Abstract
Raman spectroscopy is a rapid nondestructive technique providing spectroscopic and structural information on both organic and inorganic molecular compounds. Extensive applications for the method in the characterization of pigments have been found. Due to the high sensitivity of Raman spectroscopy for the detection of chlorophylls, carotenoids, scytonemin, and a range of other pigments found in the microbial world, it is an excellent technique to monitor the presence of such pigments, both in pure cultures and in environmental samples. Miniaturized portable handheld instruments are available; these instruments can be used to detect pigments in microbiological samples of different types and origins under field conditions.
INTRODUCTION
A great diversity of pigments is found in the microbial world. There are many different classes of pigments present in prokaryotic and eukaryotic microorganisms, and these compounds have very different functional roles. The best known are light-harvesting pigments for photoautotrophic and photoheterotrophic growth and compounds that serve to protect cells against harmful radiation in the visible and UV wavelength range. In other cases, the function of the pigmentation is still unknown. Among the best-known pigments are the chlorophylls (Chls) of oxygenic phototrophs, bacteriochlorophylls (Bchls) in anoxygenic phototrophic prokaryotes, pheophytins (chlorophyll molecules depleted of the magnesium atom in their tetrapyrrole ring), and the phycobiliproteins in cyanobacteria. Some halophilic Archaea possess the retinal-containing pigments bacteriorhodopsin and halorhodopsin that serve as light-driven proton and chloride pumps; similar light-driven proton pumps (proteorhodopsin) are known in the Bacteria domain. In many groups of prokaryotes, carotenoids and flexirubins absorb radiation in the blue range of the spectrum, while scytonemin and mycosporin-like amino acids protect the cells against harmful UV radiation (1–6).
In recent years, Raman spectroscopy has become a popular analytical tool for the qualitative and quantitative assessment of inorganic and organic compounds, including biomolecules and microbial pigments. The Raman effect, discovered by Sir Chandrasekhar Venkata Raman in 1928 for which he was awarded the Nobel Prize in 1930, arises from the inelastic scattering of photons giving rise to energy changes. When a sample is illuminated with electromagnetic radiation, the light can be scattered by a molecule or by a crystal. In Raman scattering, the scattered photons have an energy which is either smaller (Stokes) or greater (anti-Stokes) than the energy of the incident light. The Raman effect occurring in a sample upon irradiation with a monochromatic laser is related to the population of the molecular rotational and vibrational energy levels. The Raman spectrum reflects the molecular transitions and contains Raman bands whose wavenumber shifts from the laser excitation wavenumber are indicative of particular chemical bonds and their molecular environments, molecular interactions, and overall molecular structures. This forms the basis of diagnostic qualitative and quantitative analytical Raman spectroscopy. A particular advantage of Raman spectroscopy for quantitative studies is the linear relationship between species concentration and band intensity. Raman scattering is not perceived to be a highly sensitive molecular scattering analytical technique compared with some other techniques; nevertheless, it has several advantages to make it the first choice for combined analytical procedures, and these will be described below.
Raman microspectrometers, i.e., systems in which an optical microscope is coupled to a spectrometer, were developed as the MOLE (molecular optical laser examiner) by Delhaye and Dhamelincourt in 1975 (7). These provided for the first time the ability to obtain a molecular chemical signature from an optical image. The combination of Raman spectroscopy with optical microscopy for the study of single cells was pioneered by Puppels et al. (8); in microbiology, Edwards et al. in 1991 used the combination to analyze the colonization of rock substrates by extremophilic lichens (9). In recent years, Raman spectroscopy has been widely applied in microbiology research. For example, the method has enabled the differentiation of enterococci from various sources (10) and the rapid identification of Candida species (11). Many additional microbiological applications have been reported (12–17).
Recent reviews (18, 19) show the power of the applications of Raman spectroscopy in the study of microorganisms. These reviews, however, do not focus on the Raman detection of pigments. Microbial pigments generally give very strong and distinct Raman signals (Table 1). Therefore, the technique has been used extensively in recent years to monitor the presence of different types of pigments in microorganisms in pure cultures and in environmental samples. Such studies have used high-resolution laboratory Raman spectrometers (Fig. 1a) and more recently, portable handheld instruments that can be used in the field (Fig. 1b).
TABLE 1.
Main Raman bands of microbial pigments
| Pigment | Origin | Prominent Raman bands observed (band positions, cm−1)a | Reference |
|---|---|---|---|
| Carotenoids | |||
| Bacterioruberin | Halobacterium sp. strain NRC-1 culture | 1,505 s, 1,152 s, 1,000 s | 51 |
| Halococcus dombrowskii DSM14522T culture | 1,507 s, 1,152 s, 1,002 s | 101 | |
| Halorubrum sodomense ATCC 33755T culture | 1,506 s, 1,152 s, 1,001 s | 54 | |
| Haloarcula vallismortis ATCC 29715T culture | 1,506 s, 1,151 s, 1,000 s | 54 | |
| Rubrobacter radiotolerans DSM 5868T culture | 1,503 s, 1,150 s, 1,000 s | 52 | |
| Bacteriorhodopsin | Pure protein | 1,526 s, 1,199 m, 1,168 m, 1,150 m, 1,007 s | 122 |
| β-Carotene | Dunaliella tertiolecta CS175 culture | 1,524 s, 1,155 s, 1,001 m | 123 |
| Synechocystis sp. strain PCC 6803 culture | 1,518 s, 1,155 s, 1,005 m | 28 | |
| Decapreno β-carotene | Natromonas pharaonis, Wadi Natrun, Egypt | 1,503 m, 1,152 m, 1,000 m | 124 |
| Dodecapreno β-carotene | Natromonas pharaonis, Wadi Natrun, Egypt | 1,503 m, 1,447 w, 1,389 w, 1,316 w, 1,285 w, 1,210 w, 1,147 m | 124 |
| Astaxanthin | Hematococcus pluvialis CCALA 883 culture | 1,520 s, 1,275 w, 1,192 w, 1,157 s, 1,007 m | 50 |
| Salinixanthin | Salinibacter ruber DSM 13855T culture | 1,512 s, 1,155 s, 1,003 s | 54 |
| Sarcinaxanthin | Micrococcus luteus DSM 348T culture | 1,532 s, 1,157 s, 1,005 s | 114 |
| Deinoxanthin | Deinococcus radiodurans culture | 1,510 s, 1,152 s, 1,003 s | 33 |
| Xanthomonadin | Xanthomonas axonopodis pv. dieffenbachiae culture | 1,529-1,531 s, 1,135-1,136 s, 1,004 s | 41 |
| Neurosporene | Rhodobacter sphaeroides G1C culture | 1,527 s, 1,288 w, 1,214 w, 1,158 s, 1,006 s | 62 |
| Spheroidene | Rhodobacter sphaeroides RCO2 culture | 1,524 s, 1,238 w, 1,159 s, 1,170 s, 1,003 w, 951 w | 125 |
| Flexirubin | Flavobacterium johnsoniae DSM 2064T culture | 1,529 s, 1,154, 1133s 1,004 m | 57 |
| Flexibacter elegans DSM 3317T culture | 1,515 s, 1,155 s, 996 m | 57 | |
| Chlorophylls | |||
| Chlorophyll a | Dunaliella tertiolecta CS175 culture | 1,554 m, 1,437 m, 1,325 m, 1,289 m, 1,233 m, 1,186 m, 986 m, 915 m, 757 m | 123 |
| Chlorophyll | Microbial crust, Atacama Desert, Chile | 1,445 m, 1,327 m, 915 m, 745 m, 508 w | 69 |
| Bacteriochlorophyll a | Rhodobacter sphaeroides G1C culture | 1,640 w, 1,441 w, 1,371 w, 1,173 s, 1,020 m, 900 s, 773 w, 733 s | 62 |
| Phycobiliprotein | Microbial crust, Atacama Desert, Chile | 1,631 w, 1,583 w, 1,370 w, 1,283 w, 1,235 w, 874 w, 815 w, 665 w | 69 |
| Scytonemin | Chlorogleopsis culture O-89-Cgs | 1,590 w, 1,556 m, 1,549 w, 1,172 m | 80 |
| Lyngbya aestuarii from native mat, Laguna Ojo de Liebre, Guerrero Negro, Baja California Sur, Mexico | 1,595 m, 1,554 m, 1,173 m | 75 | |
| Microbial crust, Atacama Desert, Chile | 1,596 s, 1,558 s, 1,321 s, 1,172 m | 69 | |
| Nostoc sp. | 1,630 s | 126 | |
| Mycosporine-like amino acid | Jarosite superficial crust, Rio Tinto, Spain | 1,493, 1,414, 1,340, 1,293, 1,215, 1,181, 1,150, 920, 845, 485 | 68 |
| Pulvinic acid derivatives | Microbial crust, Atacama Desert, Chile | 1,484,1,414, 1,350 | 69 |
| Rhizocarpic acid | Acarospora chlorophana (lichen) | 1,031, 1,002, 945, 619, 597 | 19 |
| Calycin | Candelariella sp. (epilithic lichen) | 1,605, 1,580, 1,390, 1,365, 1,295, 1,152, 990, 965, 482 | 127 |
| Atranorin | Cladonia coniocraea (lichen) | 1,674 s, 1,629, 1,605 s, 1,453, 1,406 s, 1,379, 1,311, 1,281, 1,115, 1,033, 1,000, 980, 956, 869, 826, 743 m, 704, 615, 503, 303 | 78 |
| Lepraria spp. (epilithic lichen) | 1,674 s, 1,629, 1,605 s, 1,406 s, 1,379, 1,311, 1,281, 1,033, 1,000, 980, 956, 869, 826, 743 m, 704, 615, 503, 300 | 127 | |
| Gyrophoric acid | Dirinaria aegialita (lichen) | 1,663, 1,627, 1,615, 1,453, 1,437, 1,385, 1,302, 1,287, 1,067, 1,046, 855, 782, 587, 567 | 78 |
| Anthraquinones | |||
| Parietin | Xanthoria elegans (epilithic lichen) | 1,671, 1,605, 1,380, 1,180, 970, 926 | 128 |
| Caloplaca crosbyae (epilithic lichen) | 1,670, 1,608, 1,551, 1,370, 1,275, 1,256, 1,215, 1,198, 1,181, 978, 925, 725, 610, 570, 518, 465, 458, 398 | 78 |
s, strong bands; m, medium bands; w, weak bands.
FIG 1.
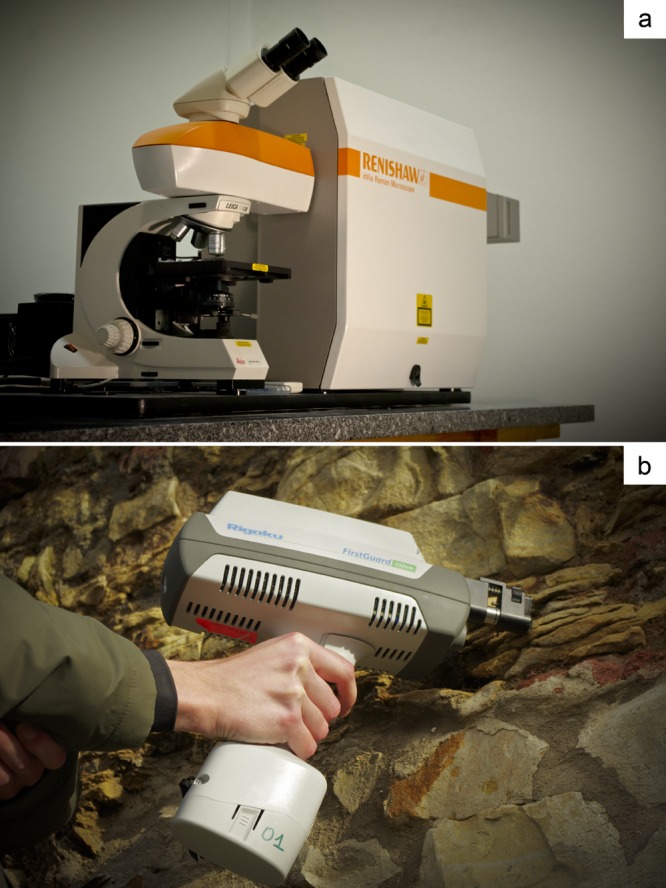
(a) Conventional confocal Raman microspectrometer. The laser irradiates the object selected in the optical microscope (laser spot ≈ 2 μm), the scattered light from the sample is collected by the optics of the microscope passing through holographic filters, pinhole, and monochromator to be detected by a charge-coupled device (CCD). (b) Handheld Raman spectrometer allows direct measurements on outcrops (cyanobacteria on siliciclastic rocks at Albertov, Prague, Czech Republic). The rugged probe head permits laser illumination of the object and collection of the scattered light.
We review here the many recent studies that have shown the power of Raman spectroscopy as a tool for the characterization of microbial pigments in a variety of settings.
Not included in this minireview are studies using enhancement of the Raman signal due to the generation of surface plasmon effects when the sample is deposited on Ag, Au, or other nanoparticles (surface enhanced Raman spectroscopy [SERS]). This technique has been applied to biomolecules (20–22), but it requires complex sample treatment, and it has mainly been used in clinical biomedical studies.
RAMAN SPECTROMETRY FOR PIGMENT ANALYSIS—HISTORICAL OVERVIEW
The application of Raman resonance spectroscopy to research on natural pigments, mainly carotenoids and chlorophylls, which became popular in the 1980s (23), dates back to the pioneer work of Euler and Hellstrom in 1932 (24). Their work came just a few years after the discovery of the Raman effect. It was followed in 1970 by the publication of Raman spectra of the carotenoid pigments lycopene and β-carotene in intact plant tissues (25). The technique was later used to assess the presence of light-harvesting bacteriochlorophyll and bacteriochlorophyll-protein complexes in purple photosynthetic bacteria (26). In 1986, it was documented that carotenoids can be easily detected in trace amounts in complex material and even within a single living cell on a very short time scale (27). Resonance Raman spectra (RRS) of astaxanthin-protein complexes published soon after showed some details of carotenoid-protein interactions (28).
RAMAN SPECTROMETRY FOR MICROBIOLOGICAL PIGMENT ANALYSIS—ADVANTAGES AND CRITICAL ISSUES
Raman spectroscopic techniques enable the direct interrogation of living colonies without detaching them from their organic or mineral substrates, affording a unique opportunity to derive molecular chemical structural information about individual components. Raman spectroscopy can thus complement pigment analysis in biological and geobiological matrices using classical techniques, such as absorption spectroscopy and high-performance liquid chromatography (HPLC), mass spectrometry (MS) including liquid chromatography (LC)-MS and gas chromatography (GC)-MS, and nuclear magnetic resonance (NMR) techniques largely used for precise conformational and structural studies. However, in these cases, extraction and sometimes extensive preparative isolation and purification work is needed, which can be quite time-consuming. Raman spectroscopy often does not require extraction and other preliminary sample preparation techniques and can additionally be employed under field conditions (with handheld mobile devices) when the use of other analytical techniques is not feasible.
The linear correlation between Raman signal intensity and chemical species concentration is very useful for the determination of relative concentrations of molecular species in mixtures, but itself has a disadvantage in that the signal response in the Raman spectrum is low compared with other techniques such as UV-visible (Vis) and Fourier transform infrared spectroscopy (FTIR), wherein the low-concentration species in a sample can be accessed using larger specimen path lengths according to the Beer-Lambert law. In Raman microspectroscopy, the use of a microscope to study micrometer-sized specimen regions obviates this disadvantage somewhat, but this usually means that many more spectral sampling points are needed to study large samples, especially when the specimens are heterogeneous. A particular advantage of Raman spectroscopy is that the bands obtained from host inorganic mineral matrices can also be accessed simultaneously with the organic moieties, which means that the molecular interactions between the biological material and their host lattices can be determined. Another advantage of Raman spectroscopy is the insensitivity of the technique to the presence of water. Other Raman spectroscopic techniques are now being explored for development and application in the microbiological field, such as spatially offset Raman spectroscopy (SORS), SERS, and RRS (29–31). Whereas these techniques may require some surface preparation, the application of RRS to microbiological pigments is well established especially for the detection of carotenoids through their signal enhancement provided by laser excitation wavelength near 500 nm, which results in a band intensity increase of several orders of magnitude over the normal nonresonance situation. Wavelength selection for excitation is extremely important for accessing quality Raman spectral data from microbiological samples, and often a multiwavelength approach is necessary to fully determine the composition of a complex specimen. It is critically important to monitor the irradiance of the specimen using different laser illumination configurations, as the focusing of only relatively small laser powers of several milliwatts into micrometer- diameter footprints for spectral interrogation can produce irradiances of milliwatts per square centimeter, enough to seriously deteriorate a living specimen or even to carbonize it. Hence, it is appropriate for the experienced Raman spectroscopist to first study the specimens using minimally small laser irradiances and then to increase the power levels as necessary to obtain the spectral data.
In some cases, the use of large incident laser irradiance levels is utilized to undertake the so-called “photobleaching” of fluorescent emission which is the bane of Raman spectroscopic studies in biology—here, bleaching or reduction of fluorescent emission intensity by imposing a high irradiance at one spot on the sample actually gives an improved Raman spectral response (32). This phenomenon is not well understood and is commonly referred to as “laser burnout.”
RAMAN SPECTROMETRY OF MICROBIAL PIGMENTS—PURE CULTURE STUDIES
Carotenoids.
Carotenoids play many vital roles in a large number of prokaryotic and eukaryotic microorganisms: they participate in energy-harvesting complexes, protect cells against damage by short-wavelength visible and UV radiation, and help repair cellular damage (33). They represent a diverse group of chemicals with a conjugated polyene skeletal backbone. The presence of this polyene backbone enhances the Raman signals, permitting the detection of extremely small amounts of carotenoids (33, 34). The first RRS of carotenoids were reported from 1970 on (23, 26). Tuning the excitation wavelength to the electronic absorption spectrum can produce selective enhancement of certain Raman bands (34, 35). These Raman bands correspond to vibrational modes that involve motions of the atoms in the chromophore, which is that portion of the molecule where the electronic transition is localized. Carotenoids have two strong Raman bands due to in-phase ν1(C=C) and ν2(C—) stretching vibrations of the polyene chain (23, 26, 36). For example, the Raman spectrum of β-carotene (11 conjugated double bonds) is characterized by the ν1 band located at 1,515 cm−1 and the ν2 band at 1,157 cm−1. A feature of medium intensity occurs at 1,008 cm−1 (ν3), corresponding to the in-plane rocking modes of the CH3 groups attached to the polyene chain. The wavenumber positions of both ν1 and ν2 bands depend on the length of the polyene chain (the number of conjugated double bonds) (37–40). The shift in band position is much more pronounced in the case of the ν1 band; a longer conjugated polyene chain causes a shift in the ν1 band to lower wavenumber positions and vice versa (37). When carotenoids are present in combination with other organic molecules, the excitation wavelength of 785 nm becomes an ideal compromise retaining a relatively high sensitivity toward carotenoids (41, 42).
A nice example of the use of Raman spectroscopy for the detection and identification of carotenoids in a heterotrophic prokaryote is the study of the pigments of Mycoplasma pneumoniae and the successful discrimination between different M. pneumoniae strains (38).
Carotenoid binding within the biomass, which affects the main polyene chain, can cause a significant shift of the ν1 band position due to change in electronic delocalization (42). Other factors affecting the band shifts in carotenoid Raman spectra are substitutions at the terminal end groups of the molecule (which result in very small wavenumber changes) and isomerism and molecular conformation in the solid and liquid state, respectively (37, 42–44). Moreover, the ν1 band position of carotenoids depends on the laser wavelength used for excitation (45, 46). This means that the unambiguous identification of particular carotenoids in complex organic material on the basis of Raman spectra alone may sometimes be difficult unless special interpretative appreciation has been given allowing for the various effects delineated above.
Binding of carotenoids to proteins in antenna complexes within photosystems leads to specific changes when carotenoid molecules are distorted from their planar configuration (46–49). In the cyanobacterium Anacystis nidulans (Synechococcus elongatus), an interaction between phycocyanin and a carotenoid was documented on the basis of a RRS investigation (48). In the case of Rhodobium marinum, preferential binding of spirilloxanthin to the photosynthetic reaction center was shown, while anhydrovibrin binds to light-harvesting system I of the pigment-protein complex (49).
Astaxanthin (3,3′-dihydroxy-β,β-carotene-4,4′-dione), found in plants, bacteria, fungi, and microalgae, has been the object of a number of Raman studies. A Fourier transform Raman spectrometer equipped with a 1,064-nm laser was used to monitor the changes in structures of pure astaxanthin standard and in situ in Haematococcus cells subjected to thermal stress. Discernible changes were monitored in the Raman spectra upon heating from −100°C systematically up to 150°C (50).
The pigmented extremely halophilic or halotolerant microorganisms that inhabit hypersaline environments such as saltern crystallizer ponds provide interesting opportunities for RRS studies of carotenoids and related pigments. Bacterioruberin is an example of a carotenoid with a long polyene chain (13 conjugated double bonds). RRS of Halobacterium salinarum pigmented pink-red by bacterioruberin (51) are characterized by the presence of bands at 1,505, 1,152, and 1,000 cm−1. There is also a published report on the possible presence of bacterioruberin, confirmed by Raman spectroscopy, as the major carotenoid present in actinobacteria related to Rubrobacter radiotolerans isolated from medieval frescoes in the Crypt of the Original Sin (Matera, Italy) (52).
A minor component of the hypersaline brine ecosystem is often Salinibacter ruber (Bacteroidetes). This red-colored bacterium produces a major carotenoid pigment named salinixanthin. It is a 40-carbon carotenoid with one terminal ring and one open end group bound to a glucose moiety which is esterified with a branched 15-carbon fatty acid (53). The Raman spectrum of this unusual pigment was recently published. The C=C stretching band is present at 1,512 cm−1, the C—CH3 mode band is observed at 1,003 cm−1, and the C—C stretching vibration lies at 1,155 cm−1 (54).
Flexirubins.
Flexibacter and Flavobacterium (Bacteroidetes) contain yellow to orange pigments called flexirubins. Flexirubin pigments are aryl polyenes containing a polyenoic acid chromophore terminated by a p-hydroxyphenyl group and esterified with a dialkylated resorcinol (55, 56). Raman spectroscopy is a suitable tool for the sensitive detection of flexirubin pigments in bacterial colonies and cell extracts of Flavobacterium johnsoniae and Flexibacter elegans, organisms that contain different flexirubins differentiated by the position of the ν1 band (1,527 to 1,529 cm−1 and 1,512 to 1,516 cm−1, respectively) and by the features in the second-order region of the spectrum (57).
Chls and Bchls.
Chlorophylls (Chls), including bacteriochlorophylls (Bchls), are the main functional and structural compounds in all photosystems of oxygenic and anoxygenic phototrophs. Raman spectra obtained from BChls from green photosynthetic bacteria are similar to those of the water-soluble Bchl-protein complexes but differ from spectra of monomeric and aggregated Bchls in vitro (58, 59). Fourier transform (FT) Raman spectroscopy using 1,064-nm laser excitation permits one to obtain Raman spectra suppressing the accompanying Bchl fluorescence emission; thus, key Raman band signatures of the chlorophyll porphyrin skeletal vibrations could be assigned, which can be used to identify chlorophylls in complex cultures (60). The conjugated aromatic pyrrole rings coordinated with the central magnesium(II) ion give characteristic Raman bands in the region of 400 to 1,600 cm−1, and these features have been adopted to identify the presence of chlorophylls in microbial colonies inhabiting rocks in cold-, UV-, and drought-stressed environments (61–63). The application of FT Raman spectroscopy at 1,064 nm to Rhodobacter sphaeroides G1C cultures demonstrated the presence of both bacteriochlorophyll a and neurosporene (64).
MAAs.
The mycosporine-like amino acids (MAAs) are condensation derivatives of a cyclohexenone ring and amino acid or imino alcohol residues (65). Based on the characteristics of UV absorption by MAAs, several studies have suggested that these compounds can act as natural screens of UV radiation and should be considered photoprotectants (66, 67). The Raman spectrum assigned to a mycosporine-like amino acid compound was reported for a jarosite matrix originating from Rio Tinto, Spain (68) and from hyperarid (Atacama, Chile) shallow subsurface layers (69).
Scytonemin.
Scytonemin is an effective UV radiation screening pigment exclusively synthesized by some cyanobacteria (70, 71). It is a yellow-brown lipid-soluble pigment, produced as part of the extracellular sheath (72). It may have originated in a common ancestor of the current groups of cyanobacteria or in an early member of this lineage (73, 74). Raman spectra of scytonemin were first investigated by Edwards et al. (75) using FT Raman spectroscopy. The spectra show characteristic corroborative bands of the molecule at 1,590 [ν(CCH) aromatic ring quadrant stretch], 1,549 [ν(CCH) p-disubstituted aromatic ring], 1,323 [ν(C=N) indole ring], and 1,172 cm−1 [ν(C=C—C=C) system (trans)].
RAMAN FEATURES OF PIGMENTS FROM NATURAL BIOGEOLOGICAL SAMPLES
Raman spectroscopy of lichen pigments.
Lichens, a symbiosis of unicellular green algae or cyanobacteria and fungi, may contain different unique pigments: rhizocarpic acid, parietin, and usnic acid. A comprehensive listing of the lichen pigments identified by Raman spectroscopy is given in Table 1. The first Raman spectrum of a lichen was reported in 1991 in a study of the biodegradation of 16th century Italian Renaissance frescoes in the Palazzo Farnese, Caprarola, Italy, by Dirina massiliensis forma sorediata (9). The major advantage of the application of Raman spectroscopy here is the ability to interrogate the lichen in situ without detachment from its substratum. Use of a Raman microprobe across a range of available laser excitation wavelengths enables the acquisition of molecular information from organic (acids and pigments) and inorganic components of the lichen encrustation with a spectral footprint of several micrometers in diameter (76–79). The distribution of pigments (zeaxanthin, gyrophoric acid, atranorin, and others) was determined in different species of lichens and reported from many areas of differing climatic and physicochemical and substrate conditions (80–86) (Table 1).
Raman spectroscopy of pigments of endoliths.
When environmental conditions do not permit microbes to survive on the rock surface, they colonize internal niches between or inside mineral grains in rocks. Under such harsh physical or chemical conditions, endoliths can accumulate special chemicals (e.g., pigments and compatible solutes) that help them to live under such extremes. Raman microspectrometry permits the direct study of pigment type and distribution in endolithic zones in the frame of natural stony samples without sample treatment or extractions. Microbial communities inhabiting rocks from various extreme environments were studied: Antarctic habitats (82–84), volcanic rocks as well as travertines on Svalbard (Norway) (80, 85) as well as halophiles from a hot desert (68, 86). Raman spectroscopy revealed differences in pigment distribution in chasmolithic and endolithic colonizations of differing lithologies, including those from dolomite, gypsum, or altered orthoquartzite (83, 86). Raman microspectrometry was able to detect scytonemin as well as chlorophyll, carotenoids and mycosporine-like amino acids in a series of samples of halite endoliths from Yungay and Salar Grande sites in the Atacama Desert in Chile (69). Detailed information on gypsum colonizations in the Atacama Desert was also gathered: in situ Raman microspectroscopy revealed differences between cyanobacteria and eukaryotic algae, without any sample preparation, and facilitated the estimation of the physiological state of the cyanobacteria, showing a depletion of phycobiliprotein signals relative to carotenoids and chlorophyll within decayed cells (87).
Identification of microbial pigments in halite inclusions by Raman spectroscopy.
Crystallizing halite can easily entrap microorganisms and organic debris in liquid inclusions, and there they can retain their viability for prolonged periods (88–92). The presence of cells resembling Dunaliella within brine inclusions in ancient halite, 9 thousand years to 1.44 millions of years ago, from borehole cores from Death Valley, Saline Valley, and Searles Lake, California, was corroborated by Raman microspectrometry (with excitation at 514.5 nm). Well-preserved carotenoids occurred within fluid inclusions as yellowish to red-brown amorphous and crystalline masses associated with spheroidal algal cells resembling Dunaliella (93). Raman microspectrometry also showed β-carotene accumulations originating from Dunaliella in halite inclusions from shallow deposits in extremely acidic and saline Lake Magic (western Australia) (94). Laboratory simulation studies have been performed to follow the entrapment of nine different strains of halophilic Archaea within growing halite crystals. Two laser excitation wavelengths were used: nonresonant 1,064 nm and resonant 514.5 nm. The main peaks collected (1,507, 1,152, and 1,002 cm−1) could be mainly assigned to bacterioruberin (95).
ADVANCED RAMAN SPECTROSCOPIC TECHNIQUES IN MICROBIOLOGY
Recently, Raman micromapping has been employed in microbiological research, as it can rapidly provide useful detailed information at the molecular level. Maps of the occurrence of a given compound can be obtained collecting huge series of individual point RS and representing the presence/intensity of a characteristic Raman band as two-dimensional maps. However, in this approach, very small sample areas are mapped, and long times of accumulation may be needed to obtain distribution maps. Planar samples are preferred, and very precise microscopic stages are needed for controlled programmed stepwise scanning of the sample (x and y directions). Generally, a z-direction automatic fast focusing to the surface at each point permits correct spectrum collection. Fluorescence effects and spectroscopic artifacts have to be avoided by proper calibration and advanced confocality of the instruments.
Advanced Raman instrumentation provides the possibility to learn more about the spatial distribution of pigments within the cell. Thus, the presence of β-carotene and chlorophyll a was demonstrated in vivo in individual cells within colonies of Dunaliella tertiolecta using confocal Raman microscopy with excitation at 532 nm (96). Confocal Raman spectroscopy was used to differentiate and map the relation between a carotene-containing yeast and Pseudomonas aeruginosa cells in living, hydrated, composite biofilms on the basis of their spectral signatures (97). The presence of carotenoids, chlorophyll, and triglycerides was monitored in healthy and starved colonies of the algae Chlorella sorokiniana and Neochloris oleoabundans (98). The distribution of carotenoids in different microalgae (Dunaliella, Phaeodactylum, Haematococcus pluvialis, Thermosynechococcus elongatus) was mapped at the cellular level (99–103) using slightly different instrumental settings (Fig. 2). Raman spectroscopy was used to monitor the production of astaxanthin in the yeast Phaffia rhodozyma in fed-batch experiments (104). The use of laser tweezers Raman spectroscopy (LTRS) provides further possibilities (105). It enabled the quantification of the carotenoid content of the yeast Rhodotorula glutinis based on the relative intensity of the ν1 band (106). Coherent anti-Stokes Raman scattering microscopy (CARS) has been identified as an additional technique for advanced imaging of carotenoids in microalgae and cyanobacteria. In this technique, interfering fluorescence effects are efficiently suppressed (107–109).
FIG 2.
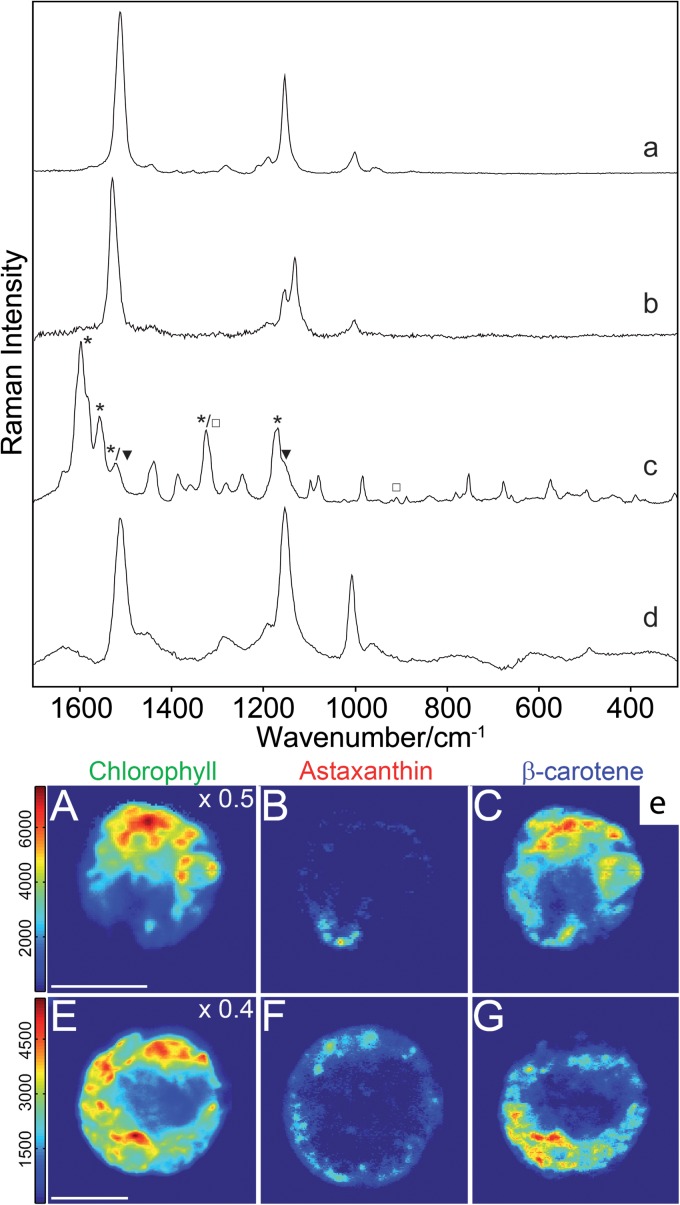
Examples of microbiological application of Raman spectroscopy using different instrumentation. (a to c) Raman spectra obtained using a laboratory Raman microspectrometer. Raman spectra of salinixanthin from a culture of Salinibacter ruber (a) (532-nm wavelength; image modified from reference 54), of flexirubin from a culture of Flavobacterium johnsonae (b) (532-nm wavelength; image modified from reference 57), and of chlorophyll (□), carotenoid (▼), and scytonemin (*) from a native gypsum crust (Yungay, Atacama, Chile) (c) (785-nm wavelength; image modified from reference 69). (d) Raman spectrum collected using a handheld Raman spectrometer of a spirilloxanthin-like carotenoid of the red layer of the bottom gypsum crust from a saltern at Eilat (Israel) colonized by Halochromatium-like microorganisms (532-nm wavelength; image modified from reference 117). (e) Example of Raman mapping–distribution of carotenoids (astaxanthin, β-carotene, chlorophyll in the frame of flagellated motile cell obtained using a confocal Raman spectrometer (532-nm wavelength; image modified from reference 100, which was published under a Creative Commons agreement). The intensities within the concentration map represent relative component concentrations. Bars, 10 μm.
Finally, Raman spectroscopy of pigments can also be a powerful tool in chemotaxonomy studies. Rösch et al. (110) proposed the use of micro-Raman analysis together with a data classification approach they called the support vector machine (SVM) technique. They tested it on a series of microorganisms originating from airborne particles of clean-room samples. Raman microspectrometry allowed the characterization of bacteria as well as their main cellular components, including pigments in a few cases.
Miniaturization/field applications of Raman spectroscopy in geobiology and astrobiology.
Raman spectroscopy is an extremely promising technique for life detection systems in space exploration. Therefore, the development of miniaturized instruments and their exploitation for the analysis of geological samples on Earth will prepare the possible future deployment of Raman instruments to test for pigments such as carotenoids deposited by extinct or extant organisms in the Mars regolith (9, 34, 35, 62, 63, 111–118). Very recent publications review the state of the art of the use of portable and handheld Raman instruments and experience using these instruments (119–121).
Raman spectrometry with 785-nm excitation is useful for the study of biological samples as that wavelength is optimal for the identification of chlorophyll a. Small and lightweight (<2 kg) handheld battery-powered Raman instruments have been developed only recently (first 785-nm diodes, more recently 532 nm), and they enable the researcher to obtain spectra outdoors under field conditions. The spectral resolution of commonly marketed portable instruments is around 8 cm−1, and the operational wavenumber shift range is generally 2,000 to 200 cm−1. Miniaturized Raman instruments have already been applied to the study of lichens in situ. Examples are the detection of different biomolecules associated with the yellow Antarctic lichen Acarospora chlorophana with 852-nm excitation, using one of the first prototype instruments (113). Portable systems with 785-nm excitation performed well in the investigation of the degradation of rhizocarpic acid, parietin, and β-carotene in Xanthoria parietina during irradiation experiments (114). Another type of handheld instrument (excitation wavelength of 785 nm) was used to probe lichen samples from a hyperarid area of the Tabernas Desert (Spain). Native lichens were investigated under wet conditions, and several photosynthetic pigments were documented in Squamarina lentigera, Diploschistes diacapsis, as well as in Lepraria crassissima (115). Portable and miniaturized Raman spectrometers were further applied to the detection of microbial biomarkers in halite from the hyperarid region at Yungay (Atacama Desert, Chile). Measurements were performed directly on the rock and on homogenized, powdered rock samples. For carotenoid analysis, excitation at 532 nm was found to be superior for the analysis of powdered specimens, but 785-nm excitation enabled the improved detection of scytonemin, another pigment present in the material examined (116). Using 1,064-nm laser excitation from a semiportable instrument facilitated the detection of scytonemin, carotenoids, and chlorophyll in evaporitic cyanobacterial colonization in the Atacama Desert and carotenes in the epilithic lichen Acarospora sp. (112).
With a handheld spectrometer (514-nm excitation) (Rigaku), Raman spectra of pigments in autotrophic (cyanobacteria and purple sulfur bacteria) and heterotrophic halophilic microorganisms (Archaea of the family Halobacteriaceae and Salinibacter) were recorded. Common and less common carotenoids, including α-bacterioruberin (Haloferax, Haloarcula, and Halobacterium) (Archaea), salinixanthin (Salinibacter) (Bacteroidetes), and spirilloxanthin-like (Ectothiorhodospira) (Gammaproteobacteria) were detected in cell pellets of laboratory cultures. Bacterioruberin was identified as the dominant carotenoid in pellets of cells collected from the saltern crystallizer ponds in Eilat, Israel. Raman band positions correspond well (within 4 cm−1) with data recorded on standards or using high-performance dispersive laboratory microspectrometers. Raman analysis of the colored microbial community layers in a benthic gypsum crust in the saltern evaporation ponds also identified myxoxanthophyll and echinenone carotenoids as well as spirilloxanthin in different layers (117).
CONCLUDING REMARKS
Raman spectroscopy has an excellent sensitivity for detecting polyenes, including carotenoids, because of the strong spectral signatures of the conjugated C—C and C=C functional modes. In particular, this is enhanced by several orders of magnitude through resonance Raman excitation effects occurring between 500- and 532-nm laser wavelengths. In spite of the limitations as outlined in this minireview, Raman spectroscopy has several advantages for pigment and carotenoid work. The technique is nondestructive and very fast, and in the microspectrometry mode, spectral information can be obtained about specific molecules of interest even in complex mixtures. Also, it can be combined with optical imaging of samples at the micrometric level, which is advantageous for the direct in situ interrogation of biological inclusions in mineralogical matrices. The astrobiological potential is now well realized, and it is planned to take miniaturized Raman spectrometers to Mars in future space missions.
ACKNOWLEDGMENTS
This study was supported by grant P210/10/0467 from the Grant Agency of the Czech Republic, by institutional support MSM0021620855 from the Ministry of Education of the Czech Republic (both to J.J.), and by grant 1103/10 from the Israel Science Foundation (to A.O.).
We thank Adam Culka and Petr Vítek (IGMMR) for technical help and pictures.
Footnotes
Published ahead of print 28 March 2014
REFERENCES
- 1.Stanier RY, Cohen-Bazire G. 1977. Phototrophic prokaryotes: the cyanobacteria. Annu. Rev. Microbiol. 31:225–274. 10.1146/annurev.mi.31.100177.001301 [DOI] [PubMed] [Google Scholar]
- 2.Oren A. 2002. Pigments of halophilic microorganisms, p 173–206 In Halophilic microorganisms and their environments: cellular origin, life in extreme habitats and astrobiology. Springer, Dordrecht, The Netherlands [Google Scholar]
- 3.Koyama Y. 1991. Structures and functions of carotenoids in photosynthetic systems. J. Photochem. Photobiol. B 9:265–280. 10.1016/1011-1344(91)80165-E [DOI] [Google Scholar]
- 4.Garcia-Pichel F, Castenholz RW. 1993. Occurrence of UV-absorbing mycosporine-like compounds among cyanobacterial isolates and an estimate of their screening capacity. Appl. Environ. Microbiol. 59:163–169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Edge R, McGarvey DJ, Truscott TG. 1997. The carotenoids as anti-oxidants—a review. Photochem. Photobiol. B Biol. 41:189–200. 10.1016/S1011-1344(97)00092-4 [DOI] [PubMed] [Google Scholar]
- 6.Frank HA, Chynwat V, Desamero RZB, Farhoosh R, Erickson J, Bautista J. 1997. On the photophysics and photochemical properties of carotenoids and their role as light-harvesting pigments in photosynthesis. Pure Appl. Chem. 69:2117–2124 [Google Scholar]
- 7.Delhaye M, Dhamelincourt P. 1975. Raman microprobe and microscope with laser excitation. J. Raman Spectrosc. 3:33–43. 10.1002/jrs.1250030105 [DOI] [Google Scholar]
- 8.Puppels GJ, Demul FFM, Otto C, Greve J, Robert-Nicoud M, Arndt-Jovin DJ, Jovin TM. 1990. Studying single living cells and chromosomes by confocal Raman microspectroscopy. Nature 347:301–303. 10.1038/347301a0 [DOI] [PubMed] [Google Scholar]
- 9.Edwards HGM, Farwell DW, Seaward MRD. 1991. Raman spectra of oxalates in lichen encrustations on Renaissance frescoes. Spectrochim. Acta A 47:1531–1539. 10.1016/0584-8539(91)80247-G [DOI] [Google Scholar]
- 10.Kirschner C, Maquelin K, Pina P, Ngo Thi NA, Choo-Smith LP, Sockalingum GD, Sandt C, Ami D, Orsini F, Doglia SM, Allouch P, Mainfait M, Puppels GJ, Naumann D. 2001. Classification and identification of enterococci: a comparative phenotypic, genotypic, and vibrational spectroscopic study. J. Clin. Microbiol. 39:1763–1770. 10.1128/JCM.39.5.1763-1770.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maquelin K, Choo-Smith LP, Endtz HP, Bruining HA, Puppels GJ. 2002. Rapid identification of Candida species by confocal Raman microspectroscopy. J. Clin. Microbiol. 40:594–600. 10.1128/JCM.40.2.594-600.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chan J, Fore S, Wachsman-Hogiu S, Huser T. 2008. Raman spectroscopy and microscopy of individual cells and cellular components. Laser Photon. Rev. 2:325–349. 10.1002/lpor.200810012 [DOI] [Google Scholar]
- 13.Harz M, Rösch P, Popp J. 2009. Vibrational spectroscopy—a powerful tool for the rapid identification of microbial cells at the single-cell level. Cytometry A 75:104–113. 10.1002/cyto.a.20682 [DOI] [PubMed] [Google Scholar]
- 14.Maquelin K, Kirschner C, Choo-Smith LP, van den Braak N, Endtz HP, Naumann D, Puppels GJ. 2002. Identification of medically relevant microorganisms by vibrational spectroscopy. J. Microbiol. Methods 51:255–271. 10.1016/S0167-7012(02)00127-6 [DOI] [PubMed] [Google Scholar]
- 15.Petry R, Schmitt M, Popp J. 2003. Raman spectroscopy—a prospective tool in the life sciences. Chem. Phys. Chem. 4:14–30. 10.1002/cphc.200390004 [DOI] [PubMed] [Google Scholar]
- 16.Wagner M. 2009. Single-cell ecophysiology of microbes as revealed by Raman microspectroscopy or secondary ion mass spectrometry imaging. Annu. Rev. Microbiol. 60:411–429. 10.1146/annurev.micro.091208.073233 [DOI] [PubMed] [Google Scholar]
- 17.Rösch P, Schmitt M, Kiefer W, Popp J. 2003. The identification of microorganisms by micro-Raman spectroscopy. J. Mol. Struct. 661-662:363–369. 10.1016/j.molstruc.2003.06.004 [DOI] [Google Scholar]
- 18.Huang WE, Li M, Jarvis RM, Goodacre R, Banwart SA. 2010. Shining light on the microbial world: the application of Raman microspectroscopy. Adv. Appl. Microbiol. 70:153–186. 10.1016/S0065-2164(10)70005-8 [DOI] [PubMed] [Google Scholar]
- 19.Lu X, Al-Qadiri HM, Lin M, Rasco BA. 2011. Application of mid-infrared and Raman spectroscopy to the study of bacteria. Food Bioprocess Technol. 4:919–935. 10.1007/s11947-011-0516-8 [DOI] [Google Scholar]
- 20.Kneipp J, Kneipp H, Kneipp K. 2008. SERS—a single-molecule and nanoscale tool for bioanalytics. Chem. Soc. Rev. 37:1052–1060. 10.1039/b708459p [DOI] [PubMed] [Google Scholar]
- 21.Wachsmann-Hogiu S, Weeks T, Huser T. 2009. Chemical analysis in vivo and in vitro by Raman spectroscopy-from single cells to humans. Curr. Opin. Biotechnol. 20:63–73. 10.1016/j.copbio.2009.02.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lombardi JR, Birke RL. 2009. A unified view of surface-enhanced Raman scattering. Acc. Chem. Res. 42:734–742. 10.1021/ar800249y [DOI] [PubMed] [Google Scholar]
- 23.Merlin JC. 1985. Resonance Raman spectroscopy of carotenoids and carotenoid-containing systems. Pure Appl. Chem. 57:785–792 [Google Scholar]
- 24.Euler HV, Hellstrom H. 1932. Raman Spektren von Carotinoiden. Z. Phys. Chem. 15:342–346 [Google Scholar]
- 25.Gill D, Kilponen RG, Rimai L. 1970. Resonance Raman scattering of laser radiation by vibrational modes of carotenoid pigment molecules in intact plant tissues. Nature 227:743–744. 10.1038/227743a0 [DOI] [PubMed] [Google Scholar]
- 26.Hayashi H, Hamaguchi H, Tasumi M. 1983. Resonance Raman spectra of light-harvesting bacteriochlorophyll a in pigment-protein complexes from purple photosynthetic bacteria. Chem. Lett. 12:1857–1860. 10.1246/cl.1983.1857 [DOI] [Google Scholar]
- 27.Wagner WD. 1986. Raman excitation profiles from pigments in vivo. J. Raman Spectrosc. 17:51–53. 10.1002/jrs.1250170111 [DOI] [Google Scholar]
- 28.Merlin JC. 1987. Resonance Raman analysis of astaxanthin-protein complexes. J. Raman Spectrosc. 18:519–523. 10.1002/jrs.1250180713 [DOI] [Google Scholar]
- 29.Duncan MD, Reintjes J, Manuccia TJ. 1982. Scanning coherent anti-Stokes Raman microscope. Opt. Lett. 7:350–352. 10.1364/OL.7.000350 [DOI] [PubMed] [Google Scholar]
- 30.Palonpon AF, Sodeoka M, Fujita K. 2013. Molecular imaging of live cells by Raman microscopy. Curr. Opin. Chem. Biol. 17:708–715. 10.1016/j.cbpa.2013.05.021 [DOI] [PubMed] [Google Scholar]
- 31.Stender AS, Marchuk K, Liu C, Sander S, Matthew W, Meyer MW, Smith EA, Neupane B, Wang G, Li J, Cheng JX, Huang B, Fang N. 2013. Single cell optical imaging and spectroscopy. Chem. Rev. 113:2469–2527. 10.1021/cr300336e [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Andreeva A, Abarova S, Stoitchkova K, Picorel R, Velitchkova M. 2007. Selective photobleaching of chlorophylls and carotenoids in photosystem I. Particles under high-light treatment. Photochem. Photobiol. 83:1301–1307. 10.1111/j.1751-1097.2007.00136.x [DOI] [PubMed] [Google Scholar]
- 33.Dartnell LR, Page K, Jorge-Villar SE, Wright G, Munshi T, Scowen IJ, Ward JM, Edwards HGM. 2012. Destruction of Raman biosignatures by ionising radiation and the implications for life detection on Mars. Anal. Bioanal. Chem. 403:131–144. 10.1007/s00216-012-5829-6 [DOI] [PubMed] [Google Scholar]
- 34.Vítek P, Osterrothová K, Jehlička J. 2009. Beta-carotene—a possible biomarker in the Martian evaporitic environment: Raman micro-spectroscopic study. Planet. Space Sci. 57:454–459. 10.1016/j.pss.2008.06.001 [DOI] [Google Scholar]
- 35.Vítek P, Jehlička J, Edwards HGM, Osterrothová K. 2009. Identification of β-carotene in an evaporitic matrix—evaluation of Raman spectroscopic analysis for astrobiological research on Mars. Anal. Bioanal. Chem. 393:1967–1975. 10.1007/s00216-009-2677-0 [DOI] [PubMed] [Google Scholar]
- 36.Carey PR. 1982. Biochemical applications of Raman and resonance Raman spectroscopies. Academic Press, New York, NY [Google Scholar]
- 37.Withnall R, Chowdhry BZ, Silver J, Edwards HGM, de Oliveira LFC. 2003. Raman spectra of carotenoids in natural products. Spectrochim. Acta A 59:2207–2212. 10.1016/S1386-1425(03)00064-7 [DOI] [PubMed] [Google Scholar]
- 38.Maquelin K, Hoogenboezem T, Jachtenberg JW, Dumke R, Jacobs E, Puppels GJ, Hartwig NG, Vink C. 2009. Raman spectroscopic typing reveals the presence of carotenoids in Mycoplasma pneumoniae. Microbiology 155:2068–2077. 10.1099/mic.0.026724-0 [DOI] [PubMed] [Google Scholar]
- 39.Koyama Y. 1995. Resonance Raman spectroscopy, p 135–146 In Britton G, Liaaen-Jensen S, Pfander H. (ed), Carotenoids, vol 1B Spectroscopy. Birkhauser, Basel, Switzerland [Google Scholar]
- 40.Kniggendorf A-K, Gaul TW, Meinhardt-Wollweber M. 2011. Effects of ethanol, formaldehyde, and gentle heat fixation in confocal resonance Raman microscopy of purple nonsulfur bacteria. Microsc. Res. Tech. 74:177–183. 10.1002/jemt.20889 [DOI] [PubMed] [Google Scholar]
- 41.Paret ML, Sharma S, Green LM, Alvarez AM. 2010. Biochemical characterization of Gram-positive and Gram-negative plant-associated bacteria with micro-Raman spectroscopy. Appl. Spectrosc. 64:433–441 [DOI] [PubMed] [Google Scholar]
- 42.de Oliveira VE, Castro HV, Edwards HGM, de Oliveira LFC. 2010. Carotenes and carotenoids in natural biological samples: a Raman spectroscopic analysis. J. Raman Spectrosc. 41:642–650 [Google Scholar]
- 43.Liaaen-Jensen S. 1997. Stereochemical aspects of carotenoids. Pure Appl. Chem. 69:2027–2038 [Google Scholar]
- 44.Barnard W, de Waal D. 2006. Raman investigation of pigmentary molecules in the molluscan biogenic matrix. J. Raman Spectrosc. 37:342–352. 10.1002/jrs.1461 [DOI] [Google Scholar]
- 45.Ruban AV, Pascal AA, Robert B, Horton P. 2001. Configuration and dynamics of xanthophylls in light-harvesting antennae of higher plants - spectroscopic analysis of isolated light-harvesting complex of photosystem II and thylakoid membranes. J. Biol. Chem. 276:24862–24870. 10.1074/jbc.M103263200 [DOI] [PubMed] [Google Scholar]
- 46.Andreeva A, Velitchkova M. 2005. Resonance Raman spectroscopy of carotenoids in photosystem I particles. Biophys. Chem. 114:129–135. 10.1016/j.bpc.2004.11.012 [DOI] [PubMed] [Google Scholar]
- 47.Gruszecki WI, Gospodarek M, Grudzinski W, Mazur R, Gieczewska K, Garstka M. 2009. Light-induced change of configuration of the LHCII-bound xanthophyll (tentatively assigned to violaxanthin): a resonance Raman study. J. Phys. Chem. B 113:2506–2512. 10.1021/jp8101755 [DOI] [PubMed] [Google Scholar]
- 48.Szalontai B, Vandeven M. 1981. Raman spectroscopic evidence for phycocyanin-carotenoid interaction in Anacystis nidulans. FEBS Lett. 131:155–157. 10.1016/0014-5793(81)80909-X [DOI] [Google Scholar]
- 49.Qian P, Saiki K, Mizoguchi T, Hara K, Sashima T, Fujii R, Koyama Y. 2001. Time-dependent changes in the carotenoid composition and preferential binding of spirilloxanthin to the reaction center and anhydrorhodovibrin to the LH1 antenna complex in Rhodobium marinum. Photochem. Photobiol. 74:444–452. 10.1562/0031-8655(2001)0740444TDCITC2.0.CO2 [DOI] [PubMed] [Google Scholar]
- 50.Kaczor A, Baranska M. 2011. Structural changes of carotenoid astaxanthin in a single algal cell monitored in situ by Raman spectroscopy. Anal. Chem. 83:7763–7770. 10.1021/ac201302f [DOI] [PubMed] [Google Scholar]
- 51.Marshall CP, Leuko S, Coyle CM, Walter MR, Burns BP, Neilan BA. 2007. Carotenoid analysis of halophilic Archaea by resonance Raman spectroscopy. Astrobiology 7:631–643. 10.1089/ast.2006.0097 [DOI] [PubMed] [Google Scholar]
- 52.Imperi F, Caneva G, Cancellieri L, Ricci MA, Sodo A, Vizca P. 2007. The bacterial aetiology of rosy discoloration of ancient wall paintings. Environ. Microbiol. 9:2894–2902. 10.1111/j.1462-2920.2007.01393.x [DOI] [PubMed] [Google Scholar]
- 53.Lutnaes BF, Oren A, Liaaen-Jensen S. 2002. New C40-carotenoid acyl glycoside as principal carotenoid of Salinibacter ruber, an extremely halophilic eubacterium. J. Nat. Prod. 65:1340–1343. 10.1021/np020125c [DOI] [PubMed] [Google Scholar]
- 54.Jehlička J, Oren A, Edwards HGM. 2013. Bacterioruberin and salinixanthin carotenoids of extremely halophilic Archaea and Bacteria: a Raman spectroscopic study. Spectrochim. Acta A 106:99–103. 10.1016/j.saa.2012.12.081 [DOI] [PubMed] [Google Scholar]
- 55.Achenbach H, Kohl W, Wachter W, Reichenbach H. 1978. New flexirubin-type pigments. Arch. Microbiol. 117:253–257. 10.1007/BF00738543 [DOI] [PubMed] [Google Scholar]
- 56.Oren A. 2011. Characterization of pigments of prokaryotes and their use in taxonomy and classification. Methods Microbiol. 38:262–283 [Google Scholar]
- 57.Jehlička J, Osterrothová K, Oren A, Edwards HGM. 2013. Raman spectrometric discrimination of flexirubin pigments from two genera of Bacteroidetes. FEMS Microbiol. Lett. 348:97–102 [DOI] [PubMed] [Google Scholar]
- 58.Lutz M. 1977. Antenna chlorophyll in photosynthetic membranes. A study by resonance Raman-spectroscopy. Biochim. Biophys. Acta 460:408–430. 10.1016/0005-2728(77)90081-0 [DOI] [PubMed] [Google Scholar]
- 59.Lutz M, Kleo J, Reisshusson F. 1976. Resonance Raman scattering of bacteriochlorophyll, bacteriopheophytin and spheroidene in reaction centers of Rhodopseudomonas speroides. Biochem. Biophys. Res. Commun. 69:711–717. 10.1016/0006-291X(76)90933-5 [DOI] [PubMed] [Google Scholar]
- 60.Koyama Y, Umemoto Y, Akamatsu A, Uehara K, Tanaka M. 1986. Raman spectra of chlorophyll forms. J. Mol. Struct. 146:273–287. 10.1016/0022-2860(86)80299-X [DOI] [Google Scholar]
- 61.Wynn-Williams DD, Edwards HGM. 2002. Environmental UV radiation: biological strategies for protection and avoidance, p 245–260 In Horneck G, Baumstark-Khan C. (ed), Astrobiology: the quest for the conditions of life. Springer-Verlag, Berlin, Germany [Google Scholar]
- 62.Edwards HGM, Hutchinson IB, Ingley R. 2013. Raman spectral signatures in the biogeological record: an astrobiological challenge, p 311–330 In de Vera JP, Seckbach J. (ed), Habitability of other planets and satellites. Cellular origin, life in extreme habitats and astrobiology series, vol 28 Springer, Dordrecht, The Netherlands [Google Scholar]
- 63.Edwards HGM, Hutchinson IB, Ingley R, Parnell J, Vítek P, Jehlička J. 2013. Raman spectroscopic analysis of geological and biogeological specimens of relevance to the ExoMars mission. Astrobiology 13:543–549. 10.1089/ast.2012.0872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Okada K, Nishawa E, Fujimoto Y, Koyama Y, Muraishi S, Ozaki Y. 1992. Nondestructive structural analysis of photosynthetic pigments in living Rhodobacter sphaeroides mutants by near-infrared Fourier transform Raman spectroscopy. Appl. Spectrosc. 46:518–523. 10.1366/0003702924125267 [DOI] [Google Scholar]
- 65.Favre-Bonvin J, Arpin N, Brevard C. 1976. Structure de la mycosporine (P310). Can. J. Chem. 54:1105–1110. 10.1139/v76-158 [DOI] [Google Scholar]
- 66.Vernet M, Brody E, Holm-Hansen O, Mitchell BG. 1994. The response of antarctic phytoplankton to ultraviolet radiation: absorption, photosynthesis and taxonomic composition, p 143–158 In Weiler S, Penhale P. (ed), Ultraviolet radiation and biological research in Antarctica, vol 62 American Geophysical Union, Washington, DC [Google Scholar]
- 67.Castenholz RW, Garcia-Pichel F. 2000. Cyanobacterial responses to UV-radiation, p 591–611 In Whitton BA, Potts M. (ed), The ecology of cyanobacteria. Kluwer Academic Publishers, Dordrecht, The Netherlands [Google Scholar]
- 68.Edwards HGM, Vandenabeele P, Villar SEJ, Carter EA, Rull Perez F, Hargreaves MD. 2007. The Rio Tinto Mars Analogue site: an extremophilic Raman spectroscopic study. Spectrochim. Acta A 68:1133–1137. 10.1016/j.saa.2006.12.080 [DOI] [PubMed] [Google Scholar]
- 69.Vítek P, Edwards HGM, Jehlička J, Ascaso C, De los Ríos A, Valea S, Villar SEJ, Davila AF, Wierzchos J. 2010. Microbial colonization of halite from the hyper-arid Atacama Desert studied by Raman spectroscopy. Philos. Trans. R. Soc. A 368:3205–3221. 10.1098/rsta.2010.0059 [DOI] [PubMed] [Google Scholar]
- 70.Proteau PJ, Gerwick WH, Garcia-Pichel F, Castenholz R. 1993. The structure of scytonemin, an ultraviolet sunscreen pigment from the sheaths of cyanobacteria. Experientia 49:825–829. 10.1007/BF01923559 [DOI] [PubMed] [Google Scholar]
- 71.Garcia-Pichel F. 1998. Solar ultraviolet and the evolutionary history of cyanobacteria. Orig. Life Evol. Biosph. 28:321–347. 10.1023/A:1006545303412 [DOI] [PubMed] [Google Scholar]
- 72.Garcia-Pichel F, Castenholz RW. 1991. Characterization and biological implications of scytonemin, a cyanobacterial sheath pigment. J. Phycol. 27:395–409. 10.1111/j.0022-3646.1991.00395.x [DOI] [Google Scholar]
- 73.Garcia-Pichel F, Lopez Cortes A, Nübel U. 2001. Phylogenetic and morphological diversity of cyanobacteria in soil desert crusts from the Colorado Plateau. Appl. Environ. Microbiol. 67:1902–1910. 10.1128/AEM.67.4.1902-1910.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Fulton JM, Arthur MA, Freeman KH. 2012. Subboreal aridity and scytonemin in the Holocene Black Sea. Org. Geochem. 49:47–55. 10.1016/j.orggeochem.2012.05.008 [DOI] [Google Scholar]
- 75.Edwards HGM, Garcia-Pichel F, Newton EM, Wynn-Williams DD. 2000. Vibrational Raman spectroscopic study of scytonemin, the UV-protective cyanobacterial pigment. Spectrochim. Acta A 56:193–200. 10.1016/S1386-1425(99)00218-8 [DOI] [PubMed] [Google Scholar]
- 76.Edwards HGM, Edwards KAE, Farwell DW, Lewis IR, Seaward MRD. 1994. An approach to stone and fresco lichen biodeterioration through Fourier transform Raman microscopic investigation of thallus-substratum encrustations. J. Raman Spectrosc. 25:99–103. 10.1002/jrs.1250250114 [DOI] [Google Scholar]
- 77.Edwards HGM, Russell NC, Seaward MRD, Slarke D. 1995. Lichen biodeterioration under different microclimates: an FT Raman spectroscopic study. Spectrochim. Acta A 51:2091–2100. 10.1016/0584-8539(95)01499-1 [DOI] [Google Scholar]
- 78.Edwards HGM, Farwell DW, Seaward MRD. 1997. FT-Raman spectroscopy of Dirina massiliensis f. sorediata encrustations growing on diverse substrata. Lichenologist 29:83–90. 10.1017/S002428299700011X [DOI] [Google Scholar]
- 79.Edwards HGM, Russell NC, Seaward MRD. 1997. Calcium oxalate in lichen biodeterioration studied using FT-Raman spectroscopy. Spectrochim. Acta A 53:99–105. 10.1016/S1386-1425(97)83013-2 [DOI] [Google Scholar]
- 80.Jorge Vilar SE, Edwards HGM. 2010. Lichen colonization of an active volcanic environment: a Raman spectroscopic study of extremophile biomolecular protective strategies. J. Raman Spectrosc. 41:63–67. 10.1002/jrs.2204 [DOI] [Google Scholar]
- 81.Holder JM, Wynn-Williams DD, Rull Perez F, Edwards HGM. 2000. Raman spectroscopy of pigments and oxalates in situ within epilithic lichens: Acarospora from the Antarctic and Mediterranean. New Phytol. 145:271–280. 10.1046/j.1469-8137.2000.00573.x [DOI] [Google Scholar]
- 82.Wynn-Williams DD, Edwards HGM, Garcia-Pichel F. 1999. Functional biomolecules of Antarctic stromatolitic and endolithic cyanobacterial communities. Eur. J. Phycol. 34:381–391. 10.1080/09670269910001736442 [DOI] [Google Scholar]
- 83.Edwards HGM, Villar SEJ, Parnell J, Cockell CS, Lee P. 2005. Raman spectroscopic analysis of cyanobacterial gypsum halotrophs and relevance for sulfate deposits on Mars. Analyst 130:917–923. 10.1039/b503533c [DOI] [PubMed] [Google Scholar]
- 84.Russell NC, Edwards HGM, Wynn-Williams DD. 1998. FT-Raman spectroscopic analysis of endolithic microbial communities from Beacon sandstone in Victoria Land, Antarctica. Antarct. Sci. 10:63–74 [Google Scholar]
- 85.Villar SEJ, Edwards HGM, Benning LG. 2006. Raman spectroscopic and scanning electron microscopic analysis of a novel biological colonisation of volcanic rocks. Icarus 184:158–169. 10.1016/j.icarus.2006.04.009 [DOI] [Google Scholar]
- 86.Edwards HGM, Villar SEJ, Pullan D, Hargreaves MD, Hofmann BA, Westall F. 2007. Morphological biosignatures from relict fossilised sedimentary geological specimens: a Raman spectroscopic study. J. Raman Spectrosc. 38:1352–1361 [Google Scholar]
- 87.Vítek P, Cámara-Gallego B, Edwards HGM, Jehlička J, Ascaso C, Wierzchos J. 2013. Phototrophic community in gypsum crust from the Atacama Desert studied by Raman spectroscopy and microscopic imaging. Geomicrobiol. J. 30:399–410. 10.1080/01490451.2012.697976 [DOI] [Google Scholar]
- 88.Reiser R, Tasch P. 1960. Investigations of the viability of osmophile bacteria of great geologic age. Trans. Kans. Acad. Sci. 63:31–34. 10.2307/3626919 [DOI] [PubMed] [Google Scholar]
- 89.Tasch P. 1963. Paleoecological considerations of growth and form of fossil protists. Ann. N. Y. Acad. Sci. 108:437–450 [DOI] [PubMed] [Google Scholar]
- 90.Dombrowski H. 1963. Bacteria from Palaeozoic salt deposits. Ann. N. Y. Acad. Sci. 108:453–460 [DOI] [PubMed] [Google Scholar]
- 91.Dombrowski H. 1966. Geological problems in the question of living bacteria in Palaeozoic salt deposits, p 215–220 In Rau JL. (ed), Second Symposium on Salt, vol 1 Geology, geochemistry, mining. Northern Ohio Geological Society, Cleveland, OH [Google Scholar]
- 92.Schubert BA, Lowenstein TK, Timofeeff MN. 2009. Microscopic identification of prokaryotes in modern and ancient halite, Saline Valley and Death Valley, California. Astrobiology 9:467–482. 10.1089/ast.2008.0282 [DOI] [PubMed] [Google Scholar]
- 93.Winters YD, Lowenstein TK, Timofeeff MN. 2013. Identification of carotenoids in ancient salt from Death Valley, Saline Valley, and Searles Lake, California using laser Raman spectroscopy. Astrobiology 13:1065–1080. 10.1089/ast.2012.0952 [DOI] [PubMed] [Google Scholar]
- 94.Conner AJ, Benison KC. 2013. Acidophilic halophilic microorganisms fluid inclusions in halite from Lake Magic, Western Australia. Astrobiology 13:850–860. 10.1089/ast.2012.0956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Fendrihan S, Musso M, Stan-Lotter H. 2009. Raman spectroscopy as a potential method for the detection of extremely halophilic archaea embedded in halite in terrestrial and possibly extraterrestrial samples. J. Raman Spectrosc. 40:1996–2003. 10.1002/jrs.2357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Heraud P, Beardall J, McNaughton D, Wood BR. 2007. In vivo prediction of the nutrient status of individual microalgal cells using Raman microspectroscopy. FEMS Microbiol. Lett. 275:24–30. 10.1111/j.1574-6968.2007.00861.x [DOI] [PubMed] [Google Scholar]
- 97.Sandt C, Smith-Palmer T, Pink J, Pink D. 2008. A confocal Raman microscopy study of the distribution of a carotene-containing yeast in a living Pseudomonas aeruginosa biofilm. Appl. Spectrosc. 62:975–983. 10.1366/000370208785793245 [DOI] [PubMed] [Google Scholar]
- 98.Huang YY, Beal CM, Cai WW, Ruoff RS, Terentjev EM. 2010. Micro-Raman spectroscopy of algae: composition analysis and fluorescence background behavior. Biotechnol. Bioeng. 105:889–898 [DOI] [PubMed] [Google Scholar]
- 99.Abbas A, Josefson M, Abrahamsson K. 2011. Characterization and mapping of carotenoids in the algae Dunaliella and Phaeodactylum using Raman and target orthogonal partial least squares. Chemometr. Intell. Lab. Syst. 107:174–177. 10.1016/j.chemolab.2011.03.004 [DOI] [Google Scholar]
- 100.Collins AM, Jones HDT, Han D, Hu Q, Beechem TE, Timlin JA. 2011. Carotenoid distribution in living cells of Haematococcus pluvialis (Chlorophyceae). PLoS One 6:e24302. 10.1371/journal.pone.0024302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kaczor A, Turnaub K, Baranska M. 2011. In situ Raman imaging of astaxanthin in a single microalgal cell. Analyst 136:1109–1112. 10.1039/c0an00553c [DOI] [PubMed] [Google Scholar]
- 102.Ando M, Sugiura M, Hayashi H, Hamaguchi H-O. 2011. 1064 nm deep near-infrared (NIR) excited Raman microspectroscopy for studying photolabile organisms. Appl. Spectrosc. 65:488–492. 10.1366/10-06196 [DOI] [PubMed] [Google Scholar]
- 103.Li M, Canniffe DP, Jackson PJ, Davison PA, Fitzerald S, Dickman MJ, Burgess JG, Hunter CN, Huang WE. 2012. Rapid resonance Raman microspectroscopy to probe carbon dioxide fixation by single cells in microbial communities. ISME J. 6:875–885. 10.1038/ismej.2011.150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Cannizzaro C, Rhiel M, Marison I, von Stockar U. 2003. On-line monitoring of Phaffia rhodozyma fed-batch process with in situ dispersive Raman spectroscopy. Biotechnol. Bioeng. 83:668–680. 10.1002/bit.10698 [DOI] [PubMed] [Google Scholar]
- 105.Li MQ, Xu J, Romero Gonzales M, Banwart SA, Huang WE. 2012. Single cell Raman spectroscopy for cell sorting and imaging. Curr. Opin. Biotechnol. 23:56–63. 10.1016/j.copbio.2011.11.019 [DOI] [PubMed] [Google Scholar]
- 106.Tao Z, Wang G, Xu X, Yuan Y, Wang X, Li Y. 2011. Monitoring and rapid quantification of total carotenoids in Rhodotorula glutinis cells using laser tweezers Raman spectroscopy. FEMS Microbiol. Lett. 314:42–48. 10.1111/j.1574-6968.2010.02139.x [DOI] [PubMed] [Google Scholar]
- 107.Brackmann C, Bengtsson A, Alminger ML, Svanbergb U, Enejdera A. 2011. Visualization of β-carotene and starch granules in plant cells using CARS and SHG microscopy. J. Raman Spectrosc. 42:586–592. 10.1002/jrs.2778 [DOI] [Google Scholar]
- 108.Chen JX, Volkmer A, Book LD, Xie XS. 2002. Multiplex coherent anti-stokes Raman scattering microspectroscopy and study of lipid vesicles. J. Phys. Chem. 106:8493–8498. 10.1021/jp025771z [DOI] [Google Scholar]
- 109.Dementjev A, Kostkeviciene J. 2013. Applying the method of Coherent Anti-stokes Raman microscopy for imaging of carotenoids in microalgae and cyanobacteria. J. Raman Spectrosc. 44:973–979. 10.1002/jrs.4321 [DOI] [Google Scholar]
- 110.Rösch P, Harz M, Schmitt M, Peschke K-D, Ronneberger O, Burkhardt H, Motzkus H-W, Lankers M, Hofer S, Thiele H, Popp J. 2005. Chemotaxonomic identification of single bacteria by micro-Raman spectroscopy: application to clean-room-relevant biological contaminations. Appl. Environ. Microbiol. 71:1626–1637. 10.1128/AEM.71.3.1626-1637.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Böttger U, de Vera JP, Fritz J, Weber I, Hubers H-W, Schulze-Makuch D. 2012. Optimizing the detection of carotene in cyanobacteria in a martian regolith analogue with a Raman spectrometer for the ExoMars mission. Planet Space Sci. 60:356–362. 10.1016/j.pss.2011.10.017 [DOI] [Google Scholar]
- 112.Vítek P, Ali EMA, Edwards HGM, Jehlička J, Cox R, Page K. 2012. Evaluation of portable Raman spectrometer with 1064 nm excitation for geological and forensic applications. Spectrochim. Acta A 86:320–327. 10.1016/j.saa.2011.10.043 [DOI] [PubMed] [Google Scholar]
- 113.Dickensheets DL, Wynn-Williams DD, Edwards HGM, Schoen C, Crowder C, Newton EM. 2000. A novel miniature confocal microscope/Raman spectrometer system for biomolecular analysis on future Mars missions after Antarctic trials. J. Raman Spectrosc. 31:633–635. [DOI] [Google Scholar]
- 114.Som SM, Foing BH. 2012. Thermal degradation of organic material by portable laser Raman spectrometry. Int. J. Astrobiol. 11:177–186. 10.1017/S1473550412000079 [DOI] [Google Scholar]
- 115.Miralles I, Jorge-Villar SE, Canton Y, Domingo F. 2012. Using a mini-Raman spectrometer to monitor the adaptive strategies of extremophile colonizers in arid deserts: relationships between signal strength, adaptive strategies, solar radiation, and humidity. Astrobiology 12:743–753. 10.1089/ast.2011.0763 [DOI] [PubMed] [Google Scholar]
- 116.Vítek P, Edwards HGM, Jehlička J, Hutchinson I, Ascaso C, Wierzchos J. 2012. The miniaturized Raman system and detection of traces of life in halite from the Atacama Desert: some considerations for the search for life signatures on Mars. Astrobiology 12:1095–1099. 10.1089/ast.2012.0879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Jehlička J, Oren A. 2013. Use of a handheld Raman spectrometer for fast screening of microbial pigments in cultures of halophilic microorganisms and in microbial communities in hypersaline environments in nature. J. Raman Spectrosc. 44:1285–1291. 10.1002/jrs.4362 [DOI] [Google Scholar]
- 118.Parnell J, McMahona S, Blamey NJF, Hutchinson IB, Harrisa LV, Ingley R, Edwards HGM, Lynch E, Feely M. 25 October 2013. Detection of reduced carbon in a basalt analogue for martian nakhlite: a signpost to habitat on Mars. Int. J. Astrobiol. 10.1017/S1473550413000360 [DOI] [Google Scholar]
- 119.Colomban P. 2012. The on-site/remote Raman analysis with mobile instruments: a review of drawbacks and success in cultural heritage studies and other associated fields. J. Raman Spectrosc. 43:1529–1535. 10.1002/jrs.4042 [DOI] [Google Scholar]
- 120.Vandenabeele P, Edwards HGM, Jehlička J. 2014. The role of mobile instrumentation in novel applications of Raman spectroscopy: archaeometry, geosciences, and forensics. Chem. Soc. Rev. 43:2628–2649. 10.1039/c3cs60263j [DOI] [PubMed] [Google Scholar]
- 121.Sorak D, Herberholz L, Iwascek S, Altinpinar S, Pfeifer F, Siesler HW. 2012. New developments and applications of handheld Raman, mid-infrared, and near-infrared spectrometers. Appl. Spectrosc. Lett. 47:83–115. 10.1080/05704928.2011.625748 [DOI] [Google Scholar]
- 122.Marshall CP, Carter EA, Leuko S, Javaux EJ. 2006. Vibrational spectroscopy of extant and fossil microbes: relevance for the astrobiological exploration of Mars. Vibrat. Spectrosc. 41:182–189. 10.1016/j.vibspec.2006.01.008 [DOI] [Google Scholar]
- 123.Wood BR, Heraud P, Stojkovic S, Morrison D, Beardall J, McNaughton D. 2005. A portable Raman acoustic levitation spectroscopic system for the identification and environmental monitoring of algal cells. Anal. Chem. 77:4955–4961. 10.1021/ac050281z [DOI] [PubMed] [Google Scholar]
- 124.Edwards HGM, Currie KJ, Ali HRH, Villar SEJ, David AR, Denton J. 2007. Raman spectroscopy of natron: shedding light on ancient Egyptian mummification. Anal. Bioanal. Chem. 388:683–689. 10.1007/s00216-007-1249-4 [DOI] [PubMed] [Google Scholar]
- 125.Gall A, Ridge JP, Robert B, Cogdell RJ, Jones MR, Fyfe PK. 1999. Effects of mutagenesis on the detailed structure of spheroidenone in the Rhodobacter sphaeroides reaction centre examined by resonance Raman spectroscopy. Photosynth. Res. 59:223–230. 10.1023/A:1006168118363 [DOI] [Google Scholar]
- 126.Wang GH, Hao ZJ, Huang ZB, Chen LZ, Li XY, Hu CX, Liu YD. 2010. Raman spectroscopic analysis of a desert cyanobacterium Nostoc sp. in response to UVB radiation. Astrobiology 10:783–788. 10.1089/ast.2009.0407 [DOI] [PubMed] [Google Scholar]
- 127.Edwards HGM, Newton EM, Wynn-Williams DD, Lewis-Smith RI. 2003. Nondestructive analysis of pigments and other organic compounds in lichens using Fourier-transform Raman spectroscopy: a study of Antarctic epilithic lichens. Spectrochim. Acta A 59:2301–2309. 10.1016/S1386-1425(03)00073-8 [DOI] [PubMed] [Google Scholar]
- 128.Edwards HGM, Russell NC, Wynn-Williams DD. 1997. Fourier transform Raman spectroscopic and scanning electron microscopic study of cryptoendolithic lichens from Antarctica. J. Raman Spectrosc. 28:685–690. [DOI] [Google Scholar]