Abstract
Due to the emergence of highly virulent community-associated methicillin-resistant Staphylococcus aureus (CA-MRSA) infections, S. aureus has become a major threat to public health. A majority of CA-MRSA skin and soft tissue infections in the United States are caused by S. aureus USA300 strains that are known to produce high levels of alpha hemolysin (Hla). Therefore, vaccines that contain inactivated forms of this toxin are currently being developed. In this study, we sought to determine the immune mechanisms of protection for this antigen using a vaccine composed of a genetically inactivated form of Hla (HlaH35L). Using a murine model of skin and soft tissue infections (SSTI), we found that BALB/c mice were protected by vaccination with HlaH35L; however, Jh mice, which are deficient in mature B lymphocytes and lack IgM and IgG in their serum, were not protected. Passive immunization with anti-HlaH35L antibodies conferred protection against bacterial colonization. Moreover, we found a positive correlation between the total antibody concentration induced by active vaccination and reduced bacterial levels. Animals that developed detectable neutralizing antibody titers after active vaccination were significantly protected from infection. These data demonstrate that antibodies to Hla represent the major mechanism of protection afforded by active vaccination with inactivated Hla in this murine model of SSTI, and in this disease model, antibody levels correlate with protection. These results provide important information for the future development and evaluation of S. aureus vaccines.
INTRODUCTION
Staphylococcus aureus is an opportunistic pathogen that colonizes 30% of the population (1). This bacterium has a plethora of virulence factors that it uses to evade the immune system, scavenge for needed nutrients, and attach to and destroy host tissue (2, 3). S. aureus is a common cause of uncomplicated skin infections (4) but can also cause more invasive infections, like sepsis, endocarditis, and osteomyelitis (2). Methicillin-resistant S. aureus (MRSA), which is resistant to all β-lactam antibiotics, has historically been associated with nosocomial infections; however, community-acquired MRSA (CA-MRSA) has emerged in recent years (5). CA-MRSA infections, a majority of which in the United States are caused by the highly virulent USA300 genotype (6, 7), often occur in immunocompetent patients lacking obvious risk factors (4). Vancomycin is the drug of choice for treating MRSA infections (8, 9). However, due to its extensive use (5, 9), vancomycin-resistant S. aureus (VRSA) has been increasingly isolated since the first reported vancomycin-intermediate S. aureus (VISA) infection in 1997 (10, 11). The emergence of hypervirulent CA-MRSA and VRSA strains is an alarming threat to public health that highlights the need for an S. aureus vaccine.
Others have suggested that prophylactic therapies that target CA-MRSA should include inactivated alpha hemolysin (Hla), since Hla is believed to contribute to the severity of USA300 skin infections (12) and is expressed at high levels by USA300 strains (13). Hla is a secreted toxin that binds to the eukaryotic cell surface protein ADAM10 and then oligomerizes to generate a heptameric pore that is cytolytic to multiple cell types, including erythrocytes and endothelial cells (14). Passive immunization with anti-Hla monoclonal antibodies has been shown to reduce lesion size in a mouse dermonecrosis model (15). Inactivated forms of Hla have been included in vaccine candidates that have been studied in the clinic setting (16). A better understanding of the mechanism(s) of protection for this antigen would improve our ability to evaluate these vaccines. For example, information regarding the immune mechanisms of protection for Hla-based vaccines ensures that appropriate immune responses to vaccination are measured both in preclinical and clinical studies. Moreover, the development of correlates of protection can be useful for monitoring vaccine consistency and the susceptibilities of specific populations postvaccination (17).
In this study, we examined the mechanisms of protection of a vaccine based on the genetically inactivated alpha hemolysin mutant HlaH35L, in which histidine 35 was substituted with leucine, in a mouse model of S. aureus skin and soft tissue infections (SSTI). HlaH35L binds to the cell membrane; however, it is unable to form a pore and is therefore not toxic (18, 19). We determined the important role that antibodies play in the protection afforded by active immunization with this genetically inactivated Hla-based vaccine and demonstrate that anti-Hla antibody levels correlate with protection.
MATERIALS AND METHODS
Ethics statement.
For all mouse studies, the protocols were reviewed and approved by the Institutional Animal Care and Use Committee (IACUC) of the Center for Biologics Evaluation and Research.
Reagents.
Purified recombinant HlaH35L was generated as described by Brady et al. (20).
Rabbit anti-HlaH35L polyclonal antibody production and IgG purification.
The production of Rabbit anti-HlaH35L polyclonal antibody was performed by Cocalico Biologicals, Inc. (Reamstown, PA), subject to their IACUC approval. New Zealand White rabbits (2 to 3 months old, male, 1.8 to 2.3 kg) were immunized with 100 μg HlaH35L at day 0. The rabbits were then immunized again on days 21 and 42 with 50 μg HlaH35L. For each dose, the antigen was diluted to 0.5 ml in 0.9% saline and adsorbed to an equal volume of Alhydrogel (Brenntag Biosector, Frederikssund, Denmark). Bleeds were obtained from the rabbits at days 56 and 70. IgG was purified by affinity chromatography. Rabbit preimmune and immune sera were diluted 1:1 in protein A IgG binding buffer (provided by Thermo Scientific, Rockford, IL) and applied to 5 ml protein A columns (NAb Spin columns, Thermo Scientific, Rockford, IL). The columns were placed on a rotator and incubated for 10 min at room temperature. The columns were then washed three times with 10 ml of binding buffer, and IgG was eluted three times with 5 ml of IgG elution buffer (provided by Thermo Scientific, Rockford, IL), followed by immediate neutralization of the pH using 0.5 ml of 1 M Tris-HCl (pH 8) (Fisher Scientific, Fair Lawn, NJ). Pooled fractions were diafiltered into phosphate-buffed saline (PBS) (Quality Biological, Gaithersburg, MD) using Amicon Ultra-15-ml 30,000 molecular weight cutoff (MWCO) centrifugal filters (EMD Millipore, Billerica, MA). IgG concentration was measured using the GE NanoVue (GE Healthcare, Piscataway, NJ) IgG function (which measures the A280 and calculates protein concentration using the extinction coefficient of IgG). The purified IgG was characterized on NuPAGE 4 to 12% Bis-Tris gels in morpholinepropanesulfonic acid (MOPS) buffer (Invitrogen, Grand Island, NY). The final IgG concentration was brought to 22 to 24 mg/ml.
Active immunization studies.
Purified recombinant HlaH35L was thawed and diafiltered into PBS using Amicon Ultra-15-ml 30,000 MWCO centrifugal filters. The HlaH35L concentration was determined by the bicinchoninic acid (BCA) Protein Assay (Pierce, Rockford, IL) according to the manufacturer's protocol. HlaH35L was then adsorbed to Alhydrogel with CpG for 1 h on ice (CBER Core Facility, Bethesda, MD) to obtain final concentrations of 200 μg/ml HlaH35L, 2 mg/ml Alhydrogel, and 150 μg/ml CpG. We used this antigen/adjuvant combination because we have shown that this formulation is protective in a murine SSTI model of S. aureus infection. Six-week-old female BALB/c mice or Jh mice were immunized subcutaneously in the flank with 100 μl formulated vaccine containing 20 μg of adjuvanted HlaH35L on days 0 and 14. All mice were purchased from Taconic, Hudson, NY. The control mice received 100 μl of adjuvant only, which was prepared as described above but without HlaH35L. The mice were bled at day 26 to determine antibody levels and were challenged with S. aureus at day 28, as described below. The dose-response study was conducted as described above except that 6-week-old female BALB/c mice (NCI, Frederick, MD) were immunized only once (day 0), and the highest vaccine dose was prepared as described above at a volume sufficient to allow for 1:4 serial dilutions following adsorption. Extra control (adjuvant only) vaccine was prepared and used as a diluent in these serial dilutions.
Passive immunization study.
Eight-week-old female BALB/c mice (NCI, Frederick, MD) were passively immunized intraperitoneally with 200 μl of concentrated IgG at days 0 and 3. The mice were challenged on day 1 with S. aureus, as described below.
Skin and soft tissue challenge.
Two days prior to challenge, S. aureus strain SAP149 (21) was streaked on tryptic soy agar (TSA) with 5% sheep blood (BD, Franklin Lakes, NJ). The following day, an overnight culture was initiated using a single hemolytic colony. On the day of challenge, four baffled Erlenmeyer flasks (Corning, Corning, NY) containing 50 ml of tryptic soy broth (TSB) (MP Biomedicals, Solon, OH) were inoculated at a dilution of 1:50 using the overnight growth. The cultures were then placed in a 37°C shaking water bath and incubated until they reached an absorbance at 600 nm (A600) between 0.75 and 0.8. The bacteria were pelleted by centrifuging at 4,000 × g for 10 min. All four pellets were resuspended in 50 ml of PBS and the concentration was determined using a Petroff-Hausser counting chamber (Hausser Scientific Partnership, Horsham, PA). The bacteria were centrifuged again and resuspended with PBS to a final concentration of 1 × 1011 CFU/ml. The concentration was verified by serial dilution and plating on TSA. The mice were anesthetized and challenged as previously described by Brady et al. (20). Seven days postchallenge, each mouse was euthanized, and its affected ears were imaged when indicated. The affected ears were then gently cleaned with alcohol pads (PDI, Orangeburg, NY), removed, and homogenized in sterile PBS. The CFU count per ear was determined by serial dilutions and plating on TSA. In addition, the two individuals scored the ears for tissue damage based on a scale of 1 to 3, and these scores were averaged. Mice that received a score of 1 had no missing ear pieces, no scabs, little to no redness, and no or small holes. Mice that received a score of 2 had no missing ear pieces, small to medium scabs, moderate redness, and small to medium holes. Mice that received a score of 3 had missing pieces or large holes, medium to large scabs, and moderate to severe redness.
Enzyme linked immunosorbent assay.
Enzyme-linked immunosorbent assays (ELISAs) to measure mouse antibodies were performed as described by Brady et al. (20). ELISAs to measure rabbit antibodies were performed according to the same protocol; however, the conjugate antibody used was goat anti-rabbit IgG conjugated to horseradish peroxidase (HRP) (Bio-Rad Laboratories, Hercules, CA). To determine IgG titers, the data were plotted using GraphPad Prism software (version 5, GraphPad Software, La Jolla, CA) with a 4-parameter logistic (4PL) curve fit of A405 (y axis) versus the logarithm of the reciprocal serum dilution (x axis). The ELISA titers were defined as the 50% effective dose (ED50) of a 4PL curve.
Hla neutralization assay.
The assay was designed based on one described by Adhikari et al. (22). Briefly, serial dilutions of mouse serum were preincubated with 20 ng Hla (List Biological Laboratories, Campbell, CA) for 30 min at 37°C in 100 μl total volume of PBS supplemented with 2.5% fetal bovine serum (Gibco, Life Technologies, Grand Island, NY) in a 96-well plate (MaxiSorp; Thermo Fisher, Waltham, MA). An equal volume of 1% rabbit erythrocytes (CBER Animal Facility, Bethesda, MD) was then added to each well, and incubation at 37°C was continued for another 30 min. Each row of diluted antiserum included a no-toxin control that served as a blank, and every plate included a toxin-only control for lysis, as well as serially diluted rabbit anti-Hla polyclonal antibody (the same as used for passive studies; see above) as a positive control for neutralization. After incubation, the plates were centrifuged at 500 × g for 5 min, and 100 μl of the supernatant from each well was transferred to a new plate. The degree of erythrocyte lysis was determined by reading the A416 on a VersaMax microplate reader (Molecular Devices, Sunnyvale, CA). For each antibody dilution, the corresponding blank reading was subtracted to remove background absorbance, and the corrected absorbance readings were plotted against the log of the reciprocal antibody dilution using a 4PL-curve fit determined using GraphPad Prism. The 50% neutralization titer (NT50) is defined as the reciprocal dilution of serum that neutralizes 50% of the hemolytic activity of Hla in the assay.
Statistical analyses.
GraphPad Prism was used for statistical analyses. To assess the correlation between antibody titers and protection, the ELISA ED50 was plotted as a function of the CFU/ear, and a linear best-fit curve was used to determine the r2 value and whether the slope of the line was significantly different than zero.
RESULTS
Passive immunization with rabbit anti-HlaH35L IgG decreases bacterial colonization.
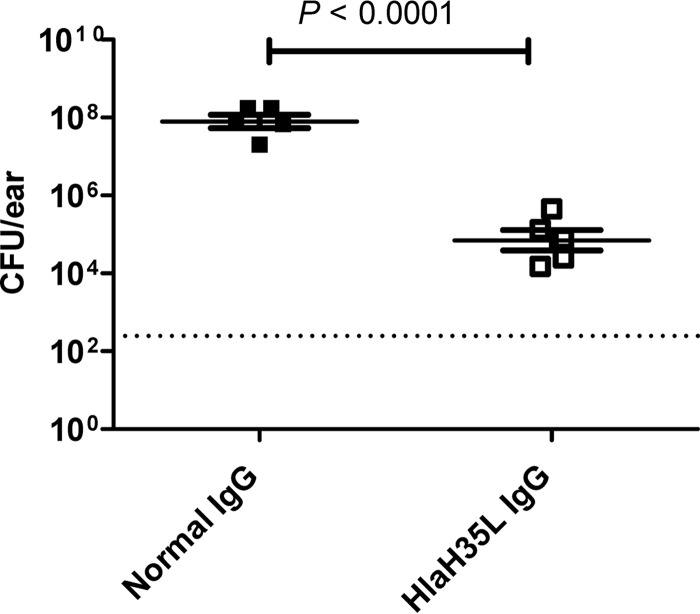
Previously, others demonstrated in a murine skin infection model that anti-Hla polyclonal antibodies can passively protect against dermonecrotic lesions (12). However, because the model used in that study involved subcutaneous administration of S. aureus rather than the epicutaneous administration method used in our model, we sought to confirm that antibodies can passively protect in the epicutaneous model of skin infections used in this study. The mice were passively immunized with purified rabbit anti-HlaH35L IgG and normal rabbit IgG and then challenged using the epicutaneous model of skin infections developed by Prabhakara et al. (23). We found that passive immunization with anti-Hla polyclonal antibodies protected mice against lesions (data not shown), as was reported for the subcutaneous model (12). Moreover, passively immunized mice had significantly lower CFU counts/ear than control mice that received normal rabbit IgG (Fig. 1), indicating that passive immunization with anti-HlaH35L IgG not only protects against tissue damage but also reduces bacterial loads. These data indicate that antibodies are sufficient for protection in an epicutaneous model of S. aureus disease. However, the data do not exclude the possibility that other immune responses might contribute to protection or even be the overriding mechanisms of protection in an active immunization model.
FIG 1.
Protection provided by passive immunization using rabbit anti-HlaH35L IgG in a murine model of S. aureus SSTI. Mice were passively immunized with either anti-HlaH35L IgG (□) or normal rabbit IgG (■) and challenged with 1 × 109 CFU S. aureus SAP149 as described in Materials and Methods. CFU/ear are plotted with the mean ± standard error of the mean (SEM) for each group indicated. The P value was determined by a two-tailed Student's t test. The dashed line indicates the limit of detection for the assay. The results are representative of two independent experiments.
B-cell immunity is necessary in order to decrease disease severity and bacterial colonization.
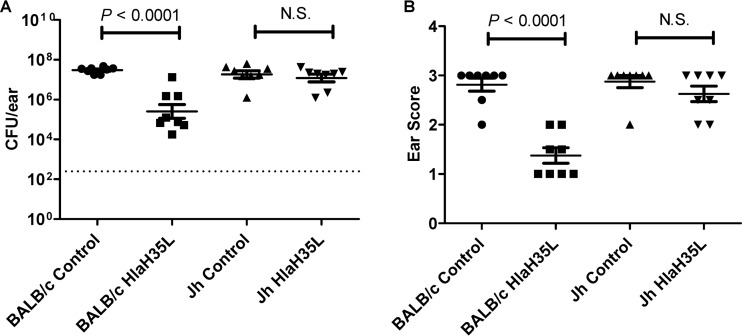
We therefore sought to determine whether antibodies are necessary for protection mediated by active immunization with an inactivated Hla-based vaccine, or whether other immune responses, such as T-cell responses, might represent the major protective mechanism(s). To this end, we examined the ability of genetically inactivated Hla to protect Jh mice, which are devoid of mature B lymphocytes and have no detectable IgM or IgG in their blood serum (24) against S. aureus infection in the SSTI model. Jh mice were immunized with either HlaH35L formulated with adjuvant or adjuvant only and were subsequently challenged with S. aureus. The effect of vaccination on the protection of Jh mice compared to the protection of wild-type BALB/c mice was then assessed. As was observed previously (20), at 14 days postchallenge, BALB/c mice immunized with adjuvanted HlaH35L had significantly less bacterial colonization (Fig. 2A) and visibly less tissue damage of the affected ear (Fig. 2B) than did BALB/c mice that received the adjuvant control. In contrast, HlaH35L-immunized Jh mice did not exhibit a significant difference in bacterial colonization or in damage to the affected ear compared to control Jh mice immunized with adjuvant only (Fig. 2A and B). These results demonstrate that B-cell-based immunity is necessary in order to decrease disease severity and bacterial colonization in the SSTI model.
FIG 2.
Analysis of protection of mice devoid of mature B lymphocytes provided by active immunization with HlaH35L vaccine. Jh mice which are devoid of mature B lymphocytes and have no detectable IgM or IgG in their sera (24) and BALB/c mice were immunized with either HlaH35L vaccine or adjuvant control and then challenged with 1 × 109 CFU S. aureus SAP149 as described in Materials and Methods. (A) CFU counts/ear were compared between BALB/c control (●) and BALB/c HlaH35L-immunized (■) mice, as well as Jh control (▲) and Jh HlaH35L-immunized (▼) mice. The dashed line indicates the limit of detection for the assay. (B) Ears were scored for tissue damage, and the averages were plotted to compare BALB/c control (●) and BALB/c HlaH35L-immunized (■) mice, as well as Jh control (▲) and HlaH35L-immunized (▼) mice (B). Each bar indicates the mean ± SEM for each group. P values were determined using a two-tailed Student's t test. The results are representative of three independent experiments. N.S., not significant.
Anti-HlaH35L antibody levels correlate with reduction in bacterial colonization.
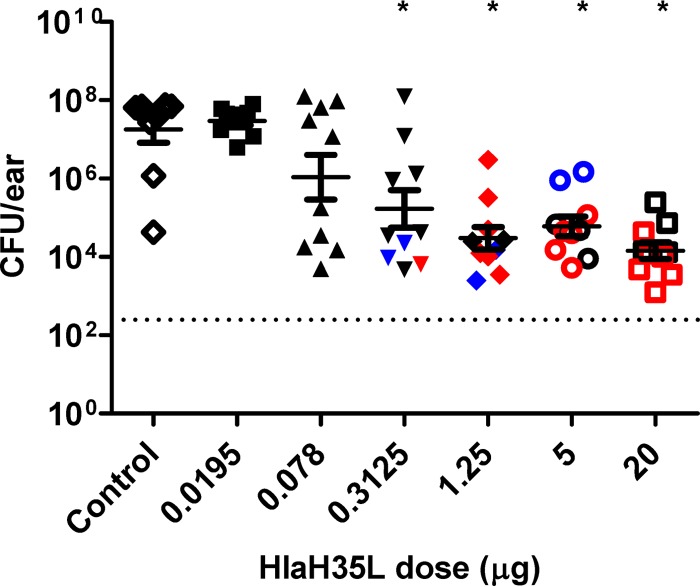
In order to determine if antibody levels correlate with bacterial colonization in the SSTI model, the mice were immunized once with either various doses of HlaH35L vaccine or adjuvant control, bled 2 days before challenge to measure antibody levels, and then were challenged with S. aureus. A clear dose response was observed, with significant protection achieved with a dose of HlaH35L vaccine as low as 0.3125 μg (Fig. 3).
FIG 3.
Protection provided by active immunization with titrated doses of HlaH35L vaccine using a mouse model of S. aureus SSTI. Mice immunized with adjuvant control (♢) or titrated doses of HlaH35L vaccine of 0.0195 μg (■), 0.078 μg (▲), 0.3125 μg (▼), 1.25 μg (◆), 5 μg (○), or 20 μg (□) were challenged with S. aureus SAP149, and the CFU count/ear was determined as described in Materials and Methods. *, groups with a statistically significant decrease in CFU/ear compared to the adjuvant control group as determined by 1-way analysis of variance (ANOVA) with a Tukey's multiple comparisons posttest; a P value of <0.05 was considered significant. The dashed line indicates the limit of detection for the assay. The bars represent the mean ± SEM. The results are representative of two independent experiments. The black symbols represent mice with nonneutralizing sera, blue symbols represent mice with partially neutralizing sera, and red symbols represent mice with fully neutralizing sera, as determined using the Hla neutralization assay described in Materials and Methods.
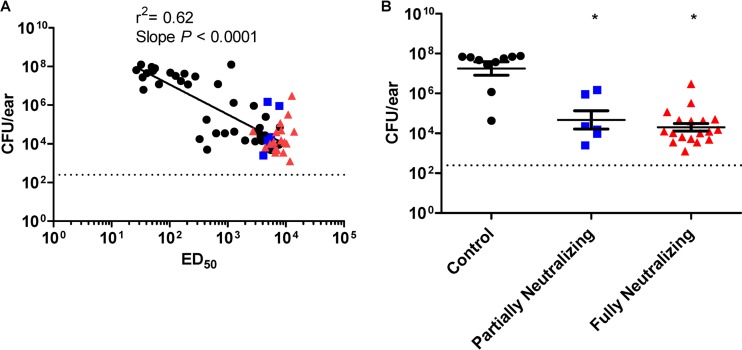
We next examined the correlation between antibody titer and bacterial levels at 14 days postchallenge. We observed a moderate correlation (r2 = 0.62) between total IgG titer (ED50) and bacterial levels at 14 days postchallenge (Fig. 4A). The slope of the best-fit line was significantly different from zero. These results indicate that anti-HlaH35L antibodies play a role in reducing bacterial colonization in the SSTI model, with a positive relationship between total IgG level and bacterial reduction. However, the moderate correlation suggests that total IgG titer may not fully explain the protective mechanisms of this antigen.
FIG 4.
Correlation analysis for antibody level and CFU/ear. (A) Total IgG titers (ED50) were plotted versus CFU/ear for serum samples from individual mice from the study shown in Fig. 3A. The slope of the line was determined to be significantly different from 0 (P < 0.0001), and the coefficient of determination (r2) was calculated to be 0.62. The neutralizing titers were also determined for sera from each of the individual mice. The black symbols represent mice with nonneutralizing sera, blue symbols represent mice with partially neutralizing sera, and red symbols represent mice with fully neutralizing sera, as determined using the Hla neutralization assay described in Materials and Methods. (B) CFU counts/ear were compared between mice with partially neutralizing (■), fully neutralizing (▲), and adjuvant control serum (●). *, groups with a statistically significant decrease in CFU/ear compared to the adjuvant control group, as determined by 1-way ANOVA with a Tukey's multiple comparisons posttest; a P value of <0.05 was considered significant. The dashed line indicates the limit of detection for the assay. Each bar represents the mean ± SEM.
Therefore, we also examined the relationship between toxin neutralizing antibody titer and reduction in bacterial levels. To do this, we examined the ability of serum samples from individual mice to neutralize Hla using a rabbit erythrocyte toxin neutralization assay. When these serum samples were titrated with fixed concentrations of erythrocytes and Hla, we noted a differential ability of individual samples to neutralize erythrocyte lysis. Unfortunately, due to the lack of sensitivity of the assay, we were unable to achieve full neutralization curves for all sera and were therefore not able to quantitatively determine the NT50 values for a number of the serum samples. Instead, we assigned the neutralization capabilities of each the serum samples to one of three categories based on the extent of neutralization observed: (i) fully neutralizing (serum can completely inhibit hemolysis such that the neutralization curve reaches 0), (ii) partially neutralizing (the lowest absorbance reading of the neutralization curve is at less than one-half of the upper asymptote), and (iii) nonneutralizing (the lowest absorbance of the neutralization curve is more than one-half of the upper asymptote). We first qualitatively examined the relationship between vaccine dose and neutralization. As shown in Fig. 3, we observed that most animals that received higher doses of HlaH35L had anti-Hla antibodies that were at least partially neutralizing. We then examined which points on the ELISA ED50/CFU correlation best-fit curve fell into each neutralization category. The sera with neutralization capabilities (either fully neutralizing or partially neutralizing) tended to be correlated with higher ELISA titers and lower CFU levels in the SSTI model (Fig. 4A; see also Table S1 in the supplemental material). As shown in Fig. 4B, mice that had either partially or fully neutralizing titers had significantly lower bacterial levels than control mice that received adjuvant alone. These results suggest that toxin neutralization may be important in protecting mice from SSTI infection after active immunization with HlaH35L.
DISCUSSION
Hla is a well-studied toxin, which, in its inactivated form, has been included in vaccines under development (16); however, the immune mechanisms of Hla-based vaccine-induced protection and immune parameters that correlate with protection for active vaccination with this antigen have not been precisely defined. In this study, we used a mouse model of S. aureus SSTI to examine the immune mechanisms of protection for a genetically inactivated Hla vaccine. We found that antibodies are both necessary and sufficient for protection in an epicutaneous SSTI model of infection. Antibodies were not only important for preventing tissue damage but also for reducing bacterial levels.
We further examined the relationship between antibodies and protection in order to determine whether antibodies levels correlate with protection. We found that IgG titers showed an inverse correlation with CFU levels; that is, higher antibody levels corresponded to decreased bacterial loads in affected ears. While the slope of the best-fit line was significantly nonzero, the moderate r2 value of 0.62 suggests that total IgG levels do not fully explain the observed protection. One possibility for this observation is that only a subpopulation of antibodies confers protection. To that end, we attempted to correlate neutralizing antibody levels with protection; however, we were hampered in this effort because of the relative lack of sensitivity of the assays used to assess toxin neutralization. However, we did find that mice that had detectable neutralizing antibody levels were significantly protected, leaving open the possibility that very small amounts of neutralizing antibody might be sufficient to protect in this model. Such functional antibodies might represent the major mechanism of protection. Alternatively, other immune responses might contribute to protection; however, the major protective immune mechanism is clearly B-cell mediated, since Jh mice that are devoid of mature B lymphocytes but which have normal T-cell development (24) were not protected by active vaccination with a genetically inactivated Hla-based vaccine in this model.
Vaccine-induced protection is antibody mediated for a number of toxoid-based vaccines (17). Our work demonstrates that the protection that we observed using a genetically inactivated Hla vaccine in a murine SSTI model is likely similarly based. While we demonstrated that antibodies mediate protection in this SSTI model, the specific mechanism of how antibodies against a secreted toxin target S. aureus for pathogen clearance remains unknown. While a small amount of Hla is associated with the bacterial surface as it is secreted, the majority of toxin is not associated with the surface and, as such, it is unlikely that anti-Hla antibodies would opsonize the bacteria. Because neutrophils, which have been shown to be important in the control of S. aureus infections (23, 25–28), are a target of alpha hemolysin (14), one might theorize that immunization with HlaH35L protects neutrophils, or other cells involved in the immune response that are important for control of the bacteria, from intoxication with Hla during infection. Alternatively, because secreted toxins cause localized tissue damage, the neutralization of alpha hemolysin may decrease subsequent nutrient release from host tissues and lessen the ability of S. aureus to further invade the host. This idea is supported by decreased levels of tissue destruction in HlaH35L-immunized mice (20). Whatever the mechanism, active toxin appears to be involved in the survival of the bacteria (23), and anti-Hla antibodies clearly play a pivotal role in the protection afforded by active immunization with an inactivated Hla-based vaccine in this SSTI model.
In summary, we determined that antibodies mediate protection afforded by vaccination with a genetically inactivated Hla-based vaccine in a murine model of S. aureus SSTI. Because inactivated Hla-based vaccines are currently undergoing clinical study, knowledge of the immune parameters that correlate with protection for such vaccines would ensure that the appropriate immune markers are evaluated in clinical trials. We believe that the work described here represents an important first step in the development of correlates of protection for new S. aureus vaccines that contain an inactivated Hla component. The work reported here was conducted using a murine model of S aureus SSTI; however, we recognize that what is true for mice might not necessarily be true for humans. In this regard, Fritz et al. (29) recently found that elevated titers to Hla were significantly correlated with protection from recurrent S. aureus infection in humans. These results are consistent with the findings reported here and underscore the importance of anti-Hla antibodies in protection. Additional clinical studies are needed to confirm the role that anti-Hla antibodies play in the protection induced by active immunization against S. aureus infections in humans and to establish a protective level.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by the Intramural Research Program of the Center for Biologics Evaluation and Research, Food and Drug Administration.
We thank Lindsey Zimmerman for assistance with animal experiments.
Footnotes
Published ahead of print 26 February 2014
Supplemental material for this article may be found at http://dx.doi.org/10.1128/CVI.00051-14.
REFERENCES
- 1. Gorwitz RJ, Kruszon-Moran D, McAllister SK, McQuillan G, McDougal LK, Fosheim GE, Jensen BJ, Killgore G, Tenover FC, Kuehnert MJ. 2008. Changes in the prevalence of nasal colonization with Staphylococcus aureus in the United States, 2001–2004. J. Infect. Dis. 197:1226–1234. 10.1086/533494 [DOI] [PubMed] [Google Scholar]
- 2. Lowy FD. 1998. Staphylococcus aureus infections. N. Engl. J. Med. 339:520–532. 10.1056/NEJM199808203390806 [DOI] [PubMed] [Google Scholar]
- 3. Foster TJ. 2005. Immune evasion by staphylococci. Nat. Rev. Microbiol. 3:948–958. 10.1038/nrmicro1289 [DOI] [PubMed] [Google Scholar]
- 4. Nygaard TK, DeLeo FR, Voyich JM. 2008. Community-associated methicillin-resistant Staphylococcus aureus skin infections: advances toward identifying the key virulence factors. Curr. Opin. Infect. Dis. 21:147–152. 10.1097/QCO.0b013e3282f64819 [DOI] [PubMed] [Google Scholar]
- 5. Chambers HF, Deleo FR. 2009. Waves of resistance: Staphylococcus aureus in the antibiotic era. Nat. Rev. Microbiol. 7:629–641. 10.1038/nrmicro2200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Moran GJ, Krishnadasan A, Gorwitz RJ, Fosheim GE, McDougal LK, Carey RB, Talan DA, EMERGEncy ID Net Study Group 2006. Methicillin-resistant S. aureus infections among patients in the emergency department. N. Engl. J. Med. 355:666–674. 10.1056/NEJMoa055356 [DOI] [PubMed] [Google Scholar]
- 7. McDougal LK, Steward CD, Killgore GE, Chaitram JM, McAllister SK, Tenover FC. 2003. Pulsed-field gel electrophoresis typing of oxacillin-resistant Staphylococcus aureus isolates from the United States: establishing a national database. J. Clin. Microbiol. 41:5113–5120. 10.1128/JCM.41.11.5113-5120.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Liu C, Bayer A, Cosgrove SE, Daum RS, Fridkin SK, Gorwitz RJ, Kaplan SL, Karchmer AW, Levine DP, Murray BE, Michael JR, Talan DA, Chambers HF. 2011. Clinical practice guidelines by the Infectious Diseases Society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children: executive summary. Clin. Infect. Dis. 52:285–292. 10.1093/cid/cir034 [DOI] [PubMed] [Google Scholar]
- 9. van Hal SJ, Fowler VG., Jr 2013. Is it time to replace vancomycin in the treatment of methicillin-resistant Staphylococcus aureus infections? Clin. Infect. Dis. 56:1779–1788. 10.1093/cid/cit178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hiramatsu K, Hanaki H, Ino T, Yabuta K, Oguri T, Tenover FC. 1997. Methicillin-resistant Staphylococcus aureus clinical strain with reduced vancomycin susceptibility. J. Antimicrob. Chemother. 40:135–136. 10.1093/jac/40.1.135 [DOI] [PubMed] [Google Scholar]
- 11. Askari E, Tabatabai SM, Arianpoor A, Nasab MN. 2013. VanA-positive vancomycin–resistant Staphylococcus aureus: systematic search and review of reported cases. Infect. Dis. Clin. Pract. 21:91–93. 10.1097/IPC.0b013e31826e8199 [DOI] [Google Scholar]
- 12. Kennedy AD, Bubeck Wardenburg J, Gardner DJ, Long D, Whitney AR, Braughton KR, Schneewind O, DeLeo FR. 2010. Targeting of alpha-hemolysin by active or passive immunization decreases severity of USA300 skin infection in a mouse model. J. Infect. Dis. 202:1050–1058. 10.1086/656043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Li M, Diep BA, Villaruz AE, Braughton KR, Jiang X, DeLeo FR, Chambers HF, Lu Y, Otto M. 2009. Evolution of virulence in epidemic community-associated methicillin-resistant Staphylococcus aureus. Proc. Natl. Acad. Sci. U. S. A. 106:5883–5888. 10.1073/pnas.0900743106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Berube BJ, Bubeck Wardenburg J. 2013. Staphylococcus aureus α-toxin: nearly a century of intrigue. Toxins (Basel) 5:1140–1166. 10.3390/toxins5061140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Tkaczyk C, Hua L, Varkey R, Shi Y, Dettinger L, Woods R, Barnes A, MacGill RS, Wilson S, Chowdhury P, Stover CK, Sellman BR. 2012. Identification of anti-alpha toxin monoclonal antibodies that reduce the severity of Staphylococcus aureus dermonecrosis and exhibit a correlation between affinity and potency. Clin. Vaccine Immunol. 19:377–385. 10.1128/CVI.05589-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ohlsen K, Lorenz U. 2010. Immunotherapeutic strategies to combat staphylococcal infections. Int. J. Med. Microbiol. 300:402–410. 10.1016/j.ijmm.2010.04.015 [DOI] [PubMed] [Google Scholar]
- 17. Plotkin SA. 2010. Correlates of protection induced by vaccination. Clin. Vaccine Immunol. 17:1055–1065. 10.1128/CVI.00131-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Menzies BE, Kernodle DS. 1994. Site-directed mutagenesis of the alpha-toxin gene of Staphylococcus aureus: role of histidines in toxin activity in vitro and in a murine model. Infect. Immun. 62:1843–1847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Menzies BE, Kernodle DS. 1996. Passive immunization with antiserum to a nontoxic alpha-toxin mutant from Staphylococcus aureus is protective in a murine model. Infect. Immun. 64:1839–1841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Brady RA, Mocca CP, Prabhakara R, Plaut RD, Shirtliff ME, Merkel TJ, Burns DL. 2013. Evaluation of genetically inactivated alpha toxin for protection in multiple mouse models of Staphylococcus aureus infection. PLoS One 8:e63040. 10.1371/journal.pone.0063040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Plaut RD, Mocca CP, Prabhakara R, Merkel TJ, Stibitz S. 2013. Stably luminescent Staphylococcus aureus clinical strains for use in bioluminescent imaging. PLoS One 8:e59232. 10.1371/journal.pone.0059232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Adhikari RP, Karauzum H, Sarwar J, Abaandou L, Mahmoudieh M, Boroun AR, Vu H, Nguyen T, Devi VS, Shulenin S, Warfield KL, Aman MJ. 2012. Novel structurally designed vaccine for S. aureus α-hemolysin: protection against bacteremia and pneumonia. PLoS One 7:e38567. 10.1371/journal.pone.0038567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Prabhakara R, Foreman O, De Pascalis R, Lee GM, Plaut RD, Kim SY, Stibitz S, Elkins KL, Merkel TJ. 2013. Epicutaneous model of community-acquired Staphylococcus aureus skin infections. Infect. Immun. 81:1306–1315. 10.1128/IAI.01304-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Chen J, Trounstine M, Alt FW, Young F, Kurahara C, Loring JF, Huszar D. 1993. Immunoglobulin gene rearrangement in B cell deficient mice generated by targeted deletion of the JH locus. Int. Immunol. 5:647–656. 10.1093/intimm/5.6.647 [DOI] [PubMed] [Google Scholar]
- 25. Verdrengh M, Tarkowski A. 1997. Role of neutrophils in experimental septicemia and septic arthritis induced by Staphylococcus aureus. Infect. Immun. 65:2517–2521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Rigby KM, DeLeo FR. 2012. Neutrophils in innate host defense against Staphylococcus aureus infections. Semin. Immunopathol. 34:237–259. 10.1007/s00281-011-0295-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kim MH, Granick JL, Kwok C, Walker NJ, Borjesson DL, Curry FR, Miller LS, Simon SI. 2011. Neutrophil survival and c-kit(+)-progenitor proliferation in Staphylococcus aureus-infected skin wounds promote resolution. Blood 117:3343–3352. 10.1182/blood-2010-07-296970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mölne L, Verdrengh M, Tarkowski A. 2000. Role of neutrophil leukocytes in cutaneous infection caused by Staphylococcus aureus. Infect. Immun. 68:6162–6167. 10.1128/IAI.68.11.6162-6167.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Fritz SA, Tiemann KM, Hogan PG, Epplin EK, Rodriguez M, Al-Zubeidi DN, Bubeck Wardenburg J, Hunstad DA. 2013. A serologic correlate of protective immunity against community-onset Staphylococcus aureus infection. Clin. Infect. Dis. 56:1554–1561. 10.1093/cid/cit123 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.