Abstract
Antivector immunity may limit the immunogenicity of adenovirus vector vaccines. We tested sera from individuals immunized with adenovirus type 4 and 7 (Ad4 and Ad7, respectively) vaccine or naturally infected with Ad4 for their ability to neutralize a panel of E1-deleted human and chimpanzee adenoviruses (ChAd). Small statistically significant increases in titers to ChAd63, ChAd3, human Ad35, and human Ad5 were observed. Neutralizing antibodies elicited by Ad4 infection or immunization results in a small amount of adenovirus cross-reactivity.
TEXT
Adenoviruses (Ad) are promising candidates as vectors for infectious disease and cancer vaccines designed to elicit T-cell activation and cellular immune responses (1–3). However, preexisting immunity may limit their usefulness as vaccines, which has been described for recombinant human Ad type 5 (rAd5) (4–8) and was attributed to the presence of neutralizing antibodies (nAb). As a result, an alternative approach has been to utilize uncommon human or closely related animal adenovirus types as potential vectors (9). Chimpanzee adenovirus (ChAd) vectors demonstrated efficacy in a nonhuman primate model of Ebola virus infection and for hepatitis C and Plasmodium falciparum malaria (10, 11), and they appear to be unaffected by preexisting Ad5 immunity (N. Sullivan, unpublished observations). However, the concern that cross-reactive neutralizing activity may impair the immunogenicity of these alternative vectors persists (12). Specifically, ChAd63 and Ad4 are classified within the species E serogroup of adenoviruses. Ad4 infection is fairly common in adult populations (34 to 45% adult seroprevalence) (9, 13, 14) and is a common cause of acute febrile respiratory illness (AFRI) among military recruits (15, 16). Currently, all U.S. military recruits receive the licensed live oral types 4 and 7 adenovirus vaccine during basic training. To assess for potential antivector immunity, we tested sera from vaccinated and naturally infected subjects against a panel of E1-deleted adenoviruses that included Ad4, Ad5, Ad26, Ad35, ChAd3, and ChAd63.
We obtained fifty paired serum samples from a clinical trial of oral Ad4 and Ad7 vaccine (n = 19) in military recruits and in civilians (17) and from a prospective study of AFRI with Ad4 infection at a U.S. Army recruit training center (n = 31) (18). All samples were tested at the time of the initial studies and were negative for Ad4 nAb at baseline and either positive or negative for Ad7 nAb. Blood specimens were obtained prior to vaccination or the onset of illness and from 2 to 8 weeks afterward. All sera were tested concurrently in a luciferase reporter gene virus neutralization assay as previously described (19). Briefly, A549 human lung carcinoma cells (or 2393/T17 cells for ChAd63) were plated at a density of 1 × 104 cells per well in 96-well plates and infected with E1-deleted replication-incompetent rAd-luciferase reporter constructs of different serotypes (Table 1) at a multiplicity of infection of 500, with 2-fold serial dilutions of serum in 200-μl reaction volumes. Following a 24-hour incubation, the luciferase activity in the cells was measured using the Steady-Glo luciferase reagent system (Promega). Ninety-percent neutralization titers were defined as the maximum serum dilution that neutralized 90% of luciferase activity. We calculated the frequencies of samples exhibiting cross-neutralization and geometric mean titers (GMTs) for pre- and postexposure samples. An Ad nAb titer of >200 was used to stratify the analyses in a phase IIb HIV vaccine trial (20) and was considered potentially detrimental to use of the vector in this study. In addition, we defined a positive response as a ≥4-fold increase in the titer over baseline. We used nonparametric tests to compare GMTs and the Chi-square test for categorized titers (<12, 12 to 100, 101 to 1,000, and >1,000). The participating institutional review boards (IRBs) approved all the studies from which the samples were collected. The Walter Reed Army Institute of Research and National Institutes of Health IRBs approved this study.
TABLE 1.
Pre- and postinfection or vaccination titers by adenovirus serotype
| Serotype and time point (no. of samples)a | Prevalence of titers ≥200 (% [95% CI])b | GMT (95% CI)c | Fold change in GMT | No. (%) of respondersd | Pe |
|---|---|---|---|---|---|
| Ad4 | |||||
| Pre (50) | 0 (0–7.1) | 11.3 (10.7–12.0) | |||
| Post (50) | 70.0 (55.4–82.1) | 578.0 (400.5–834.1) | 51.2 | 50 (100) | <0.0001 |
| Ad5 | |||||
| Pre (31) | 40.6 (23.7–59.4) | 135.2 (54.3–336.4) | |||
| Post (31) | 46.9 (29.1–65.2) | 250.4 (99.5–630.4) | 1.9 | 5 (16.1) | 0.0001 |
| Ad26 | |||||
| Pre (32) | 6.3 (0.8–20.8) | 15.3 (10.4–22.4) | |||
| Post (32) | 12.5 (3.5–29.0) | 18.4 (11.1–30.4) | 1.2 | 1 (3.1) | 0.25 |
| Ad35 | |||||
| Pre (29) | 0 (0–11.9) | 12.1 (9.9–14.8) | |||
| Post (29) | 13.8 (3.9–31.7) | 30.3 (15.8–58.2) | 2.5 | 6 (20.7) | 0.0005 |
| ChAd3 | |||||
| Pre (43) | 27.9 (15.3–43.7) | 62.0 (35.8–107.3) | |||
| Post (43) | 51.2 (35.4–66.7) | 181.9 (96.7–342.3) | 2.9 | 12 (27.9) | <0.0001 |
| ChAd63 | |||||
| Pre (38) | 0 (0–9.3) | 11.1 (10.9–11.2) | |||
| Post (38) | 7.9 (1.7–21.4) | 21.4 (14.3–31.9) | 1.93 | 4 (10.5) | 0.0002 |
Ad, adenovirus; Ch, chimpanzee.
CI, confidence interval.
Eleven was used as minimum for calculations based on negative cutoff value of 12. GMT, geometric mean titer.
Defined as 4-fold increase in titer.
P values calculated based on Wilcoxon matched-pairs test.
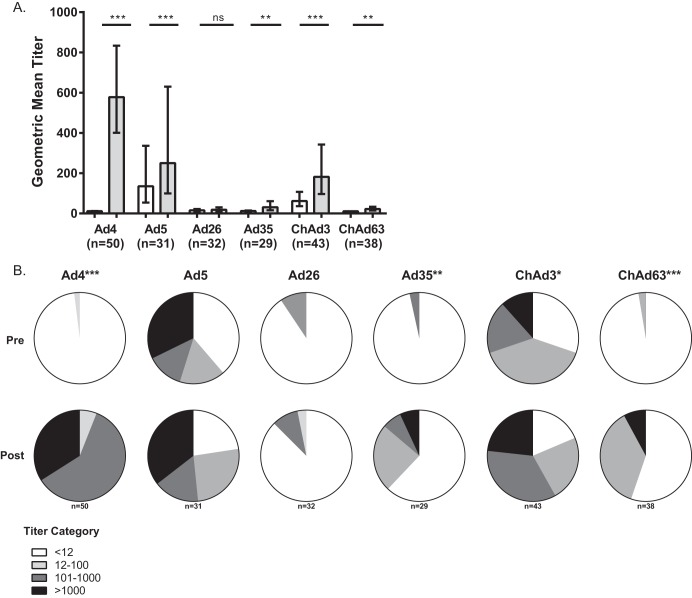
The postinfection GMTs for Ad4 and other adenoviruses in the AFRI cohort (n = 31) were higher than the postvaccination cohort (n = 19) GMTs (for Ad4, 652.6 [95% confidence interval (CI), 412.1 to 1,033] versus 474.2 [95% CI, 246.5 to 912.2], respectively), but the differences were not statistically significant (P = 0.41 for Ad4). There were no statistically significant differences in the GMTs between the infection and vaccination groups for the other adenoviruses tested (data not shown); therefore, we combined data from both groups for our analyses. We observed small (2- to 3-fold) but statistically significant increases in the GMTs after vaccination or infection with Ad4 for all viruses tested except for Ad26 (Fig. 1A, Table 1). The GMTs observed were mostly of low magnitude (<200), except for ChAd3 (for which 51.2% of GMTs were ≥200 postinfection/vaccination). When the GMTs were grouped by category, statistically significant differences were observed for Ad4, Ad35, ChAd3, and ChAd63 (Fig. 1B). Evaluation of paired titers demonstrated boosting of cross-reactive responses and new responses (defined as a 4-fold rise in titer) (Table 1). Ad5 neutralization responses were not correlated with the responses to ChAd3, which is from the same serogroup C (P = 0.34 for preinfection titers and P = 0.46 for postinfection or vaccination titers, Spearman's correlation test). However, the responses to other Ad not tested might have been associated with this cross-reactivity (9). Overall, these results indicate a small but consistent cross-neutralization of several adenovirus serotypes after Ad4 immunization or infection.
FIG 1.
Vaccination or infection with Ad4 is associated with a small increase in neutralizing activity against common and rare human and primate adenoviruses (white bars represent pre-Ad exposure and gray bars post-Ad exposure titers). (A) Geometric mean titers (GMTs) and 95% confidence intervals (CI) for all subjects. The Kruskal-Wallis test was used to assess statistical significance. (B) Pie charts depicting frequency of pre-Ad exposure and post-Ad exposure titers by titer range. The Chi-square test was used to test statistical significance. There was insufficient sample to test sera against all isolates. *, P < 0.05; **, P < 0.001; ***, P < 0.0001. ns, not significant.
Adenovirus type identification by neutralization has historically been associated with cross-reactivity, sometimes requiring further testing using agglutination or molecular methods (13, 21, 22). Adenovirus infection may also produce smaller, heterotypic antibody responses to other Ad types (23). We observed small nAb responses in the same serogroup (E, ChAd63) and in serogroups B (Ad35) and C (Ad5, ChAd3). The largest heterotypic response was to ChAd3 (28%). We did not detect any differences between vaccination and infection, suggesting that these results are likely attributable to Ad4 rather than to Ad7. Previous studies have demonstrated decreased T-cell responses to Ad5 vector vaccines with preexisting Ad5 neutralization (8, 20, 24). In one study, preimmunization Ad5 titers of <12 were associated with a 3.29-times-higher enzyme-linked immunosorbent spot assay (ELISpot) response that that of seropositive subjects (25). The high prevalence of nAb to ChAd3 (27.9%) and the increase in GMTs following vaccination or infection suggest that this may not be a good candidate vector in this study population. One caveat to our study is that vaccination with a replication-deficient Ad vector may induce less cross-reactivity than we observed with a live replication-competent vaccine.
In summary, reporter gene-based neutralization assays can be used to quickly detect potential antivector immunity when there is concern for cross-reactivity. Our results are consistent with previous prevalence studies of antibodies to human and chimpanzee adenoviruses (4, 12, 26) and demonstrate that infection or vaccination with a more common adenovirus serotype may generate or boost cross-reactive antibodies. Further study is needed to establish the ability, if any, of these levels of neutralization activity to impair vector immunity.
ACKNOWLEDGMENTS
This research was supported by the Intramural Research Program of the Vaccine Research Center, National Institute of Allergy and Infectious Diseases, National Institutes of Health.
The opinions herein are those of the authors and should not be construed as official or representing the views of the U.S. Department of Health and Human Services, the National Institute for Allergy and Infectious Diseases, the Department of Defense, or the Department of the Army.
Footnotes
Published ahead of print 12 March 2014
REFERENCES
- 1. Altaras NE, Aunins JG, Evans RK, Kamen A, Konz JO, Wolf JJ. 2005. Production and formulation of adenovirus vectors. Adv. Biochem. Eng. Biotechnol. 99:193–260. 10.1007/10_008 [DOI] [PubMed] [Google Scholar]
- 2. Tatsis N, Ertl HC. 2004. Adenoviruses as vaccine vectors. Mol. Ther. 10:616–629. 10.1016/j.ymthe.2004.07.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Barouch DH, Nabel GJ. 2005. Adenovirus vector-based vaccines for human immunodeficiency virus type 1. Hum. Gene Ther. 16:149–156. 10.1089/hum.2005.16.149 [DOI] [PubMed] [Google Scholar]
- 4. Mast TC, Kierstead L, Gupta SB, Nikas AA, Kallas EG, Novitsky V, Mbewe B, Pitisuttithum P, Schechter M, Vardas E, Wolfe ND, Mste-Amezaga Casimiro DR, Coplan P, Straus WL, Shiver JW. 2010. International epidemiology of human pre-existing adenovirus (Ad) type-5, type-6, type-26 and type-36 neutralizing antibodies: correlates of high Ad5 titers and implications for potential HIV vaccine trials. Vaccine 28:950–957. 10.1016/j.vaccine.2009.10.145 [DOI] [PubMed] [Google Scholar]
- 5. McCoy K, Tatsis N, Korioth-Schmitz B, Lasaro MO, Hensley SE, Lin SW, Li Y, Giles-Davis W, Cun A, Zhou D, Xiang Z, Letvin NL, Ertl HC. 2007. Effect of preexisting immunity to adenovirus human serotype 5 antigens on the immune responses of nonhuman primates to vaccine regimens based on human- or chimpanzee-derived adenovirus vectors. J. Virol. 81:6594–6604. 10.1128/JVI.02497-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. O'Brien KL, Liu J, King SL, Sun YH, Schmitz JE, Lifton MA, Hutnick NA, Betts MR, Dubey SA, Goudsmit J, Shiver JW, Robertson MN, Casimiro DR, Barouch DH. 2009. Adenovirus-specific immunity after immunization with an Ad5 HIV-1 vaccine candidate in humans. Nat. Med. 15:873–875. 10.1038/nm.1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Paris RM, Kim JH, Robb ML, Michael NL. 2010. Prime-boost immunization with poxvirus or adenovirus vectors as a strategy to develop a protective vaccine for HIV-1. Expert Rev. Vaccines 9:1055–1069. 10.1586/erv.10.106 [DOI] [PubMed] [Google Scholar]
- 8. Priddy FH, Brown D, Kublin J, Monahan K, Wright DP, Lalezari J, Santiago S, Marmor M, Lally M, Novak RM, Brown SJ, Kulkarni P, Dubey SA, Kierstead LS, Casimiro DR, Mogg R, DiNubile MJ, Shiver JW, Leavitt RY, Robertson MN, Mehrotra DV, Quirk E. 2008. Safety and immunogenicity of a replication-incompetent adenovirus type 5 HIV-1 clade B gag/pol/nef vaccine in healthy adults. Clin. Infect. Dis. 46:1769–1781. 10.1086/587993 [DOI] [PubMed] [Google Scholar]
- 9. Colloca S, Barnes E, Folgori A, Ammendola V, Capone S, Cirillo A, Siani L, Naddeo M, Grazioli F, Esposito ML, Ambrosio M, Sparacino A, Bartiromo M, Meola A, Smith K, Kurioka A, O'Hara GA, Ewer KJ, Anagnostou N, Bliss C, Hill AVS, Traboni C, Klenerman P, Cortese R, Nicosia A. 2012. Vaccine vectors derived from a large collection of simian adenoviruses induce potent cellular immunity across multiple species. Sci. Transl. Med. 4:115ra2. 10.1126/scitranslmed.3002925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Barnes E, Folgori A, Capone S, Swadling L, Aston S, Kurioka A, Meyer J, Huddart R, Smith K, Townsend R, Brown A, Antrobus R, Ammendola V, Naddeo M, O'Hara GA, Willberg C, Harrison A, Grazioli F, Esposito ML, Siani L, Traboni C, Oo Y, Adams D, Hill A, Colloca S, Nicosia A, Cortese R, Klenerman P. 2012. Novel adenovirus-based vaccines induce broad and sustained T cell responses to HCV in man. Sci. Transl. Med. 4:115ra1. 10.1126/scitranslmed.3003155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. O'Hara GA, Duncan CJ, Ewer KJ, Collins KA, Elias SC, Halstead FD, Goodman AL, Edwards NJ, Reyes-Sandoval A, Bird P, Rowland R, Sheehy SH, Poulton ID, Hutchings C, Todryk S, Andrews L, Folgori A, Berrie E, Moyle S, Nicosia A, Colloca S, Cortese R, Siani L, Lawrie AM, Gilbert SC, Hill AV. 2012. Clinical assessment of a recombinant simian adenovirus ChAd63: a potent new vaccine vector. J. Infect. Dis. 205:772–781. 10.1093/infdis/jir850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dudareva M, Andrews L, Gilbert SC, Bejon P, Marsh K, Mwacharo J, Kai O, Nicosia A, Hill AV. 2009. Prevalence of serum neutralizing antibodies against chimpanzee adenovirus 63 and human adenovirus 5 in Kenyan children, in the context of vaccine vector efficacy. Vaccine 27:3501–3504. 10.1016/j.vaccine.2009.03.080 [DOI] [PubMed] [Google Scholar]
- 13. Madisch I, Harste G, Pommer H, Heim A. 2005. Phylogenetic analysis of the main neutralization and hemagglutination determinants of all human adenovirus prototypes as a basis for molecular classification and taxonomy. J. Virol. 79:15265–15276. 10.1128/JVI.79.24.15265-15276.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Barouch Dan H, Kathryn Stephenson E, Erica Borducchi N, Smith K, Stanley K, Anna McNally G, Liu J, Abbink P, Lori Maxfield F, Michael Seaman S, Dugast A-S, Alter G, Ferguson M, Li W, Patricia Earl L, Moss B, Elena Giorgi E, James Szinger J, Leigh Eller A, Erik Billings A, Rao M, Tovanabutra S, Sanders-Buell E, Weijtens M, Maria Pau G, Schuitemaker H, Merlin Robb L, Jerome Kim H, Bette Korber T, Nelson Michael L. 2013. Protective efficacy of a global HIV-1 mosaic vaccine against heterologous SHIV challenges in rhesus monkeys. Cell 155:531–539. 10.1016/j.cell.2013.09.061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Barraza EM, Ludwig SL, Gaydos JC, Brundage JF. 1999. Reemergence of adenovirus type 4 acute respiratory disease in military trainees: report of an outbreak during a lapse in vaccination. J. Infect. Dis. 179:1531–1533. 10.1086/314772 [DOI] [PubMed] [Google Scholar]
- 16. Hendrix RM, Lindner JL, Benton FR, Monteith SC, Tuchscherer MA, Gray GC, Gaydos JC. 1999. Large, persistent epidemic of adenovirus type 4-associated acute respiratory disease in U. S. army trainees. Emerg. Infect. Dis. 5:798–801. 10.3201/eid0506.990609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lyons A, Longfield J, Kuschner R, Straight T, Binn L, Seriwatana J, Reitstetter R, Froh IB, Craft D, McNabb K, Russell K, Metzgar D, Liss A, Sun X, Towle A, Sun W. 2008. A double-blind, placebo-controlled study of the safety and immunogenicity of live, oral type 4 and type 7 adenovirus vaccines in adults. Vaccine 26:2890–2898. 10.1016/j.vaccine.2008.03.037 [DOI] [PubMed] [Google Scholar]
- 18. Kolavic-Gray SA, Binn LN, Sanchez JL, Cersovsky SB, Polyak CS, Mitchell-Raymundo F, Asher LV, Vaughn DW, Feighner BH, Innis BL. 2002. Large epidemic of adenovirus type 4 infection among military trainees: epidemiological, clinical, and laboratory studies. Clin. Infect. Dis. 35:808–818. 10.1086/342573 [DOI] [PubMed] [Google Scholar]
- 19. Sprangers MC, Lakhai W, Koudstaal W, Verhoeven M, Koel BF, Vogels R, Goudsmit J, Havenga MJ, Kostense S. 2003. Quantifying adenovirus-neutralizing antibodies by luciferase transgene detection: addressing preexisting immunity to vaccine and gene therapy vectors. J. Clin. Microbiol. 41:5046–5052. 10.1128/JCM.41.11.5046-5052.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Buchbinder SP, Mehrotra DV, Duerr A, Fitzgerald DW, Mogg R, Li D, Gilbert PB, Lama JR, Marmor M, Del RC, McElrath MJ, Casimiro DR, Gottesdiener KM, Chodakewitz JA, Corey L, Robertson MN. 2008. Efficacy assessment of a cell-mediated immunity HIV-1 vaccine (the Step Study): a double-blind, randomised, placebo-controlled, test-of-concept trial. Lancet 372:1881–1893. 10.1016/S0140-6736(08)61591-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wigand R. 1987. Pitfalls in the identification of adenoviruses. J. Virol. Methods 16:161–169. 10.1016/0166-0934(87)90001-2 [DOI] [PubMed] [Google Scholar]
- 22. Gall JGD, Crystal RG, Falck-Pedersen E. 1998. Construction and characterization of hexon-chimeric adenoviruses: specification of adenovirus serotype. J. Virol. 72:10260–10264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Grayston JT, Johnston PB, Loosli CG, Smith ME, Woolridge RL. 1956. Neutralizing and complement fixing antibody response to adenovirus infection. J. Infect. Dis. 99:199–206. 10.1093/infdis/99.2.199 [DOI] [PubMed] [Google Scholar]
- 24. McElrath MJ, De Rosa SC, Moodie Z, Dubey S, Kierstead L, Janes H, Defawe OD, Carter DK, Hural J, Akondy R, Buchbinder SP, Robertson MN, Mehrotra DV, Self SG, Corey L, Shiver JW, Casimiro DR. 2008. HIV-1 vaccine-induced immunity in the test-of-concept step study: a case-cohort analysis. Lancet 372:1894–1905. 10.1016/S0140-6736(08)61592-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Catanzaro AT, Koup RA, Roederer M, Bailer RT, Enama ME, Moodie Z, Gu L, Martin JE, Novik L, Chakrabarti BK, Butman BT, Gall JGD, King CR, Andrews CA, Sheets R, Gomez PL, Mascola JR, Nabel GJ, Graham BS, Vaccine Res C. 2006. Phase 1 safety and immunogenicity evaluation of a multiclade HIV-1 candidate vaccine delivered by a replication-defective recombinant adenovirus vector. J. Infect. Dis. 194:1638–1649. 10.1086/509258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Quinn KM, Da Costa A, Yamamoto A, Berry D, Lindsay RWB, Darrah PA, Wang L, Cheng C, Kong W-P, Gall JGD, Nicosia A, Folgori A, Colloca S, Cortese R, Gostick E, Price DA, Gomez CE, Esteban M, Wyatt LS, Moss B, Morgan C, Roederer M, Bailer RT, Nabel GJ, Koup RA, Seder RA. 2013. Comparative analysis of the magnitude, quality, phenotype, and protective capacity of simian immunodeficiency virus gag-specific CD8+ T cells following human-, simian-, and chimpanzee-derived recombinant adenoviral vector immunization. J. Immunol. 190:2720–2735. 10.4049/jimmunol.1202861 [DOI] [PMC free article] [PubMed] [Google Scholar]