Abstract
Primary amenorrhea is defined as the absence of menarche by 16-18 years of age in the presence of well-developed secondary sexual characters. An incidence of 1-3% has been reported in women of reproductive age group. The etiology varies with anatomical, genetic and hormonal factors implicated in the causation of primary amenorrhea. We present a case of absent endometrium due to balanced reciprocal translocation (RCPTR), 46 XX t (4;20)(q12;q13.1) as primary amenorrhea.
KEY WORDS: Amenorrhea, infertility, translocation
INTRODUCTION
The association of primary amenorrhea with chromosomal translocations has long been observed. The usual translocations reported are between X chromosome and an autosome. The most frequently involved autosomes are 4, 6, 9, 12, 15 and 18.[1] We present a case with unique balanced autosomal translocation of t (4;20)(q12;q13.1).
CASE REPORT
A 21-year-old female patient reported to infertility clinic with an inability to conceive for 2 years. On detailed history taking, it was elicited that she had primary amenorrhea. There was no history of any cyclical symptoms or any chronic medical or surgical illness or consanguinity. She had already received progesterone with no withdrawal bleeding. Menstrual cycles in mother and sister were normal. Parental reproductive history showed no family history of abortions, bad obstetrics history or children with mental retardation. On examination, general condition was fair and intelligence was normal. Her height was 158 cm and weight 54 kg. She had well-developed secondary sexual characters (breast and pubic hairs belonged to tanner stage IV). Speculum examination revealed a normal cervix; vaginal examination revealed a normal size, retroverted uterus.
Her hormonal profile showed normal parameters (folllicle stimulating hormone = 4.4 mIU/ml, luteinizing hormone = 3.78 mIU/ml, thyroid stimulating hormone = 4.78 μIU/ml, prolactin = 21.5 ng/ml, testosterone = 0.12 ng/dl, anti mullerian hormone = 2.88 ng/ml). Her chest X-Ray was normal. Her pelvic ultrasonography showed uterus size of 6.5 × 5 × 2.7 cm with inconspicuous endometrium. Both ovaries were normal. Hysteroscopy evaluation of the uterine cavity revealed uterocervical length of 6.25 cm with smooth uterine cavity. Endometrial curettage obtained no endometrial tissue. Endometrial aspirate for acid fast bacilli culture was negative. Chromosomal analysis was conducted on heparinized whole blood by 72 h stimulated cultures with appropriate serum and antibiotics. RPMI − 1640 was used as the medium with banding resolution of 450-550. Chromosomal preparations were subjected to GTG-banding and karyotyped according to ISCN 2005 (G bands by trypsin and Giemsa). A karyotype of 46 XX t (4;20)(q12;q13.1) was revealed [Figure 1]. The patient was administered oral conjugate estrogen tablets (0.625 mg thrice day) for 3 months, but there was no increase in endometrium thickness on ultrasonographic examination during treatment and there was no vaginal bleeding. Thus, it was diagnosed as absent endometrium due to balanced translocation of chromosome 4 and 20 causing primary amenorrhea. Counseling was done for her reproductive potential and adoption was advised.
Figure 1.
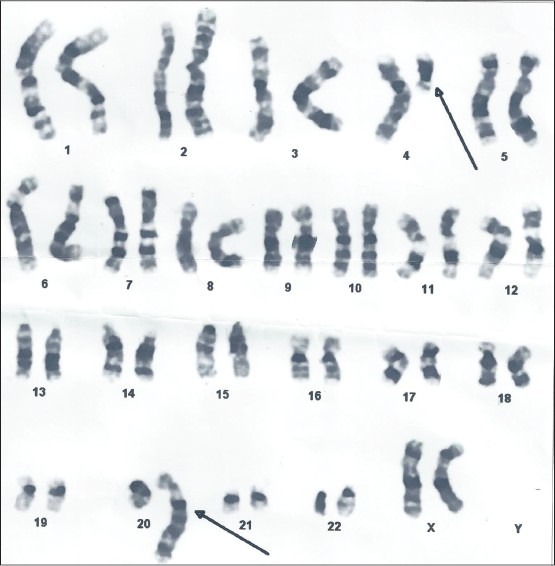
Karyogram of the patient showing the 46 XX t(4;20)(q12;q13.1)
DISCUSSION
The most common causes of primary amenorrhea are ovarian failure, congenital absence of uterus, gonadotropin-releasing hormone (GnRH) deficiency and constitutional delay in puberty.[2] The chromosomal aberrations in case of primary amenorrhea were reported in 20.7% cases in Indian subcontinent and autosomal structural abnormalities were found in only 1% of these cases.[3]
It is estimated that every 1 in 200 live born infants suffers from chromosomal anomalies; amongst these, the incidence of RCPTR is reportedly 1 in 500 live births. RCPTRs result when two non-homologous chromosomes exchange genetic material. The breakpoint of a translocation is where the two chromosomes are joined. A person who is heterozygous for a RCPTR will appear normal phenotypically because although there is a re-arrangement of genes, the genetic pool remains intact. It is only when gametes undergo meiosis that problems arise. RCPTR can occur de-novo or be genetically transmitted from parental carriers. The manifestations of RCPTR might range from none at all, to different degrees of mental retardation. Rarely, there may be associated amenorrhea. It has been reported that the percentage of chromosomal abnormalities varies from 20 to 31 in patients with primary amenorrhea.[4] Importantly, most of the studies in primary amenorrhea patients have observed the involvement of sex chromosomes. Translocation between X and autosome have been reported as sporadic findings.[1] This area still needs to be investigated. Primary amenorrhea has also been reported in women with balanced autosomal translocations and double Robertsonian translocations. Incidence of 1.4% was reported in women presenting as primary amenorrhea.[5] It is hypothesized that amenorrhea is a result of change in the pattern of genes located at the breakpoint area in balanced translocations.[6] Congenital absence of the endometrium without chromosomal aberration has been reported once.[7]
To the best of our knowledge, the association of balanced translocation of chromosome 4;20 with absent endometrium presenting as primary amenorrhea has not been reported in the literature. We reiterate that all such novel cases be reported in order to enable us attain better insight into the intricacies involving chromosomal abnormalities.
CONCLUSION
The most common causes of primary amenorrhea are ovarian failure, congenital absence of uterus, GnRH deficiency and constitutional delay in puberty. In the presence of secondary sexual characters and normal genital tract evaluation on ultrasonography, hysteroscopy and estrogen therapy must be given before labeling it as a case of end organ failure or absent endometrium even in the presence of chromosomal anomalies.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Tullu MS, Arora P, Parmar RC, Muranjan MN, Bharucha BA. Ovarian dysgenesis with balanced autosomal translocation. J Postgrad Med. 2001;47:113–5. [PubMed] [Google Scholar]
- 2.Timmreck LS, Reindollar RH. Contemporary issues in primary amenorrhea. Obstet Gynecol Clin North Am. 2003;30:287–302. doi: 10.1016/s0889-8545(03)00027-5. [DOI] [PubMed] [Google Scholar]
- 3.Dutta UR, Ponnala R, Pidugu VK, Dalal AB. Chromosomal abnormalities in amenorrhea: A retrospective study and review of 637 patients in South India. Arch Iran Med. 2013;16:267–70. [PubMed] [Google Scholar]
- 4.Laxmi KV, Babu SJ, Dayakar S, Mehrothra RN, Goud KI. Cytogenetic investigation of patients with primary amenorrhea. Indian J Hum Genet. 2012;18:112–6. doi: 10.4103/0971-6866.96676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kopakka N, Dalvi R, Shetty DL, Das BR, Mandava S. Balanced autosomal translocation and double Robertsonian translocation in cases of primary amenorrhea in an Indian population. Int J Gynaecol Obstet. 2012;116:253–7. doi: 10.1016/j.ijgo.2011.09.029. [DOI] [PubMed] [Google Scholar]
- 6.Karakus N, Kara N, Tural S, Kocak I, Elbistan M. A retrospective study of balanced chromosomal translocations in a Turkish population. Int J Hum Genet. 2012;12:319–23. [Google Scholar]
- 7.Berker B, Taşkin S, Taşkin EA. Absence of endometrium as a cause of primary amenorrhea. Fertil Steril. 2008;89:723e.1–3. doi: 10.1016/j.fertnstert.2007.03.076. [DOI] [PubMed] [Google Scholar]


