Abstract
Despite many years of clinical experience with cefepime, data regarding the outcome of patients suffering from bloodstream infections (BSIs) due to Enterobacter cloacae (Ecl) are scarce. To address the gap in our knowledge, 57 Ecl responsible for 51 BSIs were analysed implementing phenotypic and molecular methods (microarrays, PCRs for bla and other genes, rep-PCR to analyse clonality). Only two E. cloacae (3.5%) were ESBL-producers, whereas 34 (59.6%) and 18 (31.6%) possessed inducible (Ind-Ecl) or derepressed (Der-Ecl) AmpC enzymes, respectively. All isolates were susceptible to imipenem, meropenem, gentamicin and ciprofloxacin. Der-Ecl were highly resistant to ceftazidime and piperacillin/tazobactam (both MIC90 ≥ 256 µg/mL), whereas cefepime retained its activity (MIC90 of 3 µg/mL). rep-PCR indicated that the isolates were sporadic, but Ecl collected from the same patients were indistinguishable. In particular, three BSIs initially due to Ind-Ecl evolved (under ceftriaxone or piperacillin/tazobactam treatment) into Der-Ecl because of mutations or a deletion in ampD or insertion of IS4321 in the promoter. These last two mechanisms have never been described in Ecl. Mortality was higher for BSIs due to Der-Ecl than Ind-Ecl (3.8% vs. 29.4%; P = 0.028) and was associated with the Charlson co-morbidity index (P = 0.046). Using the following directed treatments, patients with BSI showed a favourable treatment outcome: cefepime (16/18; 88.9%); carbapenems (12/13; 92.3%); ceftriaxone (4/7; 57.1%); piperacillin/tazobactam (5/7; 71.4%); and ciprofloxacin (6/6; 100%). Cefepime represents a safe therapeutic option and an alternative to carbapenems to treat BSIs due to Ecl when the prevalence of ESBL-producers is low.
Keywords: Cefepime, Bacteraemia, Outcome, Enterobacter, AmpC, AmpD
1. Introduction
Among the pathogens that are difficult to treat, Enterobacter cloacae (Ecl) represents a unique challenge as it is naturally resistant to penicillins, first- and second-generation cephalosporins and amoxicillin/clavulanic acid (AMC) owing to the production of chromosomal AmpC β-lactamases (cAmpC) [1]. Moreover, under the selective pressure of most β-lactams, this organism can rapidly hyperproduce the cAmpC enzymes, developing further resistance to third-generation cephalosporins (3GCs) [e.g. ceftriaxone (CRO) and ceftazidime] and monobactams. This phenomenon can be transient [‘inducible’ isolates (Ind-Ecl)] or constitutive [‘stably derepressed’ isolates (Der-Ecl)] generally due to mutations in the ampD gene [2–4]. As a result, use of monotherapy with 3GCs is discouraged, even if the organism tests susceptible [1,3,5]. In contrast, cefepime (FEP), a fourth-generation cephalosporin, is a poor inducer of cAmpC enzymes and is weakly hydrolysed by these enzymes [6].
Resistance to 3GCs in Ecl can also arise as a result of production of plasmid-mediated AmpC (pAmpC) enzymes, whereas resistance both to 3GCs and FEP is associated with the expression of extended-spectrum β-lactamases (ESBLs). Furthermore, reduced permeability of the outer membrane and/or production of carbapenemases can render Ecl resistant to carbapenems [7]. Up to now, the prevalence of pAmpC enzymes in Ecl is low, and carbapenemase-producers are found only in some regions [7]. In contrast, the spread of ESBL-producing Ecl (ESBL-Ecl) has reached significant levels worldwide [8,9]. However, data regarding the frequency of the abovementioned multidrug-resistant Ecl in Switzerland are unknown. This information is crucial to choose the correct empirical treatment for severe infections.
Ecl is frequently responsible for nosocomial infections, including urinary tract infections, pneumonia and bloodstream infections (BSIs) [2]. In particular, Ecl is responsible for 3–6% of BSIs, with crude mortality rates ranging from 27% to 61% [10,11]. Several reports have described clinical features and the outcome of BSIs due to Ecl (BSI-Ecl). Unfortunately, these studies were unable to capture a comprehensive picture of the impact of BSI-Ecl. First, host- and healthcare-related risk factors for the development of BSI-Ecl are often not considered. Second, the treatment success of various antimicrobials, in particular the association with minimum inhibitory concentration (MIC) values, is usually unreported [12–15]. Finally, the links between the molecular characterisation of isolates, the treatment paradigm and their outcome are lacking. For instance, data regarding the clinical efficacy of FEP or piperacillin/tazobactam (TZP) for the treatment of BSI-Ecl when different levels of cAmpC enzymes (i.e. Ind-Ecl vs. Der-Ecl) are expressed are very scarce [3,11,12].
In the present study, we investigated the antibiotic resistance phenotypes and resistance genes of Ecl causing BSIs and tried to elucidate host-, healthcare- and treatment-related factors that have a potential influence on the clinical outcome. We conclude that in populations where ESBL-Ecl is low (<5%), FEP is a safe therapeutic option for the treatment of BSI. This finding bodes well for infection control and stewardship programmes that aim to spare carbapenem-containing regimens.
2. Materials and methods
2.1. Sample collection and microbiological methods
All Ecl responsible for bacteraemia during the period January 2008 to July 2011 were included in the analysis. Blood cultures were processed at the Clinical Microbiology Laboratory of the University of Bern (Switzerland). The study was conducted in accordance with the local requirements of the ethics committee.
Blood culture bottles were incubated in a BacT/ALERT® 3D System (bioMérieux, Marcy l’Étoile, France). Species identification (ID) was routinely achieved using the VITEK® 2 system (bioMérieux), and antimicrobial susceptibility testing (AST) were performed by disk diffusion. ID was confirmed using matrix-assisted laser desorption/ionisation time-of-flight mass spectrometry (MALDI-TOF/MS; Bruker Daltonik GmbH, Leipzig, Germany), whereas the MICs for several antibiotics (see Table 1) were determined by Etest (bioMérieux). Results were interpreted according to European Committee on Antimicrobial Susceptibility Testing (EUCAST) criteria [16].
Table 1.
Phenotypic and molecular background of resistance for Enterobacter cloacae (Ecl) isolates responsible for bloodstream infections during the 3.5-year study period.
| No. | MIC (µg/mL)a | SXT (mm) | DDST | DAA | Microarray analysesb (DNA sequencing for bla genes) |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TZP | CRO | CAZ | CTX | FEP | IPM | MEM | ETP | CIP | GEN | AMK | |||||
| 1 | 4 | 0.094 | 0.75 | 0.125 | 0.064 | 0.25 | 0.064 | 0.016 | 0.016 | 1 | 2 | 24 | − | Ind | TEMwt |
| 2 | 3 | 0.125 | 0.5 | 0.19 | 0.064 | 0.38 | 0.047 | 0.016 | 0.012 | 1 | 3 | 25 | − | Ind | – |
| 3-Ac | 4 | 0.25 | 0.5 | 0.25 | 0.094 | 0.38 | 0.047 | 0.023 | 0.008 | 1 | 2 | 22 | − | Ind | – |
| 4d | 2 | 0.125 | 0.25 | 0.125 | 0.064 | 0.5 | 0.094 | 0.094 | 0.047 | 0.75 | 2 | 26 | − | Ind | dfrV |
| 5-Ie | 8 | 24 | 12 | 8 | 0.125 | 0.5 | 0.125 | 0.75 | 0.023 | 0.75 | 2 | 22 | − | Ind | – |
| 6 | 3 | >32 | 64 | 8 | 1 | 0.38 | 0.094 | 0.094 | 0.25 | 0.75 | 2 | 0 | + | Ind | SHV:G238S/E240K (SHV-12), TEMwt (TEM-1), qnrB, tet(D), dfrA, sul2, strB |
| 7 | 4 | 0.75 | 1 | 1.5 | 0.25 | 0.38 | 0.064 | 0.016 | 0.38 | 1 | 3 | 17 | − | Ind | TEMwt, qnrB, dfrV |
| 8 | 2 | 0.19 | 0.5 | 0.19 | 0.047 | 1 | 0.125 | 0.032 | 0.012 | 1 | 2 | 0 | − | Ind | TEMwt, tet(D), floR, dfrA, sul2 |
| 9 | 6 | 1.5 | 3 | 2 | 0.094 | 0.5 | 0.094 | 0.125 | 0.012 | 0.75 | 2 | 26 | − | Ind | – |
| 10 | 3 | 0.125 | 0.5 | 0.125 | 0.064 | 0.38 | 0.064 | 0.19 | 0.016 | 0.75 | 2 | 25 | − | Ind | – |
| 11 | 1.5 | 0.094 | 0.25 | 0.094 | 0.047 | 0.25 | 0.047 | 0.016 | 0.012 | 0.5 | 2 | 0 | − | Ind | – |
| 12 | 1 | 0.032 | 0.125 | 0.047 | 0.032 | 0.38 | 0.125 | 0.094 | 0.006 | 0.75 | 2 | 22 | − | Ind | – |
| 13 | 0.75 | 0.094 | 0.125 | 0.064 | 0.032 | 0.25 | 0.064 | 0.064 | 0.008 | 0.5 | 1.5 | 30 | − | Ind | TEMwt |
| 14 | 2 | 0.125 | 1 | 0.125 | 0.19 | 0.25 | 0.047 | 0.023 | 0.023 | 1 | 2 | 28 | − | Ind | – |
| 15 | 2 | 0.125 | 0.38 | 0.125 | 0.047 | 0.25 | 0.047 | 0.032 | 0.016 | 0.75 | 2 | 22 | − | Ind | – |
| 17 | 1.5 | 0.125 | 0.25 | 0.125 | 0.032 | 0.25 | 0.047 | 0.023 | 0.008 | 0.75 | 2 | 29 | − | Ind | – |
| 18 | 2 | 0.125 | 0.38 | 0.25 | 0.047 | 0.25 | 0.047 | 0.047 | 0.016 | 0.75 | 2 | 25 | − | Ind | – |
| 19 | 1.5 | 0.094 | 0.38 | 0.094 | 0.047 | 0.38 | 0.125 | 0.19 | 0.023 | 1 | 3 | 21 | − | Ind | – |
| 20 | 24 | >32 | 24 | 64 | 0.5 | 0.38 | 0.094 | 0.25 | 0.012 | 0.75 | 2 | 24 | − | Ind | – |
| 21 | 1.5 | 0.125 | 0.38 | 0.125 | 0.047 | 0.38 | 0.064 | 0.032 | 0.012 | 1 | 2 | 26 | − | Ind | TEMwt |
| 24 | 3 | 0.38 | 1 | 0.5 | 0.064 | 0.25 | 0.094 | 0.19 | 0.023 | 0.5 | 1.5 | 22 | − | Ind | TEMwt |
| 25 | 2 | 0.19 | 0.38 | 0.19 | 0.064 | 0.38 | 0.094 | 0.047 | 0.016 | 1 | 2 | 25 | − | Ind | – |
| 26-Ac | 3 | 6 | 2 | 3 | 0.094 | 0.38 | 0.064 | 0.094 | 0.008 | 1 | 3 | 24 | − | Ind | dfrV |
| 27 | 2 | 0.5 | 0.75 | 0.75 | 0.047 | 0.25 | 0.023 | 0.016 | 0.012 | 0.75 | 2 | 28 | − | Ind | dfrV |
| 28 | 2 | 0.19 | 0.38 | 0.25 | 0.047 | 0.38 | 0.094 | 0.125 | 0.016 | 1 | 2 | 27 | − | Ind | – |
| 29 | 3 | 0.75 | 1 | 1 | 0.064 | 0.25 | 0.125 | 0.38 | 0.016 | 0.75 | 2 | 27 | − | Ind | – |
| 30-Ie | 2 | 0.094 | 0.38 | 0.125 | 0.047 | 0.38 | 0.047 | 0.032 | 0.008 | 0.75 | 2 | 27 | − | Ind | – |
| 31-Ac | 4 | 0.25 | 0.75 | 0.38 | 0.064 | 0.38 | 0.094 | 0.19 | 0.016 | 1 | 2 | 25 | − | Ind | – |
| 36 | 2 | 0.125 | 0.38 | 0.125 | 0.047 | 0.25 | 0.047 | 0.016 | 0.012 | 0.5 | 2 | 29 | − | Ind | – |
| 38 | 3 | 2 | 2 | 2 | 0.094 | 0.38 | 0.125 | 0.25 | 0.016 | 1 | 2 | 25 | − | Ind | TEMwt |
| 40 | 3 | 0.125 | 0.38 | 0.19 | 0.047 | 0.38 | 0.064 | 0.032 | 0.012 | 0.75 | 2 | 26 | − | Ind | TEMwt |
| 42 | 2 | 0.25 | 0.5 | 0.38 | 0.064 | 0.25 | 0.064 | 0.125 | 0.016 | 0.75 | 3 | 27 | − | Ind | – |
| p45-Ie,f | 2 | 0.094 | 0.38 | 0.125 | 0.064 | 0.38 | 0.064 | 0.032 | 0.012 | 1 | 1.5 | 0 | − | Ind | TEMwt, sul2, tet(D), dfrA, strA, int1 |
| p48f,g | 16 | >32 | 24 | 16 | 0.125 | 0.5 | 0.25 | 0.75 | 0.023 | 1.5 | 2 | 28 | − | Ind | – |
| p49f,g | 12 | >32 | 16 | 16 | 0.125 | 0.5 | 0.25 | 1 | 0.023 | 1 | 2 | 29 | − | Ind | – |
| Ind-Ecl (n = 34; 59.6%)h | |||||||||||||||
| MIC50 | 2 | 0.19 | 0.5 | 0.19 | 0.064 | 0.38 | 0.064 | 0.047 | 0.016 | 0.75 | 2 | NA | |||
| MIC90 | 16 | ≥32 | 12 | 8 | 0.125 | 0.5 | 0.125 | 0.38 | 0.023 | 1 | 3 | NA | |||
| S (%) | 85.3 | 76.5 | 79.4 | 76.5 | 100 | 100 | 100 | 91.2 | 100 | 100 | 100 | 91.2 | |||
| 3-Bc | 96 | >32 | >256 | >256 | 2 | 0.25 | 0.094 | 0.25 | 0.016 | 1 | 2 | 25 | − | Der | – |
| 5-IIe | 24 | >32 | 128 | 48 | 0.75 | 0.25 | 0.19 | 0.5 | 0.047 | 0.75 | 2 | 25 | − | Der | – |
| 16 | 64 | >32 | 32 | 192 | 0.75 | 0.094 | 0.023 | 0.38 | 0.016 | 0.75 | 2 | 26 | − | Der | – |
| 22 | >256 | >32 | >256 | >256 | 3 | 0.38 | 0.19 | 1 | 0.023 | 1 | 2 | 24 | − | Der | – |
| 23 | 64 | >32 | >256 | 96 | 32 | 0.19 | 0.094 | 0.125 | 0.023 | 1 | 3 | 26 | + | Der | CTX-M-grI (CTX-M-1), TEMwt, dfrV |
| 26-Bc | >256 | >32 | >256 | >256 | 0.75 | 0.38 | 0.094 | 0.38 | 0.023 | 1.5 | 3 | 22 | − | Der | dfrV |
| 30-IIe | >256 | >32 | >256 | >256 | 1 | 0.25 | 0.064 | 0.38 | 0.016 | 0.75 | 2 | 26 | − | Der | – |
| 31-Bc | 96 | >32 | >256 | >256 | 2 | 0.19 | 0.094 | 0.38 | 0.032 | 1 | 2 | 26 | − | Der | – |
| 32 | >256 | >32 | >256 | >256 | 2 | 0.38 | 0.19 | 1 | 0.032 | 1 | 3 | 22 | − | Der | – |
| 33 | 48 | >32 | 128 | 48 | 0.38 | 0.75 | 0.25 | 0.5 | 0.064 | 0.75 | 2 | 22 | − | Der | tet(B), cmlA |
| 37 | >256 | >32 | >256 | >256 | 3 | 0.38 | 0.125 | 1 | 0.032 | 0.5 | 2 | 23 | − | Der | – |
| 39 | 96 | >32 | 48 | 64 | 0.38 | 0.75 | 0.25 | 1 | 0.064 | 0.5 | 2 | 24 | − | Der | – |
| 43d | >256 | >32 | >256 | >256 | 96 | 0.5 | 0.19 | 0.75 | 0.012 | 0.75 | 2 | 26 | − | Der | – |
| 44 | 32 | >32 | 64 | 96 | 1 | 0.25 | 0.125 | 0.38 | 0.016 | 0.75 | 1.5 | 26 | − | Der | TEMwt |
| p45-IIe,f | >256 | >32 | >256 | >256 | 4 | 0.25 | 0.125 | 0.75 | 0.032 | 0.75 | 2 | 0 | − | Der | TEMwt, sul2, tet(D), dfrA, strA, int1 |
| 46 | 64 | >32 | 192 | 128 | 0.75 | 0.75 | 0.5 | 1 | 0.032 | 0.75 | 1.5 | 28 | − | Der | TEMwt |
| 47 | 24 | >32 | 96 | 32 | 0.25 | 0.38 | 0.094 | 0.38 | 0.016 | 1.5 | 3 | 0 | − | Der | TEMwt, sul2, int1, aadA1, dfrA |
| 50 | 96 | >32 | 192 | 192 | 1 | 0.19 | 0.064 | 0.25 | 0.016 | 0.75 | 1.5 | 27 | − | Der | TEMwt |
| 51 | 12 | >32 | 48 | 24 | 0.38 | 0.19 | 0.094 | 0.19 | 0.032 | 0.75 | 2 | 26 | − | Der | – |
| Der-Ecl (n = 18; 31.6%)h | |||||||||||||||
| MIC50 | 96 | ≥32 | 192 | 192 | 1 | 0.25 | 0.125 | 0.38 | 0.023 | 0.75 | 2 | NA | |||
| MIC90 | ≥256 | ≥32 | ≥256 | ≥256 | 3 | 0.75 | 0.25 | 1 | 0.047 | 1.5 | 3 | NA | |||
| S (%) | 0.0 | 0.0 | 0.0 | 0.0 | 61.1 | 100 | 100 | 61.1 | 100 | 100 | 100 | 88.9 | |||
| 34 | 2 | 0.047 | 0.19 | 0.064 | 0.064 | 0.25 | 0.064 | 0.012 | 0.032 | 0.75 | 2 | 23 | − | Un | – |
| 35 | 1.5 | 0.19 | 0.75 | 0.19 | 0.25 | 0.25 | 0.032 | 0.016 | 0.032 | 0.75 | 3 | 29 | − | Un | – |
| p41f | 1.5 | 0.047 | 0.38 | 0.125 | 0.125 | 0.25 | 0.064 | 0.008 | 0.023 | 0.5 | 2 | 25 | − | Un | TEMwt, SHVwt |
| All Ecl (n = 57) | |||||||||||||||
| MIC50 | 3 | 6 | 1 | 1 | 0.094 | 0.38 | 0.094 | 0.19 | 0.016 | 0.75 | 2 | NA | |||
| MIC90 | ≥256 | ≥32 | ≥256 | ≥256 | 2 | 0.5 | 0.19 | 0.75 | 0.032 | 1 | 3 | NA | |||
| S (%) | 61.4 | 45.6 | 52.6 | 50.9 | 86.0 | 100 | 100 | 84.2 | 100 | 100 | 100 | 89.5 | |||
MIC, minimum inhibitory concentration; TZP, piperacillin/tazobactam; CRO, ceftriaxone; CAZ, ceftazidime; CTX, cefotaxime; FEP, cefepime; IPM, imipenem; MEM, meropenem; ETP, ertapenem; CIP, ciprofloxacin; GEN, gentamicin; AMK, amikacin; SXT, trimethoprim/sulfamethoxazole; DDST, double-disk synergy test; DAA, disk approximation assay; Ind, inducible; Der, derepressed; MIC50,90, MIC for 50% and 90% of the organisms, respectively; NA, not applicable; S, susceptible; Un, unidentified (i.e. non-inducible and non-derepressed); +, positive; −, negative.
Interpretation of MICs according to 2012 EUCAST criteria [16]: TZP (S, ≤8 µg/mL); CRO (S, ≤1 µg/mL); CAZ (S, ≤1 µg/mL); CTX (S, ≤1 µg/mL); FEP (S, ≤1 µg/mL); IPM (S, ≤2 µg/mL); MEM (S, ≤2 µg/mL); ETP (S, ≤0.5 µg/mL); CIP (S, ≤0.5 µg/mL); GEN (S, ≤2 µg/mL); AMK (S, ≤8 µg/mL); and SXT (evaluated by disk diffusion; S, ≥16 mm).
TEMwt and SHVwt indicate that only wild-type blaTEM or blaSHV genes (e.g. blaTEM-1, blaSHV-1) were present, as defined by the amino acid sites assayed for by Check-Points (Wageningen, The Netherlands); cmlA, chloramphenicol efflux gene; dfrA1 and dfrV, trimethoprim resistance dihydrofolate reductase genes; floR, florfenicol efflux gene; tet(B) and tet(D), tetracycline efflux genes; aadA1, streptomycin adenyltransferase gene; strA and strB, streptomycin phosphotransferase genes; sul2, sulfonamide resistance dihydropteroate synthase gene; intI1, class 1 integrase gene; qnrB, plasmid-mediated quinolone resistance determinant.
For these BSI cases, two Ecl strains (i.e. one inducible indicated with ‘-A’ and one derepressed indicated with ‘-B’) were simultaneously isolated in the same blood culture.
These patients initially had a BSI episode due to Ind-Ecl (i.e. strains indicated with ‘-I’) that evolved to Der-Ecl (i.e. strains indicated with ‘-II’).
These Ecl isolates were responsible for BSI in paediatric patients.
Cases p48 and p49 occurred in the same patient (>1 month apart) (see Table 4).
The extended-spectrum β-lactamase (ESBL)-producers (6 and 23) and three unidentified isolates (34, 35 and p41) were excluded for the calculation of MIC50/90 and % S isolates.
2.2. Screening for extended-spectrum β-lactamase-producers and categorisation of inducible and derepressed isolates
To screen for ESBL production, Ecl were tested using the double-disk synergy test (DDST) on Mueller–Hinton agar plates (Oxoid Ltd., Pratteln, Switzerland) containing cloxacillin (Sigma, Buchs, Switzerland) (concentration 200 µg/mL): aztreonam, ceftazidime, cefotaxime and FEP disks (Becton Dickinson AG, Basel, Switzerland) were placed 20 mm apart around a disk containing AMC. The disk approximation assay was implemented to recognise Ind-Ecl [17].
2.3. Molecular studies
Genomic extraction was performed using a DNA Bacteria Plus Kit (QIAGEN, Hombrechtikon, Switzerland). The microarray CT-101 (Check-Points, Wageningen, The Netherlands) was used to detect class A, B and C β-lactamase genes [18]. An Identibac microarray AMR-ve v.05m (Alere Technologies GmbH, Jena, Germany) was also used to identify genes conferring resistance to β-lactams (including blaOXA types), quinolones, aminoglycosides, tetracyclines, sulfonamides and trimethoprim as well as class I/II integrases.
For selected Ecl, partial DNA sequencing for the chromosomal blaAmpC was performed as previously described [19]. The ampD gene and its promoter were also studied with primers ampD-F (5′-TATTAATACGTTCCAGAAGC-3′) and ampD-R (5′-CATGGTAAACAACGTCATGT-3′). Analysis of the IS4321 element was carried out with ampD-F/-R along with primers IS-F1 (5′-CGACTG AGTACCATTCTTGAG-3′), IS-F2 (5′-AAAGCAACTTTGTCGTCACTT-3′) and IS-F3 (5′-CGGAGCGTCTATATGGGACAG-3′). blaSHV and blaCTX-M were analysed as previously reported [20]. DNA sequencing was done using an ABI 3130 Sequencer (Applied Biosystems, Carlsbad, CA). Sequences were analysed using MEGA 4 and were compared with those described (http://www.lahey.org/Studies/). Results of blaAmpC and ampD sequencing were compared with the wild-type genes (GenBank accession nos. X07274 for blaP99 and Z14003 for ampD).
2.4. Analysis of clonal relatedness
Genetic relatedness was determined using repetitive extragenic palindromic PCR (rep-PCR) [20]. rep-PCR products were separated by electrophoresis using an Agilent 2100 Bioanalyzer (Agilent Technologies, Basel, Switzerland) and band patterns were analysed using GelCompar II software (Applied Maths, Sint-Martens-Latem, Belgium). Clonally related isolates were defined as those sharing a genetic homology ≥90%.
2.5. Clinical data
Patient charts of individuals with BSI-Ecl were reviewed retrospectively. The severity of underlying diseases was categorised using the McCabe–Jackson classification and the Charlson weighted index [21,22], whereas the severity of septicaemia was classified as previously described [23]. The estimated glomerular filtration rate prior to (the closest measurement within 14 days) and during the BSI episode (lowest value within 7 days from BSI onset) were obtained for adult patients [24].
The following predisposing conditions were investigated: bladder and intravascular catheters, drainages, mechanical ventilation, neutropenia, biopsies, oesophagogastroscopy and bronchoscopy when present for ≥72 h before the BSI onset. Previous use of antibiotics, immunosuppressive drugs (e.g. corticosteroids, anti-neoplastic agents), parenteral feeding, dialysis and surgery were taken into account when present during the 4 weeks before the BSI.
2.6. Definitions
Isolates obtained from blood cultures of the same patient >7 days after a previous BSI were considered as causing a new BSI if between the two episodes there was a period of clinical improvement and/or negative cultures. Secondary BSIs occurred when laboratory evidence showed a previous infection at a distant body site with an Ecl possessing the same AST results of that found in blood.
Empirical treatment was defined as the antibiotic(s) given before ID/AST results; it was considered adequate when the causative Ecl was subsequently found to be susceptible to the administered drug(s) (with the exception of 3GCs that were always considered inadequate) [1]. Treatment outcome was classified as ‘responders’ (including complete response and partial response), ‘non-responders’ (including relapse and treatment failure) and ‘not assessable’, as previously described [25]. Notably, the treatment outcome was attributed to the drug administered after the ID/AST results (i.e. directed treatment).
2.7. Statistical analysis
Statistical analysis was performed using STATA 10 (StataCorp LP, College Station, TX) only for adult (≥18 years) patients with Ind-Ecl or Der-Ecl BSI. Paediatric patients and those with ESBL-Ecl or unidentified isolates were excluded to avoid interferences in the interpretation of the results (see Table 2). Student’s unpaired t-test was used to compare continuous variables and the Mann–Whitney U-test was used to compare non-normally distributed continuous variables. Fisher’s exact test was used to compare patients infected by Ind-Ecl versus those with Der-Ecl and to evaluate factors associated with mortality. Differences were considered statistically significant when the two-tailed P-value was ≤0.05.
Table 2.
Clinical data of investigated adult bloodstream infection (BSI) cases due to Enterobacter cloacae (Ecl) isolates: difference between cases due to inducible (Ind-Ecl) and derepressed (Der-Ecl) isolates and factors associated with mortality.a,b
| Data | All BSI-Ecl cases | Ind-Ecl | Der-Ecl | P-value | No. deaths/no. cases |
P-value |
|---|---|---|---|---|---|---|
| Adult BSI cases analysedc | 43 | 26 | 17 | NA | 6 | NA |
| Female sex | 18 (41.9) | 6 (23.1) | 12 (70.6) | 0.004 | 4/18 (22.2) | NS |
| Age (years) (mean ± S.D.) | 59.5 ± 13.6 | 58.3 ± 11.9 | 61.4 ± 16.2 | NS | 65.3 | NS |
| McCabe–Jackson classification | ||||||
| Non-fatal | 20(46.5) | 10(38.5) | 10(58.8) | NSd | 2/20 (10.0) | NSd |
| Ultimately fatal | 22(51.2) | 15 (57.7) | 7 (41.2) | 3/22 (13.6) | ||
| Rapidly fatal | 1 (2.3) | 1 (3.8) | – | 1/1 (100) | ||
| Charlson weighted index (mean ± S.D.) | 4.3 ± 3.6 | 4.0 ± 3.8 | 4.6 ± 3.3 | NS | 9.5 ± 2.1 | 0.046 |
| Previous hospitalisation (during the last 12 months) | 28 (65.1) | 16 (61.5) | 12 (70.6) | NS | 4/28 (14.3) | NS |
| Hospital-acquired BSI | 29 (67.4) | 16 (61.5) | 13 (76.5) | NS | 4/29 (13.8) | NS |
| MLHS (days) (mean ± S.D.) | 30.2 ± 22.8 | 27.5 ± 24.5 | 34.3 ± 19.9 | 0.174 | 18.5 ± 2.1 | NS |
| MLHS before BSI diagnosis (days) (mean ± S.D.) | 12.4 ± 16.1 | 10.2 ± 17.8 | 15.7 ± 12.9 | 0.139 | 3.7 ± 6.4 | NS |
| MLHS after the BSI (days) (mean ± S.D.) | 17.7 ± 13.7 | 17.5 ± 14.4 | 18.1 ± 13.1 | NS | 15.3 ± 4.7 | NS |
| Ward of hospitalisation | ||||||
| ICU | 5 (11.6) | 4 (15.4) | 1 (5.9) | NS e | – | NS e |
| Medicine | 22(51.2) | 12(46.2) | 10 (58.8) | 5/22 (22.7) | ||
| Surgery | 16 (37.2) | 10 (38.5) | 6 (35.3) | 1/16 (6.3) | ||
| Severity of septicaemia | ||||||
| Sepsis | 32 (74.4) | 21 (80.8) | 11 (64.7) | NSf | 3/32 (9.4) | 0.164f |
| Severe sepsis | 10 (23.3) | 5 (19.2) | 5 (29.4) | 2/10 (20.0) | ||
| Septic shock | 1(2.3) | – | 1(5.9) | 1/1 (100) | ||
| Predisposing factors | ||||||
| Bladder catheter | 23 (53.5) | 12 (46.2) | 11 (64.7) | NS | 3/23 (13.0) | NS |
| Intravascular catheter | 31 (72.1) | 16 (61.5) | 15 (88.2) | 0.085 | 4/31 (12.9) | NS |
| Previous use of antibiotics | 29 (67.4) | 16 (61.5) | 13 (76.5) | NS | 4/29 (13.8) | NS |
| AMC | 15 (34.9) | 8 (30.8) | 7 (41.2) | NS | 2/15 (13.3) | |
| 3GCs | 3 (7.0) | 1 (3.8) | 2 (11.8) | NS | 1/3 (33.3) | |
| TZP | 5(11.6) | 3 (11.5) | 2 (11.8) | NS | – | |
| Cefepime | 7 (16.3) | 3 (11.5) | 4 (23.5) | NS | 1/7 (14.3) | |
| Carbapenems | 4 (9.3) | 2 (7.7) | 2 (11.8) | NS | – | |
| Other β-lactams | 4 (9.3) | 2 (7.7) | 2 (11.8) | NS | – | |
| Quinolones | 5 (11.6) | 2 (7.7) | 3 (17.6) | NS | – | |
| Aminoglycosides | – | – | – | – | – | |
| Anti-Gram-positivesg | 10 (23.3) | 7 (26.9) | 3 (17.6) | NS | – | |
| Metronidazole | 5 (11.6) | 3 (11.5) | 2 (11.8) | NS | – | |
| Immunosuppressive therapy | 18 (41.9) | 12 (46.2) | 6 (35.3) | NS | 2/18 (11.1) | NS |
| Previous surgery | 15 (34.9) | 7 (26.9) | 8 (47.1) | NS | 2/15 (13.3) | NS |
| Biopsies | 4 (9.3) | 2 (7.7) | 2 (11.8) | NS | – | NS |
| Dialysis | 6 (14.0) | 2 (7.7) | 4 (23.5) | 0.193 | 1/6 (16.7) | NS |
| Parenteral feeding | 14(32.6) | 10(38.5) | 4(23.5) | NS | 2/14 (14.3) | NS |
| Drainages | 8 (18.6) | 4 (15.4) | 4 (23.5) | NS | 1/8 (12.5) | NS |
| Mechanical ventilation | 11 (25.6) | 8 (30.8) | 3 (17.6) | NS | 2/11 (18.2) | NS |
| Oesophagogastroscopy | 8 (18.6) | 4 (15.4) | 4 (23.5) | NS | 1/8 (12.5) | NS |
| Neutropenia | 13 (30.2) | 9 (34.6) | 4 (23.5) | NS | 1/13 (7.7) | NS |
| Overall secondary BSI (only if culture positive) | 8 (18.6) | 6 (23.1) | 2 (11.8) | NS | – | |
| Urinary tract | 6 (14.0) | 5 (19.2) | 1 (5.9) | NS | – | |
| Lower respiratory tract | – | – | – | NS | – | |
| Intravenous catheter | 3 (7.0) | 2 (7.7) | 1 (5.9) | NS | – | |
| eGFR prior the BSI episode | ||||||
| ≥90 mL/min | 22 (52.4)h | 16 (64.0) | 6 (35.3) | 0.115h | 1/22 (4.5) | 0.087h |
| 51–89 mL/min | 15 (35.7)h | 8 (32.0) | 7 (41.2) | 3/15 (20.0) | ||
| ≤50 mL/min | 5 (11.9)h | 1 (4.0) | 4 (23.5) | 2/5 (40.0) | ||
| eGFR during the BSI episode | ||||||
| ≥90 mL/min | 15 (34.9) | 11 (42.3) | 4 (23.5) | NSh | 2/15 (13.3) | NSh |
| 51–89 mL/min | 10 (23.3) | 7 (26.9) | 3 (17.6) | – | ||
| ≤50 mL/min | 18 (41.9) | 8 (30.8) | 10 (58.8) | 4/18 (22.2) | ||
| Empirical treatment provided | 42 (97.7) | 26 (100) | 16 (94.1) | NS | 5/42 (11.9) | 0.140 |
| Not adequate | 17 (39.5) | 9 (34.6) | 8 (47.1) | NSi | 3/17 (17.6) | NSi |
| AMC | 3 (7.0) | 2 (7.7) | 1 (5.9) | 1/3 (33.3) | ||
| 3GCs | 10 (23.3) | 7 (26.9) | 3 (17.6) | 1/10 (10.0) | ||
| TZP | 3 (7.0) | – | 3 (17.6) | 1/3 (33.3) | ||
| Cefepime | 1(2.3) | – | 1 (5.9) | – | ||
| Adequate | 25 (58.1) | 17 (65.4) | 8 (47.1) | 3/25 (12.0) | ||
| Cefepime | 17 (39.5) | 10 (38.5) | 7 (41.2) | 2/17 (11.8) | ||
| TZP | 1 (2.3) | 1 (3.8) | – | – | ||
| Carbapenems | 5 (11.6) | 4 (15.4) | 1 (5.9) | 1/5 (20.0) | ||
| Quinolones | 1 (2.3) | 1 (3.8) | – | – | ||
| Aminoglycosides | 1 (2.3) | 1 (3.8) | – | – | ||
| Treatment after ID/AST results (i.e. directed treatment) | ||||||
| Non-adequate | 8 (18.6) | 4 (15.4) | 4 (23.5) | NSi | 3/8 (37.5) | 0.067i |
| Adequate | 35 (81.4) | 22 (84.6) | 13 (76.5) | 3/35 (8.6) | ||
| 28-day mortality | 6(14.0) | 1 (3.8) | 5 (29.4) | 0.028 | NA | NA |
| Mortality attributable to BSI | 2 (4.7) | – | 2 (11.8) | 0.151 | NA | NA |
NA, not applicable; NS, not significant (results reported only when P ≤ 0.200); S.D., standard deviation; MLHS, mean length of hospital stay; ICU, Intensive Care Unit; AMC, amoxicillin/clavulanic acid; 3GC, third-generation cephalosporins; TZP, piperacillin/tazobactam; eGFR, estimated glomerular filtration rate; ID, identification; AST, antimicrobial susceptibility testing.
BSI cases initially due to Ind-Ecl but then evolving to Der-Ecl (5, 30 and p45) and those simultaneously having Ind-Ecl and Der-Ecl isolates (3, 26 and 31) were included only in the group of Der-Ecl.
Data are expressed as n (%) of patients unless otherwise indicated; – indicates n = 0.
One adult patient had two BSIs (4 and 43). Three paediatric patients (p41, p45 and p48/p49) were excluded; cases due to unidentified (34 and 35) and ESBL-Ecl (6 and 23) were also excluded.
Calculated as patients with non-fatal versus those with ultimately fatal plus rapidly fatal diseases.
Calculated as ICU versus non-ICU patients (i.e. those from medicine plus surgery).
Calculated as patients with sepsis versus those with severe sepsis and septic shock.
Includes vancomycin, clindamycin, daptomycin and flucloxacillin.
Calculated as patients with eGFR ≥90 mL/min versus those with eGFR <90 mL/min (data for one case due to Ind-Ecl were not available).
Calculated as cases receiving non-adequate versus those receiving adequate treatment.
2.8. Nucleotide sequence accession numbers
The sequences regarding the analysis of the ampD gene have been submitted to GenBank under accession nos. JX482621–JX482632.
3. Results and discussion
In this study, 51 BSI-Ecl were analysed that occurred in 49 patients, of which 3 were paediatric patients (p41, p45 and one with two BSIs, p48 and p49). Three cases (3, 26 and 31) were due to a simultaneous infection of Ind-Ecl and Der-Ecl, whereas three patients (5, 30 and p45) had an initial BSI due to an Ind-Ecl that evolved in a few days to a Der-Ecl during the same infectious episode. Overall, 57 Ecl were available for phenotypic and molecular studies (Table 1). The prevalence of BSI-Ecl at the University Hospital of Bern in 2010 was 3.4%.
3.1. Characterisation of isolates
Phenotypic tests identified 34 Ind-Ecl, 18 Der-Ecl, 3 unidentified and only 2 ESBL-Ecl (isolate 6 is SHV-12, whereas isolate 23 is CTX-M-1; Table 1). The small number of ESBL-producers in this collection (3.5%) was consistent with the low national prevalence for Escherichia coli (4.8%) and Klebsiella pneumoniae (4.6%) observed in 2010 (http://www.anresis.ch). In contrast, it was surprising to observe that all Ecl were uniformly susceptible to ciprofloxacin (CIP) and aminoglycosides (Table 1). For ESBL-negative Ecl, resistance rates of ca. 20% (but up to 67%) are reported for both CIP and gentamicin [9]. These epidemiological differences may be due to the limited use of quinolones in our area [26]. Moreover, since previous use of quinolones is a well-known risk factor associated with the selection of ESBL-Ecl [8], our low prevalence of ESBL-producers is also probably secondary to this different antibiotic prescribing practice.
Ind-Ecl tested more susceptible than Der-Ecl to 3GCs (e.g. CRO, 76.5% vs. 0%, respectively) and TZP (85.3% vs. 0%), whereas FEP, imipenem and meropenem (MEM) were the most active drugs in vitro regardless of the inducible or derepressed phenotypes (susceptibilities of 86%, 100% and 100%, respectively; Table 1). Based on the findings, empirical treatment with FEP is justified when BSI-Ecl is suspected and this may help to limit the overuse of carbapenems. In this context, rapid identification of Ecl (e.g. using MALDI-TOF/MS directly on positive blood cultures) may play a key role in shortening the interval from empirical treatment to directed therapy [27]. Nevertheless, one of the most important factors in choosing an antimicrobial for empirical treatment (e.g. carbapenem vs. FEP) remains the local epidemiology (e.g. prevalence of ESBL-Ecl).
Consistent with the phenotypic data, molecular analyses indicated that the isolates carried a limited number of resistance genes: 17 Ecl carried wild-type TEM β-lactamases, several harboured genes conferring resistance to other antibiotic classes (e.g. dfrV, dfrA, tetD, sul2, aadA1) and only 2 (isolates 6 and 7) carried the quinolone resistance determinant qnrB. The qnrB-containing isolates exhibited only decreased susceptibility to CIP, whose MICs remained below the recommended resistance breakpoints (Table 1). Usually, the above resistance genes are carried on plasmid(s) along with the blaESBLs. Thus, the limited number of resistance determinants observed in this collection is not surprising.
Interestingly, in one Ecl detected in a patient who underwent prolonged treatment with FEP before the second BSI (case 43; Table 3), a high MIC for the drug was recorded that was lowered when tested in the presence of cloxacillin (96 µg/mL and 2 µg/mL, respectively). Moreover, the DDST was negative (indicating ESBL production was not present) and common class A and D bla genes were not detected (Table 1). Analysis of blaP99 revealed the following amino acid substitutions: P169S, S247T, E285G and A292P. Amino acid substitutions in position 291 and 293 increase the ability of cAmpC enzymes to hydrolyse FEP [28,29]. It can be speculated that the A292P substitution found in our Ecl plays a similar role. However, this needs to be confirmed with more appropriate studies.
Table 3.
Clinical characteristics, antimicrobial regimens and treatment outcome for patients with bloodstream infection (BSI) due to Enterobacter cloacae (Ecl) isolates with derepressed AmpC (Der-Ecl) and those with unidentified phenotypes.a
| No. | Age (years)/sex |
Admitted for (ward) |
McCabe– Jackson class, Charlson index |
Severity of septicaemia |
Primary source of BSIb |
eGFR prior/during BSI (mL/min)c |
Empirical treatment (total days) |
Therapy administered after ID/AST results |
Treatment outcome (comments) |
|
|---|---|---|---|---|---|---|---|---|---|---|
| Agent (timing from BSI onset) |
Daily dose (total days) |
|||||||||
| 3-A/-B | 65/M | AML (oncology) | UF, 4 | Sepsis | (Neutropenic colitis) | ≥90/≥90 | FEP (12) | MEM (1 day after) | 1 g × 3 (10) | PR (death 11 days after BSI) |
| 5-I | 71/F | Aortic valve stenosis (nephrology) | NF, 5 | Septic shock | Unknown | NA/<10 | MEM (5) | TZP (2 days after) | NA (11) | Failure (Ind-Ecl evolved to Der-Ecl after 3 days, see #5-II) |
| 5-II | – | – | – | – | – | <10 | – | FEP (13 days after) | 2 g × 2 (4) | Relapse (death 17 days after the first BSI) |
| 16 | 64/F | Breast Ca (oncology) | UF, 10 | Sepsis | Unknown | ≥90/≥90 | AMC | AMC (25 days before) | 625 mg × 3 (43) | Not assessable |
| 22 | 74/M | Rupture AAA (vascular surgery) | NF, 4 | Sepsis | (Infected vascular prosthesis) | 57/<10 | CFZ (1) | MEM (1 day after) | 1 g × 1 (30) | CR |
| 23 | 70/F | Valve replacement (vascular surgery/ICU) | NF, 3 | Severe sepsis | LRTI, IVC | 78/≥90 | FEP | Unchanged (11 days before) | 2 g × 3 (31) | Failure (ESBL-Ecl; death 69 days after BSI) |
| 26-A/-B | 85/M | Endocarditis (medicine) | NF, 4 | Sepsis | (Endocarditis) | 30/18 | CRO | Unchanged (1 day before) | 2 g × 1 (18) | Failure (both Ind-Ecl and Der-Ecl; death 18 days after BSI) |
| 30-I | 26/F | Gastroparesis (abdominal surgery) | NF, 0 | Sepsis | IVC | PH | AMC (1); TZP (1) | CRO (2 days after) | 2 g × 1 (5) | Failure (Ind-Ecl evolved to Der-Ecl after 6 days, see #30-II) |
| 30-II | – | – | – | – | – | ≥90/≥90 | TZP (2) | MEM (3 days after) | 2 g × 3 (13) | CR |
| 31-A/-B | 78/M | Valve replacement (vascular surgery) | NF, 5 | Severe sepsis | (Colitis) | 55/22 | FEP (3) | MEM (3 days after) | 1 g × 3 (17) | CR (both Ind-Ecl and Der-Ecl) |
| 32 | 41/F | Haemoptysis, fever (medicine) | NF, 7 | Sepsis | (IVC) | 15/13 | FEP (1) | MEM (4 days after) | 1 g × 3 (6) | CR |
| 33 | 81/M | Wound infection, PAVD (vascular surgery) | NF, 6 | Sepsis | (Central venous catheter) | 68/55 | FEP | Unchanged (first day) | 2 g × 1 (10) | Not assessable |
| 37 | 64/F | Cardiac decompensation (nephrology) | NF, 7 | Sepsis | UTI | 10/23 | CAZ (4) | CIP (5 days after) | NA (9) | CR |
| 39 | 57/F | Stromal tumour, sepsis (medicine) | UF, 11 | Severe sepsis | (Small intestine) | 61/45 | FEP (4) | CIP (4 days after) | 750 mg × 2 (6) | PR (death 17 days after the first BSI) |
| 43 | 51/F | Neutropenic fever, AML (oncology) | UF, 2 | Sepsis | (Neutropenic colitis) | ≥90/≥90 | FEP (41) | MEM (first day) | 2 g × 3 (3) | PR (previous BSI due to Ind-Ecl, see #4) |
| 44 | 33/F | Pre-eclampsia (gynaecology) | NF, 0 | Severe sepsis | (UTI) | ≥90/44 | AMC (1); FEP (2) | MEM (3 days after) | 1 g × 3 (3) | CR |
| p45-I | <1/M | Pyelonephritis (paediatric surgery) | NF, NA | na | UTI | na | CRO (2) | FEP (3 days after) | NA (4) | CR (Ind-Ecl evolved to Der-Ecl after 8 days, see #p45-II) |
| p45-II | – | Persistent UTIs (paediatric) | – | na | UTI | na | None | SXT (first day) | NA (NA) | Not assessable |
| 46 | 81/M | Abdominal abscess (ICU) | NF, 1 | Severe sepsis | (Abdominal abscess) | ≥90/84 | TZP | Unchanged (first day) | 4.5 g × 3 (14) | PR |
| 47 | 64/F | ALL (oncology) | UF, 2 | Sepsis | Unknown | 89/42 | FEP | Unchanged (first day) | 2 g × 3 (46) | PR |
| 50 | 63/F | AML (oncology) | UF, 2 | Sepsis | (Neutropenic colitis) | 80/86 | FEP (4) | MEM (4 days after) | 1 g × 3 (25) | CR |
| 51 | 62/F | Pancreatic Ca (abdominal surgery) | UF, 8 | Severe sepsis | (Cholangiosepsis) | 88/21 | TZP | Unchanged (9 days before) | 4.5 g × 3 (16) | Failure (transferred to LTCF; death in a few days because of BSI) |
| 34d | 62/F | AML (oncology) | UF, 2 | Severe sepsis | (Neutropenic colitis) | ≥90/≥90 | FEP | Unchanged (first day) | 2 g × 3 (25) | CR |
| 35d | 33/M | AML (oncology) | UF, 2 | Sepsis | (Neutropenic colitis) | ≥90/≥90 | FEP | Unchanged (first day) | 2 g × 3 (6) | CR |
| p41d | <1/F | Fever, intestinal ataxia (paediatric) | NF, 0 | NA | (Colitis) | na | FEP (5) | MEM (5 days after) | 35 mg/kg × 3 (12) | CR |
ID, identification; AST, antimicrobial susceptibility test; AML, acute myeloid leukaemia; Ca, carcinoma; AAA, abdominal aortic aneurysm; ICU, Intensive Care Unit; PAVD, peripheral arterial vascular disease; UTI, urinary tract infection; ALL, acute lymphoblastic leukaemia; UF, ultimately fatal; NF, non-fatal; NA, not available; na, not applicable; LRTI, lower respiratory tract infection; IVC, intravascular catheter colonisation; PH, previously healthy; FEP, cefepime; MEM, meropenem; AMC, amoxicillin/clavulanic acid; CFZ, cefazolin; CRO, ceftriaxone; TZP, piperacillin/tazobactam; CAZ, ceftazidime; CIP, ciprofloxacin; SXT, trimethoprim/sulfamethoxazole; PR, partial response; CR, complete response; ESBL, extended-spectrum β-lactamase; LTCF, long-term care facility.
BSI cases initially due to inducible Ecl (Ind-Ecl) but then evolving to Der-Ecl (5, 30 and p45) and those simultaneously having Ind-Ecl and Der-Ecl strains (3, 26 and 31) were included only in this table.
Primary sources based only on clinical suspicion (and not culture results) are reported in parentheses.
Estimated glomerular filtration rate (eGFR) prior to BSI (defined as closest measurement within 14 days) and during sepsis (defined as lowest value within 7 days from date of BSI onset).
The three cases of BSI due to unidentified (i.e. non-inducible and non-derepressed) Ecl isolates are also presented in this table.
3.2. Analysis of clonality and ampD gene
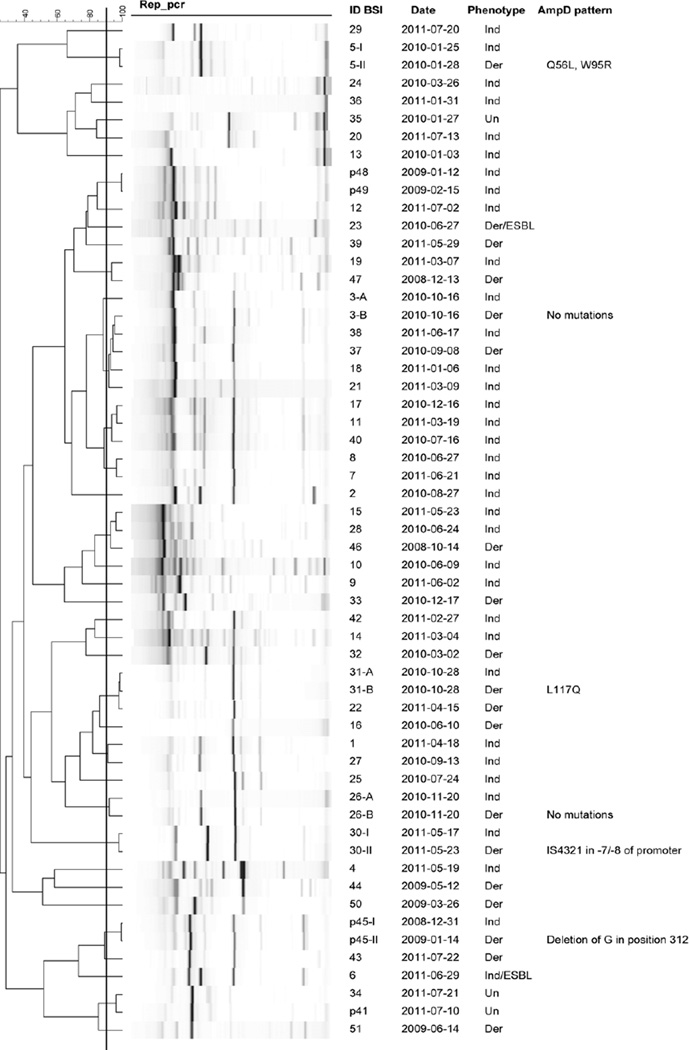
Most Ecl possessed a genetic homology <90% by rep-PCR, indicating a non-outbreak situation. However, the Ind-Ecl and Der-Ecl simultaneously detected in the same blood cultures (3-A/-B, 26-A/-B and 31-A/-B) were genetically indistinguishable or highly related (Fig. 1). Such a phenomenon was also observed for the Ecl detected in three patients who had an initial BSI due to Ind-Ecl (5-I, 30-I and p45-I) that evolved to Der-Ecl (5-II, 30-II and p45-II).
Fig. 1.
Results of repetitive extragenic palindromic PCR (rep-PCR) analysis performed for all Enterobacter cloacae (Ecl) isolates (n = 57) responsible for 51 cases of bloodstream infection (BSI) in 49 patients (46 adult and 3 paediatric patients). Overall, the majority of Ecl were not clonally related (i.e. genetic homology <90%). However, regardless of the inducible (Ind) or derepressed (Der) phenotype, Ecl isolated from the same patient were indistinguishable or highly related. In particular, Der-Ecl 3-B, 26-B and 31-B were found in the same blood culture along with those that were inducible (3-A, 26-A and 31-A), whereas Der-Ecl 5-II, 30-II and p45-II were detected after a few days of treatment implemented to cure the BSI due to Ind-Ecl (5-I, 30-I and p45-I). The figure illustrates the molecular mechanism of resistance affecting the ampD gene and leading to the derepressed phenotype in the above six cases. Un, unknown; ESBL, extended-spectrum β-lactamase.
Interestingly, the ampD of several of the above Der-Ecl showed sequence differences compared with the initial Ind-Ecl (Fig. 1). In particular, isolates 31-B and 5-II developed amino acid substitutions, whereas p45-II had a frameshift (leading to a stop codon) due to a single nucleotide deletion of a G in position 312. More intriguingly, isolate 30-II had a wild-type ampD gene as observed in its original Ind-Ecl (30-I), but possessed an IS4321 inserted between positions 7 and 8 of the promoter. These last two mechanisms of resistance have never been reported in Ecl and indicate that there are more ways to select for Der-Ecl. For the remaining Der-Ecl without a modification of ampD (3-B and 26-B), we speculate that there may be mutations in ampR [4].
Taken together, the above results illustrate the dynamic evolution of Ind-Ecl to Der-Ecl when patients are under certain β-lactam regimens (e.g. CRO for cases 30-I and p45-I, and TZP for 5-I) (Table 3). To our knowledge, this is the first study where such a phenomenon is shown in vivo and established using molecular techniques. These findings raise the question whether or not AST results for 3GCs in Ecl should even be reported [3,5].
3.3. Clinical characteristics of patients and empirical treatment
As previously observed [2,14], BSI-Ecl in adults were frequently hospital-acquired (68%) and occurred in patients with severe underlying diseases (mean Charlson index of 4.2), previous hospitalisations (67%), previous use of antibiotics (68%) and risk factors for infection (e.g. intravascular and bladder catheters, 73% and 55%, respectively) (Table 2). As shown in Tables 3 and 4, the most frequent underlying diseases were leukaemia/lymphoma (n = 15) and solid tumours (n = 10). These patient characteristics can help to guide the choice of empirical treatment for a defined population (Tables 3 and 4). In this regard, the most frequent empirical treatments were FEP (n = 23), CRO (n = 10), MEM (n = 6) and TZP (n = 6). In particular, FEP was frequently used in oncology (13/15 patients) according to the international guidelines for neutropenic patients.
Table 4.
Clinical characteristics, antimicrobial regimens and treatment outcome for bloodstream infection (BSI) cases due to Enterobacter cloacae (Ecl) isolates with inducible AmpC phenotype (Ind-Ecl).a
| No. | Age (years)/sex |
Admitted for (ward) |
McCabe– Jackson class, Charlson index |
Severity of septicaemia |
Primary source of BSIb |
eGFR prior/during BSI (mL/min)c |
Empirical treatment (total days) |
Therapy administered after ID/AST results |
Treatment outcome (comments) |
|
|---|---|---|---|---|---|---|---|---|---|---|
| Agent (timing from BSI onset) |
Daily dose (total days) |
|||||||||
| 1 | 66/M | Pancreatic Ca (abdominal surgery) | UF, 13 | Severe sepsis | (UTI) | ≥90/≥90 | CRO (4) | AMC (4 days after); CIP (4 days after) | 625 mg × 3 (11); 500 mg × 2 (11) | PR |
| 2 | 63/M | Gallbladder Ca (abdominal surgery) | UF, 11 | Severe sepsis | (UTI) | ≥90/55 | IPM | Unchanged (20 days before) | 500 mg × 4 (37) | CR |
| 4 | 51/F | Neutropenic fever, AML (oncology) | UF, 2 | Sepsis | (Neutropenic colitis) | ≥90/≥90 | AMC (17), CIP (17) | FEP (1 day after); CIP (5 days after) | 2 g × 3 (5); 500 mg × 2 (10) | PR (new BSI, see #43) |
| 6 | 44/F | Subarachnoid bleeding (neurosurgery) | NF, 3 | Sepsis | (Cholangiosepsis) | ≥90/≥90 | TZP (1), CRO (1) | FEP (2 days after) | 1 g × 3 (14) | CR (ESBL-Ecl) |
| 7 | 60/F | Ureteral Ca (urology) | UF, 6 | Sepsis | UTI, IVC | ≥90/50 | AMC (4), TOB (4) | CIP (4 days after) | 500 mg × 2 (20) | CR |
| 8 | 50/M | ALL (oncology) | UF, 3 | Sepsis | UTI | 71/79 | FEP | Unchanged (first day) | 1 g × 3 (15) | CR |
| 9 | 60/M | Polytrauma (ICU) | NF, 0 | Sepsis | (Wound infection) | PH/21 | SXT (1), FEP | FEP (first day) | 1 g × 2 (8) | PR |
| 10 | 50/M | ALL (oncology) | UF, 2 | Sepsis | (Neutropenic colitis) | 51/86 | FEP | Unchanged (first day) | 1 g × 3 (22) | CR |
| 11 | 57/M | ALL (oncology) | UF, 3 | Sepsis | Unknown | ≥90/86 | FEP (3) | CRO (4 days after) | 2 g × 1 (7) | CR |
| 12 | 51/M | Polytrauma (ICU) | NF, 0 | Severe sepsis | Unknown | PH/24 | TZP | Unchanged (first day) | 2.25 g × 4 (9) | CR |
| 13 | 70/M | Cardiogenic shock (vascular surgery) | NF, 5 | Sepsis | (Central venous catheter) | 53/35 | MEM (3) | FEP (4 days after) | 1 g × 3 (8) | CR |
| 14 | 55/M | Cardiogenic shock (vascular surgery) | NF, 3 | Sepsis | (UTI) | NA/64 | CRO | Unchanged (first day) | 2 g × 1 (11) | CR |
| 15 | 74/F | Neutropenic fever (oncology) | UF, 4 | Sepsis | (Neutropenic colitis) | 88/85 | FEP | Unchanged (first day) | 2 g × 3 (12) | PR |
| 17 | 39/M | AML (oncology) | UF, 2 | Sepsis | Unknown | ≥90/≥90 | FEP | Unchanged (first day) | 1 g × 3 (20) | CR |
| 18 | 44/F | AML (oncology) | UF, 2 | Sepsis | UTI | PH/≥90 | FEP (3) | TZP (3 days after) | 4.5 mg × 3 (4) | PR |
| 19 | 67/F | Vaginal Ca (abdominal surgery) | UF, 3 | Severe sepsis | (Fistula in small intestine) | ≥90/61 | IPM, TOB | Unchanged (first day) | 1 g × 3 (9); 5 mg/kg × 1 (1) | Relapse |
| 20 | 67/M | Polytrauma (orthopaedic) | NF, 0 | Sepsis | (Cholecystitis) | PH/≥90 | CRO (2) | FEP (3 days after); MEM (7 days after) | 1 g × 3 (4); 1 g × 3 (28) | CR |
| 21 | 68/M | Lung Ca (medicine) | RF, 8 | Sepsis | Unknown | 79/≥90 | AMC (7) | CRO (1 day after) | 2 g × 1 (9) | Failure (death 22 days after BSI onset) |
| 24 | 75/M | Urosepsis, prostate Ca (urology) | NF, 4 | Severe sepsis | UTI | 48/38 | CRO | Unchanged (first day) | 2 g × 1 (4) | CR |
| 25 | 73/M | Choledocolithiasis (ICU) | NF, 5 | Sepsis | (Cholangiosepsis) | 51/26 | CRO (1) | TZP (2 days after) | 4.5 g × 3 (4) | PR |
| 27 | 70/M | Heart bypass (ICU) | NF, NA | Sepsis | IVC | 65/27 | FEP | Unchanged (first day) | 2 g × 1 (14) | CR |
| 28 | 27/M | Acute appendicitis (abdominal surgery) | NF, 0 | Sepsis | (Appendicitis) | ≥90/≥90 | AMC (1) | CIP (3 days after) | 500 mg × 2 (6) | CR |
| 29 | 65/F | Hepatic Ca (abdominal surgery) | UF, 13 | Sepsis | Unknown | 68/30 | TZP | Unchanged (first day) | 4.5 g × 3 (13) | CR |
| 36 | 59/M | Lung Ca, pneumonia (medicine) | UF, 7 | Sepsis | (LRTI) | ≥90/≥90 | FEP | Unchanged (first day) | 1 g × 3 (10) | CR |
| 38 | 49/M | AML (oncology) | UF, 2 | Sepsis | UTI | ≥90/≥90 | MEM (2) | FEP (3 days after) | 2 g × 3 (10) | CR |
| 40 | 62/M | Neutropenic fever (abdominal surgery) | NF, 1 | Sepsis | (Cholangiosepsis) | ≥90/≥90 | CRO (7) | CIP (8 days after) | 500 mg × 2 (15) | CR |
| 42 | 44/M | Fever, T-cell lymphoma (oncology) | UF, 2 | Sepsis | Unknown | ≥90/≥90 | FEP (NA) | MEM (NA) | NA, NA | CR |
| p48 | 8/M | Severe aplastic anaemia (paediatric) | RF, 0 | na | Unknown | na | MEM (4) | CRO (4 days after) | NA (7) | PR (new BSI, see #p49) |
| p49 | – | – | – | na | Unknown | na | CRO (5), AMK (10) | MEM (1 day after); CIP (5 days after) | NA (11); NA (7) | CR |
ID, identification; AST, antimicrobial susceptibility test; Ca, carcinoma; AML, acute myeloid leukaemia; ALL, acute lymphoblastic leukaemia; ICU, Intensive Care Unit; UF, ultimately fatal; NF, non-fatal; RF, rapidly fatal; na, not applicable; UTI, urinary tract infection; IVC, intravascular catheter colonisation; LRTI, lower respiratory tract infection; PH, previously healthy; NA, not available; CRO, ceftriaxone; IPM, imipenem; AMC, amoxicillin/clavulanic acid; CIP, ciprofloxacin; TZP, piperacillin/tazobactam; TOB, tobramycin; FEP, cefepime; SXT, trimethoprim/sulfamethoxazole; MEM, meropenem; AMK, amikacin; PR, partial response; CR, complete response; ESBL, extended-spectrum β-lactamase.
BSI cases initially due to Ind-Ecl but then evolving to derepressed Ecl (Der-Ecl) (5, 30 and p45) and those simultaneously having Ind-Ecl and Der-Ecl strains (3, 26 and 31) are included only in Table 3.
Primary sources based only on clinical suspicion (and not culture results) are reported in parentheses.
Estimated glomerular filtration rate (eGFR) prior to BSI (defined as closest measurement within 14 days) and during sepsis (defined lowest value within 7 days from date of BSI).
3.4. Comparison of bloodstream infection episodes due to Ind-Ecl and Der-Ecl isolates
Adult patients with BSIs due to Der-Ecl tended to be older, more often immunocompromised and to have more risk factors for Gram-negative sepsis than those with BSIs due to Ind-Ecl (Table 2). Among the mentioned risk factors, the use of intravascular catheters tended to be significant (P < 0.10). Female sex was also significantly more frequent in BSIs due to Der-Ecl (P = 0.004) but we cannot explain this finding.
In contrast with other reports [2,3], we were surprised to see that patients with BSIs due to Der-Ecl did not have a significant association with previous use of β-lactams (e.g. 3GCs). This might be due to the following reasons. First, we recorded only the use of antibiotics during the 4 weeks preceding the BSI and not that during previous hospitalisations. In fact, the majority of patients (ca. 60%) had an earlier hospitalisation with potential acquisition/selection of Der-Ecl. Second, according to our in-house strategy, most patients included in the study qualified for FEP treatment, potentially making the absolute number with previous β-lactam exposure too small for statistical significance.
3.5. Factors associated with mortality
In this study, the 28-day and attributable mortality in adults were slightly lower (i.e. 14.0% and 4.7%, respectively) than usually observed [2,13], but 28-day mortality was significantly higher for patients with Der-Ecl than that for those with Ind-Ecl (29.4% vs. 3.8%; P = 0.028). We also observed that patients who died had a Charlson index significantly higher than the overall average (P = 0.046). These two findings might be related to the previously discussed exposure in a healthcare institution and acquisition of Der-Ecl.
The adequateness of empirical treatment (57% of the overall cases) was not significantly related to mortality, but the directed antimicrobial treatment likely played a role in determining the outcome (P < 0.10). In this context, we noted that two patients who died because of the BSI (26 and 51) were managed with CRO or TZP (Table 3).
3.6. Directed therapy and treatment outcome
Analysis of treatment outcome is summarised in Table 5. Overall, after the ID/AST results, 18 cases were managed with FEP, 13 with carbapenems, 7 with CRO, 7 with TZP and 6 with CIP. Responders were observed in ≥89% of cases managed with FEP, carbapenems and CIP, whereas only 57–71% of patients treated with CRO and TZP presented a favourable treatment outcome (Table 5).
Table 5.
Treatment outcome [n (%)] of all bloodstream infection (BSI) cases due to Enterobacter cloacae (Ecl) isolates: overall data and comparison between cases due to inducible (Ind-Ecl) and derepressed (Der-Ecl) isolates.
| Antimicrobial administered | Phenotype and BSI casesa,b,c | All BSIs due to E. cloacae (n = 51)d | BSIs due to Ind-Ecl (n = 31) | BSIs due to Der-Ecl (n = 15)c | |||
|---|---|---|---|---|---|---|---|
| Responders | Non-responders | Responders | Non-responders | Responders | Non-responders | ||
| CRO | Ind-Ecl: 11, 14, 21, 24, 30-I, p48 | 4 (57.1)e | 3 (42.9) | 4 (66.7) | 2 (33.3) | – | 1 (100) |
| Der-Ecl: 26-B | |||||||
| FEP | Ind-Ecl: 4, 8, 9, 10, 13, 15, 17, 20, 27, 36, 38, p45-I | 16 (88.9) | 2 (11.1)f | 12 (100) | – | 1 (50.0) | 1 (50.0) |
| Der-Ecl: 5-II, 47 | |||||||
| ESBL-Ecl: 6, 23 | |||||||
| Unidentified: 34, 35 | |||||||
| TZP | Ind-Ecl: 12, 18, 25, 29, 5-I | 5(71.4) | 2(28.6) | 4(80.0) | 1(20.0) | 1(50.0) | 1(50.0) |
| Der-Ecl: 46, 51 | |||||||
| MEM | Ind-Ecl: 42, p49 | 11 (100) | – | 2 (100) | – | 8 (100) | – |
| Der-Ecl: 3-B, 22, 30-II, 31-B, 32, 43, 44, 50 | |||||||
| Unidentified: p41 | |||||||
| IPM | Ind-Ecl: 2, 19 | 1 (50.0) | 1 (50.0) | 1 (50.0) | 1 (50.0) | – | – |
| CIP | Ind-Ecl: 1, 7, 28, 40 | 6 (100) | – | 4 (100) | – | 2 (100) | – |
| Der-Ecl: 37, 39 | |||||||
| Total | 43(84.3) | 8(15.7) | 27(87.1) | 4(12.9) | 12(80.0) | 3(20.0) | |
CRO, ceftriaxone; FEP, cefepime; TZP, piperacillin/tazobactam; MEM, meropenem; IPM, imipenem; CIP, ciprofloxacin; ESBL, extended-spectrum β-lactamase.
For patients treated with two active antibiotics after identification/antimicrobial susceptibility testing results (i.e. directed treatment), only the drug started earlier was taken into consideration (e.g. FEP for cases 4 and 20 and MEM for case p49; see Table 4).
BSI cases 5-I/-II and 30-I/-II (i.e. due to Ind-Ecl that evolved to Der-Ecl) were taken into account as different BSI episodes.
The treatment outcomes of three BSI cases due to Der-Ecl (16, 33 and p45-II) were not assessable (see Table 3).
Three BSI cases due to unidentified Ecl (34, 35 and p41) and cases due to ESBL-Ecl (6 and 23) were included (see Tables 3 and 4).
Two of these BSI cases were empirically treated with FEP (case 11 for 3 days) or MEM (case p48 for 4 days) before implementing CRO.
Case 5-II received FEP treatment 13 days after the BSI onset, whereas case 23 was due to an ESBL-Ecl (see Table 3).
Considering the low prevalence of ESBL-producers in our institution, FEP is administered empirically for bacteraemia in immunosuppressed hosts, patients who were recently exposed to antibiotics, patients with multiple risk factors for Pseudomonas spp. infection or, as presented here, when Ecl is suspected. In this last specific situation, after identification of Ecl and verification of a FEP MIC ≤ 1 µg/mL (the EUCAST susceptibility breakpoint), the drug is continued as directed therapy [16]. However, in these cases, and to the best of our knowledge, there are no uniformly accepted recommendations [3,16,30]. Lee et al. analysed 13 BSI-Ecl, including 1 due to an ESBL-Ecl, in neutropenic patients managed with FEP. In three cases treatment failure was observed, of which two subsequently cured with imipenem [12]. Qureshi et al. noted that FEP treatment failed in 7 of 9 BSIs due to ESBL-Ecl, including those with MICs lower than the EUCAST cut-off [11,16]. Hence, on the basis of local prevalence of ESBL-producers, carbapenems might be more adequate for empirical treatment. The results of the current study showed that a favourable treatment outcome was obtained with FEP in 16 episodes in which the isolate had a MIC ≤ 1 µg/mL (including case 6 due to an ESBL-Ecl). The second case due to an ESBL-Ecl (isolate 23; MIC of 32 µg/mL) showed treatment failure, whereas although Der-Ecl isolate 5-II was fully sensitive to FEP (MIC of 0.75 µg/mL), the drug was unable to resolve the BSI probably because the treatment started too late (13 days after the onset of the infection) (Table 3). Overall, based on our local resistance patterns, our treatment strategy for BSI-Ecl appears to be safe and reasonable.
Carbapenems are the drugs of choice for BSIs due to ESBL-producing Enterobacteriaceae. However, these data are in large part limited to E. coli and K. pneumoniae, whereas only a few studies focused on ESBL-Ecl [8,11]. Twelve BSIs were treated and cured with carbapenems; only episode 19 presented a relapse of infection probably due to the severity of infection and underlying diseases (Table 4). However, the choice of compound was not only based on the MICs of the pathogens but also on postulated side effects of FEP and the corresponding physician’s decision. In our study, only two ESBL-Ecl were found. Nevertheless, considering that the MICs of carbapenems for ESBL- and non-ESBL-Ecl are comparable (Table 1 and [11]), these antibiotics are the best options for the treatment of BSI-Ecl [8]. Yet in order to avoid the selection of carbapenem-resistant isolates [7], we discourage the use of carbapenems if therapy with FEP is possible.
Despite the fact that 3GCs are not recommended for severe infections due to Ecl, in this series not all BSIs treated with CRO showed treatment failure. Four cases due to Ind-Ecl received directed therapy with CRO and experienced a favourable treatment outcome. However, we note that two patients (11 and p48) were managed with an empirical treatment with FEP and MEM for ≥3 days before implementing CRO. Moreover, all adults received 2 g/day of drug leading to high trough levels. Whether or not these plasma levels supported the clearing of the infection remains speculation.
In four BSIs due to Ind-Ecl the treatment outcome with TZP was favourable (all MICs ≤ 3 µg/mL), whereas in two of three cases due to Der-Ecl treatment failed (case 5 evolved from Ind-Ecl to Der-Ecl, whereas patient 51 died because of bacteraemia). Given these small numbers and limited clinical data previously published [3], we are unable to draw any conclusions about the use of TZP for BSI-Ecl. However, since Qureshi et al. also observed treatment failures with TZP [11], we are reluctant to encourage TZP as first-line therapy instead of FEP.
Data regarding the treatment outcome of BSI-Ecl managed with quinolones are still limited [8]. In our study, all six patients receiving oral treatment with CIP had a favourable outcome (including case 7 where the Ecl possessed the qnr element). This small set of results indicates that CIP may be a safe therapeutic alternative to FEP and carbapenems, especially for patients with allergy to β-lactams or those who are released early from the hospital.
4. Conclusions
In this work, we described the in vivo development of two new molecular mechanisms leading to constitutive hyperexpression of cAmpC enzymes in Ecl. Moreover, the clinical data proved that FEP is a reasonable alternative to carbapenems for BSI-Ecl, irrespective of the inducible or derepressed phenotypes, when the prevalence of ESBL-Ecl is low (<5%) as observed in our institution. Implementation of antibiotic treatment for BSI-Ecl should be based on clear knowledge of the patients’ characteristics and on local epidemiological analyses describing the mechanisms of resistance possessed by the isolates.
Acknowledgments
The authors thank Dr Carlo Casanova, Irene Lendenmann, Carmen Perroulaz, Regula Tinguely, Sylvia Selvini, Alexandra Collaud and Gabriele Zenger for their assistance. The authors dedicate this work to their beloved colleague Prof. Kathrin Mühlemann who died on 1 November 2012.
Funding: RAB is supported by the National Institutes of Health (R01AI063517), VISN 10 GRECC and the Veterans Health Administration and also receives grant support form Pfizer, Rib-X and AstraZeneca.
Footnotes
Competing interests: None declared.
Ethical approval: Not required.
References
- 1.Leclercq R, Canton R, Brown DF, Giske CG, Heisig P, Macgowan AP, et al. EUCAST expert rules in antimicrobial susceptibility testing. Clin Microbiol Infect. 2011 doi: 10.1111/j.1469-0691.2011.03703.x. http://dx.doi.org/10.1111/j.1469-0691.2011.03703.x [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 2.Sanders WE, Jr, Sanders CC. Enterobacter spp.: pathogens poised to flourish at the turn of the century. Clin Microbiol Rev. 1997;10:220–241. doi: 10.1128/cmr.10.2.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Harris PN, Ferguson JK. Antibiotic therapy for inducible AmpC β-lactamase-producing Gram-negative bacilli: what are the alternatives to carbapenems, quinolones and aminoglycosides? Int J Antimicrob Agents. 2012;40:297–305. doi: 10.1016/j.ijantimicag.2012.06.004. [DOI] [PubMed] [Google Scholar]
- 4.Kaneko K, Okamoto R, Nakano R, Kawakami S, Inoue M. Gene mutations responsible for overexpression of AmpC β-lactamase in some clinical isolates of Enterobacter cloacae. J Clin Microbiol. 2005;43:2955–2958. doi: 10.1128/JCM.43.6.2955-2958.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Livermore DM, Brown DF, Quinn JP, Carmeli Y, Paterson DL, Yu VL. Should third-generation cephalosporins be avoided against AmpC-inducible Enterobacteriaceae? Clin Microbiol Infect. 2004;10:84–85. doi: 10.1111/j.1469-0691.2004.00831.x. [DOI] [PubMed] [Google Scholar]
- 6.Endimiani A, Perez F, Bonomo RA. Cefepime: a reappraisal in an era of increasing antimicrobial resistance. Expert Rev Anti Infect Ther. 2008;6:805–824. doi: 10.1586/14787210.6.6.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Walsh TR. Emerging carbapenemases: a global perspective. Int J Antimicrob Agents. 2010;36(Suppl. 3):S8–S14. doi: 10.1016/S0924-8579(10)70004-2. [DOI] [PubMed] [Google Scholar]
- 8.Lee CC, Lee NY, Yan JJ, Lee HC, Chen PL, Chang CM, et al. Bacteremia due to extended-spectrum-β-lactamase-producing Enterobacter cloacae: role of carbapenem therapy. Antimicrob Agents Chemother. 2010;54:3551–3556. doi: 10.1128/AAC.00055-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bell JM, Turnidge JD, Jones RN. Prevalence of extended-spectrum β-lactamase-producing Enterobacter cloacae in the Asia-Pacific region: results from the SENTRY Antimicrobial Surveillance Program, 1998 to 2001. Antimicrob Agents Chemother. 2003;47:3989–3993. doi: 10.1128/AAC.47.12.3989-3993.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marra AR, Camargo LF, Pignatari AC, Sukiennik T, Behar PR, Medeiros EA, et al. Nosocomial bloodstream infections in Brazilian hospitals: analysis of 2,563 cases from a prospective nationwide surveillance study. J Clin Microbiol. 2011;49:1866–1871. doi: 10.1128/JCM.00376-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Qureshi ZA, Paterson DL, Pakstis DL, Adams-Haduch JM, Sandkovsky G, Sordillo E, et al. Risk factors and outcome of extended-spectrum β-lactamase-producing Enterobacter cloacae bloodstream infections. Int J Antimicrob Agents. 2011;37:26–32. doi: 10.1016/j.ijantimicag.2010.09.009. [DOI] [PubMed] [Google Scholar]
- 12.Lee JA, Kang CI, Joung MK, Moon SY, Chung DR, Ko KS, et al. Efficacy of cefepime therapy for Enterobacter bacteraemia, with special emphasis on febrile neutropenic patients. Scand J Infect Dis. 2010;42:557–559. doi: 10.3109/00365541003621528. [DOI] [PubMed] [Google Scholar]
- 13.Kang CI, Kim SH, Park WB, Lee KD, Kim HB, Oh MD, et al. Bloodstream infections caused by Enterobacter species: predictors of 30-day mortality rate and impact of broad-spectrum cephalosporin resistance on outcome. Clin Infect Dis. 2004;39:812–818. doi: 10.1086/423382. [DOI] [PubMed] [Google Scholar]
- 14.Marcos M, Inurrieta A, Soriano A, Martinez JA, Almela M, Marco F, et al. Effect of antimicrobial therapy on mortality in 377 episodes of Enterobacter spp. bacteraemia. J Antimicrob Chemother. 2008;62:397–403. doi: 10.1093/jac/dkn155. [DOI] [PubMed] [Google Scholar]
- 15.Ye Y, Li JB, Ye DQ, Jiang ZJ. Enterobacter bacteremia: clinical features, risk factors for multiresistance and mortality in a Chinese university hospital. Infection. 2006;34:252–257. doi: 10.1007/s15010-006-5038-3. [DOI] [PubMed] [Google Scholar]
- 16.European Committee on Antimicrobial Susceptibility Testing. Clinical breakpoints v.20. Basel, Switzerland: EUCAST; 2012. [accessed 23.11.12]. http://www.eucast.org. [Google Scholar]
- 17.Dunne WM, Jr, Hardin DJ. Use of several inducer and substrate antibiotic combinations in a disk approximation assay format to screen for AmpC induction in patient isolates of Pseudomonas aeruginosa, Enterobacter spp., Citrobacter spp., Serratia spp. J Clin Microbiol. 2005;43:5945–5949. doi: 10.1128/JCM.43.12.5945-5949.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bogaerts P, Hujer AM, Naas T, de Castro RR, Endimiani A, Nordmann P, et al. Multicenter evaluation of a new DNA microarray for rapid detection of clinically relevant bla genes from β-lactam-resistant Gram-negative bacteria. Antimicrob Agents Chemother. 2011;55:4457–4460. doi: 10.1128/AAC.00353-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hujer AM, Page MG, Helfand MS, Yeiser B, Bonomo RA. Development of a sensitive and specific enzyme-linked immunosorbent assay for detecting and quantifying CMY-2 and SHV β-lactamases. J Clin Microbiol. 2002;40:1947–1957. doi: 10.1128/JCM.40.6.1947-1957.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hilty M, Betsch BY, Bögli-Stuber K, Heiniger N, Stadler M, Kuffer M, et al. Transmission dynamics of extended-spectrum β-lactamase-producing Enterobacteriaceae in the tertiary care hospital and the household setting. Clin Infect Dis. 2012;55:967–975. doi: 10.1093/cid/cis581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 22.McCabe WR, Jackson GG. Etiology and ecology. Arch Intern Med. 1962;110:847–862. [PubMed] [Google Scholar]
- 23.American College of Chest Physicians/Society of Critical Care Medicine Consensus Conference: definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Crit Care Med. 1992;20:864–874. [PubMed] [Google Scholar]
- 24.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130:461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 25.Endimiani A, Luzzaro F, Perilli M, Lombardi G, Coli A, Tamborini A, et al. Bacteremia due to Klebsiella pneumoniae isolates producing the TEM-52 extended-spectrum β-lactamase: treatment outcome of patients receiving imipenem or ciprofloxacin. Clin Infect Dis. 2004;38:243–251. doi: 10.1086/380645. [DOI] [PubMed] [Google Scholar]
- 26.Pluss-Suard C, Pannatier A, Kronenberg A, Muhlemann K, Zanetti G. Hospital antibiotic consumption in Switzerland: comparison of a multicultural country with Europe. J Hosp Infect. 2011;79:166–171. doi: 10.1016/j.jhin.2011.05.028. [DOI] [PubMed] [Google Scholar]
- 27.Vlek AL, Bonten MJ, Boel CH. Direct matrix-assisted laser desorption ionization time-of-flight mass spectrometry improves appropriateness of antibiotic treatment of bacteremia. PLoS ONE. 2012;7:e32589. doi: 10.1371/journal.pone.0032589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rodríguez-Martínez JM, Fernández-Echauri P, Fernández-Cuenca F, Diaz de Alba P, Briales A, Pascual A. Genetic characterization of an extended-spectrum AmpC cephalosporinase with hydrolysing activity against fourth-generation cephalosporins in a clinical isolate of Enterobacter aerogenes selected in vivo. J Antimicrob Chemother. 2012;67:64–68. doi: 10.1093/jac/dkr423. [DOI] [PubMed] [Google Scholar]
- 29.Vakulenko SB, Golemi D, Geryk B, Suvorov M, Knox JR, Mobashery S, et al. Mutational replacement of Leu-293 in the class C Enterobacter cloacae P99 β-lactamase confers increased MIC of cefepime. Antimicrob Agents Chemother. 2002;46:1966–1970. doi: 10.1128/AAC.46.6.1966-1970.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Clinical and Laboratory Standard Institute. Performance standards for antimicrobial susceptibility testing; 22nd informational supplement. Wayne, PA: CLSI; 2012. Document M100-S22. [Google Scholar]