Abstract
The vertebrate organizer can induce a complete body axis when transplanted to the ventral side of a host embryo1 by virtue of its distinct head and trunk inducing properties. Wingless/Wnt antagonists secreted by the organizer have been identified as head inducers2–4. Their ectopic expression can promote head formation, whereas ectopic activation of Wnt signalling during early gastrulation blocks head formation5–7. These observations suggest that the ability of head inducers to inhibit Wnt signalling during formation of anterior structures is what distinguishes them from trunk inducers that permit the operation of posteriorizing Wnt signals8. Here we describe the zebrafish headless (hdl) mutant and show that its severe head defects are due to a mutation in T-cell factor-3 (Tcf3), a member of the Tcf/Lef family9,10. Loss of Tcf3 function in the hdl mutant reveals that hdl represses Wnt target genes. We provide genetic evidence that a component of the Wnt signalling pathway is essential in vertebrate head formation and patterning.
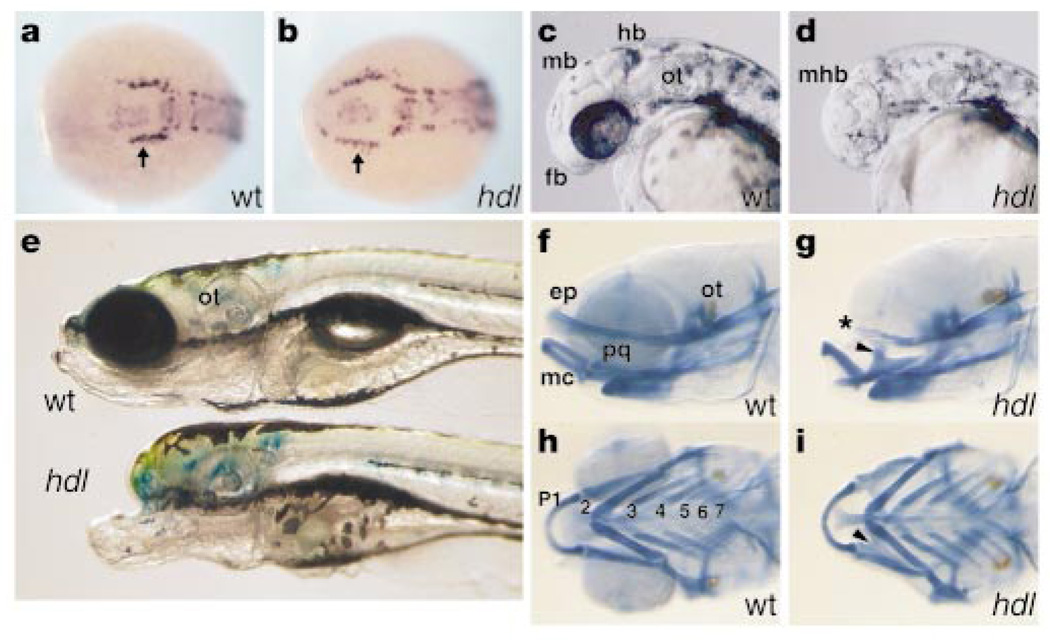
The hdl mutant was isolated as part of a screen for ethyl nitrosourea (ENU)-induced mutations that disrupt early neurogenesis in zebrafish11. Mutant embryos obtained from hdl heterozygous parents, however, display a weak phenotype and are characterized by a slight reduction in eye size. Their weak phenotype allowed a subset of homozygous hdl fish to be grown to adulthood. Here, hdl mutants refers to maternally and zygotically homozygous mutant embryos. Our examination of hdl mutants with the early neuronal marker, huC12, revealed an aberrant pattern of trigeminal neurons (Fig. 1a, b). The mutation, however, derives its name — headless — from the head defect in embryos that is characterized by complete loss of eyes, forebrain and part of the midbrain (Fig. 1c, d, e). Analysis of the head skeleton reveals cranial-specific defects (Fig. 1f, g); the pharyngeal arches appear relatively unaffected (Fig. 1h, i).
Figure 1.
Morphological analysis. a, b, huC expression at the neural plate stage shows abnormal rostral extension of trigeminal neurons (arrow). Dorsal-anterior view. c, d, 34-hour embryos show complete loss of eye, forebrain (fb) and part of the midbrain in hdl. mb, midbrain; hb, hindbrain; mhb, midbrain-hindbrain boundary; ot, otic vesicle. e, hdl larvae at 8 days show a severe head defect. f-i, Skeletal preparations of day-5 larvae stained with Alcian blue. In mutants, the ethmoid plate (ep) is reduced (asterisk), whereas ventral pharyngeal arches (p1–p7), including Meckel's cartilage (mc), are less affected. Fused pterygoid process of the palatoquadrate (pq) in hdl (arrowhead).
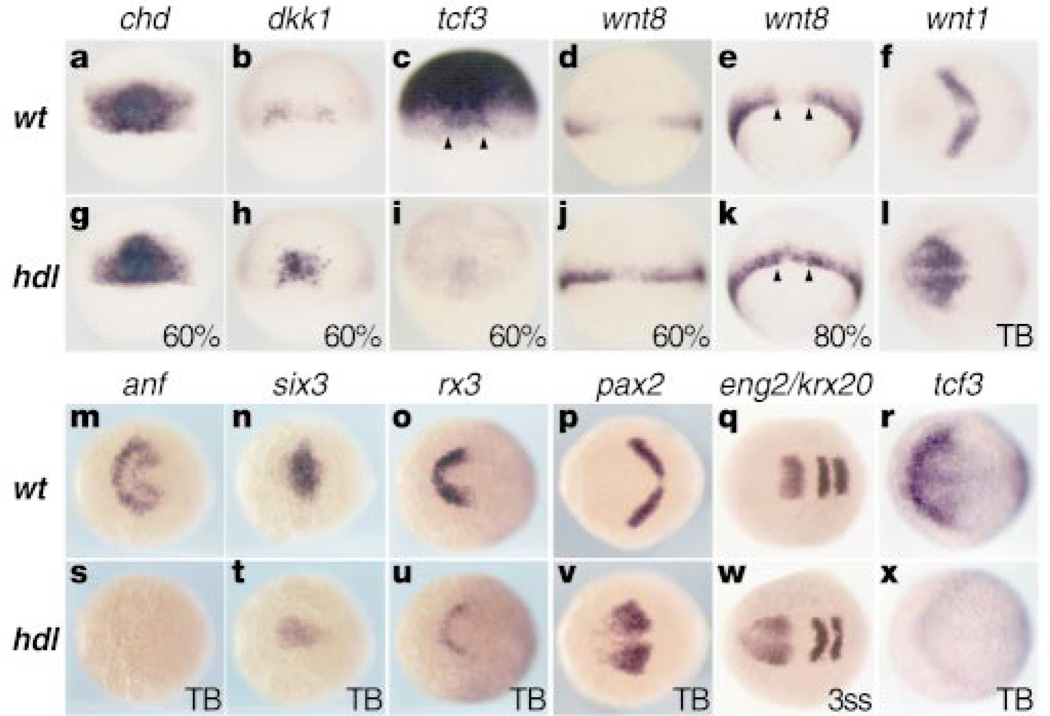
Changes in the expression of genes that are early markers for the forebrain and eye are consistent with morphological changes observed in hdl mutants. A homeobox gene anf/Hesx1, normally expressed in the anterior neural plate (Fig. 2m), is almost absent in mutants (Fig. 2s). Mutational analysis has shown that Hesx1 is required for normal forebrain development in mice and humans13. Transcripts of six3, another anterior brain marker14, are also reduced in the anterior neural plate, but its expression in the underlying prechordal plate is not changed (Fig. 2n, t). This suggests that the hdl phenotype is not related to loss of the underlying prechordal plate. Expression of rx3, a marker for the presumptive retina and ventral forebrain, is also strongly reduced in mutants (Fig. 2o, u). Mouse embryos carrying a null allele of the Rx gene have severe defects in eye and forebrain formation15. The reduction in expression of these anterior neural-specific genes is accompanied by a rostral expansion of midbrain-hindbrain boundary (MHB) genes such as pax2 (Fig. 2p, v) and engrailed2 (eng2) (Fig. 2q, w) but not krox20 (krx20), expressed in rhombomeres 3 and 5 (Fig. 2w).
Figure 2.
Gene expression analyses. Animal pole is to the top for 60–80% epiboly embryos. Anterior is to the left for tail bud (TB) and 3-somite-stage embryos (3ss). a, g, chd is relatively unaffected in the organizer. b, h, increased dkk1 expression in hdl. c, i, Widespread tcf3 expression, excluded from the ventrolateral margin in the wild type and reduced in hdl. Arrowheads indicate the organizer region. d, e and j, k, wnt8 is excluded from the dorsal margin (arrowheads) in the wild type but not in hdl. f, l, Anterior expansion of wnt1 expression in hdl. m-o and s-u, Reduced expression of anterior neural markers in hdl. p, q and v, w, Anterior expansion of MHB markers. q, w, krox20 is relatively unaffected in hdl. r, x, Graded anterior neural plate tcf3 expression, reduced in hdl.
To investigate how early changes in the vertebrate organizer contribute to the genesis of the mutant phenotype, we examined a number of genes expressed in it during early gastrulation. There is little change in the expression of goosecoid (data not shown) and chordin (Fig. 2a, g) in hdl mutants. In contrast, expression of the Wnt antagonist, dickkopf-1 (dkk1)3,16, is increased in the organizer of mutants (Fig. 2b, h). These changes suggest that the hdl mutation leads to changes in head induction that start as early as the beginning of gastrulation.
Previous studies have shown that ectopic activation of Wnt signalling after the midblastula transition can mimic some aspects of the hdl phenotype5–7, so we examined whether there is ectopic expression of Wnts in the hdl mutants. During early gastrulation, wnt8 is expressed in the ventrolateral margin and excluded from the dorsal margin7 (Fig. 2d, e). In hdl mutants, wnt8 expression expands into the dorsal margin (Fig. 2j, k). In addition, rostral expansion of wnt1 (Fig. 2f, l) and wnt8b (data not shown) expression is observed in late-gastrula-stage mutant embryos. These data suggest that the loss of anterior neural structures and the rostral expansion of MHB domain in hdl mutants may result from ectopic Wnt signalling. To test whether inhibition of Wnt signalling can reduce the hdl phenotype, we ectopically expressed the Wnt inhibitors, Cerberus4 and Dkk1 (ref. 16). Microinjection of their synthetic RNAs into hdl mutant embryos showed that neither of these Wnt inhibitors rescues the mutants (data not shown). This suggests that the normal function of hdl is necessary for effective function of these genes.
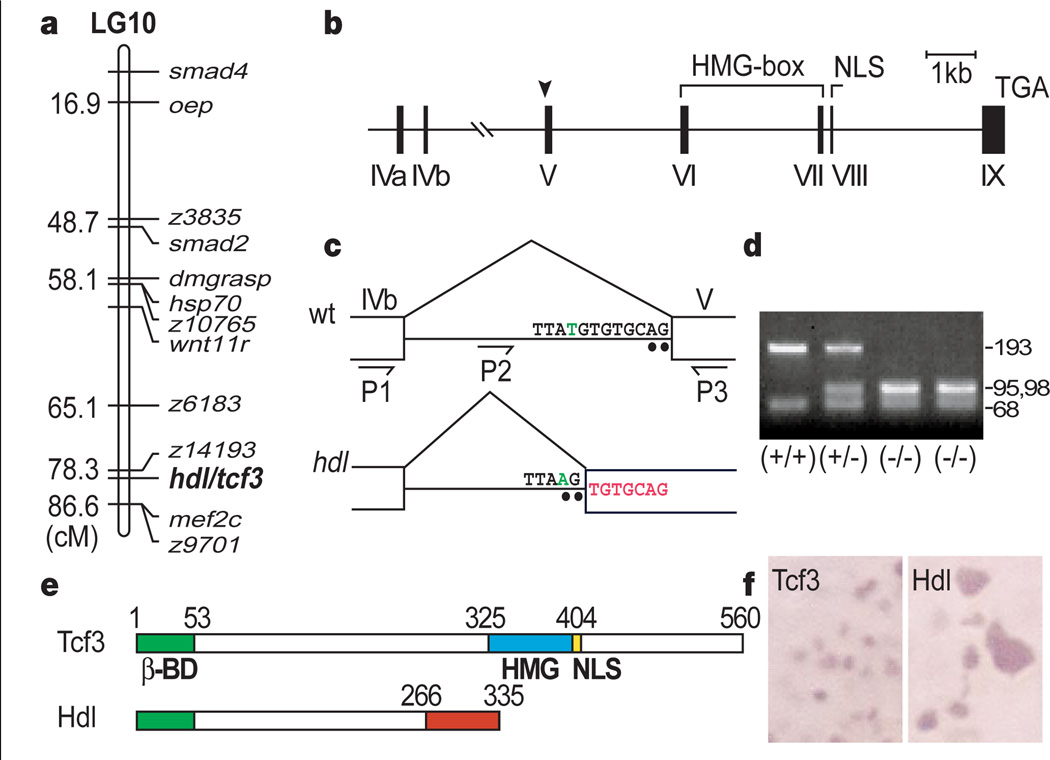
To clone the hdl mutation, we first established its position on the genetic map, using single-strand length polymorphism markers17. Bulked segregant analysis with centromeric markers localized hdl to linkage group 10 (Fig. 3a). Further genetic mapping placed the hdl locus between the SSLP markers z6183 and z9701. The proximity of hdl to z14193 revealed that the previously isolated tcf3 gene, a Tcf/Lef family high mobility group (HMG)-box-containing transcription factor10, mapped close to the hdl locus. This gene has maternal expression and is involved in the Wnt pathway, so we investigated tcf3 as a candidate for the hdl gene. We first compared the wild-type sequence of tcf3 cDNA with that isolated from hdl mutants. The wild-type tcf3 gene encodes a transcription factor with a β-catenin-binding domain, a single HMG DNA-binding domain and a nuclear localization signal (NLS) motif 10. Sequence analysis revealed a seven-base-pair (bp) insertion in the tcf3 cDNA from hdl mutants that causes a frame-shift and is predicted to produce a truncated protein. The truncated Hdl protein retains its β-catenin-binding site but lacks the HMG box as well as the NLS motif (Fig. 3e). Myc-epitope tagged versions of the wild-type and mutant Hdl show that the truncated Hdl proteins are not specifically localized in the nuclei, but the wild-type Tcf3 protein is specifically transported into nuclei (Fig. 3f).
Figure 3.
Mapping of the hdl/tcf3 gene. a, Genetic map of linkage group 10 (LG10) showing position of hdl in relation to previously mapped genes and SSLP markers. b, Structure of the tcf3 gene. Arrowhead indicates the site of the hdl mutation. c, T-to-A transversion (green) produces an aberrant splice acceptor site (AG). The 7-bp insertion is indicated in red. d, Mse I digestion of the genomic PCR product (P2–P3), showing a polymorphism. e, Structure of Headless (Hdl). The truncated Hdl protein lacks the high mobility group (HMG) box DNA-binding motif as well as the nuclear localization signal (NLS). f, Subcellular localization of the wild-type Tcf3 and truncated Hdl proteins is changed from nuclear to cytoplasmic.
As ENU-based mutagenesis is not likely to cause a 7-bp insertion directly18, we predicted that the insertion in tcf3 cDNA from hdl mutants was the result of a splicing defect caused by a point mutation. Thus, we isolated the tcf3 gene from bacterial artificial chromosome (BAC) clones and analysed its genomic structure (Fig. 3b). Sequence analysis revealed the identical 7-bp sequence in the intron flanking exon V. In addition, we identified a T-to-A transversion in the hdl mutant genomic DNA. This point mutation is expected to cause altered splicing in the hdl transcripts (Fig. 3c). We used a polymorphism associated with the novel MseI site created by the point mutation for fine linkage analysis and showed that no recombinants were seen in 1,018 mutant embryos (Fig. 3d). Sequence analysis also revealed that the structure of the zebrafish tcf3 gene (Fig. 3b) is similar to the human Tcf1 gene19.
The tcf3 gene is maternally expressed in Xenopus9 and zebrafish10, having very wide distribution during the blastula stage. However, despite the broad expression of tcf3 in the early gastrula, its expression is excluded from the ventrolateral margin (Fig. 2c), where wnt8 is expressed (Fig. 2d). Strong expression of the tcf3 transcripts is detectable in the neurectoderm during gastrulation. By the tail bud stage, tcf3 is expressed in a graded pattern in the anterior neurectoderm, with the highest levels at the anterior edge of the neural plate (Fig. 2r). Tcf3 is absent at the MHB where wnt1 (Fig. 2f) and wnt8b (data not shown) are expressed. These expression patterns of tcf3 are consistent with its potential early role in head induction and later in patterning the anterior neurectoderm. In hdl mutants, tcf3 expression is strongly reduced both at early blastula and late gastrula stages (Fig. 2i, x).
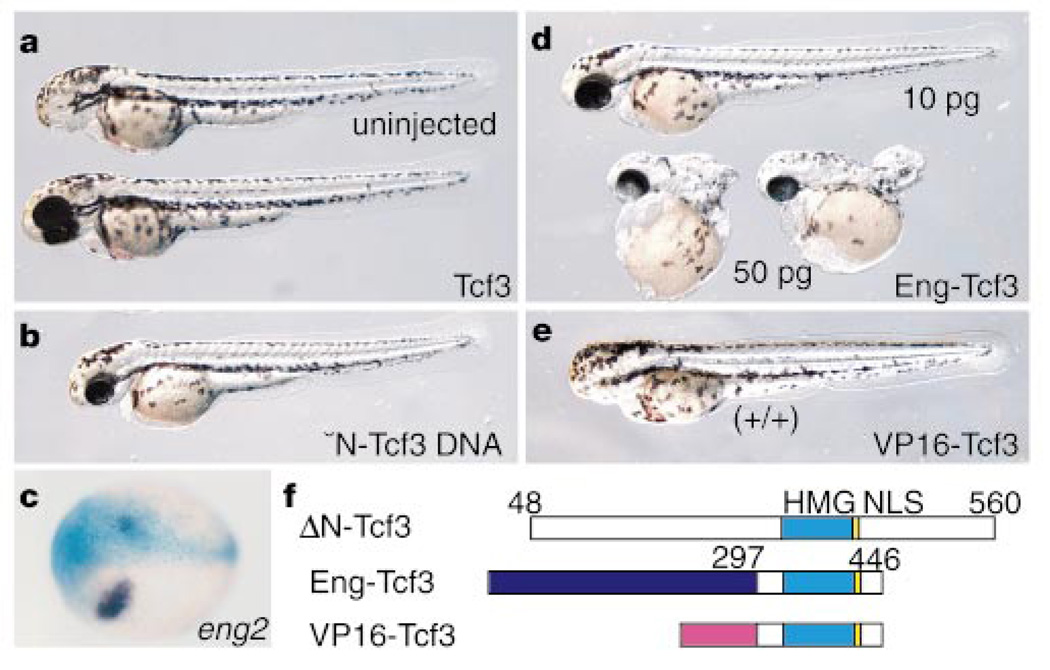
To confirm that the hdl mutation in the tcf3 gene is responsible for the mutant phenotype, we attempted to rescue the mutant embryos by expressing wild-type tcf3 RNA. The injection of wild-type tcf3 RNA (50 pg per embryo) rescued most of the mutants injected (90%; n = 50) (Fig. 4a). Ectopic expression of an equivalent amount of the mutant hdl RNA could not rescue the headless phenotype (n = 50). To test whether the truncated Hdl protein has dominant-negative effects10, we injected up to a tenfold higher concentration of hdl mutant RNA (500 pg per embryo) in both mutant and wild-type embryos. No significant effect was seen in either of these embryos, which suggests that hdl is functionally a null mutation.
Figure 4.
Rescue by Tcf3 expression. a, Two-day-old hdl mutant injected with wild-type Tcf3 RNA (bottom). Top, uninjected control mutant. b, An hdl mutant injected with the ΔNTcf3 plasmid DNA which is expressed after the MBT. c, ΔN-Tcf3 suppresses expression of eng2. Blue X-gal staining shows the injected side of embryo. d, Rescue of hdl by Eng-Tcf3 RNA injection. Higher doses (>50 pg) cause trunk defects. e, Opposite effects of VP16-Tcf3 on head formation. A wild-type embryo injected with VP16-Tcf3 RNA, showing a headless phenotype. f, Structure of proteins used for injection experiments.
It is known that Tcf can act as a repressor of Wnt target genes20,21 through its association with Groucho22,23 and/or CtBP24, or as an activator through its association with β-catenin25,26. Our functional analysis of the hdl mutants indicates, however, that the phenotype may be associated with the loss of a mechanism that is critical for repressing Wnt signalling, not facilitating it. To test this idea, we examined the effects of ΔN-Tcf3, a deletion of Tcf3 that lacks β-catenin binding (Fig. 4f) but is known to retain its repressor activity9. Injection of RNA encoding ΔN-Tcf3 (50 pg per embryo) rescued the hdl mutant phenotype (90%; n = 50). Embryos injected with plasmid DNA encoding ΔN-Tcf3 also rescued mutants (30%; n = 50, Fig. 4b), indicating that zygotic expression of ΔN-Tcf3 is sufficient for rescue. Injection of 50 pg of ΔN-Tcf3 RNA into one blastomere of two-cell-stage embryos suppressed expression of a known target of β-catenin/Tcf signalling27, eng2, demonstrating that ΔN-Tcf3 can repress Wnt target genes (Fig. 4c).
To provide further evidence that the activity of Tcf3 as a repressor is critical for head formation, the HMG box DNA-binding domain of Tcf3 was combined with the repressor domain of the Engrailed protein28 (Fig. 4f). Injection of RNA (10 pg per embryo) encoding Eng-Tcf3 rescued the hdl mutants (80%; n = 40, Fig. 4f). Conversely, we investigated whether a chimaeric transcription activator29 VP16-Tcf3 (Fig. 4f) could rescue hdl mutants. None of the mutants were rescued by injection (10 pg per embryo) of VP16-Tcf3 (n = 50), although higher doses (>50 pg per embryo) caused severe defects (data not shown). Most wild-type embryos injected with 10 pg of VP16-Tcf3 RNA display a headless phenotype (85%; n = 19, Fig. 4e), mimicking the effects of late Wnt activation5–7 and the hdl mutation.
These results indicate that function of Tcf3 as a repressor may be critical for head formation. Basal repression of Wnt targets by Tcf3 during early gastrulation seems to be essential for repressing the genes responsible for the formation of the MHB domain and for permitting the expression of genes responsible for formation of the forebrain, eyes and the midbrain. Repression of Wnt target genes in the prospective neurectoderm by Tcf3 defines a primary condition for function of head inducers. In the absence of this basal repression, secreted Wnt antagonists are ineffective, because they operate by preventing Wnt signals from de-repressing Wnt target genes in the anterior neurectoderm. The graded pattern of tcf3 expression at the anterior edge of the neurectoderm and wnt genes at the MHB suggest a continuing role for Wnts and their antagonists in patterning the anterior neurectoderm during late gastrulation.
Methods
Genetic mapping
For mapping, an AB-background heterozygous hdl carrier was crossed to two polymorphic strains, WIK and HK. Linkage analysis was performed on embryos obtained by crossing an hdl AB homozygous female and an hdl AB/HK or hdl AB/WIK heterozygous male. Genomic DNA was used for genotyping embryos by polymerase chain reaction (PCR). The genetic distance between SSLP markers and the hdl locus was determined by assaying DNA polymorphisms in individual mutant embryos. Zebrafish BAC (192G1 and 103L24, Genome Systems, Inc.) and YAC (76G7, Research Genetics, Inc.) clones were screened by PCR as pools according to the manufacturer's instructions. Restriction enzyme-digested BAC DNA was subcloned into the pBluescript vector and sequenced by standard procedures. For fine linkage analysis, we amplified genomic DNA from individual mutant embryos with specific oligonucleotide primers (P2, 5′-TCAGTCATTCGCTATCACCTCTGTC-3′ and P3, 5′-ACAATGGCGGGGTGAGGGATGC-3′) and digested it with the restriction enzyme MseI.
Cloning of mutant hdl cDNA
RNA was extracted from wild-type embryos or maternally homozygous mutant embryos at the tail bud stage and PCR was carried out after reverse transcription of RNA. Multiple clones were sequenced and compared with wild-type sequences to detect the mutation. To confirm the existence of a 7-bp insertion in the mutant hdl cDNA, we amplified a partial cDNA fragment expected to contain the mutated site using the primers P1 (5′-CCTCT TGGATGGTTGGTTCCTCAG-3′) and P3. In high-percentage gel electrophoresis, a single predicted 177-bp band, slightly larger than the 170-bp wild-type band, was detected in hdl mutant embryos. There were no additional bands detectable in the gel, indicating that the acquired 3′-splice acceptor is preferred for RNA splicing in mutant embryos.
DNA constructs
To generate Myc-tagged Tcf3 (pCS3Tcf3), first the amino-terminal region, starting from the second amino-acid proline, was generated by PCR using primers flanked by BamHI and EcoRI sequences. The PCR product was then subcloned into the BglII and EcoRI site of the pCS3 expression vector. Second, the BamHI- and SpeI-digested fragment, containing the Myc epitope and the N-terminal β-catenin-binding domain, was subcloned into the CS2+Tcf3a plasmid (a gift from R. Dorsky). pCS3ΔN-Tcf3 was created by blunt-ligation of the NcoI- and Spe-digested pCS3Tcf3, deleting the first 47 amino acids of β-catenin-binding domain. The pCS2Eng-Tcf3 construct was generated by fusing the Tcf3 DNA-binding domain (amino acids 297–446) to a fragment encoding amino acids 1–298 of the Drosophila Engrailed protein28. pCS2VP16-Tcf3 was made by fusing the same Tcf3 DNA-binding domain to the activator domain (amino acids 410–490) of the VP16 from the Herpes simplex virus29. RNA for injection was prepared from linearized plasmid DNAs using the SP6 mMessage Machine kit (Ambion).
Acknowledgements
We thank R. T. Moon, R. Dorsky, T. Hirano, M. Hibi, A. Kawahara, L. Kodjabachian, D. Turner, M. Halpern and M. Kobayashi for constructs; M. Kacergis and G. Palardy for technical assistance; R. Subramanian and members of the Dawid lab for comments on the manuscript. The mutagenesis screen was performed at CVRC/MGH/Harvard Medical School. This work was supported by the NIH.
References
- 1.Nieto MA. Reorganizing the organizer 75 years on. Cell. 1999;98:417–425. doi: 10.1016/s0092-8674(00)81971-6. [DOI] [PubMed] [Google Scholar]
- 2.Leyns L, Bouwmeester T, Kim SH, Piccolo S, De Robertis EM. Frzb-1 is a secreted antagonist of Wnt signaling expressed in the Spemann organizer. Cell. 1997;88:747–756. doi: 10.1016/s0092-8674(00)81921-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Glinka A, et al. Dickkopf-1 is a member of a new family of secreted proteins and functions in head induction. Nature. 1998;391:357–362. doi: 10.1038/34848. [DOI] [PubMed] [Google Scholar]
- 4.Piccolo S, et al. The head inducer Cerberus is a multifunctional antagonist of Nodal, BMP and Wnt signals. Nature. 1999;397:707–710. doi: 10.1038/17820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Christian JL, Moon RT. Interactions between Xwnt-8 and Spemann organizer signaling pathways generate dorsoventral pattern in the embryonic mesoderm of Xenopus. Genes Dev. 1993;7:13–28. doi: 10.1101/gad.7.1.13. [DOI] [PubMed] [Google Scholar]
- 6.Hoppler S, Brown JD, Moon RT. Expression of a dominant-negative Wnt blocks induction of MyoD in Xenopus embryos. Genes Dev. 1996;10:2805–2817. doi: 10.1101/gad.10.21.2805. [DOI] [PubMed] [Google Scholar]
- 7.Kelly GM, Greenstein P, Erezyilmaz DF, Moon RT. Zebrafish wnt8 and wnt8b share a common activity but are involved in distinct developmental pathways. Development. 1995;121:1787–1799. doi: 10.1242/dev.121.6.1787. [DOI] [PubMed] [Google Scholar]
- 8.Niehrs C. Head in the WNT: the molecular nature of Spemann’s head organizer. Trends Genet. 1999;15:314–319. doi: 10.1016/s0168-9525(99)01767-9. [DOI] [PubMed] [Google Scholar]
- 9.Molenaar M, et al. XTcf-3 transcription factor mediates beta-catenin-induced axis formation in Xenopus embryos. Cell. 1996;86:391–399. doi: 10.1016/s0092-8674(00)80112-9. [DOI] [PubMed] [Google Scholar]
- 10.Pelegri F, Maischein HM. Function of zebrafish beta-catenin and TCF-3 in dorsoventral patterning. Mech. Dev. 1998;77:63–74. doi: 10.1016/s0925-4773(98)00132-4. [DOI] [PubMed] [Google Scholar]
- 11.Artinger KB, Chitnis AB, Mercola M, Driever W. Zebrafish narrowminded suggests a genetic link between formation of neural crest and primary sensory neurons. Development. 1999;126:3969–3979. doi: 10.1242/dev.126.18.3969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim CH, et al. Zebrafish elav/HuC homologue as a very early neuronal marker. Neurosci. Lett. 1996;216:109–112. doi: 10.1016/0304-3940(96)13021-4. [DOI] [PubMed] [Google Scholar]
- 13.Dattani MT, et al. Mutations in the homeobox gene HESX1/Hesx1 associated with septo-optic dysplasia in human and mouse. Nature Genet. 1998;19:125–133. doi: 10.1038/477. [DOI] [PubMed] [Google Scholar]
- 14.Kobayashi M, Toyama R, Takeda H, Dawid IB, Kawakami K. Overexpression of the forebrain-specific homeobox gene six3 induces rostral forebrain enlargement in zebrafish. Development. 1998;125:2973–2982. doi: 10.1242/dev.125.15.2973. [DOI] [PubMed] [Google Scholar]
- 15.Mathers PH, Grinberg A, Mahon KA, Jamrich M. The Rx homeobox gene is essential for vertebrate eye development. Nature. 1997;387:603–607. doi: 10.1038/42475. [DOI] [PubMed] [Google Scholar]
- 16.Hashimoto H, et al. Zebrafish Dkk1 functions in forebrain specification and axial mesendoderm formation. Dev. Biol. 2000;217:138–152. doi: 10.1006/dbio.1999.9537. [DOI] [PubMed] [Google Scholar]
- 17.Gates MA, et al. A genetic linkage map for zebrafish: comparative analysis and localization of genes and expressed sequences. Genome Res. 1999;9:334–347. [PubMed] [Google Scholar]
- 18.Mullins MC, Hammerschmidt M, Haffter P, Nusslein-Volhard C. Large-scale mutagenesis in the zebrafish: in search of genes controlling development in a vertebrate. Curr. Biol. 1994;4:189–202. doi: 10.1016/s0960-9822(00)00048-8. [DOI] [PubMed] [Google Scholar]
- 19.van de Wetering M, et al. The human T cell transcription factor-1 gene. Structure, localization, and promoter characterization. J. Biol. Chem. 1992;267:8530–8536. [PubMed] [Google Scholar]
- 20.Lin R, Thompson S, Priess JR. pop-1 encodes an HMG box protein required for the specification of a mesoderm precursor in early C. elegans embryos. Cell. 1995;83:599–609. doi: 10.1016/0092-8674(95)90100-0. [DOI] [PubMed] [Google Scholar]
- 21.van deWetering M, et al. Armadillo coactivates transcription driven by the product of the Drosophila segment polarity gene dTCF. Cell. 1997;88:789–799. doi: 10.1016/s0092-8674(00)81925-x. [DOI] [PubMed] [Google Scholar]
- 22.Cavallo RA, et al. Drosophila Tcf and Groucho interact to repress Wingless signaling activity. Nature. 1998;395:604–608. doi: 10.1038/26982. [DOI] [PubMed] [Google Scholar]
- 23.Roose J, et al. The Xenopus Wnt effector XTcf-3 interacts with Groucho-related transcriptional repressors. Nature. 1998;395:608–612. doi: 10.1038/26989. [DOI] [PubMed] [Google Scholar]
- 24.Brannon M, Brown JD, Bates R, Kimelman D, Moon RT. XCtBP is a XTcf-3 co-repressor with roles throughout Xenopus development. Development. 1999;126:3159–3170. doi: 10.1242/dev.126.14.3159. [DOI] [PubMed] [Google Scholar]
- 25.Clevers H, van deWetering M. TCF/LEF factor earn their wings. Trends Genet. 1997;13:485–489. doi: 10.1016/s0168-9525(97)01305-x. [DOI] [PubMed] [Google Scholar]
- 26.Moon RT, Brown JD, Torres M. WNTs modulate cell fate and behavior during vertebrate development. Trends Genet. 1997;13:157–162. doi: 10.1016/s0168-9525(97)01093-7. [DOI] [PubMed] [Google Scholar]
- 27.McGrew LL, Takemaru K, Bates R, Moon RT. Direct regulation of the Xenopus engrailed-2 promoter by the Wnt signaling pathway, and a molecular screen for Wnt-responsive genes, confirm a role for Wnt signaling during neural patterning in Xenopus. Mech. Dev. 1999;87:21–32. doi: 10.1016/s0925-4773(99)00136-7. [DOI] [PubMed] [Google Scholar]
- 28.Badiani P, Corbella P, Kioussis D, Marvel J, Weston K. Dominant interfering alleles define a role for c-Myb in T-cell development. Genes Dev. 1994;8:770–782. doi: 10.1101/gad.8.7.770. [DOI] [PubMed] [Google Scholar]
- 29.Triezenberg SJ, Kingsbury RC, McKnight SL. Functional dissection of VP16, the transactivator of herpes simplex virus immediate early gene expression. Genes Dev. 1988;2:718–729. doi: 10.1101/gad.2.6.718. [DOI] [PubMed] [Google Scholar]