scanned for any ovarian cyst on the first day of the menstrual cycle and were started on injection gonal f (150 IU to 225 IU) from day 2. GnRH antagonist cetrorelix acetate (Injection Cetrotide, AEterna Zentaris, Canada). 25 mg was added on 6th day of the menstrual cycle (fixed dose regime). Follicular monitoring was done in both groups using TVS and dose of gonadotrophin was adjusted accordingly. The cycles were cancelled in patients with no follicle more than 10 mm after 10 days of gonadotropin stimulation. Ovulation was triggered when leading follicle reached 18 mm along with at least two follicles >16 mm, using 250 mg of recombinant human chorionic gonadotropin (HCG) (Injection Ovitrelle, Marck Serono, UK). Serum estrogen (E2) and ET were measured on the day of trigger. Embryo transfer was done between day 2 to day 5 depending upon the number of good quality embryo. All patients were given luteal phase support by i. m injection progesterone 100 mg/day. On the 6th day of embryo transfer, pregnancy was assessed by serum beta HCG assay and confirmed by the presence of the gestational sac on TVS after another 2 weeks. Biochemical pregnancies were not included in our analysis.
Statistical analysis
Data were computerized and analyzed using the statistical package IBM SPSS version 16.0. Descriptive statistics were computed for base-line characteristics of patients, ovarian stimulation factors, hormonal profile, ET on the day of HCG trigger and embryological variables for each study group. After determining whether the data met the normality assumption, Student t-independent two-tailed test was conducted to test whether the means of continuous variables were significantly different between the two study groups. Nominal or frequency data were analyzed using Chi-square test or Fishers's exact test as appropriate. For the entire statistical tests P < 0.05 was considered to be statistically significant.
RESULT
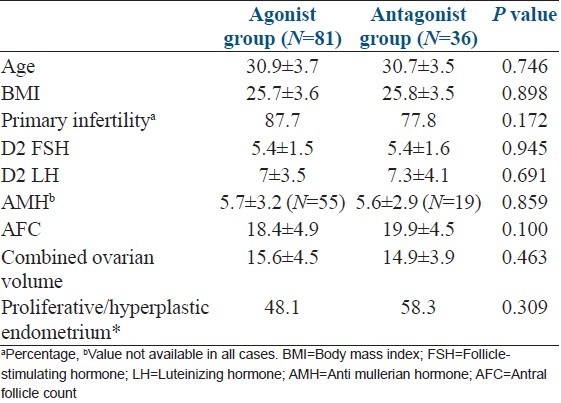
Among 944 patients enrolled in the given time period, 153 (16.2%) patients had PCOS and 117 patients met our inclusion criteria. Out of these 117, 81 (69.2%) patients had long agonist protocol and 36 (30.8%) patients had antagonist protocol. The baseline characteristics of patients enrolled in two protocol groups are summarized in Table 1. There was no significant difference in mean age, BMI, percentage of patient with primary infertility, day 2 FSH, LH, anti-Mullerian hormone, antral follicle count, combined ovarian volume, percentage of patients with pre-menstrual proliferative or hyperplastic endometrium between two groups.
Table 1.
Baseline characteristics
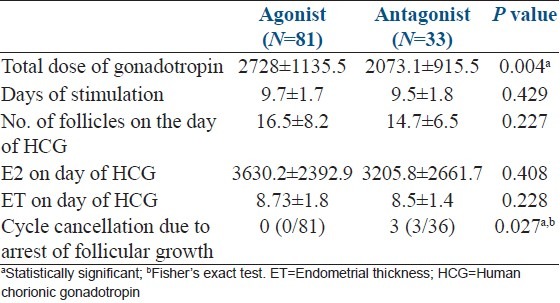
Ovarian stimulation characteristics, serum estrogen (E2) level and ET on the day of HCG trigger in two study groups are compared as shown in Table 2. In antagonist group, three patients were cancelled after 10 days of gonadotropin stimulation as no dominant follicle > 10 mm was seen on TVS. Total dose of gonadotropin was significantly lower in antagonist group however, no significant difference was found in total days of stimulation, number of follicles, E2 level and ET on the day of HCG trigger between two groups. Two patients in agonist group developed moderate OHSS after triggering for which embryo transfer was cancelled and all embryos were cryopreserved. Number of embryo transferred was between 2 and 4.
Table 2.
Ovarian stimulation characteristics, E2 level and ET on the day of trigger
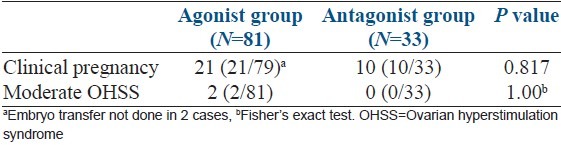
During analysis of embryological data [Table 3], we could not find any significant difference in number of oocyte retrieved, percentage of metaphage ll (M2) oocyte, fertilization rate, cleavage rate, percentage of grade 1 embryo formed between two groups. Mean number of embryo transferred was also not different in two groups. Table 4 shows number of clinical pregnancy and OHSS in two groups. Pregnancy was confirmed by USG in 21 patients (26.6%) of agonist group and in 10 patients (30.3%) of antagonist group but the difference was not significant statistically. As mentioned above 2 patients of agonist group developed moderate OHSS, but none of the patients in antagonist group had this complication.
Table 3.
Embryology data of two groups
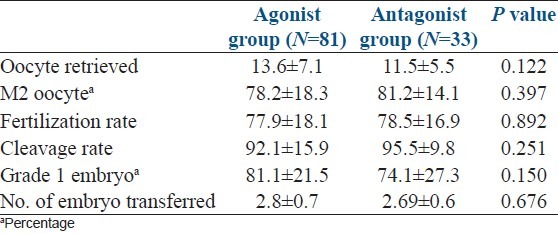
Table 4.
Clinical pregnancy and OHSS
DISCUSSION
GnRH agonist protocol is still considered as the gold-standard protocol in IVF/ICSI cycles for COH. In the recent years however, antagonist protocol is gaining popularity because of short and simple cycle and lower incidence of OHSS. Comparative studies of agonist and antagonist protocols yield conflicting results.[6,7,8,9,10] The meta-analysis by Al-Inany et al. in 2007[17] examined the first five comparative studies of fixed GnRH-ant protocol with the standard GnRH-a long protocol and showed 5% lower pregnancy rate with GnRH-ant regimen. Later, a second study by Kolibianakis et al.,[11] a meta-analytic review of 22 RCTs published as full papers in peer reviewed journals, showed that the probability of live birth between GnRH-ant and GnRH-a was not significantly different. A third study, which was an additional updated meta-analysis by Al-Inany et al.[12] also showed that there was no significant difference in pregnancy rate following GnRH ant compared with GnRH agonist regimens.
The objective of the present study was to compare the outcome of IVF cycles of PCOS patients in GnRH agonist and fixed antagonist protocol. We found significant lower dose of gonadotropin required in antagonist group. Total days of stimulation, number of follicles and E2 level on the day of triggering, total number of oocytes retrieved, percentage of M2 oocytes, fertilization rate, cleavage rate, percentage of Grade 1 embryo, all were comparable between two groups. The pregnancy rate was also not significantly different. Two patients of agonist regime and none of antagonist regime had moderate OHSS, but the difference was not statistically significant.
In literature, there are a number of studies comparing agonist and antagonist protocol in PCOS patients with highly variable results [Table 5]. Bahçeci et al. in 2005[18] did a randomized prospective pilot study which showed no difference in total dose of gonadotropin used, number of oocyte retrieved and pregnancy rate between agonist and antagonist group but the number of days of stimulation, number of M2 oocytes were significantly lower in antagonist group. They also found no significant difference in incidence of OHSS between these two groups. In the same year, Ashrafi et al.,[13] in their RCT, found that number of retrieved oocytes and M2 oocytes were significantly higher in antagonist group. There was no statistically significant difference in total dose of gonadotropin, fertilization or pregnancy rate. Interestingly, number of patients with risk of OHSS (E2 > 3000 pg/ml) was significantly higher in antagonist group (P - 0.004). Ragni et al., (2005) on the other hand, showed significant lower risk of OHSS in antagonist group.[14]
Table 5.
Previous comparative studies of GnRH agonist and antagonist in PCO patients undergoing IVF cycles

Orvieto et al. in 2009[15] found significant higher pregnancy rate in long agonist protocol (36% vs. 19%) while Hosseini et al.[19] and Kim et al.[20] in 2010, showed no difference in pregnancy rate between two regimens. Lainas et al.[21] in 2010 compared flexible GnRH antagonist protocol with long agonist protocol in PCOS patients. They found that, the total dose of gonadotropin, number of days of stimulation, incidence of OHSS were significantly lower in antagonist group but there was no difference in ongoing pregnancy rate. They concluded that, antagonist protocol should be the treatment of choice in PCOS patients. More recent studies (Haydardedeoglu et al. 2012, Onofriescu et al. 2013)[22,23] also reflect the same view.
From India, Kaur et al.[24] in 2012, published their prospective controlled study comparing long agonist protocol with flexible antagonist protocol. They found no difference in days of stimulation between two groups, but total dose of gonadotropin was significantly lower in antagonist group. We also found the same. Number of oocyte retrieved, number of mature oocyte, fertilization rate were higher in agonist group but there was no difference in clinical pregnancy rate and life birth rate. The incidence of OHSS was lower in antagonist group. We also found no difference in pregnancy rate but unlike this study, we couldn't find any difference in number of retrieved oocytes, number of mature oocytes, fertilization rate between two groups. These findings were similar to study of Lainas et al. We had two patients in agonist group and none in antagonist group with moderate OHSS, the difference being insignificant. Though this insignificant difference may be because of smaller no of patients in antagonist group, Bahçeci et al.[18] in their pilot study also found no difference in OHSS rate in two regimen. So, in our opinion, careful assessment of patients before stimulation, low starting dose of gonadotropin and careful monitoring of follicular growth with adjustment of dose of gonadotropin can reduce the incidence of OHSS in PCOS patients undergoing COH with long agonist regimen.
In our study, we also had 3 patients in antagonist group in whom no dominant follicle was formed after 10 days of stimulation. None of the patients in agonist group had follicular growth arrest, the difference being statistically significant. In the study of Bahçeci et al.[18] 3 of 27 patients (11%) in antagonist group and 1 of 25 patients (4%) in agonist group had arrest of follicular development. Though we can't draw any conclusion from two studies, these findings do evoke the old debate regarding the role of LH in follicular development. GnRH antagonist can induce a sharp decrease in serum LH level which may be detrimental if the level falls below a “threshold.” In fact, minimum threshold of LH has to be maintained for adequate steroidogenesis and folliculogenesis (European recombinant human LH study group 1998). In antagonist regimen, abrupt fall of LH at a critical stage of folliculogenesis when follicles become more and more sensitive to LH due to increased LH receptor on granulose cells hinders the combined attempt of FSH and LH to achieve complete follicular maturity and oocyte competence.[25] Another problem with antagonist regime is that, pre-existing follicle size discrepancies may hinder coordinated follicular growth during ovarian stimulation, thereby reducing the number of follicles that reach maturation.[26] Luteal phase suppression of endogenous FSH in long agonist protocol can trim down this discrepancy. Increase no of mature oocyte retrieved in patients undergoing long agonist protocol (Bahççeci et al., Kaur et al.) can be explained by these theories.
We found two meta-analysis comparing agonist and antagonist protocol in OHSS patients. The meta-analysis of Griesinger et al.[27] in 2006, compared agonist and antagonist protocol in a total of 305 patients with PCOS, and included four studies. Pregnancy rates were not significantly different in the agonist and antagonist groups but the incidence of severe OHSS was significantly lower in the antagonist group. Pundir et al.,[28] in a recent meta-analysis which included 9 RCTS with 966 women, tried to find whether GnRH antagonist protocol reduces the risk of OHSS in PCOS patients. There was no difference in severe OHSS rate but when moderate and severe OHSS cases were pooled, there was significant (P < 0.0001) lower incidence in antagonist group.
The present study analyzed a single center data of 4 years. We found comparable pregnancy rate in two regimen and lower dose of gonadotropin in antagonist group. Of importance is the fact that follicular growth arrest was significantly higher in antagonist group while OHSS rate was similar. With the limits of the retrospective study, we can still conclude that it is not the time to replace long agonist protocol with antagonist protocol in PCOS patients enrolled for IVF. In our opinion, larger RCTs with adequate sample size and more meta-analysis are required to reach a final conclusion.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Franks S. Polycystic ovary syndrome. N Engl J Med. 1995;333:853–61. doi: 10.1056/NEJM199509283331307. [DOI] [PubMed] [Google Scholar]
- 2.Tang T, Glanville J, Orsi N, Barth JH, Balen AH. The use of metformin for women with PCOS undergoing IVF treatment. Hum Reprod. 2006;21:1416–25. doi: 10.1093/humrep/del025. [DOI] [PubMed] [Google Scholar]
- 3.Sohrabvand F, Ansari Sh, Bagheri M. Efficacy of combined metformin-letrozole in comparison with metformin-clomiphene citrate in clomiphene-resistant infertile women with polycystic ovarian disease. Hum Reprod. 2006;21:1432–5. doi: 10.1093/humrep/del020. [DOI] [PubMed] [Google Scholar]
- 4.Thessaloniki ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group. Consensus on infertility treatment related to polycystic ovary syndrome. Fertil Steril. 2008;89:505–22. doi: 10.1016/j.fertnstert.2007.09.041. [DOI] [PubMed] [Google Scholar]
- 5.Delvigne A, Demoulin A, Smitz J, Donnez J, Koninckx P, Dhont M, et al. The ovarian hyperstimulation syndrome in in-vitro fertilization: A Belgian multicentric study. I. Clinical and biological features. Hum Reprod. 1993;8:1353–60. doi: 10.1093/oxfordjournals.humrep.a138260. [DOI] [PubMed] [Google Scholar]
- 6.Olivennes F, Cunha-Filho JS, Fanchin R, Bouchard P, Frydman R. The use of GnRH antagonists in ovarian stimulation. Hum Reprod Update. 2002;8:279–90. doi: 10.1093/humupd/8.3.279. [DOI] [PubMed] [Google Scholar]
- 7.Tarlatzis BC, Bili HN. Gonadotropin-releasing hormone antagonists: Impact of IVF practice and potential non-assisted reproductive technology applications. Curr Opin Obstet Gynecol. 2003;15:259–64. doi: 10.1097/00001703-200306000-00009. [DOI] [PubMed] [Google Scholar]
- 8.Del Gadillo JC, Siebzehnrübl E, Dittrich R, Wildt L, Lang N. Comparison of GnRH agonists and antagonists in unselected IVF/ICSI patients treated with different controlled ovarian hyperstimulation protocols: A matched study. Eur J Obstet Gynecol Reprod Biol. 2002;102:179–83. doi: 10.1016/s0301-2115(01)00580-2. [DOI] [PubMed] [Google Scholar]
- 9.Moraloglu O, Kilic S, Karayalçin R, Yuksel B, Tasdemir N, Işik A, et al. Comparison of GnRH agonists and antagonists in normoresponder IVF/ICSI in Turkish female patients. Adv Ther. 2008;25:266–73. doi: 10.1007/s12325-008-0028-8. [DOI] [PubMed] [Google Scholar]
- 10.Engel JB, Griesinger G, Schultze-Mosgau A, Felberbaum R, Diedrich K. GnRH agonists and antagonists in assisted reproduction: Pregnancy rate. Reprod Biomed Online. 2006;13:84–7. doi: 10.1016/s1472-6483(10)62019-6. [DOI] [PubMed] [Google Scholar]
- 11.Kolibianakis EM, Collins J, Tarlatzis BC, Devroey P, Diedrich K, Griesinger G. Among patients treated for IVF with gonadotrophins and GnRH analogues, is the probability of live birth dependent on the type of analogue used. A systematic review and meta-analysis? Hum Reprod Update. 2006;12:651–71. doi: 10.1093/humupd/dml038. [DOI] [PubMed] [Google Scholar]
- 12.Al-Inany HG, Youssef MA, Aboulghar M, Broekmans F, Sterrenburg M, Smit J, et al. Gonadotrophin-releasing hormone antagonists for assisted reproductive technology. Cochrane Database Syst Rev. 2011;5:CD001750. doi: 10.1002/14651858.CD001750.pub3. doi:10.1002/14651858. CD001750.pub 3. [DOI] [PubMed] [Google Scholar]
- 13.Ashrafi M, Moini A, Mohammadzadeh A, Ezabadi Z, Zafarani F, Baghestani AR, et al. A comparative study of GnRH antagonist and GnRH agonist in PCO patients undergoing IVF/ICSI cycles. Iran J Reprod Med. 2005;3:14–8. [Google Scholar]
- 14.Ragni G, Vegetti W, Riccaboni A, Engl B, Brigante C, Crosignani PG. Comparison of GnRH agonists and antagonists in assisted reproduction cycles of patients at high risk of ovarian hyperstimulation syndrome. Hum Reprod. 2005;20:2421–5. doi: 10.1093/humrep/dei074. [DOI] [PubMed] [Google Scholar]
- 15.Orvieto R, Meltcer S, Homburg R, Nahum R, Rabinson J, Ashkenazi J. What is the preferred GnRH analogue for polycystic ovary syndrome patients undergoing controlled ovarian hyperstimulation for in vitro fertilization? Fertil Steril. 2009;91(Suppl 4):1466–8. doi: 10.1016/j.fertnstert.2008.07.1711. [DOI] [PubMed] [Google Scholar]
- 16.Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil Steril. 2004;81:19–25. doi: 10.1016/j.fertnstert.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 17.Al-Inany HG, Abou-Setta AM, Aboulghar M. Gonadotrophin-releasing hormone antagonists for assisted conception: A Cochrane review. Reprod Biomed Online. 2007;14:640–9. doi: 10.1016/s1472-6483(10)61059-0. [DOI] [PubMed] [Google Scholar]
- 18.Bahçeci M, Ulug U, Ben-Shlomo I, Erden HF, Akman MA. Use of a GnRH antagonist in controlled ovarian hyperstimulation for assisted conception in women with polycystic ovary disease: A randomized, prospective, pilot study. J Reprod Med. 2005;50:84–90. [PubMed] [Google Scholar]
- 19.Hosseini MA, Aleyasin A, Saeedi H, Mahdavi A. Comparison of gonadotropin-releasing hormone agonists and antagonists in assisted reproduction cycles of polycystic ovarian syndrome patients. J Obstet Gynaecol Res. 2010;36:605–10. doi: 10.1111/j.1447-0756.2010.01247.x. [DOI] [PubMed] [Google Scholar]
- 20.Kim YJ, Ku SY, Jee BC, Suh CS, Kim SH, Choi YM, et al. A comparative study on the outcomes of in vitro fertilization between women with polycystic ovary syndrome and those with sonographic polycystic ovary-only in GnRH antagonist cycles. Arch Gynecol Obstet. 2010;282:199–205. doi: 10.1007/s00404-010-1401-9. [DOI] [PubMed] [Google Scholar]
- 21.Lainas TG, Sfontouris IA, Zorzovilis IZ, Petsas GK, Lainas GT, Alexopoulou E, et al. Flexible GnRH antagonist protocol versus GnRH agonist long protocol in patients with polycystic ovary syndrome treated for IVF: A prospective randomised controlled trial (RCT) Hum Reprod. 2010;25:683–9. doi: 10.1093/humrep/dep436. [DOI] [PubMed] [Google Scholar]
- 22.Haydardedeoglu B, Kilicdag EB, Parlakgumus AH, Zeyneloglu HB. IVF/ICSI outcomes of the OCP plus GnRH agonist protocol versus the OCP plus GnRH antagonist fixed protocol in women with PCOS: A randomized trial. Arch Gynecol Obstet. 2012;286:763–9. doi: 10.1007/s00404-012-2348-9. [DOI] [PubMed] [Google Scholar]
- 23.Onofriescu A, Bors A, Luca A, Holicou M, Onofriescu M, Vulpoi C. GnRH antagonist protocol in PCOS. Current Health Science Journal. 2013;39:20–5. [PMC free article] [PubMed] [Google Scholar]
- 24.Kaur H, Krishna D, Shetty N, Krishnan S, Srinivas M, Rao KA. A prospective study of GnRH long agonist versus flexible GnRH antagonist protocol in PCOS: Indian experience. J Hum Reprod Sci. 2012;5:181–6. doi: 10.4103/0974-1208.101019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hillier SG. Gonadotropic control of ovarian follicular growth and development. Mol Cell Endocrinol. 2001;179:39–46. doi: 10.1016/s0303-7207(01)00469-5. [DOI] [PubMed] [Google Scholar]
- 26.Fanchin R, Méndez Lozano DH, Schonäuer LM, Cunha-Filho JS, Frydman R. Hormonal manipulations in the luteal phase to coordinate subsequent antral follicle growth during ovarian stimulation. Reprod Biomed Online. 2005;10:721–8. doi: 10.1016/s1472-6483(10)61115-7. [DOI] [PubMed] [Google Scholar]
- 27.Griesinger G, Diedrich K, Tarlatzis BC, Kolibianakis EM. GnRH-antagonists in ovarian stimulation for IVF in patients with poor response to gonadotrophins, polycystic ovary syndrome, and risk of ovarian hyperstimulation: A meta-analysis. Reprod Biomed Online. 2006;13:628–38. doi: 10.1016/s1472-6483(10)60652-9. [DOI] [PubMed] [Google Scholar]
- 28.Pundir J, Sunkara SK, El-Toukhy T, Khalaf Y. Meta-analysis of GnRH antagonist protocols: Do they reduce the risk of OHSS in PCOS? Reprod Biomed Online. 2012;24:6–22. doi: 10.1016/j.rbmo.2011.09.017. [DOI] [PubMed] [Google Scholar]


