SUMMARY
Although several studies have examined the association between phase I/II enzyme polymorphisms and esophageal adenocarcinoma (EAC) and/or Barrett’s esophagus (BE), their overall findings remain unclear. We performed a systematic review and meta-analysis to determine whether phase I/II polymorphisms are independent risk factors for either BE or EAC. We employed keyword searches in multiple databases to identify studies published before October 1, 2007. Single-nucleotide polymorphisms (SNPs) examined in ≥3 studies were meta-analyzed to obtain a pooled estimate of effect. Meta-analysis suggested the minor allele for GSTP1 Val105 conveys modest excess risk (odds ratio [OR]BE = 1.50, 95% confidence interval [CI] 1.16–1.95; OREAC = 1.20, 95% CI 0.94–1.54). No excess risk was observed with GSTM1 null (ORBE = 0.77, 95% CI: 0.56–1.08; OREAC = 1.08, 95% CI: 0.79–1.48), GSTT1 null (ORBE = 1.35, 95% CI: 0.91–2.01; OREAC = 0.84, 95% CI: 0.48–1.49), or CYP1A Val462 (OREAC = 0.89, 95% CI: 0.40–1.97). Insufficient data existed to meta-analyze remaining SNPs. Our review identified GSTP1Ile105Val as a possible risk factor for BE and EAC in Caucasian males. No excess risk was observed for other phase I/II polymorphisms with sufficient data to meta-analyze. Additional studies are needed to determine if GSTP1 conveys excess risk in females or non-Caucasians and to evaluate other phase I/II polymorphisms.
Keywords: Barrett’s esophagus, epidemiology, esophageal adenocarcinoma, genetic association, GST, single-nucleotide polymorphism (SNP)
INTRODUCTION
The incidence of esophageal adenocarcinoma (EAC) has been dramatically increasing over the last 30 years in several regions in North America, Europe, and Oceania.1 While incidence of EAC is increasing in both genders and all ethnic groups, it is most pronounced in Caucasian males. Specifically, EAC incidence has increased 350% since the 1970s in Caucasian men in the USA, which currently results in approximately 10 000 deaths per year.2 Although increased screening practices, improved diagnostic techniques, and changes in classification likely explain some of the observed increase, the ongoing annual increases of approximately 10% suggest a real increase in the incidence of EAC.1,3
It is believed that EAC arises from Barrett’s esophagus (BE), an abnormal growth of columnar cells from normal esophageal interstitial tissue. BE has been reported to as much as 6–12% in patients screened for reflux disease, and in 10–25% asymptomatic patients undergoing endoscopy for other reasons.4,5 Patients with BE have a 50- to 100-fold increased risk of developing EAC,6 although the annual incidence of cancer in BE cases is though to be between 0.4% and 0.5%. Main risk factors for BE and EAC include chronic reflux symptoms, high body mass index (BMI), and possibly tobacco smoking, alcohol use, and low dietary intake of fruit and vegetables. Together, these risk factors suggest a strong environmental component in the development of BE and EAC.
However, due to the dramatic rise in EAC in men of a single racial group even across multiple continents and cultures, there is also strong implication that there are genetic determinants involved in disease etiology and progression, and, more specifically, that the likely genetic pathways may be associated with metabolism of environmental exposures.
The primary metabolic defense against tissue damage from xenobiotic chemicals like tobacco and alcohol is mediated through phase I and II enzymes. Phase I enzymes, including cytochrome P450 (CYP) and microsomal epoxide hydrolases (mEH), are found throughout the body, but are primarily expressed in the gastrointestinal tract, liver, kidneys, and lungs. The phase II conjugating enzymes, including quinine oxidoreductase (NQO), N-acetyltransferases (NAT), and gluthathione S-transferases (GST), are found ubiquitously and mediate reactions that can take place simultaneously or sequentially to the phase I reactions.
Several genetic association studies have evaluated the distribution of phase I and II metabolic genes in EAC or BE cases. However, it is difficult to obtain a collective view of these studies due to the lack of critical systematic reviews dedicated to this topic. Current reviews have summarized genetic associations with esophageal cancer irrespective of subtype (i.e., adeno vs. squamous).7–9 However, given the considerable differences in the distribution, temporal trends, and risk factors of these two types of esophageal cancer, it is important to focus the review specifically on EAC and its precursor, BE.
We therefore conducted a systematic review to identify studies examining phase I and phase II genetic polymorphisms as risk factors for BE or EAC. When sufficient comparable studies existed, we also performed a meta-analysis to obtain a precise estimate of overall effect and to identify potential sources of between-study heterogeneity.
METHODS
Search strategy
We followed recommended guidelines for performance and reporting of meta-analyses.10 Two investigators conducted independent searches of PubMed, Ovid, EBSCO, Web of Science, OMIM, Dissertation Abstracts Internet, and conference abstracts for the American Society for Clinical Oncology.
To decrease the potential impact of publication bias, we did not restrict the search by language, population, or sample size. We identified all studies published prior to October 2007 using key words searches with combinations of the following terms: ‘esophageal adenocarcinoma,’ ‘Barrett’s esophagus,’ ‘glutathionine S-transferase,’ ‘GSTT1,’ ‘GSTP1,’ ‘GSTM1,’ ‘cytochrome P450,’ ‘microsomal epoxide hydrolase,’ ‘oxidoreductase,’ and ‘polymorphism.’ We also reviewed bibliographies of all identified articles, as well as relevant review articles to capture studies not identified by our keyword searches.
Eligibility criteria
We included only studies in which (i) cases were clearly defined as having either BE or EAC; and (ii) there was a comparable EAC- or BE-free control group. Studies that also included cases with squamous cell carcinoma or gastric cardia were included only if the sample size and risk estimates for EAC were clearly separated from the other cancers. We excluded studies that examined somatic mutations or examined or used animal models or human cell lines.
Our searches identified 134 citations, of which 57 were reviewed in more detail. Only 11 original reports11–22 and two review papers8,9 met our eligibility criteria. Seven evaluated cases with EAC, three evaluated both BE and EAC cases, and one evaluated just BE cases in comparison with a disease-free control group. We excluded most publications because they used tumor or cell line DNA, 10 publications because they only reported combined findings for squamous cell carcinoma and EAC,23–32 and 1 because it included another cancer without separate reporting of EAC,33 and 1 publication lacked sufficient detailed data.34
Data abstraction
Data on study methods, including case and control identification, as well as all associated study findings, were abstracted and entered into a structured database. When insufficient data were reported, we used data from any earlier reports or contacted the corresponding author.
Analysis
We decided a priori to conduct a meta-analysis for any single-nucleotide polymorphism (SNP) evaluated in three or more comparable studies. To maintain independence among pooled studies, we included the most recent study when there was more than one publication based on the same dataset.
We evaluated between-study heterogeneity by using Higgins and Thompson’s I2, which is a scale-free measure of variability that calculates the proportion of total between-study variation resulting from heterogeneity as opposed to random error or chance.35 The decision to perform a random or fixed-effects meta-analysis was based on this heterogeneity test result and the number of studies available. If there was considerable between-study heterogeneity (defined as I2 = ≥50%), we employed a random effects analysis to calculate the pooled estimator.36 While if there was less than five studies available, we employed the Mantel–Haenszel method to compensate for the poor estimate of the χ2 distributions when the study number is small. We presented odds ratios (ORs) and 95% confidence intervals (CIs) for each study in a forest plot that included the pooled estimator. We used a dominant (AA vs. Aa + aa) for the meta-analysis of CYP1A and GSTP1 genes based on the information given in the studies and the probable biological model of the gene effect in the literature.37–40 For the GSTM1 and GSTT1 genes, we compared cases against controls for the contrast of the null vs. the nondeleted genotype. We assessed deviations from Hardy–Weinberg equilibrium (HWE) for control groups using a χ2 goodness-of-fit test. Publication bias was assessed by evaluation of funnel plots and Egger’s test. We also performed an analysis of influence to determine the importance of any one study on the pooled estimator. Finally, we investigated how the pooled estimator changed over time by performing a cumulative meta-analysis. All analyses were conducted with Stata, version 9.0 (Stata Corp, College Station, TX, USA).
RESULTS
All 12 original studies we identified were case-control studies, and most (n = 7) were conducted in mostly male, exclusively Caucasian populations from Europe or North America.11–14,18–22 The most common SNPs studied were in the glutathione S-transferase genes – GSTP1 in eight studies followed by GSTM1 and GSTT1 in six studies. Other SNPs examined were in the following genes: cytochrome P450 1A (CYP1A1) (n = 3 studies), microsomal epoxide hydrolase (EPHX1) (n = 2 studies), quinine oxidoreductase (NQO1) (n = 3 studies), and N-acetyltransferase I (NAT1) (n = 1 study). Characteristics of studies evaluating EAC and BE are presented in Tables 1 and 2, respectively.
Table 1.
Characteristics of eligible studies evaluating the effect of phase I and phase II metabolic enzyme polymorphisms on risk of esophageal adenocarcinoma
| Study (first author [year published], country, study time period, reference no.) |
Case ascertainment |
Sample size (case/control) |
Controls ascertainment | SNPs | Cases | Controls | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Age | Race (%) | Men (%) | Age | Race (%) | Men (%) | |||||
| van Lieshout (1999), Netherlands, unknown time, 4-year span20 |
Hospital based |
21/247 | Healthy, unmatched, source – unknown |
CYP1A1 (MspI), CYP1A1 (Ile462Val), GSTM1 (null), GSTP1 (Ile105Val), GSTT1(null) |
Mean = 64, SE 2 |
White (100) | Unknown (86) |
Mean = 52, SE 2 |
100% white | Unknown (40) |
| Casson (2003), Canada, 1992–199712 |
Hospital based |
45/45 | Healthy, population based, age and sex, matched |
CYP1A1 (MspI), GSTM1 (null), GSTP1 (Ile105Val), GSTT1(null), EPHX1 (exon 3), EPHX1 (exon 4) |
Unknown | Unknown | 38 (84) | Unknown | Unknown | 38 (84) |
| Casson (2006), Canada, 2001–200313 |
Hospital based |
56/95 | Healthy, clinic based, frequency matched but unknown on what |
GSTM1 (null, GSTT1 (null), GSTP1 (Ile105Val), EPHX1 (exon 3), EPHX1 (exon 4) |
<50 years = 4%, ≥50 years = 96% |
White (100) | 50 (89) | <50 years = 38%, ≥50 years = 62% |
White (100) | 64 (67) |
| Jain (2006), India, 2003–200615 |
Hospital based |
9/137 | Cancer free, clinic based, age and sex, matched |
GSTM1(null), GSTT1 (null), GSTP1 (Ile105Val) |
Mean = 56.5, SD 13.1 |
North Indian (100) |
Unknown (76) |
Mean = 55, SD 9.4 |
North Indian (100) |
Unknown (71) |
| Jain (2007), India, 2003–200616 |
Hospital based |
9/200 | Cancer free, clinic based, age and sex, matched |
GSTM3 (*B) | Mean = 58.5, SD 12.4 |
North Indian (Asian) |
Unknown (76) |
Mean = 55, SD 9.4 |
North Indian (Asian) |
Unknown (71) |
| Abbas (2004), France, unknown11 |
Clinic based |
27/130 | Cancer free, clinic based, age and sex, matched |
CYP1A1 Ile462Val, GSTM1(null), GSTP1 (Ile105Val), GSTT1 (null) |
Mean = 66, range (51–85) |
Unknown | 25 (93) | Mean = 56, range = 19–87 |
Unknown | 87 (67) |
| Sarbia (2003), Germany, 1983–200119 |
Hospital based |
61/252 | Healthy, population based, unmatched |
NQ01 (Pro609Ser) | Mean = 62, range (33–81) |
White (100) | 53 (86.9) | Mean = 39, range = 24–70 |
White (100) | 193, (76.6) |
| von Rahden (2005), Germany, 1991–200321 |
Hospital based |
140/260 | Cancer free, hospital based, unmatched |
NQ01(Pro609Ser) | Mean = 62.6, SD 10.8 |
White (100) | 128 (91.4) | Mean = 57.5, SD18 |
White (100) | 172, (66.2) |
| Wideroff (2007), USA, 1993–199522 |
Registry based |
67/209 | Healthy, population based, age and sex, matched |
CYP1A1 Ile462Val, GSTM1 (null), GSTP1 (Ile105Val), GSTT1 (null), NAT1 (*10, *11) |
Median = 63 | White (100) | Unknown | Median = 66 | White (100) | Unknown |
| Murphy (2007), Ireland, 2002–200418 |
Hospital based |
207/223 | Healthy, clinic bases, age and sex, matched |
GSTP1 (Ile105Val) | Mean = 64.4, range (35–85) |
White (100) | 172 (83) | Mean = 64.4, range (35–86) |
White (100) | 184 (83) |
| di Martino (2007), UK, 2000–200314 |
Hospital based |
144/94 | Symptomatic but disease free, hospital based, unmatched |
NQ01 (Pro609Ser) | Mean = 72, range (41–94) |
Unknown | 117 (81) | Mean = 56 (29–88) |
Unknown | 42 (45) |
SE, standard error; SD, standard deviation; SNPs, single-nucleotide polymorphisms.
Table 2.
Characteristics of eligible studies evaluating the effect of phase I and phase II metabolic enzyme polymorphisms on risk of Barrett’s esophagus
| Study (first author [year published], country, study time period, reference no.) |
Case ascertainment | Sample size (case/control) |
Controls ascertainment | SNPs | Cases | Controls | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Age | Race (%) | Men (%) | Age | Race (%) | Men (%) | |||||
| van Lieshout (1999), Netherlands, 4-year span20 |
Hospital based | 98/247 | Healthy, source–unknown, unmatched |
CYP1A1 (MspI) CYP1A1 (Ile462Val), GSTM1 (null), GSTP1 (Ile105Val), GSTT1 (null) |
Mean = 64, SD 2 | White (100) | (86) | Mean = 52, SD 2 |
White (100) | Unknown (40) |
| Casson (2006), Canada, 2001–200313 |
Hospital based | 125/95 | Healthy, clinic based, freq. match but unknown on what |
GSTM1(null), GSTP1 (Ile105Val), GSTT1 (null), EPHX1 (exon 3), EPHX1 (exon 4) |
<50years = 4%, ≥50years = 96% |
White (100) | 50 (89) | <50 years = 38% ≤50years = 62% |
White (100) | 64 (67) |
| Kala (2007), Czech Republic, Unknown17 |
Hospital based | 22/173 | Healthy, clinic based, age, sex and smoking, matched |
GSTM1 (null), GSTP1 (Ile105Val), GSTT1 (null) |
Mean = 46.4, SD 12.7 |
White (100) | 18 (82) | Mean = 44.7, SD 10.7 |
White (100) | 107 (62) |
| Murphy (2007), Ireland18 | Hospital based | 189/223 | Healthy, population based, | GSTP1 (Ile105Val) | Mean = 62.2 (24–85) | White (100) | 156 (83) | Mean = 64.4 (35–86) |
White (100) | 184 (83) |
| di Martino (2007), UK14 | Hospital based | 200/94 | Symptomatic but disease free, hospital based, unmatched |
NQ01 (Pro609Ser) | Mean = 65 (25–97) | Unknown | 148 (741) | Mean = 56 (29–88) |
Unknown | 42 (45) |
SD, standard deviation; SNPs, single-nucleotide polymorphisms.
Phase I enzymes
The initial metabolic defense against xenobiotic chemicals such as tobacco or other potential carcinogens is mediated through conversion of hydrophobic compounds to reactive electrophiles by phase I enzymes.
CYP 1A
CYP1A enzymes metabolize aromatic hydrocarbons found in environmental pollutants like cigarette smoke. Although expressed in most extrahepatic tissues, expression is greatest in the lung and placenta.41
Gene
The CYP1A1 gene is found on chromosome 15 in the q22–q24 region and encompasses seven exons and six introns that span 5810 bp. Although several polymorphisms have been identified in the CYP1A1 gene, the two main polymorphisms both result in increased enzymatic expression, leading individuals to be classified as extensive metabolizers. CYP1A1 Ile462Val involves a A → G substitution in exon 7 and leads to a twofold increase in enzymatic expression.33 CYP1A1 MspI is in strict linkage disequilibrium with CYP1A1 Ile462Val and involves a T → C (T6235C) substitution in the 3′ flanking region.42–44 Both polymorphisms are relatively infrequent in Caucasian populations. The frequency of the rare homozygous genotype for CYP1A1 Ile462Val (Val462/Val462) is 0.6% in Caucasians and 4.9% in Asians,45 while the frequency of the rare homozygous genotype for CYP1A1 MspI (C6235/C6235) is 1.2% in Caucasians and 14% in Asians.45
Studies
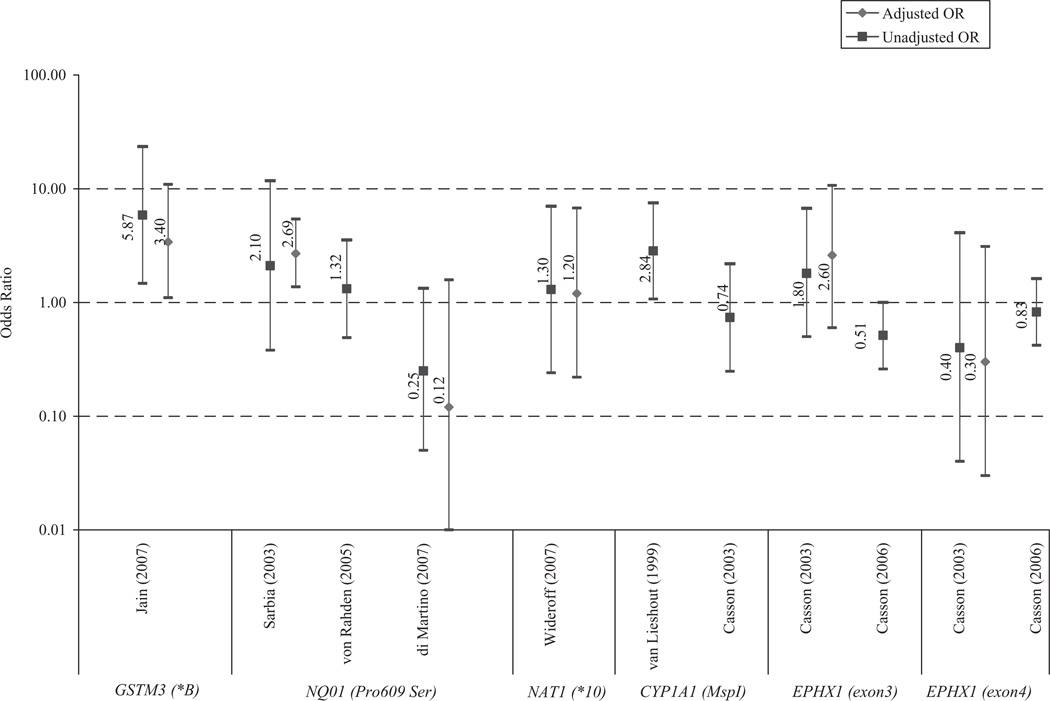
We identified three studies that examined CYP1A1 Ile462Val and two that examined CYP1A1 MspI as risk factors for EAC11,12,20,22 (see Fig. 1). One study also examined these polymorphisms as risk factors for BE20 (see Fig. 2). Findings were highly variable for both polymorphisms and across both conditions, and only one study reported a significant association.20
Fig. 1.
Odds ratios (ORs) and 95% confidence intervals reported by studies evaluating GSTM3, CYP1, EPHX1, NQ01, and NAT1 single-nucleotide polymorphisms as risk factors for esophageal adenocarcinoma. Jain adjusted for age, sex, smoking status, and alcohol use. Sarbia adjusted for age. di Martino adjusted for age, sex, reflux, body mass index, and smoking status. Wideroff adjusted for age, sex, and study center.
Fig. 2.
Odds ratios (ORs) and 95% confidence intervals reported by studies evaluating CYP1, mEH, NQ01, NAT1 and GST single-nucleotide polymorphisms as risk factors for Barrett’s esophagus. di Martino adjusted for age, sex, reflux, body mass index, and smoking status.
Meta-analysis
Sufficient comparable studies existed to perform a meta-analysis examining CYP1A1 Ile462Val in 103 EAC cases and 503 controls pooled from three studies.11,20,22 The individual study ORs ranged from 0.65 to 1.60. We independently confirmed that controls in HWE in all studies. There was no detectable heterogeneity (I2 = 0%), so we adopted a fixed-effects model. We assumed a dominant model of inheritance in the meta-analysis because there were insufficient reported cases of minor allele homozygote (Val462/Val462) in the three studies. The minor allele for CYP1A1 was not a significant risk factor for EAC (OR = 0.89, 95% CI 0.40–1.97) (Fig. 3). Our analysis of influence showed that removal of any of the studies affected the pooled estimator, as was expected by the small number of studies available for the analysis. Finally, our cumulative meta-analysis by year of publication showed no trend in risk over time (data not shown).
Fig. 3.
Forest plot of fixed-effect pooled odds ratio and corresponding 95% confidence intervals (CIs) from studies evaluating associations between CYP1A1 Ile462Val single-nucleotide polymorphisms and esophageal adenocarcinoma. Box size is proportional to the meta-analysis study weight. Dominant model used, combining heterozygote genotype with homozygote minor allele genotype.
mEH
mEH) encoded by the EPHX1 gene, hydrolyzes polycyclic aromatic hydrocarbons found in cigarette smoke, but may also subsequently produce highly toxic and mutagenic transdihydrodiols by-products. The EPHX1 gene expression is greatest in the kidney, liver, lung, and epithelial cells.46–48
Gene
The EPHX1 gene is found on chromosome 1 at position q42.1 and encompasses nine exons and eight introns that span 35.48 kb. Two SNPs known to affect enzymatic activity are EPHX1 Tyr113His (EPHX1 exon3) and EPHX1 His139Arg (EPHX1 exon4). While EPHX1 exon3 conveys a 40–50% reduction in enzymatic activity, EPHX1 exon4 conveys a 25% increase in enzymatic activity.49 Both SNPs have been associated with oropharyngeal carcinoma and esophageal squamous cell carcinoma.50,51 EPHX1 polymorphisms have also been associated with lung,52 colorectal,53 and liver cancer.54 The reported minor allele frequency (MAF) for EPHX1 exon3 is 34% in Caucasians and 51% in Asians. The reported MAFs for EPHX1 exon4 are lower – 19% in Caucasians and 14% in Asians.55
Studies
We identified two studies examining these SNPs12,13 (see Fig. 1). Casson (2006)13 included cases with BE as well as EAC, while Casson (2003)12 only included cases with EAC. While carriage of the minor allele for EPHX1 exon3 was associated with moderate increased risk of EAC, carriage of the minor allele for EPHX1 exon4 was associated with moderate decreased risk. However, neither association was significant.
Phase II enzymes
Phase II enzymes increase the hydrophilicity of potentially toxic compounds so they can be excreted in urine or bile. Phase II enzymes mediate reactions that can take place simultaneously or sequentially to the phase I reactions.
NAD(P)H: quinone oxidoreductase 1 (NQO1)
NQO1 detoxifies quinones derived from the oxidation of benzene. It also protects cells from free radicals and reactive oxygen species. It is expressed in all human tissues56 in response to oxidative stress and has been shown to be overexpressed in breast,57 pancreatic,58 colorectal,59 and esophageal squamous cell cancers.60
Gene
The NQO1 gene is located on chromosome 16 in the q22.1 region, spanning 35 kb and consisting of six exons and five introns. A −C609T substitution leads to a proline to a serine amino acid substitution. Rare homozygotes for NQO1C609T (Ser−609/Ser−609) have only 2–4% of the enzymatic activity observed in the wild type.61 The frequency for the minor allele among Caucasians ranges from 12 to 23 %, while the frequency among Asians is more than twice as great and ranges from 35 to 61%.62,63
Studies
We identified three case-control studies that examined NQO1 Ser−609 in EAC14,19,21 (Fig. 1), and one that also examined it in BE14 (Fig. 3). Although two of the EAC studies appear to be performed with the same case population,19,21 the von Rahden et al. study expanded upon the original study of Sarbia et al., including doubling in size the original case group. Given the correlation in these findings due to the partially shared case group, we had an insufficient number of comparable studies to perform a meta-analysis. The di Martino study was the only study to evaluate NQO1 Ser−609 as a risk factor for BE. Similar to their results for EAC, a strong decreased risk of BE was observed with NQO1 Ser−609 minor allele (OR = 0.22, 95% CI 0.07–0.76, P = 0.01).
NAT1
Arylamine NATs catalyze the detoxification of xenobiotics by N-acetylation. Slow acetylation has been associated with bladder cancer, while fast acetylation has been associated with colon cancer.64 NAT1 variants have also been associated with lung and breast cancer.65
Gene
The NAT1 locus is found on chromosome 8p21.3-2366 and spans a 870 bp open reading frame.67 Combinations of nucleotide insertions/deletions and substitutions result in more than 24 different allelic variants in humans. NAT1*3 (C1095A), NAT1*4 (wild-type referent), NAT1*10 (T1088A, C1095A), and NAT1*11 (Val149Ile, Ser214Ala) are the most commonly examined polymorphisms in the literature. Because of the variant polyadenylation signal, NAT1*10 is associated with increased N-acetyltransferase activity in the colon, liver, and bladder.68,69 The frequency of NAT1 wild-type referent (NAT1*4) is reported to range from 44 to 62% in Caucasians and 38% in Asians, while the frequency for the NAT1*10 is approximately 16–20% in Caucasians68,70 and 30–53% in Asians.71,72
Studies
We identified only one study that examined NAT1 variants, which only examined cases with EAC (Fig. 1).22 There was no significant association between the NAT1*10 alleles and EAC.
GST
The most frequently studied SNPs in EAC research involve genes of the GST family. GSTs are expressed ubiquitously but are especially prevalent in the liver, lung, and upper gastrointestinal tract. There are six gene families identified for GST, among them theta (GSTT1), mu (GSTM1 and GSTM3), and pi (GSTP1).73,74 GSTs have broad and overlapping substrates and have been associated with bladder, head and neck, and lung cancer.75,76
Gene
The GSTP1 gene is found on chromosome 11 at the p13 region. There are two variants of the GSTP1 gene, Ile105Val and Ala114 Val, and both produce decreased levels of enzymatic activity.37,77,78 Rare homozygotes for GSTP1Ile105Val (Ile105/Ile105) increased IgE and resulted in an immune-mediated inflammatory response, a potentially important process in EAC pathogenesis.79
The GSTT1 gene is found on chromosome 22 in the q11.2 region, while the GSTM1 and GSTM3 genes are found on chromosome 1 in the p13.3 region. GSTT1 and GSTM1 both have homozygous deletion polymorphisms (GSTT1 null and GSTM1 null) that result in loss of enzymatic activity. GSTM3, which is in tight disequilibrium with GSTM1, has a 3 bp deletion mutation on intron 6 that similarly results in a loss of function. GSTM3*A is recognized as the wild type, and GSTM3*B is the less common deletion polymorphism.
The minor allele frequency (MAF) for GSTP1Ile105Val is 31% in Caucasians and 17% in Asians. Frequency of the GSTM1 null genotype ranges from 42% to 57% in Caucasians and from 42% to 54% in Asians,45,80 while the frequency of the GSTT1 null genotype ranges from 13 to 26% in Caucasians and from 35 to 53% in Asians. GSTM3*B, the minor allele for GSTM3, is rare and occurs in only 16% of Caucasians.50,81
Studies
Our review identified seven studies that evaluated the association between EAC and GST polymorphisms, including seven that evaluated GSTP1Ile105Val,11–13,15,18,20,22 six that also evaluated GSTM1 and GSTT1 null,11–13,15,20,22 and one that also examined GSTM3 (Table 1).16 Controls were tested and were within HWE in all of the studies with the exception of Abbas et al.11 and Casson et al. (2003),12 which reported HWE among the controls in the manuscript text.
Sufficient comparable studies (n ≥ 3) existed to perform meta-analyses examining the association between GSTP1, GSTM1, and GSTT1 in BE and EAC, the results of which are described in the meta-analysis section below. The single study16 that evaluated GSTM3 found a strong and significant excess risk of EAC with the minor allele (GSTM3*B) (OR = 2.2; 95% CI 1.4–3.3) (Fig. 1).
Meta-analysis
GSTP1
EAC
There were a total of 432 genotyped EAC cases and 1086 controls pooled from seven studies.11–13,15,18,20,22 The individual study ORs ranged from 0.89 to 4.62 (Fig. 4). We used a dominant model in the meta-analysis combining Ile105/Val105 and Val105/Val105 genotypes. The pooled estimator suggested that carriage of the minor or ‘high-risk’ allele for GSTP1 results in modest excess risk of EAC (OREAC = 1.20, 95% CI 0.94–1.54). While Egger’s test indicated a potential small study or publication bias (P = 0.06), our evaluation of the funnel plot (Appendix I) suggested that much of this apparent effect may be attributable to a single study.20 Further, our analysis of influence indicated that the pooled estimator was robust and not substantially affected by removal of the smallest (Casson 2003)12 or largest (Murphy 2007)18 studies (data not shown). Finally, our cumulative meta-analysis by year of publication suggested a potential diminution in risk over time. However, this trend was not monotonic (data not shown).
Fig. 4.
Forest plot of fixed-effect pooled odds ratio and corresponding 95% confidence intervals (CIs) from studies evaluating associations between GSTP1Ile105Val single-nucleotide polymorphism and esophageal adenocarcinoma. Box size is proportional to the meta-analysis study weight. Dominant model used, combining heterozygote genotype with homozygote minor allele genotype.
BE
A total of 434 cases and 738 controls were available to pool from four studies.13,17,18,20 ORs of the individual studies varied from 0.84 to 3.86, the majority of which indicated excessive risk for EAC. The pooled estimator suggested carriage of the minor allele for GSTP1 (i.e. Ile105/Val105 and Val105/Val105) results in moderate excess risk of BE (ORBE = 1.50, 95% CI 1.16–1.95) (Fig. 5). We did not detect any evidence of publication bias (P = 0.54) (Appendix II), and our cumulative meta-analysis by year of publication did not suggest a trend in the effect reporting over time. However, our analysis of influence indicated that the pooled estimator was vulnerable to removal of the largest study (Murphy 2007),18 which is not unexpected with only four studies included in the analysis (data not shown).
Fig. 5.
Forest plot of fixed-effect pooled odds ratio and corresponding 95% confidence intervals (CIs) from studies evaluating associations between GSTP1Ile105Val SNP and Barrett’s esophagus. Box size is proportional to the meta-analysis study weight. Dominant model used, combining heterozygote genotype with homozygote minor allele genotype.
GSTM1 null
EAC
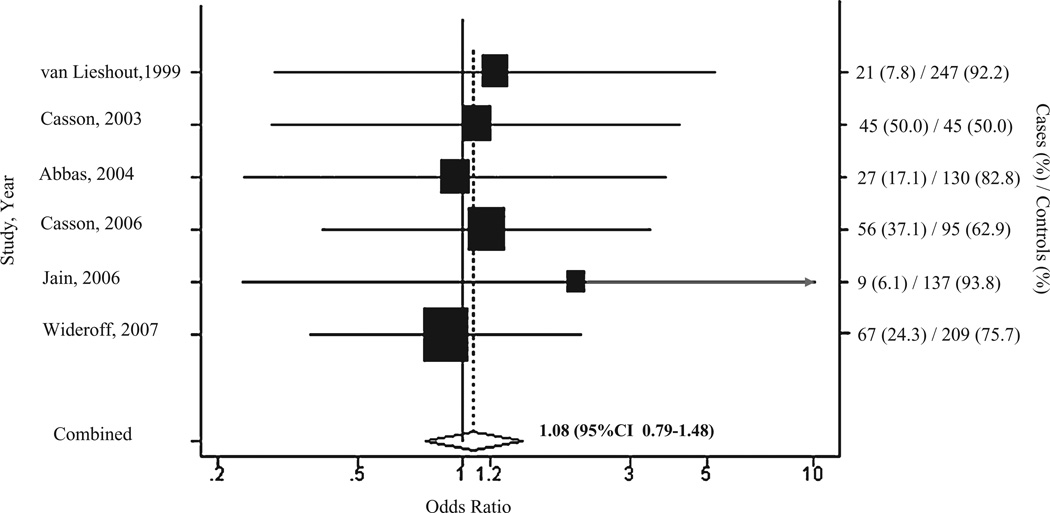
There were a total of 225 genotyped EAC cases and 863 controls pooled from six studies.11–13,15,20,22 The individual study ORs ranged from 0.90 to 2.10. Given between-study heterogeneity <30%, we employed a fixed-effects model. The pooled OR suggested neither strong nor significant risk of EAC with GSTM1 null genotype (OR = 1.08, 95% CI 0.79–1.48) (Fig. 6). While Egger’s test (P = 0.04) was significant, our evaluation of the funnel plot (Appendix III) indicated little evidence of small study or publication bias, suggesting the one outlier study15 may be influencing the result. Additionally, our analysis of influence again indicated that the pooled estimator was robust to removal of the smallest (Casson 2003)12 or largest (Widerff 2007)22 study. Finally, our cumulative meta-analysis by year of publication did not show any trend in the data over time (data not shown).
Fig. 6.
Forest plot of fixed-effect pooled odds ratio and corresponding 95% confidence intervals (CIs) from studies evaluating associations between GSTM1null variant and esophageal adenocarcinoma. Box size is proportional to the meta-analysis study weight.
BE
Three studies also examined the GSTM1 null variants in BE cases, which included 245 BE cases compared with 515 controls.13,17,20 All three studies showed that GSTM1 null variant was protective for BE, although not significantly so, and individual ORs ranged between 0.51 and 0.97. The pooled OR indicated the effect was also moderately protective (OR = 0.77 95% CI 0.56–1.08) (Fig. 7). We did not detect any evidence of publication bias (P = 0.6) (Appendix IV), and our cumulative meta-analysis by year of publication did not suggest a trend in the effect reporting over time. However, because there were only three studies included in the analysis of influence, we found that the pooled estimator was vulnerable to removal of the largest study (van Lieshout 1999)20 (data not shown).
Fig. 7.
Forest plot of fixed-effect pooled odds ratio and corresponding 95% confidence intervals (CIs) from studies evaluating associations between GSTM1null variant and Barrett’s esophagus. Box size is proportional to the meta-analysis study weight.
GSTT1 null
EAC
There was a total of 225 genotyped EAC cases and 863 controls from six studies.11–13,15,20,22 The individual study ORs ranged from 0.59 to 1.78. Given between-study heterogeneity (I2 = 38%), we employed a fixed-effects model. The pooled estimator suggested that the GSTT1 null genotype conveys neither strong nor significant risk of EAC (OR = 0.84, 95% CI 0.48–1.49) (Fig. 8). Both Egger’s test (P = 0.26) and our evaluation of the funnel plot (Appendix V) indicated that there was no substantial small study or publication bias. Our analysis of influence again suggested that the pooled estimator was robust to removal of the smallest13 or largest22 study, and our cumulative meta-analysis by year of publication did not show any trend in the data over time.
Fig. 8.
Forest plot of fixed-effect pooled odds ratio and corresponding 95% confidence intervals (CIs) from studies evaluating associations between GSTT1null variant and esophageal adenocarcinoma. Box size is proportional to the meta-analysis study weight.
BE
All three studies with BE cases in the GSTM1 null meta-analysis were included in the GSTT1 null meta-analysis, so a total of 245 cases and 515 controls were pooled.13,17,20 The individual study ORs ranged from 1.31 to 1.50, and they all trended toward excessive risk for BE (Fig. 9). The GSTT1 null genotype conveys some excess risk of BE (OR = 1.35, 95% CI 0.91–2.01). Although there were only three studies included in the analysis, we found no evidence of publication bias either from the funnel plot or by Egger’s test statistic (P = 0.2) (Appendix VI), and our cumulative meta-analysis showed no trend in the reported study affect over time. Our analysis of influence again suggested that the pooled estimator was significantly affected by the removal of any of the studies.
Fig. 9.
Forest plot of fixed-effect pooled odds ratio and corresponding 95% confidence intervals (CIs) from studies evaluating associations between GSTT1null variant and Barrett’s esophagus. Box size is proportional to the meta-analysis study weight.
DISCUSSION
This is the first meta-analysis and systematic review to examine phase I and II metabolic enzymes in EAC and its precursor condition BE, evaluating a total of 786 cases with EAC and 509 BE cases in 12 studies. Four phase I and six phase II genes were examined. Eight SNPs were examined in BE cases and ten in EAC cases. While other review studies have examined genetic variants in esophageal squamous cell carcinoma or in unspecified esophageal cancer, little work has focused in EAC or BE.
The most frequently examined SNPs were phase II GST genes. We found that carriage of GSTP1 Val105 allele was associated with a modest increase in the risk of having BE (ORBE = 1.50, 95% CI 1.16–1.95), and was also a suggestive factor in the risk of EAC (OREAC = 1.20, 95% CI 0.94–1.54). The difference in magnitude between the effect sizes found in the BE and EAC suggests GST-pi may play a greater role in the initial etiology of the precursor condition than in the transition of cells to cancer. However, increased levels of GST-pi expression have been found in malignant esophagus and gastric biopsies,82 indicating strong biological plausibility for its association with the development of EAC, and, therefore, survival bias in EAC studies is a possible explanation of the nonsignificant findings. GST-pi is the main GST enzyme expressed in the esophagus, and the GSTP1 Val105 has been associated with increase risk of reflux esophagitis83 and esophageal squamous cell carcinoma.84,85 While the mechanism by which an amino acid change from isoleucine to valine in the GSTP1 gene affects EAC or BE susceptibility is unknown, the resulting conformational change may affect the enzyme-binding site, thus changing substrate stability.86 Pandya et al. showed that the amino acid switch in the hetero and homozygotes reduce the enzymes catalytic activity more than 85% toward some alkylating agents.87 This decreased catalytic activity could result in increased risk of cancer. The second most frequently examined SNPs were the deletion polymorphisms GSTM1 null and GSTT1 null. We found that neither variant showed a strong or significant effect for EAC(ORGSTM1 null = 1.08,95%CI 0.79–1.48;ORGSTT1 null = 0.84, 95% CI 0.48–1.49) or BE (ORGSTM1 null = 0.77 95% CI 0.56–1.08; ORGSTT1 null = 1.35, 95% CI 0.91– 2.01). TheGSTM3*B SNP was also evaluated as a risk allele in EAC cases. GSTM3 gene is in tight disequilibrium with the GSTM1, and the GSTM3*B variant has been suggested to modify the GSTM1 enzyme expression, thereby effecting susceptibility to some cancers like basal cell carcinoma.88 Jain et al. found strong significant excess risk with the GSTM3*B allele in one study that evaluated EAC cases in Northern India (OR = 2.2; 95% CI 1.4–3.3). However, further evaluation examining the GSTM1 GSTM3 haplotypes is needed to determine importance of this variant.
Sufficient studies also existed to meta-analyze one of the gene variants in the CYP1A in EAC cases. We found that the CYP1A1 Val462 allele effect was neither strong nor significant for EAC (OR = 0.89, 95% CI 0.40–1.97) or BE. However, several systematic reviews found the CYP1A1 Ile462/Val462 to be a significant risk factor for the development of cancer. Hiyama et al. suggested a possible relationship between CYP1A1 Ile462/Val462 and esophageal squamous cell carcinoma, citing that seven studies showed an OR > 1.0.8 Similarly, Yang et al. also found that patients with CYP1A1Ile462/Val462 substitution were at increased risk for unspecified esophageal cancer (OR = 2.52 for Ile462/Val462 heterozygotes).9 This is again important because it emphasizes the need to differentiate between the cancer cell types.
Other phase I and phase II enzymes investigated in the literature, CYP1A1 MspI, EPHX1 His113Tyr (EH3) or EPHX1 Arg139His (EH4), and NAT1*10, NAT1*11 alleles, were not available in sufficient numbers to meta-analyze, being only identified in one or two studies. Results were generally nonsignificant and variable both within and across conditions, thereby making interpretation of the results more difficult. For example, we found the studies that examined the NQO1C609T SNPs had highly disparate results. Where one study found the minor Ser−609 allele to increase risk for EAC,19 another later study found the allele had no effect,21 and a third study found that the effect of the minor allele was protective.14 These differing results may be a result of the study limitations or a result of the Proteus phenomenon, coined by Ioannidis,89 where early studies produce extreme contradictory results. Further validation research is needed to parse out whether the discrepancy is due to chance, analytical manipulation, or other study restrictions.
Meta-analyses and reviews are affected by the quality, availability, and comparability of studies. The studies included in our review were highly comparable because they were all case-control study designs and largely included the same target population. Therefore, population admixture was not an important factor in these studies.90 The quality of the studies can in part be measured by calculating whether the alleles are within HWE. We independently confirmed HWE for each SNP, with the exception of controls in the Abbas11 and Casson (2003)12 studies, which did not provide enough data to confirm equilibrium compliance; however, HWE was reported in the manuscript. The low number and small sample size of available studies investigating either EAC or BE has affected the precision of the risk estimates even when meta-analysis was possible. Sample sizes within our review varied, with 400 enrolled in the largest study, and 90 enrolled in the smallest study. However, the sample size needed to adequately detect an allelic OR of less than 1.5 in a common variant is over a thousand.91,92 Future validation research might select cases with early onset of disease or cases with family history to select those with a strong genetic predisposition.
Our meta-analysis identified GSTP1 Val105 as a possible determinant of increased BE risk and may also effect the risk of EAC. The decreased level of enzymatic activity resulting from the Val105 may predispose individuals to BE; however, it is still unclear if the decreased activity also affects disease progression to EAC. Larger genetic association studies for BE and for EAC separate from esophageal squamous cell carcinoma are necessary to further examine possible genetic determinants. Future research could also involve more diverse populations, such as women and different racial group, and include more or newly identified variants in the phase I and phase II metabolic pathways.
Acknowledgments
The study is supported in part by a grant from the Helis Foundation.
APPENDIX I
Begg’s funnel plot of log odds ratios and pseudo 95% confidence intervals for studies evaluating associations between GSTP1Ile105Val single-nucleotide polymorphism and esophageal adenocarcinoma. Circle size is proportional to the study weight. Dominant model used, combining heterozygote genotype with homozygote minor allele genotype.
APPENDIX II
Begg’s funnel plot of unadjusted odds ratios and corresponding 95% confidence intervals for studies evaluating associations between GSTP1Ile105Val SNP and Barrett’s esophagus. Circle size is proportional to the study weight. Dominant model used, combining heterozygote genotype with homozygote minor allele genotype.
APPENDIX III
Begg’s funnel plot of log odds ratios and pseudo 95% confidence intervals for studies evaluating associations between GSTM1null variant and esophageal adenocarcinoma. Circle size is proportional to the study weight.
APPENDIX IV
Begg’s funnel plot of log odds ratios and pseudo 95% confidence intervals for studies evaluating associations between GSTM1null variant and Barrett’s esophagus. Circle size is proportional to the study weight.
APPENDIX V
Begg’s funnel plot of log odds ratios and pseudo 95% confidence intervals for studies evaluating associations between GSTT1null variant and esophageal adenocarcinoma. Circle size is proportional to the study weight.
APPENDIX VI
Begg’s funnel plot of log odds ratios and pseudo 95% confidence intervals for studies evaluating associations between GSTT1null variant and Barrett’s esophagus. Circle size is proportional to the study weight.
References
- 1.Blot WJ, Devesa SS, Kneller RW, Fraumeni JF., Jr Rising incidence of adenocarcinoma of the esophagus and gastric cardia. JAMA. 1991;265(10):1287–1289. [PubMed] [Google Scholar]
- 2.Devesa SS, Blot WJ, Fraumeni JF., Jr Changing patterns in the incidence of esophageal and gastric carcinoma in the United States. Cancer. 1998;83(10):2049–2053. [PubMed] [Google Scholar]
- 3.Younes M, Henson DE, Ertan A, Miller CC. Incidence and survival trends of esophageal carcinoma in the United States: racial and gender differences by histological type. Scand J Gastroenterol. 2002;37(12):1359–1365. doi: 10.1080/003655202762671215. [DOI] [PubMed] [Google Scholar]
- 4.Gerson LB, Shetler K, Triadafilopoulos G. Prevalence of Barrett’s esophagus in asymptomatic individuals. Gastroenterology. 2002;123(2):461–467. doi: 10.1053/gast.2002.34748. [DOI] [PubMed] [Google Scholar]
- 5.Rex DK, Cummings OW, Shaw M, et al. Screening for Barrett’s esophagus in colonoscopy patients with and without heartburn. Gastroenterology. 2003;125(6):1670–1677. doi: 10.1053/j.gastro.2003.09.030. [DOI] [PubMed] [Google Scholar]
- 6.Streitz JM., Jr Barrett’s esophagus and esophageal cancer. Chest Surg Clin N Am. 1994;4(2):227–240. [PubMed] [Google Scholar]
- 7.Vallbohmer D, Lenz HJ. Predictive and prognostic molecular markers in outcome of esophageal cancer. Dis Esophagus. 2006;19(6):425–432. doi: 10.1111/j.1442-2050.2006.00622.x. [DOI] [PubMed] [Google Scholar]
- 8.Hiyama T, Yoshihara M, Tanaka S, Chayama K. Genetic polymorphisms and esophageal cancer risk. Int J Cancer. 2007;121(8):1643–1658. doi: 10.1002/ijc.23044. [DOI] [PubMed] [Google Scholar]
- 9.Yang CX, Matsuo K, Wang ZM, Tajima K. Phase I/II enzyme gene polymorphisms and esophageal cancer risk: a meta-analysis of the literature. World J Gastroenterol. 2005;11(17):2531–2538. doi: 10.3748/wjg.v11.i17.2531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) Group. JAMA. 2000;283(15):2008–2012. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 11.Abbas A, Delvinquiere K, Lechevrel M, et al. GSTM1, GSTT1, GSTP1 and CYP1A1 genetic polymorphisms and susceptibility to esophageal cancer in a French population: different pattern of squamous cell carcinoma and adenocarcinoma. World J Gastroenterol. 2004;10(23):3389–3393. doi: 10.3748/wjg.v10.i23.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Casson AG, Zheng Z, Chiasson D, et al. Associations between genetic polymorphisms of Phase I and II metabolizing enzymes, p53 and susceptibility to esophageal adenocarcinoma. Cancer Detect Prev. 2003;27(2):139–146. doi: 10.1016/s0361-090x(03)00033-3. [DOI] [PubMed] [Google Scholar]
- 13.Casson AG, Zheng Z, Porter GA, Guernsey DL. Genetic polymorphisms of microsomal epoxide hydroxylase and glutathione S-transferases M1, T1 and P1, interactions with smoking, and risk for esophageal (Barrett) adenocarcinoma. Cancer Detect Prev. 2006;30(5):423–431. doi: 10.1016/j.cdp.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 14.di Martino E, Hardie LJ, Wild CP, et al. The NAD-(P)H:quinone oxidoreductase I C609T polymorphism modifies the risk of Barrett esophagus and esophageal adenocarcinoma. Genet Med. 2007;9(6):341–347. doi: 10.1097/gim.0b013e3180654ccd. [DOI] [PubMed] [Google Scholar]
- 15.Jain M, Kumar S, Rastogi N, et al. GSTT1, GSTM1 and GSTP1 genetic polymorphisms and interaction with tobacco, alcohol and occupational exposure in esophageal cancer patients from North India. Cancer Lett. 2006;242(1):60–67. doi: 10.1016/j.canlet.2005.10.034. [DOI] [PubMed] [Google Scholar]
- 16.Jain M, Kumar S, Lal P, Tiwari A, Ghoshal UC, Mittal B. Role of GSTM3 polymorphism in the risk of developing esophageal cancer. Cancer Epidemiol Biomarkers Prev. 2007;16(1):178–181. doi: 10.1158/1055-9965.EPI-06-0542. [DOI] [PubMed] [Google Scholar]
- 17.Kala Z, Dolina J, Marek F, Izakovicova HL. Polymorphisms of glutathione S-transferase M1, T1 and P1 in patients with reflux esophagitis and Barrett’s esophagus. J Hum Genet. 2007;52(6):527–534. doi: 10.1007/s10038-007-0148-z. [DOI] [PubMed] [Google Scholar]
- 18.Murphy SJ, Hughes AE, Patterson CC, et al. A population-based association study of SNPs of GSTP1, MnSOD, GPX2 and Barrett’s esophagus and esophageal adenocarcinoma. Carcinogenesis. 2007;28(6):1323–1328. doi: 10.1093/carcin/bgm007. [DOI] [PubMed] [Google Scholar]
- 19.Sarbia M, Bitzer M, Siegel D, et al. Association between NAD(P)H: quinone oxidoreductase 1 (NQ01) inactivating C609T polymorphism and adenocarcinoma of the upper gastrointestinal tract. Int J Cancer. 2003;107(3):381–386. doi: 10.1002/ijc.11430. [DOI] [PubMed] [Google Scholar]
- 20.van Lieshout EM, Roelofs HM, Dekker S, et al. Polymorphic expression of the glutathione S-transferase P1 gene and its susceptibility to Barrett’s esophagus and esophageal carcinoma. Cancer Res. 1999;59(3):586–589. [PubMed] [Google Scholar]
- 21.von Rahden BH, Stein HJ, Langer R, et al. C609T polymorphism of the NAD(P)H: quinone oxidoreductase I gene does not significantly affect susceptibility for esophageal adenocarcinoma. Int J Cancer. 2005;113(3):506–508. doi: 10.1002/ijc.20576. [DOI] [PubMed] [Google Scholar]
- 22.Wideroff L, Vaughan TL, Farin FM, et al. GST, NAT1, CYP1A1 polymorphisms and risk of esophageal and gastric adenocarcinomas. Cancer Detect Prev. 2007;31(3):233–236. doi: 10.1016/j.cdp.2007.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gao C, Takezaki T, Wu J, et al. Interaction between cytochrome P-450 2E1 polymorphisms and environmental factors with risk of esophageal and stomach cancers in Chinese. Cancer Epidemiol Biomarkers Prev. 2002;11(1):29–34. [PubMed] [Google Scholar]
- 24.Gao CM, Toshiro T, Wu JZ, et al. A case-control study on the polymorphisms of methylenetetrahydrofolate reductase 1298A–>C and susceptibility of esophageal cancer. Zhonghua Liu Xing Bing Xue Za Zhi. 2004;25(4):341–345. [PubMed] [Google Scholar]
- 25.Gao CM, Takezaki T, Wu JZ, et al. Polymorphisms in thymidylate synthase and methylenetetrahydrofolate reductase genes and the susceptibility to esophageal and stomach cancer with smoking. Asian Pac J Cancer Prev. 2004;5(2):133–138. [PubMed] [Google Scholar]
- 26.Huang ZG. Meta-analysis on glutathione S-transferase M1 polymorphisms and the risk of esophageal cancer. Zhonghua Liu Xing Bing Xue Za Zhi. 2004;25(10):898–901. [PubMed] [Google Scholar]
- 27.Itoga S, Nomura F, Makino Y, et al. Tandem repeat polymorphism of the CYP2E1 gene: an association study with esophageal cancer and lung cancer. Alcohol Clin Exp Res. 2002;26(Suppl 8):15S–19S. doi: 10.1097/01.ALC.0000026828.13868.B5. [DOI] [PubMed] [Google Scholar]
- 28.Li Y, Zhang JH, Guo W, Wang R, Wen DG, Wei LZ. Polymorphism of NAD(P)H dehydrogenase (quinone) 1 (NQO1) C 609 T and risk of esophageal neoplasm. Zhonghua Liu Xing Bing Xue Za Zhi. 2004;25(8):731. [PubMed] [Google Scholar]
- 29.Nimura Y, Yokoyama S, Fujimori M, et al. Genotyping of the CYP1A1 and GSTM1 genes in esophageal carcinoma patients with special reference to smoking. Cancer. 1997;80(5):852–857. doi: 10.1002/(sici)1097-0142(19970901)80:5<852::aid-cncr4>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 30.Lin DX, Tang YM, Peng Q, Lu SX, Ambrosone CB, Kadlubar FF. Susceptibility to esophageal cancer and genetic polymorphisms in glutathione S-transferases T1, P1, and M1 and cytochrome P450 2E1. Cancer Epidemiol Biomarkers Prev. 1998;7(11):1013–1018. [PubMed] [Google Scholar]
- 31.Wang AH, Sun CS, Li LS, Huang JY, Chen QS. Relationship of tobacco smoking CYP1A1 GSTM1 gene polymorphism and esophageal cancer in Xi’an. World J Gastroenterol. 2002;8(1):49–53. doi: 10.3748/wjg.v8.i1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gonzalez CA, Sala N, Capella G. Genetic susceptibility and gastric cancer risk. Int J Cancer. 2002;100(3):249–260. doi: 10.1002/ijc.10466. [DOI] [PubMed] [Google Scholar]
- 33.Bartsch H, Nair U, Risch A, Rojas M, Wikman H, Alexandrov K. Genetic polymorphism of CYP genes, alone or in combination, as a risk modifier of tobacco-related cancers. Cancer Epidemiol Biomarkers Prev. 2000;9(1):3–28. [PubMed] [Google Scholar]
- 34.Leistner-Segal S, Kaspary AP, Lopez P, Pilger DA, Segal F. TP53 gene R72P polymorphism analysis in patients with Barrett esophagus. Cancer Genet Cytogenet. 2006;170(1):76–77. doi: 10.1016/j.cancergencyto.2006.03.017. [DOI] [PubMed] [Google Scholar]
- 35.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 36.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 37.li-Osman F, Akande O, Antoun G, Mao JX, Buolamwini J. Molecular cloning, characterization, and expression in Escherichia coli of full-length cDNAs of three human glutathione S-transferase Pi gene variants. Evidence for differential catalytic activity of the encoded proteins. J Biol Chem. 1997;272(15):10004–10012. doi: 10.1074/jbc.272.15.10004. [DOI] [PubMed] [Google Scholar]
- 38.Ntais C, Polycarpou A, Ioannidis JP. Association of GSTM1, GSTT1, and GSTP1 gene polymorphisms with the risk of prostate cancer: a meta-analysis. Cancer Epidemiol Biomarkers Prev. 2005;14(1):176–181. [PubMed] [Google Scholar]
- 39.Sundberg K, Johansson AS, Stenberg G, et al. Differences in the catalytic efficiencies of allelic variants of glutathione transferase P1-1 towards carcinogenic diol epoxides of polycyclic aromatic hydrocarbons. Carcinogenesis. 1998;19(3):433–436. doi: 10.1093/carcin/19.3.433. [DOI] [PubMed] [Google Scholar]
- 40.Voho A, Impivaara O, Jarvisalo J, Metsola K, Vainio H, Hirvonen A. Distribution of glutathione S-transferase M1, P1 and T1 genotypes in different age-groups of Finns without diagnosed cancer. Cancer Detect Prev. 2006;30(2):144–151. doi: 10.1016/j.cdp.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 41.Smith CA, Smith G, Wolf CR. Genetic polymorphisms in xenobiotic metabolism. Eur J Cancer. 1994;30A(13):1921–1935. doi: 10.1016/0959-8049(94)00382-f. [DOI] [PubMed] [Google Scholar]
- 42.Cascorbi I, Brockmoller J, Roots I. A C4887A polymorphism in exon 7 of human CYP1A1: population frequency, mutation linkages, and impact on lung cancer susceptibility. Cancer Res. 1996;56(21):4965–4969. [PubMed] [Google Scholar]
- 43.Landi MT, Bertazzi PA, Shields PG, et al. Association between CYP1A1 genotype, mRNA expression and enzymatic activity in humans. Pharmacogenetics. 1994;4(5):242–246. doi: 10.1097/00008571-199410000-00002. [DOI] [PubMed] [Google Scholar]
- 44.Crofts F, Taioli E, Trachman J, et al. Functional significance of different human CYP1A1 genotypes. Carcinogenesis. 1994;15(12):2961–2963. doi: 10.1093/carcin/15.12.2961. [DOI] [PubMed] [Google Scholar]
- 45.Garte S, Gaspari L, Alexandrie AK, et al. Metabolic gene polymorphism frequencies in control populations. Cancer Epidemiol Biomarkers Prev. 2001;10(12):1239–1248. [PubMed] [Google Scholar]
- 46.Oesch F, Schmassmann H. Species and organ specificity of the trans-stilbene oxide induced effects on epoxide hydratase and benzo(a)pyrene monooxygenase activity in rodents. Biochem Pharmacol. 1979;28(2):171–176. doi: 10.1016/0006-2952(79)90498-2. [DOI] [PubMed] [Google Scholar]
- 47.Omiecinski CJ, Aicher L, Holubkov R, Checkoway H. Human peripheral lymphocytes as indicators of microsomal epoxide hydrolase activity in liver and lung. Pharmacogenetics. 1993;3(3):150–158. doi: 10.1097/00008571-199306000-00005. [DOI] [PubMed] [Google Scholar]
- 48.Seidegard J, Ekstrom G. The role of human glutathione transferases and epoxide hydrolases in the metabolism of xenobiotics. Environ Health Perspect. 1997;105(Suppl 4):791–799. doi: 10.1289/ehp.105-1470052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hassett C, Aicher L, Sidhu JS, Omiecinski CJ. Human microsomal epoxide hydrolase: genetic polymorphism and functional expression in vitro of amino acid variants. Hum Mol Genet. 1994;3(3):421–428. doi: 10.1093/hmg/3.3.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jourenkova-Mironova N, Mitrunen K, Bouchardy C, Dayer P, Benhamou S, Hirvonen A. High-activity microsomal epoxide hydrolase genotypes and the risk of oral, pharynx, and larynx cancers. Cancer Res. 2000;60(3):534–536. [PubMed] [Google Scholar]
- 51.Lin YC, Wu DC, Lee JM, et al. The association between microsomal epoxide hydrolase genotypes and esophageal squamous-cell-carcinoma in Taiwan: interaction between areca chewing and smoking. Cancer Lett. 2006;237(2):281–288. doi: 10.1016/j.canlet.2005.06.010. [DOI] [PubMed] [Google Scholar]
- 52.Lee WJ, Brennan P, Boffetta P, et al. Microsomal epoxide hydrolase polymorphisms and lung cancer risk: a quantitative review. Biomarkers. 2002;7(3):230–241. doi: 10.1080/13547500210121882. [DOI] [PubMed] [Google Scholar]
- 53.Harrison DJ, Hubbard AL, MacMillan J, Wyllie AH, Smith CA. Microsomal epoxide hydrolase gene polymorphism and susceptibility to colon cancer. Br J Cancer. 1999;79(1):168–171. doi: 10.1038/sj.bjc.6690028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tiemersma EW, Omer RE, Bunschoten A, et al. Role of genetic polymorphism of glutathione-S-transferase T1 and microsomal epoxide hydrolase in aflatoxin-associated hepatocellular carcinoma. Cancer Epidemiol Biomarkers Prev. 2001;10(7):785–791. [PubMed] [Google Scholar]
- 55.Kiyohara C, Yoshimasu K, Takayama K, Nakanishi Y. EPHX1 polymorphisms and the risk of lung cancer: a HuGE review. Epidemiology. 2006;17(1):89–99. doi: 10.1097/01.ede.0000187627.70026.23. [DOI] [PubMed] [Google Scholar]
- 56.Jaiswal AK. Regulation of genes encoding NAD(P)H: quinone oxidoreductases. Free Radic Biol Med. 2000;29(3–4):254–262. doi: 10.1016/s0891-5849(00)00306-3. [DOI] [PubMed] [Google Scholar]
- 57.Montano MM, Bianco NR, Deng H, Wittmann BM, Chaplin LC, Katzenellenbogen BS. Estrogen receptor regulation of quinone reductase in breast cancer: implications for estrogen-induced breast tumor growth and therapeutic uses of tamoxifen. Front Biosci. 2005;10:1440–1461. doi: 10.2741/1630. [DOI] [PubMed] [Google Scholar]
- 58.Lyn-Cook BD, Yan-Sanders Y, Moore S, Taylor S, Word B, Hammons GJ. Increased levels of NAD(P)H: quinone oxidoreductase-1 (NQO1) in pancreatic tissues from smokers and pancreatic adenocarcinomas: a potential biomarker of early damage in the pancreas. Cell Biol Toxicol. 2006;22(2):73–80. doi: 10.1007/s10565-006-0156-3. [DOI] [PubMed] [Google Scholar]
- 59.Lafuente MJ, Casterad X, Trias M, et al. NAD(P)H: quinone oxidoreductase-dependent risk for colorectal cancer and its association with the presence of K-ras mutations in tumors. Carcinogenesis. 2000;21(10):1813–1819. doi: 10.1093/carcin/21.10.1813. [DOI] [PubMed] [Google Scholar]
- 60.Zhang J, Schulz WA, Li Y, et al. Association of NAD(P)H: quinone oxidoreductase 1 (NQO1) C609T polymorphism with esophageal squamous cell carcinoma in a German Caucasian and a northern Chinese population. Carcinogenesis. 2003;24(5):905–909. doi: 10.1093/carcin/bgg019. [DOI] [PubMed] [Google Scholar]
- 61.Siegel D, McGuinness SM, Winski SL, Ross D. Genotype-phenotype relationships in studies of a polymorphism in NAD(P)H: quinone oxidoreductase 1. Pharmacogenetics. 1999;9(1):113–121. doi: 10.1097/00008571-199902000-00015. [DOI] [PubMed] [Google Scholar]
- 62.Kiyohara C, Yoshimasu K, Takayama K, Nakanishi Y. NQO1, MPO, and the risk of lung cancer: a HuGE review. Genet Med. 2005;7(7):463–478. doi: 10.1097/01.gim.0000177530.55043.c1. [DOI] [PubMed] [Google Scholar]
- 63.Kelsey KT, Ross D, Traver RD, et al. Ethnic variation in the prevalence of a common NAD(P)H quinone oxidoreductase polymorphism and its implications for anti-cancer chemotherapy. Br J Cancer. 1997;76(7):852–854. doi: 10.1038/bjc.1997.474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hein DW. Molecular genetics and function of NAT1 and NAT2: role in aromatic amine metabolism and carcinogenesis. Mutat Res. 2002;506–507:65–77. doi: 10.1016/s0027-5107(02)00153-7. [DOI] [PubMed] [Google Scholar]
- 65.Zheng W, Deitz AC, Campbell DR, et al. N-acetyltransferase-1 genetic polymorphism, cigarette smoking, well-done meat intake, and breast cancer risk. Cancer Epidemiol Biomarkers Prev. 1999;8(3):233–239. [PubMed] [Google Scholar]
- 66.Hickman D, Risch A, Buckle V, et al. Chromosomal localization of human genes for arylamine N-acetyltransferase. Biochem J. 1994;297(Pt 3):441–445. doi: 10.1042/bj2970441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ebisawa T, Deguchi T. Structure and restriction fragment length polymorphism of genes for human liver arylamine N-acetyltransferases. Biochem Biophys Res Commun. 1991;177(3):1252–1257. doi: 10.1016/0006-291x(91)90676-x. [DOI] [PubMed] [Google Scholar]
- 68.Bell DA, Badawi AF, Lang NP, Ilett KF, Kadlubar FF, Hirvonen A. Polymorphism in the N-acetyltransferase 1 (NAT1) polyadenylation signal: association of NAT1*10 allele with higher N-acetylation activity in bladder and colon tissue. Cancer Res. 1995;55(22):5226–5229. [PubMed] [Google Scholar]
- 69.Zenser TV, Lakshmi VM, Rustan TD, et al. Human N-acetylation of benzidine: role of NAT1 and NAT2. Cancer Res. 1996;56(17):3941–3947. [PubMed] [Google Scholar]
- 70.Bruhn C, Brockmoller J, Cascorbi I, Roots I, Borchert HH. Correlation between genotype and phenotype of the human arylamine N-acetyltransferase type 1 (NAT1) Biochem Pharmacol. 1999;58(11):1759–1764. doi: 10.1016/s0006-2952(99)00269-5. [DOI] [PubMed] [Google Scholar]
- 71.Yang M, Katoh T, Delongchamp R, Ozawa S, Kohshi K, Kawamoto T. Relationship between NAT1 genotype and phenotype in a Japanese population. Pharmacogenetics. 2000;10(3):225–232. doi: 10.1097/00008571-200004000-00003. [DOI] [PubMed] [Google Scholar]
- 72.Zhao B, Lee EJ, Yeoh PN, Gong NH. Detection of mutations and polymorphism of N-acetyltransferase 1 gene in Indian, Malay and Chinese populations. Pharmacogenetics. 1998;8(4):299–304. doi: 10.1097/00008571-199808000-00003. [DOI] [PubMed] [Google Scholar]
- 73.Hayes JD, Pulford DJ. The glutathione S-transferase supergene family: regulation of GST and the contribution of the isoenzymes to cancer chemoprotection and drug resistance. Crit Rev Biochem Mol Biol. 1995;30(6):445–600. doi: 10.3109/10409239509083491. [DOI] [PubMed] [Google Scholar]
- 74.Mannervik B, Awasthi YC, Board PG, et al. Nomenclature for human glutathione transferases. Biochem J. 1992;282(Pt 1):305–306. doi: 10.1042/bj2820305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Benhamou S, Lee WJ, Alexandrie AK, et al. Meta- and pooled analyses of the effects of glutathione S-transferase M1 polymorphisms and smoking on lung cancer risk. Carcinogenesis. 2002;23(8):1343–1350. doi: 10.1093/carcin/23.8.1343. [DOI] [PubMed] [Google Scholar]
- 76.Hashibe M, Brennan P, Strange RC, et al. Meta- and pooled analyses of GSTM1, GSTT1, GSTP1, and CYP1A1 genotypes and risk of head and neck cancer. Cancer Epidemiol Biomarkers Prev. 2003;12(12):1509–1517. [PubMed] [Google Scholar]
- 77.Board PG. Biochemical genetics of glutathione-S-transferase in man. Am J Hum Genet. 1981;33(1):36–43. [PMC free article] [PubMed] [Google Scholar]
- 78.Wiebel FA, Dommermuth A, Thier R. The hereditary transmission of the glutathione transferase hGSTT1-1 conjugator phenotype in a large family. Pharmacogenetics. 1999;9(2):251–256. [PubMed] [Google Scholar]
- 79.Gilliland FD, Li YF, Saxon A, az-Sanchez D. Effect of glutathione-S-transferase M1 and P1 genotypes on xenobiotic enhancement of allergic responses: randomised, placebo-controlled crossover study. Lancet. 2004;363(9403):119–125. doi: 10.1016/S0140-6736(03)15262-2. [DOI] [PubMed] [Google Scholar]
- 80.Geisler SA, Olshan AF. GSTM1, GSTT1, and the risk of squamous cell carcinoma of the head and neck: a mini-HuGE review. Am J Epidemiol. 2001;154(2):95–105. doi: 10.1093/aje/154.2.95. [DOI] [PubMed] [Google Scholar]
- 81.Inskip A, Elexperu-Camiruaga J, Buxton N, et al. Identification of polymorphism at the glutathione S-transferase, GSTM3 locus: evidence for linkage with GSTM1*A. Biochem J. 1995;312(Pt 3):713–716. doi: 10.1042/bj3120713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mohammadzadeh GS, Nasseri MS, Rasaee MJ, Zaree AB, Mahmoodzadeh H, Allameh A. Measurement of glutathione S-transferase and its class-pi in plasma and tissue biopsies obtained after laparoscopy and endoscopy from subjects with esophagus and gastric cancer. Clin Biochem. 2003;36(4):283–288. doi: 10.1016/s0009-9120(03)00012-2. [DOI] [PubMed] [Google Scholar]
- 83.Liu B, Fan YJ, Wang ML, et al. Genetic polymorphisms in glutathione S-transferases T1, M1 and P1 and susceptibility to reflux esophagitis. Dis Esophagus. 2006;19(6):477–481. doi: 10.1111/j.1442-2050.2006.00609.x. [DOI] [PubMed] [Google Scholar]
- 84.Lee JM, Lee YC, Yang SY, et al. Genetic polymorphisms of p53 and GSTP1, but not NAT2, are associated with susceptibility to squamous-cell carcinoma of the esophagus. Int J Cancer. 2000;89(5):458–464. doi: 10.1002/1097-0215(20000920)89:5<458::aid-ijc10>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 85.Morita S, Yano M, Tsujinaka T, et al. Association between genetic polymorphisms of glutathione S-transferase P1 and N-acetyltransferase 2 and susceptibility to squamous-cell carcinoma of the esophagus. Int J Cancer. 1998;79(5):517–520. doi: 10.1002/(sici)1097-0215(19981023)79:5<517::aid-ijc12>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 86.Zimniak P, Nanduri B, Pikula S, et al. Naturally occurring human glutathione S-transferase GSTP1-1 isoforms with isoleucine and valine in position 104 differ in enzymic properties. Eur J Biochem. 1994;224(3):893–899. doi: 10.1111/j.1432-1033.1994.00893.x. [DOI] [PubMed] [Google Scholar]
- 87.Pandya U, Srivastava SK, Singhal SS, et al. Activity of allelic variants of Pi class human glutathione S-transferase toward chlorambucil. Biochem Biophys Res Commun. 2000;278(1):258–262. doi: 10.1006/bbrc.2000.3787. [DOI] [PubMed] [Google Scholar]
- 88.Hand PA, Inskip A, Gilford J, et al. Allelism at the glutathione S-transferase GSTM3 locus: interactions with GSTM1 and GSTT1 as risk factors for astrocytoma. Carcinogenesis. 1996;17(9):1919–1922. doi: 10.1093/carcin/17.9.1919. [DOI] [PubMed] [Google Scholar]
- 89.Ioannidis JP, Trikalinos TA. Early extreme contradictory estimates may appear in published research: the Proteus phenomenon in molecular genetics research and randomized trials. J Clin Epidemiol. 2005;58(6):543–549. doi: 10.1016/j.jclinepi.2004.10.019. [DOI] [PubMed] [Google Scholar]
- 90.Wright AF, Carothers AD, Pirastu M. Population choice in mapping genes for complex diseases. Nat Genet. 1999;23(4):397–404. doi: 10.1038/70501. [DOI] [PubMed] [Google Scholar]
- 91.Hattersley AT, McCarthy MI. What makes a good genetic association study? Lancet. 2005;366(9493):1315–1323. doi: 10.1016/S0140-6736(05)67531-9. [DOI] [PubMed] [Google Scholar]
- 92.Salanti G, Sanderson S, Higgins JP. Obstacles and opportunities in meta-analysis of genetic association studies. Genet Med. 2005;7(1):13–20. doi: 10.1097/01.gim.0000151839.12032.1a. [DOI] [PubMed] [Google Scholar]