Abstract
Background
Obesity is a major public health issue and is associated with increased risk of several cancers, currently a leading cause of mortality. Obese patients undergoing bariatric surgery may allow for evaluation of the effect of intentional excess weight loss on subsequent risk of cancer. We aim to evaluate cancer risk, incidence, and mortality following bariatric surgery.
Methods
A comprehensive literature search was conducted using PubMed / MEDLINE and Embase from the inception of both databases to January 2012. Inclusion criteria incorporated all human studies examining oncologic outcomes following bariatric surgery. Two authors independently reviewed selected studies and relevant articles from their bibliographies for data extraction, quality appraisal, and meta-analysis.
Results
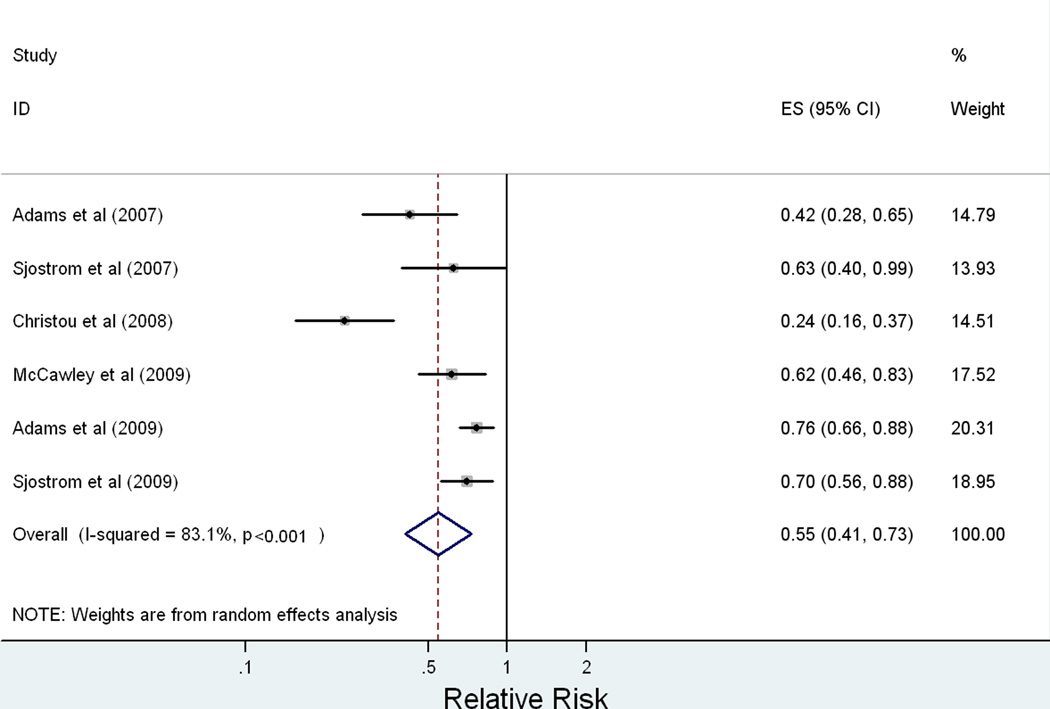
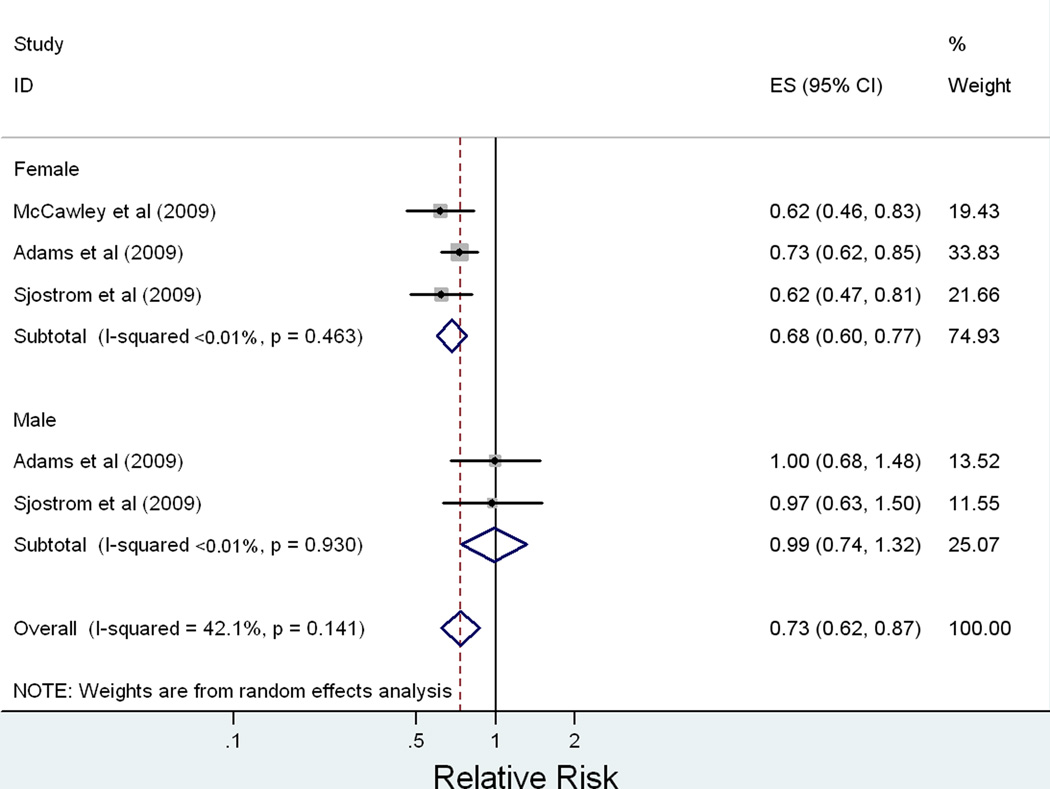
Six observational studies (N = 51,740) comparing relative risk (RR) of cancer in obese patients undergoing bariatric surgery versus obese controls were analyzed. Overall, the RR of cancer in obese patients after undergoing bariatric surgery is 0.55 [95% CI: 0.41–0.73, P < 0.0001, I2 = 83%]. The effect of bariatric surgery on cancer risk is modified by gender (P = 0.021). The pooled RR in females is 0.68 [95% CI: 0.60–0.77, P < 0.0001, I2 < 0.1%] while in males is 0.99 [95% CI: 0.74–1.32, P = 0.937, I2 < 0.1%].
Conclusions
Our results suggest that bariatric surgery reduces cancer risk and mortality in formerly obese patients. When stratifying our meta-analysis by gender, the effect of bariatric surgery on oncologic outcomes is protective in women but not in men.
Keywords: Bariatric, Surgery, Cancer, Oncology, Meta-Analysis
Introduction
Obesity is a major public health concern associated with several co-morbid conditions, early mortality, and significant health care costs (1–3). Overweight (body mass index greater than 25 kg/m2) and obesity (body mass index greater than 30 kg/m2) are important risk factors in the development of certain cancers, a leading cause of death in North America (1, 4–6). Specifically, obesity is associated with increased risk and / or mortality from cancers of the gastrointestinal tract (esophagus, stomach, colon and rectum, liver, gallbladder, pancreas), genitourinary system (kidney), male reproductive system (prostate), female reproductive system (breast, ovary, uterus, cervix), and hematopoietic system (lymphoma) (1, 4–7).
Bariatric surgery is a safe procedure that results in sustained excess weight loss and reversal of obesity-related co-morbidities and mortality (8–13). Given the association between obesity and cancer risk, and excess weight loss induced by bariatric surgery, interest has developed in examining the relationship between bariatric surgery and oncologic outcomes (14–21). The goal of this systematic review and meta-analysis is to examine the relationship between bariatric surgery and oncologic outcomes in adult patients.
Methods
Literature Search
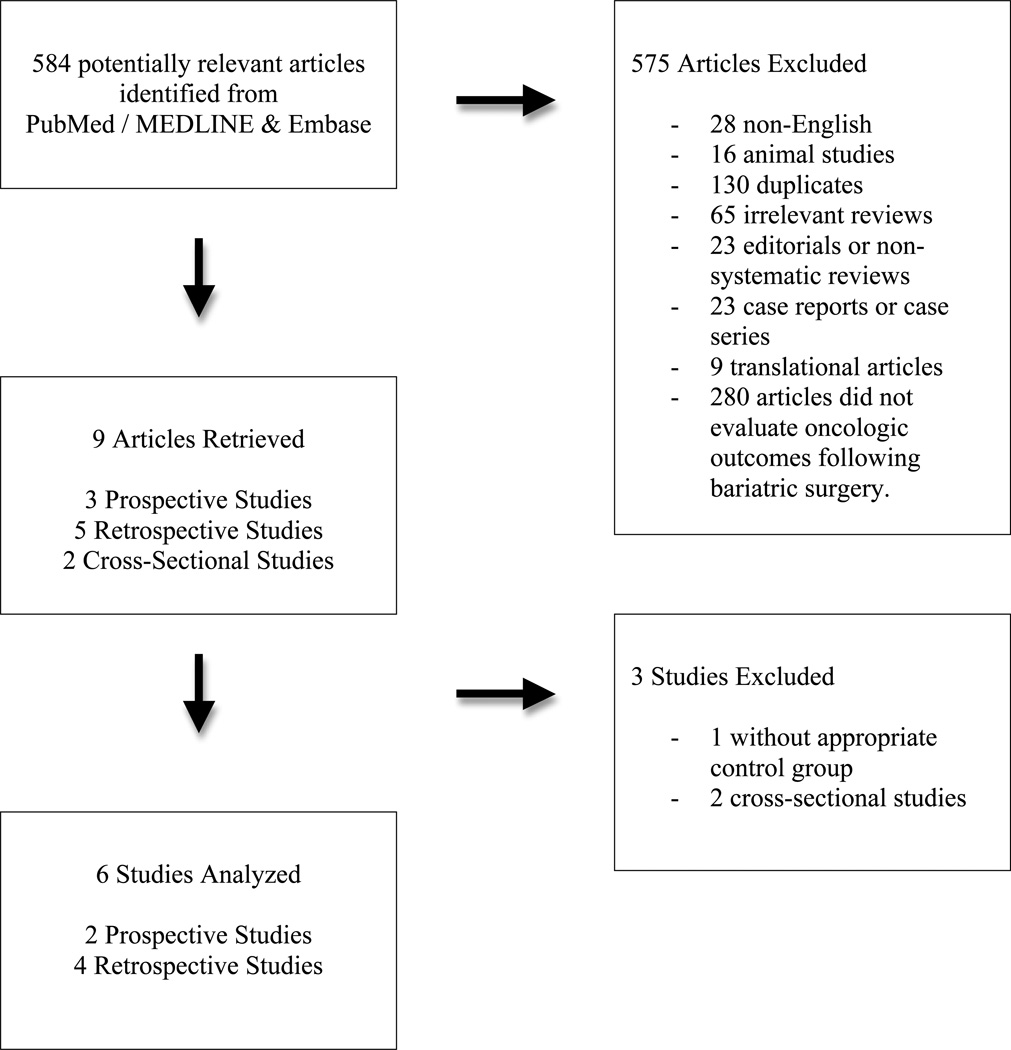
This systematic review was conducted according to the Meta-analysis of Observational Studies in Epidemiology (MOOSE) guidelines (22). PubMed / MEDLINE and Embase were systematically searched with the assistance of a reference librarian from the inception of all databases to January 2012 (Figure 1). Notable articles were probed for MeSH terms in order to encompass key phrases that would capture publications related to the overall concepts of “Bariatric Surgery”, “Obesity”, and “Neoplasms / Cancer”. Evaluation of the references of the most relevant articles retrieved supplemented the overall collection of research articles. Two authors (MCT and YC) independently evaluated all retrieved articles using pre-specified eligibility criteria detailed below. All reasons for exclusion were documented and are detailed in Figure 1.
Figure 1.
Study Selection
Study Selection
All studies based on human data relevant to the primary research question were included in this review. The exposure of interest, bariatric surgery, included restrictive and malabsorptive procedures. Outcomes were defined as cancer incidence, risk, or cancer-related mortality. Only studies with an appropriate control and intervention group in the form of a clinical trial, observational cohort, or case control study were analyzed.
We excluded studies with any of the following: non-human subjects, studies that did not include an appropriate control group, outcome that did not evaluate cancer risk, incidence or mortality, or interventions other bariatric surgery for obese patients (e.g. medical management of obesity). Studies from which raw data was not extractable were also excluded.
Data Extraction and Analysis
Two authors (MCT and YC) independently extracted data from each study. Statistical analyses were conducted with available statistical expertise using Stata version 11.2 (College Station, TX, USA). Relative risks extracted from raw data were pooled to compare overall cancer risk in obese patients undergoing bariatric surgery versus obese controls using the DerSimonian-Laird random-effects meta-analysis (23). Forest plots were generated to demonstrate the individual and pooled effect estimates and confidence intervals, and to allow visual inspection for study heterogeneity. The χ2-test of homogeneity (Cochran Q-statistic) and I2 statistic were utilized to quantify heterogeneity. Meta-regression and Galbraith plots were employed to explore potential sources of between-study heterogeneity.
Influence analysis for studies that contributed to heterogeneity of pooled meta-estimates was conducted (24). Sensitivity analysis was conducted for studies that reported cancer mortality rather than incidence rate or risk (19, 25). Additional sensitivity analyses were conducted for one study that evaluated only female bariatric surgery patients (18). Cumulative analysis was also conducted to demonstrate effect of publication year on the evolution of the pooled estimate.
Sub-group analyses were performed for cancer type and gender. Meta-analysis by cancer type was conducted to evaluate the effect of bariatric surgery on site-specific cancers. Only studies for which raw data could be extracted by cancer type were included in this sub-group analysis (14, 16). Meta-analysis by gender was also conducted based upon results of the systematic review consistently showing differential effects between men and women of bariatric surgery on oncologic outcomes. Only studies for which raw data could be extracted by gender were included in this stratified analysis (15, 18, 20). A test for homogeneity between the two gender subgroups was performed to evaluate the presence of effect modification (24).
Quality Assessment of Studies
A uniform data collection form for both data extractors was developed to summarize studies reviewed. Any study that potentially met inclusion criteria on the basis of title and abstract were obtained in full and critically reviewed based upon quality assessment criteria developed by the Enhancing the Quality and Transparency of Health Research Network (26). Publication bias was evaluated by constructing a funnel plot with visual assessment of asymmetry. The results of the funnel plot were corroborated using Begg’s adjusted rank correlation and Egger’s linear regression methods for statistical evaluation of potential publication bias (27, 28).
Results
Overall Meta-Analysis
Ten articles were selected for full-text evaluation and data extraction based upon review of title and abstract (Figure 1). There were no randomized clinical trials. Of the 9 observational studies considered for meta-analysis, 3 were prospective cohorts, 4 were retrospective cohorts, and the remaining 2 were cross-sectional studies. The two cross-sectional studies were excluded, as they examined cancer prevalence in bariatric surgery patients without an appropriate control group (29, 30). Another study was excluded because it compared the incidence rate of obesity-related cancers following bariatric surgery to the general population, rather than obese controls (31).
The characteristics of the six studies analyzed are summarized in Table I. These studies (N = 51,740) provided data on cancer incidence or mortality among obese adult patients following bariatric surgery against obese controls who did not undergo surgical treatment for obesity (15, 16, 18–20, 25). The overall effect of bariatric surgery on relative risk of cancer is presented in Figure 2. The meta-estimate for overall relative risk of any cancer following bariatric surgery is 0.55 [95% CI: 0.41 – 0.73, p < 0.0001]. L’Abbe plot of the included studies for meta-analysis confirmed that the effect measure of relative risk was appropriate for these studies. A test for between-study heterogeneity revealed an I2 = 83% [95% CI: 64% – 92%, p < 0.0001]. Given this degree of between-study heterogeneity, we identified sources of heterogeneity using meta-regression, Galbraith plots, and stratified (subgroup) analysis.
Table I.
Meta-Analysis Study Characteristics
| Study | Design | Population | Intervention | Control | Outcome |
|---|---|---|---|---|---|
| Adams et al (2007)19 | Retrospective Mean follow-up 7.1 years. |
N = 15850 USA (Utah) |
N = 7925 Obese patients undergoing roux-en-y gastric bypass. |
N = 7925 Obese controls obtained from state registry individually matched for age, sex, BMI. |
Cancer mortality. HR = 0.40 [0.25–0.65] Cox models adjusted for matching factors of age, sex, and BMI. |
| Sjostrom et al (2007)25 | Prospective Mean follow-up 10.9 years. |
N = 4047 Sweden (SOS cohort) |
N = 2010 Obese patients undergoing gastric band, vertical banded gastroplasty, or gastric bypass. |
N = 2037 Obese controls obtained from the general population, risk-set matched on multiple (18) variables. |
Cancer mortality. HR = 0.71 (all) [0.54–0.92] Univariate analyses for cancer-related mortality. |
| Christou et al (2008)21 | Retrospective Follow-up of 5 years. |
N = 6781 Canada (Montreal) |
N = 1035 Obese patients undergoing vertical banded gastroplasty or roux-en-y gastric bypass. |
N = 5746 Obese controls obtained from provincial health insurance data matched for age, gender, and diagnosis of obesity. |
Relative risk of all cancer diagnoses. RR = 0.22 [0.14–0.35] Univariate analyses for relative risk of cancer over 5 years. |
| McCawley et al (2009)23 | Retrospective Follow-up time up to 16 years. |
N = 4977 USA (Virginia) |
N = 1482 Obese female patients undergoing gastric band, vertical banded gastroplasty or roux-en-y gastric bypass. |
N = 3495 Obese female controls obtained from university database with diagnosis of morbid obesity, not matched to intervention group. |
Change in prevalence of any cancer diagnosis in women only. 3.6% in surgical group versus 5.8% (p =0.002) in controls over a 16-year period. Univariate analyses without any matching. |
| Adams et al (2009)20 | Retrospective Mean follow-up of 12.5 years. |
N = 16038 USA (Utah) |
N = 6596 Obese patients undergoing roux-en-y gastric bypass. |
N = 9442 Obese controls obtained from state registry individually matched for age, sex, BMI. |
Incidence of all cancer diagnoses. HR = 0.76 (all) [0.65–0.89] HR = 0.73 (F) [0.62–0.87] HR = 1.02 (M) [0.69–1.52] Cox models adjusted for matching factors of age, sex, and BMI. |
| Sjostrom et al (2009)24 | Prospective Mean follow-up 10.9 years. |
N = 4047 Sweden (SOS cohort) |
N = 2010 Obese patients undergoing gastric band, vertical banded gastroplasty, or gastric bypass. |
N = 2037 Obese controls obtained from the general population, risk-set matched on multiple (18) variables. |
Incidence of all first-time cancers. HR = 0.67 (all) [0·53–0·85] HR = 0·58 (F) [0·44–0·77] HR = 0·97 (M) [0·62–1·52] Cox model adjusted for statistically significant confounders by forward stepwise selection. |
Figure 2.
Meta-regression by publication year (p = 0.373), study size (p = 0.971), and prospective study design (p = 0.451) demonstrated that these factors were not significant sources of heterogeneity. A Galbraith plot showed one study to be a particular outlier to the overall effect estimate (16). We conducted influence analysis by systematically removing each included study to determine whether the meta-estimate would significantly change qualitatively and quantitatively. With this outlier study removed, the meta-estimate of relative risk was 0.72 [95% CI: 0.64 – 0.80, p < 0.0001] (16). The systematic exclusion of any one study did not significantly alter the overall meta-estimate: the effect estimate remained protective and excluded the null value of relative risk in all influence analyses.
We extended our sensitivity analyses to exclude studies that examined cancer mortality rather than risk or incidence (19, 25). With these studies removed, the meta-estimate of relative risk was 0.56 [95% CI: 0.39 – 0.80, p = 0.002] (19, 25). One study that evaluated only women was also removed in addition to the previous studies, which gave a meta-estimate of relative risk of 0.53 [95% CI: 0.32 – 0.87, p = 0.012] (18).
Meta-Analysis Stratified by Gender
Stratification by gender was pursued to evaluate gender as a source of heterogeneity and to explore possible effect modification (Figure 3). Random-effects meta-analysis stratified by gender demonstrates a protective effect of bariatric surgery on cancer risk for women with a RR = 0.68 [95% CI: 0.60 – 0.77, p < 0.0001], I2 < 0.1% for heterogeneity (p = 0.463 for between-study heterogeneity). There does not appear to be a protective effect for men in the stratified analysis, RR = 0.99 [95% CI: 0.74 – 1.32, p = 0.937], I2 < 0.1% (p = 0.930 for between-study heterogeneity). A test of homogeneity of the relative risk of cancer following bariatric surgery of men versus women was conducted, which confirmed effect modification by gender (p = 0.021).
Figure 3.
Sub-Group Analysis by Cancer Type
We attempted to evaluate the effect of bariatric surgery on cancer risk and mortality by performing cancer type sub-group analysis. Of the cancer types that were presented, we were able to perform sub-group analyses on breast, colorectal, melanoma, non-Hodgkin’s lymphoma, and pancreatic cancer (16, 25). The sub-group analyses did not reveal any statistically significant effect of bariatric surgery on any of these cancer types on oncologic outcomes (data not shown).
Evaluation of Bias and Quality Components of Studies
Publication bias was evaluated visually and quantitatively. Funnel plot of the included studies demonstrated relative symmetry. Statistical confirmation of the lack of publication bias was carried out with Begg’s adjusted rank correlation (p = 0.221) and Egger’s regression (p = 0.144), both of which were not significant for the presence of publication bias.
The six observational studies that met our specified criteria to be entered into meta-analysis all evaluated various bariatric surgeries (gastric band, vertical banded gastroplasty, or gastric bypass) and oncologic outcomes (cancer risk, incidence, or mortality) against an appropriate control group, which was either a university hospital database, state / provincial registry, or an obese control group selected from the general population (15, 16, 18–20, 25).
The two prospective studies represent the Swedish Obese Subjects cohort, which contemporaneously matched intervention subjects to controls on multiple factors (19, 20, 32). Although the initial purpose of this database was to measure mortality outcomes with a secondary interest in cardiovascular and metabolic disease profile following bariatric surgery, this clinically rich database nevertheless allowed for rigorous statistical analysis (19, 20, 32).
The four retrospective studies may have been prone to biases such as lack of blinding and inability to measure and / or adjust for potential confounders. One study, which evaluated female bariatric surgery patients compared to obese female controls from a university hospital database, did not employ matching or other methods of risk adjustment (18). The other retrospective studies were able to appropriately match intervention subjects to controls on factors such as age, gender, and body mass index (15, 16, 25). Only two of the four retrospective studies were able to conduct multivariate Cox proportional hazards models to control for several other confounders (15, 25).
Discussion
Main Findings of Meta-Analysis and Systematic Review
Our meta-analysis suggests an overall reduced risk of cancer in obese patients following bariatric surgery relative to obese individuals who do not undergo surgery (RR = 0.55 [95% CI: 0.41–0.73], p < 0.0001, I2 = 83%). The protective effect of bariatric surgery on oncologic outcomes seems more pronounced for women (RR = 0.68 [95% CI: 0.60 – 0.77], p < 0.0001, I2 < 0.1%) compared to men (RR = 0.99 [95% CI: 0.74 – 1.32], p = 0.930, I2 < 0.1%). A formal test of homogeneity confirms that the effect of bariatric surgery on oncologic outcomes is modified by gender (p = 0.021). Individual studies also observed effect modification by gender with a protective effect of bariatric surgery seen in females but not males (15, 20). In a stratified analysis, the HR for cancer incidence following bariatric surgery was 0.58 [95% CI 0.44–0.77] in women compared to a HR of 0.97 [95% 0.62–1.52] in men (20). Another study suggests the HR for cancer incidence following bariatric surgery is 0.73 [95% CI 0.62–0.87] in women and 1.02 [95% CI 0.69–1.57] in men (15).
Implications for Surgical Practice
Obesity is associated with increased mortality and several co-morbid conditions such as hypertension, dyslipidemia, diabetes, coronary artery disease, obstructive sleep apnea, and certain cancers (4, 10–12). Bariatric surgery has been shown to provide sustained excess weight loss in obese individuals, reduce mortality, and improve or resolve obesity-related co-morbidities (10, 19, 32). Compared to medical therapy alone, bariatric surgery consistently results in greater long-term weight reduction in obese individuals (17, 19, 33). Weight loss itself (without surgical intervention) has been shown to reduce risk of cancer (34). Thus, bariatric surgery may protect against cancer risk and mortality by facilitating the process of achieving healthy body weight.
The mechanisms by which bariatric surgery induces sustained excess weight loss and the probable interplay with reducing cancer risk and mortality is an evolving field of research. Gastric bypass of a hormonally active foregut has implicated ghrelin and leptin as important physiological factors for appetite control and reduced caloric intake (35–37). This is in addition to the mechanical restriction and / or malabsorption produced by bariatric surgery (38, 39).
Adipose tissue is also a hormonally active organ involved in the regulation of immune function, inflammation, insulin metabolism, peripheral aromatization of circulating androgens to estrogens, and cellular growth (6, 34, 40, 41). The association between bariatric surgery and cancer risk may also be the consequence of metabolic derangements associated with obesity that are restored by either sustained excess weight loss, surgically induced caloric restriction, and / or bypass of a hormonally active foregut.
One excluded study from our meta-analysis compared cancer incidence of obese patients who had undergone bariatric surgery with that of the general population (31). This study found that for obesity-related cancers (breast, prostate, colorectum, endometrium, kidney, pancreas, gallbladder, and esophagus), the overall incidence rate ratio was 1.04 [95% CI 0.93–1.17] for all subjects who underwent bariatric surgery compared to the general population (31). These findings suggest that bariatric surgery may decrease the risk of cancer in formerly obese subjects to a risk comparable to that of the general population. Thus, in addition to clinical effectiveness in weight reduction, resolution of co-morbid conditions, bariatric surgery may provide additional benefit to obese patients by bringing their elevated risk of cancer back down to a baseline population risk.
Study Limitations
The rigor of this systematic review and meta-analysis is limited by the available studies in the literature and the presence of any uncontrolled confounding or unmeasured biases in these studies. There was significant between-study heterogeneity in the overall meta-analysis and pooling differing measures of oncologic outcome (cancer mortality, risk, and incidence) further introduced heterogeneity. We explored sources of heterogeneity graphically with Forest and Galbraith plots in addition to meta-regression, sensitivity analyses and finally, stratified meta-analysis. Our stratified meta-analysis by gender demonstrates a far more acceptable degree of between-study heterogeneity than our overall meta-analysis.
There are some additional limitations. First, the effect of surgery itself versus induced intentional and excess weight loss on oncologic outcomes cannot be dissociated. Second, whether the effect of bariatric surgery on oncologic outcomes is the result of interfering with the initiation of carcinogenesis or halting further growth of subclinical neoplasia via surgically-induced caloric restriction and / or malabsorption leading to excess weight loss cannot be determined in a meta-analysis. Both scenarios would lead to a reduced cancer incidence and mortality, and it would not be possible to determine which particular effect is a greater contributor to the intervention-outcome association.
Finally, potential bias may result from higher cancer screening implicit in the peri-operative work-up and long-term follow-up after surgical management of obesity. While increased pre-operative cancer screening would decrease the apparent frequency of cancer in this group, increased surveillance after bariatric surgery would result in greater detection of malignancies that would not have been detected otherwise. This effect should actually bias our results toward the null and may even underestimate the true effect of bariatric surgery on oncologic outcomes.
A major strength of the meta-analysis is the independent review of retrieved articles and data extraction by two authors from the largest databases of biomedical journals (PubMed / MEDLINE and Embase). This has minimized bias and error with respect to inclusion of articles for analysis, interpretation of results, appraisal of quality of evidence, and meta-analysis.
In summary, bariatric surgery appears to decrease the risk of cancer relative to obese individuals that do not undergo surgery. This association is more marked among women than men. As bariatric surgery is a relatively new procedure, research exploring the association between surgically induced intentional weight loss in obese patients and oncologic outcomes remains an evolving field of study. Since randomized trials evaluating the role of bariatric surgery on cancer risk may not be ethically feasible, additional high quality observational studies are necessary to further examine whether bariatric surgery can indeed reduce cancer burden and whether its effects are limited to women as suggested in the current analysis.
Acknowledgements
Ms. Carol Mita, Reference Librarian, Countway Library of Medicine, Harvard University, Boston, MA, USA.
Funding: Supported in part by grants 5P30DK46200-18 and U54CA155626-01 from the National Institutes of Health.
Footnotes
Disclosures
Drs. Tee, Warnock, Hu, Chavarro and Ms. Cao (all authors) have no conflicts of interest or financial ties to disclose.
References
- 1.Anderson AS, Caswell S. Obesity management--an opportunity for cancer prevention. Surgeon. 2009 Oct;7(5):282–285. doi: 10.1016/s1479-666x(09)80005-x. [DOI] [PubMed] [Google Scholar]
- 2.Haslam DW, James WP. Obesity. Lancet. 2005 Oct 1;366(9492):1197–1209. doi: 10.1016/S0140-6736(05)67483-1. [DOI] [PubMed] [Google Scholar]
- 3.Pender JR, Pories WJ. Epidemiology of obesity in the united states. Gastroenterol Clin North Am. 2005 Mar;34(1):1–7. doi: 10.1016/j.gtc.2004.12.010. [DOI] [PubMed] [Google Scholar]
- 4.Bianchini F, Kaaks R, Vainio H. Overweight, obesity, and cancer risk. Lancet Oncol. 2002 Sep;3(9):565–574. doi: 10.1016/s1470-2045(02)00849-5. [DOI] [PubMed] [Google Scholar]
- 5.Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med. 2003 Apr 24;348(17):1625–1638. doi: 10.1056/NEJMoa021423. [DOI] [PubMed] [Google Scholar]
- 6.Calle EE, Thun MJ. Obesity and cancer. Oncogene. 2004 Aug 23;23(38):6365–6378. doi: 10.1038/sj.onc.1207751. [DOI] [PubMed] [Google Scholar]
- 7.McTiernan A. Obesity and cancer: The risks, science, and potential management strategies. Oncology (Williston Park) 2005 Jun;19(7):871–881. discussion 881-2, 885-6. [PubMed] [Google Scholar]
- 8.Buchwald H, Avidor Y, Braunwald E, Jensen MD, Pories W, Fahrbach K, et al. Bariatric surgery: A systematic review and meta-analysis. JAMA. 2004 Oct 13;292(14):1724–1737. doi: 10.1001/jama.292.14.1724. [DOI] [PubMed] [Google Scholar]
- 9.Christou NV. Impact of obesity and bariatric surgery on survival. World J Surg. 2009 Oct;33(10):2022–2027. doi: 10.1007/s00268-009-0050-2. [DOI] [PubMed] [Google Scholar]
- 10.Schauer PR, Kashyap SR, Wolski K, Brethauer SA, Kirwan JP, Pothier CE, et al. Bariatric surgery versus intensive medical therapy in obese patients with diabetes. N Engl J Med. 2012 Apr 26;366(17):1567–1576. doi: 10.1056/NEJMoa1200225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sjostrom L, Lindroos AK, Peltonen M, Torgerson J, Bouchard C, Carlsson B, et al. Lifestyle, diabetes, and cardiovascular risk factors 10 years after bariatric surgery. N Engl J Med. 2004 Dec 23;351(26):2683–2689. doi: 10.1056/NEJMoa035622. [DOI] [PubMed] [Google Scholar]
- 12.Sjostrom L, Narbro K, Sjostrom CD, Karason K, Larsson B, Wedel H, et al. Effects of bariatric surgery on mortality in swedish obese subjects. N Engl J Med. 2007 Aug 23;357(8):741–752. doi: 10.1056/NEJMoa066254. [DOI] [PubMed] [Google Scholar]
- 13.Blackburn GL, Hutter MM, Harvey AM, Apovian CM, Boulton HR, Cummings S, et al. Expert panel on weight loss surgery: Executive report update. Obesity (Silver Spring) 2009 May;17(5):842–846. doi: 10.1038/oby.2008.578. [DOI] [PubMed] [Google Scholar]
- 14.Adams TD, Hunt SC. Cancer and obesity: Effect of bariatric surgery. World J Surg. 2009 Oct;33(10):2028–2033. doi: 10.1007/s00268-009-0169-1. [DOI] [PubMed] [Google Scholar]
- 15.Adams TD, Stroup AM, Gress RE, Adams KF, Calle EE, Smith SC, et al. Cancer incidence and mortality after gastric bypass surgery. Obesity (Silver Spring) 2009 Apr;17(4):796–802. doi: 10.1038/oby.2008.610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Christou NV, Lieberman M, Sampalis F, Sampalis JS. Bariatric surgery reduces cancer risk in morbidly obese patients. Surg Obes Relat Dis. 2008 Nov-Dec;4(6):691–695. doi: 10.1016/j.soard.2008.08.025. [DOI] [PubMed] [Google Scholar]
- 17.Christou NV, Sampalis JS, Liberman M, Look D, Auger S, McLean AP, et al. Surgery decreases long-term mortality, morbidity, and health care use in morbidly obese patients. Ann Surg. 2004 Sep;240(3):416–423. doi: 10.1097/01.sla.0000137343.63376.19. discussion 423-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McCawley GM, Ferriss JS, Geffel D, Northup CJ, Modesitt SC. Cancer in obese women: Potential protective impact of bariatric surgery. J Am Coll Surg. 2009 Jun;208(6):1093–1098. doi: 10.1016/j.jamcollsurg.2009.01.045. [DOI] [PubMed] [Google Scholar]
- 19.Sjostrom L, Narbro K, Sjostrom CD, Karason K, Larsson B, Wedel H, et al. Effects of bariatric surgery on mortality in swedish obese subjects. N Engl J Med. 2007 Aug 23;357(8):741–752. doi: 10.1056/NEJMoa066254. [DOI] [PubMed] [Google Scholar]
- 20.Sjostrom L, Gummesson A, Sjostrom CD, Narbro K, Peltonen M, Wedel H, et al. Effects of bariatric surgery on cancer incidence in obese patients in sweden (swedish obese subjects study): A prospective, controlled intervention trial. Lancet Oncol. 2009 Jul;10(7):653–662. doi: 10.1016/S1470-2045(09)70159-7. [DOI] [PubMed] [Google Scholar]
- 21.Renehan AG. Bariatric surgery, weight reduction, and cancer prevention. Lancet Oncol. 2009 Jul;10(7):640–641. doi: 10.1016/S1470-2045(09)70170-6. [DOI] [PubMed] [Google Scholar]
- 22.Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, et al. Meta-analysis of observational studies in epidemiology: A proposal for reporting. meta-analysis of observational studies in epidemiology (MOOSE) group. JAMA. 2000 Apr 19;283(15):2008–2012. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 23.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986 Sep;7(3):177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 24.Egger M, Davey Smith G, Altman DG, editors. BMJ Books. 2nd edition. 2001. Systematic reviews in health care: Meta-analysis in context. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Adams TD, Gress RE, Smith SC, Halverson RC, Simper SC, Rosamond WD, et al. Long-term mortality after gastric bypass surgery. N Engl J Med. 2007 Aug 23;357(8):753–761. doi: 10.1056/NEJMoa066603. [DOI] [PubMed] [Google Scholar]
- 26.Simera I, Moher D, Hirst A, Hoey J, Schulz KF, Altman DG. Transparent and accurate reporting increases reliability, utility, and impact of your research: Reporting guidelines and the EQUATOR network. BMC Med. 2010 Apr 26;8:24. doi: 10.1186/1741-7015-8-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994 Dec;50(4):1088–1101. [PubMed] [Google Scholar]
- 28.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997 Sep 13;315(7109):629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Boru C, Silecchia G, Pecchia A, Iacobellis G, Greco F, Rizzello M, et al. Prevalence of cancer in italian obese patients referred for bariatric surgery. Obes Surg. 2005 Sep;15(8):1171–1176. doi: 10.1381/0960892055002284. [DOI] [PubMed] [Google Scholar]
- 30.Gagne DJ, Papasavas PK, Maalouf M, Urbandt JE, Caushaj PF. Obesity surgery and malignancy: Our experience after 1500 cases. Surg Obes Relat Dis. 2009 Mar-Apr;5(2):160–164. doi: 10.1016/j.soard.2008.07.013. [DOI] [PubMed] [Google Scholar]
- 31.Plecka Ostlund M, Lu Y, Lagergren J. Risk of obesity-related cancer after obesity surgery in a population-based cohort study. Ann Surg. 2010 Jun 21; doi: 10.1097/SLA.0b013e3181e33778. [DOI] [PubMed] [Google Scholar]
- 32.Sjostrom L. Bariatric surgery and reduction in morbidity and mortality: Experiences from the SOS study. Int J Obes (Lond) 2008 Dec;32(Suppl 7):S93–S97. doi: 10.1038/ijo.2008.244. [DOI] [PubMed] [Google Scholar]
- 33.O'Brien PE, Dixon JB, Laurie C, Skinner S, Proietto J, McNeil J, et al. Treatment of mild to moderate obesity with laparoscopic adjustable gastric banding or an intensive medical program: A randomized trial. Ann Intern Med. 2006 May 2;144(9):625–633. doi: 10.7326/0003-4819-144-9-200605020-00005. [DOI] [PubMed] [Google Scholar]
- 34.Imayama I, Ulrich CM, Alfano CM, Wang C, Xiao L, Wener MH, et al. Effects of a caloric restriction weight loss diet and exercise on inflammatory biomarkers in overweight/obese postmenopausal women: A randomized controlled trial. Cancer Res. 2012 May 1;72(9):2314–2326. doi: 10.1158/0008-5472.CAN-11-3092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cummings DE, Weigle DS, Frayo RS, Breen PA, Ma MK, Dellinger EP, et al. Plasma ghrelin levels after diet-induced weight loss or gastric bypass surgery. N Engl J Med. 2002 May 23;346(21):1623–1630. doi: 10.1056/NEJMoa012908. [DOI] [PubMed] [Google Scholar]
- 36.Cummings DE, Overduin J, Foster-Schubert KE. Gastric bypass for obesity: Mechanisms of weight loss and diabetes resolution. J Clin Endocrinol Metab. 2004 Jun;89(6):2608–2615. doi: 10.1210/jc.2004-0433. [DOI] [PubMed] [Google Scholar]
- 37.Hickey MS, Pories WJ, MacDonald KG, Jr, Cory KA, Dohm GL, Swanson MS, et al. A new paradigm for type 2 diabetes mellitus: Could it be a disease of the foregut? Ann Surg. 1998 May;227(5):637–643. doi: 10.1097/00000658-199805000-00004. discussion 643-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.DeMaria EJ. Bariatric surgery for morbid obesity. N Engl J Med. 2007 May 24;356(21):2176–2183. doi: 10.1056/NEJMct067019. [DOI] [PubMed] [Google Scholar]
- 39.Leff DR, Heath D. Surgery for obesity in adulthood. BMJ. 2009 Sep 22;339:b3402. doi: 10.1136/bmj.b3402. [DOI] [PubMed] [Google Scholar]
- 40.Cottam D, Fisher B, Ziemba A, Atkinson J, Grace B, Ward DC, et al. Tumor growth factor expression in obesity and changes in expression with weight loss: Another cause of increased virulence and incidence of cancer in obesity. Surg Obes Relat Dis. 2010 Sep-Oct;6(5):538–541. doi: 10.1016/j.soard.2010.04.011. [DOI] [PubMed] [Google Scholar]
- 41.Hu FB. Obesity epidemiology. New York, NY: Oxford University Press; 2008. [Google Scholar]