Abstract
Streptococcus suis is an important swine pathogen that can cause septicemia, meningitis, and pneumonia. Also recognized as an emerging zoonotic agent, it is responsible for outbreaks of human infections in Asian countries. Serotype 2 is the predominant isolate from diseased animals and humans. The aerosolization of S. suis in the air of swine confinement buildings (SCB) was studied. The presence of S. suis in bioaerosols was monitored in SCB where cases of infection had been reported and in healthy SCB without reported infections. Using a quantitative-PCR (qPCR) method, we determined the total number of bacteria (1 × 108 to 2 × 108 airborne/m3), total number of S. suis bacteria (4 × 105 to 10 × 105 airborne/m3), and number of S. suis serotype 2 and 1/2 bacteria (1 × 103 to 30 × 103 airborne/m3) present in the air. S. suis serotypes 2 and 1/2 were detected in the air of all growing/finishing SCB that had documented cases of S. suis infection and in 50% of healthy SCB. The total number of bacteria and total numbers of S. suis and S. suis serotype 2 and 1/2 bacteria were monitored in one positive SCB during a 5-week period, and it was shown that the aerosolized S. suis serotypes 2 and 1/2 remain airborne for a prolonged period. When the effect of aerosolization on S. suis was observed, the percentage of intact S. suis bacteria (showing cell membrane integrity) in the air might have been up to 13%. Finally S. suis was found in nasal swabs from 14 out of 21 healthy finishing-SCB workers, suggesting significant exposure to the pathogen. This report provides a better understanding of the aerosolization, prevalence, and persistence of S. suis in SCB.
INTRODUCTION
Bioaerosols are commonly defined as aerosolized particles with a biological component. These particles can contain any type of microorganism and are dispersed into the air by a variety of abiotic and biotic mechanisms. In swine confinement buildings (SCB), the air quality is of utmost importance. The swine and their movements release large quantities of airborne contaminants, such as odorous compounds, organic dust, and microorganisms (1, 2). Given that airborne microorganisms and their components may be hazardous, studies on air quality in SCB are fully justified (3–6). Bioaerosols in SCB are composed of microorganisms (e.g., bacteria, viruses, archaea, yeasts, and molds) and their components (e.g., endotoxins and mycotoxins), animal proteins, litter, food, fecal matter, urine, and soil (7). Moreover, Cambra-Lopez et al. have shown that most airborne particles in pig houses originate from manure and skin (8). Bioaerosol concentrations vary considerably and are influenced by farming practices, seasons, food types, and the production system (e.g., use of deep or shallow litter and solid or liquid separation systems) (4, 9). High levels of bioaerosols lead to poor indoor air quality (1–4, 6). Consequently, SCB workers and animals may be exposed to high levels of airborne dust, endotoxins, and microorganisms (2, 10). Agricultural workers are at a higher risk of developing respiratory symptoms than most other workers (11, 12).
Streptococcus suis is an important swine pathogen that is responsible for significant economic losses in the swine industry. It also represents a major health problem worldwide, particularly over the last 20 years (13). The natural habitat of S. suis is the upper respiratory tract of pigs, primarily in the tonsils and nasal cavities. It can also be found in the genital and digestive tracts (14). S. suis causes outbreaks of septicemia, meningitis, and pneumonia in neonatal piglets and adult pigs (14). Thirty-five serotypes of S. suis have been described. Serotype 2 is the most common serotype associated with diseases in pigs and humans (15, 16). Other serotypes, such as 1/2, 5, 9, and 14, have also been associated with S. suis outbreaks in pigs in North America and Europe (17, 18). S. suis is increasingly recognized as an emerging zoonotic agent, especially in Asian countries (19). Zoonotic transmission is most frequently associated with serotype 2 strains and occupational exposure to pigs or consumption of infected pork. Two major human outbreaks, which affected more than 200 people, resulting in more than 50 deaths, occurred in China in 1998 and 2005. S. suis is the leading cause of bacterial meningitis in adults in Vietnam (13, 20) and the second leading cause in Thailand (21). In the Western Hemisphere, human S. suis infection cases are less frequent and usually affect workers in the swine industry. However, Smith et al. studied swine-exposed American adults for antibodies to S. suis serotype 2, and the serologic data suggested that human infections involving S. suis are likely to occur more frequently than has been documented (22). In North America, there have been seven reported human cases of S. suis infection due to swine contamination: two in Canada (endocarditis and meningitis) and five in the United States (meningitis) (23–29). This recent increase in reported cases of human infection by S. suis is also observable in Europe (30–32). Petersen et al. showed that the risk of meningitis caused by streptococci increased significantly when workers were in close proximity to pigs (19). The number of reported infections remains low, considering the large number of worker exposed to S. suis; the true incidence might be higher, due to misidentification of the bacterium in clinical cases (19, 33). Another potential explanation for underreporting S. suis infections is that the bacteria are sensitive to antibiotics commonly used to treat infections in humans. Many cases of infection are treated with broad-spectrum antibiotics without identifying the causative pathogen.
S. suis can survive in feces for up to 104 days at 0°C, 10 days at 9°C, and 8 days at temperatures between 22 and 25°C (34). Berthelot-Herault et al. demonstrated the airborne transmission of S. suis in pigs consigned to experimental SCB (35). Their results were confirmed by Madsen et al., who successfully transmitted infections to pigs by exposure to an experimental aerosol of S. suis serotype 2, causing lesions similar to those seen in spontaneously infected animals (36). The authors suggested that the tonsils were the possible portals of entry for S. suis, with subsequent lymphogenous spread (37).
The aim of the present study was to document whether all types of S. suis, and specifically serotypes 2 and 1/2, are present in the air of unhealthy (i.e., where cases of S. suis infection had been reported) and healthy (with no report of S. suis infection) SCB, and if so, whether the bacteria persist in the air over time. Then, we were interested in evaluating the effect of aerosolization on S. suis cell membrane integrity. Lastly, we evaluated possible colonization of farmers by S. suis.
MATERIALS AND METHODS
Sampling sites. (i) Growing/finishing SCB with documented cases of S. suis infections.
Four growing/finishing SCB (SCB1, SCB2, SCB3, and SCB4) located in the Quebec City area (eastern Canada) with confirmed cases of S. suis infection (Veterinary College of Université de Montréal) were visited within 48 h after the case notification. Given the diagnosis delays, samples were done within 1 month after the first reported symptoms. These four SCB housed phase 1, 2, 3, and 4 pigs (Table 1). To evaluate the persistence over time of S. suis in the air, one growing/finishing SCB with documented cases of S. suis infections (SCB1 in this study) was monitored over a period of 5 weeks.
TABLE 1.
Correspondence between swine age, growth steps, and phases
| Phase | Growth step | Age (days) |
|---|---|---|
| 1 | Maternity | 0–20 |
| 2 | Nursery | 21–35 |
| 3 | Nursery | 36–49 |
| 4 | Fattening | ≥50 |
(ii) Healthy finishing SCB.
Twenty-one Quebec healthy finishing SCB were sampled in a previous study (3). Sample collections took place in the rooms just before the pigs were sent to the slaughterhouse (3).
(iii) Nasal flora of swine producers.
Samples were obtained from banked frozen DNA samples from a previous study by Létourneau et al. (38). Briefly, 27 hog producers from 14 previously mentioned healthy finishing SCB and 5 unexposed control subjects were recruited and signed an informed consent to participate in this part of the study.
Sampling method. (i) Growing/finishing SCB with documented cases of S. suis infection.
Sample collection took place in at least 4 rooms—maternity (2 rooms), nursery 1 (2 rooms), nursery 2 (2 rooms), and fattening rooms (2 rooms)—corresponding to phases 1, 2, 3, and 4 (Table 1). Air sampling was performed using a Coriolis cyclone sampler (Bertin Technologies, Montigny-le-Bretonneux, France). The median aerodynamic diameter (d50) is 0.5 μm for a flow of operation of 300 liters/min, meaning that 0.5-μm particles are sampled at 50% efficiency and larger particles are sampled at higher efficiency. Fifteen milliliters of sterile 50 mM phosphate-buffered saline (PBS) (pH 7.4) was placed in the sampling cone of the Coriolis sampler, which was run for a period of 10 min at 300 liters/min. According to the producers, certain rooms appeared to be more susceptible to S. suis infections than others. Consequently, these rooms were chosen for sampling, as well as one, two, or three surrounding rooms. Two samples were taken in each room of each SCB. The remaining liquid volume was determined after sampling. Each sample represented 3,000 liters of air.
For the follow-up study to assess the persistence of S. suis, we took samples from rooms that housed pigs in four different age groups (Table 1). Each room, representing one of these age groups, was sampled twice. Two or three rooms per group were sampled on the same day. Air sampling was again performed using a Coriolis cyclone sampler. At least 8 rooms were sampled in each visit with 2 Coriolis samples per room. Consequently, at least 16 samples were taken per building per visit.
To determine the percent viability of total bacteria and of total S. suis bacteria present in the air, 10 air samples, using a Coriolis cyclone sampler that was run for a period of 10 min at 300 liters/min, were taken from three nursery rooms in SCB1 (SCB1 was the first growing/finishing SCB that was visited [see above]).
(ii) Healthy finishing SCB.
Samples for the healthy finishing SCB part of the study were taken from banked air samples from a previous study (38). For that study, air samples were collected over 4 h at 2 liters/min, using Institute of Occupational Medicine (IOM) cassettes (SKC Inc., Edinburgh, United Kingdom) loaded with 25-mm gelatin membranes. The IOM gelatin membrane system was operated with a Gilair 5 pump (Sensidyne, FL, USA). The gelatin membranes were kept at 4°C until they were brought back to the laboratory. The membranes were dissolved by placing them in 5 ml 0.9% NaCl and vortexing with a Multi-Pulse Vortexer (Glas-Col, Terre Haute, IN). The resulting suspension was aliquoted (1.5 ml, corresponding to 144 liters of sampled air) and centrifuged at 8,000 × g. The supernatant was removed, and the pellets were stored at −20°C. Because the frozen DNA from the previous study was obtained from IOM samples, we needed to validate the similarity of the results obtained with the IOM system and the Coriolis sampler. We thus also used IOM cassettes loaded with 25-mm gelatin membranes in the 4 SCB with S. suis infections (within 1 month) to compare the method with the Coriolis cyclone sampler. We obtained similar results, confirming that the two methods are comparable (data not shown). The same observation was previously published by our team (39).
(iii) Nasal flora of swine producers.
The nasal secretions of swine producers were sampled by a nurse, using a calcium-alginate swab (Fisherbrand; Fisher Scientific Company, Ottawa, ON, Canada). After sampling, the tip of the swab was placed in a plastic tube containing 1 ml of PBS, the swab stick was cut off, and the plastic tube was kept on ice. This protocol was approved by the ethics committee of the Institut Universitaire de Cardiologie et de Pneumologie de Québec (CER 1090).
Determination of total culturable bacteria in air samples.
After sampling with the Coriolis cyclone sampler, 1 ml of PBS was removed and used to prepare a 10-fold dilution series (100 to 10−7). All samples were plated in duplicate on brain heart infusion agar (BHIA) (Difco, Sparks, MD) containing 5 μg/ml amphotericin B to prevent growth of molds. The plates were incubated at 25°C for 48 h. Bacterial counts were determined at the dilution where the plates showed between 30 and 300 colonies.
DNA extraction from air samples.
Aliquots (1.5 ml) of air samples were centrifuged (10 min at 14,000 × g), and the pellets were stored at −20°C until DNA extraction was performed. Total genomic DNA was extracted using the QIAamp DNA minikit (Qiagen, Mississauga, Ontario, Canada) according to the manufacturer's instructions. Total DNA was eluted in 200 μl of elution buffer, supplied with the kit.
Quantitative PCR (qPCR).
Amplification was performed using the Bio-Rad CFX 384 thermocycler (Bio-Rad Laboratories, Mississauga, Ontario, Canada). All primers and DNA probes (Table 2) were purchased from Integrated DNA Technologies (Coralville, IA, USA). The results were analyzed using Bio-Rad CFX Manager software version 3.0.1224.1015 (Bio-Rad Laboratories).
TABLE 2.
Primers used in the study
| Primer | Target | Sequence (5′–3′) | Reference |
|---|---|---|---|
| EUBf | Bacterial 16S rRNA | GGTAGTCYAYGCMSTAAACGT | 40 |
| EUBr | Bacterial 16S rRNA | GACARCCATGCASCACCTG | 40 |
| EUBp | Bacterial 16S rRNA | FAM-TKCGCGTTGCDTCGAATTAAWCCAC-IBTMFQ | 40 |
| 463f | S. suis 16S rRNA | AGAAGAGTGGAAAGTTTCTCA | This study |
| 637r | S. suis 16S rRNA | TCACAGTTTCCAAAGCGT | This study |
| 594p | S. suis 16S rRNA | FAM- CAAACCGCCTGCGCTCGCTTTACG | This study |
| CpS2Jf | cps2J gene | GGTTACTTGCTACTTTTGATGGAAATT | 42 |
| CpS2Jr | cps2J gene | CGCACCTCTTTTATCTCTTCCAA | 42 |
| CpS2Jp | cps2J gene | FAM-TCAAGAATCTGAGCTGCAAAAGTGTCAAATTGA-TAMRA | 42 |
(i) Total bacterial qPCR.
Quantification of total bacteria was performed as described by Bach et al. (40) with 16S rRNA forward primer EUBf (5′-GGTAGTCYAYGCMSTAAACG-3′), 16S rRNA reverse primer EUBr (5′-GACARCCATGCASCACCTG-3′), and 16S rRNA probe EUBp (5′-6-carboxyfluorescein [FAM]-TKCGCGTTGCDTCGAATTAAWCCAC-IBTMFQ [Iowa Black Fret Quencher]) (Table 2). The PCR mixture contained 2 μl of DNA template, 0.4 μmol/liter (each) primer, 0.08 μmol/liter probe, and 10 μl of 2× QuantiTect Probe PCR master mix (QuantiTect Probe PCR kit; Qiagen, Mississauga, Ontario, Canada) in a 20-μl reaction mixture. The qPCR thermal profile used to amplify the 245-bp amplicon was as follows: 95°C for 3 min for DNA denaturation and activation of DNA polymerase, and then 40 cycles of 95°C for 20s and 62°C for 60 s. Quantification was performed using a standard curve of a 10-fold dilution series of Escherichia coli genomic DNA preparation (41).
(ii) Total S. suis qPCR.
Quantification of total S. suis was performed using PCR primers designed in house using Beacon designer 5 software (Premier Biosoft, Palo Alto, CA). To target the 16S RNA gene, forward primer 463f (5′-AGAAGAGTGGAAAGTTTCTCA-3′), reverse primer 637r (5′-TCACAGTTTCCAAAGCGT-3′), and probe 594p (5′-FAM-CAAACCGCCTGCGCTCGCTTTACG-3′) (Table 2) were used. The PCR components were as follows: 2 μl of DNA template, 0.4 μmol/liter (each) primer, 0.1 μmol/liter probe, and 10 μl of 2× QuantiTect Probe PCR master mix (QuantiTect Probe PCR kit; Qiagen, Mississauga, Ontario, Canada) in a 20-μl reaction mixture. The PCR program was as follows: 94°C for 3 min, and then 40 cycles of 94°C for 10 s and 62°C for 30 s. A 10-fold dilution series of S. suis P1/7 (serotype 2) genomic DNA was used for the standard curve.
(iii) S. suis serotype 2 and 1/2 qPCR.
Since the method presented cannot differentiate between serotypes 2 and 1/2, all our results include these two subtypes. Quantification of S. suis serotypes 2 and 1/2 was performed as described by Nga et al. (42). The primers and probe for S. suis serotype 2 real-time PCR targeted the cps2J gene (43, 44), which is part of the operon encoding the serotype 2- and serotype 1/2-specific polysaccharide capsule of S. suis. Primers Cps2Jf (5′-GGTTACTTGCTACTTTTGATGGAAATT-3′) and Cps2Jr (5′-CGCACCTCTTTTATCTCTTCCAA-3′) and probe Cps2Jp (5′-FAM-TCAAGAATCTGAGCTGCAAAAGTGTCAAATTGA-6-carboxytetramethylrhodamine [TAMRA]-3′) were used for amplification and detection of an 88-bp amplicon (Table 2). The PCR components were as follows: 2 μl of DNA template, 0.4 μmol/liter (each) primer, 0.1 μmol/liter probe, and 10 μl of 2× QuantiTect Probe PCR master mix (QuantiTect Probe PCR kit; Qiagen, Mississauga, Ontario, Canada) in a 20-μl reaction mixture. The PCR program was as follows: 95°C for 3 min, and then 45 cycles of 94°C for 10 s and 60°C for 60 s. A 10-fold dilution series of S. suis P1/7 genomic DNA was used for the standard curve.
To determine the number of bacteria in each sample, data were analyzed (using Bio-Rad CFX Manager software version 3.0.1224.1015) by linear regression of the following function: log10 (target copy number) = f(threshold cycle). Negative controls were included to detect PCR reagent contamination in each PCR run.
(iv) Propidium monoazide qPCR.
Previously described by Fittipaldi et al., the propidium monoazide (PMA) method allows the quantification of intact bacteria (45). PMA {phenanthridium, 3-amino-8-azido-5[3-(diethylmethylammonio) propyl]-6-phenyl dichloride; Biotium Inc., Hayward, CA, USA} was dissolved in 20% dimethyl sulfoxide (DMSO) to create a stock concentration (5 mM) and stored at −20°C in the dark. To determine an appropriate PMA concentration, different amounts of PMA (final concentrations, 25, 50, and 100 μM) were added, and different light exposure times were tested (5, 10, 15, and 30 min) (data not shown). Finally, 2.5 μl of PMA was added to 250-μl aliquots of air samples to a final concentration of 25 μM. Transparent 1.5-ml microcentrifuge tubes were used (Fisher Scientific Co., Ottawa, ON, Canada). Following an incubation period of 5 min in the dark with occasional mixing, the samples were exposed to light for 15 min using a PMA-Lite LED Photolysis Device (a long-lasting LED light with 465- to 475-nm emission for efficient activation of PMA; Biotium Inc.). After photoinduced cross-linking, the cells were pelleted at 14,000 × g for 10 min prior to DNA extraction (as mentioned above). The PMA dye is a high-affinity, photoreactive, DNA binding molecule; it is cell membrane impermeable, and it can selectively modify only exposed DNA from dead cells, so PMA-modified cells cannot be amplified by normal qPCR.
Statistical analysis.
The statistical method used to perform comparisons was one-way analysis of variance (ANOVA). The results were considered significant if the P value was <0.05.
RESULTS
Four growing/finishing SCB with documented cases of S. suis infection were sampled within 1 month following the diagnosis by the veterinarian. Bacteria in the air samples were detected by quantitative PCR targeting the 16S rRNA gene for total bacteria and total S. suis. The cps2J gene was used for determination of S. suis serotypes 2 and 1/2.
In these four growing/finishing SCB with documented cases of S. suis infection sampled with a Coriolis apparatus, the concentration of culturable bacteria was about 105 CFU/m3 air (Fig. 1A). S. suis is difficult to identify on culture media from air samples, as there is no known selective medium to optimize its growth and prevent the growth of other organisms. Furthermore, even though the Andersen impactor shows high capture efficiency for aerosolized bacteria (46), the airborne bacteria collected can be stressed and, consequently, can be nonculturable. Therefore, to allow better detection and quantification of total bacteria, total S. suis, and S. suis serotypes 2 and 1/2 in our samples, qPCR was used.
FIG 1.
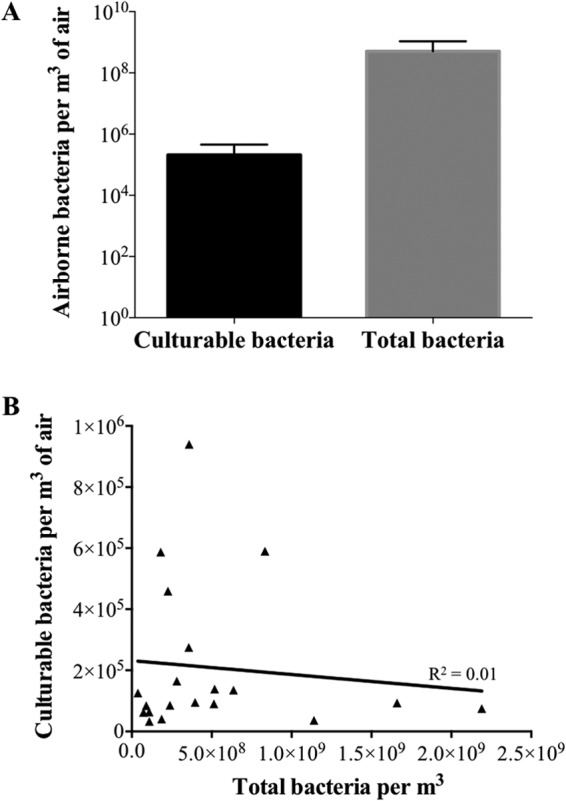
(A) Quantification of culturable airborne bacteria and total airborne bacteria determined by qPCR for SCB with reported cases of S. suis infection. The error bars indicate standard deviations. (B) Correlation between culturable and total bacterial counts.
The results, as expected (3), showed a difference of 3 orders of magnitude between the levels of total culturable bacteria versus total bacteria detected by qPCR (Fig. 1A). The correlation between culturable counts and estimated counts by qPCR was evaluated. As shown in Fig. 1B, no correlation could be established.
The concentrations of total bacteria, total S. suis, and S. suis serotypes 2 and 1/2 for these 4 growing/finishing SCB with documented cases of S. suis infection are reported in Fig. 2. The total bacteria in the four SCB ranged from 7.4 × 108 to 1.2 × 109 per m3 of air. The counts of S. suis for these SCB ranged from 4.5 × 105 to 1.1 × 106 bacteria per m3 of air. Differences in S. suis counts between the SCB were not statistically significant. S. suis serotypes 2 and 1/2 were found in all 4 SCB tested. Lower levels of S. suis serotypes 2 and 1/2 were found in SCB 4 (Fig. 2). In this building, the producer had treated the animals by adding penicillin to the food. In SCB1, the concentration of S. suis serotypes 2 and 1/2 constituted up to 11% of the total S. suis bacteria present in the air.
FIG 2.
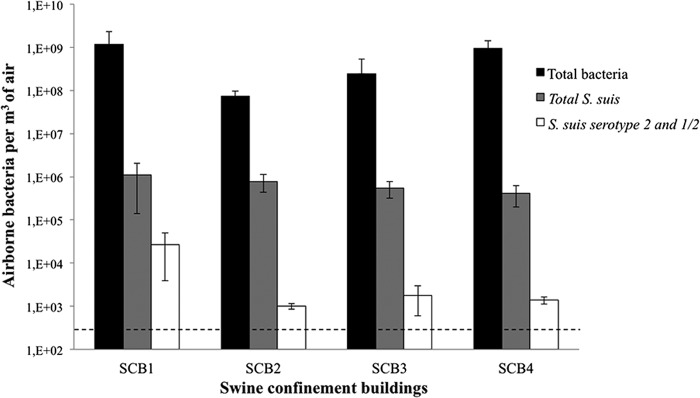
Quantification of airborne bacteria (total bacteria, total S. suis, and S. suis serotypes 2 and 1/2) by qPCR in four different growing/finishing SCB identified as having cases of S. suis infection. The dashed line represents the detection threshold. The horizontal lines represent medians. The error bars indicate standard deviations.
In the healthy finishing SCB (with no reported cases of S. suis infection) presented in Fig. 3, high concentrations of total bacteria comparable to those present in the 4 SCB described above were found. The average of total bacterial counts in finishing rooms was 1.4 × 109 total bacteria per m3 of air, which is similar to the concentrations found in SCB with known cases of infection caused by S. suis. There was no significant difference between SCB in regard to the concentration of total S. suis (which includes S. suis serotypes 1 to 35 and nontypeable strains). S. suis was a major component of bioaerosols, even in SCB without known cases of infection. S. suis represented 0.01% to 0.3% of the total bacteria. However, the concentrations of S. suis serotypes 2 and 1/2 varied from farm to farm, and the bacteria were present in only 10 out of the 21 (47.6%) SCB. S. suis serotype 2 represented from less than 0.01 to 87% of the total S. suis bacteria detected, more often corresponding to less than 0.01% of the total bacteria in the SCB.
FIG 3.
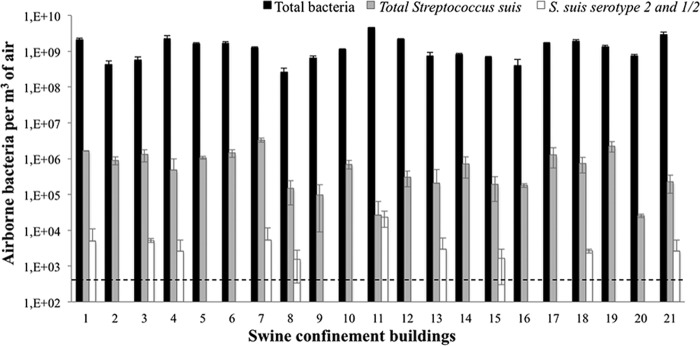
Quantification of total bacteria, total S. suis, and S. suis serotypes 2 and 1/2 by qPCR in 21 finishing SCB with no reported cases of S. suis infection. The dashed line represents the detection threshold. The error bars indicate standard deviations.
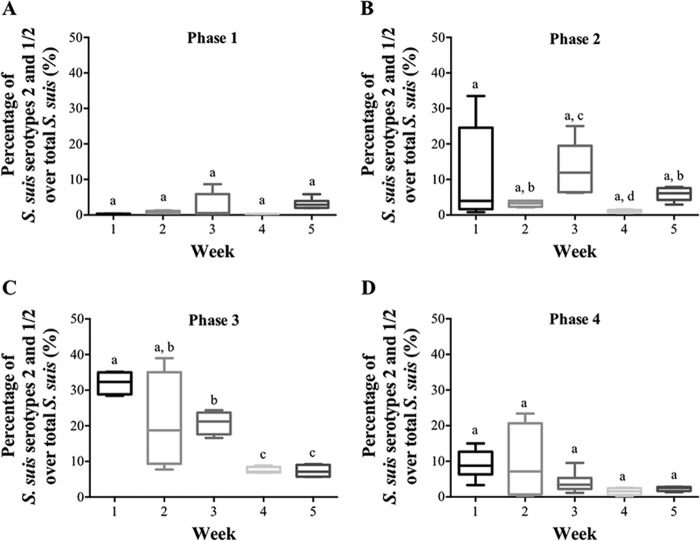
To determine whether the age of swine influences the production of S. suis bioaerosols in the air of SCB and then their persistence in the air, bioaerosols were monitored in different rooms of SCB1 during a 5-week period. For each sample, the total numbers of bacteria, total S. suis, and S. suis serotypes 2 and 1/2 were measured. The counts of total bacteria and total S. suis did not vary over time (data not shown). However, the levels of S. suis serotypes 2 and 1/2 differed over time (weeks). Figure 4 shows the ratio of S. suis serotypes 2 and 1/2 to total S. suis as a function of time (over 5 weeks) for different categories of swine growing phases. The results show that, in phase 1, there is only a small variation in the amounts of S. suis serotypes 2 and 1/2 over time (Fig. 4A). In the nursery areas (phases 2 and 3) (Fig. 4B and C), the ratio increased over time, although this increase varies between weeks. In phase 3 (Fig. 4C), the serotype 1/2-to-2 ratios range from 5 to 40%. As for phase 2, phase 3 shows the highest ratio of S. suis serotypes 2 and 1/2 to total S. suis during the first and the third weeks. The differences are statistically significant between weeks 2, 3, and 4 (Fig. 4B), as well as between weeks 1 and 3 and weeks 4 and 5 (Fig. 4C). In phase 4, levels of S. suis are stable over time with low ratios (Fig. 4D).
FIG 4.
Quantification of airborne bacteria during five consecutive weeks by PCR in one SCB (SCB1), combining different rooms for each age group. (A) Analysis of three swine maternity rooms (corresponding to phase 1). (B) Analysis of three nursery swine rooms (phase 2). (C) Analysis of two nursery swine rooms (phase 3). (D) Analysis of three swine fattening rooms (related to phase 4). The top of the box plot represents the 75th quartile, and the bottom represents the 25th quartile; the median is the horizontal line, and the lower and upper whiskers represent the minimum and maximum values, respectively. The letters a, b, c, and d represent statistical differences in the presence of S. suis serotype 2/total S. suis in the air as a function of time and depending on swine growth steps. P < 0.05.
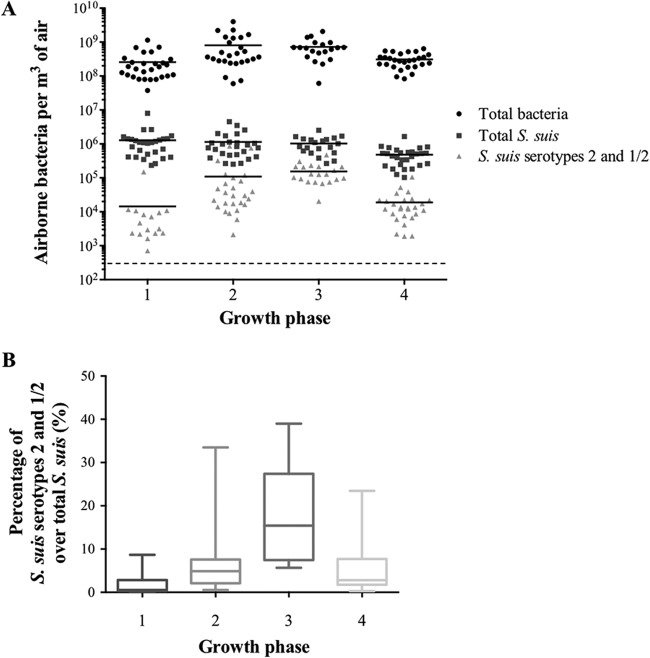
Figure 5A shows the concentrations of total bacteria, total S. suis, and S. suis serotypes 2 and 1/2 in relation to different categories of swine age. There was no statistically significant difference between the total concentration of bacteria and the total concentration of S. suis for the different categories of swine groups, unlike the concentrations of S. suis serotypes 2 and 1/2, which vary with swine age. Figure 5B shows a difference in the ratio of S. suis serotypes 2 and 1/2 to total S. suis according to the age groups. The results were significantly different for swine in phase 3.
FIG 5.
(A) Quantification of total bacteria, total S. suis, and S. suis serotypes 2 and 1/2 by qPCR in SCB1, combining different rooms for different age groups. Shown are analyses of rooms that housed four steps, corresponding to different age groups (Table 1). Each symbol on the graph represents one air sample. The dashed line represents the detection threshold. The horizontal lines represent medians. (B) Ratios of S. suis serotype 2 to total S. suis were calculated to compare the change in airborne concentrations between different age groups in the same SCB. The top of the box plot represents the 75th quartile and the bottom the 25th quartile, the median is the horizontal line, and the lower whisker represents the minimum with the upper representing the maximum.
Figure 6 shows the percentage of intact cells of total bacteria and total S. suis in the air of SCB in nursery phases, following the use of PMA dye. Using PMA qPCR to selectively detect intact cells, there was a higher percentage of live S. suis bacteria (up to 14%) than of total bacteria (up to 4%). Similar results for S. suis serotypes 2 and 1/2 are not available because the detection limit of the method for these subtypes was not reached. Because of their similar structures, there is no reason to suggest that their cell integrity would be different than that of the other S. suis serotypes.
FIG 6.
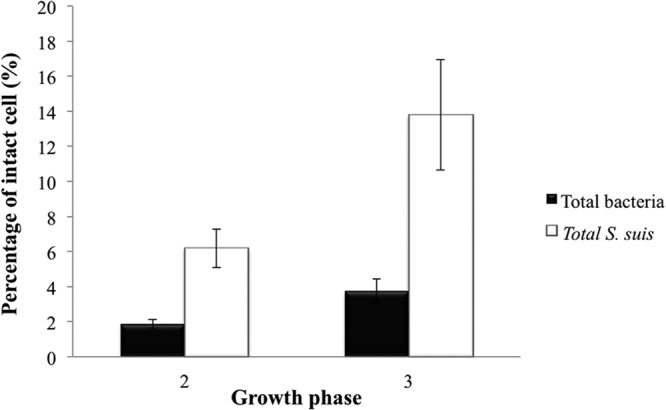
Percent cell integrity of total bacteria and total S. suis in air samples from nursery steps (SCB1) by quantification of viable cells using a PMA qPCR assay. The error bars indicate standard deviations.
Lastly, colonization of swine producers' nasal cavities with S. suis was evaluated. Using banked DNA samples obtained from swine workers (38), total S. suis was found in the nasal flora of 15 out of 27 hog producers from the healthy finishing SCB (Table 3). The 27 samples were negative for S. suis serotypes 2 and 1/2, even for hog producers working in the SCB that had S. suis serotypes 2 and 1/2 in the air; this is possibly due to the detection limit of our qPCR. All control subjects were negative for all S. suis serotypes.
TABLE 3.
Presence of total S. suis in the nasal flora of 27 hog producers from healthy finishing SCB
| Farmer | SCB | Presencea of total S. suis |
Presencea of S. suis serotypes 2 and 1/2 |
||
|---|---|---|---|---|---|
| Nasal flora | SCB | Nasal flora | SCB | ||
| 1 | 15 | + | + | − | + |
| 2 | 18 | − | + | − | + |
| 3 | 16 | + | + | − | − |
| 4 | 14 | − | + | − | − |
| 5 | 19 | + | + | − | − |
| 6 | 2 | + | + | − | − |
| 7 | 13 | − | + | − | + |
| 8 | 2 | + | + | − | − |
| 9 | 13 | − | + | − | + |
| 10 | 16 | − | + | − | − |
| 11 | 19 | + | + | − | − |
| 12 | 2 | + | + | − | − |
| 13 | 12 | + | + | − | − |
| 14 | 14 | + | + | − | − |
| 15 | 17 | − | + | − | − |
| 16 | 18 | − | + | − | + |
| 17 | 19 | + | + | − | − |
| 18 | 16 | + | + | − | − |
| 19 | 9 | − | + | − | − |
| 20 | 18 | + | + | − | + |
| 21 | 14 | + | + | − | − |
| 22 | 3 | + | + | − | + |
| 23 | 4 | − | + | − | + |
| 24 | 9 | − | + | − | − |
| 25 | 6 | − | + | − | − |
| 26 | 10 | + | + | − | − |
| 27 | 11 | − | + | − | + |
| Control 1 | − | − | NA | − | NA |
| Control 2 | − | − | NA | − | NA |
| Control 3 | − | − | NA | − | NA |
| Control 4 | − | − | NA | − | NA |
| Control 5 | − | − | NA | − | NA |
+, present; −, absent; NA, not applicable.
DISCUSSION
In this study, we were interested in extending our knowledge of the possible detection, presence, and persistence of S. suis in the air of swine confinement facilities, which may represent a potential reservoir for this important pathogen. This new information could benefit swine producers by contributing to health care of pigs and reducing important economic losses.
The detection and persistence of total bacteria, total S. suis, and S. suis serotypes 2 and 1/2 were evaluated in the air of growing/finishing SCB with reported cases of S. suis infection (4 SCB) and healthy finishing SCB (21 SCB). Previous studies have reported high concentrations of culturable bacteria in the air of SCB, independent of the structure of the SCB (3, 9, 47). No correlation between the culturable count of total bacteria and estimated counts by qPCR was found. The stress induced by aerosolization and sampling processes commonly lead to this underestimation of culture. Some bacteria can remain viable in the air even though nonculturable, and in addition, qPCR amplifies even DNA from dead cells. Nehme et al. reported the presence of Streptococcus spp. in bacterial bioaerosols in SCB, specifically Streptococcus bovis (AY324610), Streptococcus equinus (AF429765), Streptococcus macedonicus (AF459431), and Streptococcus gallolyticus (AY858648) (3). The authors did not explore the presence of the swine pathogen S. suis, and the sequencing approach did not lead to its detection. To our knowledge, this is the first report on the presence of S. suis in the bioaerosols of SCB. These results, combined with the data published by Madsen et al. and Berthelot-Herault, suggest that the air is a likely route of transmission for the pathogen. S. suis is usually transmitted nasally or orally and colonizes the palatine tonsils of both clinically ill and healthy pigs (48), and this study emphasizes that S. suis serotype 2 and 1/2 aerosol exposure could also be sufficient to initiate infections in pigs (35–37). Additionally, S. suis was detected in all tested rooms, suggesting potential transmission of aerosols over short distances.
After studying 4 SCB with recent infections, banked air samples from 21 healthy finishing SCB were used, and S. suis could also be found in SCB where no recent infections had been reported (3). These samples were acquired with the IOM cassettes loaded with 25-mm gelatin membranes. Consequently, we verified that the methods produced results comparable to those obtained in the 4 original SCB sampled by the Coriolis apparatus. S. suis serotypes 2 and 1/2 were detected in the air of half of these 21 healthy finishing SCB. This emphasizes the importance of informing farmers about the potential presence of S. suis in their buildings. S. suis is the most important reason for requesting veterinarian services, and the incidence of infection by the pathogen is likely underestimated.
Since there was no information on the frequency, concentration, and persistence over time of airborne S. suis in SCB and on the influence of swine age, one growing/finishing SCB (SCB1) where S. suis serotypes 2 and 1/2 were veterinarian diagnosed was monitored over a 5-week period. The total bacterial concentrations were very stable and did not fluctuate over time or with the age of the swine. The variations in the concentration of total S. suis over time and location were not statistically significant. However, the concentrations of serotypes 2 and 1/2 varied over time and were influenced by swine age. S. suis serotypes 2 and 1/2 persisted in the aerosols during this period, but the ratio of S. suis serotypes 2 and 1/2 to total S. suis is higher in one particular week; the increased activity while pigs were moved from stage 3 to stage 4 may explain this difference. Many risk factors exist for the development of S. suis meningitis in phase 3 pigs. More specifically, streptococcal infections in 2- to 6-week-old pigs are rather common, and they often occur during stressful events, such as vaccination, weaning, mixing of litters, weather variations, and transition to provision of solid food (49–54).
Our results reveal the presence of higher levels of S. suis serotypes 2 and 1/2 in swine age phases 2 and 3. This observation was supported by most swine producers, who reported that certain rooms seemed to be more susceptible to S. suis infections than others. This is in agreement with Robertson et al., who concluded that most cases of infection are seen in piglets and suggested that S. suis serotype 2 may be transmitted during birth (55). Nonetheless, transmission of S. suis also occurs in all age categories at farrowing farms: sows, piglets, and weaned pigs (56–58). Therefore, quantification of S. suis in bioaerosols and correlation with the health status of pigs can help predict and evaluate the exposure of pig farmers to the pathogen. The presence of S. suis serotypes 2 and 1/2 in aerosols could become a predictor of an infectious outbreak.
Dekker et al. determined that prevention of direct contact with infected animals decreases the risk of infection in susceptible pigs (59). The presence of S. suis in the air of SCB confirms their findings and the data of Berthelot-Herault (35) and showed that spatial separation of animal groups within a compartment would not prevent S. suis transmission on a farm (59). In Canada, the United States, and Europe at this time, S. suis infections in humans have most often been restricted to workers in close contact with pigs or contaminated swine products. However, in Asia, the bacterium also affects the general population, and it represents a significant public health concern (60). The presence of S. suis in the air of the SCB can explain and represent an additional risk for the entire swine herd as well as the farmers.
In this study, S. suis seemed to be more resistant to aerosolization/sampling stresses than other bacteria, a property that could be due to its thick sialic-acid-rich capsule (61).
Fifteen out of 26 (58%) nasal samples from hog producers, taken before the work shift, were positive for S. suis, suggesting workers' exposure and possible nasal cavity colonization. However, even though no S. suis serotype 2 and 1/2 bacteria were detected due to the limit of detection of the test, SCB workers could develop an infectious disease (62), especially if they are immunocompromised or if the contact between contaminated swine and swine producers increases. Cases of human S. suis infection in North America and Europe may be underestimated, since the diagnostic procedure is limited to identification to the genus level (streptococci). Efficient communication between scientists and swine producers is of utmost importance, and personal respiratory protection devices should be worn, especially during tasks linked to higher bioaerosol exposure (swine handling, moving, and vaccination) and when cases of infection are diagnosed on the farm (28, 29).
Conclusions.
The presence of viable S. suis, especially serotypes 2 and 1/2, and its persistence in SCB (over a 5-week period), combined with previous reports supporting its potential transmission via aerosols, clearly suggest that air can act as a transmission route for swine infection. It is also considered a reservoir for pathogenic S. suis that could persist over time. This study provides a better understanding of the presence and persistence of S. suis and could contribute to the knowledge required to improve the prevention of infection and the protection of swine and swine producers.
ACKNOWLEDGMENTS
L.B. is a CIHR strategic training fellow in Public Health and the Agricultural Rural Ecosystem (PHARE). C.D. is a Fonds de la Recherche en Santé du Québec (FRQ-S) Senior Scholar and a member of the Réseau en Santé Respiratoire du FRQ-S.
We are thankful to all the swine producers who participated in this study and the veterinary doctors (Clinique Demeter, St-Nicolas, Quebec, Canada) who informed us when cases of S. suis infection occurred. We also thank Serge Simard for his contributions to the statistical analysis and Phillipa Evelyn Perrott for thorough revision of the manuscript.
Footnotes
Published ahead of print 14 March 2014
REFERENCES
- 1.Cormier Y, Tremblay G, Meriaux A, Brochu G, Lavoie J. 1990. Airborne microbial contents in two types of swine confinement buildings in Quebec. Am. Ind. Hyg. Assoc. J. 51:304–309. 10.1080/15298669091369709 [DOI] [PubMed] [Google Scholar]
- 2.Kristiansen A, Saunders AM, Hansen AA, Nielsen PH, Nielsen JL. 2012. Community structure of bacteria and fungi in aerosols of a pig confinement building. FEMS Microbiol. Ecol. 80:390–401. 10.1111/j.1574-6941.2012.01305.x [DOI] [PubMed] [Google Scholar]
- 3.Nehme B, Letourneau V, Forster RJ, Veillette M, Duchaine C. 2008. Culture-independent approach of the bacterial bioaerosol diversity in the standard swine confinement buildings, and assessment of the seasonal effect. Environ. Microbiol. 10:665–675. 10.1111/j.1462-2920.2007.01489.x [DOI] [PubMed] [Google Scholar]
- 4.Letourneau V, Nehme B, Meriaux A, Masse D, Duchaine C. 2010. Impact of production systems on swine confinement buildings bioaerosols. J. Occup. Environ. Hyg. 7:94–102. 10.1080/15459620903425642 [DOI] [PubMed] [Google Scholar]
- 5.Bonlokke JH, Cormier Y, Veillette M, Radu A, Meriaux A, Duchaine C. 2013. Immunologic mechanisms in the adaptation of swine farm workers to their work environment. Innate Immun. 19:403–410. 10.1177/1753425912466576 [DOI] [PubMed] [Google Scholar]
- 6.Lee SA, Adhikari A, Grinshpun SA, McKay R, Shukla R, Reponen T. 2006. Personal exposure to airborne dust and microorganisms in agricultural environments. J. Occup. Environ. Hyg. 3:118–130. 10.1080/15459620500524607 [DOI] [PubMed] [Google Scholar]
- 7.Millner PD. 2009. Bioaerosols associated with animal production operations. Bioresour. Technol. 100:5379–5385. 10.1016/j.biortech.2009.03.026 [DOI] [PubMed] [Google Scholar]
- 8.Cambra-Lopez M, Hermosila T, Lai HTL, Aarnink AJA, Ogink NWM. 2011. Particulate matter emitted from poultry and pig houses: source identification and quantification. Trans. ASABE 54:629–642. 10.13031/2013.36466 [DOI] [Google Scholar]
- 9.Duchaine C, Grimard Y, Cormier Y. 2000. Influence of building maintenance, environmental factors, and seasons on airborne contaminants of swine confinement buildings. AIHAJ 61:56–63. [DOI] [PubMed] [Google Scholar]
- 10.Jolie R, Backstrom L, Olson L, Chase C. 1999. A 15-week experimental exposure of pigs to airborne dust with added endotoxin in a continuous flow exposure chamber. Can. J. Vet. Res. 63:129–137 [PMC free article] [PubMed] [Google Scholar]
- 11.Von Essen SG, McCurdy SA. 1998. Health and safety risks in production agriculture. West J. Med. 169:214–220 [PMC free article] [PubMed] [Google Scholar]
- 12.Dosman JA, Fukushima Y, Senthilselvan A, Kirychuk SP, Lawson JA, Pahwa P, Cormier Y, Hurst T, Barber EM, Rhodes CS. 2006. Respiratory response to endotoxin and dust predicts evidence of inflammatory response in volunteers in a swine barn. Am. J. Ind. Med. 49:761–766. 10.1002/ajim.20339 [DOI] [PubMed] [Google Scholar]
- 13.Gottschalk M, Segura M, Xu J. 2007. Streptococcus suis infections in humans: the Chinese experience and the situation in North America. Anim. Health Res. Rev. 8:29–45. 10.1017/S1466252307001247 [DOI] [PubMed] [Google Scholar]
- 14.Higgins R, Gottschalk M. 2005. Streptococcal diseases, p 769–783 In Straw BE, D'Allaire S, Mengeling WL, Taylor DJ. (ed), Diseases of swine, 9th ed. University of Iowa Press, Iowa City, IA [Google Scholar]
- 15.Staats JJ, Feder I, Okwumabua O, Chengappa MM. 1997. Streptococcus suis: past and present. Vet. Res. Commun. 21:381–407. 10.1023/A:1005870317757 [DOI] [PubMed] [Google Scholar]
- 16.Wei Z, Li R, Zhang A, He H, Hua Y, Xia J, Cai X, Chen H, Jin M. 2009. Characterization of Streptococcus suis isolates from the diseased pigs in China between 2003 and 2007. Vet. Microbiol. 137:196–201. 10.1016/j.vetmic.2008.12.015 [DOI] [PubMed] [Google Scholar]
- 17.Gottschalk M. 2012. Streptococcocis, 841–855 In Straw BE, Zimmerman JJ, D'Allaire S, Taylor DJ. (ed), Diseases of swine. Blackwell Publishing, Ames, IA [Google Scholar]
- 18.Vela AI, Goyache J, Tarradas C, Luque I, Mateos A, Moreno MA, Borge C, Perea JA, Dominguez L, Fernandez-Garayzabal JF. 2003. Analysis of genetic diversity of Streptococcus suis clinical isolates from pigs in Spain by pulsed-field gel electrophoresis. J. Clin. Microbiol. 41:2498–2502. 10.1128/JCM.41.6.2498-2502.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Petersen R, Hannerz H, Tuchsen F, Egerton JR. 2011. Meningitis, sepsis and endocarditis among workers occupationally exposed to pigs. Occup. Med. 61:437–439. 10.1093/occmed/kqr040 [DOI] [PubMed] [Google Scholar]
- 20.Wertheim HF, Nguyen HN, Taylor W, Lien TT, Ngo HT, Nguyen TQ, Nguyen BN, Nguyen HH, Nguyen HM, Nguyen CT, Dao TT, Nguyen TV, Fox A, Farrar J, Schultsz C, Nguyen HD, Nguyen KV, Horby P. 2009. Streptococcus suis, an important cause of adult bacterial meningitis in northern Vietnam. PLoS One 4:e5973. 10.1371/journal.pone.0005973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Suankratay C, Intalapaporn P, Nunthapisud P, Arunyingmongkol K, Wilde H. 2004. Streptococcus suis meningitis in Thailand. Southeast Asian J. Trop. Med. Public Health 35:868–876 [PubMed] [Google Scholar]
- 22.Smith TC, Capuano AW, Boese B, Myers KP, Gray GC. 2008. Exposure to Streptococcus suis among US swine workers. Emerg. Infect. Dis. 14:1925–1927. 10.3201/eid1412.080162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Michaud S, Duperval R, Higgins R. 1996. Streptococcus suis meningitis: first case reported in Quebec. Can. J. Infect. Dis. 7:329–331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Trottier S, Higgins R, Brochu G, Gottschalk M. 1991. A case of human endocarditis due to Streptococcus suis in North America. Rev. Infect. Dis. 13:1251–1252. 10.1093/clinids/13.6.1251 [DOI] [PubMed] [Google Scholar]
- 25.Willenburg KS, Sentochnik DE, Zadoks RN. 2006. Human Streptococcus suis meningitis in the United States. N. Engl. J. Med. 354:1325. 10.1056/NEJMc053089 [DOI] [PubMed] [Google Scholar]
- 26.Haleis A, Alfa M, Gottschalk M, Bernard K, Ronald A, Manickam K. 2009. Meningitis caused by Streptococcus suis serotype 14, North America. Emerg. Infect. Dis. 15:350–352. 10.3201/eid1502.080842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee GT, Chiu CY, Haller BL, Denn PM, Hall CS, Gerberding JL. 2008. Streptococcus suis meningitis, United States. Emerg. Infect. Dis. 14:183–185. 10.3201/eid1401.070930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fittipaldi N, Collis T, Prothero B, Gottschalk M. 2009. Streptococcus suis meningitis, Hawaii. Emerg. Infect. Dis. 15:2067–2069. 10.3201/eid1512.090825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fowler HN, Brown P, Rovira A, Shade B, Klammer K, Smith K, Scheftel J. 2013. Streptococcus suis meningitis in swine worker, Minnesota, U. S. A. Emerg. Infect. Dis. 19:330–331. 10.3201/eid1902.120918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vela AI, Aspiroz C, Fortuno B, Tirado G, Sierra J, Martinez R, Fernandez-Garayzabal JF. 2013. Meningitis caused by an unusual genotype (ST3) of Streptococcus suis. Infection 41:701–703. 10.1007/s15010-012-0382-y [DOI] [PubMed] [Google Scholar]
- 31.Camporese A, Tizianel G, Bruschetta G, Cruciatti B, Pomes A. 2007. Human meningitis caused by Streptococcus suis: the first case report from north-eastern Italy. Infez. Med. 15:111–114 [PubMed] [Google Scholar]
- 32.Zalas-Wiecek P, Michalska A, Grabczewska E, Olczak A, Pawlowska M, Gospodarek E. 2013. Human meningitis caused by Streptococcus suis. J. Med. Microbiol. 62:483–485. 10.1099/jmm.0.046599-0 [DOI] [PubMed] [Google Scholar]
- 33.Strangmann E, Froleke H, Kohse KP. 2002. Septic shock caused by Streptococcus suis: case report and investigation of a risk group. Int. J. Hyg. Environ. Health 205:385–392. 10.1078/1438-4639-00165 [DOI] [PubMed] [Google Scholar]
- 34.Clifton-Hadley FA, Enright MR. 1984. Factors affecting the survival of Streptococcus suis type 2. Vet. Rec. 114:584–586. 10.1136/vr.114.24.584 [DOI] [PubMed] [Google Scholar]
- 35.Berthelot-Herault F, Gottschalk M, Labbe A, Cariolet R, Kobisch M. 2001. Experimental airborne transmission of Streptococcus suis capsular type 2 in pigs. Vet. Microbiol. 82:69–80. 10.1016/S0378-1135(01)00376-5 [DOI] [PubMed] [Google Scholar]
- 36.Madsen LW, Nielsen B, Aalbaek B, Jensen HE, Nielsen JP, Riising HJ. 2001. Experimental infection of conventional pigs with Streptococcus suis serotype 2 by aerosolic exposure. Acta Vet. Scand. 42:303–306. 10.1186/1751-0147-42-303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Madsen LW, Bak H, Nielsen B, Jensen HE, Aalbaek B, Riising HJ. 2002. Bacterial colonization and invasion in pigs experimentally exposed to Streptococcus suis serotype 2 in aerosol. J. Vet. Med. B Infect. Dis. Vet. Public Health 49:211–215. 10.1046/j.1439-0450.2002.00491.x [DOI] [PubMed] [Google Scholar]
- 38.Létourneau V, Nehme B, Meriaux A, Masse D, Cormier Y, Duchaine C. 2010. Human pathogens and tetracycline-resistant bacteria in bioaerosols of swine confinement buildings and in nasal flora of hog producers. Int. J. Hyg. Environ. Health 213:444–449. 10.1016/j.ijheh.2010.09.008 [DOI] [PubMed] [Google Scholar]
- 39.Blais Lecours P, Veillette M, Marsolais D, Duchaine C. 2012. Characterization of bioaerosols from dairy barns: reconstructing the puzzle of occupational respiratory diseases by using molecular approaches. Appl. Environ. Microbiol. 78:3242–3248. 10.1128/AEM.07661-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bach HJ, Tomanova J, Schloter M, Munch JC. 2002. Enumeration of total bacteria and bacteria with genes for proteolytic activity in pure cultures and in environmental samples by quantitative PCR mediated amplification. J. Microbiol. Methods 49:235–245. 10.1016/S0167-7012(01)00370-0 [DOI] [PubMed] [Google Scholar]
- 41.Nehme B, Gilbert Y, Letourneau V, Forster RJ, Veillette M, Villemur R, Duchaine C. 2009. Culture-independent characterization of archaeal biodiversity in swine confinement building bioaerosols. Appl. Environ. Microbiol. 75:5445–5450. 10.1128/AEM.00726-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nga TV, Nghia HD, Tu le TP, Diep TS, Mai NT, Chau TT, Sinh DX, Phu NH, Nga TT, Chau NV, Campbell J, Hoa NT, Chinh NT, Hien TT, Farrar J, Schultsz C. 2011. Real-time PCR for detection of Streptococcus suis serotype 2 in cerebrospinal fluid of human patients with meningitis. Diagn. Microbiol. Infect. Dis. 70:461–467. 10.1016/j.diagmicrobio.2010.12.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Smith HE, Damman M, van der Velde J, Wagenaar F, Wisselink HJ, Stockhofe-Zurwieden N, Smits MA. 1999. Identification and characterization of the cps locus of Streptococcus suis serotype 2: the capsule protects against phagocytosis and is an important virulence factor. Infect. Immun. 67:1750–1756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Smith HE, de Vries R, van't Slot R, Smits MA. 2000. The cps locus of Streptococcus suis serotype 2: genetic determinant for the synthesis of sialic acid. Microb. Pathog. 29:127–134. 10.1006/mpat.2000.0372 [DOI] [PubMed] [Google Scholar]
- 45.Fittipaldi M, Nocker A, Codony F. 2012. Progress in understanding preferential detection of live cells using viability dyes in combination with DNA amplification. J. Microbiol. Methods 91:276–289. 10.1016/j.mimet.2012.08.007 [DOI] [PubMed] [Google Scholar]
- 46.Zhao Y, Aarnink AJA, Doornenbal P, Huynh HT, Groot Koerkamp PWG, Landman WJM, de Jong MCM. 2011. Investigation of the efficiencies of bioaerosol samplers for collecting aerosolized bacteria using a fluorescent tracer. II. Sampling efficiency and half-life time. Aerosol Sci. Technol. 45:432–442. 10.1080/02786826.2010.543196 [DOI] [Google Scholar]
- 47.Donham KJ. 1991. Association of environmental air contaminants with disease and productivity in swine. Am. J. Vet. Res. 52:1723–1730 [PubMed] [Google Scholar]
- 48.Wertheim HF, Nghia HD, Taylor W, Schultsz C. 2009. Streptococcus suis: an emerging human pathogen. Clin. Infect. Dis. 48:617–625. 10.1086/596763 [DOI] [PubMed] [Google Scholar]
- 49.Elliott SD. 1966. Streptococcal infection in young pigs. I. An immunochemical study of the causative agent (PM streptococcus). J. Hyg. 64:205–212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Elliott SD, Alexander TJ, Thomas JH. 1966. Streptococcal infection in young pigs. II. Epidemiology and experimental production of the disease. J. Hyg. 64:213–220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dee SA, Carlson AR, Winkelman NL, Corey MM. 1993. Effect of management practices on the Streptococcus suis carrier rate in nursery swine. J. Am. Vet. Med. Assoc. 203:295–299 [PubMed] [Google Scholar]
- 52.Dee SA, Corey MM. 1993. The survival of Streptococcus suis on farm and veterinary equipment. Swine Health Production 1:17–20 [Google Scholar]
- 53.Villani DJ. 2003. A retrospective evaluation of actions taken to control Streptococcus suis infection. Swine Health Production 11:27–30 [Google Scholar]
- 54.Johannson LM. 2006. Meningitis and septicemia in a 7-week-old piglet caused by dual streptococcal infection. Can. Vet. J. 47:796–798 [PMC free article] [PubMed] [Google Scholar]
- 55.Robertson ID, Blackmore DK, Hampson DJ, Fu ZF. 1991. A longitudinal study of natural infection of piglets with Streptococcus suis types 1 and 2. Epidemiol. Infect. 107:119–126. 10.1017/S0950268800048743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cloutier G, D'Allaire S, Martinez G, Surprenant C, Lacouture S, Gottschalk M. 2003. Epidemiology of Streptococcus suis serotype 5 infection in a pig herd with and without clinical disease. Vet. Microbiol. 97:135–151. 10.1016/j.vetmic.2003.09.018 [DOI] [PubMed] [Google Scholar]
- 57.Amass SF, San Miguel P, Clark LK. 1997. Demonstration of vertical transmission of Streptococcus suis in swine by genomic fingerprinting. J. Clin. Microbiol. 35:1595–1596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Amass SF, Clark LK, Knox KE, Wu CC, Hill MA. 1996. Streptococcus suis colonization of piglets during parturition. Swine Health Production 4:269–272 [Google Scholar]
- 59.Dekker N, Bouma A, Daemen I, Klinkenberg D, van Leengoed L, Wagenaar JA, Stegeman A. 2013. Effect of spatial separation of pigs on spread of Streptococcus suis serotype 9. PLoS One 8:e61339. 10.1371/journal.pone.0061339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gottschalk M, Xu J, Calzaas C, Segura M. 2010. Streptococcus suis: a new emerging or an old neglected zoonotic pathogen? Future Microbiol. 5:371–391. 10.2217/fmb.10.2 [DOI] [PubMed] [Google Scholar]
- 61.Charland N, Kobisch M, Martineau-Doize B, Jacques M, Gottschalk M. 1996. Role of capsular sialic acid in virulence and resistance to phagocytosis of Streptococcus suis capsular type 2. FEMS Immunol. Med. Microbiol. 14:195–203. 10.1111/j.1574-695X.1996.tb00287.x [DOI] [PubMed] [Google Scholar]
- 62.Trivedi K, Tang CM, Exley RM. 2011. Mechanisms of meningococcal colonisation. Trends Microbiol. 19:456–463. 10.1016/j.tim.2011.06.006 [DOI] [PubMed] [Google Scholar]