Abstract
The geometric mean concentration (GMC) and the proportion maintaining a protective level (150 enzyme-linked immunosorbent assay (ELISA) units [ELU]/ml) 2 years following a single dose of 25 μg of injectable Vi capsular polysaccharide typhoid vaccine was measured against that of the control hepatitis A vaccine in children 2 to 16 years old in cluster randomized trials in Karachi and Kolkata. The GMC for the Vi group (1,428 ELU/ml) was statistically significantly different from the GMC of the control hepatitis A vaccine group (86 ELU/ml) after 6 weeks. A total of 117 children (95.1%) in the Vi group and 9 (7.5%) in the hepatitis A group showed a 4-fold rise in Vi IgG antibody concentrations at 6 weeks (P < 0.01). Protective antibody levels remained significantly different between the two groups at 2 years (38% in the Vi vaccine groups and 6% in the hepatitis A group [P < 0.01]). A very small proportion of younger children (2 to 5 years old) maintained protective Vi IgG antibody levels at 2 years, a result that was not statistically significantly different compared to that for the hepatitis A group (38.1% versus 10.5%). The GMCs of the Vi IgG antibody after 2 years were 133 ELU/ml for children 2 to <5 years old and 349 ELU/ml for children 5 to 16 years old. In conclusion, Vi capsular polysaccharide typhoid vaccine is immunogenic in children in settings of South Asia where typhoid is highly endemic. The antibody levels in children who received this vaccine remained higher than those in children who received the control vaccine but were significantly reduced at 2 years of follow-up.
INTRODUCTION
Typhoid fever is a major bacterial infection in developing countries (1). Typhoid fever, until recently, was considered a disease of high incidence in children older than 5 years and endemic in South and South-East Asia. However, the data gathered in the past 10 years have shown that the disease can affect infants and has a high incidence in other parts of the world, such as Africa (2, 3). The incidence of typhoid fever ranges from 104 per 100,000 persons per year to 273 per 100,000 persons per year (4, 5).
Currently, two vaccines are licensed for typhoid fever, the live attenuated Ty21a vaccine and the Vi capsular polysaccharide vaccine. The World Health Organization (WHO) recommends the use of typhoid vaccines in areas where the disease is endemic and in high-risk populations (travelers, microbiology laboratory technologists, etc.) (6).
The Vi capsular polysaccharide typhoid vaccine contains 25 μg of purified Vi capsular polysaccharide, to be administered intramuscularly. It can be administered to nonpregnant individuals 2 years old and older (see http://public.gsk.co.uk/products/Typherix.html). Large-scale Vi capsular polysaccharide vaccine efficacy studies conducted in South Africa and Nepal established the efficacy and immunogenicity of the vaccine (7, 8). The immune response to the Vi capsular polysaccharide vaccine is elicited by the production of IgG antibodies and is T cell independent. IgG antibody levels are used for the assessment of protection against typhoid fever, as there are no direct measures of protection for Vi-based vaccines. IgG antibodies are elicited in 85 to 95% of the vaccinees after Vi capsular polysaccharide vaccine administration (9). Protective levels of antibodies are elicited after 7 days and peak at 28 days postvaccination. The antibody levels to Vi capsular polysaccharide vaccines wane with the passage of time, usually after 2 years (10–12). Revaccination every 3 years, therefore, is recommended for sustained protection against typhoid fever in settings where typhoid is endemic and in high-risk populations (13).
The correlation of Vi IgG antibody levels with Vi capsular polysaccharide vaccine protection as well as the long-term persistence of Vi IgG antibodies was documented in a study from South Africa (8). The results, however, have not been widely accepted as correlates of protection. Additionally, there is currently limited information on the immune response in young children to Vi capsular polysaccharide vaccine, in view of the immaturity of the immune response in young children from polysaccharide vaccine administration (14).
The Disease of Most Impoverished Program of the International Vaccine Institute conducted Vi capsular polysaccharide vaccine effectiveness trails in Karachi, Pakistan, targeting children 2 to 16 years old, and in Kolkata, India, targeting all those who are 2 years old or older to assess the feasibility of introducing Vi capsular polysaccharide vaccine as a regular public health program as described in earlier publications (15, 16). We present the results of the immune responses to Vi capsular polysaccharide vaccine in children 2 to 16 years old from these trials.
MATERIALS AND METHODS
Study setting and population.
Cluster randomized trials to assess the effectiveness of the Vi capsular polysaccharide vaccination were conducted between 2002 and 2007 in the urban slums of Karachi, Pakistan, and Kolkata, India (15–17). The Vi capsular polysaccharide vaccine or a control hepatitis A vaccine was administered to the target population. A computer-generated random subsample at each site was selected for the study of immune responses. In Kolkata, two individuals per cluster were selected, stratified by age group (two from the age group of 2 to 18 years and two from the group older than 18 years). In Karachi, four subjects per cluster were selected (two from the age group of 2 to <5 years and two from 5 to 16 years). We pooled the data from the two sites for this analysis and hence did not use the information for children older than 16 years from Kolkata.
Vaccines.
The vaccines (Typherix and Havrix) were supplied by GlaxoSmithKline Biologicals (GSK) (Rixensart, Belgium). Typherix was supplied as a 0.5-ml dose of vaccine containing 25 μg of the Vi capsular polysaccharide of Salmonella typhi in a clear isotonic colorless solution. A single dose of 0.5 ml is recommended for intramuscular injection in both adults and children 2 years old and older. A Havrix pediatric dose was supplied as a 0.5-ml dose consisting of 720 enzyme-linked immunosorbent assay (ELISA) units (ELU) of viral antigen adsorbed on 0.25 mg of aluminum as aluminum hydroxide. Havrix was supplied as a sterile suspension for intramuscular administration.
Data collection.
Vaccination cards with household member information were distributed prior to vaccination. The names of the children randomly selected for blood sampling were highlighted in the vaccination record books to ease identification when children visited vaccination centers. The study staff explained to the parent/guardian the purpose of the blood sampling, and after written informed consent was obtained, 5 ml of blood was collected before vaccination at the vaccination post. The same subjects were followed up at the participants' homes at 6 weeks and 2 years after vaccination for the subsequent sampling. At the field sites, the samples were allowed to clot at room temperature for 30 min to 2 h. At the end of the day, the blood samples were transported to the clinical laboratories of Aga Khan University Hospital (AKUH) in Karachi, Pakistan, and the National Institute of Cholera and Enteric Diseases (NICED) in Kolkata, India. The blood samples were centrifuged at 3,000 rpm for 15 min and the sera were separated aseptically into multiple aliquots within 24 h after the sampling. Sera were then stored at −80°C. Each blood sample and aliquot was labeled with premarked stickers. Quantitative serum Vi and hepatitis A antibody (IgG) concentrations were measured through an ELISA technique using the GSK ELISA standard (18–21). The serum analyses were performed at the end of the trials, and the technicians performing assays were blinded to the vaccine codes.
Data analysis.
The serological responses to the Vi capsular polysaccharide vaccine were expressed as the geometric mean concentration (GMC) of serum Vi IgG in ELU/ml. Descriptive statistics included calculation of percentiles and 95% confidence limits. The number of subjects who attained protective antigen levels (concentration, ≥150 ELU/ml) and the number who achieved a ≥4-fold rise in antibody levels were compared between intervention and control groups at baseline and at each follow-up using generalized estimating equations with the logit link function (22). Data were analyzed using SAS version 9.3.
The studies were approved by the Institutional Review Board (IRB) of the International Vaccine Institute, the Ethical Review Committee of the Aga Khan University (Karachi, Pakistan), the IRBs of the National Institute of Cholera and Enteric Diseases (Kolkata, India), and the Indian Council of Medical Research (Delhi, India).
RESULTS
Blood samples for 300 participants were collected at the time of vaccination, 243 (81%) 6 weeks after vaccination, and 139 (46%) at 2 years following vaccination. Sixty (39%) children in the Vi vaccine group and 52 (35%) in the control hepatitis A vaccine group were younger than 5 years of age. At least three blood samples were collected for 69 (45%) children in the Vi vaccine group and 64 (44%) in the hepatitis A vaccine group (Table 1). There was no statistically significant difference in the number of children enrolled in the two groups. Twelve children in the Vi vaccine group and 10 in the hepatitis A vaccine group had prior protective antibody levels. The baseline Vi IgG antibody GMC levels for the two groups were not statistically different. The baseline Vi IgG GMC was 81.8 ELU/ml (95% confidence limits [CL], 77.2 to 86.7 ELU/ml) in the hepatitis A vaccine group and was 83.2 ELU/ml (95% CL, 78.4 to 88.4 ELU/ml) in the Vi vaccine group (Table 2).
TABLE 1.
The distribution of sample size by site and age group for assessment of immune response as part of Vi capsular polysaccharide vaccine effectiveness trials in Karachi and Kolkata
| Blood sample collection no. and time period | Age group (yr) | Sample sizes (n [%]) in: |
Total (n [%]) |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Karachi |
Kolkata |
|||||||||
| Vi | HepAa | Total | Vi | HepA | Total | Vi | HepA | Total | ||
| 1, day 0/prevaccination | 2–5 | 57 (46.7) | 47 (40.9) | 104 (43.9) | 3 (9.7) | 5 (15.6) | 8 (12.7) | 60 (39.2) | 52 (35.4) | 112 (37.3) |
| 5–16 | 65 (53.3) | 68 (59.1) | 133 (56.1) | 28 (90.3) | 27 (84.4) | 55 (87.3) | 93 (60.8) | 95 (64.6) | 188 (62.7) | |
| 2, 6 wk after vaccination | 2–5 | 40 (43.5) | 36 (39.6) | 76 (41.5) | 2 (6.5) | 3 (10.3) | 5 (8.3) | 42 (34.2) | 39 (32.5) | 81 (33.3) |
| 5–16 | 52 (56.5) | 55 (60.4) | 107 (58.5) | 29 (93.6) | 26 (89.7) | 55 (91.7) | 81 (65.9) | 81 (67.5) | 162 (66.7) | |
| 3, 2 yr after vaccination | 2–5 | 20 (43.5) | 17 (37.8) | 37 (40.7) | 1 (4.0) | 2 (8.7) | 3 (6.3) | 21 (29.6) | 19 (27.9) | 40 (28.8) |
| 5–16 | 26 (56.5) | 28 (62.2) | 54 (59.3) | 24 (96.0) | 21 (91.3) | 45 (93.8) | 50 (70.4) | 49 (72.1) | 99 (71.2) | |
| All three samples | 2–5 | 19 (42.2) | 14 (34.2) | 33 (38.4) | 1 (4.2) | 2 (8.7) | 3 (6.4) | 20 (29.0) | 16 (25.0) | 36 (27.1) |
| 5–16 | 26 (57.8) | 27 (65.9) | 53 (61.6) | 23 (95.8) | 21 (91.3) | 44 (93.6) | 49 (71.0) | 48 (75.0) | 97 (72.9) | |
HepA, hepatitis A vaccine.
TABLE 2.
Comparison of immune response to the Vi capsular polysaccharide vaccine and the control hepatitis A vaccine in children between the ages of 2 and 16 years in Karachi and Kolkata
| Variables | Values for children receiving the indicated vaccine |
P | |
|---|---|---|---|
| Vi | Hepatitis A | ||
| Overall (age 2–16 yr) | |||
| Blood sample 1, day 0 (prevaccination)a | |||
| No. | 153 | 147 | |
| GMCb (95% confidence limits) | 83.2 (78.4–88.4) | 81.8 (77.2–86.7) | 0.692 |
| Attained protective antigen level (n [%])c | 12 (7.8) | 10 (6.8) | 0.723 |
| Blood sample 2, 6 wk since vaccination | |||
| No. | 123 | 120 | |
| GMC (95% confidence limits) (ELU/ml) | 1,428.3 (1130–1805.4) | 85.9 (78–94.6) | <0.001 |
| Attained protective antigen level (n [%]) | 117 (95.1) | 9 (7.5) | <0.001 |
| Achieved a ≥4-fold rised (n [%]) | 104 (86.7) | 2 (1.7) | <0.001 |
| Blood sample 3, 2 yr since vaccination | |||
| No. | 71 | 68 | |
| GMC (95% confidence limits) (ELU/ml) | 262.6 (190–362.8) | 90.4 (79.1–103.4) | <0.001 |
| Attained protective antigen level (n [%]) | 40 (56.3) | 8 (11.8) | <0.001 |
| Achieved a ≥4-fold rise (n [%]) | 27 (39.1) | 4 (6.1) | <0.001 |
| Age <5 yr | |||
| Blood sample 1, day 0 (prevaccination) | |||
| No. | 60 | 52 | |
| GMC (95% confidence limits) (ELU/ml) | 75 (75–75) | 76.2 (73.8–78.7) | 0.304 |
| Attained protective antigen level (n [%]) | 0 (0.0) | 1 (1.9) | e |
| Blood sample 2, 6 wk since vaccination | |||
| No. | 42 | 39 | |
| GMC (95% confidence limits) (ELU/ml) | 832.2 (546–1268.5) | 82.7 (67.8–100.8) | <0.001 |
| Attained protective antigen level (n [%]) | 36 (85.7) | 1 (2.6) | <0.001 |
| Achieved a ≥4-fold rise (n [%]) | 29 (70.7) | 1 (2.7) | <0.001 |
| Blood sample 3, 2 yr since vaccination | |||
| No. | 21 | 19 | |
| GMC (95% confidence limits) (ELU/ml) | 133.4 (93.7–190) | 96.5 (67–139) | 0.218 |
| Attained protective antigen level (n [%]) | 8 (38.1) | 2 (10.5) | 0.071 |
| Achieved a ≥4-fold rise (n [%]) | 5 (25.0) | 2 (11.1) | 0.379 |
| Age 5–15.9 yr | |||
| Blood sample 1, day 0 (prevaccination) | |||
| No. | 93 | 95 | |
| GMC (95% confidence limits) (ELU/ml) | 89 (80.8–98.1) | 85.1 (77.9–92.9) | 0.497 |
| Attained protective antigen level (n [%]) | 12 (12.9) | 9 (9.5) | 0.463 |
| Blood sample 2, 6 wk since vaccination | |||
| No. | 81 | 81 | |
| GMC (95% confidence limits) (ELU/ml) | 1,890 (1447.3–2468.1) | 87.5 (78.5–97.6) | <0.001 |
| Attained protective antigen level (n [%]) | 81 (100.0) | 8 (9.9) | e |
| Achieved a ≥4-fold rise (n [%]) | 75 (94.9) | 1 (1.3) | <0.001 |
| Blood sample 3, 2 yr since vaccination | |||
| No. | 50 | 49 | |
| GMC (95% confidence limits) (ELU/ml) | 348.9 (230.2–528.8) | 88.2 (77.3–100.5) | <0.001 |
| Attained protective antigen level (n [%]) | 32 (64.0) | 6 (12.2) | <0.001 |
| Achieved a ≥4-fold rise (n [%]) | 22 (44.9) | 2 (4.2) | <0.001 |
Blood sample 1 is from day 0 (prevaccination), 2 is from week 6 since vaccination, and 3 is from year 2 since vaccination.
GMC, geometric mean concentration. All values are expressed in ELU/ml.
Attained protective antigen level (Vi, ≥150 ELU/ml [GSK in-house standard]). We used the value 75 ELU/ml (midpoint) for those listed as <150 ELU/ml in the report.
Achieved a ≥4-fold rise in antibodies, in relation to baseline, as detected in the cited blood sample.
P value is not possible due to 0 in one of the cells.
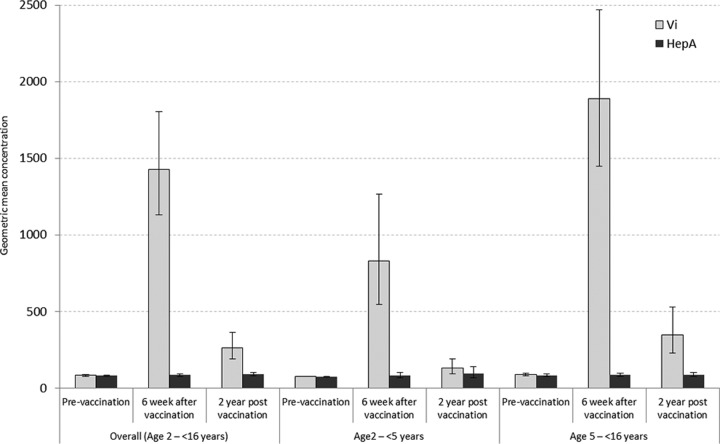
The Vi IgG GMC at 6 weeks after vaccination for the Vi vaccine group was 1,428.3 ELU/ml (95% CL, 1,130.0 to 1805.4 ELU/ml) compared to 85.9 ELU/ml (95% CL, 78.0 to 94.6 ELU/ml) in the hepatitis A vaccine group. Two years after vaccination, the Vi IgG GMC in the Vi vaccine group was 262.6 ELU/ml (95% CL, 190.0 to 362.8 ELU/ml) compared to 90.4 ELU/ml (95% CL, 79.1 to 103.4 ELU/ml) in the hepatitis A vaccine group. Within the groups vaccinated with Vi capsular polysaccharide vaccine, the Vi IgG GMC reduced to 262.6 ELU/ml compared to 1,428.3 ELU/ml at 6 weeks after vaccination (Table 2).
The proportion of children maintaining a protective level of Vi IgG antibodies 2 years after vaccination in the Vi vaccine group was statistically significantly different from the proportion in the control hepatitis A vaccine group (39.1% in Vi vaccine group and 6.1% in hepatitis A vaccine group; P < 0.001) (Fig. 1). Six children (4.9%) in the sample did not develop Vi IgG antibodies after receiving the Vi capsular polysaccharide vaccine. All nonresponders were <5 years old. The Vi IgG antibody levels dropped below the protective level in 31 (43.7%) children after 2 years. The immune response to the Vi capsular polysaccharide vaccine in younger children (<5 years old) was not statistically different compared to the response in older children (5 to 16 years old) at 6 weeks and 2 years after vaccination (Table 2).
FIG 1.
Comparison of anti-Vi antibody levels at three different time points by age group and their corresponding 95% confidence intervals from the studies of immune response to Vi capsular polysaccharide vaccine in Karachi and Kolkata. HepA, hepatitis A vaccine.
DISCUSSION
Typhoid fever incidence continues to be high in children, and in the absence of long-term interventions (such as improvements in water quality and sanitation), vaccination is an alternative short-term strategy. In Kolkata and Karachi, areas of high endemicity for typhoid fever, the Vi capsular polysaccharide vaccine is immunogenic in children aged 2 to 16 years, with a considerable proportion of the vaccinees retaining a protective level of antibodies after 2 years. However, immune responses are higher in older children (5 to 16 years old) than in younger children (2 to <5 years old).
Approximately 7% of children enrolled in our study had protective levels of Vi IgG antibodies prior to vaccination, suggesting a high prevalence of immune response due to natural infection. Natural infection with S. typhi does not confer long-term immunity (23, 24). The mechanism through which a polysaccharide vaccine may interact and boost the existing immunity due to natural infection is not well understood. However, children who had prior antibodies responded better to the Vi vaccine (data not shown).
It is important to note that the correlates of protection for typhoid fever vaccines are not established. The ELISA method has been the most common way to assess immunogenicity (8, 25, 26); however, the lack of standardized quantitative IgG reference sera makes direct comparison of immunogenicity data from different studies impossible. In our study, the Vi IgG antibody levels were measured using the ELISA method with the GSK Biologicals' reference standard, for which the threshold had been standardized for their internal use (18–21). Therefore, we were unable to compare our results with those of other studies of Vi immune responses due to differences in measurement methods and scales.
Nonetheless, the results from our study complement the effectiveness studies and support the importance of typhoid vaccination as a preventive measure for typhoid control in areas where typhoid is highly endemic. However, these results also pose a programmatic challenge due to the short durations of the presence of Vi IgG in the vaccinated population as well as of the immune responses in children <5 years old, due to the polysaccharide vaccine's T cell independent immunity and the immaturity of the immune response in younger children (27).
Even with a shorter duration of the presence of Vi IgG antibodies in younger children, the effectiveness study in Kolkata had shown a significant clinical protection in younger children when the vaccine was provided to the whole community (16). This larger vaccination coverage may have contributed to the interruption in disease transmission in the community, inducing indirect protection to the younger children. Similarly, a study of the long-term persistence of Vi IgG antibodies in South Africa supports the argument for the use of a series of vaccination campaigns in high-risk areas where, over a 10-year period, the majority of the population will be protected with Vi capsular polysaccharide vaccination (28).
In regions of the world with high endemicity, typhoid fever incidences are higher in children <5 years old compared to those in older children. The immune response elicited by natural infection as well as by the vaccine in the children younger than 5 years needs further exploration. In the absence of an effective vaccine for younger children, other methods of prevention at the household level, such as hand washing and drinking clean water, should be adopted to prevent typhoid fever in children <5 years old (29, 30). Further, after much-awaited development and introduction, the Vi conjugate vaccine is likely to overcome the limitations of the Vi capsular polysaccharide vaccine, as the conjugate vaccine was shown to be more immunogenic in younger children and to confer sustained protection (31, 32).
ACKNOWLEDGMENTS
We acknowledge the contributions of Didar Alam for providing laboratory support, Sue Kyoung Jo for providing administrative support for the project, and Raul Gomez Roman for scientific editing of the manuscript.
This study was supported through the Disease of Most Impoverished Program, awarded by the Bill and Melinda Gates Foundation. The International Vaccine Institute receives core financial support from the governments of the Republic of Korea and Sweden.
Currently, R.L.O. is an employee of Sanofi Pasteur and C.J.A. is an employee of Merck. The studies in this article were done prior to employment at these companies. Neither they nor the companies have any benefits or conflicts associated with the findings in this article. The other authors have no conflicts to declare.
R.L.O. and M.I.K. designed the study, supervised the implementation, participated in data analysis, and drafted the manuscript. S.B.S., D.S., M.A.H., B.M., S.D., S.K., and S.M.S. implemented the study, participated in the data analysis, and reviewed the manuscript. Y.A.Y. and M.A. performed the statistical analysis and helped draft the manuscript. C.J.A., S.K.B., Z.A.B., and J.D.C. conceived the study, participated in the design and coordination, reviewed the statistical analysis, and helped draft the manuscript. All authors read and approved the final manuscript.
Footnotes
Published ahead of print 5 March 2014
REFERENCES
- 1. Crump JA, Luby SP, Mintz ED. 2004. The global burden of typhoid fever. Bull. World Health Organ. 82:346–353 [PMC free article] [PubMed] [Google Scholar]
- 2. Breiman RF, Cosmas L, Njuguna H, Audi A, Olack B, Ochieng JB, Wamola N, Bigogo GM, Awiti G, Tabu CW. 2012. Population-based incidence of typhoid fever in an urban informal settlement and a rural area in Kenya: implications for typhoid vaccine use in Africa. PLoS One 7:e29119. 10.1371/journal.pone.0029119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sinha A, Sazawal S, Kumar R, Sood S, Reddaiah VP, Singh B, Rao M, Naficy A, Clemens JD, Bhan MK. 1999. Typhoid fever in children aged less than 5 years. Lancet 354:734–737. 10.1016/S0140-6736(98)09001-1 [DOI] [PubMed] [Google Scholar]
- 4. Punjabi NH, Agtini MD, Ochiai RL, Simanjuntak CH, Lesmana M, Subekti D, Oyofo BA, von Seidlein L, Deen J, Shin S, Acosta C, Wangsasaputra F, Pulungsih SP, Saroso S, Suyeti S, Sudarmono P, Syarurachman A, Suwandono A, Arjoso S, Beecham HJ, 3rd, Corwin AL, Clemens JD. 2013. Enteric fever burden in North Jakarta, Indonesia: a prospective, community-based study. J. Infect. Dev. Ctries. 7:781–787. 10.3855/jidc.2629 [DOI] [PubMed] [Google Scholar]
- 5. Ochiai RL, Acosta CJ, Danovaro-Holliday MC, Baiqing D, Bhattacharya SK, Agtini MD, Bhutta ZA, Canh do G, Ali M, Shin S, Wain J, Page AL, Albert MJ, Farrar J, Abu-Elyazeed R, Pang T, Galindo CM, von Seidlein L, Clemens JD, Domi Typhoid Study Group 2008. A study of typhoid fever in five Asian countries: disease burden and implications for controls. Bull. World Health Organ. 86:260–268. 10.2471/BLT.06.039818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.World Health Organization. 2008. Typhoid vaccines: WHO position paper. Wkly. Epidemiol. Rec. 83:49–60 [PubMed] [Google Scholar]
- 7. Acharya IL, Lowe CU, Thapa R, Gurubacharya VL, Shrestha MB, Cadoz M, Schulz D, Armand J, Bryla DA, Trollfors B. 1987. Prevention of typhoid fever in Nepal with the Vi capsular polysaccharide of Salmonella typhi. A preliminary report. N. Engl. J. Med. 317:1101–1104. 10.1056/NEJM198710293171801 [DOI] [PubMed] [Google Scholar]
- 8. Klugman KP, Koornhof HJ, Robbins JB, Le Cam NN. 1996. Immunogenicity, efficacy and serological correlate of protection of Salmonella typhi Vi capsular polysaccharide vaccine three years after immunization. Vaccine 14:435–438. 10.1016/0264-410X(95)00186-5 [DOI] [PubMed] [Google Scholar]
- 9. Plotkin SA, Bouveret-Le Cam N. 1995. A new typhoid vaccine composed of the Vi capsular polysaccharide. Arch. Intern. Med. 155:2293–2299 [PubMed] [Google Scholar]
- 10. Wong KH, Feeley JC, Pittman M, Forlines ME. 1974. Adhesion of Vi antigen and toxicity in typhoid vaccines inactivated by acetone or by heat and phenol. J. Infect. Dis. 129:501–506. 10.1093/infdis/129.5.501 [DOI] [PubMed] [Google Scholar]
- 11. Levin DM, Wong KH, Reynolds HY, Sutton A, Northrup RS. 1975. Vi antigen from Salmonella typhosa and immunity against typhoid fever. 11. Safety and antigenicity in humans. Infect. Immun. 12:1290–1294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Robbins JD, Robbins JB. 1984. Reexamination of the protective role of the capsular polysaccharide (Vi antigen) of Salmonella typhi. J. Infect. Dis. 150:436–449. 10.1093/infdis/150.3.436 [DOI] [PubMed] [Google Scholar]
- 13. Zhou WZ, Koo HW, Wang XY, Zhang J, Park JK, Zhu F, Deen J, Acosta CJ, Chen Y, Wang H, Galindo CM, Ochiai L, Park T, von Seidlein L, Xu ZY, Clemens JD. 2007. Revaccination with locally-produced vi typhoid polysaccharide vaccine among Chinese school-aged children: safety and immunogenicity findings. Pediatr. Infect. Dis. J. 26:1001–1005. 10.1097/INF.0b013e31812565bc [DOI] [PubMed] [Google Scholar]
- 14. Clemens J, Hoffman S, Ivanoff B, Klugman K, Levine MM, Neira M, Pang T. 1999. Typhoid fever vaccines. Vaccine 17:2476–2478. 10.1016/S0264-410X(99)00067-5 [DOI] [PubMed] [Google Scholar]
- 15. Khan MI, Soofi SB, Ochiai RL, Habib MA, Sahito SM, Nizami SQ, Acosta CJ, Clemens JD, Bhutta ZA, DOMI Typhoid Karachi Vi Effectiveness Study Group 2012. Effectiveness of Vi capsular polysaccharide typhoid vaccine among children: a cluster randomized trial in Karachi, Pakistan. Vaccine 30:5389–5395. 10.1016/j.vaccine.2012.06.015 [DOI] [PubMed] [Google Scholar]
- 16. Sur D, Ochiai RL, Bhattacharya SK, Ganguly NK, Ali M, Manna B, Dutta S, Donner A, Kanungo S, Park JK, Puri MK, Kim DR, Dutta D, Bhaduri B, Acosta CJ, Clemens JD. 2009. A cluster-randomized effectiveness trial of Vi typhoid vaccine in India. N. Engl. J. Med. 361:335–344. 10.1056/NEJMoa0807521 [DOI] [PubMed] [Google Scholar]
- 17. Acosta CJ, Galindo CM, Ali M, Elyazeed RA, Ochiai RL, Danovaro-Holliday MC, Page AL, Thiem VD, Jin Y, Park JK, Lee H, Puri MK, Ivanoff B, Agtini MD, Soeharno R, Simanjuntak CH, Punjabi NH, Canh DG, Sur D, Nizami Q, Manna B, DBai-qing, Anh DD, Honghui Y, Bhattacharya SK, Bhutta Z, Trach DD, Xu ZY, Pang T, Donner A, Clemens JD. 2005. A multicountry cluster randomized controlled effectiveness evaluation to accelerate the introduction of Vi polysaccharide typhoid vaccine in developing countries in Asia: rationale and design. Trop. Med. Int. Health 10:1219–1228. 10.1111/j.1365-3156.2005.01517.x [DOI] [PubMed] [Google Scholar]
- 18. Cordero-Yap L, Rivera RG, Dispo AP, Mallabo J. 2001. Evaluation of a new Vi polysaccharide typhoid vaccine in children aged 2–5 years. BioDrugs 15(Suppl 1):27. [DOI] [PubMed] [Google Scholar]
- 19. Lebacq E. 2001. Comparative tolerability and immunogenicity of Typherix or Typhim Vi in healthy adults: 0, 12-month and 0, 24-month administration. BioDrugs 15(Suppl 1):5–12. 10.2165/00063030-200115001-00002 [DOI] [PubMed] [Google Scholar]
- 20. Pelser HH. 2001. Reactogenicity and immunogenicity of a single dose of typhoid Vi polysaccharide vaccine in children aged between 4 and 14 years. BioDrugs 15(Suppl 1):13–19. 10.2165/00063030-200115001-00003 [DOI] [PubMed] [Google Scholar]
- 21. Ramkissoon A, Jugnundan P. 2001. Reactogenicity and immunogenicity of a single dose of a typhoid Vi polysaccharide vaccine in adolescents. BioDrugs 15(Suppl 1):21–26. 10.2165/00063030-200115001-00004 [DOI] [PubMed] [Google Scholar]
- 22. Liang K, Zeger S. 1986. Longitudinal data analysis using generalized linear models. Biometrika 73:13–22. 10.1093/biomet/73.1.13 [DOI] [Google Scholar]
- 23. Siler JF, Dunham GC. 1939. Duration of immunity conferred by typhoid vaccine: results of re-vaccination by intracutaneous injection of typhoid vaccine. Am. J. Public Health Nations Health 29:95–103. 10.2105/AJPH.29.2.95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gaines S, Landy M, Edsall G, Mandel AD, Trapani RJ, Benenson AS. 1961. Studies on infection and immunity in experimental typhoid fever. III. Effect of prophylactic immunization. J. Exp. Med. 114:327–342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lim SM, Jung HS, Kim MJ, Park DW, Kim WJ, Cheong HJ, Park SC, Lee KC, Shin YK, Tan HK, Kim SL, Sohn JW. 2007. Immunogenicity and safety of Vi capsular polysaccharide typhoid vaccine in healthy persons in Korea. J. Microbiol. Biotechnol. 17:611–615 [PubMed] [Google Scholar]
- 26. Tacket CO, Levine MM, Robbins JB. 1988. Persistence of antibody titres three years after vaccination with Vi polysaccharide vaccine against typhoid fever. Vaccine 6:307–308. 10.1016/0264-410X(88)90175-2 [DOI] [PubMed] [Google Scholar]
- 27. Cadoz M. 1998. Potential and limitations of polysaccharide vaccines in infancy. Vaccine 16:1391–1395. 10.1016/S0264-410X(98)00097-8 [DOI] [PubMed] [Google Scholar]
- 28. Keddy KH, Klugman KP, Hansford CF, Blondeau C, Bouveret le Cam NN. 1999. Persistence of antibodies to the Salmonella typhi Vi capsular polysaccharide vaccine in South African school children ten years after immunization. Vaccine 17:110–113. 10.1016/S0264-410X(98)00160-1 [DOI] [PubMed] [Google Scholar]
- 29. Gasem MH, Dolmans WM, Keuter MM, Djokomoeljanto RR. 2001. Poor food hygiene and housing as risk factors for typhoid fever in Semarang, Indonesia. Trop. Med. Int. Health 6:484–490. 10.1046/j.1365-3156.2001.00734.x [DOI] [PubMed] [Google Scholar]
- 30. Luby SP, Agboatwalla M, Feikin DR, Painter J, Billhimer W, Altaf A, Hoekstra RM. 2005. Effect of handwashing on child health: a randomised controlled trial. Lancet 366:225–233. 10.1016/S0140-6736(05)66912-7 [DOI] [PubMed] [Google Scholar]
- 31. Kossaczka Z, Lin FY, Ho VA, Thuy NT, Van Bay P, Thanh TC, Khiem HB, Trach DD, Karpas A, Hunt S, Bryla DA, Schneerson R, Robbins JB, Szu SC. 1999. Safety and immunogenicity of Vi conjugate vaccines for typhoid fever in adults, teenagers, and 2- to 4-year-old children in Vietnam. Infect. Immun. 67:5806–5810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Mai NL, Phan VB, Vo AH, Tran CT, Lin FY, Bryla DA, Chu C, Schiloach J, Robbins JB, Schneerson R, Szu SC. 2003. Persistent efficacy of Vi conjugate vaccine against typhoid fever in young children. N. Engl. J. Med. 349:1390–1391. 10.1056/NEJM200310023491423 [DOI] [PubMed] [Google Scholar]