Abstract
Respiratory syncytial virus (RSV) causes significant disease in elderly adults, and we have previously reported that individuals 65 years of age and older have reduced RSV F protein-specific gamma interferon (IFN-γ)-producing T cells compared to healthy younger adults. To measure RSV F-specific memory T cell responses in the elderly following infection or vaccination, we optimized and qualified an IFN-γ enzyme-linked immunospot (ELISPOT) assay. Since peripheral blood mononuclear cells (PBMC) from the elderly could be more fragile, we established optimal cryopreservation techniques and minimal viability acceptance criteria. The number of cells per well, types and concentrations of stimulation antigens, and incubation times were evaluated to maximize assay sensitivity and precision. The optimized assay uses 300,000 cells/well, 2 μg/ml of an RSV F peptide pool (RSV Fpp), and incubation for 22 ± 2 h in serum-free CTL-Test medium. The assay was qualified by 3 analysts using 3 RSV F-responding donor PBMC samples (high, medium, and low responders) tested on 5 different assay days. The assay sensitivity or limit of detection (LOD) was determined to be 21 spot-forming cells (SFC) per 106 PBMC, and the lower limit of quantitation (LLOQ) was estimated to be 63 SFC/106 PBMC. The intra- and interassay percent coefficients of variation (CV) were <10.5% and <31%, respectively. The results of the qualification study demonstrate that a robust, precise, and sensitive IFN-γ ELISPOT assay has been developed that is fit for measuring RSV F-specific IFN-γ T cell responses in subjects enrolled in a vaccine clinical trial or in epidemiology studies.
INTRODUCTION
Clinical evaluation of vaccine candidates relies on sensitive and precise immune-monitoring assays that may include antibody and T cell response measurements. Critical decisions required to select antigens, adjuvants, formulations, number of doses, and the vaccination schedule are often dependent on immunogenicity assessments conducted in the early stages of clinical development. Assays are typically designed to measure the immune response following natural infection or vaccination (1). Flow cytometry-based intracellular cytokine staining (ICS) and enzyme-linked immunospot (ELISPOT) assays are often used for immune monitoring of antigen-specific T cell responses, which is important to the understanding of viral infections and the immune response following vaccination. Vaccines with viral antigens derived from human immunodeficiency virus (HIV) and varicella-zoster virus induce relatively robust T cell responses that have been measured in a clinical setting using sensitive cytokine ELISPOT assays (2–4). Given the sensitivity and robustness of the ELISPOT assay format, we developed and qualified a gamma interferon (IFN-γ) ELISPOT assay for the measurement of respiratory syncytial virus (RSV) F antigen-specific T cell responses.
RSV is a common cause of acute respiratory illness (ARI) in adults 50 years and older (5) and a cause of significant disease in elderly adults, 65 years and older (6). Notably, the immune correlates associated with increased susceptibility to severe RSV illness in this population are not well understood, and no RSV vaccine is currently licensed for elderly adults (7, 8). Immunosenescence impairs protective immune responses to infection in the elderly (7, 9). Studies have found that the RSV-specific memory CD8+ T cells are reduced in the peripheral blood of healthy elderly adults (10, 11), and we have previously shown that RSV-specific IFN-γ T cell responses are diminished in subjects 65 years and older (12). A number of studies have found that higher neutralizing antibody levels in serum correlate with reduced risk for RSV infection at all ages (13, 14); thus, we believe that an effective vaccine in the elderly will need to induce high levels of RSV-neutralizing antibodies and elicit a T helper type 1 (Th1)-like cellular immune response denoted by IFN-γ production.
To measure the induction of IFN-γ-secreting T cells following immunization with different doses and formulations of an RSV F subunit vaccine in both young and elderly subjects, we sought to develop a sensitive, robust, and reproducible RSV F protein (RSV-F)-specific IFN-γ T cell ELISPOT assay for use in clinical trials evaluating vaccine responses. Since the viability of peripheral blood mononuclear cells (PBMC) may vary among donors and clinical sites, we developed an optimal PBMC cryopreservation method and established minimal viability acceptance criteria for PBMC from the elderly. Following identification of an optimal IFN-γ ELISPOT kit and serum-free culture medium, the ELISPOT assay was optimized for the type and concentration of antigen. The RSV F-specific IFN-γ ELISPOT assay was found to be more sensitive in recalling an RSV F-specific T cell response when using overlapping peptide pools from the RSV F protein (RSV Fpp), which can stimulate both a CD4+ and a CD8+ T cell response (15), rather than the RSV F protein antigen itself. PBMC stimulation controls were incorporated into the ELISPOT assay as well. Individual donor PBMC were stimulated with staphylococcal enterotoxin B (SEB) mitogen as well as a pool of precharacterized CD8+ peptides from cytomegalovirus (CMV), Epstein-Barr virus (EBV), and influenza A virus (referenced as CEF peptide pool or CEFpp) to ensure that donor PBMC were functional. Additionally, a control donor sample was included on each plate to serve as a trending plate control. To ensure that the assay was sufficiently sensitive to measure T cell responses over a range of magnitude, RSV F-specific IFN-γ responses were tested for reproducibility over multiple days and by multiple analysts using low-, medium-, and high-responding samples. The limit of detection (LOD) for the assay was determined using the upper 95th percentile of the distribution of the mock IFN-γ responses generated from multiple replicates, plates, days, and analysts. The lower limit of quantitation (LLOQ) was estimated as 3-fold the LOD. The upper limit of the countable range for the ImmunoSpot analyzer was determined using the upper 95th percentile of the distribution of the response to SEB at a plating density of 100,000 cells/well where spot confluence was observed. The results of these studies are presented here to document the methodology and operating characteristics of an RSV F-specific IFN-γ ELISPOT assay. The results of this qualification study demonstrate that we have developed a robust, sensitive, and reproducible IFN-γ ELISPOT assay that is fit for measuring RSV F-specific IFN-γ responses in PBMC from young or older adults.
MATERIALS AND METHODS
This section was written in compliance with the Minimal Information about T Cell Assays (MIATA) guidelines (http://miataproject.org/index.php?option=com_content&view=article&id=134&Itemid=29) (16).
PBMC isolation and freezing.
Whole blood from healthy, young (20 to 40 years) or elderly (65 to 85 years) male and female volunteers was collected in sodium heparin blood collection tubes (BD Biosciences, San Jose, CA) under informed consent approved by the Independent Institutional Review Board of Bioreclamation, LLC (Hicksville, NY). Whole blood was transported at ambient temperature from the Bioreclamation collection center in New Jersey to Pharmaceutical Product Development's (PPD, Wilmington, NC) processing site in New Jersey within 2 h of blood draw. PBMC were isolated using Accuspin tubes (Sigma, St. Louis, MO) and frozen at 107 cells/ml in serum-free CTL-Cryo ABC freeze medium (Cellular Technology, LLC, Cleveland, OH). For the freezing medium comparison study, isolated PBMC were frozen at equivalent numbers in 10% dimethyl sulfoxide (DMSO) (Sigma, St. Louis, MO) and 90% heat-inactivated fetal bovine serum (FBS) (Gibco Life Technologies, Grand Island, NY). PBMC samples were placed in Nalgene Mr. Frosty freezing containers (ThermoFisher Scientific, Inc., Waltham, MA) at −80°C overnight and transferred the next morning to liquid nitrogen (LN2) for storage. Cryopreserved samples were shipped overnight on dry ice to MedImmune within 2 weeks of freezing, stored in LN2, and thawed for ELISPOT assay evaluation within 2 weeks of arrival.
PBMC thawing and counting.
Cryovials of PBMC were quickly thawed at 37°C into CTL-Wash medium (Cellular Technology, LLC) containing 50 units/ml of Benzonase nuclease (EMD Millipore, Billerica, MA) to prevent cell clumping. Cells were washed and diluted in CTL-Test medium (Cellular Technology, LLC) without Benzonase nuclease for counting on the Guava easyCyte HT flow cytometer (Millipore, Billerica, MA) using the Guava ViaCount reagent (Millipore, Billerica, CA). In addition to measuring the total viable cell count, the percentages of viable, apoptotic, and dead cells in each sample were also measured by the Guava easyCyte instrument. Other serum-free cell culture media evaluated for PBMC dilution in our studies were AIM-V (Invitrogen, Carlsbad, CA), OpTmizer (Invitrogen), and X-Vivo 15 (Lonza, Walkersville, MD).
Antigens.
Overlapping peptides (15-mers overlapping by 11 amino acids) of a soluble form of the RSV F protein lacking the transmembrane and cytoplasmic tail (17) representing both CD4+ and CD8+ T cell epitopes were custom designed and ordered from JPT GmbH (Berlin, Germany). The purity of the custom-synthesized 141-peptide pool (pp) was >90%, and a final dilution of 1, 2, or 5 μg/ml was used for PBMC stimulation in the ELISPOT assay. Peptides covering the human leukocyte antigen (HLA) class I-restricted immunodominant T cell epitopes of CMV, EBV, and influenza A virus proteins (CEFpp) were purchased from Cellular Technology, LLC (Cleveland, OH). An RSV-soluble F protein containing amino acids 1 to 524 of the RSV A2 F sequence and lacking the transmembrane domain was immunoaffinity purified with the RSV F-specific monoclonal antibody, palivizumab (MedImmune, Inc.), from the supernatants of stably transfected Chinese hamster ovary (CHO) cells. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and Western blot analysis indicated that affinity-purified RSV F protein was >95% pure.
RSV F-specific IFN-γ ELISPOT assay.
The optimized IFN-γ ELISPOT procedure used anti-human IFN-γ antibody precoated plates and detection antibodies purchased from Mabtech, Inc. (Mariemont, OH). The manufacturer's instructions for these kits were followed exactly. Freshly thawed PBMC incubated at 25,000 to 400,000 viable cells/well were incubated with CTL-Test medium containing 0.1% DMSO (mock) or 2 μg/ml of overlapping peptide pools of RSV F (JPT GmbH, Berlin, Germany) or CEF peptide pools (CTL, Cleveland, OH) for 22 ± 2 h at 37°C. To serve as the positive control, 30,000 viable cells/well were stimulated with SEB subunit (Sigma, St. Louis, MO). All plates were developed for 4 min ± 30 s, and spots were counted using Image Acquisition 4.5 on the ImmunoSpot Analyzer 5.0.3 Pro DC (Cellular Technology, LLC) with a sensitivity setting of 160, background balance of 80, spot separation of 3, and spot size range of 0.0051 to 9.6372 mm2. These parameters were established based on optimal resolution and enumeration of sharply defined spots in the antigen-specific wells relative to mock wells. Data were expressed as spot-forming cells per million PBMC (SFC/106 PBMC) after background subtraction of mock wells.
Statistical analysis.
A variance components probability model (18) was used to estimate the variability due to assay day, analyst, and replicate repeatability for low, medium, and high responses. The percent coefficient of variation (% CV) was calculated from the estimated standard deviation and the mean IFN-γ SFC/well response for low (33 to 150 SFC/106 PBMC), medium (151 to 1,000 SFC/106 PBMC), and high (≥1,001 SFC/106 PBMC) responder samples. The LOD was calculated using the negative binomial distribution of 4 replicate mock wells from all assays (n = 360 mock wells). The upper 95th percentile limit of the IFN-γ response of individual mock wells was set as the LOD of the assay to control for a false-positive rate of <5%. The RSV F-specific assay LLOQ was estimated as 3-fold of the LOD (19). The upper limit of the countable range for the ImmunoSpot analyzer was determined by the upper 95th percentile of the distribution of the response to SEB at a plating density of 100,000 cells/well where spot confluence was observed.
Protein structure accession number.
The RSV F protein sequence is available in the GenBank database (http://www.ncbi.nlm.nih.gov/GenBank) under accession no. KJ155694.
RESULTS
Viability acceptance criteria for PBMC from elderly donors.
PBMC quality is of crucial importance to the measurement of reliable, functional antigen-specific IFN-γ responses. PBMC subjected to suboptimal temperatures (e.g., −20°C for 4 h) have been shown to have decreased viability and functional responses in the ELISPOT assay (20). Suboptimal handling and storage of frozen PBMC during transfer between the clinical site and the clinical testing laboratory can adversely impact the quality and integrity of the samples. For optimal PBMC quality upon thawing, the frozen PBMC should be stored in the vapor phase of liquid nitrogen and transferred between sites in either liquid nitrogen or dry ice (21).
Temperature fluctuations can mistakenly occur during handling and transit between the clinical site and the clinical testing laboratory, which could affect the functional responses of fragile elderly PBMC. Thus, our first goal was to set sample acceptance criteria from viability-associated IFN-γ recall response changes in PBMC from the elderly. Apoptosis and cell death were deliberately induced (using suboptimal temperature sample storage), since it was crucial to understand the IFN-γ responses generated by nonfunctional PBMC samples in comparison with those generated by viable PBMC samples. Since RSV-specific T cell IFN-γ responses in elderly PMBC are very low (12), we used CEFpp-specific T cell IFN-γ responses and SEB-specific T cell IFN-γ responses to evaluate T cell function in relation to viability. Replicate vials of cryopreserved PBMC from three healthy, elderly adult donors were either kept in liquid nitrogen (LN2), transferred to −80°C for 8 h, or transferred to −20°C for 8 h, in order to assess the effect of storage temperature on viability (20). The mean viability of cryopreserved PBMC samples as determined on the Guava easyCyte instrument was 63.2% for those samples stored in LN2, 57.7% for those samples stored at −80°C, and 51.3% for those samples stored at −20°C (Table 1). Both percent viable and percent dead values were statistically different (P = 0.005 and P = 0.023, respectively) for samples stored at −20°C compared to those stored in LN2 (Table 1). CEFpp- and SEB-specific IFN-γ ELISPOT assay responses for the PBMC samples stored in LN2 or at −80°C were comparable, whereas the IFN-γ ELISPOT assay responses of the samples stored at −20°C with reduced viability were lower (Fig. 1A and B). These results indicate that IFN-γ ELISPOT assay function diminishes with decreased PBMC viability. Based on these results, the acceptance criterion for including a PBMC sample in the IFN-γ ELISPOT assay was determined to be ≤35% dead cells.
TABLE 1.
Effect of storage temperature on viability
| Cell status | % of cells at indicated status when stored: |
P valuea |
|||
|---|---|---|---|---|---|
| In LN2 | At −80°C | At −20°C | −80°C versus LN2 | −20°C versus LN2 | |
| Viable | 63.2 ± 5.5 | 57.7 ± 10.4 | 51.3 ± 8.2 | P = 0.2857 | P = 0.0055 |
| Apoptotic | 8.5 ± 5.2 | 11.5 ± 7.9 | 10.8 ± 6.2 | P = 0.3644 | P = 0.2236 |
| Dead | 28.2 ± 1.7 | 30.8 ± 3.8 | 38.0 ± 3.8 | P = 0.4478 | P = 0.0230 |
Significance determined using the mixed-effects model (Dunnett's adjusted P value).
FIG 1.
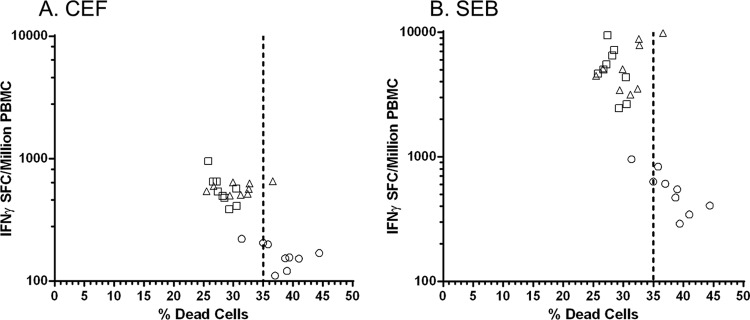
Setting PBMC viability acceptance criteria for the IFN-γ ELISPOT assay. Freshly isolated and frozen PBMC vials from random elderly donors were placed in LN2 (□) or at −80°C (△) or −20°C (○) storage for 8 h to induce cellular apoptosis at the lower temperatures. All vials were simultaneously thawed and viabilities measured. Cells were plated in the IFN-γ ELISPOT assay based on the viable-cell concentration with 2 μg/ml of CEF peptide pools (A) or 1 μg/ml SEB (B).
Determination of stimulation antigen.
The selection of the form of antigen to use in an RSV F-specific IFN-γ ELISPOT assay is important because the antigen type and source can affect the sensitivity and reproducibility of an ELISPOT assay. Both whole RSV F protein and an RSV Fpp were evaluated at multiple antigen concentrations in the IFN-γ ELISPOT assay (Fig. 2). RSV F IFN-γ ELISPOT assay responses were higher using the peptide pool and increased with increasing peptide concentrations. Incubation of PBMC with 2 μg/ml of RSV Fpp for 22 ± 2 h was chosen for the optimized assay to conserve peptide. A 48-h incubation time was also evaluated but did not provide an advantage in assay sensitivity (data not shown).
FIG 2.
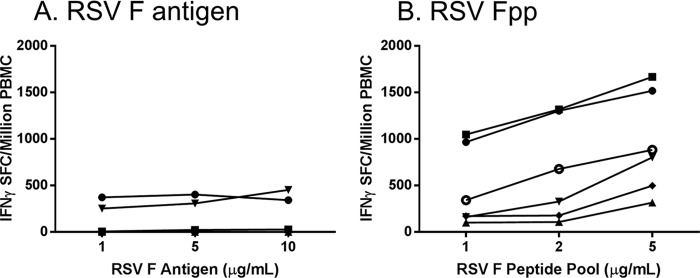
Determination of stimulation antigen. Various concentrations of RSV F antigen (A) or RSV Fpp (B) were evaluated in the IFN-γ ELISPOT assay using a standardized assay procedure (each symbol indicates an individual donor).
Optimization of serum-containing versus serum-free culture and freezing media.
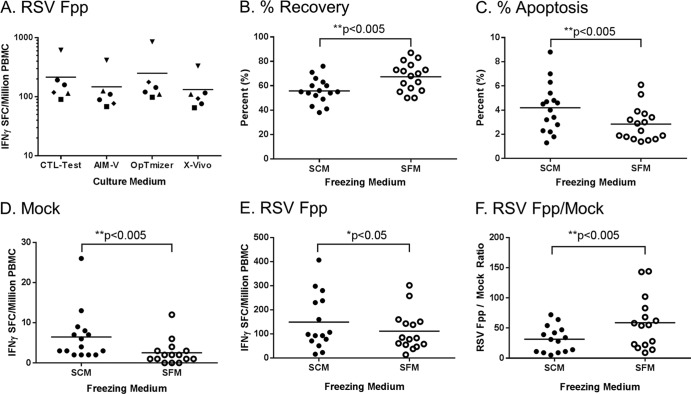
Minimization of nonspecific background responses is key to ensuring a high signal-to-noise ratio in an assay and is especially important in a setting where a low signal is expected. Avoidance of serum components in cellular processing has been recommended for T cell-based assays, as antigen-specific responses to serum components have been observed (22–25). The performances of serum-free culture media (X-Vivo, AIM-V, and OpTmizer), previously described by Mander et al. (26) and Janetzki et al. (24), were evaluated against CTL-Test medium in the RSV F-specific IFN-γ ELISPOT assay using PBMC from 6 individual donors. No significant differences were found in the performances of these four serum-free culture media with respect to background or RSV F-specific IFN-γ responses (Fig. 3A). The CTL-Test medium was selected due to the advantages of requiring fewer constituent additives and a longer shelf life than X-Vivo, AIM-V, or OpTmizer.
FIG 3.
Comparison of PBMC frozen or cultured in serum-containing or serum-free freezing medium in the RSV Fpp-specific IFN-γ ELISPOT assay. (A) Comparison of RSV Fpp-specific IFN-γ responses of PBMC samples tested in different serum-free supplemented culture mediums: CTL-Test, AIM-V, OpTmizer, and X-Vivo. No significance was observed by one-way analysis of variance (ANOVA). Freshly isolated PBMC from 14 random young and elderly donors were frozen in serum-containing (10% FBS) or serum-free (CryoABC) medium using a standardized protocol. Viability of the thawed PBMC samples was measured on a Guava easyCyte cytometer to determine % recovery (B) and % apoptosis (C) of the frozen PBMC. (D to F) Medium background (mock) (D) and RSV Fpp-specific IFN-γ response without background subtraction (E) and signal (F): background ratio of RSV Fpp-specific IFN-γ response over mock, comparing PBMC samples frozen in SCM versus SFM.
While cryopreservation of PBMC often uses serum-containing cryopreservation medium (SCM), serum-free cryopreservation medium (SFM) is also available. Fresh whole blood from 15 donors aged 18 to 45 was split into two parts and processed in parallel to generate paired samples of SFM-cryopreserved PBMC and SCM-cryopreserved PBMC. After thawing, the number of viable cells and percent apoptotic cells were determined for each. PBMC frozen in SFM demonstrated better recovery (Fig. 3B) and contained fewer apoptotic cells (Fig. 3C) than PBMC frozen in SCM. PBMC frozen in either SCM or SFM were then compared in an IFN-γ ELISPOT assay with either mock or RSV Fpp stimulation (Fig. 3D and E). Background and RSV Fpp-specific IFN-γ responses were lower for PBMC samples frozen in SFM than for PBMC samples frozen in SCM (Fig. 3D and E); however, the signal-to-background (noise) ratio was significantly higher using SFM (Fig. 3F). Furthermore, samples cryopreserved in SFM retained their RSV F-specific and SEB IFN-γ response levels following storage up to 60 days under LN2 conditions (data not shown). Based on these results, PBMC to be tested in the RSV F-specific IFN-γ ELISPOT assay were cryopreserved in SFM and stored in LN2.
Cell density optimization.
A cell-based assay such as the ELISPOT assay requires optimal cell numbers to ensure contact between antigen-presenting cells and the responding memory T cells. Too few or too many cells can negatively impact the quality or frequency of the spots formed. Therefore, we evaluated the optimal number of cells that should be plated per well for sensitivity and reproducibility. PBMC from two random donors were plated in 10 replicate wells at cell densities of 400,000, 300,000, 200,000, 100,000, 50,000, and 25,000 cells/well and stimulated with mock or RSV Fpp. Measured spot counts across the 10 replicate wells were used to calculate the mean, standard deviation, and % CV for each plating density (Fig. 4). A linear relationship between SFC and cell number was observed at 300,000 to 50,000 cells/well. As expected, the variability of the response increased as the plating cell density decreased and the mean spot counts declined. The variability of the response exceeded 20% at cell plating densities below 200,000 cells/well, while cell plating densities between 200,000 and 300,000 cells/well yielded an acceptable intra-assay variation of <20% CV (Fig. 4). Based on both optimal sensitivity and acceptable intra-assay reproducibility, the 300,000-cells/well concentration was chosen for this RSV F-specific IFN-γ ELISPOT assay.
FIG 4.
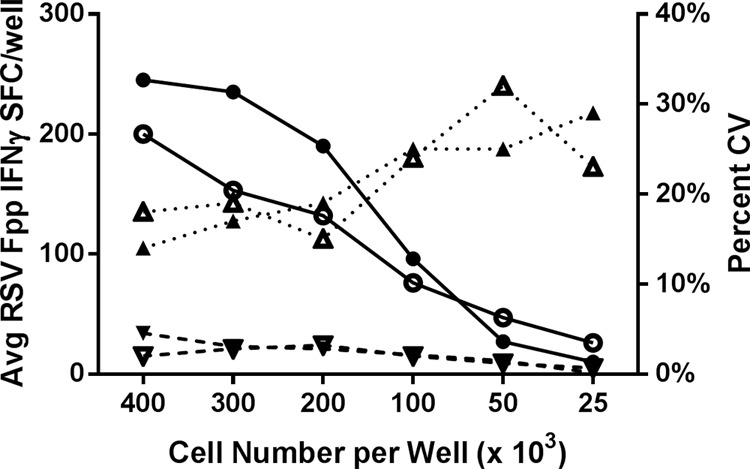
Linear relationship of the RSV F-specific IFN-γ response with cell density. PBMC from two random donors (open and closed symbols) were plated as 10 replicates at the indicated cell densities in the ELISPOT assay. The linear relationship between the average RSV Fpp-specific IFN-γ spot counts (solid line with circles) of the replicate wells versus the plating cell density is shown. The average spot count in mock wells (dashed line with inverted triangles) and % CV of the RSV Fpp-specific spot counts (dotted line with upright triangles) at each plating cell density are also depicted on the same graph.
The countable range of the IFN-γ ELISPOT assay needs to be sufficient to encompass the expected range of observable RSV F-specific responses. To calculate the upper limit of the countable range of the ImmunoSpot analyzer, spots were quantitated from an SEB stimulation of an ascending cell titration of PBMC (data not shown). SEB was chosen for this purpose since as a mitogen it could easily stimulate sufficient cells to achieve spot confluence above the upper limit of the assay. The upper limit of the countable range was set at 890 spots/well (2,964 spots/106 PBMC) based on the upper 95th percentile of the counts where individual discrete spots could still be detected by the ImmunoSpot analyzer.
Performance trending of a PBMC control in the IFN-γ ELISPOT assay.
Appropriate and robust controls are integral to assay qualification in order to ensure consistent assay performance over time and between analysts. We evaluated whether incorporation of a precharacterized PBMC sample with known CEF-specific responses could serve as an appropriate plate performance control for this ELISPOT assay. The CEF-specific IFN-γ frequency of PBMC aliquots from this PBMC sample was found to be consistent (% CV ≤ 33) over a period of 16 weeks as determined on 12 different days by a single analyst. Moreover, the background IFN-γ response was low (8 ± 7 SFC/106 PBMC) and the SEB-specific IFN-γ response high (5,303 ± 987 SFC/106 PBMC). Due to its consistent performance in our assay, this precharacterized PBMC sample was implemented as an assay performance standard in our qualification study to gauge analyst-to-analyst and day-to-day variability of the IFN-γ responses of test samples (Fig. 5).
FIG 5.

Performance trending of the PBMC reference control in the ELISPOT assay. IFN-γ SFC/106 PBMC of a reference PBMC control to medium (mock), CEFpp (360 ± 121, 33% CV), RSV Fpp (105 ± 36, 34% CV), or SEB (5,303 ± 987, 19% CV) measured over a 16-week period using an optimized and standardized protocol. Results shown were produced by a single analyst in 12 different assays.
Assay qualification.
The sensitivity (LOD), lower limit of quantitation (LLOQ), and intra-assay and interassay precision of the RSV Fpp ELISPOT assay were evaluated by using 3 different donor PBMC samples that exhibited a low (33 to 150 SFC/106 PBMC), medium (151 to 1,000 SFC/106 PBMC), and high (≥1,001 SFC/106 PBMC) IFN-γ response to RSV Fpp. Three analysts performed the assay on 5 different days using the standardized procedure. All plates were scanned and counted by a single skilled analyst using identical CTL analyzer settings. Based on data from PBMC with adequate viability (≤35% dead) upon thaw, acceptable mock and SEB responses were statistically defined as ≤80 SFC/106 PBMC and ≥470 SFC/106 PBMC, respectively. The sensitivity or limit of detection (LOD) of the RSV F-specific IFN-γ ELISPOT assay was statistically determined to be 21 SFC/106 PBMC, and the lower limit of quantitation (LLOQ) for an RSV F-specific IFN-γ response was established to be 63 SFC/106 PBMC, 3-fold the LOD (19). RSV F-specific IFN-γ responses among low-, medium-, and high-responding donor PBMC measured across 6 replicate wells by 3 analysts in a single assay were reproducible (Fig. 6). The data were analyzed using a variance components model to determine the intra- and interassay variability. Table 2 lists the standard deviation and the % CV for the intra-assay, interassay, and interanalyst reproducibility achieved using RSV Fpp-specific IFN-γ low-, medium-, and high-responding samples. Variability was greatest in samples with low responses, though intra-assay variability was low at 3.9% CV to 10.5% CV. Interassay variability ranged from 10.3% CV to 31% CV. Total variability, including both intra- and interassay precision, ranged from 11.0% for the high-responding sample to 30.3% and 32.5% for the low- and medium-responding samples, respectively. Based on the sensitivity and precision, this assay is fit for our intended purpose of evaluating RSV F-specific IFN-γ responses in vaccine clinical trials.
FIG 6.
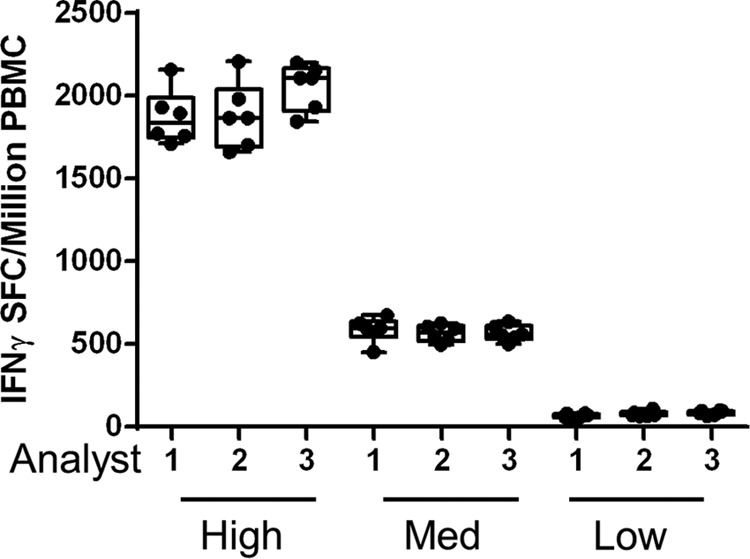
Determination of IFN-γ ELISPOT assay precision. RSV F-specific IFN-γ SFC/106 PBMC of low-, medium-, and high-responding donor PBMC measured across 6 replicate wells by 3 analysts in a single assay, demonstrating the intra-assay precision.
TABLE 2.
Variability and percent CV of the RSV F-specific low, medium, and high IFN-γ responder controls
| Level of response | Mean no. of SFC/106 PBMC | Intra-assay precision |
Interassay precision (day and analyst) |
Total |
|||
|---|---|---|---|---|---|---|---|
| Variability (SD) | % CV | Variability (SD) | % CV | Variability (SD) | % CV | ||
| Low | 127.1 | 13.3 | 10.5 | 35.3 | 28.4 | 38.5 | 30.3 |
| Medium | 619.5 | 59.4 | 9.6 | 158.8 | 31.0 | 201.3 | 32.5 |
| High | 2,118.5 | 83.6 | 3.9 | 208.6 | 10.3 | 232.8 | 11.0 |
Measurement of antigen-specific IFN-γ T cell responses in young and elderly adults using the qualified ELISPOT assay.
The qualified IFN-γ ELISPOT assay was used to measure antigen-specific T cell responses in young and elderly adult PBMC samples. RSV F-specific IFN-γ T cell responses were significantly lower in PBMC from elderly individuals than from young adults (P < 0.005) (Fig. 7A), while the IFN-γ T cell responses to CEFpp and SEB were not statistically different (P = 0.056 and P = 0.178, respectively) between PBMC from the young and the elderly (Fig. 7B and C). These data confirm our previous findings that the RSV F-specific IFN-γ T cell response is diminished in the elderly (12).
FIG 7.
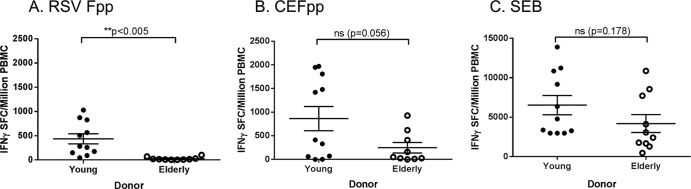
RSVpp-, CEFpp-, and SEB-specific IFN-γ T cell responses in PBMC from young and elderly adult subjects. PBMC isolated from young and elderly adult donors and frozen in serum-free medium were stimulated with RSV F peptide pool (A), CEF peptide pool (B), or SEB (C) to measure the frequency of IFN-γ-secreting T cells (horizontal lines and error bars indicate the mean ± SEM). There were statistically significant (P < 0.005) differences in the RSV Fpp-specific responses between young and elderly adult PBMC samples.
DISCUSSION
Accurate and reproducible quantification of antigen-specific T cell-mediated immune responses during vaccine or immunotherapy clinical trials has remained elusive and a major challenge until recently. The work presented here describes the design and step-by-step qualification of an RSV F-specific human IFN-γ ELISPOT assay that complies with current recommendations and guidelines established for similar assays in the field, and this assay is now ready to measure the T cell immune response elicited by a candidate RSV vaccine in either young or elderly adults. Notably, the elucidation of an RSV vaccine's potential mechanism of action will require a characterization of both the cellular and humoral immune responses elicited in participants of future RSV vaccine trials. Clinical T cell responses measured using the IFN-γ ELISPOT assay may constitute a functional biomarker of vaccine-induced cellular immune response and may enhance our understanding of the immune correlates associated with protection from severe RSV disease.
In this report, we describe the development and qualification of a sensitive and robust human IFN-γ ELISPOT assay that enables reproducible quantification of ex vivo RSV F-specific T cell immune responses in PBMC. Preanalytical variables including freezing medium, storage temperature, viability criteria, plate type, plating cell density, antigen type, antigen concentration, and incubation times were optimized. To demonstrate the reproducibility of this assay, the optimized RSV F-specific IFN-γ ELISPOT assay qualification was performed by 3 analysts over 5 days using samples exhibiting low, medium, and high responses to RSV Fpp.
The health of PBMC used in an ELISPOT assay is critically important in developing an ELISPOT assay for use in clinical trials, as reduced viability can result in a decreased T cell response measured ex vivo. Since using fresh PBMC in multicentric clinical trials is impractical, we standardized our process to have PBMC isolated within 8 h or less from blood draw, based on previous reports showing that time from blood draw to PBMC isolation is a critical factor for detecting antigen-specific T cell responses (22). We also set viability acceptance criteria for PBMC derived from the elderly using an IFN-γ ELISPOT assay response to a CEFpp and SEB. The results from our study showed a significant difference in the percent viable and percent dead populations when comparing −20°C to LN2 overnight storage (P = 0.005 and P = 0.023, respectively). Our study also showed that elderly PBMC with >35% dead cells had a diminished response to CEFpp and SEB compared to elderly PBMC with ≤35% dead cells. Based on these findings, we set an acceptance threshold of sample viability at ≤35% dead cells. The relatively low viability of PBMC from elderly donors in our study is consistent with other reports showing greater numbers of apoptotic and dead cells in PBMC derived from the elderly (21, 27, 28). Based on these observations, we standardized our process to store samples in LN2 for the long term with up to 3 days of storage at −80°C at clinical sites (21). In addition to examining storage temperature on recovery and viability of PBMC, we also examined the effects of using different freezing media on the recovery and viability of PBMC. Our results suggest that freezing PBMC in SFM has an advantage compared to using SCM, as it improves recovery and reduces background responses. Additionally, samples frozen in SFM maintain stable responses for at least 60 days when stored in LN2. These results are consistent with other reports that suggest it is beneficial to freeze freshly isolated PBMC in SFM rather than SCM (22–25).
After optimizing the collection and storage process for PBMC, the assay was further developed to define the optimal plate manufacturers, antigen for stimulation (RSV Fpp versus RSV F protein), antigen stimulation concentration, serum-free culture media, and cell plating densities. IFN-γ ELISPOT plates from Mabtech, Millipore, and Becton, Dickinson were assessed, and Mabtech plates were selected as they had the optimal signal-to-background ratio (data not shown). Following the selection of the plates, 4 different serum-free culture media were characterized in the assay, and all 4 were shown to be equivalent. Serum-containing culture medium was not evaluated since it has been shown that equivalent or better results with lower background can be obtained using serum-free culture medium (26). Next, RSV Fpp and the RSV F proteins were compared as a stimulation antigen, and the RSV Fpp was found to be more effective at eliciting a robust IFN-γ T cell response detectable in the ELISPOT assay. The RSV Fpp may have elicited a higher frequency of IFN-γ-secreting cells, as it is more likely to activate both CD4+ and CD8+ RSV F-specific T cells since antigen processing is not required ex vivo. To determine if antigen-specific responses were positive, the average spot counts from the mock wells were used as a negative control in our experiments, which is consistent with the analyses in previous reports (20). An irrelevant peptide antigen control was not included in these experiments due to the potential for cross-reactivity in a heterogeneous human population. We next optimized the number of PBMC per well to use in the ELISPOT assay. We examined a range of cell densities ranging from 25,000 to 400,000 and determined 300,000 cells/well to have an acceptable signal-to-background ratio and high overall precision. The optimized assay was standardized to use Mabtech plates, RSV Fpp at 2 μg/ml, and 300,000 cells/well in serum-free medium. Prior to qualifying the assay, we evaluated a precharacterized donor PBMC as recommended in the field (29–31). The control donor PBMC was assessed for its SEB, CEFpp, and RSV Fpp IFN-γ ELISPOT assay responses routinely over a period of 140 days. The results of this study showed that the control donor PBMC response was consistent over this period and that the assay was sufficiently robust and reproducible for qualification.
The RSV Fpp IFN-γ ELISPOT assay was qualified by 3 analysts testing a panel of 3 samples measured on 5 different days over a period of 2 weeks. The intra-assay precisions of the low, medium, and high RSV responder PBMC were determined to be 10.5% CV, 9.6% CV, and 3.9% CV. The interassay precisions that combined assay day and analyst were determined to be 28.4% CV, 31.0% CV, and 10.3% CV, and the total precisions of the assay combining intra- and interassay precisions were determined to be 30.3% CV, 32.5% CV, and 11.0% CV for the low, medium, and high RSV-responder PBMC, respectively. A total assay variability of ≤41% CV is required for this assay to detect a >2.5-fold rise in RSV F-specific T cell responses in individuals pre- and postvaccination. This would enable defining clinical responders with a false-positive rate of <5%. Thus, the assay is fit for the intended purpose of measuring RSV F-specific IFN-γ responses in future vaccine trials. The LOD was calculated to be 21 SFC/106 PBMC, and the LLOQ was estimated to be 63 SFC/106 PBMC. The acceptance criteria for a functional PBMC sample were determined to be a mock response of <80 SFC/106 PBMC and an SEB response of ≥470 SFC/106 PBMC. Lastly, the qualified RSV Fpp-specific IFN-γ ELISPOT assay was used to compare the T cell immune responses in PBMC from young (18- to 45-year-old) and elderly (≥65-year-old) adults. The results show that the elderly have a reduced T cell response to RSV Fpp. These results are consistent with previous findings showing a reduced RSV F protein-specific response in the elderly compared to the young (12). In contrast, the CEFpp-specific and SEB-specific responses were more comparable between the young and the elderly, suggesting that the reduced RSV Fpp-specific IFN-γ response is unique to the elderly.
In summary, we have optimized both preanalytical and analytical variables to develop a robust ELISPOT assay to measure RSV Fpp-specific IFN-γ-secreting T cells. Special attention was paid to elderly PBMC freezing medium and storage conditions. The assay has been optimized and is sufficiently sensitive, precise, and robust for use in clinical studies. The assay is fit for its intended purpose of measuring changes in RSV F-specific memory T cell responses following infection or vaccination in subjects enrolled in epidemiology studies or vaccine clinical trials.
ACKNOWLEDGMENTS
We acknowledge Barbara Meyer and Holly Lash of PPD Vaccine and Biologics Laboratory (Wayne, PA) for processing and freezing the PBMC samples used in the serum-containing and serum-free medium studies described here. We thank Lokesh Agrawal for his assistance with managing material transfer agreements and contracts. We thank Nancy Craighead for her editorial support. We thank Heather Lawlor for her assistance with the RSV F protein sequence submission to GenBank. We also thank Kavita Mathi and Darren Heeke for their technical support of the described studies.
Footnotes
Published ahead of print 26 February 2014
REFERENCES
- 1. Kostense S, Hendriks J. 2012. Challenges of immunogenicity assays for vaccines. Bioanalysis 4:397–406. 10.4155/bio.11.327 [DOI] [PubMed] [Google Scholar]
- 2. Dubey S, Clair J, Fu TM, Guan L, Long R, Mogg R, Anderson K, Collins KB, Gaunt C, Fernandez VR, Zhu L, Kierstead L, Thaler S, Gupta SB, Straus W, Mehrotra D, Tobery TW, Casimiro DR, Shiver JW. 2007. Detection of HIV vaccine-induced cell-mediated immunity in HIV-seronegative clinical trial participants using an optimized and validated enzyme-linked immunospot assay. J. Acquir. Immune Defic. Syndr. 45:20–27. 10.1097/QAI.0b013e3180377b5b [DOI] [PubMed] [Google Scholar]
- 3. Levin MJ, Oxman MN, Zhang JH, Johnson GR, Stanley H, Hayward AR, Caulfield MJ, Irwin MR, Smith JG, Clair J, Chan IS, Williams H, Harbecke R, Marchese R, Straus SE, Gershon A, Weinberg A. 2008. Varicella-zoster virus-specific immune responses in elderly recipients of a herpes zoster vaccine. J. Infect. Dis. 197:825–835. 10.1086/528696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Tyring SK, Stek JE, Smith JG, Xu J, Pagnoni M, Chan IS, Silber JL, Parrino J, Levin MJ. 2012. Varicella-zoster virus-specific enzyme-linked immunospot assay responses and zoster-associated pain in herpes zoster subjects. Clin. Vaccine Immunol. 19:1411–1415. 10.1128/CVI.00095-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Schanzer DL, Langley JM, Tam TW. 2008. Role of influenza and other respiratory viruses in admissions of adults to Canadian hospitals. Influenza Other Respir. Viruses 2:1–8. 10.1111/j.1750-2659.2008.00035.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Murata Y, Falsey AR. 2007. Respiratory syncytial virus infection in adults. Antivir. Ther. 12:659–670. 10.1097/MCP.0b013e3282f79651 [DOI] [PubMed] [Google Scholar]
- 7. Falsey AR, Hennessey PA, Formica MA, Cox C, Walsh EE. 2005. Respiratory syncytial virus infection in elderly and high-risk adults. N. Engl. J. Med. 352:1749–1759. 10.1056/NEJMoa043951 [DOI] [PubMed] [Google Scholar]
- 8. Olson MR, Varga SM. 2008. Pulmonary immunity and immunopathology: lessons from respiratory syncytial virus. Expert Rev. Vaccines 7:1239–1255. 10.1586/14760584.7.8.1239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Walsh EE, Peterson DR, Falsey AR. 2004. Risk factors for severe respiratory syncytial virus infection in elderly persons. J. Infect. Dis. 189:233–238. 10.1086/380907 [DOI] [PubMed] [Google Scholar]
- 10. Cusi MG, Martorelli B, Di Genova G, Terrosi C, Campoccia G, Correale P. 2010. Age related changes in T cell mediated immune response and effector memory to Respiratory Syncytial Virus (RSV) in healthy subjects. Immun. Ageing 7:14. 10.1186/1742-4933-7-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Looney RJ, Falsey AR, Walsh E, Campbell D. 2002. Effect of aging on cytokine production in response to respiratory syncytial virus infection. J. Infect. Dis. 185:682–685. 10.1086/339008 [DOI] [PubMed] [Google Scholar]
- 12. Cherukuri A, Patton K, Gasser RA, Jr, Zuo F, Woo J, Esser MT, Tang RS. 2013. Adults 65 years old and older have reduced numbers of functional memory T cells to respiratory syncytial virus fusion protein. Clin. Vaccine Immunol. 20:239–247. 10.1128/CVI.00580-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Falsey AR, Walsh EE, Capellan J, Gravenstein S, Zambon M, Yau E, Gorse GJ, Edelman R, Hayden FG, McElhaney JE, Neuzil KM, Nichol KL, Simoes EA, Wright PF, Sales VM. 2008. Comparison of the safety and immunogenicity of 2 respiratory syncytial virus (rsv) vaccines—nonadjuvanted vaccine or vaccine adjuvanted with alum—given concomitantly with influenza vaccine to high-risk elderly individuals. J. Infect. Dis. 198:1317–1326. 10.1086/592168 [DOI] [PubMed] [Google Scholar]
- 14. Kurzweil V, Tang R, Galinski M, Wang K, Zuo F, Cherukuri A, Gasser RA, Jr, Malkin E, Sifakis F, Mendel DB, Esser MT. 2013. Translational sciences approach to RSV vaccine development. Expert Rev. Vaccines 12:1047–1060. 10.1586/14760584.2013.824706 [DOI] [PubMed] [Google Scholar]
- 15. Maecker HT, Dunn HS, Suni MA, Khatamzas E, Pitcher CJ, Bunde T, Persaud N, Trigona W, Fu TM, Sinclair E, Bredt BM, McCune JM, Maino VC, Kern F, Picker LJ. 2001. Use of overlapping peptide mixtures as antigens for cytokine flow cytometry. J. Immunol. Methods 255:27–40. 10.1016/S0022-1759(01)00416-1 [DOI] [PubMed] [Google Scholar]
- 16. Britten CM, Janetzki S, Butterfield LH, Ferrari G, Gouttefangeas C, Huber C, Kalos M, Levitsky HI, Maecker HT, Melief CJ, O'Donnell-Tormey J, Odunsi K, Old LJ, Ottenhoff TH, Ottensmeier C, Pawelec G, Roederer M, Roep BO, Romero P, van der Burg SH, Walter S, Hoos A, Davis MM. 2012. T cell assays and MIATA: the essential minimum for maximum impact. Immunity 37:1–2. 10.1016/j.immuni.2012.07.010 [DOI] [PubMed] [Google Scholar]
- 17. Tang RS, MacPhail M, Schickli JH, Kaur J, Robinson CL, Lawlor HA, Guzzetta JM, Spaete RR, Haller AA. 2004. Parainfluenza virus type 3 expressing the native or soluble fusion (F) protein of respiratory syncytial virus (RSV) confers protection from RSV infection in African green monkeys. J. Virol. 78:11198–11207. 10.1128/JVI.78.20.11198-11207.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Searle SR, Casella G, McCulloch CE. 1992. Variance components. John Wiley & Sons, New York, NY [Google Scholar]
- 19. Walsh PN, Friedrich DP, Williams JA, Smith RJ, Stewart TL, Carter DK, Liao HX, McElrath MJ, Frahm N. 2013. Optimization and qualification of a memory B-cell ELISpot for the detection of vaccine-induced memory responses in HIV vaccine trials. J. Immunol. Methods 394:84–93. 10.1016/j.jim.2013.05.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Smith JG, Joseph HR, Green T, Field JA, Wooters M, Kaufhold RM, Antonello J, Caulfield MJ. 2007. Establishing acceptance criteria for cell-mediated-immunity assays using frozen peripheral blood mononuclear cells stored under optimal and suboptimal conditions. Clin. Vaccine Immunol. 14:527–537. 10.1128/CVI.00435-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mallone R, Mannering SI, Brooks-Worrell BM, Durinovic-Bello I, Cilio CM, Wong FS, Schloot NC. 2011. Isolation and preservation of peripheral blood mononuclear cells for analysis of islet antigen-reactive T cell responses: position statement of the T-Cell Workshop Committee of the Immunology of Diabetes Society. Clin. Exp. Immunol. 163:33–49. 10.1111/j.1365-2249.2010.04272.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bull M, Lee D, Stucky J, Chiu YL, Rubin A, Horton H, McElrath MJ. 2007. Defining blood processing parameters for optimal detection of cryopreserved antigen-specific responses for HIV vaccine trials. J. Immunol. Methods 322:57–69. 10.1016/j.jim.2007.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Germann A, Schulz JC, Kemp-Kamke B, Zimmermann H, von Briesen H. 2011. Standardized serum-free cryomedia maintain peripheral blood mononuclear cell viability, recovery, and antigen-specific T-cell response compared to fetal calf serum-based medium. Biopreserv. Biobank. 9:229–236. 10.1089/bio.2010.0033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Janetzki S, Price L, Britten CM, van der Burg SH, Caterini J, Currier JR, Ferrari G, Gouttefangeas C, Hayes P, Kaempgen E, Lennerz V, Nihlmark K, Souza V, Hoos A. 2010. Performance of serum-supplemented and serum-free media in IFNgamma Elispot assays for human T cells. Cancer Immunol. Immunother. 59:609–618. 10.1007/s00262-009-0788-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Weinberg A, Song LY, Wilkening CL, Fenton T, Hural J, Louzao R, Ferrari G, Etter PE, Berrong M, Canniff JD, Carter D, Defawe OD, Garcia A, Garrelts TL, Gelman R, Lambrecht LK, Pahwa S, Pilakka-Kanthikeel S, Shugarts DL, Tustin NB. 2010. Optimization of storage and shipment of cryopreserved peripheral blood mononuclear cells from HIV-infected and uninfected individuals for ELISPOT assays. J. Immunol. Methods 363:42–50. 10.1016/j.jim.2010.09.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mander A, Gouttefangeas C, Ottensmeier C, Welters MJ, Low L, van der Burg SH, Britten CM. 2010. Serum is not required for ex vivo IFN-gamma ELISPOT: a collaborative study of different protocols from the European CIMT Immunoguiding Program. Cancer Immunol. Immunother. 59:619–627. 10.1007/s00262-009-0814-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Deng Y, Jing Y, Campbell AE, Gravenstein S. 2004. Age-related impaired type 1 T cell responses to influenza: reduced activation ex vivo, decreased expansion in CTL culture in vitro, and blunted response to influenza vaccination in vivo in the elderly. J. Immunol. 172:3437–3446 [DOI] [PubMed] [Google Scholar]
- 28. Koch S, Larbi A, Derhovanessian E, Ozcelik D, Naumova E, Pawelec G. 2008. Multiparameter flow cytometric analysis of CD4 and CD8 T cell subsets in young and old people. Immun. Ageing 5:6. 10.1186/1742-4933-5-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Janetzki S, Britten CM. 2012. The impact of harmonization on ELISPOT assay performance. Methods Mol. Biol. 792:25–36. 10.1007/978-1-61779-325-7_2 [DOI] [PubMed] [Google Scholar]
- 30. Kelley M. 2008. Considerations while setting up cell-based assays, p 1–9 In Prabhakar U, Kelley M. (ed), Validation of cell-based assays in the GLP setting: a practical guide. John Wiley & Sons, Ltd., Chichester, United Kingdom. 10.1002/9780470987810 [DOI] [Google Scholar]
- 31. van der Burg SH. 2008. Therapeutic vaccines in cancer: moving from immunomonitoring to immunoguiding. Expert Rev. Vaccines 7:1–5. 10.1586/14760584.7.1.1 [DOI] [PubMed] [Google Scholar]