Abstract
With continuing occurrence of varicella despite increasing vaccine coverage for the past 20 years, a case-based study, a case-control study, and an immunogenicity and safety study were conducted to address the impact of varicella vaccination in South Korea. Varicella patients under the age of 16 years were enrolled for the case-based study. For the case-control study, varicella patients between 12 months and 15 years of age were enrolled with one control matched for each patient. For the immunogenicity and safety study, otherwise healthy children from 12 to 24 months old were immunized with Suduvax (Green Cross, South Korea). Fluorescent antibody to membrane antigen (FAMA) varicella-zoster virus (VZV) antibody was measured before and 6 weeks after immunization. In the case-based study, the median age of the patients was 4 years. Among 152 patients between 1 and 15 years of age, 139 children received varicella vaccine and all had breakthrough infections. Clinical courses were not ameliorated in vaccinated patients, but more vaccinated patients received outpatient rather than inpatient care. In the case-control study, the adjusted overall effectiveness of varicella vaccination was 54%. In the immunogenicity and safety study, the seroconversion rate and geometric mean titer for FAMA antibody were 76.67% and 5.31. Even with increasing varicella vaccine uptake, we illustrate no upward age shift in the peak incidence, a high proportion of breakthrough disease, almost no amelioration in disease presentation by vaccination, and insufficient immunogenicity of domestic varicella vaccine. There is need to improve the varicella vaccine used in South Korea.
INTRODUCTION
Varicella caused by varicella-zoster virus (VZV) is a highly infectious disease, and the secondary attack rates may reach up to 90% for susceptible household contacts. In South Korea, a live attenuated Biken Oka strain varicella vaccine was first introduced in 1988, and since then a few more imported and domestic vaccines have been on the market for different lengths of time. Among the few domestic vaccines which were developed in the early 1990s, only Suduvax (Green Cross, South Korea) is currently on the market and is the most popular varicella vaccine in South Korea. With the increase of varicella vaccine coverage in South Korea, varicella became a nationally notifiable disease, and the vaccine was mandated for universal immunization at government expense in 2005; routine varicella immunization for infants at 12 to 15 months of age was recommended. These measures should have reduced the burden of varicella in South Korea. However, the nationwide occurrence of varicella has not decreased substantially, unlike the successful experience in other countries (1–5). We report a short-term case-based study, a case-control study, and an immunogenicity and safety study to evaluate the effectiveness of varicella vaccination in South Korea.
MATERIALS AND METHODS
A case-based study was conducted prospectively from August 2006 to March 2007, when 176 children with varicella who were younger than 16 years of age were recruited from eight hospitals located in Seoul and one hospital each in Gyeonggi-do, Chungcheongnam-do, and Jeonbuk-do in South Korea. The enrolled patients were not particularly selected but were enrolled in the order of diagnosis at each participating hospital with parental or legal-guardian consent. Demographic characteristics, social history, varicella vaccination (the date, the site, and the product's name), patient disposition (outpatient care versus hospitalization), clinical presentation (fever, chills, malaise, toxic looking, irritability, poor oral intake, pruritus, headache, cough, rhinorrhea, conjunctival injection, pharyngeal injection, lymphadenopathy, abnormal chest auscultation findings, abdominal pain, back pain), various characteristics of rash (onset, pruritus, course, site, number, and duration), and complications, including secondary bacterial infection of the lesion, hemorrhagic varicella, pneumonia, cellulitis, necrotizing fasciitis, thrombocytopenia, invasive group A streptococcal infections, bacteremia/sepsis, cerebellar ataxia, encephalitis, dehydration, Reye syndrome, other neurological conditions, and death, were obtained. For assigning patients into four groups according to the number of skin rashes, each parent or guardian was asked whether the child had numerous lesions. When the answer was “not many,” each parent or guardian was asked whether the child had <50 lesions, and if the answer was no, investigators went on to ask in sequence to ≥500 lesions. When a parent or guardian answered the child had numerous lesions, investigators asked whether the child had ≥500 lesions, and if the answer was no, we proceeded to ask in sequence to <50 lesions. As the study continued, members of research teams in collaborating hospitals had communicated better with parents or guardians who seemed to follow the instructions well. Mother and father were also asked whether any of them missed work for the patients and the duration of missing work. Parents or guardians were asked to subjectively interpret the severity of varicella by choosing one of the three categories of very severe, moderately severe, and mild. Past medical history revealed asthma in 5 patients, atopic dermatitis in 5 patients, and bronchiolitis in one patient. Vaccination was verified by checking immunization records or directly contacting the health care providers in 69.4% of the study subjects. A case of varicella was defined as an acute, generalized, maculopapulovesicular rash without other apparent cause (6). Breakthrough disease was defined as varicella that occurred more than 42 days after vaccination (7).
A case-control study was conducted prospectively from September 2007 to March 2008 at six hospitals located in Seoul, two hospitals in Gyeonggi-do, and one hospital in Pusan. The case group consisted of healthy children between 12 months and 15 years of age in whom varicella was diagnosed in the participating study hospitals. The enrolled patients were not particularly selected but were enrolled in the order of diagnosis at each participating hospital with parental or legal-guardian consent. For each child with varicella, one control matched by age (within 3 months) was selected at the participating hospitals. Demographic characteristics, social history, varicella vaccination status (the date, the site, the product's name), the date of measles, mumps, rubella (MMR) vaccination, and patient disposition (outpatient care versus hospitalization) were collected. Among the varicella group, 2 patients had a history of asthma, 1 patient had a history of acute bronchiolitis, and 1 patient had atopic dermatitis. Vaccination was verified by checking immunization records or directly contacting the health care providers for 66.0% of the case group and 72.6% of the control group. Cases of varicella and breakthrough disease were defined as described above.
An immunogenicity and safety study was conducted from September 2008 to November 2009 in children recruited from three hospitals each in Seoul and Gyeonggi-do, two hospitals in Incheon, and one hospital in Gyeongsangnam-do in South Korea. Entry criteria were as follows: age of 12 to 24 months; no clinical history of varicella; no history of neoplasia, current steroid use, or other immunosuppression in the child or siblings; no exposure to varicella within the past 4 weeks; no administration of vaccine or immunoglobulin within 1 month before the varicella vaccine was given; and no previous diagnosis of Kawasaki disease. Each study subject was vaccinated with a single dose of Suduvax (greater than 1,350 PFU/dose, varicella virus vaccine, live, attenuated [MAV/06], lyophilized; Green Cross, South Korea). Blood samples were obtained before and 6 weeks after immunization in each subject. Any local or systemic adverse events during the first 7 days following vaccination were collected by telephone interviews, and a daily diary card was given to each parent or guardian to record any symptoms of illness for 4 weeks, during which time the parent or guardian was asked to take the child's temperature daily. If parents or guardians reported rash or fever, a complete examination was performed by a physician. Fluorescent antibody to membrane antigen (FAMA) assay was performed using previously described methods with minor modification (8, 9). Briefly, heat-inactivated serum samples were diluted serially in 2-fold aliquots (from 1:2 to 1:128 of each sample) in 96-well U-bottom plates (Nunc, Inc., Roskilde, Denmark), to which unfixed VZV-infected MRC-5 cells were added and incubated at room temperature for 30 min. Following incubation, fluorescein-5-isothiocyanate (FITC)-conjugated goat IgG fraction to human IgG (Cappel, Inc., Ohio, USA) was added to the washed cell pellets and incubated for 1 h. Membrane fluorescence was read under the fluorescence microscope (Microscope Research digital camera, Axioskop 40 Axiocam Mrc5; Carl Zeiss, Inc., Goettingen, Germany). A bright fluorescent ring around the surface of cells was graded on a scale of 1+ to 4+ on the basis of width and intensity. Grade 1 was defined as cells with specific but vague fluorescent staining, grade 2 as specific and weak fluorescence, grade 3 as bright and specific fluorescence, and grade 4 as a thick, brilliant fluorescent membrane. Damaged cells with cytoplasmic fluorescence and no distinct membranous fluorescence were regarded negative. Grade 2+ or greater fluorescence is considered seropositive. Diluted anti-VZV antibody (3 × 10−3 IU/ml; National Institute for Biological Standards and Control, Hertfordshire, United Kingdom) was used as a grade 2 positive standard, and phosphate-buffered solution was used as a negative standard for the FAMA assay. In addition, the FAMA assay was regarded as reliable when the FAMA titer of a seropositive adult serum, for which the FAMA antibody titer is known, had a variation in the ≤2-fold range. Seropositivity of the FAMA assay was defined by a positive fluorescence at a serum dilution of 1:4 or greater.
Each participating center received institutional review board approval before initiation of the studies, and written informed consents were obtained from the parent or legal guardian of each patient and control before enrollment.
Statistical analysis was done by using a chi-square test to compare categorical variables, including sex distribution, weekday location, characteristic rash, fever, illness severity as reported by parents, and parents' missed work, by using a Mann-Whitney U test to compare continuous variables, including age distribution and number of days with rash, by using an age-adjusted Cochran-Mantel-Haenszel test to compare the proportion of inpatient care and outpatient care between vaccinated varicella patients and unvaccinated varicella patients, by using McNemar's test to compare between varicella group and control group for the rate of varicella vaccination, MMR vaccination, and varicella vaccination within 28 days of MMR vaccination, and by using a paired t test to compare age at varicella vaccination between the varicella group and the control group. The odds ratio for vaccination with 95% confidence intervals in the case-control study was calculated by unconditional logistic regression analysis adjusted for the effects of potential confounders as sex, age, the confirmation method of vaccination, and the duration between varicella vaccination and onset of varicella (date of control enrollment) in the varicella group (control group). The effectiveness of the vaccine was calculated as 1 minus the odds ratio. Results were considered statistically significant if the P value was <0.05. Data were analyzed with SAS version 9.1 (SAS Institute, Inc., Cary, NC).
RESULTS
Case-based study.
One hundred and seventy-six children with varicella younger than 16 years of age were enrolled in the study. Among the study groups, 80 subjects (45.5%) were girls and 19 subjects (11.1%) were younger than 1 year of age. The largest number of the patients belonged to the 1-to-4-years age group, with the mean age of 4.6 ± 3.2 years and median age of 4 years. The number of enrolled patients was the lowest in the first month of the study in August 2006, increased to the highest in January 2007, and then decreased as the end of the study was reached in March 2007.
Among 152 varicella patients who were 1 year of age or older, 139 patients (91.4%) had been vaccinated; 143 patients were cared for at the outpatient clinics and the rest were hospitalized. The median age at varicella vaccination was 13 months (mean, 1.13 ± 0.29 years), and the median time from vaccination to the onset of disease was 3 years (mean, 3.98 ± 2.91 years). The varicella vaccination rate among varicella patients in out-of-home care was 94.4% (118 of 125 patients), and the rate among varicella patients cared for at home was 76.9% (20 of 26 patients). The proportion of breakthrough infection was 91.4% among patients older than 11 months of age and was not different among various age groups.
Varicella rashes appeared predominantly on the trunk (91.3%), scalp (89.1%), upper extremities (70.7%), lower extremities (70.1%), and the oral cavity (19.6%) and consisted of vesicles (84.2%), papules (84.2%), macules (83.3%), crust (77.4%), pustules (33.3%), and petechiae (2.2%). The most common clinical presentation other than skin rash was pruritus followed by fever, poor oral intake, cough, and rhinorrhea, but there was no difference between the vaccinated group and the nonvaccinated group. The severity of the disease in vaccinated patients was not statistically different from that in unvaccinated patients for the appearance, duration, extent in distribution, and the number of skin lesions, fever, and the number of parents who had missed work. However, parents of vaccinated varicella patients appraised the illness less seriously than parents of unvaccinated patients and had their children cared for more in the outpatient clinics (Table 1). All the study patients survived. Comparison of clinical characteristics in enrollees of four distinct geographic regions of the participating hospitals did not show a difference.
TABLE 1.
Characteristics of children with varicella according to vaccination statusj
| Clinical characteristic | Value |
P value | |
|---|---|---|---|
| Vaccinated patients (n = 139) | Unvaccinated patients (n = 13) | ||
| Age (mo) | |||
| Mean ± SD | 4.63 ± 2.89 | 3.85 ± 3.16 | 0.2264i |
| Median | 4 | 3 | |
| Range (min–max) | 1–14 | 1–9 | |
| No. (%) of females | 67 (48.2) | 4 (30.8) | 0.2598 |
| No. (%) with a weekday location of: | 0.0411 | ||
| Day care | 84 (60.9) | 4 (30.8) | |
| School | 29 (21.0) | 3 (23.1) | |
| Home | 20 (14.5) | 6 (46.1) | |
| Cramming school | 5 (3.6) | 0 (0.0) | |
| Median no. of days with rash (range) | 5 (1–24)a | 6 (4–14)b | 0.0547i |
| No. (%) with lesions | |||
| <50 | 65 (47.4)c | 4 (30.7) | 0.4606 |
| 50–249 | 56 (40.9) | 7 (53.8) | |
| 250–499 | 13 (9.5) | 1 (7.7) | |
| >500 | 3 (2.1) | 1 (7.7) | |
| No. (%) with itchy rash | 94 (68.1)d | 9 (69.2) | 0.9342 |
| No. (%) who developed scabs | 104 (74.8) | 11 (84.6) | 0.4313 |
| No. (%) with fever | 24 (17.2) | 3 (23.0) | 0.6001 |
| No. (%) with a moderate illness severity reported by parents | 62 (50.0)e | 10 (83.3)f | 0.0026 |
| No. (%) with parents who missed work | 8 (6.0)g | 1 (7.6) | |
| No. (%) cared for at outpatient clinics | 133 (95.6) | 10 (76.9) | 0.0079h |
For vaccinated patients, total n = 124.
For unvaccinated patients, total n = 11.
For vaccinated patients, total n = 137.
For vaccinated patients, total n = 138.
For vaccinated patients, total n = 124.
For unvaccinated patients, total n = 12.
For vaccinated patients, total n = 133.
Cochran-Mantel-Haenszel test was used.
P value was obtained by a Mann-Whitney U test, and the rest of the P values were obtained by a chi-square test.
Clinical severity was not available for all cases.
Case-control study.
One hundred and thirty-five varicella patients younger than 16 years of age were enrolled, their mean age was 4.8 ± 3.2 years, and varicella vaccination coverage rate was 91.7% in patients older than 1 year of age. Breakthrough varicella was confirmed in 82.2% of all enrolled varicella cases, and all of the vaccinated varicella cases were breakthrough infection. One control was matched for each of 106 patients; 103 patients were cared for in the outpatient clinics, and the remaining three patients were hospitalized in participating institutions. The name of the varicella vaccine product was provided in 48.1% (51 subjects) of 106 varicella patients and in 51.9% (55 subjects) of 106 controls. Varicella vaccination rates were above 90% for both case and control groups. The proportions of subjects younger than 15 months of age who received varicella vaccine were not different in the two groups, and the proportion of subjects who received varicella vaccine within 28 days of MMR vaccination was greater in the control group than in the case group (Table 2). The effectiveness of varicella vaccination was 54%, with an adjusted matched odds ratio of 0.46 (95% confidence interval, 0.10 to 2.05). Each subject of both groups received one of eight varicella vaccine products: four domestic ones, Suduvax (Green Cross; 22 cases and 27 controls), Suduvaccine (Cheil Jedang [CJ]; 10 cases and 4 controls), Varimmune (SK Chemicals Co.; 3 cases and 10 controls), and Varicella Kovax (Korea Vaccine; 2 cases and 2 controls); and four imported ones, Varilrix (GlaxoSmithKline [GSK]; 8 cases and 7 controls), Vari-L (Changchun Institute of Biological Products, 3 cases and 2 controls), Biken (Biken Institute; 2 cases and 3 controls), and Varivax (Merck Sharp & Dohme Corp. [MSD]; 1 case). The proportions were not statistically different between the two groups.
TABLE 2.
Characteristics of children with varicella and matched controls
| Clinical characteristics | Value |
P valuea | |
|---|---|---|---|
| Children with varicella (n = 106) | Controls (n = 106) | ||
| Age (yr) | |||
| Mean ± SD | 5.2 ± 2.9 | 5.2 ± 3.0 | |
| Median | 4 | 4 | |
| Range | 1–13 | 1–14 | |
| No. (%) of females | 50 (47.2) | 50 (47.2) | |
| No. (%) who received varicella vaccine | 100 (94.3) | 103 (97.2) | 0.3173 |
| No. (%) with varicella vaccination before 12 months of age | 0 (0.0) | 1 (1.2) | 0.4885 |
| No. (%) with varicella vaccination before 15 months of age | 75 (84.3) | 76 (90.5) | 0.2585 |
| Mean ± SD age at varicella vaccination (mo)b | 14.9 ± 9.2 | 13.3 ± 4.2 | 0.1403 |
| No. (%) who received varicella vaccine within 28 days of MMR vaccinec | 15 (21.7) | 41 (58.5) | <.0001 |
P values were obtained by McNemar's test except age at varicella vaccination, which was obtained by a paired t test.
The date of varicella vaccination was obtained in 89 children in the varicella group and 84 children in the control group.
Duration from MMR vaccination to varicella vaccination was obtained in 69 children in the varicella group and 70 children in the control group.
Immunogenicity and safety study.
A total of 126 healthy children were vaccinated with a single dose of Suduvax (Green Cross, South Korea). Four children lost for follow-up and two children with positive prevaccination FAMA titers were excluded from further analysis. Of the remaining 120 children, the seroconversion rate and geometric mean titers for FAMA antibody were 76.67% and 5.31, respectively (Tables 3 and 4). Adverse reactions were analyzed for a total of 126 children. Local adverse reactions were observed in 16 children (12.7%), including 12 cases of erythema, 4 cases of swelling, 6 cases of tenderness, and 3 cases of petechiae. Systemic adverse reactions were observed in 15 children (11.9%), including 12 cases of fever, 2 cases of chills, 3 cases of lassitude, and 3 cases of rash which didn't look like varicella. Serious adverse events occurred in three children (2, rotaviral enteritis; 1, acute pharyngitis) but were not judged to be vaccine related.
TABLE 3.
Geometric mean titers following immunization with the MAV/06 varicella vaccine measured by the antivaricella FAMA assay
| Pre- or postvaccination | GMT (mean) | 95% CI for GMT | FAMA antibody titer (range) |
|---|---|---|---|
| Prevaccination (n = 120) | 1.1 | 1.0, 1.1 | 1–2 |
| Postvaccination (n = 120) | 5.3 | 4.4, 6.3 | 1–64 |
TABLE 4.
Seroconversion rate following immunization with the MAV/06 varicella vaccine measured by the antivaricella FAMA assay
| Seroconversion after vaccinationa | No. (%) |
|---|---|
| Yes | 92 (76.7) |
| No | 28 (23.3) |
| Total | 120 (100.0) |
95% CI is 69.1 to 84.2.
DISCUSSION
A live attenuated varicella vaccine began to be developed in Japan in 1974. Biken Oka strain varicella vaccine has been recommended for susceptible children in Japan since 1986 and in South Korea since 1988. Worldwide, varicella vaccine has currently been recommended as a universal vaccination in approximately a dozen countries, a few of which have also implemented a routine two-dose schedule (10). The United States is the first country that endorsed Varivax (Merck/Oka varicella vaccine) in 1995 for routine universal childhood immunization. Ten years of implementation of the 1-dose vaccination program caused the varicella incidence to decline by 90%, the peak age to be shifted from 4 to 5 years to >10 years, significant declines of varicella-related hospitalization (VRH) and complications during VRH, and a sharp decline in the rate of death due to varicella (11–13). Despite an effective vaccination program, report of breakthrough varicella of much milder clinical presentation in smaller scale than natural varicella continued, and the likelihood of the spread of the virus from patients with breakthrough disease to others, resulting in varicella outbreaks in day care centers and schools, led the Advisory Committee on Immunization Practices to adopt implementation of a routine two-dose varicella vaccination program for children in June 2006 (14).The two-dose varicella vaccination program has substantially decreased varicella incidence, hospitalizations, and outbreaks. Declined varicella incidence has also been observed in infants not eligible for varicella vaccination and adults with low levels of vaccination (15).
The results of the current prospective case-based study performed in South Korea for 9 months from August 2006 to March 2007 were not different from two previous retrospective studies performed in the middle of the 1990s: one on patients diagnosed in private pediatricians' offices (16) and the other on patients diagnosed in one university hospital (17). The approximate average age of varicella was 4 years old, with slightly over 10% of patients younger than 1 year old and with bimodal peaks of varicella outbreaks in June to July and November to January. However, the vaccine coverage was quite different in the two groups of studies; approximately 25% in the earlier ones and 91.4% in the current one. Vaccine utilization was reported as 73.1% in a Korea Centers for Disease Control and Prevention (KCDC) document from one rural area in 2005 (18) and 88.3% in a study for nationwide vaccine coverage in 2007 (19). The most recent survey in 2011 revealed varicella vaccine coverage above 97% (20). The unchanged peak incidence at 4 to 5 years of age has also been delineated in the 2011 KCDC Infectious Diseases Surveillance Report (21).
The number of varicella patients reported to KCDC has continued to increase from 22.6 cases per 100,000 population in 2006 to 71.6 cases per 100,000 population in 2011 (21), and the number of patients who filed health insurance claims for varicella has also not shown a substantial decrease for the past 13 years (Fig. 1) (22). Although the increase in reported cases of varicella to KCDC may be due to the fact that mandatory varicella notification began in 2005, no decrease in the number of varicella patients does not harmonize with the fact that the varicella vaccine coverage increased to above 97% in 2011. Although it can be asserted that the annual number of cases of varicella might have been higher with greater morbidity in the prevaccine era, the high vaccine uptake, the lack of upward age shift in the peak incidence, and the high proportion of breakthrough disease, with almost no amelioration in disease presentation among vaccinated patients, strongly suggest that varicella vaccination has not been effective in preventing varicella in South Korea and is in great need of improvement.
FIG 1.
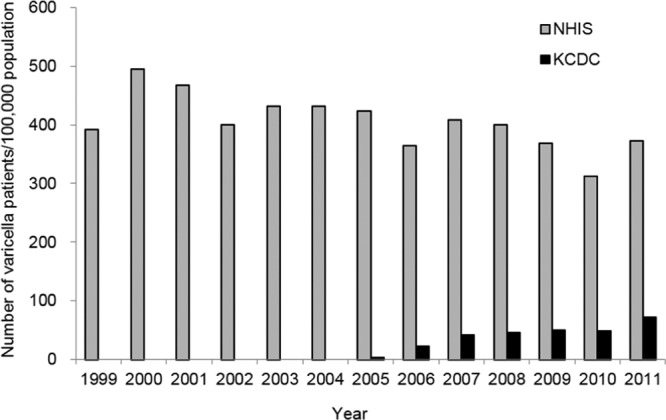
Annual number of varicella patients in Korea. NHIS, National Health Insurance Service; KCDC, Korea Centers for Disease Control and Prevention.
The current prospective case-based study and case-control study revealed high proportions of breakthrough varicella, suggesting that most cases of varicella occurring in South Korea seem to be breakthrough infection, although the breakthrough varicella rate has not been elucidated. These observations are contrary to the situations in other countries with routine varicella immunization programs. In the United States, the vaccine failure or breakthrough varicella rate was initially reported at 2% to 4% of vaccinees per year and recently reported as 14% cumulative incidence over 7 years, which is consistent with an estimated vaccine effectiveness of ∼85% in community settings (1, 23). Considering the previous reports of an increased risk of varicella breakthrough when varicella vaccine was given before 14 months (24) or 15 months (25, 26) of age, varicella vaccination at a median age of 13 months in our case-based study may suggest the necessity to look into the appropriate timing for varicella vaccination in South Korea. Contrary to the previously reported risk factor of varicella breakthrough related with the timing of MMR vaccination, the proportion of subjects who received varicella vaccine within 28 days of MMR vaccination was greater in the control group than in the case group in our case-control study (25).
Breakthrough disease is allegedly milder than varicella in unvaccinated individuals (27), but our study showed that varicella vaccination did not seem to alleviate clinical symptoms, although vaccinated patients were cared for more in the outpatient clinics with less serious appraisal of the disease by their parents. However, the lack of a difference in clinical manifestations between vaccinated and unvaccinated patients in the current study may be due to the fact that the size of the study population was inadequate. This notion is disclosed further with the fact that vaccinated cases appeared to have fewer skin lesions; even though not statistically significant, this could suggest that a decreased number of skin lesions in vaccinated patient might have been hidden because of the small number of unvaccinated patients or the possible mistake in grouping of skin lesions in study subjects despite careful guidance by investigators for parents or guardians to estimate the number of the lesions.
The effectiveness of varicella vaccines estimated in the case-control study was 54%, with a 95% confidence interval of 0.10 to 2.05, which fit the almost-unchanged annual number of reported varicella patients and the lack of an upward age shift in the peak incidence. The postlicensure studies on Varivax in the United States showed 97% to 100% effectiveness against severe varicella disease and 80% to 85% effectiveness against any disease presentation (1, 28–34). But one study on the outbreak of varicella at a day care center estimated the effectiveness of the vaccine at 44.0% against disease of any severity and 86.0% against moderate or severe disease (35). In other countries, the effectiveness of one dose of Oka strain-based varicella vaccines was good; adjusted effectiveness in a prospective case-control study was 86.4% against any severity and 97.7% against moderate or severe varicella in Germany (36), vaccine effectiveness against varicella based on the data of Taiwan's National Immunization Information System was 82.6% (37), vaccine effectiveness against varicella assessed in a case-control study was 86.4% for Varilrix, 79.6% for Changchun Institutes of Biologic Products, and 92.6% for Shanghai Institutes of Biologic Products in China (38), and in Israel, the effectiveness of varicella vaccine was 71 to 100% in preventing disease of any severity and 95 to 100% in preventing moderate/severe disease; however, a study of an outbreak of varicella among children attending a day care center demonstrated vaccine efficacy of only 44% (39). Significant decline of varicella cases and VRH seen in the United States has also been reported in Germany (2), Canada (3), Australia (4), and Taiwan (5). The current case-control study indicates that varicella vaccination is relatively ineffective in South Korea.
Since the introduction of the Biken Oka strain varicella vaccine in 1988 in South Korea, several domestic vaccines containing mainly the Oka strain were developed in the 1990s, when strict licensing processes were evolving. Suduvax (Green Cross, South Korea), which did not contain the Oka strain, was licensed in 1993, has remained the only domestic vaccine on the market, and is the most popular varicella vaccine. Suduvax contains the MAV/06 varicella strain that was isolated from a 33-month-old South Korean boy with chickenpox in 1989 in Seoul, South Korea. Initially, seven varicella-zoster virus strains isolated from seven South Korean patients with chickenpox were tested for restriction fragment length polymorphism, and the MAV/06 strain, which was different from the Oka strain, was chosen to be the vaccine strain (40, 41). The isolated virus had been proliferated in normal human lung diploid cells (HEL) and attenuated by subculturing in HEL, normal guinea pig lung diploid cells (GEL), and MRC-5 (41, 42). A prelicensure safety study and the immunogenicity study on 161 subjects using the FAMA assay which used varicella virus-infected cells treated with 1% glutaraldehyde showed that Suduvax (>1,000 PFU of MAV/06; Green Cross, South Korea) was safe and highly immunogenic, with a postvaccination geometric mean titer (GMT) of 173.7 ± 29.8 and seroconversion rate of 100% (43). Clinical trials for efficacy have not been undertaken.
Continual varicella outbreaks despite high vaccination coverage and disappointing results of our observational studies led us to do the immunogenicity study on Suduvax (Green Cross, South Korea), which was the most common vaccine (46.2% of the subjects who provided the name of the vaccine products) among the total eight vaccines received by the patients in our case-control study. The classic FAMA assay using varicella-zoster virus-infected live cells was chosen and established with the help of Anne Gershon at Columbia University, since the classic FAMA assay has been known as the optimal test to measure immunogenicity of varicella vaccine, and glycoprotein (gp)-based enzyme-linked immunosorbent assay (gpELISA), which has mostly been used in the development of varicella vaccine, was not available. Our data on Suduvax (Green Cross, South Korea) revealed a seroconversion rate of 76.67% and a GMT of 5.31, which seemed a bit lower than the data by Michalik et al., who used sera which were obtained longer than 3 months following vaccination with the Merck/Oka varicella vaccine and had been kept frozen for several years (9). It seemed much lower than the above-mentioned high postvaccination GMT and seroconversion rate of prelicensure immunogenicity on Suduvax measured by the FAMA assay, which used glutaraldehyde-treated cells (43). These findings indicate that classic FAMA assay using live cells has revealed the actuality and explains the current situation of varicella and varicella vaccination in South Korea and that Suduvax (Green Cross, South Korea) may not be immunogenic enough to be effective in preventing varicella in South Korea.
Limitations of these studies are that the enrolled subjects in both the case-based study and case-control study may not be representative of the South Korean population, because convenience samples were used and the coverage rates in the areas of the hospitals that joined the studies were not known. The case-based study was initially not based on representative design, and the study subjects were enrolled in the order of diagnosis at each participating hospital with parental or legal-guardian consent and not particularly selected. However, the facts that varicella is a common disease, that the ages and seasonal distributions of varicella patients in our data are comparable to those generated by KCDC and National Health Insurance Service, and that comparison of clinical characteristics in enrollees of four distinct geographic regions of the participating hospitals to the case-based study did not show a difference render our study results based on a convenience sample valuable in understanding the varicella situation in South Korea. Another limitation is that a larger study population as well as a larger number of controls might have resulted in favorable results of varicella vaccination in South Korea. However, it was very difficult to meet patients with varicella who were not vaccinated in populations with high vaccination coverage. Furthermore, it cannot be overstated that the effectiveness study with each vaccine product on the market is mandatory to disclose the effectiveness of the varicella vaccine in South Korea. As a whole, the results of the studies reported herein and in previously reported literature and the prospective and retrospective epidemiology data of varicella strongly indicate the need for thorough investigation to elucidate the situation of varicella and the vaccine in South Korea.
ACKNOWLEDGMENTS
Korea Centers for Disease Control and Prevention provided the funding for the case-based study. Korean Ministry of Food and Drug Safety provided the funding for the case-control study and the immunogenicity and safety study. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
We gratefully acknowledge the expert assistance provided by Anne Gershon at Columbia University.
Footnotes
Published ahead of print 26 March 2014
REFERENCES
- 1. Vázquez M, LaRussa PS, Gershon AA, Steinberg SP, Freudigman K, Shapiro ED. 2001. The effectiveness of the varicella vaccine in clinical practice. N. Engl. J. Med. 344:955–960. 10.1056/NEJM200103293441302 [DOI] [PubMed] [Google Scholar]
- 2. Streng A, Grote V, Carr D, Hagemann C, Liese JG. 2013. Varicella routine vaccination and the effects on varicella epidemiology—results from the Bavarian Varicella Surveillance Project (BaVariPro), 2006-2011. BMC Infect. Dis. 13:303. 10.1186/1471-2334-13-303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Waye A, Jacobs P, Tan B. 2013. The impact of the universal infant varicella immunization strategy on Canadian varicella-related hospitalization rates. Vaccine 31:4744–4748. 10.1016/j.vaccine.2013.08.022 [DOI] [PubMed] [Google Scholar]
- 4. Marshall HS, McIntyre P, Richmond P, Buttery JP, Royle JA, Gold MS, Wood N, Elliott EJ, Zurynski Y, Toi CS, Dwyer DE, Booy R. 2013. Changes in patterns of hospitalized children with varicella and of associated varicella genotypes after introduction of varicella vaccine in Australia. Pediatr. Infect. Dis. J. 32:530–537. 10.1097/INF.0b013e31827e92b7 [DOI] [PubMed] [Google Scholar]
- 5. Chang LY, Huang LM, Chang IS, Tsai FY. 2011. Epidemiological characteristics of varicella from 2000 to 2008 and the impact of nationwide immunization in Taiwan. BMC Infect. Dis. 11:352. 10.1186/1471-2334-11-352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Centers for Disease Control and Prevention. 1997. Case definitions for infectious conditions under public health surveillance. MMWR Morb. Mortal. Wkly. Rep. 46:1–55 [PubMed] [Google Scholar]
- 7. Bernstein HH, Rothstein EP, Watson BM, Reisinger KS, Blatter MM, Wellman CO, Chartrand SA, Cho I, Ngai A, White CJ. 1993. Clinical survey of natural varicella compared with breakthrough varicella after immunization with live attenuated Oka/Merck varicella vaccine. Pediatrics 92:833–837 [PubMed] [Google Scholar]
- 8. Williams V, Gershon A, Brunell PA. 1974. Serologic response to varicella-zoster membrane antigens measured by direct immunofluorescence. J. Infect. Dis. 130:669–672. 10.1093/infdis/130.6.669 [DOI] [PubMed] [Google Scholar]
- 9. Michalik DE, Steinberg SP, Larussa PS, Edwards KM, Wright PF, Arvin AM, Gans HA, Gershon AA. 2008. Primary vaccine failure after 1 dose of varicella vaccine in healthy children. J. Infect. Dis. 197:944–949. 10.1086/529043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.World Health Organization. 2013. Vaccine schedule selection form. WHO, Geneva, Switzerland: http://apps.who.int/immunization_monitoring/globalsummary/schedules [Google Scholar]
- 11. Guris D, Jumaan AO, Mascola L, Watson BM, Zhang JX, Chaves SS, Gargiullo P, Perella D, Civen R, Seward JF. 2008. Changing varicella epidemiology in active surveillance sites—United States, 1995-2005. J. Infect. Dis. 197(Suppl 2):S71–S75. 10.1086/522156 [DOI] [PubMed] [Google Scholar]
- 12. Nguyen HQ, Jumaan AO, Seward JF. 2005. Decline in mortality due to varicella after implementation of varicella vaccination in the United States. N. Engl. J. Med. 352:450–458. 10.1056/NEJMoa042271 [DOI] [PubMed] [Google Scholar]
- 13. Lopez AS, Zhang J, Brown C, Bialek S. 2011. Varicella-related hospitalizations in the United States, 2000-2006: the 1-dose varicella vaccination era. Pediatrics 127:238–245. 10.1542/peds.2010-0962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kroger AT, Atkinson WL, Marcuse EK, Pickering LK, Advisory Committee on Immunization Practices (ACIP) Centers for Disease Control and Prevention (CDC) 2006. General recommendations on immunization: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm. Rep. 55:1–48 [PubMed] [Google Scholar]
- 15. Bialek SR, Perella D, Zhang J, Mascola L, Viner K, Jackson C, Lopez AS, Watson B, Civen R. 2013. Impact of a routine two-dose varicella vaccination program on varicella epidemiology. Pediatrics 132:e1134–e1140. 10.1542/peds.2013-0863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kim DJ, Park HS, Lee SY, Park KS, Kim TK, Song YH, Choi J, Han JW, Song YS, Park TJ, Kim HK, Lee SY, Lee SH, Kim HW, Oh SH. 1997. Epidemiology of varicella in Korea based on pediatrician's office practice. J. Korean Pediatr. Soc. 40:620–628 [Google Scholar]
- 17. Kim MR, Park JS, Kim DH, Lee HR, Park CY. 1998. A clinical and epidemiologic study on varicella in children. Korean J. Pediatr. Infect. Dis. 5:88–95 [Google Scholar]
- 18.Korea Centers for Disease Control and Prevention Shin YC, Lee MS, Kwon SS, Ki M, Kim K, Na B, Nam H, Lee SW, Shin E, Choi BJ, Kim SI, Park YM. 2005. Development of vaccination coverage estimation methods and evaluation indicators of national immunization program in Korea. Korea Centers for Disease Control and Prevention, Chungcheongnam-do, South Korea [Google Scholar]
- 19. Lee HK, Lee HJ, Kim SH, Chun BC, Kim KH, Lee HJ. 2007. Vaccination coverage in Korean children of six years old and younger, abstr O-016, p 90 Abstr. 57th Annu. Fall Meet. Korean Pediatr. Soc Seoul, South Korea [Google Scholar]
- 20. Lee SG, Ki M. 2011. Korea national immunization survey. Korea Centers for Disease Control and Prevention, Chungcheongnam-do, South Korea [Google Scholar]
- 21.Korea Centers for Disease Control and Prevention. 2011. Infectious diseases surveillance yearbook 2011 public health weekly report. Korea Centers for Disease Control and Prevention, Ministry of Health and Welfare, Chungcheongnam-do, South Korea: http://www.bokjiro.go.kr/data/statusView.do?board_sid=297&data_sid=5998874 [Google Scholar]
- 22.National Health Insurance Service. 2011. Statistical yearbook. National Health Insurance Service, Health Insurance Review & Assessment Service. http://www.nhis.or.kr/portal/site/main/MENU_WBDDG02/
- 23. White CJ, Kuter BJ, Ngai A, Hildebrand CS, Isganitis KL, Patterson CM, Capra A, Miller WJ, Krah DL, Provost PJ, Ellis RW, Calandra GB. 1992. Modified cases of chickenpox after varicella vaccination: correlation of protection with antibody response. Pediatr. Infect. Dis. J. 11:19–23. 10.1097/00006454-199201000-00006 [DOI] [PubMed] [Google Scholar]
- 24. Galil K, Fair E, Mountcastle N, Britz P, Seward J. 2002. Younger age at vaccination may increase risk of varicella vaccine failure. J. Infect. Dis. 186:102–105. 10.1086/341089 [DOI] [PubMed] [Google Scholar]
- 25. Verstraeten T, Jumaan AO, Mullooly JP, Seward JF, Izurieta HS, DeStefano F, Black SB, Chen RT, Vaccine Safety Datalink Research Group 2003. A retrospective cohort study of the association of varicella vaccine failure with asthma, steroid use, age at vaccination, and measles-mumps-rubella vaccination. Pediatrics 112(2):e98–e103 [DOI] [PubMed] [Google Scholar]
- 26. Vázquez M, LaRussa PS, Gershon AA, Niccolai LM, Muehlenbein CE, Steinberg SP, Shapiro ED. 2004. Effectiveness over time of varicella vaccine. JAMA 291:851–855. 10.1001/jama.291.7.851 [DOI] [PubMed] [Google Scholar]
- 27. Bernstein HH, Rothstein EP, Watson BM, Reisinger KS, Blatter MM, Wellman CO, Chartrand SA, Cho I, Ngai A, White CJ. 1993. Clinical survey of natural varicella compared with breakthrough varicella after immunization with live attenuated Oka/Merck varicella vaccine. Pediatrics 92:833–837 [PubMed] [Google Scholar]
- 28. Izurieta HS, Strebel PM, Blake PA. 1997. Postlicensure effectiveness of varicella vaccine during an outbreak in a child care center. JAMA 278:1495–1499. 10.1001/jama.278.18.1495 [DOI] [PubMed] [Google Scholar]
- 29. Clements DA, Moreira SP, Coplan PM, Bland CL, Walter EB. 1999. Postlicensure study of varicella vaccine effectiveness in a day-care setting. Pediatr. Infect. Dis. J. 18:1047–1050. 10.1097/00006454-199912000-00004 [DOI] [PubMed] [Google Scholar]
- 30. Buchholz U, Moolenaar R, Peterson C, Mascola L. 1999. Varicella outbreaks after vaccine licensure: should they make you chicken? Pediatrics 104:561–563 [DOI] [PubMed] [Google Scholar]
- 31. Tugwell BD, Lee LE, Gillette H, Lorber EM, Hedberg K, Cieslak PR. 2004. Chickenpox outbreak in a highly vaccinated school population. Pediatrics 113:455–459 [DOI] [PubMed] [Google Scholar]
- 32.Centers for Disease Control and Prevention (CDC). 2004. Outbreak of varicella among vaccinated children—Michigan, 2003. MMWR Morb. Mortal. Wkly. Rep. 53:389–392 [PubMed] [Google Scholar]
- 33. Lopez AS, Guris D, Zimmerman L, Gladden L, Moore T, Haselow DT, Loparev VN, Schmid DS, Jumaan AO, Snow SL. 2006. One dose of varicella vaccine does not prevent school outbreaks: is it time for a second dose? Pediatrics 117:e1070–e1077. 10.1542/peds.2005-2085 [DOI] [PubMed] [Google Scholar]
- 34. Seward JF, Marin M, Vázquez M. 2008. Varicella vaccine effectiveness in the U.S. vaccination program: a review. J. Infect. Dis. 197(Suppl 2):S82–S89. 10.1086/522145 [DOI] [PubMed] [Google Scholar]
- 35. Galil K, Lee B, Strine T, Carraher C, Baughman AL, Eaton M, Montero J, Seward J. 2002. Outbreak of varicella at a day-care center despite vaccination. N. Engl. J. Med. 347:1909–1915. 10.1056/NEJMoa021662 [DOI] [PubMed] [Google Scholar]
- 36. Liese JG, Cohen C, Rack A, Pirzer K, Eber S, Blum M, Greenberg M, Streng A. 2013. Thee effectiveness of varicella vaccination in children in Germany: a case-control study. Pediatr. Infect. Dis. J. 32:998–1004. 10.1097/INF.0b013e31829ae263 [DOI] [PubMed] [Google Scholar]
- 37. Huang WC, Huang LM, Chang IS, Tsai FY, Chang LY. 2011. Varicella breakthrough infection and vaccine effectiveness in Taiwan. Vaccine 29:2756–2760. 10.1016/j.vaccine.2011.01.092 [DOI] [PubMed] [Google Scholar]
- 38. Fu C, Wang M, Liang J, Xu J, Wang C, Bialek S. 2010. The effectiveness of varicella vaccine in China. Pediatr. Infect. Dis. J. 29:690–693. 10.1097/INF.0b013e3181d7380e [DOI] [PubMed] [Google Scholar]
- 39. Sheffer R, Segal D, Rahamani S, Dalal I, Linhart Y, Stein M, Shohat T, Somekh E. 2005. Effectiveness of the Oka/GSK attenuated varicella vaccine for the prevention of chickenpox in clinical practice in Israel. Pediatr. Infect. Dis. J. 24:434–437. 10.1097/01.inf.0000160947.89942.30 [DOI] [PubMed] [Google Scholar]
- 40. Hwang KK, Park SY, Kim SJ, Ryu YW, Kim KH. 1991. Restriction fragment length polymorphism analysis of varicella-zoster virus isolated in Korea. J. Korean Soc. Virol. 21:201–210 [Google Scholar]
- 41. Hwang KK, Chun BH, Park HS, Park SY, Kim KH, Moon HM. 1992. Marker test for attenuation of varicella-zoster viruses isolated in Korea. J. Korean Soc. Virol. 22:105–109 [Google Scholar]
- 42. Sohn YM, Park CY, Hwang KK, Woo GJ, Park SY. 1994. Safety and immunogenicity of live attenuated varicella virus vaccine (MAV/06 strain). J. Korean Pediatr. Soc. 37:1405–1413 [Google Scholar]
- 43. Sohn YM, Yu GJ, Kim PK, Kim KY, Park CY, Kim MR, Jeung WK, Hwang KK, Woo GJ, Park SY. 1995. Immunogenicity and safety of live attenuated varicella vaccine (MAV/06 strain) on healthy children and immunocompromised children. J. Korean Pediatr. Soc. 38:771–777 [Google Scholar]


