Abstract
A Gram-negative pathogen Haemophilus influenzae has a truncated endotoxin known as lipooligosaccharide (LOS). Recent studies on H. influenzae LOS highlighted its structural and compositional implications for bacterial virulence; however, the role of LOS in the activation of innate and adaptive immunity is poorly understood. THP-1 monocytes were stimulated with either lipopolysaccharide (LPS) from Escherichia coli or LOS compounds derived from H. influenzae Eagan, Rd, and Rd lic1 lpsA strains. Cell surface expression of key antigen-presenting, costimulatory, and adhesion molecules, as well as gene expression of some cytokines and pattern recognition receptors, were studied. Eagan and Rd LOS had a lower capacity to induce the expression of ICAM-1, CD40, CD58, tumor necrosis factor alpha (TNF-α), and interleukin-1β (IL-1β) compared to LPS. In contrast, antigen-presenting (HLA-ABC or HLA-DR) and costimulatory (CD86) molecules and NOD2 were similarly upregulated in response to LOS and LPS. LOS from a mutant Rd strain (Rd lic1 lpsA) consistently induced higher expression of innate immune molecules than the wild-type LOS, suggesting the importance of phosphorylcholine and/or oligosaccharide extension in cellular responses to LOS. An LOS compound with a strong ability to upregulate antigen-presenting and costimulatory molecules combined with a low proinflammatory activity may be considered a vaccine candidate to immunize against H. influenzae.
INTRODUCTION
Haemophilus influenzae is a human-restricted Gram-negative pathogen commonly found in the upper respiratory tract. All H. influenzae strains are classified as either encapsulated or nonencapsulated (nontypeable [NTHI]), contingent on the expression of a polysaccharide capsule. Among six antigenically distinct encapsulated serotypes (a to f), type b (Hib) is the most virulent and was historically one of the leading causes of meningitis and other invasive infections in young children (1). Since the introduction of the Hib polysaccharide conjugate vaccine in the late 1980s, invasive diseases caused by Hib have been virtually eliminated in the countries where this vaccine became a part of the pediatric immunization program (2). However, the Hib vaccine does not prevent infections caused by non-type b strains. Recent studies have documented increased rates of invasive NTHI disease in North America and Europe, especially among the elderly and immunocompromised individuals (3, 4).
Although the polysaccharide capsule is the major virulence factor of H. influenzae (5), NTHI is a common causative agent of respiratory tract infections. It is one of the leading causes of otitis media (OM) in children, and exacerbations of chronic obstructive pulmonary disease (COPD) (6). High carriage rates of NTHI suggest its commensal nature; however, the specific mechanisms that mediate the transition of NTHI from a commensal to pathogen are poorly understood.
Haemophilus influenzae has been found to express an O-deacylated lipooligosaccharide (LOS) rather than lipopolysaccharide (LPS) typical for most Gram-negative bacteria. The LOS consists of a largely invariant triheptose oligosaccharide backbone covalently attached to a 3-deoxy-d-manno-oct-2-ulosonic acid moiety (Kdo) known as the core region. Similar to LPS, the core region is covalently linked to lipid A, which has endotoxic properties. The lipid A region is embedded in the outer membrane consisting of an acylated glucosamine disaccharide backbone. Lipid A is a cognate ligand for Toll-like receptor 4 (TLR4) and plays a key role in the activation of the inflammatory response (7). Despite the truncated structure, there is extensive inter- and intrastrain heterogeneity in the LOS glycoform populations; this is due to the variability of the oligosaccharide extensions that emanate from the triheptose core region, which can be attributed to the molecular phenomenon known as phase variation (8, 9).
Although H. influenzae LOS has been a subject of significant research, its role in activation of innate and adaptive immunity is poorly understood. An earlier study by Khair et al. found an increase in the expression of ICAM-1 and cytokines tumor necrosis factor alpha (TNF-α), interleukin-6 (IL-6), and IL-8 in human bronchial epithelial cells in response to LOS stimulation (10). It has also been found that LOS of Histophilus somni is able to engage the NF-κB transcription factor, a potent activator of the innate immune response (11). However, the capacity of LOS to induce key costimulatory and antigen-presenting molecules essential for the activation of adaptive immunity is unknown.
In this study, we assessed the immunostimulatory capacities of three LOS compounds derived from 2 different strains of H. influenzae (Fig. 1) by measuring the expression of key costimulatory and antigen-presenting molecules in human monocytic cells. Furthermore, the effect of LOS on proinflammatory and anti-inflammatory cytokine responses was measured. The results were compared to the effects of Escherichia coli LPS, which has been previously studied and served as a reference for data interpretation. An LOS compound with a strong ability to stimulate the expression of costimulatory and antigen-presenting molecules combined with a low potential to stimulate proinflammatory responses may be considered a potential vaccine candidate.
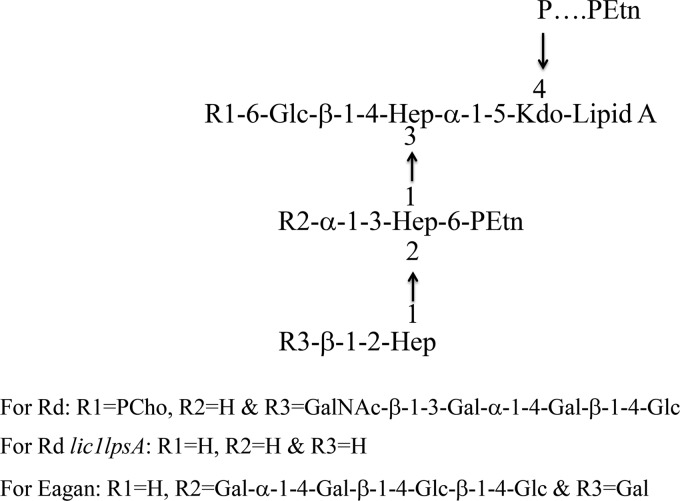
FIG 1.
Schematic representation of LOS from H. influenzae strains Eagan, Rd, and Rd lic1 lpsA as determined by MALDI. Represented in the LOS structure are 2-keto-3-deoxyoctulosonic acid (Kdo), heptose (Hep), glucose (Glc), galactose (Gal), N-acetylgalactosamine (GalNac), phosphate (P), phosphorylcholine (PCho), and phosphoethanolamine (PEtn).
MATERIALS AND METHODS
Cell culture conditions.
Human THP-1 monocytic leukemia cell line was obtained from ATCC (TIB-202) and stored in liquid nitrogen until thawed for culturing. Cells were maintained at 37°C in 5% CO2 in RPMI 1640 medium (Sigma-Aldrich, Oakville, Ontario, Canada) supplemented with 10% heat-inactivated fetal bovine serum (SAFC Biosciences, Lenexa, KS) and 200 μl antibiotic-antimycotic (Life Technologies, Inc., Burlington, Ontario, Canada). Cells were passaged when the culture density reached 1.0 ×106 cells/ml; viability was determined by the trypan blue exclusion method using a ViCell XR cell viability analyzer (Beckman Coulter, Brea, CA).
Purification of LOS and analysis.
LOS compounds were purified from H. influenzae strains Eagan (Hib), Rd (unencapsulated type d), and Rd lic1 lpsA (mutant) (Fig. 1) as previously described (12). The Eagan LOS was derived as described previously (13). The Rd LOS was derived from the genetically sequenced strain RM 118, and two individual mutants of the Rd strain were combined into a double mutant, Rd lic1 lpsA, which was grown and purified at the National Research Council of Canada (14). Profiling of fatty acid additions to lipid A was done via matrix-assisted laser desorption ionization (MALDI) analysis (12). E. coli LPS O111:B4 was obtained from Sigma-Aldrich. All compounds were solubilized in sterile distilled H2O at a concentration of 1 mg/ml, aliquoted, and stored at −20°C until use.
Cell stimulation and flow cytometry analysis.
THP-1 cells were plated at 0.5 × 106 cells/2 ml/well in 12-well plates (Fisher Scientific, Fairlawn, NJ), incubated in 37°C in 5% CO2 for 24 h, and stimulated with LOS compounds at concentrations of 1, 5, 10, and 15 μg/ml for an additional 24 h at 37°C in 5% CO2. Following stimulation, the cells were washed once with sterile phosphate-buffered saline (PBS), centrifuged at 400 × g for 10 min, and resuspended in PBS supplemented with 1% bovine serum albumin (Sigma-Aldrich). Cells were immunostained with fluorochrome-conjugated antibodies against CD54 (ICAM-1), CD40, CD86 (B7-2) (BD Biosciences, Mississauga, Ontario, Canada), CD58 (LFA-3) (Cedarlane, Burlington, Ontario, Canada), HLA-DR (major histocompatibility complex [MHC] class II) (Biolegend, San Diego, CA), or mouse IgG1 isotype control at a concentration of 2.5 μg/ml. Primary unconjugated HLA-ABC (MHC class I) antibody (BD Biosciences) was added to the cell suspension at a concentration of 5 μg/ml. All samples were incubated in the dark for 1 h at 4°C. Samples with directly conjugated primary antibodies were washed once with PBS, centrifuged at 1,000 × g for 5 min, and resuspended in 500 μl PBS for flow cytometry analysis. Samples with unconjugated primary antibodies were washed as described above, resuspended in 1% bovine serum albumin (BSA), and incubated with secondary fluorescein isothiocyanate (FITC)-IgG1 rat anti-mouse antibody (BD Biosciences) at a concentration of 2.5 μg/ml for 1 h at 4°C. Following incubation with the secondary antibody, samples were washed with PBS and resuspended in 500 μl PBS for flow cytometry analysis. All samples were analyzed using FACSCalibur with CELLQUEST PRO software (BD Biosciences), acquiring 15,000 total events. The results were expressed as the mean fluorescence intensity (MFI) on FL-1 and FL-2 channels for FITC- and phycoerythrin (PE)-conjugated antibodies, respectively.
Gene expression analysis.
RNA was isolated from THP-1 cells using an Aurum total RNA minikit (Bio-Rad, Hercules, CA) following stimulation with LPS or LOS compounds for 4 h. RNA integrity was determined using the Experion system (Bio-Rad); products with “RNA quality indicator” values greater than 9 were used for downstream applications. Five hundred nanograms of total RNA was reverse transcribed using the First Strand cDNA synthesis kit (Fisher Scientific) as per the manufacturer's instructions. Twenty-five nanograms of the synthesized cDNA was used for each reaction well, including 10 μl of RT2 SYBR green Fluor quantitative PCR (qPCR) Mastermix (Qiagen, Toronto, Ontario, Canada), and 1 μl of the primer sets' TNF-α, IL-1β, IL-10, TLR4, NOD1, and NOD2 (SA Biosciences, Mississauga, Ontario, Canada) for a total reaction volume of 20 μl. PCR was performed using the iQ5 real-time PCR detection systems (Bio-Rad); samples were preheated at 95°C for 10 min followed by 40 cycles of the two-step cycling program. The denaturing temperature was set for 15 s at 95°C followed by 1 min at 60°C, where annealing, fluorescence detection, and elongation occurred. The melt curve program immediately followed from 55°C to 95°C with plate reads every 0.5°C. The cycle threshold (CT) values were used to compare relative amounts of measured transcripts, calculated as ΔCT = 2−(CT target gene – CT housekeeping gene). To account for variability in the starting template amount this expression was normalized to housekeeping gene Peptidylprolyl isomerase B (PPIB) and relative gene expression was quantified and calculated as ΔΔCT = 2−(ΔCT experimental – ΔCT control).
Statistical analysis.
Data are expressed as means of 3 independent experiments. Statistical significance was determined (GraphPad Prism 5.0, La Jolla, CA) using one-way analysis of variance (ANOVA) with Newman-Keuls multiple comparison post hoc test. P values of <0.05 were considered significant.
RESULTS
Characterization of lipid A of LOS isoforms by matrix-assisted laser desorption ionization analysis.
The lipid A structure consists of an invariant diphosphorylated glucosamine disaccharide with a varying number of acyl chains. The MALDI analysis was performed on the lipid A of each LOS compound, and the relative intensity of acylation was determined (Table 1). The major ion with the highest intensity for each compound was given an arbitrary unit value of 1.0; all other ions were expressed relative to that. For each compound, lipid A structures acylated with 4 fatty acid chains exhibited the highest intensity. LOS from the Eagan and Rd strains had comparable ion intensities for all fatty acid additions; however, Rd lic1 lpsA LOS exhibited different intensities at both 3 and 6 fatty acid additions at 0.25 and 0.7, respectively. This suggests that there is a larger population of 6-acylated lipid A structures in LOS Rd lic1 lpsA and a smaller population of 3-acylated lipid A compared to both Eagan and Rd LOS compounds.
TABLE 1.
MALDI data expressed as relative intensity of lipid A molecule from LOS compounds derived from the Eagan, Rd, and Rd lic1 lpsA strains
| Strain | [M-H]− (m/z) | Relative intensity | Proposed compositiona | No. of acylations |
|---|---|---|---|---|
| Eagan | 1,161.7 | 0.9 | 2 GlcN, 2 P, 2 C14:0 3-OH, 1 C14:0 | 3 |
| 1,387.9 | 1 | 2 GlcN, 2 P, 3 C14:0 3-OH, 1 C14:0 | 4 | |
| 1,598.1 | 0.2 | 2 GlcN, 2 P, 3 C14:0 3-OH, 2 C14:0 | 5 | |
| 1,824.3 | 0.3 | 2 GlcN, 2 P, 4 C14:0 3-OH, 2 C14:0 | 6 | |
| Rd | 1,161.7 | 0.7 | 2 GlcN, 2 P, 2 C14:0 3-OH, 1 C14:0 | 3 |
| 1,387.9 | 1 | 2 GlcN, 2 P, 3 C14:0 3-OH, 1 C14:0 | 4 | |
| 1,598.1 | 0.1 | 2 GlcN, 2 P, 3 C14:0 3-OH, 2 C14:0 | 5 | |
| 1,824.3 | 0.2 | 2 GlcN, 2 P, 4 C14:0 3-OH, 2 C14:0 | 6 | |
| Rd lic1 lpsA | 1,161.7 | 0.25 | 2 GlcN, 2 P, 2 C14:0 3-OH, 1 C14:0 | 3 |
| 1,387.9 | 1 | 2 GlcN, 2 P, 3 C14:0 3-OH, 1 C14:0 | 4 | |
| 1,598.1 | 0.1 | 2 GlcN, 2 P, 3 C14:0 3-OH, 2 C14:0 | 5 | |
| 1,824.3 | 0.7 | 2 GlcN, 2 P, 4 C14:0 3-OH, 2 C14:0 | 6 |
The following are represented in lipid A: glucosamine (GlcN), phosphate (P), and C14:0 3-OH and C14:0 fatty acid chains.
Haemophilus influenzae LOS exhibits decreased immunostimulatory abilities compared to E. coli LPS.
The capacity of LOS from H. influenzae to activate the innate immune response or induce costimulatory signals for adaptive immunity has not been studied. To elucidate this question, cell surface expression of CD54 (ICAM-1), CD40, and CD58 (LFA-3) was measured on THP-1 monocytic cells in response to LOS stimulation by flow cytometry analysis. LPS from E. coli strain O111:B4, a potent innate immune activator served as a positive control (15). Unstimulated THP-1 cells were used as a negative control.
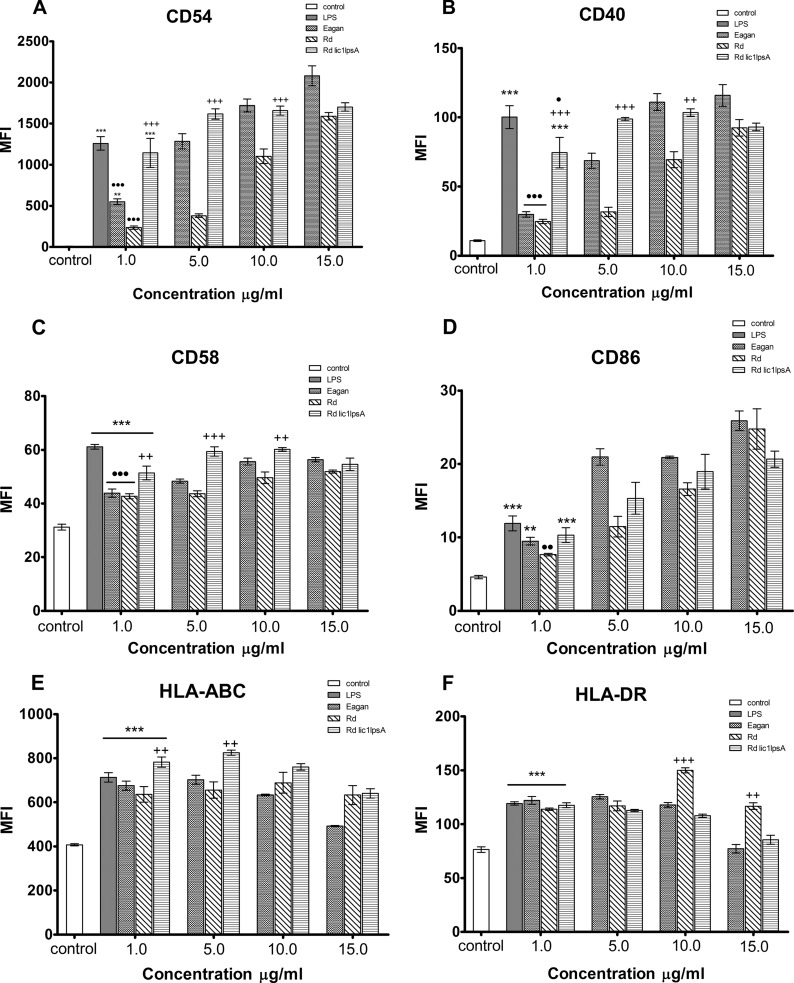
The cell surface expression of ICAM-1 was significantly upregulated in response to stimulation with both Eagan and Rd lic1 lpsA LOS compounds at 1 μg/ml (Fig. 2A) (P < 0.01 and P < 0.001, respectively). LOS from the wild-type Rd strain did not induce a significant increase over the negative control at 1 μg/ml; however, a positive dose response was observed at higher concentrations (5 to 15 μg/ml). Both Rd and Eagan LOS compounds induced lower expression of ICAM-1 compared to E. coli LPS at 1 μg/ml (P < 0.001); LOS of the Rd lic1 lpsA induced ICAM-1 expression comparable with the effect of LPS. The effect of the Rd lic1 lpsA LOS was consistently higher than that of the wild-type Rd LOS (up to 10 μg/ml). Similar results were observed with regard to the expression of CD40 and CD58 (Fig. 2B and C), where LOS compounds from both Eagan and Rd strains induced significantly less expression than LPS, whereas Rd lic1 lpsA LOS induced comparable expression.
FIG 2.
Flow cytometry analysis of costimulatory and antigen-presenting molecule expression in response to LOS stimulation on THP-1 cells. The negative control was untreated medium alone, and the positive control was LPS stimulation at 1 μg/ml. THP-1 cells were stimulated with LOS compounds for 24 h at concentrations of 1, 5, 10, and 15 μg/ml, and cell surface expression of CD54 (A), CD40 (B), CD58 (C), CD86 (D) HLA-ABC (E), and HLA-DR (F) was measured. Data represent the mean fluorescence intensity (MFI) ± the standard error of the mean (SEM) (n = 3). Significant differences are indicated as follows: *, P < 0.05 compared to the unstimulated control; +, P < 0.05 between Rd lic1 lpsA and Rd wild-type compounds; ●, P < 0.05 between LPS and LOS compounds.
In summary, LOS from various strains of H. influenzae induced significant upregulation of the studied cell surface molecules on a human monocytic cell line compared to unstimulated cells. The Rd and Eagan LOS compounds induced significantly less expression of ICAM-1, CD40, and CD58 compared to LPS, whereas Rd lic1 lpsA induced expression comparable to the effect of LPS. The data suggest that LOS of H. influenzae exhibits a lower immunostimulatory potential that may be dependent on the LOS structure.
LOS significantly upregulates antigen-presenting and costimulatory molecules.
To address whether LOS would activate the adaptive immune response, the expression of antigen-presenting molecules, HLA-ABC (MHC class I) and HLA-DR (MHC class II), as well as the major costimulatory molecule in the T cell–antigen-presenting cell axis, CD86 (B7-2), was measured in THP-1 cells stimulated with either H. influenzae LOS or E. coli LPS.
The CD86 expression was significantly upregulated in response to Eagan and Rd lic1 lpsA LOS compounds used at the concentration of 1 μg/ml. For all compounds, a positive dose response was observed (Fig. 2D). With exception of Rd LOS, there was no observed difference in expression between LPS and LOS compounds at 1 μg/ml; furthermore, LOS from Rd lic1 lpsA did not confer an increased expression of CD86 compared to the wild type, even at higher concentrations.
LOS compounds from Eagan, Rd, and Rd lic1 lpsA induced significant expression of HLA-ABC and HLA-DR that was comparable to that of the positive-control LPS at 1 μg/ml (Fig. 2E and F) (P < 0.001). At higher concentrations of all compounds (5 to 15 μg/ml), a plateau effect was observed.
Our findings show that LOS of H. influenzae is able to significantly increase expression of both the antigen-presenting molecules and B7-2 that is similar to the effect of LPS, suggesting that LOS can act as a potent activator of the adaptive immune response.
Haemophilus influenzae LOS has decreased capacity to activate proinflammatory response.
To further address the immunostimulatory capacity of LOS, gene expression of proinflammatory cytokines TNF-α and IL-1β was measured following 4 h of stimulation at 0.1 and 1 μg/ml. LPS from E. coli was used as a positive control. Gene expression of anti-inflammatory cytokine IL-10 was also measured via real-time PCR. Gene expression was normalized to the housekeeping gene coding for PPIB, and the results are presented as fold change relative to the unstimulated control.
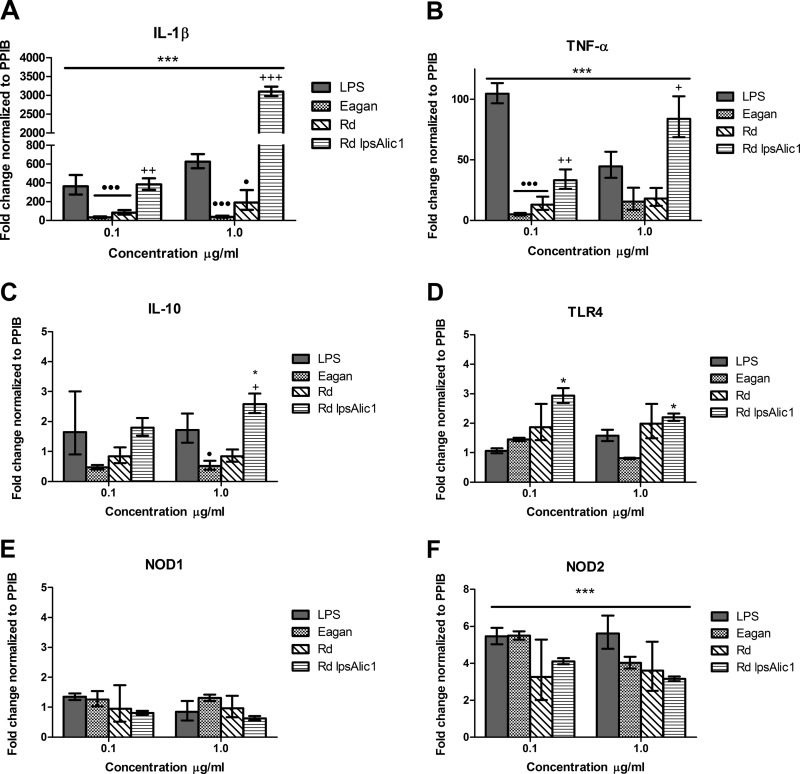
All LOS compounds induced significant upregulation of proinflammatory cytokines TNF-α and IL-1β (Fig. 3A and B) (P < 0.001). However, similar to the effect on ICAM-1, CD40, and CD58, LOS of the Eagan and Rd strains induced significantly less expression of both TNF-α and IL-1β compared to LPS. In contrast, Rd lic1 lpsA LOS stimulation resulted in a 5-fold increase in IL-1β expression compared to LPS at a concentration of 1 μg/ml. Furthermore, Rd lic1 lpsA LOS stimulation at the concentrations of 0.1 and 1 μg/ml resulted in consistently higher gene expression of both proinflammatory cytokines compared to the wild type.
FIG 3.
Relative gene expression of THP-1 cells in response to LOS stimulation. THP-1 cells were stimulated with LOS compounds for 4 h at concentrations of 0.1 and 1 μg/ml. RNA was extracted, and genetic expression was measured using real-time PCR and is presented as fold change relative to the unstimulated control. Expression of the genes coding for IL-1β (A), TNF-α (B), IL-10 (C), TLR4 (D), NOD1 (E), and NOD2 (F) was measured. Data represent means ± SEM (n = 3). Significant differences are indicated as follows: *, P < 0.05 compared to unstimulated control; +, P < 0.05 between Rd lic1 lpsA and Rd wild-type compounds; ●, P < 0.05 between LPS and LOS compounds.
Out of all LOS compounds, only Rd lic1 lpsA LOS was able to induce a significant upregulation of the anti-inflammatory cytokine IL-10 gene expression (P < 0.05) (Fig. 3C). Both Eagan and Rd LOS induced less expression of IL-10 than LPS; however, only the effect of Eagan LOS was statistically significant (P < 0.05).
Our findings show Eagan and Rd LOS compounds were able to elicit transcriptional upregulation of key proinflammatory cytokines TNF-α and IL-1β; however, the response was significantly attenuated compared to that of E. coli LPS. Rd lic1 lpsA LOS was shown to consistently induce higher cytokine gene expression compared to the wild type or LPS at 1 μg/ml.
H. influenzae LOS induces upregulation of intracellular pattern recognition receptors.
In addition to inflammatory markers, we wanted to address whether LOS of H. influenzae would affect the genetic regulation of pattern recognition receptors (PRRs), such as TLR4, the cognate LOS receptor, and intracellular PRRs, such as NOD1 and NOD2. Gene expression was determined as described above.
TLR4 gene expression in THP-1 cells did not significantly change upon stimulation with any compound, except for that from Rd lic1 lpsA at the concentrations of 0.1 and 1 μg/ml (P < 0.05) (Fig. 3D). The gene expression of NOD1 was not affected by any of the compounds (Fig. 3E), whereas NOD2 expression was significantly upregulated in response to all compounds at both concentrations (P < 0.001) (Fig. 3F).
Our findings show that NOD2 expression was increased by 4- to 6-fold with no observed differences between LPS or LOS stimulation. This suggests a possible relationship between the recognition of LOS and further downstream activation of the inflammasome complex.
DISCUSSION
Although invasive disease caused by H. influenzae has decreased considerably since the introduction of Hib conjugate vaccines, there is a concern about emerging infections caused by non-type b serotypes. This can partly be explained by the serotype replacement phenomenon where there is less competition for non-type b strains to colonize the ecological niche once occupied by Hib (16). Furthermore, there is a large body of work that corroborates the propensity of non-type b H. influenzae, namely, NTHI, to cause disease (6, 17–21).
H. influenzae lipooligosaccharide and its role in virulence have been of particular interest, especially in NTHI strains, where the effect of LOS may be more prevalent than in encapsulated strains. Interestingly, an earlier study had shown that LOS composition contributed to the pathogenesis of invasive infection caused by encapsulated H. influenzae (22), suggesting that the role of LOS as a virulence factor may not be limited to NTHI. Several studies have highlighted the role of LOS in bacterial virulence—i.e., in complement resistance (9, 23, 24), adherence (21), and host mimicry mechanisms (19, 20).
More importantly, LOS is invariably present in all H. influenzae strains irrespective of their serotype; thus, it has the potential to be considered a vaccine candidate to prevent infections caused by various strains, including NTHI. It is noteworthy that there have been earlier studies that investigated the potential use of LOS-conjugate-based vaccines; to our knowledge, clinical trials were not conducted beyond phase I (25). With increasing incidences of invasive disease caused by NTHI, there is a growing need for a vaccine to prevent this infection (26). Additionally, it is unknown whether LOS derived from different strains of H. influenzae may have different immunostimulatory activities. The composition of LOS may have significant implications for its immunostimulatory capacity and therefore the overall efficacy of a vaccine. Moreover, lipid A of LOS can potentially act as its own vaccine adjuvant as well as a general adjuvant that may be a more suitable candidate than current monophosphoryl lipid A (MPL) adjuvant. Although in the past, inclusion of lipid A in a vaccine was considered to be associated with an unacceptable rate of side effects, more recent work, however, suggests that the toxicity of lipid A largely depends on the species of bacteria; moreover, some chemical modifications may result in nontoxic compounds with high adjuvant activity (15, 27). Studies have shown that the monophosphorylated nature of the MPL compound selectively activates the TRIF pathway of the TLR4 signaling cascade (28, 29). However, it may be more beneficial for a lipid A compound to activate both TRIF and MyD88 pathways without the toxic proinflammatory side effects.
Our findings show that LOS derived from the Eagan and Rd strains of H. influenzae were able to elicit an in vitro innate immune response resulting in the upregulation of costimulatory and antigen-presenting molecules HLA-ABC, HLA-DR, and CD86, comparable to that of LPS. These surface molecules play critical roles in the priming of naive T cells essential for the activation of the adaptive immune response.
We also found that Eagan and Rd LOS had a decreased capacity to stimulate the surface expression of CD40, CD58, and ICAM-1, as well as gene expression of TNF-α and IL-1β, compared to LPS. ICAM-1, TNF-α, and IL-1β are critical mediators of inflammation in which high expression has been correlated with potent proinflammatory responses via the recruitment of immune cells and the propagation of inflammatory cytokines (30, 31). This suggests that Eagan and Rd LOS elicit a decreased proinflammatory effect compared to LPS, making them attractive candidates for vaccine development.
All compounds were found to significantly upregulate NOD2, which has been found to directly interact with NLRP1, NLRP3, and NLRP12, inflammasomes (32). In contrast, NOD1 was unaffected by either LOS or LPS; indeed, no relationship between the inflammasome complexes and NOD1 have been previously identified (32). The exact role of NOD2 in bacterial infections has yet to be fully elucidated; however, the activation of NOD2 has been implicated in T cell differentiation and host response to pulmonary infection (32). This suggests that the role of LOS during infection may not be limited to just the inflammatory response.
To address whether LOS stimulation had a toxic effect on the cells at various concentrations, cell viability was measured. It was found that LOS had no effect on viability with exception of the highest concentration, i.e., 15 μg/ml (Table 2). However, in our experiments, this concentration did not have any significant biological impact; a plateau effect was observed for the majority of surface molecules at 10 μg/ml.
TABLE 2.
THP-1 cell viability following treatment with Eagan, Rd, and Rd lic1 lpsA LOS after 24 h
| Concn (μg/ml) | % viability after treatmenta |
||||
|---|---|---|---|---|---|
| Controlb | LPS | LOS |
|||
| Eagan | Rd | Rd lic1 lpsA | |||
| 0 | 97.2 ± 1.5 | ||||
| 1 | 97.5 ± 1.4 | 97.2 ± 0.6 | 97.9 ± 0.5 | 97.6 ± 1.5 | |
| 5 | 96.7 ± 1.6 | 97.3 ± 1.7 | 97.27 ± 1.6 | ||
| 10 | 94.6 ± 2.6 | 97.3 ± 0.6 | 93.3 ± 0.6 | ||
| 15 | 81.47 ± 6.4 | 84.9 ± 3.2 | 81.6 ± 5.4 | ||
Viability was determined by the trypan blue exclusion test. Results are means ± standard deviations (n = 3).
Unstimulated.
Rd lic1 lpsA LOS was found to consistently induce a stronger effect than the wild type on the surface molecules ICAM-1, CD40, and CD58 and proinflammatory cytokines TNF-α and IL-1β. The main compositional differences between Rd lic1 lpsA and Rd are the deletions of phosphorycholine (PCho) at heptose I and oligosaccharide extension at heptose III (Fig. 1). Previous work has shown that the loss of this oligosaccharide extension in this strain resulted in a decrease in complement resistance (24); our findings add to this by suggesting that the oligosaccharide extension may also have a role in attenuating the expression of aforementioned inflammatory markers, as can be observed by the wild-type Rd LOS. Moreover, PCho has been implicated in numerous studies as a virulence factor and adhesion molecule that enhances immune evasion by H. influenzae (21, 33). Whether PCho also has a role in reducing the inflammatory response in vivo has yet to be elucidated.
The number of acyl chains on the lipid A of each compound is of interest and should be addressed. Although Eagan and Rd lipid A structures exhibited some variability, Rd lic1 lpsA LOS was found to have markedly less triacylated lipid A isoforms and considerably larger amounts of hexa-acylated lipid A compared to both Eagan and Rd lipid A (Table 1). As all compounds were prepared and isolated identically, the observed variability between Eagan and Rd lipid A may be a reflection of natural heterogeneity between the strains. This does not, however, explain the significant difference in acylation between Rd and Rd lic1 lpsA; this suggests the possible role of the lic1 and/or lpsA mutations to affect acylation. While the specific mechanism is unknown, we speculate the Rd lic1 lpsA mutant compensates for these deletions with acylation. Previous studies have shown that the immunostimulatory abilities of LPS molecules are determined by the structure of lipid A (34). Furthermore, a correlation can be found with acylation of lipid A and an inflammatory response, where increased acylation is generally associated with more potent inflammatory responses and low acylation is associated with a weaker response (27). This suggests that the proinflammatory capacity of Rd lic1 lpsA LOS observed in our experiments may also be due to the acylation state of its lipid A. Thus, further research on Rd lic1 lpsA LOS is warranted to determine how much of the observed immunostimulatory effect is attributed to acylation and/or compositional motifs.
It is important to note that LOS compounds isolated from NTHI strains were not used in our model; however, structural analysis of purified LOS of the 375 NTHI otitis media isolate shows an almost identical composition to that of the Eagan LOS (35). Furthermore, preliminary results show the NTHi LOS' immunostimulatory capacity was analogous with that of Eagan and Rd LOS compounds (unpublished observations). This suggests that the LOS structure of H. influenzae, regardless of encapsulation, has an intrinsically lower immunostimulatory potential than the toxic LPS of E. coli.
The balance between inflammatory and anti-inflammatory cytokines has been well described. It has been found that a strong inflammatory response is correlated with a strong anti-inflammatory response in order to maintain equilibrium (36). Unlike other LOS compounds, Rd lic1 lpsA LOS alone was able to significantly increase IL-10 expression, which was expected since it also induced the most proinflammatory markers out of all the LOS compounds.
In conclusion, we have shown that Eagan and Rd LOS of H. influenzae are able to induce the upregulation of key costimulatory and antigen-presenting molecules in human monocytic cells, as well as the production of inflammatory cytokines. Although these compounds were able to stimulate the expression of proinflammatory markers, their effect was markedly diminished in comparison to the toxic LPS compound of E. coli. Furthermore, all LOS compounds were found to upregulate NOD2, which has further downstream implications for the inflammasome complexes. The fact that LOS is invariably present in all H. influenzae strains makes it an attractive candidate for vaccine development. Furthermore, the moderate immunostimulatory ability of LOS may be of consideration for potential adjuvant development and may offer a more robust response than current TLR4 agonist adjuvants.
ACKNOWLEDGMENTS
This work was supported by a National Science and Engineering Research Council (NSERC) Discovery Grant and Northern Ontario School of Medicine (NOSM) Faculty Association Research Development Award to M.U.
We thank Simon Lees for kind help with optimization of gene expression methodology.
Footnotes
Published ahead of print 26 March 2014
REFERENCES
- 1. Turk DC. 1984. The pathogenicity of Haemophilus influenzae. J. Med. Microbiol. 18:1–16. 10.1099/00222615-18-1-1 [DOI] [PubMed] [Google Scholar]
- 2. Kelly DF, Moxon ER, Pollard AJ. 2004. Haemophilus influenzae type b conjugate vaccines. Immunology 113:163–174. 10.1111/j.1365-2567.2004.01971.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ladhani S, Slack MP, Heath PT, von Gottberg A, Chandra M, Ramsay ME, European Union Invasive Bacterial Infection Surveillance 2010. Invasive Haemophilus influenzae disease, Europe, 1996–2006. Emerg. Infect. Dis. 16:455–463. 10.3201/eid1603.090290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Dworkin MS, Park L, Borchardt SM. 2007. The changing epidemiology of invasive Haemophilus influenzae disease, especially in persons ≥65 years old. Clin. Infect. Dis. 44:810–816. 10.1086/511861 [DOI] [PubMed] [Google Scholar]
- 5. Moxon ER, Vaughn KA. 1981. The type b capsular polysaccharide as a virulence determinant of Haemophilus influenzae: studies using clinical isolates and laboratory transformants. J. Infect. Dis. 143:517–524. 10.1093/infdis/143.4.517 [DOI] [PubMed] [Google Scholar]
- 6. Erwin AL, Smith AL. 2007. Nontypeable Haemophilus influenzae: understanding virulence and commensal behavior. Trends Microbiol. 15:355–362. 10.1016/j.tim.2007.06.004 [DOI] [PubMed] [Google Scholar]
- 7. Lee MS, Kim Y-J. 2007. Signaling pathways downstream of pattern-recognition receptors and their cross talk. Annu. Rev. Biochem. 76:447–480. 10.1146/annurev.biochem.76.060605.122847 [DOI] [PubMed] [Google Scholar]
- 8. Swords WE, Jones PA, Apicella MA. 2003. The lipo-oligosaccharides of Haemophilus influenzae: an interesting array of characters. J. Endotoxin Res. 9:131–144. 10.1177/09680519030090030101 [DOI] [PubMed] [Google Scholar]
- 9. Schweda EK, Richards JC, Hood DW, Moxon ER. 2007. Expression and structural diversity of the lipopolysaccharide of Haemophilus influenzae: implication in virulence. Int. J. Med. Microbiol. 297:297–306. 10.1016/j.ijmm.2007.03.007 [DOI] [PubMed] [Google Scholar]
- 10. Khair OA, Devalia JL, Abdelaziz MM, Sapsford RJ, Tarraf H, Davies RJ. 1994. Effect of Haemophilus influenzae endotoxin on the synthesis of IL-6, IL-8, TNF-alpha and expression of ICAM-1 in cultured human bronchial epithelial cells. Eur. Respir. J. 7:2109–2116. 10.1183/09031936.94.07122109 [DOI] [PubMed] [Google Scholar]
- 11. Howard MD, Willis L, Wakarchuk W, St. Michael F, Cox A, Horne WT, Hontecillas R, Bassaganya-Riera J, Lorenz E, Inzana TJ. 2011. Genetics and molecular specificity of sialylation of Histophilus somni lipooligosaccharide (LOS) and the effect of LOS sialylation on Toll-like receptor-4 signaling. Vet. Microbiol. 153:163–172. 10.1016/j.vetmic.2011.02.054 [DOI] [PubMed] [Google Scholar]
- 12. Zhou P, Altman E, Perry MB, Li J. 2010. Study of matrix additives for sensitive analysis of lipid A by matrix-assisted laser desorption ionization mass spectrometry. Appl. Environ. Microbiol. 76:3437–3443. 10.1128/AEM.03082-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Weiser JN, Maskell DJ, Butler PD, Lindberg AA, Moxon ER. 1990. Characterization of repetitive sequences controlling phase variation of Haemophilus influenzae lipopolysaccharide. J. Bacteriol. 172:3304–3309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hood DW, Cox AD, Wakarchuk WW, Schur M, Schweda EK, Walsh SL, Deadman ME, Martin A, Moxon ER, Richards JC. 2001. Genetic basis for expression of the major globotetraose-containing lipopolysaccharide from H. influenzae strain Rd (RM118). Glycobiology 11:957–967. 10.1093/glycob/11.11.957 [DOI] [PubMed] [Google Scholar]
- 15. Lewicky JD, Ulanova M, Jiang ZH. 2011. Synthesis of a dimeric monosaccharide lipid A mimic and its synergistic effect on the immunostimulatory activity of lipopolysaccharide. Carbohydr. Res. 346:1705–1713. 10.1016/j.carres.2011.05.018 [DOI] [PubMed] [Google Scholar]
- 16. Berndsen MR, Erlendsdottir H, Gottfredsson M. 2012. Evolving epidemiology of invasive Haemophilus infections in the post-vaccination era: results from a long-term population-based study. Clin. Microbiol. Infect. 18:918–923. 10.1111/j.1469-0691.2011.03700.x [DOI] [PubMed] [Google Scholar]
- 17. Hong W, Peng D, Rivera M, Gu XX. 2010. Protection against nontypeable Haemophilus influenzae challenges by mucosal vaccination with a detoxified lipooligosaccharide conjugate in two chinchilla models. Microbes Infect. 12:11–18. 10.1016/j.micinf.2009.09.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Schachern PA, Tsuprun V, Wang B, Apicella MA, Cureoglu S, Paparella MM, Juhn SK. 2009. Effect of lipooligosaccharide mutations of Haemophilus influenzae on the middle and inner ears. Int. J. Pediatr. Otorhinolaryngol. 73:1757–1760. 10.1016/j.ijporl.2009.09.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Johnston JW, Zaleski A, Allen S, Mootz JM, Armbruster D, Gibson BW, Apicella MA, Munson RS., Jr 2007. Regulation of sialic acid transport and catabolism in Haemophilus influenzae. Mol. Microbiol. 66:26–39. 10.1111/j.1365-2958.2007.05890.x [DOI] [PubMed] [Google Scholar]
- 20. Bouchet V, Hood DW, Li J, Brisson JR, Randle GA, Martin A, Li Z, Goldstein R, Schweda EK, Pelton SI, Richards JC, Moxon ER. 2003. Host-derived sialic acid is incorporated into Haemophilus influenzae lipopolysaccharide and is a major virulence factor in experimental otitis media. Proc. Natl. Acad. Sci. U. S. A. 100:8898–8903. 10.1073/pnas.1432026100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Swords WE, Buscher BA, Ver Steeg Ii K, Preston A, Nichols WA, Weiser JN, Gibson BW, Apicella MA. 2000. Non-typeable Haemophilus influenzae adhere to and invade human bronchial epithelial cells via an interaction of lipooligosaccharide with the PAF receptor. Mol. Microbiol. 37:13–27. 10.1046/j.1365-2958.2000.01952.x [DOI] [PubMed] [Google Scholar]
- 22. Zwahlen A, Rubin LG, Moxon ER. 1986. Contribution of lipopolysaccharide to pathogenicity of Haemophilus influenzae: comparative virulence of genetically-related strains in rats. Microb. Pathog. 1:465–473. 10.1016/0882-4010(86)90008-2 [DOI] [PubMed] [Google Scholar]
- 23. Langereis JD, Stol K, Schweda EK, Twelkmeyer B, Bootsma HJ, de Vries SP, Burghout P, Diavatopoulos DA, Hermans PW. 2012. Modified lipooligosaccharide structure protects nontypeable Haemophilus influenzae from IgM-mediated complement killing in experimental otitis media. mBio 3(4):e00079–12. 10.1128/mBio.00079-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Griffin R, Cox AD, Makepeace K, Richards JC, Moxon ER, Hood DW. 2005. Elucidation of the monoclonal antibody 5G8-reactive, virulence-associated lipopolysaccharide epitope of Haemophilus influenzae and its role in bacterial resistance to complement-mediated killing. Infect. Immun. 73:2213–2221. 10.1128/IAI.73.4.2213-2221.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gu XX, Rudy SF, Chu C, McCullagh L, Kim HN, Chen J, Li J, Robbins JB, Van Waes C, Battey JF. 2003. Phase I study of a lipooligosaccharide-based conjugate vaccine against nontypeable Haemophilus influenzae. Vaccine 21:2107–2114. 10.1016/S0264-410X(02)00768-5 [DOI] [PubMed] [Google Scholar]
- 26. Bamberger EE, Ben-Shimol S, Abu Raya B, Katz A, Givon-Lavi N, Dagan R, Srugo I, Israeli Pediatric Bacteremia and Meningitis Group 20 January 2014. Pediatric invasive Haemophilus influenzae infections in Israel in the era of Haemophilus influenzae type b vaccine: a nationwide prospective study. Pediatr. Infect. Dis. J. 10.1097/INF.0000000000000193 [DOI] [PubMed] [Google Scholar]
- 27. Maaetoft-Udsen K, Vynne N, Heegaard PM, Gram L, Frokiaer H. 2013. Pseudoalteromonas strains are potent immunomodulators owing to low-stimulatory LPS. Innate Immun. 19:160–173. 10.1177/1753425912455208 [DOI] [PubMed] [Google Scholar]
- 28. Mata-Haro V, Cekic C, Martin M, Chilton PM, Casella CR, Mitchell TC. 2007. The vaccine adjuvant monophosphoryl lipid A as a TRIF-biased agonist of TLR4. Science 316:1628–1632. 10.1126/science.1138963 [DOI] [PubMed] [Google Scholar]
- 29. Bowen WS, Minns LA, Johnson DA, Mitchell TC, Hutton MM, Evans JT. 2012. Selective TRIF-dependent signaling by a synthetic Toll-like receptor 4 agonist. Sci. Signal. 5:ra13. 10.1126/scisignal.2001963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lisby S, Ralfkiaer E, Rothlein R, Vejlsgaard GL. 1989. Intercellular adhesion molecule-1 (ICAM-1) expression correlated to inflammation. Br. J. Dermatol. 120:479–484. 10.1111/j.1365-2133.1989.tb01320.x [DOI] [PubMed] [Google Scholar]
- 31. Cromwell O, Hamid Q, Corrigan CJ, Barkans J, Meng Q, Collins PD, Kay AB. 1992. Expression and generation of interleukin-8, IL-6 and granulocyte-macrophage colony-stimulating factor by bronchial epithelial cells and enhancement by IL-1 beta and tumour necrosis factor-alpha. Immunology 77:330–337 [PMC free article] [PubMed] [Google Scholar]
- 32. Moreira LO, Zamboni DS. 2012. NOD1 and NOD2 signaling in infection and inflammation. Front. Immunol. 3:328. 10.3389/fimmu.2012.00328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Clark SE, Snow J, Li J, Zola TA, Weiser JN. 2012. Phosphorylcholine allows for evasion of bactericidal antibody by Haemophilus influenzae. PLoS Pathog 8:e1002521. 10.1371/journal.ppat.1002521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Schromm AB, Brandenburg K, Loppnow H, Moran AP, Koch MH, Rietschel ET, Seydel U. 2000. Biological activities of lipopolysaccharides are determined by the shape of their lipid A portion. Eur. J. Biochem. 267:2008–2013. 10.1046/j.1432-1327.2000.01204.x [DOI] [PubMed] [Google Scholar]
- 35. Li J, Cox AD, Hood DW, Schweda EK, Moxon ER, Richards JC. 2005. Electrophoretic and mass spectrometric strategies for profiling bacterial lipopolysaccharides. Mol. Biosyst. 1:46–52. 10.1039/b501686j [DOI] [PubMed] [Google Scholar]
- 36. Ostrowski K, Rohde T, Asp S, Schjerling P, Pedersen BK. 1999. Pro- and anti-inflammatory cytokine balance in strenuous exercise in humans. J. Physiol. 515:287–291. 10.1111/j.1469-7793.1999.287ad.x [DOI] [PMC free article] [PubMed] [Google Scholar]