Abstract
RATIONALE:
Bacterial flora can be isolated from many semen samples of subfertile males. Bacteriospermia can compromise the outcome of intra uterine insemination (IUI) by contaminating the post-processed sperm sample.
OBJECTIVES:
The objective of the present study is to determine the efficacy of penicillin and streptomycin in eliminating the bacteria from semen samples in the sperm processing procedure, and to assess the effects of antibiotics on sperm motility, survivability, and pregnancy rates.
DESIGN AND SETTINGS:
A prospectively controlled study was carried out using couples undergoing IUI with their informed consent.
INTERVENTION:
Sperm processing using the swim-up technique in penicillin and streptomycin supplemented culture medium.
SUBJECTS AND METHODS:
Couples were consecutively allocated in two groups for sperm processing (a) Group AB+ (antibiotics supplemented culture medium, n = 33) and (b) Group AB− (antibiotic free culture medium, n = 33). Semen culture was performed before and after sperm processing. Sperm motility was assessed immediately after processing and after 24 h of incubation.
RESULTS:
Bacterial isolates were found in 20 (60.6%) and 22 (66.1%) of samples before processing in Groups AB+ and AB− respectively. Addition of antibiotics resulted in completely eliminating non-specific bacteria from semen samples without affecting sperm motility. In vitro survival rate of sperm enhanced in AB+ group compared with AB− group (motile sperm after 24 h), 62.21% (standard deviation [SD]: 37.27) versus 41.36% (SD: 30.78), P = 0.012. Pregnancy rate, was comparable between two groups (9% in Group AB+ vs. 6% in Group AB−, P = 0.45).
CONCLUSION:
Penicillin streptomycin combination could completely eliminate non-specific bacteria from semen samples during sperm processing in this population. The types of antibiotics and dosage used did not seem to have any harmful effects on human sperm.
KEY WORDS: Bacteriospermia, penicillin and streptomycin, sperm processing
INTRODUCTION
Intra uterine insemination (IUI) using processed sperm is a well-established treatment modality for subfertile couples with a broad range of defined indications. Quality of the initial semen sample and post-processed sperm is a significant predictor of prospective pregnancies following this procedure.[1,2] The quality should not refer only to the semen parameters, but sterility of the sample too. Studies on the capacity to eliminate bacteria with routinely used sperm preparation methods (swim-up or density gradient) have produced variable results. Non-specific bacteria were isolated from 23% to 36% of samples processed with swim-up and density gradients methods in our previous study.[3] Nicholson et al. have reported almost bacteria abolished samples using density gradients method with strict aseptic conditions.[4] Samples with significantly lower bacterial counts were obtained with modified density gradients centrifugation using an inner tube insertion compared with the conventional method by Fourie et al.[5] A similar method employed an inner column of Percoll has been reported by Kaneko et al. in 1986 with a greater success.[6] Sperm preparation methods are not guaranteed options for complete elimination of bacteria.
Effects of addition of antibiotics into sperm culture media is an unanswered question. Adverse effects of antibiotics on biological functions of gametes and embryo are major concerns. It has been reported that antibiotic supplementation of culture media has an adverse effect on the growth rate of pre-implantation embryos.[7] Hence, ready to use culture media are available with antibiotics or without antibiotics.
Our aims of this study were to assess the efficacy of penicillin and streptomycin supplemented culture media in clearing the non-specific bacteria from baceriospermic samples, and to determine the effect of antibiotics on sperm motility, long term survivability and pregnancy rates in a subfertile population.
SUBJECTS AND METHODS
Study subjects
A prospectively controlled study was carried out at the Infertility Unit, Department of Obstetrics and Gynecology, Faculty of Medicine, Ragama, Sri Lanka. Ethical clearance for the work was obtained from the ethics committee in the institute. Couples referred for IUI and voluntarily consented to participate to the study were recruited. They had already been clinically assessed and prepared for treatment at the time of recruitment. They were allocated consecutively into two groups; AB+ and AB−. Sperm in group AB+ were processed with antibiotic added culture medium (n = 33). The same medium without antibiotics was used to prepare the sperm in group AB− (n = 33). Semen samples were processed using the swim-up method. Samples prepared by the density gradients method for specific indications were excluded.
Sample collection and preparation
Semen samples were collected in to sterile, polystyrene, wide mouthed containers with strict aseptic conditions. Men were instructed to pass urine ½ h before the sample collection. Samples were assessed for initial semen parameters according to the World Health Organization guidelines 2010.[8] An aliquot of 0.1 ml was set aside initially for the microbial culture.
Samples were processed by swim-up method using Ferticult Flushing medium (FertiPro NV, Belgium). Culture medium used for the group AB+ was supplemented with 1% v/v penicillin streptomycin solution (10,000 U/ml penicillin and 10 mg/l streptomycin, Sigma Aldrich). After the final wash procedure sperm pellets in both groups were diluted with antibiotic free culture medium, and final volume was adjusted to 0.8 ml. From the processed sample, 0.1 ml was allotted to bacterial culture, and 0.2 ml was incubated (37°C, 5% CO2 ) for further 24 h to assess the sperm survivability. Female partners were inseminated with 0.5 ml of processed sperm samples using soft catheters within 1 h of sample preparation. Outcome of the treatment process was obtained over the phone after 4 weeks of insemination.
Bacterial culture
Aliquot of raw semen sample and processed sample was sent immediately to the microbiology laboratory. They were cultured in culture plates containing Blood agar, Nutrient agar and MacConkey agar using calibrated loop method. Plates were incubated aerobically at 37°C and read in 24 h.
Statistical analysis was done with SPSS 16.0 version (SPSS Inc.) of computer software. Comparison of data between two groups was done using Chi-squared and independent samples t-tests.
RESULTS
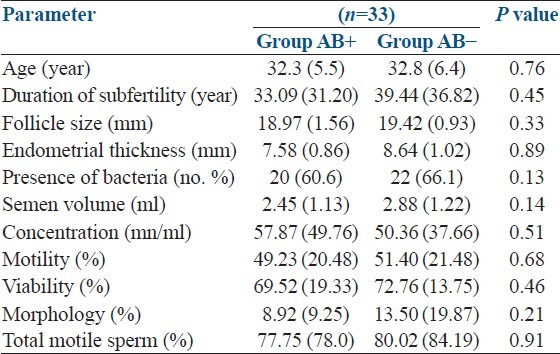
General characteristics of the study population were as follows. Age of the male partner 32.5 years (standard deviation [SD]: 5.8), female partner 28.3 years (SD: 4.9), duration of subfertility 35.4 months (SD: 23.2), size of the prominent follicle 19.19 mm (SD: 1.3), endometrial thickness 8.11 mm (SD: 0.91), semen volume 2.61 ml (SD: 1.17), sperm count 55.07 mn/ml (SD: 45.46), motility 50.04% (SD: 20.72) and normal morphology 10.61% (SD: 14.15). Comparison of observed parameters between two groups is summarized in Table 1. According to the results, all parameters are comparable at the initial level.
Table 1.
Comparison of initial characteristics between two groups (n=66)
Bacterial isolates were found in 42 (63.6%) samples. Types of bacteria, respective number and percentages of samples in which they grown were; Streptococcus spp. 24 (36.3%), coliforms, including Escherichia coli 22 (33.3%), diptheroids 10 (15.1%) Staphylococcus spp. 9 (13.6%), Neisseria spp. 3 (4.5%), and Acinetobacter spp. 1 (1.5%).
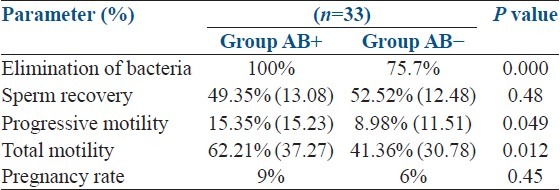
Results revealed that the total elimination of bacteria was possible with the addition of combination of penicillin and streptomycin (observed in AB+ group cultured after processing), but this was observed only in 25 (75.7%) samples processed with antibiotic free medium (group AB−). Sreptococcus spp. were the most abundant species found in post-wash samples (found in six samples, 18.1%) followed by coliforms (found in four samples, 12.1%), Neisseria spp. (found in two samples, 6.0%) and diptheroids (found in one sample 3.0%) respectively.
There was no difference of sperm recovery rates (harvesting active sperm from raw sample) between two groups 49.35% (SD: 13.08) versus 52.52% (SD: 12.48), P = 0.48. Post-wash motility (immediately after harvesting) also comparable between two groups, 98.5% (SD: 2.45) versus 97.8% (SD: 2.72). However, motile sperm counts were significantly reduced in antibiotic free group after incubating for 24 h in 5% CO2 at 37°C, whereas the progressive motility 15.35% (SD: 15.23) versus 8.98% (SD: 11.51) (P = 0.049) and total motility 62.21% (SD: 37.27) versus 41.36% (SD: 30.78) (P = 0.012) respectively, compared with the AB+ group. Pregnancy rate was 9% in AB+ group and 6% in AB− group, the difference was not statistically significantly (P = 0.48) [Table 2].
Table 2.
Benefits and risks in a clinical trial
DISCUSSION
Presence of bacterial flora (aerobic and anaerobic) in cultured semen samples from fertile and infertile males have been reported by many authors. Around 45-100% of samples from infertile males are positive for non-specific bacterial isolates.[9,10,11,12] In the present study, non-specific gram positive bacteria were isolated from 63% of samples. Majority of them are commensals of skin and mucous membranes, and few of them are rarely pathogenic. The cause-effect relationship between bacteriospermia and male infertility is still being debated. Some of the studies have reported lack of associations between bacteria and ejaculate quality[13] and fertilization rate.[9] Others argue that the simple presence of bacteria in semen samples may compromise the sperm quality even without leukospermia and clinical signs of infection.[14,15] The presence of bacteria in semen may be an early warning signal for altered reproductive potential of men.[5] The harmful effects of bacteria on spermatozoa may depend on the type and species of microorganisms invading, colonizing, or infecting the male genital tract.[16] Antibiotic treatment of leukocytospermic men without diagnosed genital tract infections reported a significant improvement of ejaculate quality.[17]
Contamination of the post-processed samples with bacteria is another aspect of clinical importance. Originating of bacteria could be from raw semen sample or occur accidentally during the collection process or from instruments in the laboratory. Significance of elimination of these bacteria has been questioned by some authors as lack of effects on fertilization or embryo quality in in vitro fertilization (IVF).[18] However, supportive evidence to the contrary is also available; e.g., Inoculation of E. coli caused adhesion to the sperm membrane and subsequent destruction leading to reducing motility and viability in washed samples.[19] Study with porcine semen has shown that E. coli caused a reduction of motility, sperm agglutination and spermicidal effects.[20] In IVF procedures, if embryo culture dishes were contaminated with bacteria the quality of the developing embryos was poor.[21] Since incubation temperature is a determining factor, incubation at 37°C could have a greater influence on bacterial growth and activity.
Semen extenders with single or combination of antibiotics such as, kanamycin, ampicillin, gentamicin, linomycin, penicillin streptomycin, terramycin, tylosin, linco spectin have been tried with different success rates in eliminating microbes in animal studies.[22] Penicillin streptomycin combination or gentamicin is abundantly used antibiotics in human semen culture media. We supplemented the recommended dose of solution for cell culture media (1% v/v containing 100 U/ml penicillin and 100 mcg/ml streptomycin) specified by the manufacturer. According to some authors the recommended dose for human semen is 100 IU penicillin and 50 mcg streptomycin.[7] The long term use of the same antibiotic may lead to emergence antibiotic resistant varieties. The resistance of E. coli to both penicillin and streptomycin used in culture media has been reported.[21] Cottell et al. could only eliminate 95% of bacteria from semen samples using penicillin streptomycin rich media with the swim-up procedure.[23] However, we were able to totally clear the non-specific bacteria from semen without affecting sperm motility, which is a prominent parameter predicting the sperm fertilization capacity.[1] Some laboratories are reluctant to use penicillin mainly because of its very short half-life.[21] Furthermore, penicillin allergy has been reported in few cases after IUI.[24] Hence, sensitivity checking before insemination or complete elimination of antibiotics at the final wash procedure should be employed as a remedy. Allergic reactions were not reported in our study as precautions were taken to remove antibiotics at the final wash.
There were no clinical signs of infections reported from women inseminated with samples processed with antibiotic free culture medium in this study. Lack of clinical symptoms among women received treatment with microbial positive post-wash samples reported by Karlström et al.[25] We cannot exclude the possibility of infection with contaminated samples. If occurred, the women's fertility would be further compromised.[26] An additional advantage observed with antibiotics was the ability to maintain the motility long time in vitro. This prolonged survivability can be mainly related to the absence of bacteria and their adverse effects. Similar behavior may me expected within the uterus as well. Because of the pregnancy rates in both groups are comparable between both groups, relating these beneficial effects on fertilization are difficult at this level. Though the two groups used were comparatively similar, smaller population size is hypothesized to produce inconsistent results. Number of studies has been done with animal semen regarding the effectiveness of antibiotics in clearing bacteria and toxicity effects. The extrapolation of those results to human semen is not valid. Additional research including a large cohort of patients would answer this question.
CONCLUSION
Despite special aseptic precautions carried out before ejaculation, bacteriospermia was found in 63% of samples. Penicillin streptomycin combination could completely eliminate bacteria from post-wash samples in this population. Addition of antibiotics seems to have no adverse effects on sperm motility, harvesting power or long term survival IVF. Laboratories should maintain high standards of hygienic conditions when collecting and preparing the samples. In addition, use of effective antibiotic or cocktails of antibiotics in sperm preparation for IUI may reduce the risk of infection and sequelae in women. Individual laboratories should determine the most suitable antibiotic/s and dosage depending on their experience on the type of bacteria grown and antibiotics sensitivity pattern.
ACKNOWLEDGEMENT
Financial support by National Research Council of Sri Lanka under the grant No. 09/69 is acknowledged.
Footnotes
Source of Support: Financial support by National Research Council of Sri Lanka under the grant No. 09/69 is acknowledged
Conflict of Interest: None declared.
REFERENCES
- 1.Merviel P, Heraud MH, Grenier N, Lourdel E, Sanguinet P, Copin H. Predictive factors for pregnancy after intrauterine insemination (IUI): An analysis of 1038 cycles and a review of the literature. Fertil Steril. 2010;93:79–88. doi: 10.1016/j.fertnstert.2008.09.058. [DOI] [PubMed] [Google Scholar]
- 2.Mangoli V, Dandekar S, Desai S, Mangoli R. The outcome of ART in males with impaired spermatogenesis. J Hum Reprod Sci. 2008;1:73–6. doi: 10.4103/0974-1208.44114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abeysundara PK, Dissanayake D, Wijesinghe PS, Perera R, Nishad A. Efficacy of two sperm preparation techniques in reducing non-specific bacterial species from human semen. J Hum Reprod Sci. 2013;6:152–7. doi: 10.4103/0974-1208.117169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nicholson CM, Abramsson L, Holm SE, Bjurulf E. Bacterial contamination and sperm recovery after semen preparation by density gradient centrifugation using silane-coated silica particles at different g forces. Hum Reprod. 2000;15:662–6. doi: 10.1093/humrep/15.3.662. [DOI] [PubMed] [Google Scholar]
- 5.Fourie J, Loskutoff N, Huyser C. Elimination of bacteria from human semen during sperm preparation using density gradient centrifugation with a novel tube insert. Andrologia. 2012;44(Suppl 1):513–7. doi: 10.1111/j.1439-0272.2011.01217.x. [DOI] [PubMed] [Google Scholar]
- 6.Kaneko S, Oshio S, Kobanawa K, Kobayashi T, Mohri H, Iizuka R. Purification of human sperm by a discontinuous Percoll density gradient with an innercolumn. Biol Reprod. 1986;35:1059–63. doi: 10.1095/biolreprod35.4.1059. [DOI] [PubMed] [Google Scholar]
- 7.Magli MC, Gianaroli L, Fiorentino A, Ferraretti AP, Fortini D, Panzella S. Improved cleavage rate of human embryos cultured in antibiotic-free medium. Hum Reprod. 1996;11:1520–4. doi: 10.1093/oxfordjournals.humrep.a019430. [DOI] [PubMed] [Google Scholar]
- 8.World Health Organization. Laboratory Manual for the Examination and Processing of Human Semen. 5th ed. Cambridge: Cambridge University Press; 2010. [Google Scholar]
- 9.Liversedge NH, Jenkins JM, Keay SD, McLaughlin EA, Al-Sufyan H, Maile LA, et al. Antibiotic treatment based on seminal cultures from asymptomatic male partners in in-vitro fertilization is unnecessary and may be detrimental. Hum Reprod. 1996;11:1227–31. doi: 10.1093/oxfordjournals.humrep.a019361. [DOI] [PubMed] [Google Scholar]
- 10.Mogra N, Dhruva A, Kothari LK. Non-specific seminal tract infection and male infertility: A bacteriological study. J Postgrad Med. 1981;27:99–104. [PubMed] [Google Scholar]
- 11.Onemu SO, Ibeh IN. Studies on the significance of positive bacterial semen cultures in male fertility in Nigeria. Int J Fertil Womens Med. 2001;46:210–4. [PubMed] [Google Scholar]
- 12.Gregoriou O, Botsis D, Papadias K, Kassanos D, Liapis A, Zourlas PA. Culture of seminal fluid in infertile men and relationship to semen evaluation. Int J Gynaecol Obstet. 1989;28:149–53. doi: 10.1016/0020-7292(89)90475-x. [DOI] [PubMed] [Google Scholar]
- 13.Gdoura R, Kchaou W, Ammar-Keskes L, Chakroun N, Sellemi A, Znazen A, et al. Assessment of Chlamydia trachomatis, Ureaplasma urealyticum, Ureaplasma parvum, Mycoplasma hominis, and Mycoplasma genitalium in semen and first void urine specimens of asymptomatic male partners of infertile couples. J Androl. 2008;29:198–206. doi: 10.2164/jandrol.107.003566. [DOI] [PubMed] [Google Scholar]
- 14.Moretti E, Capitani S, Figura N, Pammolli A, Federico MG, Giannerini V, et al. The presence of bacteria species in semen and sperm quality. J Assist Reprod Genet. 2009;26:47–56. doi: 10.1007/s10815-008-9283-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lackner JE, Herwig R, Schmidbauer J, Schatzl G, Kratzik C, Marberger M. Correlation of leukocytospermia with clinical infection and the positive effect of antiinflammatory treatment on semen quality. Fertil Steril. 2006;86:601–5. doi: 10.1016/j.fertnstert.2006.01.032. [DOI] [PubMed] [Google Scholar]
- 16.Fraczek M, Szumala-Kakol A, Jedrzejczak P, Kamieniczna M, Kurpisz M. Bacteria trigger oxygen radical release and sperm lipid peroxidation in in vitro model of semen inflammation. Fertil Steril. 2007;88:1076–85. doi: 10.1016/j.fertnstert.2006.12.025. [DOI] [PubMed] [Google Scholar]
- 17.Skau PA, Folstad I. Do bacterial infections cause reduced ejaculate quality. A meta-analysis of antibiotic treatment of male infertility? Behav Ecol. 2003;14:40–7. [Google Scholar]
- 18.Krissi H, Orvieto R, Ashkenazi J, Gilboa Y, Shalev J, Moscovitch I, et al. Effect of contaminated preprocessed semen on fertilization rate and embryo quality in assisted reproductive techniques. Gynecol Endocrinol. 2004;18:63–7. doi: 10.1080/09513590310001651821. [DOI] [PubMed] [Google Scholar]
- 19.Kala S, Singh A, Prabha V, Singh R, Sharma P. Escherichia coli attaches to human spermatozoa: Affecting sperm parameters. Arch Appl Sci Res. 2011;3:618–23. [Google Scholar]
- 20.So KM, Sa SJ, Jin Kim H, Chung KH, Jung BY, Son JH, et al. Effects of Escherichia coli contamination on extended porcine semen parameters. Reprod Dev Biol. 2011;35:479–83. [Google Scholar]
- 21.Kastrop PM, de Graaf-Miltenburg LA, Gutknecht DR, Weima SM. Microbial contamination of embryo cultures in an ART laboratory: Sources and management. Hum Reprod. 2007;22:2243–8. doi: 10.1093/humrep/dem165. [DOI] [PubMed] [Google Scholar]
- 22.Hasan S, Andrabi SM, Muneer R, Anzar M, Ahmad N. Effects of a new antibiotic combination on post-thaw motion characteristics and membrane integrity of buffalo and sahiwal bull spermatozoa and on the bacteriological quality of their semen. Pak Vet J. 2001;21:6–12. [Google Scholar]
- 23.Cottell E, Lennon B, McMorrow J, Barry-Kinsella C, Harrison RF. Processing of semen in an antibiotic-rich culture medium to minimize microbial presence during in vitro fertilization. Fertil Steril. 1997;67:98–103. doi: 10.1016/s0015-0282(97)81863-8. [DOI] [PubMed] [Google Scholar]
- 24.Smith YR, Hurd WW, Menge AC, Sanders GM, Ansbacher R, Randolph JF., Jr Allergic reactions to penicillin during in vitro fertilization and intrauterine insemination. Fertil Steril. 1992;58:847–9. doi: 10.1016/s0015-0282(16)55343-6. [DOI] [PubMed] [Google Scholar]
- 25.Karlström PO, Hjelm E, Lundkvist O. Comparison of the ability of two sperm preparation techniques to remove microbes. Hum Reprod. 1991;6:386–9. doi: 10.1093/oxfordjournals.humrep.a137346. [DOI] [PubMed] [Google Scholar]
- 26.Sanam M. A ruptured tubo-ovarian abscess after intrauterine insemination; a case report. Iran J Reprod Med. 2009;7:41–3. [Google Scholar]


