Conspectus
Despite 70 years of clinical use, β-lactam antibiotics still remain at the forefront of antimicrobial chemotherapy. The major challenge to these life-saving therapeutics is the presence of bacterial enzymes (i.e., β-lactamases) that can hydrolyze the β-lactam bond and inactivate the antibiotic. These enzymes can be grouped into 4 classes (A-D). Among the most genetically diverse are the class D β-lactamases. In this class are β-lactamases that can inactivate the entire spectrum of β- lactam antibiotics (penicillins, cephalosporins, and carbapenems).
Class D β-lactamases are mostly found in Gram-negative bacteria such as Pseudomonas aeruginosa, Escherichia coli, Proteus mirabilis, and Acinetobacter baumannii. The active-sites of class D β-lactamases contain an unusual N-carboxylated lysine post-translational modification. A strongly hydrophobic active-site helps create the conditions that allow the lysine to combine with CO2, and the resulting carbamate is stabilized by a number of hydrogen bonds. The carboxy-lysine plays a symmetric role in the reaction, serving as a general base to activate the serine nucleophile in the acylation reaction, and the deacylating water in the second step.
There are more than 250 class D β-lactamases described, and the full set of variants shows remarkable diversity with regard to substrate binding and turnover. Narrow-spectrum variants are most effective against the earliest generation penicillins and cephalosporins such as ampicillin and cephalothin. Extended-spectrum variants (also known as extended-spectrum β-lactamases, ESBLs) pose a more dangerous clinical threat as they possess a small number of substitutions that allow them to bind and hydrolyze later generation cephalosporins that contain bulkier side-chain constituents (eg. cefotaxime, ceftazidime, and cefepime). Mutations that permit this versatility seem to cluster in the area surrounding an active-site tryptophan resulting in a widened active-site to accommodate the oxyimino side-chains of these cephalosporins. More concerning are the class D β-lactamases that hydrolyze clinically-important carbapenem β-lactam drugs (e.g., imipenem). Whereas carbapenems irreversibly acylate and inhibit narrow- spectrum β-lactamases, class D carbapenemases are able to recruit and activate a deacylating water. The rotational orientation of the C6 hydroxyethyl group found on all carbapenem antibiotics likely plays a role in whether the deacylating water is effective or not.
Inhibition of class D β-lactamases is a current challenge. Commercially available inhibitors that are active against other classes of β-lactamases are ineffective against class D enzymes. On the horizon are several compounds, consisting of both β-lactam derivatives and non-β-lactams, that have the potential of providing novel leads to design new mechanism-based inactivators that are effective against the class D enzymes. Several act synergistically when given in combination with a β-lactam antibiotic, and others show a unique mechanism of inhibition that is distinct from the traditional β-lactamase inhibitors. These studies will bolster structure-based inhibitor design efforts to facilitate the optimization and development of these compounds as class D inactivators.
Introduction
The introduction of β-lactam antibiotics into clinical medicine has had a profound impact on our civilization. As a therapeutic category of drugs, β-lactams have increased our life expectancy by almost 10 years1. Each new class has addressed a significant clinical challenge in the surgical and medical treatment of adults and children. Regrettably, bacteria have acquired mechanisms that allow them to fend off these life-saving drugs. Perhaps the most important resistance mechanism involves acquisition of β-lactamases (EC 3.5.2.6), drug-inactivating enzymes that have seriously challenged the therapeutic efficacy of each generation of novel β-lactams. In response to multiple drug modification strategies, β-lactamases have altered their substrate spectrum by introducing amino acid changes in or near the active site that alter the kinetic profile to confer resistance to novel β-lactams. This evolutionary strategy has been coupled with the introduction of novel genetic elements that increase production and regulate synthesis of these enzymes2.
Presently, the medicinal chemist is challenged by the diverse β-lactamases that are present in nature (>1400 unique enzymes). Of the four distinct structural classes (designated A-D), three (A, C, and D) proceed by a serine nucleophile based hydrolysis. Class B enzymes hydrolyze β-lactams by employing 1 or 2 Zn+2 ions as part of the catalytic machinery. In keeping with their status as a family of proteins subject to direct drug selection, β-lactamases have altered their kinetic characteristics such that they can hydrolyze cephalosporins and carbapenems, the most potent β-lactam therapeutic agents.
Members of the class D β-lactamase sub-family are named using the “OXA” nomenclature (ie. OXA-1, OXA-2, etc.). This convention arose when it was observed that enzymes of this class were shown to possess strong hydrolytic activity against the semi-synthetic penicillin oxacillin. As the sub-family grew, it was discovered that many class D enzymes are active against cephalosporins, β-lactam/β-lactamase inhibitor combinations, and carbapenems as well. Unfortunately class D β-lactamases are often found among the most clinically challenging species including Acinetobacter baumannii, Pseudomonas aeruginosa, Escherichia coli andProteus mirabilis. In Acinetobacter spp. certain class D β-lactamases (ie. OXA-23, -24, -58, -143) confer resistance to carbapenems while in P. aeruginosa, other class D enzymes (ie. OXA-14, -28, -35) render resistance to extended-spectrum cephalosporins. Moreover, OXA-23 and OXA-24/40 were largely responsible for carbapenem resistance in isolates that infected members of the US military in Iraq and Afghanistan3. Recently, an OXA carbapenemase believed to originate in Turkey (OXA-48) has been reported in Klebsiella pneumonia, has caused considerable mortality in Europe4 and is now in the US5.
The > 250 members of the class D β-lactamase family are highly diverse in sequence, and display very low levels of homology with the enzymes of class A and C6. Despite this, crystal structures reveal that the topological fold is conserved among the three classes, and highly so within class D7. This fold is composed of two domains: one a fairly flat central β-sheet surrounded by α-helices, and another all-helical domain8-12. Interestingly, this basic fold is also found in a several other families of proteins, including β-lactam sensors13, transpeptidases6 and serine proteases such as chymotrypsin. The sensors bind and covalently link to β-lactam antibiotics, but in line with their role as signal transducers, the complexes enjoy longevity that lasts longer than the duration of a bacterial generation14.
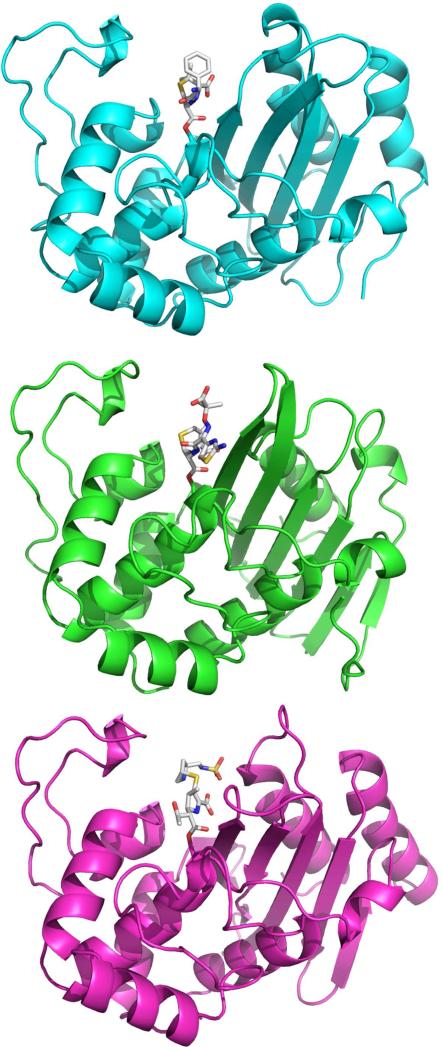
The X–ray structures of OXA-10, OXA-24/40 and the sensor protein BlaR1 (a structurally related sensor/signal transducer protein of Staphylococcus aureus) each with a β-lactam bound in the active-site, are shown in Figure 1. The deep active-site, found at the interface of the two domains, is formed mainly from a short 310 helix, a loop between α-helices α4 and α5 (115-117; all residue numbers correspond to OXA-10 unless otherwise indicated), and β-strand β5. A wall of the active-site, also observed in class A and class C β-lactamases, is made from a loop of variable length commonly referred to as the “omega loop”. Not surprisingly, those residues that are highly conserved in class D (ie. S67, K70, S115, V117, W154, L155, K205, G207), and those few that are ubiquitous in all class A, C and D enzymes, are found amongst these structural elements.
Figure 1.
Comparison of the overall topology of a narrow spectrum class D β-lactamase (OXA-10) with the penicillin ampicillin forming an acyl-enzyme intermediate (cyan; PDB 2WKH), a class D carbapenemase (OXA-24/40) acylated with the carbapenem doripenem (green; PDB 3PAE) and one of the related β-lactamase sensors (BlaR1) acylated with the cephalosporin ceftazidime (magenta; PDB 1HKZ). In each case, the drug is found near the interface of an all helical domain (left) and a mixed α/β domain (right).
In contrast to the exclusively monomeric class A and C β-lactamases, class D enzymes can be either monomeric or dimeric. Extensive structural analysis of dimeric enzymes such as OXA-10, OXA-46 and OXA-48 show a similar mode of dimerization mediated by α9 and β49,10. OXA-1 and OXA-24/40, which are members of distinct subfamilies in the class D group, are shown to be monomeric8,11 .
Active-site chemistry and the catalytic mechanism
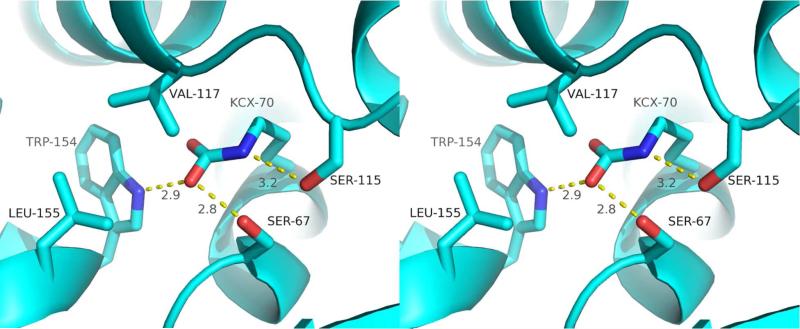
Key active-site residues of a typical class D β-lactamase (OXA-10) are shown in Figure 2. Class D β-lactamases are much more hydrophobic than those of class A and C; most notably, the nearly ubiquitous asparagine in the latter two classes (N132 in TEM-1 and N152 in AmpC) is replaced by valine (V117), isoleucine or (rarely) leucine in class D15. Other examples of highly conserved non-polar residues are W102, Y/F141, W154 and L/I155. Two other positions that are often hydrophobic (Y112 and M223 in OXA-24/40) have been shown to reside on the opposing rims of the active-site, or to extend across it forming a hydrophobic bridge8.
Figure 2.
An expanded view of the active-site of OXA-10 (PDB 1E4D).
The hydrophobic nature of class D β-lactamases is necessary to promote the formation of an unusual post-translational modification: the carboxylation of an active-site lysine to form a carbamate functional group9. Carbamates are known to bind and orient metal ions16, but in rare cases, they are implicated in substrate binding (alanine racemase)17 or directly in catalysis (ribulose-5-phosphate carboxylase)18. Lysine carboxylation was first observed in the crystal structure of OXA-109, and has since been observed in OXA-1, OXA-24/40, OXA-46 and OXA-48 structures11,12,19,20. BlaR1, though not a β-lactamase itself, conserves most of the carbamate- stabilizing active-site residues, and indeed was shown to possess a carboxylated lysine in a recent crystal structure21. Interestingly, it has been shown that β-lactam acylation of BlaR1 leads to lysine decarboxylation, thus removing the general base required for hydrolytic deacylation and allowing to the long-lived acyl-species necessary for β-lactam-sensing signal transduction22.
The hydrophobic residues of class D β-lactamase active-sites (most notably V117) presumably lower the pKa of the lysine enough to allow the deprotonation that is necessary for the attack on CO2. The carbamate moiety is stabilized by hydrogen bonds from the side-chains of W154 and the serine that serves as the attacking nucleophile, S67. Residues that differ throughout the class (e.g. S120 in OXA-1, H78 in OXA-46), also contribute hydrogen bonds or salt bridges. A second active-site serine (S115) hydrogen bonds with the carbamate nitrogen, further stabilizing and orienting the functional group. Unstabilized carbamates can easily decompose into CO2 and a free amine, but these active-site interactions push the equilibrium toward stable carboxylation (Kd values of 1-10 QM)23-25. With such low Kdvalues, class D β-lactamases are expected to be fully carboxylated under physiological conditions (~ pH 7 and > 15 mM bicarbonate). Not surprisingly, the pH of crystallization conditions can affect the degree of carboxylation, with little or no carbamate observed at pH < 6, partial occupancy observed from pH 6-7 and full carboxylation observed at more alkaline pH values8,24,26.
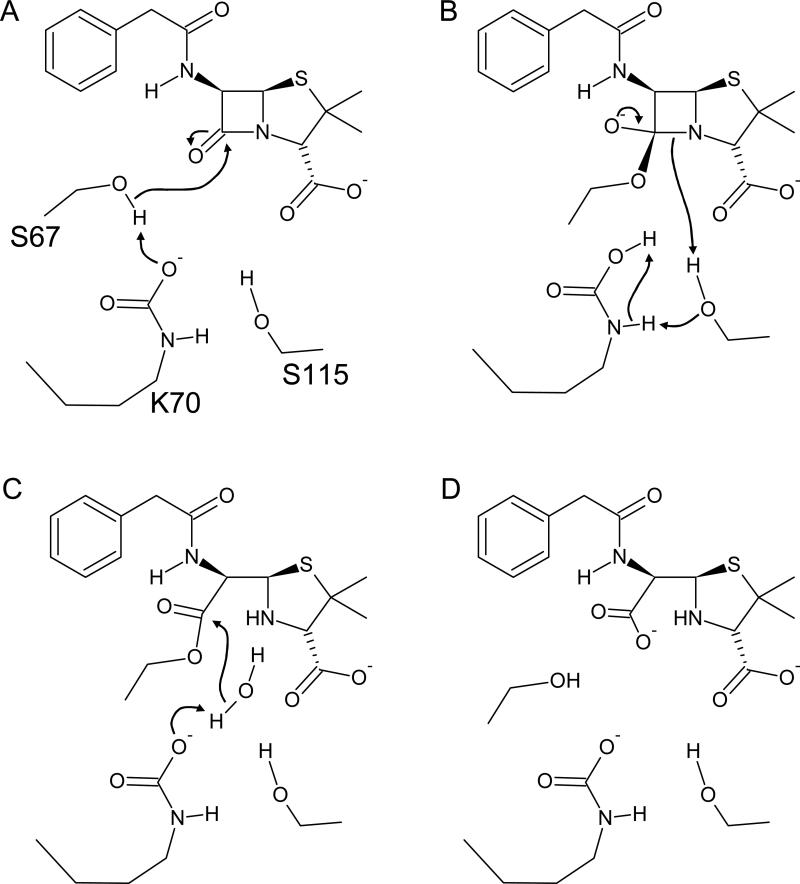
Class D enzymes use a covalent catalysis mechanism reminiscent of that seen with classic serine proteases such as chymotrypsin. In the first step, a serine nucleophile attacks the lactam carbonyl yielding a covalent acyl-intermediate (Figure 3a). The tetrahedral oxyanion transition state (Figure 3b) for this acylation reaction is stabilized by two main-chain amides (S67 and F/A/W208) and electrostatically by a second active-site lysine (K205). The ester linkage is then hydrolyzed by an active-site water (Figure 3c) allowing the release of the inactivated β-lactam (Figure 3d). Studies with hydroxyalkyl penicillinate inhibitors revealed that this deacylating water attacks the α face of the ester carbonyl of the acyl intermediate (ie. the side facing the reader in Figure 3)27,28. This matches the direction of water attack in class A β-lactamases, and contrasts with the β face attack in class C enzymes7.
Figure 3.
A proposed mechanism for class D β-lactamases. The initial acylation step is aided by carboxy-lysine-mediated deprotonation of S67. The carbamate also activates the deacylating water in the second step.
The discovery of the active-site carboxy-lysine suggests that it participates in catalysis as a general base, activating both the serine acylation nucleophile and later the deacylating water24,28. This proposal was supported by the observation that one of the carbamate oxygens overlays closely with the general base carboxylate (E166) of TEM-1, a class A β-lactamase29. Secondly, β-lactamase activity can be eliminated by exposure to conditions that favor decarboxylation (low pH), and subsequently restored by the addition of bicarbonate23,24. Mutation of the hydrophobic residues surrounding K70, most notably V117 and W154, also leads to decarboxylation and loss of activity30,31. Substitutions for K70 itself have been shown to greatly slow down acylation rates and nearly eliminate deacylation altogether32,33. Thus, as with mutation of the general base of class A (E166)34 and class C (Y150)35, K70 substitution yields an enzyme that arrests at the acyl-intermediate stage25,32.
The symmetric role of carboxy-K70 acting as a general base in both acylation and deacylation requires a mechanism by which the protonated carbamate can be restored to basicity. The simplest scenario would be to have the proton transferred to the leaving group (the β-lactam nitrogen in acylation and the S67 alcohol in deacylation) via S11511,36,37. The positive charge of a second active-site lysine (K205) may encourage the role of S115 as an intermediary in a proton relay10. While S115 is almost always found within hydrogen bonding distance of S67, most structures do not place it close enough to hydrogen bond with either of the oxygen atoms of carboxy-K70. S115 is hydrogen bonded to the carbamate nitrogen, however, and it was proposed that its hydroxyl may transfer a proton via that group11.
As noted above, the “OXA” name used for all class D β-lactamases recalls that many of the early identified β-lactamases efficiently hydrolyzed oxacillin, a semi-synthetic isoxazolyl pencillin. As more class members were identified by the presence of common sequence motifs, it became clear that efficient oxacillinase activity is not a defining property of the class. The >250 currently identified class D enzymes are classified as narrow-spectrum, ESBL (extended- spectrum β-lactamase), or CHDL (carbapenem-hydrolyzing class D β-lactamase)15,38,39. While there has been a great number of both ESBL and CHDL enzymes discovered, a class D enzyme has not yet been found that possesses both ESBL and carbapenemase activities15.
The reason for the strong preference by class D β-lactamases for oxacillin is a matter of interest. If the core structure of oxacillin binds in a manner similar to that seen for ampicillin and benzylpenicillin in OXA-1025, oxacillin's hydrophobic bicyclic side chain would likely present itself to a nonpolar patch composed of F208 and G210. In OXA-46 and OXA-48, two other proficient oxacillinases, this patch is composed of W213 and G215 in the former and Y211 and T213 in the latter. In OXA-1, the sequentially-unrelated F217 and L255 occupy the same space. As first noted in the structure of OXA-24/40 and later expanded in the analysis of OXA-48, the loop containing these non-polar residues in narrow spectrum enzymes does not extend very far into the active-site, leaving plenty of room for the bulky isoxazolyl ring8,12. In OXA-24/40 however, this loop (and the M223 side-chain emanating from it) extends so far over the top of the active-site as to form an “occluding bridge”, thereby providing an explanation for the weak binding affinity of oxacillin8,31.
Extended-spectrum activity in class D typically arises from a small number of point mutations occurring in the background of a parental narrow-spectrum enzyme. OXA-10, for instance, has given rise to approximately 10 enzymes that confer resistance to one or more advanced-generation cephalosporins15. Paetzel, et al. note that many point mutations common to OXA-10 ESBLs (G157D, N73S/T, A124T) are near the carbamate-stabilizing tryptophan W15410. Indeed, mutation of this tryptophan, or the leucine next to it (L155), leads to robust ceftazidime hydrolysis activity40-42. Mutation of W154 leads to increased “omega loop” flexibility and a more open active site30 which suggests a reason for increased activity against the bulky ceftazidime. The ceftazidime-hydrolyzing variant OXA-145 is derived from the deletion of L165 (homologous to L155 in OXA-10) from an otherwise narrow-spectrum OXA-3540. A recent crystal structure of OXA-145 shows that the loss of L165 also results in an expanded active-site cavity43.
Most of the originally identified class D β-lactamases were incapable of hydrolyzing clinically important carbapenems such as impenem and meropenem. As with class A and class C enzymes, carbapenems arrest as acyl-enzyme intermediates in OXA-1 and OXA-10. The structure of OXA-1 with doripenem bound suggests an intriguing mechanism of inhibition: the 6α-hydroxyethyl moiety adopts a rotational position that allows a hydrogen bond between its alcohol and the carbamate general base of carboxy-K7044. The lack of any water near the acyl- carbonyl indicates that this hydrogen bond prevents the hydrolytic release of product. Another earlier proposed inhibition mechanism posited that the carbapenem undergoes a post-acylation tautomerization event and adopts a new conformation that is incapable of undergoing hydrolysis45. This tautomerization event has been observed in some class A enzyme/carbapenem complexes46-48, as well as the OXA-1/doripenem structure44.
The emergence of class D β-lactamases that can hydrolyze carbapenems began in 1995 with the identification of OXA-23 (also known as ARI-1) by Amyes and colleagues49. Since that time, > 75 novel variants that confer carbapenem resistance can be grouped into five sequence- based sub-families: OXA-23, OXA-24/40, OXA-48, OXA-51 and OXA-5815. In addition to the serious clinical threat posed by these enzymes, interest in how class D active-sites adapt to deacylate carbapenems is the subject of much scrutiny. The structures of OXA-24/40 and OXA- 48 showed that different sub-families achieve that feat by different mechanisms. Santillana, et al. suggested that the same hydrophobic bridge that prevents binding of oxacillin to OXA-24/40, also anchors the side-chain of meropenem through non-polar interactions8. While this enzyme has relatively modest kcat values (< 1 s-1) for carbapenems, the extraordinarily low KM (< 20 nM) leads to clinically-relevant resistance levels8. In a telling case report, an A. baumannii strain became more resistant to meropenem as its OXA-164 CHDL mutated to create a larger non-polar bridge (L114F)50. The active-site of OXA-48, on the other hand, is much more “open”, with no occluding bridge over the top12. Docquier et. al. suggested that differences in the positioning of a highly-conserved leucine near the β5-β6 loop (L158 in OXA-48) may cause the carbapenem hydroxyethyl group to adopt different rotamer positions in CHDL versus narrow-spectrum enzymes12. If the alcohol of that group rotated away from the position observed in the OXA-1/doripenem structure, it could leave space for the deacylating water to enter12.
The first structure of a carbapenem bound to a class D carbapenemase (OXA-24/40/doripenem) confirms these predictions. The pyrrolidine ring of the doripenem side chain does indeed interact with the hydrophobic bridge, although the majority of the van der Waals contact area is made with Y112 rather than M22331. Schneider, et al. also note that the alcohol of doripenem's hydroxyethyl group points away from the carbamate of carboxy-K70, likely allowing it to bind and activate the deacylating water. More recently, Docquier, et al. confirmed the importance of the β5-β6 loop region through the construction of hybrid enzymes51. The transfer of the ten-residue loop from the CHDL enzymes OXA-48 or OXA-23 into the narrow spectrum OXA-10, conferred carbapenemase activity on the latter.
In general, class D β-lactamases are not inhibited by commercially available β-lactam-based inhibitors like clavulanic acid, tazobactam and sulbactam52,53. However, a few exceptions are observed. OXA-18 and OXA-24 are both inhibited by clavulanic acid54,55, and OXA-29 is inhibited by tazobactam, but not clavulanic acid56. The Carey group investigated the mechanistic reasons for the poor inhibition of OXA-10 by tazobactam and sulbactam using Raman crystallography and compared these results with those observed for class A β-lactamases, which are inhibited by these compounds57. Similar to the class A enzymes, OXA-10 forms an initial imine species upon acylation. However, the data suggest that this imine form in OXA-10 undergoes rapid deacylation and regenerates active enzyme, in contrast to formation of the key cis and/or trans enamine species that leads to inhibition of the class A enzymes57.
Prospects for drug development
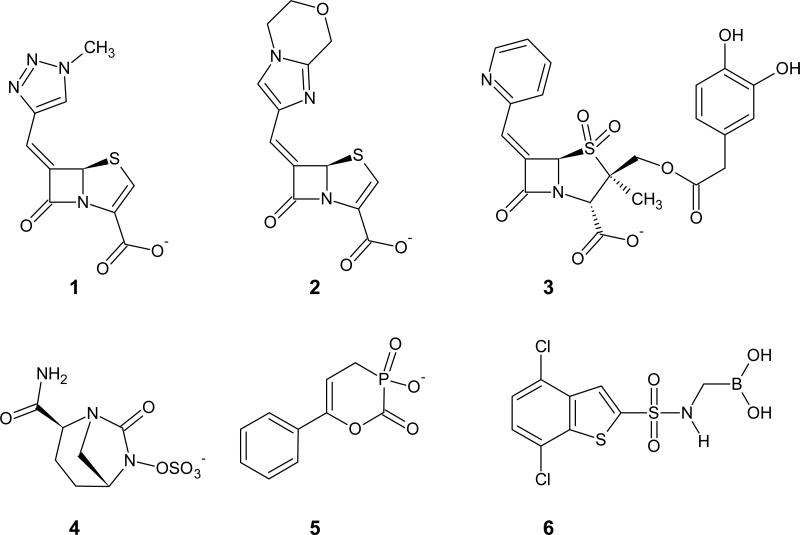
Inhibition of class D β-lactamases is an area of intense research. Whereas lead compounds that inhibit other classes of β-lactamases have been identified, few of them exhibit activity against the class D enzymes2,52,53. With extensive sequence and structural variability found within the class D β-lactamases, the discovery and design of inhibitors that act on all class D enzymes is a challenge. Figure 4 shows representative examples of the different classes of novel inhibitors. The first set (methylidene penems, penicillin sulfones) is derived from β-lactams, and the second (avibactam, phosphonates, boronic acids) are non-β-lactams.
Figure 4.
Inhibitors with activity against class D β-lactamases. Methylidine penem, BRL 42715 (1). Bicyclic penem derivative, BLI-489 (2). Penicillin sulfone, LN-1-255 (3).Avibactam (4). 4-phenylphosphonate (5). 4,7-dichloro-1-benzothien-2-yl sulfonylaminomethyl boronic acid (DSABA) (6).
Methylidine penems (parent compound, BRL 42715 and derivative BLI-489) show activity as effective class D β-lactamase inhibitors when given in combination with β-lactams58-60. Based on these leads, two novel derivatives were shown to be especially potent mechanism- based inhibitors of OXA-1, with KM values ranging from 12-45 nM61. Molecular modeling studies noted interactions between the inhibitor, penem 2, and several conserved residues of OXA-1 (e.g. W102, F114, S115, and T213).
The penicillin sulfone inhibitors were designed by Buynak as novel inactivators for class D enzymes62. Of the compounds tested, the C-2 substituted 6-alkylidene penicillin sulfones were better than C-3 substituted 7-alkylidene cephalosporin sulfones. The best inhibitor identified in this series is LN-1-255, with a KM value of 0.7 μM for the penicillinase OXA-1, 0.65 μM for the carbapenemase OXA-24, and values ranging from 0.20-8.0 μM for the ESBLs OXA-10, -14 and -1763.
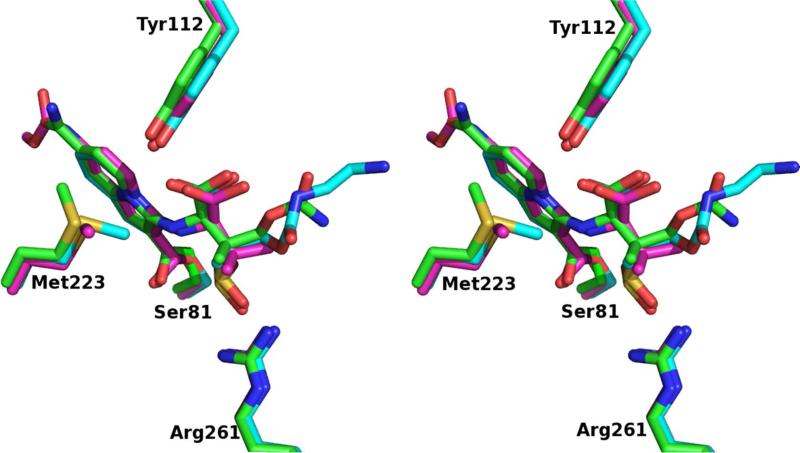
The reaction mechanism for the penicillin sulfones is unique from that of the clinical inhibitors tazobactam, clavulanic acid, and sulbactam. Crystal structures of OXA-24/40 in complexes with four of these inhibitors reveal key insights into inhibition by penicillin sulfones (Figure 5)20. The observed electron density supports rearrangement of the inhibitors into a substituted 2-indolizinecarboxylate serine ester that may obstruct the path of attack by the deacylating water molecule. The negatively charged sulfinate group mimics the C3/C4 carboxylate group and interacts with carboxylate-recognizing residue R261. The carboxylate of the inhibitors hydrogen bonds with the bridge residue Y112 to further stabilize binding. In every case, the carbonyl oxygen occupied the oxyanion hole, in contrast to what was observed in complexes with the class A enzymes, SHV-1 and SHV-2.
Figure 5.
Overlay of the X-ray crystal structures of OXA-24/40 with the final products obtained following rearrangement of the acyl-enzyme intermediate derived from the reaction with several penicillin sulfone inhibitors (PDB 3FYZ, carbon atoms green; 3FV7, carbon atoms magenta; and 3FZC, carbon atoms cyan)20. Residues in this figure are labeled with OXA-24/40 numbering.
Phosphonates and boronic acids are both non-β-lactam compounds that are known to inhibit class A and C β-lactamases64,65. These molecules act as transition state analog inhibitors and form a reversible, covalent bond between Oγ of the catalytic serine and the phosphorus or boron atom of the inhibitor, essentially acylating the enzyme. The advantage of these molecules is that they are structurally unique from current clinical inhibitors and do not resemble β-lactams themselves. Pratt and coworkers are studying phosphonates as novel inhibitors for class D β-lactamases66. The addition of hydrophobic substituents to these compounds decreases Ki values and also increases acylation rates. More recently several thiophenyl oxime-derived phosphonates were reported as inhibitors for OXA-24/40 (IC50s 17 μM and 31 μM) that exhibited synergy in combination with imipenem67. A recent paper reported 4,7-dichloro-1-benzothien-2-yl-sufonyl-aminomethyl boronic acid as the first boronic acid-based class D β-lactamase inhibitor with activity against OXA-24 (IC50 5.6 μM)68.
Avibactam (formerly known as NXL104) is another interesting inhibitor that has activity against OXA-48-producing isolates when given in combinations with ceftazidime, ceftaroline, or aztreonam69,70. Structures of avibactam in complex with OXA-10 and OXA-48 show that the drug forms a covalent complex with S67 and undergoes a ring opening reaction71.
Polycarboxylates act as inhibitors for class D enzymes. The apo structure of the non- carbapenemase OXA-46 was shown to have a tartrate molecule from the crystallization buffer bound in the active-site (PDB 3IF6)19. The two carboxylate groups hydrogen bond extensively with active-site residues (S72, K75, K210, and T211). One of the carboxylates makes an ionic interaction with R249, the residue that recognizes the C3/4 carboxylate group found on β-lactam antibiotics. Docquier et al. suggests that tartrate may promote its own binding through decarboxylation of the lysine general base (K75). Additionally, other polycarboxylates, lipophilic aminocitrate and aminoisocitrate derivatives, are reported to inhibit OXA-1072. This class of molecules may provide a new scaffold for novel inhibitor design and discovery efforts.
Summary
A better understanding of class D β-lactamases may aid in the discovery of novel, non-β-lactam inhibitors for these enzymes. Non-β-lactam compounds may avoid existing resistance mechanisms that have evolved to β-lactam-based compounds as bacteria cannot simply rely on modification of existing mechanisms for inactivation of novel compounds. Therefore, the identification of a novel inhibitor that has the ability to inhibit across the varying active-sites of the β-lactamases that have the highest clinical impact (ie. OXA-23, -24, and -48) would be highly desirable since there appears to be diverse mechanisms for carbapenemase resistance.
Acknowledgments
This work was supported by National Institutes of Health grants R01AI072219 and R01AI063517 (to R.A.B.), R15AI082416 (to D.A.L.) and R15AI094489 (to R.A.P) and the U.S. Department of Energy Joint Genome Institute through the Office of Science of the U.S. Department of Energy under Contract No. DE-AC02-05CH11231, and funds and/or facilities provided by the Cleveland Department of Veterans Affairs, the Veterans Affairs Merit Review Program and the Geriatric Research Education and Clinical Center VISN 10 to R.A.B.
Biography
David A. Leonard received his PhD in Biochemistry from Cornell University and served as a post-doctoral scientist at the University of Michigan. He joined Grand Valley State University in 1998, where he currently serves as Professor of Chemistry.
Robert A. Bonomo received his MD from Case Western Reserve University in 1983. He currently serves as Chief of Medicine at the Louis Stokes Cleveland Department of Veterans Affairs Medical Center in Cleveland and Professor of Medicine at Case Western.
Rachel A. Powers received her PhD in Structural Biology and Biochemistry from Northwestern University and served as a post-doctoral scientist at Michigan State University. She joined Grand Valley State University in 2006, where she holds the position of Associate Professor of Chemistry.
References
- 1.McDermott W, Rogers DE. Social ramifications of control of microbial disease. Johns Hopkins Med. J. 1982;151:302–12. [PubMed] [Google Scholar]
- 2.Drawz SM, Bonomo RA. Three decades of β-lactamase inhibitors. Clin. Micro. Rev. 2010;23:160–201. doi: 10.1128/CMR.00037-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hujer KM, Hujer AM, Hulten EA, Bajaksouzian S, Adams JM, Donskey CJ, Ecker DJ, Massire C, Eshoo MW, Sampath R, Thomson JM, Rather PN, Craft DW, Fishbain JT, Ewell AJ, Jacobs MR, Paterson DL, Bonomo RA. Analysis of antibiotic resistance genes in multidrug-resistant Acinetobacter sp. isolates from military and civilian patients treated at the Walter Reed Army Medical Center. Antimicrob. Agents Chemother. 2006;50:4114–23. doi: 10.1128/AAC.00778-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Poirel L, Potron A, Nordmann P. OXA-48-like carbapenemases: the phantom menace. J. Antimicrob. Chemother. 2012;67:1597–606. doi: 10.1093/jac/dks121. [DOI] [PubMed] [Google Scholar]
- 5.Lascols C, Peirano G, Hackel M, Laupland KB, Pitout JD. Surveillance and molecular epidemiology of Klebsiella pneumoniae that produce carbapenemases; the first report of OXA-48-like enzymes in North America. Antimicrob. Agents Chemother. 2012 doi: 10.1128/AAC.01686-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Massova I, Mobashery S. Kinship and diversification of bacterial penicillin-binding proteins and β-lactamases. Antimicrob. Agents Chemother. 1998;42:1–17. doi: 10.1128/aac.42.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fisher JF, Meroueh SO, Mobashery S. Bacterial resistance to β-lactam antibiotics: compelling opportunism, compelling opportunity. Chem. Rev. 2005;105:395–424. doi: 10.1021/cr030102i. [DOI] [PubMed] [Google Scholar]
- 8.Santillana E, Beceiro A, Bou G, Romero A. Crystal structure of the carbapenemase OXA-24 reveals insights into the mechanism of carbapenem hydrolysis. Proc. Natl. Acad. Sci. U.S.A. 2007;104:5354–9. doi: 10.1073/pnas.0607557104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maveyraud L, Golemi D, Kotra LP, Tranier S, Vakulenko S, Mobashery S, Samama JP. Insights into class D β-lactamases are revealed by the crystal structure of the OXA10 enzyme from Pseudomonas aeruginosa. Structure. 2000;8:1289–98. doi: 10.1016/s0969-2126(00)00534-7. [DOI] [PubMed] [Google Scholar]
- 10.Paetzel M, Danel F, de Castro L, Mosimann SC, Page MG, Strynadka NC. Crystal structure of the class D β-lactamase OXA-10. Nat. Struct. Biol. 2000;7:918–25. doi: 10.1038/79688. [DOI] [PubMed] [Google Scholar]
- 11.Sun T, Nukaga M, Mayama K, Braswell EH, Knox JR. Comparison of β-lactamases of classes A and D: 1.5-Å crystallographic structure of the class D OXA-1 oxacillinase. Protein Sci. 2003;12:82–91. doi: 10.1110/ps.0224303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Docquier JD, Calderone V, De Luca F, Benvenuti M, Giuliani F, Bellucci L, Tafi A, Nordmann P, Botta M, Rossolini GM, Mangani S. Crystal structure of the OXA-48 β-lactamase reveals mechanistic diversity among class D carbapenemases. Chem. Biol. 2009;16:540–7. doi: 10.1016/j.chembiol.2009.04.010. [DOI] [PubMed] [Google Scholar]
- 13.Kerff F, Charlier P, Colombo ML, Sauvage E, Brans A, Frère JM, Joris B, Fonzé E. Crystal structure of the sensor domain of the BlaR penicillin receptor from Bacillus licheniformis. Biochemistry. 2003;42:12835–43. doi: 10.1021/bi034976a. [DOI] [PubMed] [Google Scholar]
- 14.Golemi-Kotra D, Cha JY, Meroueh SO, Vakulenko SB, Mobashery S. Resistance to β-lactam antibiotics and its mediation by the sensor domain of the transmembrane BlaR signaling pathway in Staphylococcus aureus. J. Biol. Chem. 2003;278:18419–25. doi: 10.1074/jbc.M300611200. [DOI] [PubMed] [Google Scholar]
- 15.Poirel L, Naas T, Nordmann P. Diversity, Epidemiology, and Genetics of Class D β-lactamases. Antimicrob. Agents Chemother. 2010;54:24–38. doi: 10.1128/AAC.01512-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li Y, Yu X, Ho J, Fushman D, Allewell NM, Tuchman M, Shi D. Reversible post-translational carboxylation modulates the enzymatic activity of N-acetyl-L-ornithine transcarbamylase. Biochemistry. 2010;49:6887–95. doi: 10.1021/bi1007386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morollo AA, Petsko GA, Ringe D. Structure of a Michaelis complex analogue: propionate binds in the substrate carboxylate site of alanine racemase. Biochemistry. 1999;38:3293–301. doi: 10.1021/bi9822729. [DOI] [PubMed] [Google Scholar]
- 18.Cleland WW, Andrews TJ, Gutteridge S, Hartman FC, Lorimer GH. Mechanism of Rubisco: The Carbamate as General Base. Chem. Rev. 1998;98:549–562. doi: 10.1021/cr970010r. [DOI] [PubMed] [Google Scholar]
- 19.Docquier JD, Benvenuti M, Calderone V, Giuliani F, Kapetis D, De Luca F, Rossolini GM, Mangani S. Crystal structure of the narrow-spectrum OXA-46 class D β-lactamase: relationship between active-site lysine carbamylation and inhibition by polycarboxylates. Antimicrob. Agents Chemother. 2010;54:2167–74. doi: 10.1128/AAC.01517-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bou G, Santillana E, Sheri A, Beceiro A, Sampson JM, Kalp M, Bethel CR, Distler AM, Drawz SM, Pagadala SR, van den Akker F, Bonomo RA, Romero A, Buynak JD. Design, synthesis, and crystal structures of 6-alkylidene-2′-substituted penicillanic acid sulfones as potent inhibitors of Acinetobacter baumannii OXA-24 carbapenemase. J. Am. Chem. Soc. 2010;132:13320–31. doi: 10.1021/ja104092z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Borbulevych O, Kumarasiri M, Wilson B, Llarrull LI, Lee M, Hesek D, Shi Q, Peng J, Baker BM, Mobashery S. Lysine Nζ-decarboxylation switch and activation of the β-lactam sensor domain of BlaR1 protein of methicillin-resistant Staphylococcus aureus. J. Biol. Chem. 2011;286:31466–72. doi: 10.1074/jbc.M111.252189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kumarasiri M, Llarrull LI, Borbulevych O, Fishovitz J, Lastochkin E, Baker BM, Mobashery S. An amino-acid position at the crossroads of evolution of protein function: antibiotic-sensor domain of the BlaR1 protein from Staphylococcus aureus vs. class D β-lactamases. J. Biol. Chem. 2012 doi: 10.1074/jbc.M111.333179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Leonard DA, Hujer AM, Smith BA, Schneider KD, Bethel CR, Hujer KM, Bonomo RA. The role of OXA-1 β-lactamase Asp(66) in the stabilization of the active-site carbamate group and in substrate turnover. Biochem. J. 2008;410:455–62. doi: 10.1042/BJ20070573. [DOI] [PubMed] [Google Scholar]
- 24.Golemi D, Maveyraud L, Vakulenko S, Samama JP, Mobashery S. Critical involvement of a carbamylated lysine in catalytic function of class D β-lactamases. Proc. Natl. Acad. Sci. U.S.A. 2001;98:14280–5. doi: 10.1073/pnas.241442898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vercheval L, Bauvois C, di Paolo A, Borel F, Ferrer JL, Sauvage E, Matagne A, Frère JM, Charlier P, Galleni M, Kerff F. Three factors that modulate the activity of class D β-lactamases and interfere with the post-translational carboxylation of Lys70. Biochem. J. 2010;432:495–504. doi: 10.1042/BJ20101122. [DOI] [PubMed] [Google Scholar]
- 26.Pernot L, Frénois F, Rybkine T, L'Hermite G, Petrella S, Delettré J, Jarlier V, Collatz E, Sougakoff W. Crystal structures of the class D β-lactamase OXA-13 in the native form and in complex with meropenem. J. Mol. Biol. 2001;310:859–74. doi: 10.1006/jmbi.2001.4805. [DOI] [PubMed] [Google Scholar]
- 27.Verma V, Testero SA, Amini K, Wei W, Liu J, Balachandran N, Monoharan T, Stynes S, Kotra LP, Golemi-Kotra D. Hydrolytic Mechanism of OXA-58 Enzyme, a Carbapenem-hydrolyzing Class D β-Lactamase from Acinetobacter baumannii. J. Biol. Chem. 2011;286:37292–37303. doi: 10.1074/jbc.M111.280115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Golemi D, Maveyraud L, Ishiwata A, Tranier S, Miyashita K, Nagase T, Massova I, Mourey L, Samama JP, Mobashery S. 6-(hydroxyalkyl)penicillanates as probes for mechanisms of β-lactamases. J. Antibiot. (Tokyo) 2000;53:1022–7. doi: 10.7164/antibiotics.53.1022. [DOI] [PubMed] [Google Scholar]
- 29.Maveyraud L, Golemi-Kotra D, Ishiwata A, Meroueh O, Mobashery S, Samama JP. High-resolution X-ray structure of an acyl-enzyme species for the class D OXA-10 β-lactamase. J. Am. Chem. Soc. 2002;124:2461–5. doi: 10.1021/ja016736t. [DOI] [PubMed] [Google Scholar]
- 30.Baurin S, Vercheval L, Bouillenne F, Falzone C, Brans A, Jacquamet L, Ferrer JL, Sauvage E, Dehareng D, Frère JM, Charlier P, Galleni M, Kerff F. Critical role of tryptophan 154 for the activity and stability of class D β-lactamases. Biochemistry. 2009;48:11252–63. doi: 10.1021/bi901548c. [DOI] [PubMed] [Google Scholar]
- 31.Schneider KD, Ortega CJ, Renck NA, Bonomo RA, Powers RA, Leonard DA. Structures of the Class D Carbapenemase OXA-24 from Acinetobacter baumannii in Complex with Doripenem. J. Mol. Biol. 2011;406:583–594. doi: 10.1016/j.jmb.2010.12.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schneider KD, Bethel CR, Distler AM, Hujer AM, Bonomo RA, Leonard DA. Mutation of the active site carboxy-lysine (K70) of OXA-1 β-lactamase results in a deacylation-deficient enzyme. Biochemistry. 2009;48:6136–45. doi: 10.1021/bi900448u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Buchman JS, Schneider KD, Lloyd AR, Pavlish SL, Leonard DA. Site-saturation mutagenesis of position V117 in OXA-1 β-lactamase: effect of side chain polarity on enzyme carboxylation and substrate turnover. Biochemistry. 2012;51:3143–50. doi: 10.1021/bi201896k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Strynadka NC, Adachi H, Jensen SE, Johns K, Sielecki A, Betzel C, Sutoh K, James MN. Molecular structure of the acyl-enzyme intermediate in β-lactam hydrolysis at 1.7 Å resolution. Nature. 1992;359:700–5. doi: 10.1038/359700a0. [DOI] [PubMed] [Google Scholar]
- 35.Patera A, Blaszczak LC, Shoichet BK. Crystal Structures of Substrate and Inhibitor Complexes with AmpC β-Lactamase: Possible Implications for Substrate-Assisted Catalysis. J. Am. Chem. Soc. 2000;122:10504–10512. [Google Scholar]
- 36.Maveyraud L, Pratt RF, Samama JP. Crystal structure of an acylation transition-state analog of the TEM-1 β-lactamase. Mechanistic implications for class A β-lactamases. Biochemistry. 1998;37:2622–8. doi: 10.1021/bi972501b. [DOI] [PubMed] [Google Scholar]
- 37.Lietz EJ, Truher H, Kahn D, Hokenson MJ, Fink AL. Lysine-73 Is Involved in the Acylation and Deacylation of β-Lactamase. Biochemistry. 2000;39:4971–4981. doi: 10.1021/bi992681k. [DOI] [PubMed] [Google Scholar]
- 38.Naas T, Nordmann P. OXA-type β-lactamases. Curr. Pharm. Des. 1999;5:865–79. [PubMed] [Google Scholar]
- 39.Naas T, Poirel L, Nordmann P. Minor extended-spectrum β-lactamases. Clin. Microbiol. Infect. 2008;14(Suppl 1):42–52. doi: 10.1111/j.1469-0691.2007.01861.x. [DOI] [PubMed] [Google Scholar]
- 40.Hocquet D, Colomb M, Dehecq B, Belmonte O, Courvalin P, Plésiat P, Meziane-Cherif D. Ceftazidime-hydrolysing β-lactamase OXA-145 with impaired hydrolysis of penicillins in Pseudomonas aeruginosa. J. Antimicrob. Chemother. 2011;66:1745–1750. doi: 10.1093/jac/dkr187. [DOI] [PubMed] [Google Scholar]
- 41.Danel F, Hall LM, Livermore DM. Laboratory mutants of OXA-10 β-lactamase giving ceftazidime resistance in Pseudomonas aeruginosa. J. Antimicrob. Chemother. 1999;43:339–44. doi: 10.1093/jac/43.3.339. [DOI] [PubMed] [Google Scholar]
- 42.Fournier D, Hocquet D, Dehecq B, Cholley P, Plésiat P. Detection of a new extended-spectrum oxacillinase in Pseudomonas aeruginosa. J. Antimicrob. Chemother. 2010;65:364–5. doi: 10.1093/jac/dkp438. [DOI] [PubMed] [Google Scholar]
- 43.Meziane-Cherif D, Bonnet R, Haouz A, Plésiat P, Courvalin P. Structure of OXA-145, a ceftazidime hydrolysing class D β-lactamase with impaired hydrolysis of penicillins.. 51st Intersci. Conf. on Antimicrob. Agents and Chemother., abstr C1-599; Chicago, Il. 2011. [Google Scholar]
- 44.Schneider KD, Karpen ME, Bonomo RA, Leonard DA, Powers RA. The 1.4 Å crystal structure of the class D β-lactamase OXA-1 complexed with doripenem. Biochemistry. 2009;48:11840–7. doi: 10.1021/bi901690r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Easton CJ, Knowles JR. Inhibition of the RTEM β-lactamase from Escherichia coli. Interaction of the enzyme with derivatives of olivanic acid. Biochemistry. 1982;21:2857–62. doi: 10.1021/bi00541a008. [DOI] [PubMed] [Google Scholar]
- 46.Hugonnet JE, Tremblay LW, Boshoff HI, Barry CE, 3rd, Blanchard JS. Meropenem-clavulanate is effective against extensively drug-resistant Mycobacterium tuberculosis. Science. 2009;323:1215–8. doi: 10.1126/science.1167498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tremblay LW, Fan F, Blanchard JS. Biochemical and Structural Characterization of Mycobacterium tuberculosis β-lactamase with the Carbapenems Ertapenem and Doripenem. Biochemistry. 2010;49:3766–3773. doi: 10.1021/bi100232q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nukaga M, Bethel CR, Thomson JM, Hujer AM, Distler A, Anderson VE, Knox JR, Bonomo RA. Inhibition of class A β-lactamases by carbapenems: crystallographic observation of two conformations of meropenem in SHV-1. J. Am. Chem. Soc. 2008;130:12656–62. doi: 10.1021/ja7111146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Scaife W, Young HK, Paton RH, Amyes SG. Transferable imipenem-resistance in Acinetobacter species from a clinical source. J. Antimicrob. Chemother. 1995;36:585–6. doi: 10.1093/jac/36.3.585. [DOI] [PubMed] [Google Scholar]
- 50.Higgins PG, Schneiders T, Hamprecht A, Seifert H. In vivo selection of a missense mutation in adeR and conversion of the novel blaOXA-164 gene into blaOXA-58 in carbapenem-resistant Acinetobacter baumannii isolates from a hospitalized patient. Antimicrob. Agents Chemother. 2010;54:5021–7. doi: 10.1128/AAC.00598-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.De Luca F, Benvenuti M, Carboni F, Pozzi C, Rossolini GM, Mangani S, Docquier JD. Evolution to carbapenem-hydrolyzing activity in noncarbapenemase class D β-lactamase OXA-10 by rational protein design. Proc. Natl. Acad. Sci. U.S.A. 2011;108:18424–9. doi: 10.1073/pnas.1110530108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Page MG. β-Lactamase inhibitors. Drug Resist. Updat. 2000;3:109–125. doi: 10.1054/drup.2000.0137. [DOI] [PubMed] [Google Scholar]
- 53.Payne DJ, Cramp R, Winstanley DJ, Knowles DJ. Comparative activities of clavulanic acid, sulbactam, and tazobactam against clinically important β-lactamases. Antimicrob. Agents Chemother. 1994;38:767–72. doi: 10.1128/aac.38.4.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Philippon LN, Naas T, Bouthors AT, Barakett V, Nordmann P. OXA-18, a class D clavulanic acid-inhibited extended-spectrum β-lactamase from Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 1997;41:2188–95. doi: 10.1128/aac.41.10.2188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Toleman MA, Rolston K, Jones RN, Walsh TR. Molecular and biochemical characterization of OXA-45, an extended-spectrum class 2d' β-lactamase in Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 2003;47:2859–63. doi: 10.1128/AAC.47.9.2859-2863.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Franceschini N, Boschi L, Pollini S, Herman R, Perilli M, Galleni M, Frère JM, Amicosante G, Rossolini GM. Characterization of OXA-29 from Legionella (Fluoribacter) gormanii: molecular class D β-lactamase with unusual properties. Antimicrob. Agents Chemother. 2001;45:3509–16. doi: 10.1128/AAC.45.12.3509-3516.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Totir MA, Cha J, Ishiwata A, Wang B, Sheri A, Anderson VE, Buynak J, Mobashery S, Carey PR. Why clinically used tazobactam and sulbactam are poor inhibitors of OXA-10 β-lactamase: Raman crystallographic evidence. Biochemistry. 2008;47:4094–101. doi: 10.1021/bi702348w. [DOI] [PubMed] [Google Scholar]
- 58.Petersen PJ, Jones CH, Venkatesan AM, Bradford PA. Efficacy of piperacillin combined with the Penem β-lactamase inhibitor BLI-489 in murine models of systemic infection. Antimicrob. Agents Chemother. 2009;53:1698–700. doi: 10.1128/AAC.01549-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Farmer TH, Page JW, Payne DJ, Knowles DJ. Kinetic and physical studies of β-lactamase inhibition by a novel penem, BRL 42715. Biochem. J. 1994;303( Pt 3):825–30. doi: 10.1042/bj3030825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Matagne A, Ledent P, Monnaie D, Felici A, Jamin M, Raquet X, Galleni M, Klein D, Francois I, Frère JM. Kinetic study of interaction between BRL 42715, β-lactamases, and D-alanyl-alanine peptidases. Antimicrob. Agents Chemother. 1995;39:227–31. doi: 10.1128/aac.39.1.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bethel CR, Distler AM, Ruszczycky MW, Carey MP, Carey PR, Hujer AM, Taracila M, Helfand MS, Thomson JM, Kalp M, Anderson VE, Leonard DA, Hujer KM, Abe T, Venkatesan AM, Mansour TS, Bonomo RA. Inhibition of OXA-1 β-lactamase by penems. Antimicrob. Agents Chemother. 2008;52:3135–43. doi: 10.1128/AAC.01677-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Drawz SM, Bethel CR, Doppalapudi VR, Sheri A, Pagadala SR, Hujer AM, Skalweit MJ, Anderson VE, Chen SG, Buynak JD, Bonomo RA. Penicillin sulfone inhibitors of class D β-lactamases. Antimicrob. Agents Chemother. 2010;54:1414–24. doi: 10.1128/AAC.00743-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pattanaik P, Bethel CR, Hujer AM, Hujer KM, Distler AM, Taracila M, Anderson VE, Fritsche TR, Jones RN, Pagadala SR, van den Akker F, Buynak JD, Bonomo RA. Strategic design of an effective β-lactamase inhibitor: LN-1-255, a 6-alkylidene-2′-substituted penicillin sulfone. J. Biol. Chem. 2009;284:945–53. doi: 10.1074/jbc.M806833200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rahil J, Pratt RF. Phosphonate monoester inhibitors of class A β-lactamases. Biochem. J. 1991;275( Pt 3):793–5. doi: 10.1042/bj2750793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Beesley T, Gascoyne N, Knott-Hunziker V, Petursson S, Waley SG, Jaurin B, Grundstrom T. The inhibition of class C β-lactamases by boronic acids. Biochem. J. 1983;209:229–33. doi: 10.1042/bj2090229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Majumdar S, Adediran SA, Nukaga M, Pratt RF. Inhibition of class D β-lactamases by diaroyl phosphates. Biochemistry. 2005;44:16121–9. doi: 10.1021/bi051719s. [DOI] [PubMed] [Google Scholar]
- 67.Tan Q, Ogawa AM, Raghoobar SL, Wisniewski D, Colwell L, Park YW, Young K, Hermes JD, Dininno FP, Hammond ML. Thiophenyl oxime-derived phosphonates as nano-molar class C β-lactamase inhibitors reducing MIC of imipenem against Pseudomonas aeruginosa and Acinetobacter baumannii. Bioorg. Med. Chem. Lett. 2011;21:4363–5. doi: 10.1016/j.bmcl.2011.04.122. [DOI] [PubMed] [Google Scholar]
- 68.Tan Q, Ogawa AM, Painter RE, Park YW, Young K, DiNinno FP. 4,7-Dichloro benzothien-2-yl sulfonylaminomethyl boronic acid: first boronic acid-derived β-lactamase inhibitor with class A, C, and D activity. Bioorg. Med. Chem. Lett. 2010;20:2622–4. doi: 10.1016/j.bmcl.2010.02.065. [DOI] [PubMed] [Google Scholar]
- 69.Livermore DM, Mushtaq S, Warner M, Zhang J, Maharjan S, Doumith M, Woodford N. Activities of NXL104 combinations with ceftazidime and aztreonam against carbapenemase-producing. Enterobacteriaceae. Antimicrob. Agents Chemother. 2011;55:390–4. doi: 10.1128/AAC.00756-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mushtaq S, Warner M, Williams G, Critchley I, Livermore DM. Activity of chequerboard combinations of ceftaroline and NXL104 versus β-lactamase-producing Enterobacteriaceae. J. Antimicrob. Chemother. 2010;65:1428–32. doi: 10.1093/jac/dkq161. [DOI] [PubMed] [Google Scholar]
- 71.Docquier JD, Benvenuti M, De Luca F, Rossolini GM, Mangani S, Miossec C, Black MT. X-Ray Crystal Structure of the Klebsiella pneumoniae OXA-48 Class D Carbapenemase Inhibited by NXL104.. 50th Intersci. Conf. on Antimicrob. Agents and Chemother., abstr C1-640; Boston, MA. 2010. [Google Scholar]
- 72.Beck J, Vercheval L, Bebrone C, Herteg-Fernea A, Lassaux P, Marchand-Brynaert J. Discovery of novel lipophilic inhibitors of OXA-10 enzyme (class D β-lactamase) by screening amino analogs and homologs of citrate and isocitrate. Bioorg. Med. Chem. Lett. 2009;19:3593–7. doi: 10.1016/j.bmcl.2009.04.149. [DOI] [PubMed] [Google Scholar]