Abstract
Head and neck (H&N) radiation therapy (RT) can induce irreversible damage to the salivary glands thereby causing long-term xerostomia or dry mouth in 68%–85% of the patients. Not only does xerostomia significantly impair patients’ quality-of-life (QOL) but it also has important medical sequelae, incurring high medical and dental costs. In this article, we review various measures to assess xerostomia and evaluate current and emerging solutions to address this condition in H&N cancer patients. These solutions typically seek to accomplish 1 of the 4 objectives: (1) to protect the salivary glands during RT, (2) to stimulate the remaining gland function, (3) to treat the symptoms of xerostomia, or (4) to regenerate the salivary glands. For each treatment, we assess its mechanisms of action, efficacy, safety, clinical utilization, and cost. We conclude that intensity-modulated radiation therapy is both the most widely used prevention approach and the most cost-effective existing solution and we highlight novel and promising techniques on the cost-effectiveness landscape.
In the United States (US), 24 million persons are suffering from xerostomia, or dry mouth, of which, 8 million present with moderate to severe symptoms.1,2 More than 400 medications are known to be associated with xerostomia as a side effect.2,3 This is the leading cause of xerostomia which affects in majority the elderly, a population more likely to suffer from chronic diseases necessitating polymedication.3 Other medical etiologies, such as immune syndrome (e.g., Sjögren’s syndrome) and poorly controlled diabetes, can also lead to xerostomia. Xerostomia is the most common complaint of head and neck (H&N) cancer survivors that have received radiation therapy (RT), with a prevalence of 93% during RT and 74%–85% following RT.4 Importantly, in those cases, xerostomia cannot be attributed to concomitant chemotherapy (CHT), often used to treat advanced stage cancers, as CHT-induced xerostomia has been shown to be reversible at the end of treatment.4 Given 75,000 new H&N cancer patients per year,5 90% of which receive RT6 and 85% of which consequently develop xerostomia,7–9 the incidence of xerostomia as a consequence of H&N RT in the US can be estimated to 30–50,000 new patients each year.
Xerostomia is clinically defined as the subjective complaint of dry mouth and can be related to salivary gland hypofunction, the objective evidence of decrease in salivary secretion (unstimulated whole mouth salivary flow rates <0.1 mL/min or stimulated salivary flow rates <0.7 mL/min).4,10 However, studies are contradictory as to whether there is an actual relationship between the patient’s subjective perception of dry mouth and the clinician’s objective measure of salivary flow rates.11 Patients might experience xerostomia even without clinical evidence of mouth dryness or hyposalivation, perhaps due to a change in saliva composition. 3 There have been several attempts in the literature to define mild, moderate, and severe xerostomia, according to various subjective and/or objective evaluation criteria (Table I). Despite those attempts, the grading of xerostomia remains nonuniform and there is still no standardized definition. As an example, as recently as 2010, groups have continued to develop and validate new questionnaires to quantify subjective oral dryness (Table I).14 Other groups have attempted to measure xerostomia based on downstream xerostomia sequelae such as oral pain using the visual analog scale.15,16 Physicians often use a simple 4-point scale evaluating their patient’s xerostomia, with 0 corresponding to no dryness and 4 corresponding to nonfunctional salivary glands. Under these guidelines, levels 2 and 3 are generally categorized as moderate and severe xerostomia, respectively. However, clinician- reported assessment of xerostomia often drastically underestimates the severity of subjective xerostomia.32 Therefore, to evaluate a patient’s suffering from dry mouth, it is important to assess the following 3 parameters: (1) the subjective feeling of xerostomia symptoms via patient self-administered tests; (2) xerostomia-related quality-of-life (QOL), as evaluated by short questionnaires (Table I); and (3) the clinical evidence of dryness by measuring salivary gland hypofunction using sialometry (objective measures of salivary flow rates).
Table I.
Measures of xerostomia
| Type of measure | Name | Year | Performed by |
Description | Scale | Ref. |
|---|---|---|---|---|---|---|
| Subjective | Vanderbilt Head and Neck Cancer Survey | 2010–2012 | 28-Item questionnaire, with 5 symptom subscales: “Nutrition,” “Pain,” “Voice,” “Swallow,” and “Mucous/Dry Mouth” | Score from 0–10 | 12,13 | |
| Subjective | Groningen Radiotherapy-Induced Xerostomia questionnaire (GRIX) | 2010 | Patient | 14-Item questionnaire, with 4 subscales: xerostomia during day and night and sticky saliva during day and night | Crohnbach’s α calculated for all subscales is converted to a 0–100 score, higher scores = worse xerostomia | 14 |
| Subjective | Visual Analog Scale (VAS) | 2002 | Patient | Mouth burning and/or pain intensity is evaluated on a 10-cm long VAS | 0–10 cm scale, 10 cm being the highest toxicity | 15,16 |
| Subjective | Xerostomia-related QOL questionnaire (XQoLQ) | 2001 | Patient | Five questions relating xerostomia to QOL | Scale from 0–10 | Several studies referenced in4 |
| Subjective | Eisbruch’s Xerostomia Questionnaire (XQ), also called University of Michigan XQ (UMXQ) | 2001 | Patient | 8-Item questionnaire evaluating dryness while eating or chewing and while not eating or chewing | 0–100 score, higher scores = worse xerostomia | 17 |
| Subjective | Xerostomia Inventory (XI) | 1999 | Patient | 11-Item survey | Below 14.5: normal 55: worse toxicity | 18,19 |
| Subjective | Patient Benefit Questionnaire (PBQ) | 1999 | Patient | 8-Item questionnaire: difficulty speaking and eating, sleep problems, use of oral comfort aids or fluids, mouth and tongue soreness, and mouth dryness | 1–10 Likert scale: 1 = severe negative impact; 10 = no negative impact |
18,20 |
| Subjective | Functional assessment of cancer therapy-head and neck (FACT-H&N) questionnaire | 1997 | Patient | 38-Item survey on QOL, 11 of these questions are specific to H&N cancer | QOL score based on the sum of question scores, each rated 0–4 on a Likert scale | 18,21 |
| Subjective | Oral Impacts on Daily Performance (OIDP) | 1997 | Patient | 8 Items (eating and enjoying food; speaking and pronouncing; cleaning teeth; sleeping and relaxing; smiling; laughing and showing teeth without embarrassment; maintaining one’s usual emotional state; carrying out one’s major work or social role and enjoying contact with people) | Likert scale for each question, that is summed to a score for each of the 8 categories | 22,23 |
| Subjective | Oral Health Impact Profile (OHIP): long form (OHIP49) and short form (OHIP14) | 1994, 1997 | Patient | 49-Item or 14-item (short version) survey, in 7 domains (functional limitation, pain, psychological discomfort, physical disability, psychological disability, social disability, and handicap) | Questions are scored on a 5-point Likert scale and then added to a normalized score | 23–25 |
| Subjective | University of Washington Quality of Life questionnaire (UWQoL) | 1993–2010 | Patient | The questionnaire covers 12 domainsdpain, appearance, activity, recreation, swallowing, chewing, speech, shoulder function, taste, saliva, mood, and anxiety | Questions are scaled from 0 (worst) to 100 (best), and 3 global questions are on a Likert scale (0–5 or 0–6) | 26,27 |
| Subjective | Fox’s simple questionnaire (FOX) | 1987 | Patient | 4 Simple questions concerning the patient’s perceptions of oral dryness, oral functions and comfort, and side effects | 28 | |
| Subjective | Radiation Therapy Oncology Group (RTOG) scoring | 1995 | Clinician | Evaluating acute xerostomia, based on dryness of mouth and saliva thickness | From 0 (no xerostomia) to 4 (acute salivary gland necrosis) | 29 |
| Subjective | RTOG- European Organization for Research and Treatment of Cancer (EORTC) scoring | 1995 | Clinician | Evaluating late xerostomia, based on mouth dryness evaluation and response to stimulation | From 0 (no xerostomia) to 4 (fibrosis) | 29 |
| Subjective | Clinician rating of xerostomia | N/A | Clinician | Based on discussion with patient and/or above questionnaires | 0 = no dryness to 3 = nonfunctional salivary | N/A |
| Objective | Quantitative salivary gland scintigraphy | 2000 | Clinician | Sequential imaging of the H&N region with a gamma camera after intravenous injection of the radioactive isotope 99mTc-pertechnetate and stimulation of the glands with citric acid | Two measures: maximum tracer uptake within the gland and excretion rate of tracer after stimulation | 7,30 |
| Objective | Sialometry: unstimulated whole salivary flow rate (uWSFR) | N/A | Clinician | For 5 min, patient is expectorating periodically into a measuring container | <0.1 mL/min = xerostomia | 31 |
| Objective | Sialometry: stimulated salivary flow rate (sSFR) | N/A | Clinician | Patient is chewing paraffin for 5 min, expectorating periodically into a measuring container | <0.7 mL/min = at risk; >1 mL/ min = normal | 10 |
| Objective | Clinician rating | N/A | Clinician | Based on uWSFR and sSFR | Grade I (mild), II (moderate), III (severe) | N/A |
| Objective | Common Terminology Criteria for Adverse Events (CTCAE) v.3.0. scoring | 2003 | Clinician | Based on a combination of symptomatic evaluation and uWSFR evaluation | Grade 0 (no xerostomia) to 3 (inability to aliment orally, uWSFR < 0.1 mL/min) | 29 |
These various scales and questionnaires can be used to assess a patient’s xerostomia level and they have also been used to evaluate treatment effectiveness in several clinical trials. This list is not exhaustive.
N/A, not available.
Saliva is a complex and versatile bodily fluid that serves a wide range of physiological needs and plays an essential role in oral health. The biochemical composition of saliva includes electrolytes, peptides, proteins, lipids, and antimicrobial substances that coat and protect the oral mucosa from trauma and dehydration, provide an antibacterial, antiviral, and antifungal barrier, maintain a proper pH balance, and prevent demineralization of teeth. Indeed, during early carious lesions, calcium and phosphate ions in saliva can help remineralize the tooth surface. As a consequence, reduction in salivary flow can lead to a broad range of medical sequelae including dental caries, oral infections and sores, difficulties with eating, talking, swallowing, and with function of dental prosthesis as well as altered taste sensation.33
Xerostomia and salivary gland hypofunction (of all etiologies) have been shown to strongly affects QOL related to daily activities, speech, swallowing, sleeping, and emotional function.9,34 For example, in a study by Dirix et al., 45% of patients felt that dry mouth invaded every part of their everyday life, 44% of patients felt depressed, and no less than 39% of patients said that dry mouth “diminished their will to live.” In fact, at 6 months post-RT treatment, 80% of patients felt that it was a dire prospect to live with their level of xerostomia for the remainder of their lives.35
The impact of hyposalivation on dental health is of particularly high importance for both dentate and edentulous patients. Dentate xerostomic patients are more likely to have long-term dental and gum disease, extractions, and more invasive dental procedures.34 Therefore, it is expected that higher dental care costs are a direct consequence of xerostomia and that these costs accumulate over time with persistence of hyposalivation and diet change. In the case of Sjögren syndrome-induced xerostomia, Christensen et al.36 demonstrated higher expenses for dental treatment in patients with primary Sjögren syndrome than controls. However, cost of dental care for patients who underwent H&N cancer therapy has not been specifically evaluated, even in studies focusing on the cost of care for H&N.37,38 Edentulous xerostomia patients may experience extreme discomfort wearing dentures, denture sores, and denture dislodgement (causing social discomfort) because saliva plays a major role in adhesion, cohesion, and surface tension of a denture.39 This clearly impacts QOL and should be further studied. These additional costs of care for dentate xerostomic patients may suggest a need for new guidelines for preventing the consequences of hyposalivation. It may perhaps lead to revisiting the previous recommendations of full mouth extractions for patients undergoing radiotherapy to the oral cavity (instead of the current practice of removing only diseased teeth prior to radiotherapy).
To make a treatment decision for their xerostomia patients, clinicians are contemplating a complex and heterogenous landscape of treatment solutions. Here, we review the current and emerging solutions to address xerostomia in H&N cancer patients and assess their cost-effectiveness. Available treatments for xerostomia typically seek to accomplish 1 of the 3 objectives: (1) to protect the salivary gland during RT, (2) to stimulate the remaining salivary gland function, or (3) to treat the symptoms of xerostomia. Research is also directed toward new methods (4) to regenerate the damaged salivary glands. Within each of these categories, a variety of lifestyle changes, pharmacologic treatments, devices, and surgical procedures are used (Table II).
Table II.
Overview of the competitive landscape: current and emerging solutions to address radiation-induced xerostomia in H&N cancer patients
| Type of solution | Type of treatment | Existing solutions | Emerging solutions |
|---|---|---|---|
| Prevention | Intensity-modulated RT Intensity-modulated proton RT Salivary gland transfer Radioprotective drugs (amifostine) |
Radioprotective drugs (tempol) | |
| Treatment | Palliation | Use of water Saliva substitutes, gels Hydration pack device |
|
| Treatment | Stimulation | Cholinergic muscarinic receptor agonist drugs Chewing-gum and bitter substances Acupuncture |
Electrical stimulation devices Gene therapy Growth factors BoNT |
| Treatment | Regeneration | Stem cell transfer Artificial glands |
The solutions as classified according to their goal—prevention or treatment of xerostomia—as well as their stage in the clinical use—existing solutions in the clinics versus emerging solution, still under preclinical or clinical research investigation.
PROTECTION OF THE SALIVARY GLANDS DURING RT
The most attractive way to address xerostomia in H&N cancer patients is to prevent its occurrence in the first place. Several solutions meet this objective: new RT techniques, radioprotective drugs, and surgical procedures (Table III).
Table III.
Current protective treatments
| Protecting the salivary glands |
IMRT | Amifostine | Salivary gland transfer |
|---|---|---|---|
| % Patients still experiencing xerostomia post-RT |
25%–40%8,40 | 51%41 | ~20%42 |
| Treatment planning time | 15–26 h43 | None | None |
| Treatment delivery time | 15–30 min44 per session, in 35 sessions over 7 weeks | None | 45 min45 |
| Cost calculation | 2006 MP for whole breast IMRT = $29,79046 2010 MP for prostate cancer IMRT = $42K47 2011 MP for: IMRT planning, (CPT code 77301), $2088.19 þ IMRT delivery (CPT code 77418), $519.84 per treatment session × 35 sessions = $20,282.5948 |
Calculated assuming the patients gets 8 mg of odansetron (anti-nausea drug) before each of 35 subcutaneous injections of 500 mg amifostine, before each RT session, over a 7-week period. 2011 MP for: odansetron (HCPCS code Q1079), $0.676 × 35 sessions + amifostine (HCPCS code J0207), $322.019 × 35 sessions = $11,30049 |
2010 MP for “unlisted procedure, salivary glands or ducts” (CPT code: 42699), $1335 (RVU = 0)*; 2010 MP for “excision of submandibular glands” (CPT code: 42440), $1725 (RVU = 7.13)* |
| Cost estimate per treatment course |
$20-$100K | $10-$30K | $10-$20K |
| Invasiveness | None | None | High |
| Contraindications | None | Allergies to aminothiol compounds | Oral cavity malignancies requiring RT to submental area + any H&N cancer not requiring surgical resection |
| Side effects | None | Nausea, vomiting, hypotension | None |
| Protecting the salivary glands | BoNT (emerging treatment option) | IGF-1 growth factor (emerging treatment option) |
|---|---|---|
| % Patients still experiencing xerostomia post-RT |
N/A. Preclinical stage | N/A. Preclinical stage |
| Treatment Planning time | None | None |
| Treatment delivery time | 1 min | 1 min |
| Cost calculation | Less than 20 U of BoNT per patient will be necessary and up to 100 U per bottle may be charged if 1 bottle is used for only 1 patient, amounting the cost of the drug to $125-$600 (BoNT type A, HCPCS code: J0585, $5.48/U).49,50 Treatment delivered in a single injection, estimated to $200, based on CPT codes for a similar procedure (CPT code 64613 for injection of BoNT to the neck to treat muscular pain, is $164.11 in non-facility setting),48,50 |
Based on an FDA-approved daily dose of 0.24 mg/kg, adapted to a 70 kg adult and an average 2006 wholesale price of $562.50 per vial of 40 mg Increlex,51 the annual cost of an IGF-1 treatment for xerostomia can be expected to be in the $100K range |
| Cost estimate per treatment course | $325-$800 | $100K |
| Invasiveness | None | None |
| Contraindications | None | Unknown |
| Side effects | None | N/A |
The current treatments aimed at protecting the salivary glands are summarized. For each treatment, details are provided about the following parameters: xerostomia rate after RT, treatment planning duration, treatment delivery duration, cost estimate per treatment course, with detailed cost calculation, invasiveness, contraindications, and side-effects.
MP, medicare payment; RVU, relative value unit; N/A, not available.
2010 Otolaryngology fee schedule.
Newer RT techniques are aimed at delivering a spatially selective therapy, which minimizes dosage to normal tissues when irradiating cancerous lesions. Introduced around 2000, intensity-modulated radiation therapy (IMRT) achieves this goal by creating a highly sculpted radiation dose distribution using multiple beams, each with its own spatially varying intensity profile.7 The clinical approach is as follows: wearing a custom-fit thermoplastic immobilization mask, the patient undergoes CT imaging, which will be used by the radiation oncologist to (1) delineate tumor volumes and margins and (2) compute beam intensities. Subsequently, the treatment is delivered in approximately 35 daily fractions over 7 weeks. IMRT can better spare the parotid gland adjacent to crucial lymphatics that need to receive a high dose of radiation.7 IMRT results in a mean parotid radiation dose of 30 Gy,52 a dose close to the threshold of 26 Gy suggested for severe parotid injury.7 Due to the proximity of submandibular salivary glands to the primary tumor and/or crucial lymphatics in the oral cavity (lymph node group 1B or submandibular triangle), as well as due to primary tumor size, IMRT often might not be able to effectively protect these glands, which are responsible for unstimulated saliva production. Consequently, in many cases, IMRT may not be able to protect against xerostomia at rest, especially at night. In selected patients harboring more lateralized primary tumors, recent studies have demonstrated that it is possible to spare the contralateral submandibular gland using IMRT.7,53,54 However, this practice is limited to early stage cancers that do not require radiation to bilateral necks. Most advanced cancers (stages 3–4) require bilateral necks irradiation and the addition of CHT to the radiation, potentiating the effects of radiotherapy to eliminate cancer cells. As a side effect, this synergistic effect might also cause more damage to salivary tissue resulting in xerostomia. A study is ongoing combining the surgical submandibular gland transfer technique (see later description of the Seikaly-Jha procedure for submandibular salivary gland transfer) with tomotherapy-based IMRT,55 a very promising solution, which might provide a better sparing of the submandibular glands, but remains invasive, time-consuming, and very costly. Newer rotational IMRT techniques, such as volumetric modulated arc therapy and helical tomotherapy, can improve delivered radiation dose, reduce radiation delivery time, and carry potential for a better conformation to the salivary glands: preliminary results in terms of salivary function seem encouraging,56,57 but there is still a debate as to whether the dosimetric improvements translate into clinical outcome, as compared with standard step-and-shoot IMRT.58 As a consequence, IMRT certainly reduces xerostomia as per patient survey—from 80% down to 25%–40% incidence, according to 3 recent prospective randomized studies conducted in the UK and in Hong Kong.7,59–61 This has been confirmed in a systematic review by the Oral Care Study Group of the Multinational Association of Supportive Care in Cancer and International Society of Oral Oncology (MASCC/ISOO) demonstrating that parotid sparing IMRT has the potential to reduce the prevalence and severity of xerostomia and improve xerostomia-related QOL after therapy.4 On the practical side, IMRT is very labor and time intensive and requires a skilled radiation oncologist, radiation physicist, and dosimetrist. IMRT planning is lasting 5–16 h longer than typical RT planning (~10 h) and daily IMRT treatment delivery lasts approximately 20–30 min. IMRT is currently the recommended standard treatment for H&N cancer8 Indeed, parotid-sparing IMRT was recommended by the MASCC/ISOO for the prevention of xerostomia and salivary gland hypofunction.9 It has been shown that saliva secretion has the potential of increasing following IMRT,9 suggesting that sparing of salivary glands by IMRT could make them more amenable to treatments stimulating their function (see later). IMRT is available at most RT centers in the US, where it is increasingly used. As an example, almost 90% of H&N cancer patients are treated with IMRT at our institution’s Hospital and Clinics. However, it is only available to 10% of the global population.40 We estimated the cost of H&N IMRT treatment to $20K-$40K (Table III). However, we acknowledge that this is a simplified cost addressing the whole IMRT treatment including planning time for multiple vital structure sparing and tumor coverage, as it is very challenging to estimate cost applicable only to salivary gland sparing.
Proton RT can potentially achieve a higher sparing of salivary glands than IMRT due to a more localized radiation dose delivery. Although there are dosimetric studies examining protons for salivary gland sparing,62,63 there is no clinical trials for the use of Proton RT in H&N cancer management.
In 1995, amifostine (Ethyol; MedImmune Pharma, Nijmegen, The Netherlands), a radioprotective drug, was approved by the Food and Drug Administration (FDA) for the prevention of xerostomia in patients undergoing H&N RT. This pharmacologic treatment mainly protects the parotid glands from radiation-induced apoptotic death by acting as an oxygen-radical scavenger. The treatment is administered by subcutaneous injection (500 mg) before each RT session for 6 weeks.64 Amifostine significantly decreases xerostomia by 22%–27% up to 12 months post-RT.36,119 However, amifostine has many detrimental side effects, the major one being nausea/vomiting,41 mandating the concomitant use of a nausea-preventing drug. Still, 21% of patients drop out of amifostine treatment regimens due to the side effects.65 We estimate the amifostine treatment cost to $10K-$30K (Table III). Amifostine does not specifically target salivary glands, so there is a theoretical concern that amifostine could protect the tumor from radiation through the same mechanism by which it protects the salivary glands; however, there is no evidence in favor of this hypothesis.9 The MASCC/ISOO Study Group could not recommend any guidelines for the use of amifostine to prevent xerostomia during RT, due to lack of consensus on the interpretation of existing evidence.9 Many oncologists also avoid prescribing amifostine because of the lack of agreement that the benefits of salivary glands protection are outweighing the side effects.
Botulinum toxin (BoNT) is a promising emerging pharmacologic treatment for radiation-induced xerostomia. A preclinical rat model has recently demonstrated that intraglandular injection of BoNT before RT reduces glandular injury.66 The mechanism of action of this treatment is unclear and two hypotheses have been proposed: (1) a temporary glandular involution leading to reduction of saliva production during RT, avoiding concentration of radiation where inorganic solutes from saliva are located or (2) an action on the nitric oxide pathway. Furthermore, BoNT has been shown to increase tumor response to RT in an experimental model, suggesting that this treatment would not have the risk of tumor protection.67 Because BoNT is already an FDA-approved drug for many applications, clinicians would more likely be inclined to prescribe this potential cytoprotective treatment before the start of radiotherapy, and the regulatory hurdles are correspondingly lower. We can estimate the total treatment cost to $325-$800 (Table III).
Other emerging preventive treatments include systemic administration of growth factors like insulin growth factor 1 (IGF-1) or keratinocyte growth factor, both of which have been shown to preserve gland functions in preclinical mouse studies.68,69 These growth factors are believed to protect the salivary glands by two possible mechanisms: (1) improving the survival and proliferation of salivary acinar cells and stem cells and (2) suppressing apoptosis of those cells after radiation.8 FDA approval has not been granted for IGF-1 injections in humans for several major applications (diabetes and amyotrophic lateral sclerosis). The only approval given to date for this therapy is for severe IGF-1 deficiency inducing dwarfism (Increlex (mecasermin); Ipsen, Paris, France),70 so it seems unlikely to receive approval for xerostomia treatment. In addition, radiation oncologists are cautious in using growth factors as a preventive agent for xerostomia because of a possible protumor effect. Treatment cost is estimated in the $100K range (Table III).
Another emerging radioprotective drug is tempol (4-hydroxy-2,2,6,6-tetramethylpiperidine 1-oxyl; Sigma-Aldrich, St. Louis, MO, USA), which has been shown, after systemic or topical application, to protect salivary glands but not tumor tissue from radiation damage in a mouse model71 tempol is a stable nitroxide that has several possible mechanisms of action including (1) mimicking superoxide dismutase activity, (2) oxidizing transition metals, and (3) scavenging free radicals.8 Tempol is a promising drug: it is currently tested in its gel formulation, also called MTS-01, in Phase-II clinical trials for the treatment of alopecia induced by brain RT and for the treatment of dermatitis induced by radiation and CHT for anal cancer.72 However, a clinical trial on the use of tempol to treat xerostomia has not yet been initiated.
An approach to surgical preventive care for xerostomia is the Seikaly-Jha procedure for submandibular salivary gland transfer. In this procedure, 1 submandibular salivary gland is surgically transferred into the submental space on the contralateral side of the primary tumor, which is typically shielded during RT.42 The surgical procedure is safe, does not require any microvasculature expertise, and does not involve a dedicated surgery because it can be performed during surgical resection of the primary tumor. Performing the Seikaly-Jha procedure adds approximately 45 min to the 2–10 h total surgical time,73 and the level of sparing obtained by this procedure has been suggested by the MASCC/ISOO study group to possibly be of clinical significance. 9 It has been reported to prevent xerostomia in 83% of patients in the long term (2 years).42 However, this invasive procedure is typically only applicable to patients with specific cancers requiring surgical resection as part of their H&N treatment (e.g., oropharyngeal carcinoma). It is also contraindicated in oral cavity malignancies because the submandibular nodal basin and the submental space are often irradiated in that case. This procedure was developed in Canada and has been clinically tested in Canada74 and China.75 It is currently under clinical trial in the US under the auspices of the Radiation Therapy Oncology Group.76 Information on cost is unavailable but we estimated it to $2-$20K (Table III).
STIMULATION OF THE SALIVARY GLANDS
In many cases, prevention of xerostomia cannot be achieved efficiently (e.g., because the salivary glands are located close to the primary tumor or lymph nodes suspected of tumor involvement) and the patient is left with hypofunctioning salivary glands. Data pooled from a large number of clinical studies has shown that, during or following RT, stimulated saliva secretion is consistently higher than unstimulated secretion, suggesting that stimulation of the salivary glands might be an effective strategy to treat xerostomia.4 Several pharmaceutical, alternative medicine, or devices treatments aim at stimulating the glands to “squeeze out” more saliva in these instances (Table IV).
Table IV.
Current stimulation and palliation treatments
| Stimulation and palliation | Pilocarpine or Cevimeline | Acupuncture | Saliva substitutes | Hydration pack (Xeros) |
Electrical stimulation (GenNarino) |
|---|---|---|---|---|---|
| % Patients experiencing partial reduction in xerostomia | 80%77 | 55%78 | 40%8 | Not documented | N/A |
| Treatment duration | Lifelong | 2 × 20 min/week for 4 weeks18 | Lifelong | Lifelong | Lifelong |
| Cost calculation | 3–4 of 5 mg Salagen pills per day, costing $3.95-$5.27 per day. Or 4 of 30 mg Evoxac capsules per day, costing $3.93/day79 | Average cost of an acupuncture session, $50-$7080 × 8 sessions | 40–150 mL of saliva substitute per day81 × $0.2/mL approximate US retail price for Biotene Oral Balance Liquid | Device retail price = $79982 | Device retail price = $575† |
| Cost estimate per treatment* | $5/day | $400-$600 | $0.5-$2/day | $79982 | $575† |
| Invasiveness | None | Minimally | None | None | None |
| Contraindications | Asthma, chronic obstructive pulmonary disease, cardiovascular disease, and glaucoma7 | N/R | N/R | N/R | N/R |
| Side effects | Sweating, hot flashes7 | N/R | Sticky mouth, mucosal damage | N/D | N/R |
The current treatments aimed at stimulating the salivary glands or treating xerostomia symptoms are summarized. For each treatment, details are provided about the following parameters: xerostomia rate after treatment, treatment duration, cost estimate per treatment course, with detailed cost calculation, invasiveness, contraindications, and side effects.
N/A, not available, N/D, not documented, N/R, none reported.
Cost estimates are for the entire treatment duration, except for lifelong treatments, where the cost estimate is given on a daily basis.
A. Wolff, President of Saliwell, Ltd, written communication.
There is a long list of pharmacologic sialogogues (drugs that increase saliva flow) but only 2 are approved for xerostomia treatment: pilocarpine hydrochloride (Salagen; MGI Pharma, Bloomington, MN, USA) and cevimeline (Evoxac; Daiichi-Sankyo, Tokyo, Japan).8 These cholinergic muscarinic receptor agonists mimic saliva-inducing nervous signals. In the case of pilocarpine, the mechanisms of action are not fully understood and might be attributed to stimulatory action on minor salivary glands.9 The results of several studies with pilocarpine have been inconsistent,83,84 and the beneficial effect of both drugs on xerostomia is debated. Although salivary flow increase has been shown,85 there may be no difference in the subjective perception of xerostomia.8 Among the 42%–51% of patients responding to pilocarpine, it was shown that the response can be delayed up to 12 weeks.86 Furthermore, the positive effects disappear as soon as the patient stops the treatment, so these drugs would need to be taken for a patient’s entire lifetime. Both drugs have many contraindications, such as asthma, and moderate adverse effects including intense sweating and hot flashes (Table IV). The treatment is administered orally as a dose of 5 mg 3 times daily and costs $4-$5/day (Table IV). The MASCC/ISOO group cannot recommend the use of pilocarpine during RT due to “equivocal results of the various randomized clinical trials” but, however, recommends its use following RT to improve xerostomia.9 Oncologists routinely prescribe pilocarpine to patients complaining of xerostomia but many patients (6%–15%)86 drop out of the pilocarpine treatments because of the low benefit-to-cost ratio, both in terms of side effects and treatment cost.
Acupuncture, an “alternative medicine” treatment believed to work through neuronal activation,87 has been shown to stimulate residual salivary gland function. Pilot studies have demonstrated a sustained effect on saliva secretion for up to 3 years and improvements in subjective symptoms (55% decrease in xerostomia in IMRT patients).78 A related proprietary technique called Codetron (Ehm Rehabilitation Technologies, Inc., Ontario, Canada) based on transcutaneous electrical nerve stimulation has showed 6-month improvements in whole saliva production.88 However, acupuncture and related techniques might not be considered a clinical treatment option by clinicians and their scientific value may be subject to debate. Nevertheless, the use of acupuncture to stimulate salivary secretion and alleviate xerostomia was suggested by the MASCC/ISOO study group.9 The cost is estimated at $400-$600 per treatment course based on current practice rates in the US (Table IV).
Electrical salivary stimulators are emerging devices that can be fixed or placed inside the mouth and apply electrical currents to the nervous extensions in the oral mucosa that innervate the salivary glands and stimulate salivation.89,90 Among them, the Salitron device (Biosonics, Fort Washington, PA, USA) is an intra-oral handheld stimulation probe linked to a current generator that was FDA approved in 1988. This cumbersome apparatus caused a sustained increase of salivary flow rate, as well as subjective improvement in xerostomia symptoms.91 Because this device was limited by its large size, high price, and lack of user-friendliness, it was replaced by miniaturized devices: GenNarino (Saliwell Ltd, Saarbruecken, Germany), a remotecontrolled removable intra-oral splint appliance92 and Saliwell Crown (Saliwell Ltd), an osteointegrated dental implant.93 Short-term (10 min) higher mouth moistening and reduced xerostomia have been demonstrated in both cases.92 Clinical trials are ongoing to evaluate the long-term effect of these devices on xerostomia.94 Saliwell GenNarino has been granted approval for marketing in Europe (CE mark) and is sold at a price of $575 per device (by A. Wolff, President of Saliwell, Ltd, written communication, February 2011).
Small studies have evaluated the use of gustatory and masticatory stimulants (gums, acidic candy, and salivastimulating lozenges) for stimulating salivary gland function in xerostomia patients following RT, but the results are not consistent: although chewing gum can increase saliva production for patients with remaining salivary function, there is no evidence that gum is more or less effective than salivary substitutes (see later), which do not seem to be more efficient than placebo.3 Therefore, no consensus can be extracted from the results of these studies.9 Furthermore, the use of gum induces stickiness of the mouth, and the use of acidic candy can cause erosion of the tooth enamel.3
REDUCTION OF XEROSTOMIA SYMPTOMS
In most cases of moderate to severe xerostomia following RT, the remaining nonfunctioning or hypofunctioning salivary glands cannot be stimulated efficiently to alleviate the subjective feeling of dry mouth. In those instances, supportive and palliative treatments aimed at moistening the mouth are the only options (Table IV).
Clinicians recommend their xerostomia patients adopt a change of lifestyle to treat their symptoms. This includes the frequent use of water, sucking of ice chips, antibacterial saliva substitutes, and moistening agents to palliate mouth dryness, together with avoidance of irritants such as spicy foods, alcohol, caffeine, or smoking.3 Saliva substitutes are solutions that mimic the essential properties of normal saliva, including its viscosity, lubrication, wetting properties, and antimicrobial effects.95 The majority of those solutions, based on mucin, carboxymethylcellulose, hydroxyethylcellulose, or xanthan gum,95 seem to relieve xerostomia for approximately 40% of patients,96 but the relief is only temporary.67 Oral gel formulations, which harbor a thicker texture that can line up oral mucosa and enamel, provide a longer lasting moisture sensation and are recommended for night use.8 Under these palliative treatments, xerostomia patients still have to wake up many times at night to reapply the treatment, drink water, or go to the bathroom due to the resulting polyuria. This short lasting effect is possibly the reason why the MASCC/ISOO panel recommended the use of these lubricants and saliva substitutes for short-term xerostomia improvement following RT.9 Conversely, the Cochrane review on the subject states that there is insufficient evidence that saliva substitutes are better or worse than placebo in reducing xerostomia symptoms and does not provide recommendations on this matter.3 Large volumes of saliva substitute need to be applied every day: an average of 40 mL for mucin- and 150 mL for carboxymethylcellulose-based saliva substitutes, as reported by Vissink et al.81 Except for the lifelong use of water, the other solutions are expensive, in the range of several dollars per day (Table IV). Moreover, a strong compliance is needed for treatments that require frequent applications.
Reservoir-based devices such as a hydration device called the Xeros hydration pack (Lorin Technologies Corporation, Swansboro, NC, USA) are trying to address this compliance issue. The device is an automated pump system for delivering water or saliva substitute to the mouth from a reservoir of liquid carried in a fanny pack via tubes going to the patient’s mouth. This device, previously commercialized for hydration during hiking, received FDA approval in April 2011 and is currently commercialized for xerostomia treatment at a price of $799 per device.82 Other oral reservoir devices (mouthguards or dentures) have been clinically tested in pilot trials,97–99 but there is insufficient evidence at present to recommend their use,3 and information on commercialization or cost of these devices is unavailable.
REGENERATION OF THE SALIVARY GLANDS
As an attempt to reverse the damage caused by RT to the salivary glands, several research strategies are emerging, aimed at regenerating salivary glands either via gene therapy or by transplantation of salivary gland stem cells. Devices containing saliva-secreting cells are also envisioned.
Preclinical studies in rats and miniature pigs have demonstrated that gene therapy using the gene coding for a transmembrane water channel protein, human aquaporin-1 (hAQP1), helps recover salivary function by up to 80% of pre-irradiation baseline saliva production.100–102 The mechanism of action is believed to be an increase in water secretion by duct cells in the salivary glands. However, this adenoviral (adeno-associated virus, AAV) treatment has been shown to induce an inflammatory response in the targeted salivary glands in a preclinical model,103,104 an effect that might be reversible.105 Recent studies showed that AAV transfer of the human keratinocyte growth factor gene reduced postirradiation xerostomia in a mouse model,106 whereas AAV transfer of tousled-like kinase 1B, a prosurvival gene, helped prevent radiationinduced damage to the salivary glands in a rat model.107 A new method, based on ultrasound-assisted gene transfer has recently demonstrated that those genes could be transferred to the salivary glands without the use of a viral vector.108 The only clinical trial for gene transfer to the salivary glands is a phase I clinical trial that will determine the safety and effectiveness of an adenoviral (AAV) therapy encoding hAQP1 in humans AdhAQP1.101,109,110 However, obtaining FDA-approval for such a therapy might be very difficult, especially because the outcomes of previous gene therapy trials have revealed a risk of fatal outcome.111 The cost of gene therapy is extremely high, in the order of $100K/year.112
Recently, the potential of specific stem cells identified by their expression of several progenitor markers to regenerate salivary glands was reported using a radiation-damage preclinical model.113,114 Salivary gland stem cells research holds tremendous promise for the future of xerostomia research.115 However, results are still preliminary and stem cells are not expected to be used in the clinics in the near future.
Several patents have been filed regarding artificial salivary glands consisting of biomaterial scaffolds containing cells capable of secreting saliva.116–118 Like the stem cell approach, the research related to this treatment option is still very immature and not likely to be applicable in the clinics in the near future.
DISCUSSION AND CONCLUSION
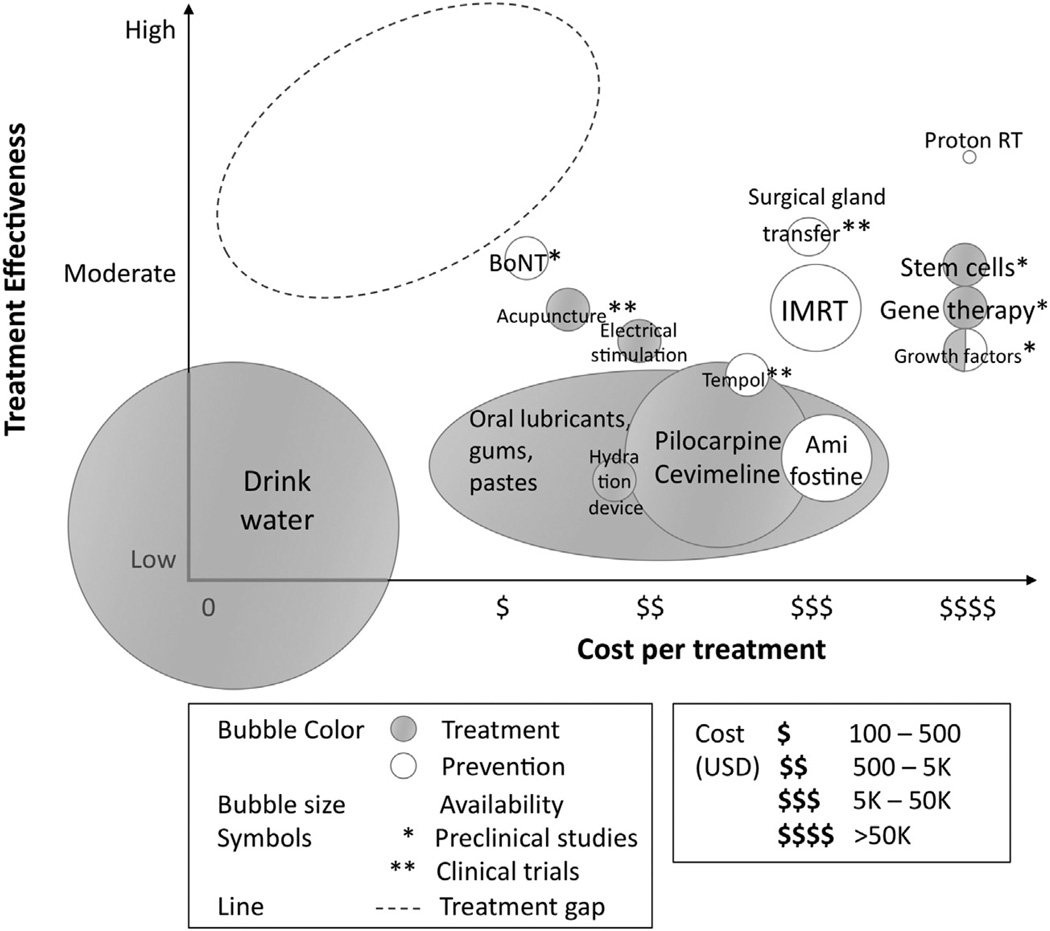
Although partial recovery may happen during the first year after H&N RT, the majority of H&N cancer patients experience long-term xerostomia, significantly impairing their QOL and potentially incurring extensive dental care costs. Here we presented an overview of the different treatment solutions for radiation-induced xerostomia, summarized as a cost-effectiveness gap analysis (Figure 1). These solutions have very heterogenous utilization profiles, with half of them still in preclinical or clinical trials and the other half being currently used or recommended in clinical practice: IMRT, proton therapy, various oral lubricants, pilocarpine, amifostine, and hydration and electrical stimulation devices. The degree of adoption of each of these treatments can vary a lot, depending on their cost, availability, and risk-to-benefit ratio. Prescribing pharmacologic treatments has demonstrated only moderate results, counterbalanced by possible QOL-impairing side effects (e.g., pilocarpine). We conclude that the most widely used strategy in the clinics is a combination of xerostomia prevention by IMRT followed by post-RT treatment of xerostomia symptoms by frequent hydration and saliva substitutes. Due to their antibacterial properties, these saliva substitutes have the potential to reduce the high dental care costs incurred by xerostomia patients. However, due to relatively short lasting moisturization, these solutions possibly have a poor efficacy at treating xerostomia at resting state and nighttime.
Fig. 1.
Treatment gap analysis. Synthesis of the solutions for addressing xerostomia in H&N cancer patients undergoing RT. The solutions are summarized in a graph showing the effectiveness of the solution at treating or preventing xerostomia versus the cost per treatment. The solutions are categorized according to the following criteria: treatment versus prevention and preclinical versus clinical stage. Relative clinical availability is also estimated and represented as bubble size. Highlighted by the dashed circle is the treatment gap, which corresponds to the area of greatest innovation opportunity.
The solutions described here occupy very different locations on the cost-effectiveness landscape (Figure 1). In our analysis, we observed a gap in treating xerostomia in H&N cancer patients with both a high effectiveness and a relatively low cost. BoNT and acupuncture treatments appear as the most cost-effective existing strategies and seem very promising because both techniques are already widely available for different applications. However, these are emerging solutions still being tested in preclinical and clinical research respectively: their efficacy at treating xerostomia need to be further studied and confirmed. IMRT appear to be the most cost-effective existing solution that is already widely available and in use in the clinics. Surgical gland transfer harbors a similar cost-effectiveness ratio and could be easily implemented in the clinics (during tumor resection surgery, when applicable) but remains very invasive and costly. Combining those two techniques seems very promising but will likely incur very high costs.
It is important to note that the cost of care for patients suffering from hyposalivation also includes extensive dental care costs, which could potentially be reduced by the solutions mentioned above. Furthermore, those costs could be further reduced if H&N cancer patients are treated in multidisciplinary settings where cancer care and dental care are integrated.
On the basis of our analysis, the ideal solution addressing resting-state xerostomia in H&N cancer patients is still missing, which would be able to reduce the perception of mouth dryness at resting state, achieve the protective function of saliva, be noninvasive, and cost-effective, at least with respect to current pharmaceuticals (in the order of $1-$2/day). In our opinion, the current clinical landscape is lacking such a medical solution and there is room for innovators to design novel solutions to meet the need of RT-induced xerostomia in H&N cancer patients. It is worth noticing that such a solution would have the potential to serve the needs and improve QOL of H&N cancer patients suffering from radiation-induced xerostomia, as well as a broader population of patients suffering from xerostomia of various etiologies, such as Sjögren’s syndrome and polypharmacy.
Statement of Clinical Relevance.
Xerostomia is the major complaint of patients receiving H&N RT. This manuscript is a practical and comprehensive review of solutions for prevention and treatment of radiation-induced xerostomia with emphasis on cost-effectiveness to help guide clinical treatment decisions.
Acknowledgments
This work was supported by a National Collegiate Inventors and Innovators Alliance (NCIIA) Advanced E-team grant. L.S.S. was supported by a Fulbright Science and Technology Fellowship, a Stanford Weiland Fellowship, a Student Fellowship from the Society for Nuclear Medicine (SNM), an International Student Fellowship from the Howard Hughes Medical Institute (HHMI).
The authors are the recipients of an Advanced E-team grant from NCIIA (National Collegiate Inventors and Innovators Alliance) to work on prototyping a device addressing xerostomia (this device is not described in this manuscript).
The authors thank Prof. Stefanos Zenios (Stanford Graduate School of Business) for his help.
REFERENCES
- 1. [Accessed May 17, 2011];WHO World Health Statistics. 2011 Available at: http://www.who.int/whosis/whostat/2011/en/index.html.
- 2.Anon. [Accessed March 3, 2011];14.1 Xerostomia Annual Reportd—NIDCR/CDC Dental, Oral and Craniofacial Data Resource Center (DRC) Available at: http://drc.hhs.gov/report/14_1.htm#14_1_1.
- 3.Furness S, Worthington HV, Bryan G, Birchenough S, McMillan R. Interventions for the management of dry mouth: topical therapies. Cochrane Database of Systematic Reviews. 2011;(Issue 12) doi: 10.1002/14651858.CD008934.pub2. Art. No. CD008924. [DOI] [PubMed] [Google Scholar]
- 4.Jensen S, Pedersen A, Vissink A, et al. A systematic review of salivary gland hypofunction and xerostomia induced by cancer therapies: prevalence, severity and impact on quality of life. Support Care Cancer. 2010;18:1039–1060. doi: 10.1007/s00520-010-0827-8. [DOI] [PubMed] [Google Scholar]
- 5.Davies L, Welch H. Epidemiology of head and neck cancer in the United States. Otolaryngol Head Neck Surg. 2006;135:451.e3–457.e3. doi: 10.1016/j.otohns.2006.01.029. [DOI] [PubMed] [Google Scholar]
- 6.Matsuba H, Katada C, Masaki T, et al. Diagnosis of the extent of advanced oropharyngeal and hypopharyngeal cancers by narrow band imaging with magnifying endoscopy. Laryngoscope. 2011;121:753–759. doi: 10.1002/lary.21553. [DOI] [PubMed] [Google Scholar]
- 7.Scrimger R. Salivary gland sparing in the treatment of head and neck cancer. Expert Rev Anticancer Ther. 2011;11:1437–1448. doi: 10.1586/era.11.101. [DOI] [PubMed] [Google Scholar]
- 8.Vissink A, Mitchell JB, Baum BJ, et al. Clinical management of salivary gland hypofunction and xerostomia in head-and-neck cancer patients: successes and barriers. Int J Radiat Oncol Biol Phys. 2010;78:983–991. doi: 10.1016/j.ijrobp.2010.06.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jensen S, Pedersen A, Vissink A, et al. A systematic review of salivary gland hypofunction and xerostomia induced by cancer therapies: management strategies and economic impact. Support Care Cancer. 2010;18:1061–1079. doi: 10.1007/s00520-010-0837-6. [DOI] [PubMed] [Google Scholar]
- 10.Navazesh M, Christensen C, Brightman V. Clinical criteria for the diagnosis of salivary gland hypofunction. J Dent Res. 1992;71:1363–1369. doi: 10.1177/00220345920710070301. [DOI] [PubMed] [Google Scholar]
- 11.Suh K-I, Lee J-Y, Chung J-W, Kim Y-K, Kho H-S. Relationship between salivary flow rate and clinical symptoms and behaviours in patients with dry mouth. J Oral Rehabil. 2007;34:739–744. doi: 10.1111/j.1365-2842.2006.01712.x. [DOI] [PubMed] [Google Scholar]
- 12.Murphy BA, Dietrich MS, Wells N, et al. Reliability and validity of the Vanderbilt Head and Neck Symptom Survey: a tool to assess symptom burden in patients treated with chemoradiation. Head Neck. 2010;32:26–37. doi: 10.1002/hed.21143. [DOI] [PubMed] [Google Scholar]
- 13.Cooperstein E, Gilbert J, Epstein JB, et al. Vanderbilt Head and Neck Symptom Survey version 2.0: report of the development and initial testing of a subscale for assessment of oral health. Head Neck. 2012;34:797–804. doi: 10.1002/hed.21816. [DOI] [PubMed] [Google Scholar]
- 14.Beetz I, Burlage FR, Bijl HP, et al. The Groningen Radiotherapy- Induced Xerostomia questionnaire: development and validation of a new questionnaire. Radiother Oncol. 2010;97:127–131. doi: 10.1016/j.radonc.2010.05.004. [DOI] [PubMed] [Google Scholar]
- 15.Vidović Juras D, Lukac J, Cekić-Arambasin A, et al. Effects of low-level laser treatment on mouth dryness. Coll Antropol. 2010;34:1039–1043. [PubMed] [Google Scholar]
- 16.Wijers OB, Levendag PC, Braaksma MMJ, Boonzaaijer M, Visch LL, Schmitz PIM. Patients with head and neck cancer cured by radiation therapy: a survey of the dry mouth syndrome in long-term survivors. Head Neck. 2002;24:737–747. doi: 10.1002/hed.10129. [DOI] [PubMed] [Google Scholar]
- 17.Eisbruch A, Kim HM, Terrell JE, Marsh LH, Dawson LA, Ship JA. Xerostomia and its predictors following parotid-sparing irradiation of head-and-neck cancer. Int J Radiol Oncol Biol Phys. 50:695–704. doi: 10.1016/s0360-3016(01)01512-7. [DOI] [PubMed] [Google Scholar]
- 18.Garcia MK, Chiang JS, Cohen L, et al. Acupuncture for radiation-induced xerostomia in patients with cancer: a pilot study. Head Neck. 2009;31:1360–1368. doi: 10.1002/hed.21110. [DOI] [PubMed] [Google Scholar]
- 19.Thomson WM, Chalmers JM, Spencer AJ, Williams SM. The Xerostomia Inventory: a multi-item approach to measuring dry mouth. Community Dent Health. 1999;16:12–17. [PubMed] [Google Scholar]
- 20.Mackowiak J, Scott-Lennox J, Lennox R, Wasserman T, Jackson S, Peeples P. PCD13: Patient Benefit Questionnaire (PBQ) for xerostomia: development and validation report. Value Health. 1999;2:197. [Google Scholar]
- 21.Ringash J, Bezjak A. A structured review of quality of life instruments for head and neck cancer patients. Head Neck. 2001;23:201–213. doi: 10.1002/1097-0347(200103)23:3<201::aid-hed1019>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 22.Adulyanon S, Vourapukjaru J, Sheiham A. Oral impacts affecting daily performance in a low dental disease Thai population. Community Dent Oral Epidemiol. 1996;24:385–389. doi: 10.1111/j.1600-0528.1996.tb00884.x. [DOI] [PubMed] [Google Scholar]
- 23.Robinson PG, Gibson B, Khan FA, Birnbaum W. Validity of two oral health-related quality of life measures. Community Dent Oral Epidemiol. 2003;31:90–99. doi: 10.1034/j.1600-0528.2003.00051.x. [DOI] [PubMed] [Google Scholar]
- 24.Locker D, Slade G. Association between clinical and subjective indicators of oral health status in an older adult population. Gerodontology. 1994;11:108–114. doi: 10.1111/j.1741-2358.1994.tb00116.x. [DOI] [PubMed] [Google Scholar]
- 25.Slade GD. Derivation and validation of a short-form oral health impact profile. Community Dent Oral Epidemiol. 1997;25:284–290. doi: 10.1111/j.1600-0528.1997.tb00941.x. [DOI] [PubMed] [Google Scholar]
- 26.Hassan SJ, Weymuller EA., Jr Assessment of quality of life in head and neck cancer patients. Head Neck. 1993;15:485–496. doi: 10.1002/hed.2880150603. [DOI] [PubMed] [Google Scholar]
- 27.Rogers SN, Lowe D. The University of Washington Quality of Life Scale. In: Preedy VR, Watson RR, editors. Handbook of Disease Burdens and Quality of Life Measures. New York, NY: Springer New York; 2010. [Accessed July 20, 2012]. pp. 101–128. Available at: http://rd.springer.com/referenceworkentry/10.1007/978-0-387-78665-0_6. [Google Scholar]
- 28.Fox PC, Busch KA, Baum BJ. Subjective reports of xerostomia and objective measures of salivary gland performance. J Am Dent Assoc. 1987;115:581–584. doi: 10.1016/s0002-8177(87)54012-0. [DOI] [PubMed] [Google Scholar]
- 29.Chambers MS, Garden AS, Kies MS, Martin JW. Radiation-induced xerostomia in patients with head and neck cancer: pathogenesis, impact on quality of life, and management. Head Neck. 2004;26:796–807. doi: 10.1002/hed.20045. [DOI] [PubMed] [Google Scholar]
- 30.Münter MW, Karger CP, Hoffner SG, et al. Evaluation of salivary gland function after treatment of head-and-neck tumors with intensity-modulated radiotherapy by quantitative pertechnetate scintigraphy. Int J Radiat Oncol Biol Phys. 2004;58:175–184. doi: 10.1016/s0360-3016(03)01437-8. [DOI] [PubMed] [Google Scholar]
- 31.Speight PM, Kaul A, Melsom RD. Measurement of whole unstimulated salivary flow in the diagnosis of Sjögren’s syndrome. Ann Rheum Dis. 1992;51:499–502. doi: 10.1136/ard.51.4.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Meirovitz A, Murdoch-Kinch CA, Schipper M, Pan C, Eisbruch A. Grading xerostomia by physicians or by patients after intensity-modulated radiotherapy of head-and-neck cancer. Int J Radiat Oncol Biol Phys. 2006;66:445–453. doi: 10.1016/j.ijrobp.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 33.Mandel ID. The role of saliva in maintaining oral homeostasis. J Am Dent Assoc. 1989;119:298–304. doi: 10.14219/jada.archive.1989.0211. [DOI] [PubMed] [Google Scholar]
- 34.Ettinger RL. Xerostomia—a complication of ageing. Aust Dent J. 1981;26:365–371. doi: 10.1111/j.1834-7819.1981.tb03992.x. [DOI] [PubMed] [Google Scholar]
- 35.Dirix P, Nuyts S, Vander Poorten V, Delaere P, Bogaert W. The influence of xerostomia after radiotherapy on quality of life. Support Care Cancer. 2007;16:171–179. doi: 10.1007/s00520-007-0300-5. [DOI] [PubMed] [Google Scholar]
- 36.Christensen LB, Petersen PE, Thorn JJ, Schiødt M. Dental caries and dental health behavior of patients with primary Sjögren syndrome. Acta Odontol. Scand. 2001;59:116–120. doi: 10.1080/000163501750266684. [DOI] [PubMed] [Google Scholar]
- 37.Jacobson JJ, Epstein JB, Eichmiller FC, et al. The cost burden of oral, oral pharyngeal, and salivary gland cancers in three groups: commercial insurance, medicare, and medicaid. Head Neck Oncol. 2012;4:15. doi: 10.1186/1758-3284-4-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Epstein JD, Knight TK, Epstein JB, Bride MA, Nichol MB. Cost of care for early- and late-stage oral and pharyngeal cancer in the California Medicaid population. Head Neck. 2008;30:178–186. doi: 10.1002/hed.20670. [DOI] [PubMed] [Google Scholar]
- 39.Turner M, Jahangiri L, Ship JA. Hyposalivation, xerostomia and the complete denture: a systematic review. J Am Dent Assoc. 2008;139:146–150. doi: 10.14219/jada.archive.2008.0129. [DOI] [PubMed] [Google Scholar]
- 40.Trotti A, Eisbruch A. Reducing xerostomia through advanced technology. Lancet Oncol. 2011;12:110–111. doi: 10.1016/S1470-2045(11)70009-2. [DOI] [PubMed] [Google Scholar]
- 41.Anon. [Accessed December 11, 2011];Ethyol (amifostine) product information sheet. 2011 Available at: http://www.ethyol.com.
- 42.Seikaly H, Jha N, Harris JR, et al. Long-term outcomes of submandibular gland transfer for prevention of postradiation xerostomia. Arch Otolaryngol Head Neck Surg. 2004;130:956–961. doi: 10.1001/archotol.130.8.956. [DOI] [PubMed] [Google Scholar]
- 43.Miles EA, Clark CH, Urbano MTG, et al. The impact of introducing intensity modulated radiotherapy into routine clinical practice. Radiother Oncol. 2005;77:241–246. doi: 10.1016/j.radonc.2005.10.011. [DOI] [PubMed] [Google Scholar]
- 44.Domrzalski D. [Accessed December 11, 2011];Blasting cancer precisely is now a local medical option. New Mexico Business Weekly. 2002 Available at: http://www.bizjournals.com/albuquerque/stories/2002/01/28/focus2.html?page=all.
- 45.Rieger J, Seikaly H, Jha N, et al. Submandibular gland transfer for prevention of xerostomia after radiation therapy: swallowing outcomes. Arch Otolaryngol Head Neck Surg. 2005;131:140–145. doi: 10.1001/archotol.131.2.140. [DOI] [PubMed] [Google Scholar]
- 46.Strauss JB, Chen SS, Dickler AT, Griem KL. Cost effectiveness of whole breast IMRT for reduction of moist desquamation. ASCO Annual Meeting Proceedings. 2007 [Google Scholar]
- 47.Arzt L. NAPT Responds to “Running a Hospital” Blog by Paul Levy, CEO of a large Boston Hospital: Protons Killing Cancer and Our Budget. [Accessed December 5, 2011];Community Forum—The National Association for Proton Therapy. Available at: http://www.proton-therapy.org/forum.htm.
- 48.Anon. [Accessed December 5, 2011];“N-00000-00 National” Medicare payment rates for CPT codes. American Medical Association. 2011 Available at: https://ocm.ama-assn.org/OCM/CPTRelativeValueSearch.do.
- 49.Anon. [Accessed December 5, 2011];HCPCS Codes & Medicare Payment, Effective October 1, 2011-December 31, 2011. BDI Pharma. Available at: http://www.bdipharma.com/Services-HCPCS-Codes-Medicare-Payment.aspx.
- 50.Anon. [Accessed December 7, 2011];New Botox Coding Opens Door to Added Reimbursement. Supercoder. Available at: http://www.supercoder.com/articles/articles-alerts/pmc/new-botox-coding-opens-door-to-added-reimbursement-39949/
- 51.Anon. [Accessed December 11, 2011];Tercica’s Increlex Pricing Provides a Significant Competitive Advantage. Business Wire. 2006 Available at: http://files.shareholder.com/downloads/TRCA/0x0x111203/6a795f39-548d-4a27-b33d-053a41cda36d/199466.pdf.
- 52.Chao KS, Deasy JO, Markman J, et al. A prospective study of salivary function sparing in patients with head-and-neck cancers receiving intensity-modulated or three-dimensional radiation therapy: initial results. Int J Radiat Oncol Biol Phys. 2001;49:907–916. doi: 10.1016/s0360-3016(00)01441-3. [DOI] [PubMed] [Google Scholar]
- 53.Saarilahti K, Kouri M, Collan J, et al. Sparing of the submandibular glands by intensity modulated radiotherapy in the treatment of head and neck cancer. Radiother Oncol. 2006;78:270–275. doi: 10.1016/j.radonc.2006.02.017. [DOI] [PubMed] [Google Scholar]
- 54.Wang Z-H, Yan C, Zhang Z-Y, et al. Impact of salivary gland dosimetry on Post-IMRT recovery of saliva output and xerostomia grade for head-and-neck cancer patients treated with or without contralateral submandibular gland sparing: a longitudinal study. Int J Radiat Oncol Biol Phys. 2011;81:1479–1487. doi: 10.1016/j.ijrobp.2010.07.1990. [DOI] [PubMed] [Google Scholar]
- 55.Saibishkumar EP, Jha N, Scrimger RA, et al. Sparing the parotid glands and surgically transferred submandibular gland with helical tomotherapy in post-operative radiation of head and neck cancer: a planning study. Radiother Oncol. 2007;85:98–104. doi: 10.1016/j.radonc.2007.09.014. [DOI] [PubMed] [Google Scholar]
- 56.Thariat J, Guevara N, Marcy P-Y, Bensadoun RJ, Bardet E, Giraud P. Conservation of salivary function and new external head and neck radiation techniques. Eur Ann Otorhinolaryngol Head Neck Dis. 2010;127:197–203. doi: 10.1016/j.anorl.2010.09.004. [DOI] [PubMed] [Google Scholar]
- 57.Berges O, Henni M, Gilliot O, et al. Tomothérapie des cancers ORL: évaluation scintigraphique prospective de la préservation des glandes salivaires. Cancer/Radiothérapie. 2008;12:704. [Google Scholar]
- 58.Chen AM, Marsano J, Perks J, et al. Comparison of IMRT techniques in the radiotherapeutic management of head and neck cancer: is tomotherapy “better” than step-and-shoot IMRT? Technol Cancer Res Treat. 2011;10:171–177. doi: 10.7785/tcrt.2012.500192. [DOI] [PubMed] [Google Scholar]
- 59.Kam MKM, Leung S-F, Zee B, et al. Prospective randomized study of intensity-modulated radiotherapy on salivary gland function in early-stage nasopharyngeal carcinoma patients. J Clin Oncol. 2007;25:4873–4879. doi: 10.1200/JCO.2007.11.5501. [DOI] [PubMed] [Google Scholar]
- 60.Nutting CM, Morden JP, Harrington KJ, et al. Parotid-sparing intensity modulated versus conventional radiotherapy in head and neck cancer (PARSPORT): a phase 3 multicentre randomised controlled trial. Lancet Oncol. 2011;12:127–136. doi: 10.1016/S1470-2045(10)70290-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pow EHN, Kwong DLW, McMillan AS, et al. Xerostomia and quality of life after intensity-modulated radiotherapy vs. conventional radiotherapy for early-stage nasopharyngeal carcinoma: initial report on a randomized controlled clinical trial. Int J Radiat Oncol Biol Phys. 2006;66:981–991. doi: 10.1016/j.ijrobp.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 62.Widesott L, Pierelli A, Fiorino C, et al. Intensity-modulated proton therapy versus helical tomotherapy in nasopharynx cancer: planning comparison and NTCP evaluation. Int J Radiat Oncol Biol Phys. 2008;72:589–596. doi: 10.1016/j.ijrobp.2008.05.065. [DOI] [PubMed] [Google Scholar]
- 63.van de Water TA, Lomax AJ, Bijl HP, Schilstra C, Hug EB, Langendijk JA. Using a reduced spot size for intensity-modulated proton therapy potentially improves salivary gland-sparing in oropharyngeal cancer. Int J Radiat Oncol Biol Phys. 2012;82:e313–e319. doi: 10.1016/j.ijrobp.2011.05.005. [DOI] [PubMed] [Google Scholar]
- 64.Bardet E, Martin L, Calais G, et al. Subcutaneous compared with intravenous administration of amifostine in patients with head and neck cancer receiving radiotherapy: final results of the GORTEC2000-02 phase III randomized trial. J Clin Oncol. 2011;29:127–133. doi: 10.1200/JCO.2009.25.5638. [DOI] [PubMed] [Google Scholar]
- 65.Brizel DM, Wasserman TH, Henke M, et al. Phase III randomized trial of amifostine as a radioprotector in head and neck cancer. J Clin Oncol. 2000;18:3339–3345. doi: 10.1200/JCO.2000.18.19.3339. [DOI] [PubMed] [Google Scholar]
- 66.Teymoortash A, Müller F, Juricko J, et al. Botulinum toxin prevents radiotherapy-induced salivary gland damage. Oral Oncol. 2009;45:737–739. doi: 10.1016/j.oraloncology.2008.11.014. [DOI] [PubMed] [Google Scholar]
- 67.Ansiaux R, Baudelet C, Cron GO, et al. Botulinum toxin potentiates cancer radiotherapy and chemotherapy. Clin Cancer Res. 2006;12:1276–1283. doi: 10.1158/1078-0432.CCR-05-1222. [DOI] [PubMed] [Google Scholar]
- 68.Lombaert IMA, Brunsting JF, Wierenga PK, Kampinga HH, de Haan G, Coppes RP. Keratinocyte growth factor prevents radiation damage to salivary glands by expansion of the stem/ progenitor pool. Stem Cells. 2008;26:2595–2601. doi: 10.1634/stemcells.2007-1034. [DOI] [PubMed] [Google Scholar]
- 69.Limesand KH, Avila JL, Victory K, et al. Insulin-like growth factor-1 preserves salivary gland function after fractionated radiation. Int J Radiat Oncol Biol Phys. 2010;78:579–586. doi: 10.1016/j.ijrobp.2010.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rosenbloom AL. The role of recombinant insulin-like growth factor I in the treatment of the short child. Curr Opin Pediatr. 2007;19:458–464. doi: 10.1097/MOP.0b013e3282094126. [DOI] [PubMed] [Google Scholar]
- 71.Vitolo JM, Cotrim AP, Sowers AL, et al. The stable nitroxide tempol facilitates salivary gland protection during head and neck irradiation in a mouse model. Clin Cancer Res. 2004;10:1807–1812. doi: 10.1158/1078-0432.ccr-03-0194. [DOI] [PubMed] [Google Scholar]
- 72.Anon. [Accessed December 11, 2011];Mitos Pharmaceuticals clinical trials webpage. Mitos Pharmaceuticals. Available at: http://www.mitospharma.com/research/product/clinical_research/
- 73.Seikaly H, Jha N, McGaw T, Coulter L, Liu R, Oldring D. Submandibular gland transfer: a new method of preventing radiation-induced xerostomia. Laryngoscope. 2001;111:347–352. doi: 10.1097/00005537-200102000-00028. [DOI] [PubMed] [Google Scholar]
- 74.Anon. [Accessed December 11, 2011];Emerging technology list: submandibular gland transfer for preservation of salivary gland function. Canadian Coordinating Office for Health Technology Assessment. 2004 Available at: http://www.cadth.ca/media/pdf/152_No21_submandibularglandtransfer_etech_e.pdf.
- 75.Zhang Y, Guo C-B, Zhang L, et al. [Accessed December 8, 2011];Prevention of radiation-induced xerostomia by submandibular gland transfer. Head Neck. 2011 doi: 10.1002/hed.21859. Available at: http://www.ncbi.nlm.nih.gov/pubmed/22083885. [DOI] [PubMed]
- 76.Anon. [Accessed November 7, 2011];Phase II study of salivary gland surgery before radiation therapy in preventing radiation-caused xerostomia in patients with head and neck cancer. ClinicalTrials.gov. Available at: http://clinicaltrials.gov/show/NCT00068237.
- 77.Berk L. Systemic pilocarpine for treatment of xerostomia. Expert Opin Drug Metab Toxicol. 2008;4:1333–1340. doi: 10.1517/17425255.4.10.1333. [DOI] [PubMed] [Google Scholar]
- 78.Blom M, Lundeberg T. Long-term follow-up of patients treated with acupuncture for xerostomia and the influence of additional treatment. Oral Dis. 2000;6:15–24. doi: 10.1111/j.1601-0825.2000.tb00316.x. [DOI] [PubMed] [Google Scholar]
- 79.Taylor SE. Efficacy and economic evaluation of pilocarpine in treating radiation-induced xerostomia. Expert Opin Pharmacother. 2003;4:1489–1497. doi: 10.1517/14656566.4.9.1489. [DOI] [PubMed] [Google Scholar]
- 80.Anon. [Accessed December 12, 2011];Acupuncture cost—how much does acupuncture cost? CostHelper.com. Available at: http://www.costhelper.com/cost/health/acupuncture.html.
- 81.Vissink A, s-Gravenmade EJ, Panders AK, et al. A clinical comparison between commercially available mucin- and CMC-containing saliva substitutes. Int J Oral Surg. 1983;12:232–238. doi: 10.1016/s0300-9785(83)80048-9. [DOI] [PubMed] [Google Scholar]
- 82.Anon. [Accessed December 11. 2011];Xeros dry mouth pump, dry mouth therapy for chronic dry mouth caused by radiation therapy treatment. Available at: http://drymouthpump.com/
- 83.Scarantino C, LeVeque F, Swann RS, et al. Effect of pilocarpine during radiation therapy: results of RTOG 97-09, a phase III randomized study in head and neck cancer patients. J Support Oncol. 2006;4:252–258. [PubMed] [Google Scholar]
- 84.Burlage FR, Roesink JM, Kampinga HH, et al. Protection of salivary function by concomitant pilocarpine during radiotherapy: a double-blind, randomized, placebo-controlled study. Int J Radiat Oncol Biol Phys. 2008;70:14–22. doi: 10.1016/j.ijrobp.2007.06.016. [DOI] [PubMed] [Google Scholar]
- 85.Petrone D, Condemi JJ, Fife R, Gluck O, Cohen S, Dalgin P. A double-blind, randomized, placebo-controlled study of cevi-meline in Sjögren’s syndrome patients with xerostomia and keratoconjunctivitis sicca. Arthritis Rheum. 2002;46:748–754. doi: 10.1002/art.510. [DOI] [PubMed] [Google Scholar]
- 86.Davies AN, Shorthose K. Cochrane Database of Systematic Reviews. John Wiley & Sons, Ltd; 2007. [Accessed July 20, 2012]. Parasympathomimetic drugs for the treatment of salivary gland dysfunction due to radiotherapy. Available at: http://onlinelibrary.wiley.com/doi/10.1002/14651858.CD003782.pub2/abstract. [DOI] [PubMed] [Google Scholar]
- 87.Deng G, Hou B, Holodny A, Cassileth B. Functional magnetic resonance imaging (fMRI) changes and saliva production associated with acupuncture at LI-2 acupuncture point: a randomized controlled study. BMC Complement Altern Med. 2008;8:37. doi: 10.1186/1472-6882-8-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wong RKW, Jones GW, Sagar SM, Babjak A-F, Whelan T. A Phase I-II study in the use of acupuncture-like transcutaneous nerve stimulation in the treatment of radiation-induced xerostomia in head-and-neck cancer patients treated with radical radiotherapy. Int J Radiat Oncol Biol Phys. 2003;57:472–480. doi: 10.1016/s0360-3016(03)00572-8. [DOI] [PubMed] [Google Scholar]
- 89.Pines E, Fenster M. [Accessed December 12, 2011];United States Patent: 7477947-System and method for electrical stimulation of salivation. 2009 Available at: http://patft.uspto.gov/netacgi/nph-Parser?Sect1=PTO2&Sect2=HITOFF&p=1&u=%2Fnetahtml%2FPTO%2Fsearch-adv.htm&r=2&f=G&l=50&d=PTXT&S1=7,477,947&OS=7,477,947&RS=7,477,947.
- 90.Brenman HS, Katz P, Beatty GE. [Accessed December 12, 2011];United States Patent: 4637405-Apparatus for stimulating salivation. 1987 Available at: http://patft.uspto.gov/netacgi/nph-Parser?Sect1=PTO2&Sect2=HITOFF&u=%2Fnetahtml%2FPTO%2Fsearch-adv.htm&r=13&f=G&l=50&d=PTXT&p=1&p=1&S1=4,637,405&OS=4,637,405&RS=4,637,405.
- 91.Talal N, Quinn JH, Daniels TE. The clinical effects of electro-stimulation on salivary function of Sjogren’s syndrome patients. Rheumatol Int. 1992;12:43–45. doi: 10.1007/BF00300975. [DOI] [PubMed] [Google Scholar]
- 92.Strietzel FP, Martín-Granizo R, Fedele S, et al. Electro-stimulating device in the management of xerostomia. Oral Dis. 2007;13:206–213. doi: 10.1111/j.1601-0825.2006.01268.x. [DOI] [PubMed] [Google Scholar]
- 93.Ami S, Wolff A. Implant-supported electrostimulating device to treat xerostomia: a preliminary study. Clin Implant Dent Relat Res. 2010;12:62–71. doi: 10.1111/j.1708-8208.2009.00180.x. [DOI] [PubMed] [Google Scholar]
- 94.Anon. [Accessed December 11, 2011];Clinical trial: evaluation of an electro-stimulator for the treatment of xerostomia (GenNarino). ClinicalTrials.gov. 2010 Available at: http://clinicaltrials.gov/ct2/show/NCT00509808.
- 95.Hahnel S, Behr M, Handel G, Bürgers R. Saliva substitutes for the treatment of radiation-induced xerostomia—a review. Support Care Cancer. 2009;17:1331–1343. doi: 10.1007/s00520-009-0671-x. [DOI] [PubMed] [Google Scholar]
- 96.Warde P, Kroll B, O’Sullivan B, et al. A phase II study of Biotene in the treatment of postradiation xerostomia in patients with head and neck cancer. Support Care Cancer. 2000;8:203–208. doi: 10.1007/s005200050286. [DOI] [PubMed] [Google Scholar]
- 97.Frost P, Shirlaw P, Challacombe S, Fernandes-Naglik L, Walter J, Ide M. Impact of wearing an intra-oral lubricating device on oral health in dry mouth patients. Oral Dis. 2006;12:57–62. doi: 10.1111/j.1601-0825.2005.01161.x. [DOI] [PubMed] [Google Scholar]
- 98.McMillan AS, Peter Tsang CS, Wong MCM, Kam AYL. Efficacy of a novel lubricating system in the management of radiotherapy-related xerostomia. Oral Oncol. 2006;42:842–848. doi: 10.1016/j.oraloncology.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 99.Robinson PG, Pankhurst CL, Garrett EJ. Randomized-controlled trial: effect of a reservoir biteguard on quality of life in xerostomia. J Oral Pathol Med. 2005;34:193–197. doi: 10.1111/j.1600-0714.2005.00305.x. [DOI] [PubMed] [Google Scholar]
- 100.Shan Z, Li J, Zheng C, et al. Increased fluid secretion after adenoviral-mediated transfer of the human aquaporin-1 cDNA to irradiated miniature pig parotid glands. Mol Ther. 2005;11:444–451. doi: 10.1016/j.ymthe.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 101.Samuni Y, Baum BJ. Gene delivery in salivary glands: from the bench to the clinic. Biochim Biophys Acta. 2011;1812:1515–1521. doi: 10.1016/j.bbadis.2011.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Gao R, Yan X, Zheng C, et al. AAV2-mediated transfer of the human aquaporin-1 cDNA restores fluid secretion from irradiated miniature pig parotid glands. Gene Ther. 2011;18:38–42. doi: 10.1038/gt.2010.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Wang S, Baum BJ, Yamano S, et al. Adenoviral-mediated gene transfer to mouse salivary glands. J. Dent. Res. 2000;79:701–708. doi: 10.1177/00220345000790020201. [DOI] [PubMed] [Google Scholar]
- 104.Zheng C, Goldsmith CM, Mineshiba F, et al. Toxicity and bio-distribution of a first-generation recombinant adenoviral vector, encoding aquaporin-1, after retroductal delivery to a single rat submandibular gland. Hum Gene Ther. 2006;17:1122–1133. doi: 10.1089/hum.2006.17.1122. [DOI] [PubMed] [Google Scholar]
- 105.Elmore S, Lanning L, Allison N, Vallant M, Nyska A. The transduction of rat submandibular glands by an adenoviral vector carrying the human growth hormone gene is associated with limited and reversible changes at the infusion site. Toxicol Pathol. 2006;34:385–392. doi: 10.1080/01926230600815189. [DOI] [PubMed] [Google Scholar]
- 106.Zheng C, Cotrim AP, Rowzee A, et al. Prevention of radiation-induced salivary hypofunction following hKGF gene delivery to murine submandibular glands. Clin Cancer Res. 2011;17:2842–2851. doi: 10.1158/1078-0432.CCR-10-2982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Palaniyandi S, Odaka Y, Green W, et al. Adenoviral delivery of Tousled kinase for the protection of salivary glands against ionizing radiation damage. Gene Ther. 2011;18:275–282. doi: 10.1038/gt.2010.142. [DOI] [PubMed] [Google Scholar]
- 108.Passineau MJ, Zourelias L, Machen L, Edwards PC, Benza RL. Ultrasound-assisted non-viral gene transfer to the salivary glands. Gene Ther. 2010;17:1318–1324. doi: 10.1038/gt.2010.86. [DOI] [PubMed] [Google Scholar]
- 109.Baum BJ, Zheng C, Alevizos I, et al. Development of a gene transfer-based treatment for radiation-induced salivary hypofunction. Oral Oncology. 2010;46:4–8. doi: 10.1016/j.oraloncology.2009.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Anon. [Accessed November 7, 2011];Effect of AdhAQP1 on salivary flow in patients treated with radiation for head and neck cancer. ClinicalTrials.gov. Available at: http://clinicaltrials.gov/ct2/show/NCT00372320.
- 111.Anon. [Accessed December 7, 2011];Institute for Human Gene Therapy Responds to FDA—Almanac Between Issues. University of Pennsylvania. Available at: http://www.upenn.edu/almanac/between/FDAresponse.html.
- 112.Lane A. [Accessed December 7, 2011];Healing the wounds that never heal. Santa Clara University Markula Center for Applied Ethics, Issues in Ethics. Available at: http://www.scu.edu/ethics/publications/iie/v10n1/wounds.html.
- 113.Lombaert IMA, Brunsting JF, Wierenga PK, et al. Rescue of salivary gland function after stem cell transplantation in irradiated glands. PLoS One. 2008;3:e2063. doi: 10.1371/journal.pone.0002063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Nanduri LSY, Maimets M, Pringle SA, van der Zwaag M, van Os RP, Coppes RP. Regeneration of irradiated salivary glands with stem cell marker expressing cells. Radiother Oncol. 2011;99:367–372. doi: 10.1016/j.radonc.2011.05.085. [DOI] [PubMed] [Google Scholar]
- 115.Kagami H, Wang S, Hai B. Restoring the function of salivary glands. Oral Dis. 2008;14:15–24. doi: 10.1111/j.1601-0825.2006.01339.x. [DOI] [PubMed] [Google Scholar]
- 116.Pradhan S, Liu C, Zhang C, Jia X, Farach-Carson MC, Witt RL. Lumen formation in three-dimensional cultures of salivary acinar cells. Otolaryngol Head Neck Surg. 2010;142:191–195. doi: 10.1016/j.otohns.2009.10.039. [DOI] [PubMed] [Google Scholar]
- 117.David R, Shai E, Aframian DJ, Palmon A. Isolation and cultivation of integrin alpha(6)beta(1)-expressing salivary gland graft cells: a model for use with an artificial salivary gland. Tissue Eng Part A. 2008;14:331–337. doi: 10.1089/tea.2007.0122. [DOI] [PubMed] [Google Scholar]
- 118.Baum BJ, Yamada KM, Cukierman E, Mooney D. [Accessed December 12, 2011];United States Patent: 6743626-Artificial salivary gland. 2004 Available at: http://patft.uspto.gov/netacgi/nph-Parser?Sect1=PTO2&Sect2=HITOFF&p=1&u=%2Fnetahtml%2FPTO%2Fsearch-bool.html&r=7&f=G&l=50&co1=AND&d=PTXT&s1=6743626&OS=6743626&RS=6743626.
- 119.Wasserman TH, Brizel DM, Henke M, et al. Influence of intravenous amifostine on xerostomia, tumor control, and survival after radiotherapy for head-and- neck cancer: 2-year follow-up of a prospective, randomized, phase III trial. Int J Radiat Oncol Biol Phys. 2005;63:985–990. doi: 10.1016/j.ijrobp.2005.07.966. [DOI] [PubMed] [Google Scholar]