Abstract
The pathogenesis of Sjögren’s syndrome (SS) likely involves complex interactions between genes and the environment. While the candidate gene approach has been previously used to identify several genes associated with SS, two recent large-scale genome-wide association studies (GWAS) have implicated many more loci as genetic risk factors. Of particular relevance, was the significant association of SS with additional immune-related genes including IL12A, BLK, and CXCR5. GWAS has also uncovered other loci and suggestive gene associations in SS, but none are related to genes encoding salivary or lacrimal components, secretion machinery and neuronal proteins involved in innervations of the glands, respectively. In this review, we discuss these genetic findings with particular attention paid to the genes identified, the strength of associations, and how the SS-associated genes compare to what has been discovered previously in systemic lupus erythematosus (SLE). We also summarize the potential impact of these associated gene products on NFκB and immune pathways and describe how this new information might be integrated further for identifying clinical subsets and understanding the pathogenesis of SS.
Keywords: Autoimmune disease, Candidate genes, Genetics, Genome-wide association studies, Polymorphisms, Sjögren’s syndrome, SNP
1. Introduction
Sjögren’s syndrome (SS) is characterized by an autoimmune attack on the salivary and lacrimal glands leading to decreased saliva and tear production, respectively [1]. A key feature of this syndrome is focal lymphocytic infiltrate in the salivary and lacrimal glands resulting in oral and ocular dryness. The American European Consensus Group criteria is the most commonly used criteria for SS diagnosis [2], but new proposed criteria are simplified and based on two of three objective tests: a focus score (≥1) in the salivary biopsy, evidence of ocular keratoconjunctivitis, and the presence of autoantibodies against SSA and SSB [3]. While the exact cause of SS is not known, women are nearly nine times more likely than men to be affected with SS, suggesting gender-based differences such as the female-associated microbiome, hormones, and other factors may be responsible.
In many SS patients, interferon activation plays an important part of the immune attack and destruction of salivary and lacrimal glands at some stage in the course of the disease. Gene expression studies in SS patients have demonstrated altered gene expression patterns consistent with interferon activation in the salivary gland [4, 5] and plasmacytoid dendritic cells [6] Enriched levels of interferon-α-producing cells in the salivary gland have also been detected in vitro [7]. The exact mechanism causing the interferon activation signature seen in SS remains unknown.
The genetic factors contributing to the pathogenesis of SS are still unclear and are an active area of research. While epidemiologic studies have shown moderate concordance (25–40%) between monozygotic twins with SLE and other autoimmune diseases [8], the rate of concordance among monozygotic twins with SS has not been systematically studied. Instead, the focus in SS has been to explore the role of specific genes as risk factors. The goal of this review is to summarize these genetic findings and describe the disease relevance of many newly identified genes and their corresponding pathways. In particular, we discuss how new information obtained from genome-wide association studies (GWAS) might be used as a framework for identifying clinical subsets of SS patients and understanding the pathogenesis of SS.
2. Candidate gene studies in SS
In the first genetic studies in SS, the roles of candidate genes previously known to be important in immune function or other autoimmune conditions were explored by linkage disequilibrium and RFLP analysis. In a seminal study in 1977, HLA genes were identified as risk factors for SS [9]. Since then, there has been much in-depth analysis of HLA class II associations in SS, however, this area will not be covered in detail here since this topic has been covered in-depth in a recent review [10]. In this section, we describe gene polymorphisms identified by the candidate gene approach in SS. Generally, the gene polymorphisms chosen for candidate gene studies are immune-related based on previous associations with other autoimmune conditions including SLE and rheumatoid arthritis. In these genetic studies, the allele frequency for the SNP-disease association is calculated in the controls and SS cases and compared using a variety of statistical tools. For interpreting the relevance of these genetic findings, there are the two important values: the statistical significance of the findings, expressed as a P value, and the pertinent association expressed as an odds ratio (OR), which should be distinguished from relative risk [11]. From these case control studies, an OR between 1.2–1.5 is generally considered a weak association and an OR greater than 1.5 is generally considered a moderate association.
In 2008, a known STAT4 polymorphism, rs7574865, associated with SLE and RA was examined in a Caucasian cohort of 124 SS cases and 1143 healthy controls [12]. From the analysis, 29.6% of the SS cases vs. 22.3% of controls showed the presence of the STAT4 polymorphism with a weak statistical association (P=0.01) and an OR of 1.47 was detected. Another study confirmed the association of the rs7574865 STAT4 polymorphism in both a Columbian and German cohort of SS patients [13]. However, a third study identified a different STAT4 polymorphism, rs7582694, as a risk factor in SS [14]. This alternative polymorphism demonstrated an OR of 1.57 and the presence of this polymorphism correlated weakly with the mRNA levels of several interferon-induced genes in peripheral blood mononuclear cells from the SS patients.
Another known autoimmune risk gene, IRF5, has also been examined in SS [15, 16]. One polymorphism, a CGGGG insertion/deletion within the IRF5 promoter, was moderately associated with SS (OR of 2.0) [15]. SS patients harboring this promoter polymorphism showed higher levels of IRF5 mRNA in their PBMCs. Additional studies exploring the impact of epigenetic DNA modifications in SS examined the DNA methylation profile of the IRF5 promoter in T cells, B cells, and other immune cells, however, no difference in the methylation pattern of the IRF5 promoter was detected between controls and SS patients [17].
NCR3/NKp30 is an important receptor found on natural killer cells regulating the cross-talk between NK and dendritic cells [18]. Recently, a minor allele SNP (rs11575837) within the promoter of NCR3 was identified as protective from SS with an OR of 2.2, and this polymorphism was found to reduce gene transcription of NCR3 [19]. Compared to controls, SS patients who lacked this polymorphism demonstrated higher circulating levels of the NCR3 ligand, which also weakly correlated with a higher focus score.
Polymorphisms within four signaling proteins from the NFκB pathway, TNFAIP3, TNIP1, NFκB, and IKBKE, were evaluated for their association with SS [20]. Part of the rationale for testing these genes was because TNFAIP3 and TNIP1 were previously known to be associated with other autoimmune conditions. In total, 12 SNPs within these four genes were analyzed in a large cohort of Scandinavian and UK controls and SS cases. Interestingly, a polymorphism within TNIP1, but not the other three genes, was found to be moderately associated (OR of 2.1) with SSA/SSB antibodies in seropositive SS patients.
It is important to point out that none of the identified polymorphisms from the published candidate gene studies alter the coding sequences of these genes. Most of these candidate studies have only analyzed single SNPs, but individuals harboring multiple risk alleles may be at greater risk. Along these lines, polymorphisms within both IRF5 and STAT4 showed an additive effect for their association with SS [14, 21].
3. Genome-wide association studies (GWAS) in SS
There is marked genomic variation in individual human genomes including differences in gene copy number, insertions, deletions, and SNPs. Genome-wide association studies (GWAS) is a powerful molecular method offering the ability to screen thousands of regions of DNA simultaneously to determine if any loci are associated with a certain disease phenotype. GWAS employs arrays of SNPs corresponding to different polymorphic alleles spanning the entire genome. The key to a successful GWAS experiment is the analysis of large numbers of patients and controls well matched for race to ensure the observed association is not a polymorphism linked to this factor. Additionally, since SS clinically encompasses a spectrum of conditions, a better selection of participants for the studies, based on clinical characteristics is required.
Unlike the extensive GWAS experiments in SLE and other autoimmune conditions, there have only been two studies in SS. One of the studies by Lessard et al analyzed over 10,000 subjects of European descent containing controls and SS cases [22]. All of the SS patients fulfilled the European-American consensus criteria for the diagnosis of SS [2]. The study utilized multiple sets of subject samples for discovery and validation, in which over a million polymorphic regions were screened initially by Illumina® SNP arrays. Seven genetic loci were identified surpassing the statistical threshold of P < 5×10−8 and included MHC-II loci, IRF5, STAT4, IL12A, BLK, CXCR5, and TNIP1 (Table 1). The strongest association was with the HLA-II locus, which was followed by STAT4 and IRF5. Of note, the HLA-II locus, STAT4, and IRF5 have all been previously identified by the candidate gene approach, but this study demonstrated more robust statistical significance due to the larger sample size and non-biased technique. Excitingly, IL12A, BLK, and CXCR5, three new genes that play important roles in immune signaling were recognized as being associated with SS (Table 1). Another gene newly identified as being associated with SS, TNIP1, plays a role in NFκB signaling. While all seven identified gene loci showed high statistical association (P < 10−8), the actual impact as assessed by the OR score were weak to moderate and ranged from 1.7 to 2.5 (Table 1). In addition, six other SS-associated genes including TNFAIP3, DGKQ, and FCGRN2, were identified, but these genes only bordered on statistical significance with P values greater than 10−5 [22]. It is important to point out that in contrast to many of the candidate gene studies examining single SNPs for each gene, multiple, different SNPs for many of the genes were identified via GWAS to be associated with SS. For example, 67 and 12 different SNPs were identified for IRF5 and STAT4 genes, respectively, that exceeded the threshold of P < 5×108 [22]. Taken together, this study suggests that there are at least eight different loci/genes associated with SS, many of which play important roles in immune function.
Table 1.
Sjögren’s syndrome-associated non-HLA genes discovered by GWAS
| Gene | Function | Top Alleles Identified |
Average OR |
Reference |
|---|---|---|---|---|
| STAT4 | Transcription Factor | Two | 1.38 | [22] |
| One | 1.44 | [23] | ||
| IRF5 | Transcription Factor | Five | 1.36 | [22] |
| IL12A | Cytokine | Two | 1.29 | [22] |
| BLK | B cell kinase | Three | 1.29 | [22] |
| CXCR5 | Chemokine | Two | 0.75 | [22] |
| TNIP | NFκB signaling | Two | 1.39 | [22] |
| GTF2I | Transcription Factor | One | 2.20 | [23] |
| TNFAIP3 | NFκB signaling | One | 1.67 | [23] |
The other large scale GWAS of SS examined a cohort of SS patients from Han, China [23]. In this study, 1090 healthy controls and 597 SS cases were analyzed using Affymetrix® genome wide arrays containing a total of 642,832 SNPs. In addition to MHC class II genes, three other genes were identified. The most strongly associated gene in the Chinese cohort was GTF2I, located at 7q21, which acts as a general transcription factor (Table 1). The association of the Chinese SS patients with GTF2I was moderate (OR = 2.2), and had higher OR scores than other SS-associated identified genes including MHC-II genes, STAT4, and TNFAIP3 [23].
Comparison of the published GWAS for the Chinese [23] and European descent cohorts [22], revealed some notable differences (Table 1). First, GTF2I was only a risk factor in the Chinese cohort and was not found to be associated with SS in the European descent study. Second, IRF5 polymorphisms and several of the other immune genes were not detected in the Chinese study. Lastly, two NFκB-related genes showed different associations between the two cohorts. TNFAIP3 was found as a statistically significant association in the Chinese cohort, but was only weakly associated in the European descent cohort, while TNIP1 was only found to be associated in the European descent cohort. Although additional confirmatory studies are needed, these results suggest the possibility that the Chinese and European descent SS patients may have different risk associated genes.
One of the important findings from GWAS of the European descent and Chinese cohorts was that none of the identified genes encode salivary or lacrimal components, secretion machinery, or neuronal proteins involved in innervation of the glands. There were also no associated genes found on the X chromosome potentially linking SS to the female gender. Rather, most of the SS-associated genes play important roles in immune function (Table 1). These results highlight the likelihood that altered immune activity is the major driving factor in SS pathogenesis. A potential framework for understanding the role of these SS-associated immune genes is that the gene polymorphisms enhance the likelihood of autoimmunity, but none by itself is sufficient to cause disease. The exact causative agents that trigger the abnormal immune activity remain to be defined.
4. Functional Studies on the Gene Polymorphisms Associated with SS
Since all of the gene polymorphisms associated with SS discovered to date are located in non-coding sequences, there is great interest in understanding their potential functional significance. In addition to testing the regulatory regions containing different nucleotide polymorphisms by in vitro transcription assays, expression quantitative trait loci (eQTL) experiments represent a relatively new approach to directly study the effect of identified SNPs on transcriptional activity in the study group [24]. In eQTL experiments, peripheral blood lymphocytes from the subjects participating in the GWAS study are used to measure the mRNA expression for the different genes. The expression of a particular gene is then analyzed in stratified samples from those with and without the polymorphisms in the sample population. In subjects with SS, higher levels of expression of IRF5 and HLA class II genes were observed when there were polymorphisms in potential transcriptional regulatory regions of these genes [22]. Interestingly, some of the polymorphisms within the HLA gene promoters appeared to be binding sites for the known transcription factor RFX5, which plays a known role in MHC class II gene expression [25]. Despite these efforts using eQTL, many of the associated polymorphisms had no detectable effect on gene regulation including GTF2I [23] and other SS-associated genes [22].
5. Comparison of the Gene Associations of SS with SLE
SS shows many clinical similarities with SLE including the presence of SSA and SSB autoantibodies, a similar interferon-α gene signature, and other aspects of immune activation [26]. Based on the substantial published gene association data in SLE identifying over 40 susceptibility loci [27, 28], comparison with SS reveals some interesting similarities and differences. First, there are a number of SS-associated gene polymorphisms including the MHC-II, STAT4, IRF5, BLK, and TNIP1 genes that are shared with SLE and other autoimmune conditions. However, there are some genes such as CXCR5 and GTF2I, which have only been detected in SS and have not been found in SLE. Finally, there are many genes associated with risk of SLE that are not found in SS. Of these SLE-associated genes, many are also involved in immune function and signaling including additional transcription factors (IRF7, IRF8), B-cell signaling proteins (LYN and BANK1), and other cytokines and receptors (IL-10, CD44, TNFSF4) [27, 28]. While future studies in SS may uncover additional genetic associations with some of these genes, it is also possible that SS and SLE represent a spectrum of autoimmune diseases with both shared and exclusive risk factors.
6. Potential pathways identified through GWAS for understanding SS pathogenesis
Although studies using the candidate gene approach and GWAS have identified novel gene associations with SS, none of the genes are strongly associated with disease (OR>2.5) and none alter the coding sequence of the corresponding protein. An alternative strategy for gaining insight into pathogenesis is to harness the genetic information for understanding altered pathways in the disease [29]. Using this approach, analysis of the SS-associated genes suggests that dysregulation might alter at least three mechanistic pathways contributing to pathogenesis (Figure 1). The most common pathway, belonging to the three genes IRF5, STAT4, and IL12A, involve increased interferon signaling and cytokine production and is consistent with the observed interferon gene signature seen in SS. It is known that the interferon regulatory transcription factors including IRF5 are important for expression of a number of cytokines. Similarly, STAT4 belongs to a family of transcription factors well established to play a role in IL12 signaling, as well as in regulation of the NFκB pathway. The other target gene, IL12A is an important cytokine and acts with the IL12B subunit to signal as a dimer through STAT4 to induce the differentiation of naïve CD4+ T cells into T-helper cells, thereby causing these cells to produce interferon-γ [30]. Curiously one of the major autoantigens in SS, Ro52/TRIM-21 is known to bind and ubiquinate another IRF member, IRF8, and has been proposed along with the STATs to transcriptionally up-regulate IL12 and other interferons [31, 32].
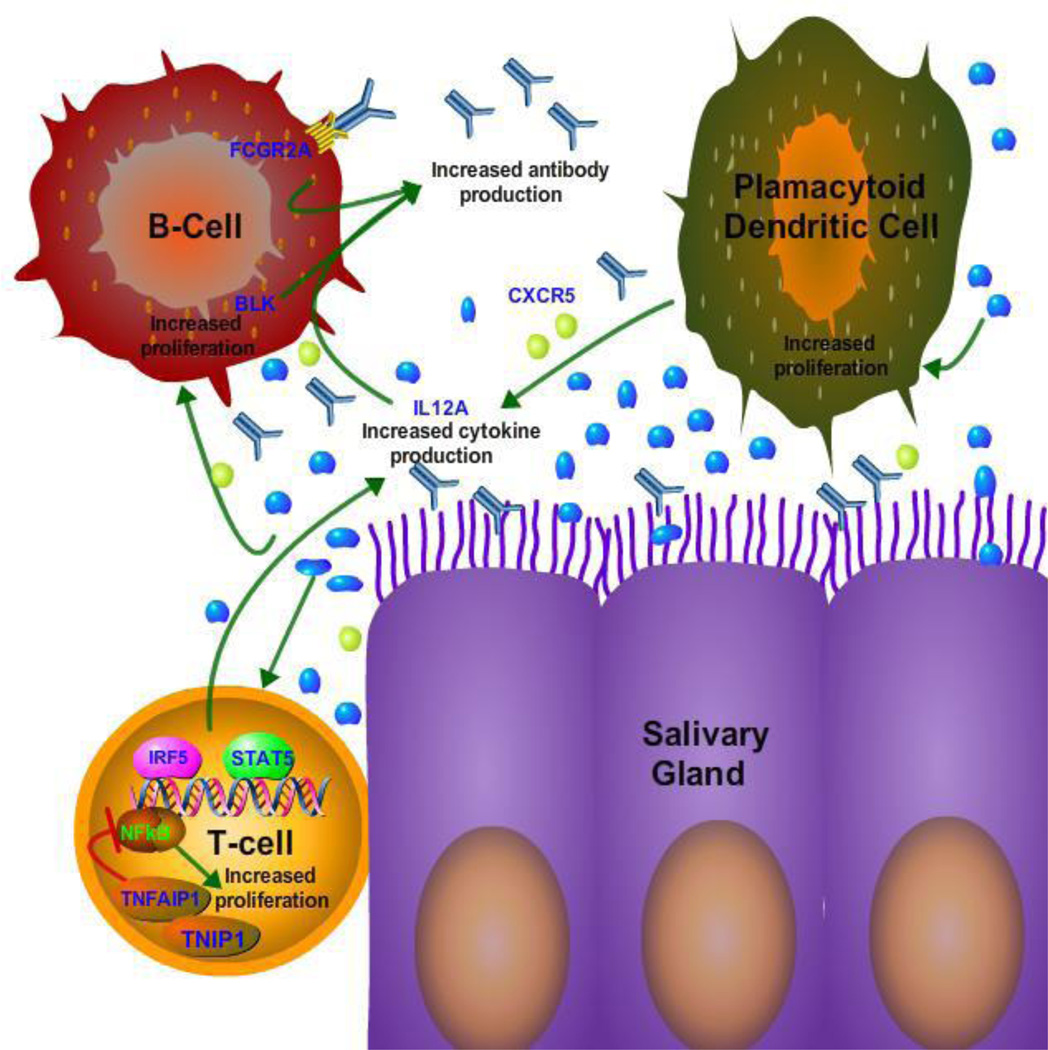
Figure 1. Functional pathways potentially altered in SS identified by GWAS.
The eight genes associated with SS that were identified by GWAS (labeled in blue) play a role in immune function and NFkB signaling. Alterations of the eight genes likely impact at least three key cellular activities: antibody production/clearance, cytokine production and proliferation of immune cells. In this model, subjects harboring these polymorphisms are more likely to develop an exaggerated inflammatory response following infection and other environmental triggers. Consequently, the activation of plasmacytoid dendritic cells, T-cells, B–cells and other immune cells cause the destruction of the salivary gland and its normal function.
The second pathway appears to involve genes important for B-cell function, antibody production, and antibody clearance (Figure 1). One gene belonging to this pathway is the BLK tyrosine kinase, which is involved in B cell signaling and differentiation. BLK is known to be associated with SLE[28], which like SS also demonstrates high levels of autoantibodies against SSA and SSB and shows abnormal B-cell activity. CXCR5 also shows association with SS and functions as a circulating CXC chemokine receptor, which is known to promote antibody responses upon antigen re-exposure [33]. The identification of the CXCR5 polymorphism as a newly identified risk factor may be in part responsible for altered B cell activity in SS [34]. One of the other genes, FCGR2, which shows suggestive association in SS, functions as a receptor for clearance of immune complexes and could be responsible for elevation of immune complexes in SS.
The third pathway derived from two SS-associated genes, TNIP1 and TFFAIP3, involves signaling to NFκB (Figure 1). TNFAIP-1 is known to dampen NFκB and is in a complex with TNIP1 [35]. Interestingly, a recent study discovered a polymorphism within TNFAIP-1, which is enriched in SS patients who develop lymphoma [36]. Analysis of this allele, RS22310926G, showed that it was significantly associated with lymphoma when compared to SS patients without lymphoma (OR 3.36) or healthy controls (OR 3.26). This polymorphism within TNFAIP-1 increased expression of the NFκB pathway as determined by luciferase reporter transcriptional activity. Moreover as previously discussed, a contemporaneous candidate gene study found TNIP1 polymorphisms that were associated with SSA/SSB autoantibody seropositive SS [20]. These two findings highlight how polymorphisms may be useful in identifying important clinical subsets of SS patients. It is possible that further study of the NFκB pathway may lead to more clues about SS pathogenesis and/or become potential drug targets for therapy.
Although we have separated the SS-associated genes into three distinct pathways, there is likely overlap between them. For example, TNIP1 appears to play a role in cell growth and apoptosis, as well as antibody production. It is important to point out that the functional impact of several associated genes are not currently known, including the role GITI2C polymorphisms might play in promoting SS in Chinese patients.
7. Conclusion
More than 15 robust susceptibility loci have now been associated with SS reflecting the potential heterogeneous nature of the disease. At present, it is not clear how many of these genes with low risk could be used for genetic screening or diagnosis. The one exception is the finding of a polymorphism in TBNP1, which is associated with lymphoma in SS. It is possible that this information, along with newer imaging technologies [37], might be exploited for better monitoring of patients at risk of lymphoma. The associated NFκB pathway might also be useful for potentially developing new drug targets for SS-associated lymphoma.
Since studies performed thus far using GWAS have only focused on the presence or absence of SS as made by clinical diagnosis, it is possible that additional insights might be gleaned if the data was analyzed in respect to objective clinical testing including the focus score, ocular score, and/or autoantibody seropositive status. In the light of the importance of SSA autoantibodies as important diagnostic tests for SS and their association with complications of pregnancy outcome in woman with SS [38], analysis of the GWAS data with these autoantibodies might also yield new clinical correlates. Since most but not all SS patients show an interferon signature [5], it might also be interesting to determine if some of the polymorphisms identified in immune genes correlate better with patients who show the gene signature. Lastly, future studies using whole genome sequencing are likely to identify additional risk genes, but will require more carefully chosen cohorts and further data analysis.
To date, there have been many mouse models of SS targeting candidate cytokines, signaling molecules, and transcription factors [39, 40]. Despite the large number of mouse models, it is unclear if any are truly representative of the human condition given its heterogeneity and uncertain pathogenesis. Based on these genetic studies in SS, it is possible that mouse models of SS more closely approximating human disease might be constructed. Along these lines, an IL-12 transgenic mouse model expressing both subunits of IL12 was previously shown to have many of the same features as SS in humans including decreased salivary flow, lymphocytic infiltrate in the gland, and increased SSB autoantibody production [41]. Since IL12 was one of the genes associated with SS in GWAS [22], it would be of interest to see if mouse models derived from other SS-risk genes such as IRF-5, STAT4, and CXCR5, also demonstrated features of human SS.
In light of the limited information that has been obtained from these large-scale genetic studies in SS, additional complementing research studies are needed to systematically explore the possible role of other factors such as epigenetics, microRNAs [42], and infectious agents in SS pathogenesis. The finding of autoantibodies years before the onset of SS [43] provides a new framework for designing studies exploring the role of environmental factors. In particular, based on the known role of many of the SS-associated genes, including HLA class II molecules, IRF5, and STAT4 in immune responses against infectious agents, studies examining viruses and bacteria that potentially trigger SS in susceptible subjects’ years before the clinical onset of disease are needed.
Take-home messages.
Pathogenesis of SS likely involves complex interactions between genes and the environment.
New genome-wide association studies have identified multiple risk loci and signal pathways.
Many of the identified risk genes play important roles in immune activity and many are often shared with systemic lupus erythematosus.
Despite these studies, most of the risk genes are only weakly associated suggesting that other epigenetic and environmental factors may be important for triggering the disease.
Acknowledgments
This research was supported by the intramural research program of NIH, NIDCR.
Abbreviations
- eQTL
expression quantitative trait loci
- GWAS
genome-wide association studies
- OR
odds ratio
- SS
Sjögren’s syndrome
- SLE
systemic lupus erythematosus
- SNP
single nucleotide polymorphisms
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Fox RI. Sjogren's syndrome. Lancet. 2005;366:321–331. doi: 10.1016/S0140-6736(05)66990-5. [DOI] [PubMed] [Google Scholar]
- 2.Vitali C, Bombardieri S, Moutsopoulos HM, Coll J, Gerli R, Hatron PY, Kater L, Konttinen YT, Manthorpe R, Meyer O, et al. Assessment of the European classification criteria for Sjogren's syndrome in a series of clinically defined cases: results of a prospective multicentre study. The European Study Group on Diagnostic Criteria for Sjogren's Syndrome. Ann Rheum Dis. 1996;55:116–121. doi: 10.1136/ard.55.2.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shiboski SC, Shiboski CH, Criswell L, Baer A, Challacombe S, Lanfranchi H, Schiodt M, Umehara H, Vivino F, Zhao Y, et al. American College of Rheumatology classification criteria for Sjogren's syndrome: a data-driven, expert consensus approach in the Sjogren's International Collaborative Clinical Alliance cohort. Arthritis Care Res (Hoboken) 2012;64:475–487. doi: 10.1002/acr.21591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gottenberg JE, Cagnard N, Lucchesi C, Letourneur F, Mistou S, Lazure T, Jacques S, Ba N, Ittah M, Lepajolec C, et al. Activation of IFN pathways and plasmacytoid dendritic cell recruitment in target organs of primary Sjogren's syndrome. Proc Natl Acad Sci U S A. 2006;103:2770–2775. doi: 10.1073/pnas.0510837103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hjelmervik TO, Petersen K, Jonassen I, Jonsson R, Bolstad AI. Gene expression profiling of minor salivary glands clearly distinguishes primary Sjogren's syndrome patients from healthy control subjects. Arthritis Rheum. 2005;52:1534–1544. doi: 10.1002/art.21006. [DOI] [PubMed] [Google Scholar]
- 6.Wildenberg ME, van Helden-Meeuwsen CG, van de Merwe JP, Drexhage HA, Versnel MA. Systemic increase in type I interferon activity in Sjogren's syndrome: a putative role for plasmacytoid dendritic cells. Eur J Immunol. 2008;38:2024–2033. doi: 10.1002/eji.200738008. [DOI] [PubMed] [Google Scholar]
- 7.Bave U, Nordmark G, Lovgren T, Ronnelid J, Cajander S, Eloranta ML, Alm GV, Ronnblom L. Activation of the type I interferon system in primary Sjogren's syndrome: a possible etiopathogenic mechanism. Arthritis Rheum. 2005;52:1185–1195. doi: 10.1002/art.20998. [DOI] [PubMed] [Google Scholar]
- 8.Bogdanos DP, Smyk DS, Rigopoulou EI, Mytilinaiou MG, Heneghan MA, Selmi C, Gershwin ME. Twin studies in autoimmune disease: genetics, gender and environment. J Autoimmun. 2012;38:J156–J169. doi: 10.1016/j.jaut.2011.11.003. [DOI] [PubMed] [Google Scholar]
- 9.Chused TM, Kassan SS, Opelz G, Moutsopoulos HM, Terasaki PI. Sjogren's syndrome association with HLA-Dw3. N Engl J Med. 1977;296:895–897. doi: 10.1056/NEJM197704212961602. [DOI] [PubMed] [Google Scholar]
- 10.Cruz-Tapias P, Rojas-Villarraga A, Maier-Moore S, Anaya JM. HLA and Sjogren's syndrome susceptibility. A meta-analysis of worldwide studies. Autoimmun Rev. 2012;11:281–287. doi: 10.1016/j.autrev.2011.10.002. [DOI] [PubMed] [Google Scholar]
- 11.Panagiotou OA, Ioannidis JP, Genome-Wide Significance P. What should the genome-wide significance threshold be? Empirical replication of borderline genetic associations. Int J Epidemiol. 2012;41:273–286. doi: 10.1093/ije/dyr178. [DOI] [PubMed] [Google Scholar]
- 12.Korman BD, Alba MI, Le JM, Alevizos I, Smith JA, Nikolov NP, Kastner DL, Remmers EF, Illei GG. Variant form of STAT4 is associated with primary Sjogren's syndrome. Genes Immun. 2008;9:267–270. doi: 10.1038/gene.2008.1. [DOI] [PubMed] [Google Scholar]
- 13.Palomino-Morales RJ, Diaz-Gallo LM, Witte T, Anaya JM, Martin J. Influence of STAT4 polymorphism in primary Sjogren's syndrome. J Rheumatol. 2010;37:1016–1019. doi: 10.3899/jrheum.091007. [DOI] [PubMed] [Google Scholar]
- 14.Gestermann N, Mekinian A, Comets E, Loiseau P, Puechal X, Hachulla E, Gottenberg JE, Mariette X, Miceli-Richard C. STAT4 is a confirmed genetic risk factor for Sjogren's syndrome and could be involved in type 1 interferon pathway signaling. Genes Immun. 2010;11:432–438. doi: 10.1038/gene.2010.29. [DOI] [PubMed] [Google Scholar]
- 15.Miceli-Richard C, Gestermann N, Ittah M, Comets E, Loiseau P, Puechal X, Hachulla E, Gottenberg JE, Lebon P, Becquemont L, Mariette X. The CGGGG insertion/deletion polymorphism of the IRF5 promoter is a strong risk factor for primary Sjogren's syndrome. Arthritis Rheum. 2009;60:1991–1997. doi: 10.1002/art.24662. [DOI] [PubMed] [Google Scholar]
- 16.Miceli-Richard C, Comets E, Loiseau P, Puechal X, Hachulla E, Mariette X. Association of an IRF5 gene functional polymorphism with Sjogren's syndrome. Arthritis Rheum. 2007;56:3989–3994. doi: 10.1002/art.23142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gestermann N, Koutero M, Belkhir R, Tost J, Mariette X, Miceli-Richard C. Methylation profile of the promoter region of IRF5 in primary Sjogren's syndrome. Eur Cytokine Netw. 2012;23:166–172. doi: 10.1684/ecn.2012.0316. [DOI] [PubMed] [Google Scholar]
- 18.Wehner R, Dietze K, Bachmann M, Schmitz M. The bidirectional crosstalk between human dendritic cells and natural killer cells. J Innate Immun. 2011;3:258–263. doi: 10.1159/000323923. [DOI] [PubMed] [Google Scholar]
- 19.Rusakiewicz S, Nocturne G, Lazure T, Semeraro M, Flament C, Caillat-Zucman S, Sene D, Delahaye N, Vivier E, Chaba K, et al. NCR3/NKp30 contributes to pathogenesis in primary Sjogren's syndrome. Sci Transl Med. 2013;5:195ra196. doi: 10.1126/scitranslmed.3005727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nordmark G, Wang C, Vasaitis L, Eriksson P, Theander E, Kvarnstrom M, Forsblad-d'Elia H, Jazebi H, Sjowall C, Reksten TR, et al. Association of Genes in the NF-kappaB Pathway with Antibody-Positive Primary Sjogren's Syndrome. Scand J Immunol. 2013;78:447–454. doi: 10.1111/sji.12101. [DOI] [PubMed] [Google Scholar]
- 21.Nordmark G, Kristjansdottir G, Theander E, Eriksson P, Brun JG, Wang C, Padyukov L, Truedsson L, Alm G, Eloranta ML, et al. Additive effects of the major risk alleles of IRF5 and STAT4 in primary Sjogren's syndrome. Genes Immun. 2009;10:68–76. doi: 10.1038/gene.2008.94. [DOI] [PubMed] [Google Scholar]
- 22.Lessard CJ, Li H, Adrianto I, Ice JA, Rasmussen A, Grundahl KM, Kelly JA, Dozmorov MG, Miceli-Richard C, Bowman S, et al. Variants at multiple loci implicated in both innate and adaptive immune responses are associated with Sjogren's syndrome. Nat Genet. 2013;45:1284–1292. doi: 10.1038/ng.2792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li Y, Zhang K, Chen H, Sun F, Xu J, Wu Z, Li P, Zhang L, Du Y, Luan H, et al. A genome-wide association study in Han Chinese identifies a susceptibility locus for primary Sjogren's syndrome at 7q11.23. Nat Genet. 2013;45:1361–1365. doi: 10.1038/ng.2779. [DOI] [PubMed] [Google Scholar]
- 24.Kliebenstein D. Quantitative genomics: analyzing intraspecific variation using global gene expression polymorphisms or eQTLs. Annu Rev Plant Biol. 2009;60:93–14. doi: 10.1146/annurev.arplant.043008.092114. [DOI] [PubMed] [Google Scholar]
- 25.Steimle V, Durand B, Barras E, Zufferey M, Hadam MR, Mach B, Reith W. A novel DNA-binding regulatory factor is mutated in primary MHC class II deficiency (bare lymphocyte syndrome) Genes Dev. 1995;9:1021–1032. doi: 10.1101/gad.9.9.1021. [DOI] [PubMed] [Google Scholar]
- 26.Wahren-Herlenius M, Dorner T. Immunopathogenic mechanisms of systemic autoimmune disease. Lancet. 2013;382:819–831. doi: 10.1016/S0140-6736(13)60954-X. [DOI] [PubMed] [Google Scholar]
- 27.Cui Y, Sheng Y, Zhang X. Genetic susceptibility to SLE: recent progress from GWAS. J Autoimmun. 2013;41:25–33. doi: 10.1016/j.jaut.2013.01.008. [DOI] [PubMed] [Google Scholar]
- 28.Deng Y, Tsao BP. Genetic susceptibility to systemic lupus erythematosus in the genomic era. Nat Rev Rheumatol. 2010;6:683–692. doi: 10.1038/nrrheum.2010.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang K, Li M, Hakonarson H. Analysing biological pathways in genome-wide association studies. Nat Rev Genet. 2010;11:843–854. doi: 10.1038/nrg2884. [DOI] [PubMed] [Google Scholar]
- 30.Chan SH, Perussia B, Gupta JW, Kobayashi M, Pospisil M, Young HW, Wolf SF, Young D, Clark SC, Trinchieri G. Induction of Interferon-Gamma Production by Natural-Killer- Cell Stimulatory Factor - Characterization of the Responder Cells and Synergy with Other Inducers. Journal of Experimental Medicine. 1991;173:869–879. doi: 10.1084/jem.173.4.869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kong HJ, Anderson DE, Lee CH, Jang MK, Tamura T, Tailor P, Cho HK, Cheong J, Xiong H, Morse HC, 3rd, Ozato K. Cutting edge: autoantigen Ro52 is an interferon inducible E3 ligase that ubiquitinates IRF-8 and enhances cytokine expression in macrophages. J Immunol. 2007;179:26–30. doi: 10.4049/jimmunol.179.1.26. [DOI] [PubMed] [Google Scholar]
- 32.Ozato K, Shin DM, Chang TH, Morse HC., 3rd TRIM family proteins and their emerging roles in innate immunity. Nat Rev Immunol. 2008;8:849–860. doi: 10.1038/nri2413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.He J, Tsai LM, Leong YA, Hu X, Ma CS, Chevalier N, Sun X, Vandenberg K, Rockman S, Ding Y, et al. Circulating precursor CCR7(lo)PD-1(hi) CXCR5(+) CD4(+) T cells indicate Tfh cell activity and promote antibody responses upon antigen reexposure. Immunity. 2013;39:770–781. doi: 10.1016/j.immuni.2013.09.007. [DOI] [PubMed] [Google Scholar]
- 34.Hansen A, Lipsky PE, Dorner T. B cells in Sjogren's syndrome: indications for disturbed selection and differentiation in ectopic lymphoid tissue. Arthritis Res Ther. 2007;9:218. doi: 10.1186/ar2210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ramirez VP, Gurevich I, Aneskievich BJ. Emerging roles for TNIP1 in regulating post-receptor signaling. Cytokine Growth Factor Rev. 2012;23:109–118. doi: 10.1016/j.cytogfr.2012.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nocturne G, Boudaoud S, Miceli-Richard C, Viengchareun S, Lazure T, Nititham J, Taylor KE, Ma A, Busato F, Melki J, et al. Germline and somatic genetic variations of TNFAIP3 in lymphoma complicating primary Sjogren's syndrome. Blood. 2013;122:4068–4076. doi: 10.1182/blood-2013-05-503383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ziakas PD, Poulou LS, Thanos L. Towards integrating positron emission tomography for work-up of patients with Sjogren's syndrome and associated lymphomas. Autoimmun Rev. 2014;13:327–329. doi: 10.1016/j.autrev.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 38.De Carolis S, Salvi S, Botta A, Garofalo S, Garufi C, Ferrazzani S, De Carolis MP. The impact of primary Sjogren's syndrome on pregnancy outcome: our series and review of the literature. Autoimmun Rev. 2014;13:103–107. doi: 10.1016/j.autrev.2013.09.003. [DOI] [PubMed] [Google Scholar]
- 39.Lee BH, Gauna AE, Pauley KM, Park YJ, Cha S. Animal models in autoimmune diseases: lessons learned from mouse models for Sjogren's syndrome. Clin Rev Allergy Immunol. 2012;42:35–44. doi: 10.1007/s12016-011-8288-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Delaleu N, Nguyen CQ, Peck AB, Jonsson R. Sjogren's syndrome: studying the disease in mice. Arthritis Res Ther. 2011;13:217. doi: 10.1186/ar3313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vosters JL, Landek-Salgado MA, Yin H, Swaim WD, Kimura H, Tak PP, Caturegli P, Chiorini JA. Interleukin-12 induces salivary gland dysfunction in transgenic mice, providing a new model of Sjogren's syndrome. Arthritis Rheum. 2009;60:3633–3641. doi: 10.1002/art.24980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Singh RP, Massachi I, Manickavel S, Singh S, Rao NP, Hasan S, Mc Curdy DK, Sharma S, Wong D, Hahn BH, Rehimi H. The role of miRNA in inflammation and autoimmunity. Autoimmun Rev. 2013;12:1160–1165. doi: 10.1016/j.autrev.2013.07.003. [DOI] [PubMed] [Google Scholar]
- 43.Jonsson R, Theander E, Sjostrom B, Brokstad K, Henriksson G. Autoantibodies present before symptom onset in primary Sjogren syndrome. JAMA. 2013;310:1854–1855. doi: 10.1001/jama.2013.278448. [DOI] [PubMed] [Google Scholar]