Abstract
In situ reactions of metal ions or their compounds are important mechanisms by which particles alter lung immune responses. We hypothesized that major determinants of the immunomodulatory effect of any metal include its redox behavior/properties, oxidation state, and/or solubility, and that the toxicities arising from differences in physicochemical parameters are manifest, in part, via differential shifts in lung iron (Fe) homeostasis. To test the hypotheses, immunomodulatory potentials for both penta-valent vanadium (VV; as soluble metavanadate or insoluble vanadium pentoxide) and hexavalent chromium (CrVI; as soluble sodium chromate or insoluble calcium chromate) were quantified in rats after inhalation (5 hr/d for 5 d) of each at 100 μg metal/m3. Differences in effects on local bacterial resistance between the two VV, and between each CrVI, agents suggested that solubility might be a determinant of in situ immunotoxicity. For the soluble forms, VV had a greater impact on resistance than CrVI, indicating that redox behavior/properties was likely also a determinant. The soluble VV agent was the strongest immunomodulant. Regarding Fe homeostasis, both VV agents had dramatic effects on airway Fe levels. Both also impacted local immune/airway epithelial cell Fe levels in there were significant increases in production of select cytokines/chemokines). Our findings contribute to a better understanding of the role that metal compound properties play in respiratory disease pathogenesis and provide a rationale for differing pulmonary immunotoxicities of commonly-encountered ambient metal pollutants.
Keywords: vanadium, chromium, iron, transferrin, ferritin, Listeria, pulmonary, immunomodulation
Introduction
Select metal ions or their compounds in polluted air may be responsible for development of respiratory diseases (EPA, 2004). Local cell populations in the lung are exposed to many of these metals that, in turn, can trigger a variety of biological effects involved in disease pathogenesis. For example, modifications of alveolar macrophage [PAM] or neutrophil [PMN] properties could lead to impaired immunocompetence and, ultimately, increases in infectious disease (Cohen et al., 2000; Cohen, 2004). Many in vivo and in vitro studies have addressed the extent and the means by which individual metals induce these effects (reviewed in Cohen, 2004, 2005). While those studies showed that dose was a determinant in the extent of immunomodulation, the potential for effects also depended on the agent itself. This suggested that inherent physicochemical properties of the metals or their complexes (i.e., solubility, redox behavior/properties, valency, electrophilicity, structural geometry, or hydrolytic activity) could determine in vitro/in situ toxicities.
Solubility of a metal agent depends on molecule size, ligand type, charge, and nuclearity. Historically, most inhalation studies with metals have generally investigated only soluble compounds. Several studies have reported that solubility can have a dramatic effect on the biological effect and toxicity of a metal (i.e., Cohen et al., 1997, 1998; Tanaka, 2004). Similarly, redox behavior and valency are also likely to impact toxicities of metals that can exist in several oxidation states. Though the fundamental difference between an Mn and an Mn+1 or Mn−1 valence state is only an electron, effects on properties are profound (McCleverty and Meyer, 2004). The availability of ligands can also affect which valence state predominates. Metals change valence (i.e. redox behavior) during shuttling or unidirectional processes; the latter are abundant in biology as metals have one preferred valence. The oxidation number (valency) also determines types of coordinating ligands, solubility, extra-/intracellular reactivity, and means of cell entry.
In defining immunotoxic potentials of any inhaled metal agent, evaluating changes in lung immune cell functions is essential. For example, changes in phagocytosis, intracellular killing, reactive oxygen intermediate (ROI)/nitric oxide production, and cytokine/chemokine formation will impact on the incidence or severity of infections in an exposed host’s lungs. Still, rather than solely pursuing a traditional approach to define which physicochemical property of a metal agent affects one or more of these functions, our recent research has also begun to examine the extent to which an induced altered iron homeostasis (AIH) in the lungs (which can lead to altered host resistance and immune cell function) is also related to a metal agent’s properties. During AIH, modifications in iron (Fe3+) transport functions (via either oxidation/reduction events or competition with Fe for carrier protein binding) lead to changes in Fe delivery/uptake by immune (and epithelial) cells (see review by Ghio and Cohen, 2005).
In the airways and lung cells, iron (as Fe3+) is sequestered primarily in ferritin to limit generation of free radicals. As Fe in the airways must first be transported across the cell membrane prior to sequestration in ferritin, functional Fe-carrier proteins, i.e., transferrin (Tf) and lactoferrin (Lf) are critical (Ward et al., 2005). Like ferritin, both Tf and Lf are present in lining fluid; however, the Fe-carrying capacity of Lf is limited (compared to Tf) due to its lower levels (Ghio et al., 1999). There are also alternate means of Fe transport into lung cells (e.g.. anion exchange proteins, divalent metal transporters, etc.). Should a metal agent affect Fe transport at any/all of these levels, this could lead to AIH in the lung and, accordingly, decreased Fe being delivered to local immune cells as well as an increased presence of free (available) catalytically-active Fe in the airways.
We tested here the hypotheses that metal compounds that differ in redox behaviors and solubilities (1) induce distinct effects on pulmonary immune responses and (2) cause concurrent shifts in Fe homeostasis in the lungs that might contribute to changes in resistance to infection. Among metals routinely found at significant levels in urban atmospheres (as well as defined occupational settings), vanadium (V) and chromium (Cr) have been among the most studied for pulmonary\immunotoxicologic effects in vivo and in vitro. By examining the extent to which physicochemical properties of these metals might influence toxicities in the lung in general, and in Fe homeostasis in particular, these studies contribute to understanding the role that compound properties play in respiratory disease pathogenesis, provided a rationale for differing pulmonary immunotoxicities of commonly-encountered ambient metal pollutants, and yielded clues that may lead to a better appreciation for potential reactions of metals in living systems.
Materials and Methods
Experimental Animals
Ten-week-old pathogen-free male F344 rats (≈ 225 g, Charles River, Wilmington, MA) were used in all exposures. On arrival, rats were quarantined 2 wk and then housed individually in plastic cages in temperature (20°C)- and humidity (50% RH)-controlled rooms, and provided Purina Rodent Chow and water ad libitum. Rats underwent routine clinical screening under veterinary supervision prior to initiation of exposures. All facilities and experimental protocols were approved by the NYU Medical Committee on Animal Care and Use.
For each study, rats were exposed to each agent or filtered air for 5 hr/d on five consecutive days. Twenty-four hr after the final exposure, cohorts of rats in each regimen were either euthanized to permit assessment of several pre-infection endpoints or infected with bacteria to subsequently determine any induced shifts in antibacterial functions in the lungs.
Chemical Agents
To analyze effects of soluble vs. an insoluble pentavalent V (VV) agents, sodium meta-vanadate (NaVO3) and vanadium pentoxide (V2O5) were employed. To analyze effects from soluble vs. insoluble hexavalent Cr (CrVI) agents, sodium chromate (Na2CrO4) and calcium chromate (CaCrO4) were used. Solubility values were: 0.70 g/L (in H2O at 25°C; NTP, 2002) for V2O5; 211 g/L for NaVO3; 0.11 g/L (in H2O at 25°C; Katz and Salem, 1993) for CaCrO4; and, 843 g/L (Dean, 1999) for Na2CrO4. All compounds were purchased from Sigma (St. Louis, MO).
Generation and Characterization of Exposure Atmospheres and Exposure System
Atmospheres of each soluble agent were generated by nebulizing a dilute solution (pH 7.2–7.4) via a Collison nebulizer (BGI, Waltham, MA) as described previously (Cohen et al., 2006, 2007). Insoluble Cr particle atmospheres were similarly generated using CaCrO4 bought in the appropriate size; insoluble V2O5 atmospheres were generated via a Wright dust feeder. As V2O5 was not placed in water to facilitate nebulization, the majority of material reaching each rat’s nose was V2O5 and not a mixture of V2O5 and associated vanadates (data not shown).
Each aerosol was mixed with filtered air and directly introduced into the radial, flow-past design nose-only exposure system. Because of the design, all particles reaching the nose of rats only differed in parent chemical composition and not in any overt way due to size, moisture content, or agglomeration. Target concentration was 100 μg metal/m3; if needed, subsequent exposures atmospheres down to 0.001 μg/m3 would to be employed. This range encompassed metal levels representative of those measured in urban air (Cohen et al., 2007; Doherty et al., 2007; Prophete et al., 2006). Aerodynamic size distribution of each aerosol was confirmed via 8-stage multiple orifice impactors (MSP, St. Paul, MN) after exposure (due to flow requirements). Mass concentration was assessed during exposure from particles collected on 47 mm filters (Type FG, 0.2 μm pore, Millipore, Bedford, MA). Rats were housed in plastic restraint tubes during each exposure; earlier work has shown the rats do not undergo undue stress under these conditions. Delivery of aerosol to each port was highly reproducible within and between groups (a 2% CV).
Studies of Pre-Infection Status of BAL Total Iron and Iron-Binding Protein(s) Levels
One day after the final exposure (this timepoint = 24 hr following the final exposure, and heretofore called Day 0), five rats/regimen were euthanized by pentobarbital (Nembutal; 100 mg/kg, IP) overdose and their lungs processed (without lavaging) for metal analyses; another five had their lungs lavaged to obtain concentrated BAL and free (immune) cells. Each of the latter rats had its trachea cannulated and lungs lavaged once with 7 ml warm (37°C) PBS/instillation (4–5 infusion-recovery exchanges). This first BAL was centrifuged (400 × g, 15 min, 4°C) and the resulting acellular supernatant was frozen at −70°C for later use in measures of the products detailed below. The lungs were then lavaged six more times to maximize recovery of immune cells. These lavages were pooled and centrifuged to recover cells present. This cell pellet was combined with that of the first BAL preparation, washed with PBS, and characterized by differential staining with Diff-Quick (Sigma) and assessed for viability by trypan blue exclusion.
Assessment of Lung Metal Burden
NIEHS Center Analytical Core procedures used earlier (Cohen et al., 2006, 2007) were applied to determine the amount of each metal in the lungs at Day 0 and at 72 hr post-infection. Each final isolate was analyzed using inductively coupled plasma optical emission spectroscopy (ICPOES-Model Optima 4300-DV; Perkin Elmer, Norwalk, CT). All materials used were reagent grade. All standards were made up in ultrapure water. Standard curves consisted of 6-point calibration with a standard blank to assure accurate baselines.
Assessment of BAL Total Iron Burden
Aliquots of BAL recovered on Day 0 (i.e., pre-infection) were analyzed using ICPOES operating at 238.204 nm. Minimal detectable Fe in this system was less than 1 ppb.
Assessment of BAL Ferritin and Transferrin
BAL recovered on Day 0 (i.e., pre-infection) was analyzed for total ferritin using an ELISA kit (R&D Systems, Minneapolis, MN). Total Tf levels in BAL samples were determined using an immunoprecipitin analysis kit (INCSTAR, Stillwater, MN).
Assessment of BAL TNFα, MIP-2, and MCP-1 Levels
BAL recovered on Day 0 (i.e., pre-infection) was analyzed for total tumor necrosis factor (TNF)-α, macrophage inflammatory protein [MIP]-2, and monocyte chemoattractant protein [MCP]-1 levels using ELISA kits (R&D Systems). Additional ELISAs were performed to measure levels of interleukin (IL)-6, -10, and -12 in the BAL aliquots (R&D Systems).
Host Resistance/In Situ Bacterial (Listerial) Clearance After Agent Exposure
Gram-positive Listeria monocytogenes (strain L242/73 Type 4b) was used for assessing changes in in situ antibacterial responses. Listeria was grown 16 hr in trypticase soy (TS) broth at 37°C, its concentration was determined spectrophotometrically at 540 nm, and an aliquot then diluted with saline to the needed concentration for intratracheal instillation (110 μl/rat) under light halothane anesthesia. Extrapolation to predict Listeria concentration is known to be within 90% of predicted values (Cohen et al., 2002, 2006).
A day after their final exposure, 10 metal- and 5 air-exposed controls rats were infected with 4 × 106 bacteria/rat (LD10 in F344 rats of this age). Six naïve rats were also infected; three were analyzed immediately to establish baseline bacterial burdens and the rest 72 hr later to monitor virulence. At 72 hr post-infection, each infected rat was euthanized and its lungs isolated en bloc; the trachea and extrapulmonary bronchi were removed and the tissue weighed and processed for estimation of listerial burden (i.e., homogenization and plating of serial dilutions on TS agar/0.6% yeast extract plates for 24 hr incubation at 37°C). The remaining homogenate was placed at 4°C for later use in determining lung metal burden at sacrifice. Differences in total Listeria/lung (vs. control rat lung values) at 72 hr were used as an index of modulated resistance.
Data Analysis
Effects from each agent on each test endpoint were analyzed by one-way ANOVA (analysis of variance) with the individual factor being the exposure group (air or metal agent). All data were tested to assure assumptions of normality and homogeneity of variance were met, and transformations applied as needed. Data were also screened for outliers using Dixon and Grubb’s analyses (Taylor, 1990). Significant effects were also sub-tested using t-tests corrected for multiple comparisons. Outcomes were considered significant at p < 0.05.
Results
Lung Metal Burdens after Exposures to the Cr or V Compounds
Analyses of filter samples collected over the 5-d exposure period indicated that rats provided V2O5 received an equivalent amount of VV relative to NaVO3-exposed rats (Table 1). Rats provided CaCrO4 received slightly more CrVI than those exposed to Na2CrO4. Mass median aerodynamic diameters [MMAD] of each agent’s particles were consistent during the studies.
Table 1.
Exposure Parameters from Each Study.
| Exposure Regimen | a Actual Exposure Level | b MMAD |
|---|---|---|
| Sodium Vanadate (NaVO3) | 110.14 ± 3.59 | 0.21 μm (σg =2.1) |
| Vanadium Pentoxide (V2O5) | 132.62 ± 37.19 | 0.74 μm (σg =2.8) |
| Sodium Chromate (Na2CrO4) | 110.08 ± 3.52 | 0.34 μm (σg =1.7) |
| Calcium Chromate (CaCrO4) | 118.57 ± 2.88 | 0.27 μm (σg =2.7) |
Values in terms of μg parent metal/m3 over the entire 5-d exposure period (mean ± SE).
Mass median aerodynamic diameters.
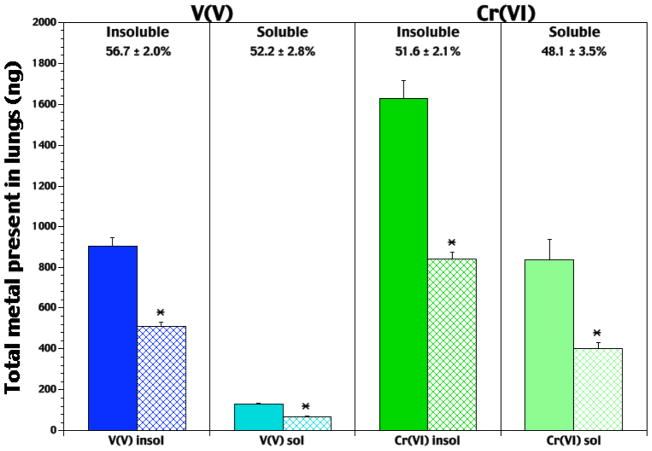
On Days 0 and 3 of infection, rats in each group were analyzed for lung metal burdens. On Day 0, rats exposed to V2O5 had a 7.0-fold higher lung V content than rats in the NaVO3 group (Figure 1). On Day 3, V2O5-exposed rats had levels 7.6-fold greater than NaVO3-exposed hosts. Net changes in V levels (reflecting retention over 3 d period) were very similar between the groups (i.e., average change 52–56%). CaCrO4-Exposed rats had a higher (96%) lung Cr content on Day 0 than Na2CrO4-treated rats. After 3 d of infection, Cr levels in the lungs of rats in the CaCrO4 group were still significantly greater (108%) than in rats that inhaled Na2CrO4. As with the V agents, retention of Cr was similar between the groups (i.e., 48–52%).
Figure 1.
Lung V and Cr burdens at Day 0 (i.e., pre-infection) and Day 3 of infection with Listeria. Each bar represents the average burden (ng V or ng Cr; ± SE) in the lungs of n = 5 (Day 0; solid bar) or n = 8–10 (Day 3; hatched bar) rats/exposure (5 hr/d, for 5 consecutive days) to insoluble V2O5, soluble NaVO3, insoluble CaCrO4, or soluble Na2CrO4. *Value significantly (p < 0.05) different from that in rats analyzed on Day 0.
For the air-exposed control rats, average lung V levels never were at/above the minimal detectable limit of the detection system (i.e., ≈ 1 ppb). In contrast, levels of Cr in the lungs of these rats were measurable, but quite low, and did not differ between Days 0 and 3; average Cr levels in these lungs were ≈ 55 ng (± ≈26 [SD]).
BAL Total Iron (Fe) Burden as a Function of the Metal Compound
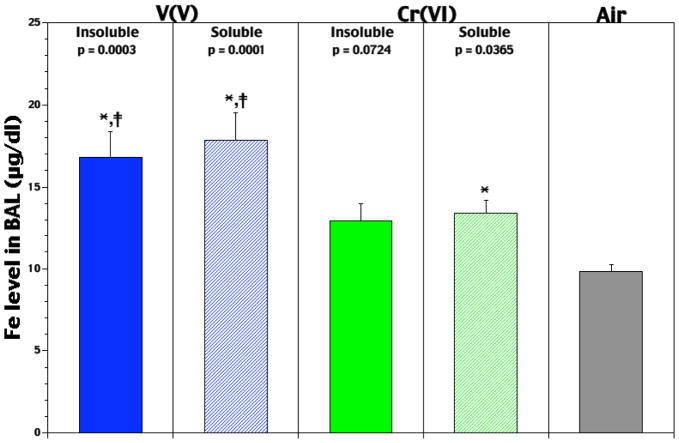
BAL Fe levels were assessed on Day 0 to determine compound-related effects on airway Fe content at time of infection. Studies on Day 3 were not done due to potential effects from the bacteria itself. Among Day 0 rats, exposure to either VV agent caused significant increases in BAL Fe levels (Figure 2); NaVO3 and V2O5 led to, respectively, 82 and 72% higher values than in air controls. While the soluble CrVI agent also had a significant effect on BAL Fe levels (36% rise), the effect from its insoluble counterpart did not (31% rise, p = 0.07). Comparisons between the soluble agents indicated that the VV effect was significantly greater (33%) than that of CrVI. The effect from insoluble VV was also significantly greater (31%) than that from insoluble CrVI.
Figure 2.
Pre-infection levels of lavagable Fe in lungs of rats that had been exposed for 5 hr/d for 5 days to soluble or insoluble forms of V or Cr. Each value reported is the mean (± SE) obtained from 5–10 rats/subset. *Level significantly differs from air control value (p-value indicated above each bar); ‡level significantly differs from solubility-matched Cr counterpart at p < 0.05.
BAL Ferritin and Transferrin Levels as Function of the Metal Compound
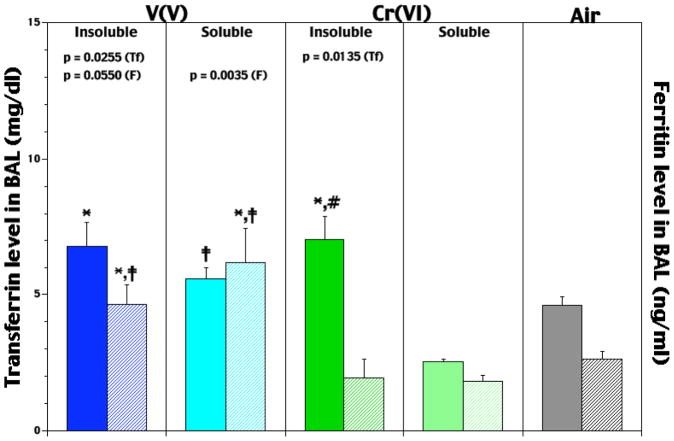
To assess any compound-related differences in the pre-infection airway presence of the Fe-related proteins transferrin (Tf) and ferritin, levels of each were assessed in the BAL from rats on Day 0. Each insoluble VV and CrVI agent caused significant increases in Tf levels (47 and 53%, respectively) compared to those in control rats (Figure 3); neither soluble form induced significant increases. Comparisons between the soluble agents indicated the VV-induced level was significantly greater (119%) than that from CrVI. The level of Tf after insoluble VV was not different from insoluble CrVI. Thus, with respect to effects upon lung Tf, the role of solubility was found to be significant for these two metals.
Figure 3.
Pre-infection levels of lavagable transferrin (solid bar/specific agent set) and ferritin (hatched bar/specific agent set) in lungs of rats that had been exposed for 5 hr/d for 5 days to soluble or insoluble forms of V or Cr. Each value reported is the mean (± SE) obtained from 5–10 rats/subset. At p < 0.05, *level significantly differs from air control value; ‡level significantly differs from solubility-matched Cr counterpart; #level significantly differs from soluble counterpart.
Effects of the metal agents on BAL ferritin levels trended somewhat differently, i.e., with the CrVI agents, there were consistent (albeit not significant) decrements relative to control rat values (Figure 3). In contrast, NaVO3 and V2O5 caused significant respective 133 and 75%, increases in the amounts of ferritin present. Analyses of the data among each metal compound indicated that each VV effect was significantly different from that due to its corresponding CrVI counterpart. Specifically, values attributable to NaVO3 and V2O5 were 237 and 138% greater than those associated with Na2CrO4 and Na2CrO4, respectively.
BAL TNFα, MIP-2, and MCP-1 Levels as Function of the Metal Compound
To assess any compound-related differences in pre-infection amounts of select cytokines and/or chemokines critical to antibacterial responses against Listeria, airway levels of TNFα, MIP-2, MCP-1, IL-6, -10, and -12 were assessed in BAL from rats on Day 0. In these rats, it was seen that levels of each interleukin were consistently below kit levels of detection. In contrast, measurable levels of MIP-2 and TNFα were seen in the majority of rat samples.
In conjunction with their significant effects on airway Fe levels, both NaVO3 and V2O5 exposures caused significant increases in BAL TNFα (4.5- and 5.3-fold, respectively) and MIP-2 (1.9- and 2.5-fold, respectively) relative to air control levels (Figure 4). The data also indicate that for VV, the role of solubility with regard to MIP-2 induction was significant. Unexpectedly, the CrVI exposures led to no change in TNFα and MIP-2 levels. It is interesting to note that the VV agents were the only ones that induced measurable amounts of MCP-1 (data not shown).
Figure 4.
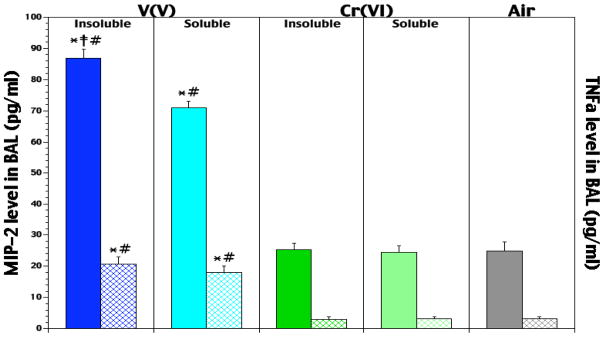
Pre-infection levels of MIP-2 (solid bar/specific agent set) and TNF-α (hatched bar/specific agent set) in lungs of rats that had been exposed for 5 hr/d for 5 days to soluble or insoluble forms of V or Cr. Each value reported is the mean (± SE) obtained from 5–10 rats/subset. At p < 0.05, *level significantly differs from air control levels; ‡level significantly differs from soluble counterpart; #level significantly differs from solubility-matched Cr counterpart
Pre-Infection Lung Immune Cell Profiles as Function of the Metal Compound
Day 0 BAL immune cell profiles were examined. The results indicated that while each CrVI agent caused decreases in AM percentages relative to control (and VV counterpart) levels, only the Na2CrO4-induced reduction was significant (i.e., 88% vs. 94–96%). In all CrVI-exposed rats, PMN levels were also increased; here, only CaCrO4 yielded values significantly different from controls (i.e., 1.92% vs. 0.77%). Oddly, though the CaCrO4-treated rats had the highest PMN levels, their AM levels did not significantly differ from the controls. In contrast to the CrVI effects, rats exposed to either VV agent had the lowest PMN levels (i.e., 0.08–0.22%). As a function of solubility, significant differences were only found to occur between the two CrVI forms.
Lung Listeria Burdens (3-Day Post-Infection) as Function of the Metal Compound
Following the 5 d of exposures and subsequent infection with Listeria, rats in each group were assessed for bacterial burdens at 72 hr post-infection. All data were then compared to burdens in the lungs of air-exposed infected rats to determine if a particular metal compound induced significant immunomodulation. In no cases were significant differences in mortality over the 72 hr period or in body weight due to pre-infection regimen detected between the air- or rats treated with metal compounds.
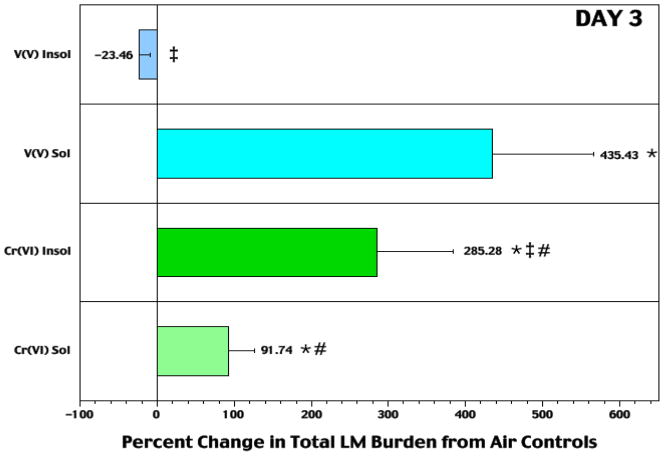
While exposures to either VV agent caused no significant effects on Listeria burdens in the first 48 hr post-infection (Cohen et al., 2006), at 72 hr it became clear that soluble NaVO3 had caused greatly reduced pathogen clearance (Figure 5). These rats had significantly greater (435%) total lung Listeria burdens than air controls; this percent change was also significantly different from that due to soluble CrVI. At 10 μg V/m3, the effect was lost. Exposure to insoluble V2O5 led to a non-significant 24% decrease in Listeria levels; this outcome significantly differed from that of soluble NaVO3. Corresponding patterns were apparent when data were analyzed in the context of lung weight at sacrifice (data not shown). These analyses accounted for changes in lung size reflecting increased mass due to bacterial presence, edema, immune cell migration, etc.
Figure 5.
Relative change (at 72 hr) from air control rat bacterial levels in lungs of rats that had been exposed 5 hr/d for 5 days to soluble or insoluble forms of V or Cr prior to infection. Each bar represents mean (± SE) of 10 rats/subset. At p < 0.05: *percent (%) change from air control levels was significant; ‡% change significantly differs from opposing solubility counterpart (within metal set); #% change significantly differs from solubility-matched V counterpart.
Neither Na2CrO4 nor CaCrO4 caused any significant effect on bacterial burdens the first 48 hr post-infection (Cohen et al., 2007). However, each did induce significant reductions in pathogen clearance by 72 hr. Rats that received the insoluble CrVI displayed a significantly (i.e., 286%) greater total lung burden of Listeria compared to air control counterparts. These levels were also significantly greater than those in the soluble CrVI-exposed rats that displayed a 92% increase compared to controls. Neither agent retained its effect at a dose of 10 μg Cr/m3. Again, corresponding patterns were apparent when data were analyzed in the context of lung weight at sacrifice.
Estimation of Relative Immunomodulatory Potential of Each Metal Compound
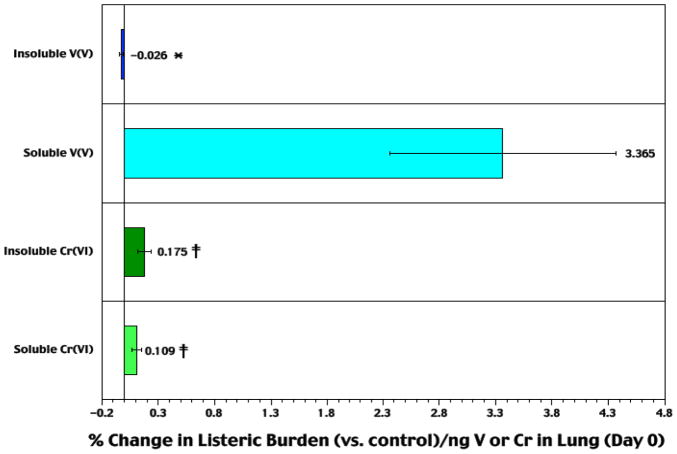
Analyses for each rat of the percentage change in bacterial burden at 72 hr (as either total burden or burden/g lung compared to that in infected control counterparts) in the context of the amount of Cr or V present in the lungs yielded data that permitted estimates of the relative immunomodulatory potentials of each agent (Figure 6).
Figure 6.
Relative difference in Listeria burden in lungs of rats at Day 3 post-infection as a function of Day 0 lung metal burdens. Each bar represents mean (± SE) (from n = 10 Day 3 rats/agent) average percentage difference in Listeria levels compared to respective values in air controls, in the context of ng V (or Cr) in lungs at Day 0. ‡Value significantly (p < 0.05) different from that in rats in V counterparts matched for relative solubilities; *value significantly different from that of opposing solubility counterpart (within metal set).
For reasons outlined previously (Cohen et al., 2006, 2007), we found that use of Day 3 lung Cr or V burdens as a factor in assessing each agent’s relative immunomodulatory potential was unreliable. Specifically, we arrived at that conclusion because there: (A) appeared to be a weak negative correlation between lung Cr or V burden on Day 3 and relative change in Listeria burden, bacterial burden/g lung, or lung weight on Day 3; (B) was a correlation between the extent of infection and lung weight; and, (C) was an increase in the loss of Cr or V as the mass of/damage to the lung during the infection-to-resolution process. In contrast, if the initial burden (i.e., ng V or Cr on Day 0) was used as a predictor for ultimate change in host resistance to the Listeria challenge, very clear patterns of potential immunomodulation become apparent.
When the percentage change in bacterial burdens from air control levels were estimated in the context of pre-infection burdens of each metal, the results clearly showed that soluble NaVO3 had the greatest effect on resistance of all four compounds tested. Among the remaining three agents, insoluble CaCrO4 had a greater effect upon resistance (at a per ng pre-infection Cr burden) than soluble Na2CrO4 as well as a significantly greater effect than insoluble V2O5. From this data, it seems likely that certain attributes of NaVO3 contribute to the strong immunotoxic potency of this metal compound (as a potential model for other soluble VV agents) in the lungs.
Discussion
We hypothesized that physicochemical properties are likely determinants of the immunomodulatory potentials of metal agents in the lung and that the extent to which each metal could induce altered iron homeostasis (AIH) was a factor contributing to altered responses to bacteria. Using soluble and insoluble forms of vanadium and chromium in their commonly-encountered oxidation states (i.e., VV and CrVI), we sought to ascertain the extent to which solubility and redox behavior/properties might govern each agent’s modulating potential and ability to induce AIH.
Solubility as Determinant for Induction of Immunomodulation in the Lungs
Soluble and insoluble particles are handled differently in the lungs. Here, following ingestion by local AM, the V2O5 or CaCrO4 particles localize in phagosomes and undergo slow dissolution to free ions. Thus, rapid diffusion by the particles through epithelia does not occur, and their clearance relies on mucociliary transport. The soluble VV and CrVI ions, if not reduced or complexed by lining fluid constituents, can readily enter epithelia/AM via portals used by anions like phosphate (PO43−) or sulfate (SO42−). Our retention results reflect these differences, i.e., rats provided insoluble agents had greater lung metal levels than rats inhaling soluble forms.
Still, other data here suggest an inconsistent impact of solubility on induction of immunomodulation. For VV, though V2O5 led to a greater lung V level at time of infection, NaVO3 had a far greater effect on resistance. These differing impacts on resistance are in keeping with earlier findings comparing each form’s ability to affect: (1) AM cytokine formation; (2) lung inflammation; and, (3) AM phagocytic activity (reviewed in Cohen, 2004 and NTP, 2002). With regard to adverse effects on lung immune cell profiles, this predisposing factor for altered resistance was, unexpectedly, not induced by either VV agent. For CrVI, CaCrO4 exposure led to both a greater lung Cr level and change in resistance than Na2CrO4. However, when data were normalized to reflect Day 0 lung Cr burdens, solubility-associated differences were no longer evident. These observations run counter to earlier findings that showed that, for CrVI, solubility influences the ability to induce inflammation and affect AM functions (Cohen et al., 1998), including several critical to host resistance to Listeria.
Why V2O5 did not affect resistance while CaCrO4 did is curious. Both could induce reductions in AM phagocytic function (i.e., as phagosomes “fill” with particles, the cell cannot readily ingest Listeria) and killing, (i.e., after ions are liberated from particles, the cell can ingest but not eliminate the bacteria). The lower effect of V2O5 is even more odd given that it is more soluble in water than CaCrO4 and should be “easier” to solubilize to its toxic ion in AM. We surmise that since our V2O5 particles: had an MMAD ≈3X that of CaCrO4; yielded lung metal levels about half that from CaCrO4; and, induced reductions in Listeria levels, the AM in V2O5-exposed rats likely ingested fewer particles than AM in CaCrO4-exposed rats. With fewer particles engulfed, more V2O5 remained in the airways (and was lethal to the Listeria as it can create acidic microenvirons [Bell et al., 2004]) and less VV overall was able to enter AM and eventually exert toxicity.
Due to this lack of a consistent pattern among the CrVI and VV agents (and with soluble/insoluble lead (Pb) and zinc (Zn) agents as well [data not shown]), it is not possible to firmly declare (for now) solubility as a determinant of in situ immunotoxic potential for all airborne metals.
Redox Behavior as Determinant for Induction of Immunomodulation in the Lungs
In cells, VV ions are recycled (shuttled) through cellular redox processes while CrVI ions are unidirectionally-reduced. On entering cells, soluble VV ions interact with reductants (e.g., NAD(P)H and GSH [glutathione]; Baran, 2000; Crans et al., 2004) and are reduced to VIV ions; these, in turn, can interact with O2/ROI (Stern et al., 1992) and revert to VV. Apart from redox-related damage from those events, VV and VIV ions both affect key enzymes and signal transduction pathways critical to cell function and viability (Chien et al., 2006; Prophete et al., 2006; Riley et al., 2003; Wang et al., 2003). Fortunately, the detrimental cycling process can be broken via (1) VIV stabilization by PO43−-based ligands that prevent reduction of O2/ROI (Nechay et al., 1986) and/or (2) loss of VV from the cell (Barac-Nieto et al., 2002; Elmariah and Gunn, 2003). In contrast to the VV, soluble CrVI ions that enter cells are quickly reduced (Standeven and Wetterhan, 1989). As the CrV and CrIV species formed are unstable intracellularly (Lay and Lavina, 2004; Nag and Bose, 1985), the ion ultimately present is CrIII. Nevertheless, though unidirectionally-reduced, CrVI ions are strong oxidants and can undergo redox/ligand displacement reactions with GSH, NAD(P)H, or other HS-bearing entities.
As both VV and CrVI could induce these effects in AM, it is fair to compare the oxidative potential or redox process displayed by each metal is a better determinant of immunomodulatory potential. Electrochemical potentials clearly predict that CrVI is the stronger oxidant. Under physiological conditions, the electrochemical potential of CrVI (as it reduces to CrIII) is (E∘(CrVI/CrIII) = +1.41V; E∘(CrV/CrIV) = +1.34 V; E∘(CrIV/CrIII) = +2.10 V; Katz and Salem, 1993); in contrast, the potential of VV (depending on parent compound) is E∘(VV/VIV) = +1.02 to +1.31 V; Rehder, 1992). However, while VV is a ‘good’ oxidant, unlike with CrVI, there is an added risk of toxicity due to reducing reactions as VIV re-converts to VV. This “double impact” from redox shuttling may be amplified by self-perpetuating ROS← →VV reactions (unless cycling is broken). These dual potential redox effects of VV suggest that - at equal burdens of each metal - VV would likely present a greater risk to an AM, and therefore be more immunomodulatory, than CrVI. Indeed, our data indicates this is so.
When our results with VV and CrVI are analyzed in the context of other studies showing that neither soluble PbII (“weakly-changeable” behavior) nor ZnII (“redox inert”) affected resistance to Listeria (data not shown), we can conclude that redox behavior/properties is likely to be a critical determinant of in situ immunotoxicity for soluble forms of metals that may be in the air.
Ability to Induce AIH as Determinant for Induction of Immunomodulation in the Lungs
Metal ions or their compounds can disrupt normal Fe homeostasis in the lungs and its cells via several pathways. Some can compete with endogenous FeIII ions for reducing equivalents prior to cell uptake or binding to/transport by transferrin (Tf) or lactoferrin (Lf). Some may also compete with FeII for: alternate import pathways (e.g., DMT1); storage in ferritin; export pathways (e.g., ferroportin); and, oxidation (i.e., ferroxidation). Such disruptions could lead to accumulation of Fe in cells to metabolically unsound levels or significant decreases in Fe delivery to the cells. In AM, while the former would lead to increased oxidative stress, cell signaling/transcription factor activation, and mediator release, the latter would yield Fe-deficient AM less able to generate ROI.
Both Cr and V agents here caused increased airway Fe levels, with the effect of the VV compounds being far greater than the CrVI agents. Analyses of Tf, a protein released (above basal levels) into airways in response to elevated Fe levels, showed that soluble and insoluble VV - but only insoluble CrVI - caused significant increases in this parameter. Conversely, airway ferritin levels were only significantly increased by VV. Similar selective effects by VV were also noted regarding levels of TNFα, MIP-2, and MCP-1, whose genes bear a hypoxia-responsive element (HRE).
Effects of both VV agents on each Fe-based parameter analyzed were anticipated. Among defined metal immunomodulants, vanadium (Harrington, 1992; Mazurier, et al., 1983; Nagaoka et al., 2004) is known to compete with Fe for binding with, or to displace Fe from, Tf (and Lf) in vivo or in vitro. Our recent in vitro studies clearly demonstrated this competition and subsequent reductions in Fe delivery to AM (Doherty et al., 2007; Prophete et al., 2006). Chromium also is known to bind with Tf in vivo and in vitro (Ani and Moshtaghie, 1992; Moshtaghie et al., 1992). However, it is unclear if it is CrVI that is binding; studies have indicated that, in fact, it is CrIII that is actually bound (Clodfelder et al., 2001; Harris, 1967). Thus, for induction of AIH to have occurred here via reduced Tf transport activity, the entrained CrVI would have to have first undergone reduction in the airways. As the airways would then have had increased levels of CrIII ions (that do not readily enter cells), observed effects on AM function would likely not be so much attributable to CrVI but, instead, to an Fe insufficiency. Lastly, a basic critical difference in the abilities of V and Cr to potentially modify Tf function provides a basis to explain why AIH would more likely to evolve after a VV (rather than CrVI) exposure. Harris (1967) showed that with Tf, most FeIII binds at “Site A” (≈90% selectivity) and then at “Site B” only during states of Fe excess. Harris also showed that when V or Cr was present, FeIII and V - individually - preferentially bind at Site A, while Cr does so at Site B. Thus, while V and Cr each could bind to Tf, V would have a more detrimental impact as it blocks FeIII binding at its preferential site.
While both VV and CrVI might “eventually” bind with Tf and so alter Fe delivery, it was still important to verify if AM Fe levels were affected here. Direct analysis by ICPOES was found to be technically difficult; therefore, product levels of HRE-bearing genes were analyzed to indirectly verify any Fe-deficiencies. We recognize that there are many factors that could lead to the transcription of pro-inflammatory mediators (such as TNFα, MIP-2, and MCP-1) by these (and other types of lung) cells. However, in the context of AIH, any agent-induced Fe insufficiency would lead to an increased presence of HIF-1 (hypoxia-inducible factor) in the cells that, in turn, would lead to the activation of multiple HRE-containing genes, including several coding for these three/other key pro-inflammatory cytokines/chemokines (Jeong et al., 2003, 2005; Lee et al., 2004).
That the VV agents affected levels of TNFα, MIP-2, and MCP-1 was in keeping with other observations here regarding induced AIH. In contrast, a lack of any increase in TNFα/MIP-2 in the CrVI-exposed rats was puzzling. As a similar confounding outcome was noted in earlier studies of hosts that had inhaled Pb (Cohen et al., 1994), concern arose that a “lack” of increase in BAL levels of both proteins may have been attributable to our use of ELISA kits. In a follow-on study performed using TNFα standards incubated with CrVI (250 μM Na2CrO4; a level equal to that of entrained CrVI expected in lining fluid after single exposure), incubations of even 5 min led to 25–30% reductions in expected absorbance values. We thus conclude that the lack of any measurable change in TNFα/MIP-2 levels in CrVI-exposed rats’ BAL was likely a result of the entrained CrVI reacting with the proteins present during the exposure and/or with these/newly-secreted entities after an exposure was complete, rather than a failure of the CrVI agents to induce effects predicted to evolve from the AM receiving less Fe.
Despite the increased levels of these three assayed pro-inflammatory mediators following the V agent exposures, an obvious PMN influx was not evident in those exposed rats. This may reflect the time of sampling or uncertainties in dose-response relationships. Further, as our own studies have shown that leukocyte responsiveness to response modifiers (both protein and non-protein) following in vivo or in vitro exposures to V are reduced through effects on receptors and/or post-modifier binding/processing steps (Cohen et al., 1996, 1999), it is also plausible that PMN in the lungs of these rats may simply not have been able to respond to the increased presence of the chemokines. Lastly, Wang et al. (2002) showed that PMN in the lungs of V-exposed hosts undergo significant apoptosis within 24 hr of any given exposure; as our sampling occurred 24 hr after the final exposure, a “lack” of increased PMN numbers in the presence of increased chemokine levels could just be a reflection of this apoptotic process having occurred during that interim period.
After analyzing the data regarding effects of each agent on Fe transport-/status-associated endpoints in the context of concurrent changes in resistance to Listeria, and accounting for the earlier-noted Listeria survival with V2O5 “problem”, we conclude that an ability to induce AIH in the lungs is a determinant of in situ immunotoxic potential for an airborne metal (compound).
Conclusion
Our studies show that the ability of an inhaled metal agent to induce AIH and its redox behavior/properties (but not electrochemical potential, per se) are likely critical determinants of its capability to induce immunomodulation in the lungs. We are unable to firmly conclude that solubility is also a critical determinant at this time. These assignations are made while viewing each characteristic as a stand-alone entity; we are mindful that it is important to recognize that no metal agent evinces only one characteristic at a time. Our ongoing studies will further refine the role that each property/ability plays in determining the in situ potential for immunomodulation by these four agents, and determine if our findings here are applicable to other Cr or V agents, as well as to other metals (in varying forms) that might be inhaled as constituents of air pollution.
References
- Ani M, Moshtaghie AA. The effect of chromium on parameters related to iron metabolism. Biol Trace Elem Res. 1992;32:57–64. doi: 10.1007/BF02784588. [DOI] [PubMed] [Google Scholar]
- Barac-Nieto M, Alfred M, Spitzer A. Basolateral phosphate transport in renal proximal tubule-like OK cells. Exp Biol Med. 2002;227:626–631. doi: 10.1177/153537020222700811. [DOI] [PubMed] [Google Scholar]
- Baran EJ. Oxovanadium(IV) and oxovanadium(V) complexes relevant to biological systems. J Inorg Biochem. 2000;80:1–10. doi: 10.1016/s0162-0134(00)00032-5. [DOI] [PubMed] [Google Scholar]
- Bell JM, Philp JC, Kuyukina MS, Ivshina IB, Dunbar SA, Cunningham CJ, Anderson P. Methods evaluating vanadium tolerance in bacteria isolated from crude oil contaminated land. J Micro Meth. 2004;58:87–100. doi: 10.1016/j.mimet.2004.03.006. [DOI] [PubMed] [Google Scholar]
- Chien PS, Mak OT, Huang HJ. Induction of COX-2 protein expression by vanadate in A549 human lung carcinoma cell line through EGF receptor and p38 MAPK-mediated pathway. Biochem Biophys Res Commun. 2006;339:562–568. doi: 10.1016/j.bbrc.2005.11.045. [DOI] [PubMed] [Google Scholar]
- Clodfelder BJ, Emamaullee J, Hepburn DD, Chakov NE, Nettles HS, Vincent JB. The trail of chromium(III) in vivo from the blood to the urine: The roles of transferrin and chromodulin. J Biol Inorg Chem. 2001;6:608–617. doi: 10.1007/s007750100238. [DOI] [PubMed] [Google Scholar]
- Cohen MD. Other metals: Aluminum, copper, manganese, selenium, vanadium, and zinc. In: Cohen MD, Zelikoff JT, Schlesinger RB, editors. Pulmonary Immunotoxicology. Kluwer Academic Publishers; Norwell, MA: 2000. pp. 267–300. [Google Scholar]
- Cohen MD. Pulmonary immunotoxicology of select metals: Aluminum, arsenic, cadmium, chromium, copper, manganese, nickel, vanadium, and zinc. J Immunotoxicol. 2004;1:39–70. doi: 10.1080/15476910490438360. [DOI] [PubMed] [Google Scholar]
- Cohen MD. Pulmonary immunotoxicology. Chapter 9. In: Gardner D, editor. Toxicology of the Lung. 4. Taylor and Francis/CRC Press; Boca Raton, FL: 2005. pp. 351–420. [Google Scholar]
- Cohen MD, Chen LC, Zelikoff JT, Schlesinger RB. Pulmonary retention and distribution of inhaled chromium: Effects of particle solubility and ozone co-exposure. Inhal Toxicol. 1997;9:843–865. [Google Scholar]
- Cohen MD, Prophete C, Sisco M, Chen L, Zelikoff J, Smee J, Holder A, Crans D. Pulmonary immunotoxic potentials of metals are governed by select physicochemical properties: Chromium agents. J Immunotoxicol. 2006;3:69–81. doi: 10.1080/15476910600718434. [DOI] [PubMed] [Google Scholar]
- Cohen MD, Schook LB, Oppenheim JJ, Freed BM, Rodgers KE. Alterations in cytokine receptors by xenobiotics. Toxicol Sci. 1999;48:163–169. doi: 10.1093/toxsci/48.2.163. [DOI] [PubMed] [Google Scholar]
- Cohen MD, Sisco M, Baker K, Li Y, Lawrence D, van Loveren H, Zelikoff JT, Schlesinger RB. Effect of inhaled ozone on pulmonary immune cells critical to antibacterial responses in situ. Inhal Toxicol. 2002;14:599–619. doi: 10.1080/08958370290084520. [DOI] [PubMed] [Google Scholar]
- Cohen MD, Sisco M, Prophete C, Chen L, Zelikoff JT, Smee JJ, Holder AA, Ghio AJ, Stonehuerner JD, Crans DC. Pulmonary immunotoxic potentials of metals are governed by select physicochemical properties: Vanadium agents. J Immunotoxicol. 2007;4:49–60. doi: 10.1080/15476910601119350. [DOI] [PubMed] [Google Scholar]
- Cohen MD, Yang Z, Qu Q, Schlesinger RB, Zelikoff JT. Vanadium affects macrophage interferon-γ-binding and -inducible responses. Toxicol Appl Pharmacol. 1996;138:110–120. doi: 10.1006/taap.1996.0104. [DOI] [PubMed] [Google Scholar]
- Cohen MD, Yang Z, Zelikoff JT. Immunotoxicity of particulate lead: In vitro exposure alters pulmonary macrophage tumor necrosis factor-α release and activity. J Toxicol Environ Health. 1994;42:377–392. doi: 10.1080/15287399409531889. [DOI] [PubMed] [Google Scholar]
- Cohen MD, Zelikoff JT, Chen LC, Schlesinger RB. Immunotoxicologic effects of inhaled chromium: Role of particle solubility and co-exposure to ozone. Toxicol Appl Pharmacol. 1998;152:30–40. doi: 10.1006/taap.1998.8502. [DOI] [PubMed] [Google Scholar]
- Crans DC, Smee JJ, Gaidamauskas E, Yang L. The chemistry and biochemistry of vanadium and the biological activities exerted by vanadium compounds. Chem Rev. 2004;104:849–902. doi: 10.1021/cr020607t. [DOI] [PubMed] [Google Scholar]
- Dean JA, editor. Lange’s Handbook of Chemistry. 15. McGraw Hill; New York: 1999. p. 3.22. [Google Scholar]
- Doherty SP, Prophete C, Maciejczyk P, Salnikow K, Gould T, Larson T, Koenig J, Jaques P, Sioutas C, Zelikoff J, Lippmann M, Cohen MD. Detection of changes in alveolar macrophage iron status induced by select PM2.5-associated components using iron response protein binding activity. Inhal Toxicol. 2007;19:553–562. doi: 10.1080/08958370701280481. [DOI] [PubMed] [Google Scholar]
- Elmariah S, Gunn RB. Kinetic evidence that the Na-PO4 co-transporter is the molecular mechanism for Na/Li exchange in human red blood cells. Am J Physiol Cell Physiol. 2003;285:C446–C456. doi: 10.1152/ajpcell.00606.2002. [DOI] [PubMed] [Google Scholar]
- Ghio AJ, Cohen MD. Disruption of iron homeostasis as a mechanism of biologic effect by particles. Inhal Toxicol. 2005;17:709–716. doi: 10.1080/08958370500224482. [DOI] [PubMed] [Google Scholar]
- Ghio AJ, Carter JD, Dailey LA, Devlin RB, Samet JM. Respiratory epithelial cells demonstrate lactoferrin receptors that increase after metal exposure. Am J Physiol. 1999;276:L933–L940. doi: 10.1152/ajplung.1999.276.6.L933. [DOI] [PubMed] [Google Scholar]
- Harrington JP. Spectroscopic analysis of the unfolding of transition metal-ion complexes of human lactoferrin and transferrin. Int J Biochem. 1992;24:275–280. doi: 10.1016/0020-711x(92)90258-3. [DOI] [PubMed] [Google Scholar]
- Harris DC. Different metal-binding properties of the two sites of human transferrin. Biochemistry. 1977;16:560–564. doi: 10.1021/bi00622a033. [DOI] [PubMed] [Google Scholar]
- Jeong HJ, Hong SH, Park RK, Shin T, An NH, Kim HM. Hypoxia-induced IL-6 production is associated with activation of MAP kinase, HIF-1, and NF-κB on HEI-OC1 cells. Hearing Res. 2005;207:59–67. doi: 10.1016/j.heares.2005.04.003. [DOI] [PubMed] [Google Scholar]
- Jeong HJ, Chung HS, Lee BR, Kim SJ, Yoo SJ, Hong SH, Kim HM. Expression of pro-inflammatory cytokines via HIF-1α and NF-κB activation on desferrioxamine-stimulated HMC-1 cells. Biochem Biophys Res Commun. 2003;306:805–811. doi: 10.1016/s0006-291x(03)01073-8. [DOI] [PubMed] [Google Scholar]
- Katz SA, Salem H. The toxicology of chromium with respect to its chemical speciation: A review. J Appl Toxicol. 1993;13:217–224. doi: 10.1002/jat.2550130314. [DOI] [PubMed] [Google Scholar]
- Lay PA, Levina A. Chromium. In: McCleverty JA, Meyer TJ, editors. Comprehensive Coordination Chemistry II – From Biology and Nanotechnology, Volume 4 Transition Metal Groups 3–6. Elsevier Pergamon; San Diego, CA: 2004. pp. 313–413. [Google Scholar]
- Lee JW, Bae SH, Jeong JW, Kim SH, Kim KW. Hypoxia-inducible factor (HIF-1)α: Its protein stability and biological functions. Exp Mol Med. 2004;36:1–12. doi: 10.1038/emm.2004.1. [DOI] [PubMed] [Google Scholar]
- Liochev SI, Fridovich I. Vanadate-stimulated oxidation of NAD(P)H in the presence of biological membranes and other sources of ·O2−. Arch Biochem Biophys. 1990;279:1–7. doi: 10.1016/0003-9861(90)90454-7. [DOI] [PubMed] [Google Scholar]
- Mazurier J, L’Hoste J, Montreuil J, Spik G. Comparative study of the iron-binding properties of human transferrins. II Electron paramagnetic resonance of mixed-metal complexes of human lactotransferrin. Biochim Biophys Acta. 1983;745:44–49. doi: 10.1016/0167-4838(83)90168-1. [DOI] [PubMed] [Google Scholar]
- McCleverty JA, Meyer TJ, editors. Comprehensive Coordination Chemistry II—From Biology and Nanotechnology, Volume 4 Transition Metal Groups 3–6. San Diego, CA: Elsevier Pergamon; 2004. [Google Scholar]
- Moshtaghie AA, Ani M, Bazrafshan MR. Comparative binding study of aluminum and chromium to human transferrin. Effect of iron. Biol Trace Elem Res. 1992;32:39–46. doi: 10.1007/BF02784585. [DOI] [PubMed] [Google Scholar]
- Nag K, Bose SN. Chemistry of tetra- and pentavalent chromium. Struct Bond. 1985;63:153–197. [Google Scholar]
- Nagaoka MH, Yamazaki T, Maitani T. Binding patterns of vanadium ions with different valence states to human serum transferrin studied by HPLC/high-resolution ICP-MS. Biochem Biophys Res Commun. 2002;296:1207–1214. doi: 10.1016/s0006-291x(02)02067-3. [DOI] [PubMed] [Google Scholar]
- Nechay BR, Nanninga LB, Nechay PS. Vanadyl (IV) and vanadate (V) binding to selected endogenous phosphate, carboxyl, and amino ligands; Calculations of cellular vanadium species distribution. Arch Biochem Biophys. 1986;251:128–138. doi: 10.1016/0003-9861(86)90059-7. [DOI] [PubMed] [Google Scholar]
- NTP. National Toxicology Program. NTP Toxicology and Carcinogenesis Studies of Vanadium Pentoxide (CAS No. 1314-62-1) in F344/N Rats and B6C3F1 Mice (Inhalation) National Toxicology Program Technical Report Series. 2002;507:1–343. [PubMed] [Google Scholar]
- Prophete C, Maciejczyk P, Salnikow K, Gould T, Larson T, Koenig J, Jaques P, Sioutas C, Lippmann M, Cohen MD. Effects of PM-associated metals on alveolar macrophage phosphorylated ERK-1 and -2 and iNOS expression during ongoing alteration in iron homeostasis. J Toxicol Environ Health. 2006;69:935–951. doi: 10.1080/15287390500362030. [DOI] [PubMed] [Google Scholar]
- Rehder D. Inorganic considerations on the function of vanadium in biological systems. In: Sigel H, Sigel A, editors. Metal Ions in Biological Systems, Vol. 31. Vanadium and Its Role in Life. New York: Marcel Dekker; 1992. pp. 1–44. [PubMed] [Google Scholar]
- Riley MR, Boesewetter DE, Kim AM, Sirvent FP. Effects of metals Cu, Fe, Ni, V, and Zn on rat lung epithelial cells. Toxicology. 2003;190:171–184. doi: 10.1016/s0300-483x(03)00162-8. [DOI] [PubMed] [Google Scholar]
- Sigel H, Sigel A, editors. Metal Ions in Biological Systems, Vol. 31. Vanadium and Its Role in Life. New York: Marcel Dekker; 1992. [Google Scholar]
- Standeven AM, Wetterhahn KE. Chromium(VI) toxicity: Uptake, reduction, and DNA damage. J Am Coll Toxicol. 1989;8:1275–1283. [Google Scholar]
- Stern A, Davison AJ, Wu Q, Moon J. Effects of ligands on reduction of oxygen by vanadium(IV) and vanadium(III) Arch Biochem Biophys. 1992;299:125–128. doi: 10.1016/0003-9861(92)90253-s. [DOI] [PubMed] [Google Scholar]
- Tanaka A. Toxicity of indium arsenide, gallium arsenide, and aluminium gallium arsenide. Toxicol Appl Pharmacol. 2004;198:405–411. doi: 10.1016/j.taap.2003.10.019. [DOI] [PubMed] [Google Scholar]
- Wang L, Medan D, Mercer R, Shi X, Huang C, Castranova V, Ding M, Rojanasakul Y. Role of neutrophil apoptosis in vanadium-induced pulmonary inflammation in mice. J Environ Pathol Toxicol Oncol. 2002;21:343–350. [PubMed] [Google Scholar]
- Ward RJ, Wilmet S, Legssyer R, Crichton RR. The influence of iron homoeostasis on macrophage function. Biochem Soc Trans. 2002;30:762–765. doi: 10.1042/bst0300762. [DOI] [PubMed] [Google Scholar]
- Willsky GR, Goldfine AB, Kostyniak PJ, McNeill JH, Yang L, Khan AR, Crans DC. Effect of vanadium (IV) compounds in the treatment of diabetes: In vivo and in vitro studies with vanadyl sulfate and bis(maltolato)oxovandium(IV) J Inorg Biochem. 2001;85:33–42. doi: 10.1016/s0162-0134(00)00226-9. [DOI] [PubMed] [Google Scholar]