Abstract
Vaccination for Johne's disease with killed inactivated vaccine in cattle herds has shown variable success. The vaccine delays the onset of disease but does not afford complete protection. Johne's disease vaccination has also been reported to interfere with measurements of cell-mediated immune responses for the detection of bovine tuberculosis. Temporal antibody responses and fecal shedding of Mycobacterium avium subsp. paratuberculosis, the causative agent of Johne's disease, were measured in 2 dairy cattle herds using Johne's disease vaccine (Mycopar) over a period of 7 years. Vaccination against Johne's disease resulted in positive serum M. avium subsp. paratuberculosis antibody responses in both herds, and the responses persisted in vaccinated cattle up to 7 years of age. Some vaccinated animals (29.4% in herd A and 36.2% in herd B) showed no serological reactivity to M. avium subsp. paratuberculosis. M. avium subsp. paratuberculosis-specific antibody responses were also detected in milk from Johne's disease-vaccinated animals, but fewer animals (39.3% in herd A and 49.4% in herd B) had positive results with milk than with serum samples. With vaccination against M. avium subsp. paratuberculosis, fecal shedding in both dairy herds was reduced significantly (P < 0.001). In addition, when selected Johne's disease-vaccinated and -infected animals were investigated for serological cross-reactivity to Mycobacterium bovis, no cross-reactivity was observed.
INTRODUCTION
Johne's disease in cattle is a chronic disease caused by Mycobacterium avium subsp. paratuberculosis. In the United States, the disease causes estimated losses of $200 million every year (1). Control of Johne's disease is achieved by testing, culling, and improving biosecurity and herd management (2). Vaccination, using killed inactivated vaccines, has also been attempted for disease control. The vaccines are said to afford protection by delaying the onset of clinical disease, but the protection against infection in cattle is not complete (3, 4). Vaccination with a calfhood vaccine is prescribed for replacement heifers and male calves. Currently available vaccines have the major disadvantages that they cause granulomas, can result in accidental vaccination of humans, and are said to interfere with the bovine tuberculosis (TB) skin test (4). Furthermore, the true cost benefits of vaccinations are unknown, although vaccinations historically have been shown to have economic value (1, 5).
Information regarding the efficiency of Johne's disease vaccination in cattle herds is scarce, and cross-reactivity in bovine TB tests has been shown to be a problem in vaccinated cattle, small ruminant, and cervid herds (6–12). Additional testing using the comparative cervical test (CCT) or gamma interferon (IFN-γ) measurement helps to determine whether reactivity seen with skin-based screening is specific, but the follow-up testing is often laborious and time-consuming (4, 6).
To study the effects of vaccination in cattle, we selected two dairy herds receiving Johne's disease vaccination in the wake of natural disease, and we studied M. avium subsp. paratuberculosis-specific antibody responses and fecal shedding of M. avium subsp. paratuberculosis in these two herds. Vaccination or infection with M. avium subsp. paratuberculosis has been shown to result in interference in cell-based bovine TB assays (6–12). With the recent availability of new serological assays to detect bovine TB, the cross-reactivity of M. avium subsp. paratuberculosis-specific antibodies in response to Johne's disease infection or vaccination was investigated using the IDEXX Mycobacterium bovis antibody enzyme-linked immunosorbent assay (ELISA), which is based on two antigens (MPB83 and MPB70) (13).
MATERIALS AND METHODS
Sample populations.
Two herds (designated A and B) that were receiving vaccinations and were part of the Pennsylvania Johne's Disease Demonstration Herd Project were selected and were studied for 7 years (2004 to 2010).
Sampling plan.
Paired blood and fecal samples were collected from all ≥24-month-old animals, both lactating and nonlactating, in both herds, in order to evaluate the M. avium subsp. paratuberculosis antibody responses and shedding status of the individual animals. The two types of samples were collected from each animal in the herd on the same day, identified using unique nontransferable identification numbers, and sent on ice to the laboratory in Harrisburg, Pennsylvania, for processing. Sampling was carried out annually for 7 years, 11 to 13 months after the previous sampling. A total of 952 animal samplings were carried out, 594 from vaccinated animals and 358 from unvaccinated animals.
In select years, Dairy Herd Improvement Association (DHIA)-collected milk samples were also obtained from all lactating cows. These samples were obtained from the DHIA test date closest to the time of the fecal and blood sampling in selected years. Since both herds were on a monthly sampling schedule, the milk samples were always obtained within 2 weeks of the blood and fecal samples but not on the same day. The milk samples were tested to evaluate the levels of M. avium subsp. paratuberculosis antibody responses.
Initial and yearly assessments were conducted for both herds, and management changes were recorded. Both farms were tested annually beginning in 2004 (year 1) and continuing through 2010 (year 7). Vaccination was included as part of disease control measures on both farms.
Vaccination and management changes.
According to state regulations, caudal fold tuberculin testing was performed on all calves prior to the initiation of vaccination. All test results were negative. Calves were vaccinated by the herd veterinarians with 0.5 ml of killed whole-cell vaccine (Mycopar), administered subcutaneously in the brisket area, before 35 days of age. Herd A began receiving vaccination against Johne's disease prior to the initiation of this study; therefore, by year 1 of the study, there were 116 vaccinated animals >24 months of age in the herd. The proportions of vaccinated animals (versus unvaccinated animals) sampled in this herd ranged from 63.7% (74/116 animals) in 2004 to 100% (122/122 animals) in 2007. Herd B initiated M. avium subsp. paratuberculosis vaccination late in 2002 and did not have any vaccinated animals >24 months of age by the time of the first sampling in 2004. In year 7, 98% of the animals (49/50 animals) that underwent sampling had received the calfhood vaccination.
In addition to vaccination, herd A implemented numerous changes designed to minimize the probability of new infections, based on a Johne's disease risk assessment (http://www.johnesdisease.org/Risk%20Assessment%20&%20Management%20Plans%20for%20Johne%27s.pdf). The most significant changes included improved hygiene of the maternity area, prompt removal of the calves from the maternity area, and development of a protocol to calve any test-positive cows in a separate location. In addition, sick animals were not housed in the maternity area, and colostrum from any test-positive or untested animals was not used for heifer calves. Almost all fecal culture-positive animals were culled from the herd shortly after diagnosis, although animals with very low levels of shedding occasionally were retained for longer periods. Special attention was paid to these animals, to monitor them for clinical signs, and precautions (see above) were taken to minimize the risk of new infections resulting from these animals.
Numerous risk areas were identified in herd B by means of the risk assessment. However, this farm elected to make very few substantive changes in their management practices, although positive animals were identified. Some attempts were made to not have heavy shedders or clinically ill animals calve in the maternity area. Sick animals were generally not housed in the maternity area, although this did occur. For financial reasons, herd B retained many of its serum- and fecal-culture-positive animals, unless there were concurrent reasons to remove the animals (e.g., high somatic cell counts or other disease concerns) or they began showing clinical signs. Most but not all replacements for herds A and B were home-raised.
ELISAs.
The IDEXX Mycobacterium paratuberculosis ELISA kit (HerdChek M. pt. kit) for detection of M. avium subsp. paratuberculosis-specific antibodies in serum and the IDEXX Mycobacterium bovis ELISA kit for M. bovis (IDEXX, Westbrook, ME) were used in this study. ELISAs were conducted following the manufacturer's directions, with a 1:20 dilution of 100 μl of serum for the IDEXX HerdChek M. pt. kit and a 1:50 dilution of 100 μl of serum for the M. bovis ELISA kits. Antibody responses against M. avium subsp. paratuberculosis in milk and serum samples from vaccinated cattle were compared. Milk samples (100 μl at a 1:20 dilution) from selected animals from both herds (440 samples from herd A and 190 samples from herd B) were tested using the Prionics Paracheck ELISA (Prionics, Switzerland). This kit is approved for testing both serum and milk samples. Positive and negative results were determined according to the kit instructions. Quantitative data were expressed as sample/positive (S/P) ratios for the IDEXX assays or sample/negative values for the Prionics assay (Prionics, Switzerland).
Agar slant cultures.
Two grams of feces was decontaminated by the double incubation-centrifugation method, as described previously (14). This material was used to inoculate 4 slants of Herrold's egg yolk medium containing 2 mg/liter mycobactin J (Becton, Dickinson & Co., MD). M. avium subsp. paratuberculosis colonies were confirmed by using acid-fast staining and an IS900 PCR assay.
Statistical analyses.
Proportions of vaccinated and unvaccinated animals were calculated for each category in different study years. The effects of vaccination on serological responses and M. avium subsp. paratuberculosis shedding were analyzed with chi-square analysis. Relative risks from M. avium subsp. paratuberculosis exposure of vaccinated and unvaccinated groups were calculated. Correlation coefficients (r) were calculated for correlations between the serum and milk ELISA results, to understand the relationship. Serum S/P ratios for selected animals (n = 14) were plotted as scatter or line plots, to show antibody responses following vaccination.
RESULTS
Antibody responses and fecal shedding.
Chi-square statistical analysis showed that vaccination was strongly associated with positive serum responses and reduction of fecal shedding of M. avium subsp. paratuberculosis in both herds (P < 0.001). Unvaccinated cattle in herd A showing no evidence of infection were predominately seronegative for M. avium subsp. paratuberculosis antibodies, as indicated by comparing positive ELISA results for the total and vaccinated animal categories (Table 1). Significant proportions of vaccinated animals showed antibody responses (ranging from 32.7% to 70.9% in different years) and were found to be fecal culture negative for M. avium subsp. paratuberculosis (P < 0.001). The rate of seropositivity was highest in 2006, when >70.4% of animals tested positive (Table 1). A substantial number of vaccinated animals remained serologically negative (29.4% in herd A). At the start of the study, the within-herd prevalence rate was 6%. In the vaccinated group, fewer animals shed M. avium subsp. paratuberculosis in feces, and those that were ELISA positive shed fewer organisms (maximum, 3.2%). The whole-herd fecal culture-positive rate was also maintained at a low level. Some animals were fecal culture positive but remained seronegative in response to both M. avium subsp. paratuberculosis infection and vaccination. Table 1 shows the total fecal culture-positive and ELISA-positive results in response to either vaccination or infection. For some animals that showed seropositive responses, antibody levels did drop below the cutoff value of 0.25 or to the negative range, as determined by S/P ratios (Fig. 1A). However, for many other animals that could be traced for several years in both herds A and B, animals remained positive for serum M. avium subsp. paratuberculosis antibodies in the fifth year of the study or 7 years following vaccination (Fig. 1B).
TABLE 1.
Mycobacterium avium subsp. paratuberculosis annual antibody responses and fecal shedding in M. avium subsp. paratuberculosis-infected cattle herds receiving M. avium subsp. paratuberculosis vaccination with management changes (herd A) or without management changes (herd B)
| Herd and yr | No. in herda | No. (%)b |
||||||
|---|---|---|---|---|---|---|---|---|
| Vaccinated | Total |
Vaccinated |
ELISA positive |
|||||
| ELISA positive | Fecal culture positive | ELISA positive | Fecal culture positive | Fecal culture positive | Vaccinated fecal culture positive | |||
| A | ||||||||
| 2004 | 116 | 74 (63.7) | 49 (42.2) | 7 (6) | 47 (63.7) | 0 | 2 | 0 |
| 2005 | 116 | 105 (90.5) | 74 (63.7) | 1 (0.8) | 73 (69.5) | 0 | 0 | 0 |
| 2006 | 115 | 110 (95.6) | 78 (67.8) | 3 (2.4) | 78 (70.9) | 1 (0.9) | 0 | 0 |
| 2007 | 122 | 122 (100) | 40 (32.7) | 2c/3 (2.4) | 40 (32.7) | 2c/3 (2.4) | 2 | 2 |
| 2008 | 125 | 125 (100) | 65 (52.0) | 3c/4 (3.2) | 65 (52) | 3c/4 (3.2) | 2 | 2 |
| 2009 | 128 | 94 (73.4) | 49 (38.2) | 2c/3 (2.3) | 49 (52.2) | 1 (1) | 0 | 0 |
| 2010 | 128 | 66 (51.5) | 35 (27.3) | 1 (0.7) | 34 (51.5) | 0 | 0 | 0 |
| B | ||||||||
| 2004 | 43 | 0 | 3 (6.6) | 10 (23.2) | 0 | 0 | 3 | 0 |
| 2005 | 45 | 2 (4.4) | 7 (15.5) | 8c/11 (24.4) | 2 (100) | 1 (50) | 3 | 1 |
| 2006 | 52 | 30 (57.6) | 30 (57.6) | 8c/11 (21.1) | 25 (83.3) | 4c/5 (16.6) | 9 | 3 |
| 2007 | 61 | 49 (80.3) | 27 (44.2) | 3c/4 (7.2) | 26 (53.0) | 2c/3 (6.1) | 3 | 3 |
| 2008 | 55 | 52 (94.5) | 33 (60) | 3c/5 (9.0) | 32 (61.5) | 3c/4 (7.6) | 4 | 2 |
| 2009 | 52 | 51 (98) | 32 (61.5) | 5c/6 (11.5) | 31 (60.7) | 5c/6 (11.7) | 6 | 4 |
| 2010 | 50 | 49 (98) | 25 (50) | 2 (4.0) | 25 (51.0) | 2 (4.0) | 2 | 1 |
The numbers of animals >24 months of age are indicated for each study year.
Total and vaccinated ELISA- and M. avium subsp. paratuberculosis fecal culture-positive animals are indicated as ELISA positive and fecal culture positive, respectively. Animals that were ELISA positive and were M. avium subsp. paratuberculosis fecal culture positive in the total and vaccinated groups are indicated as fecal culture positive and vaccinated fecal culture positive, respectively, under ELISA positive.
New infections for each year are indicated, showing totals for new infections and total positive results.
FIG 1.
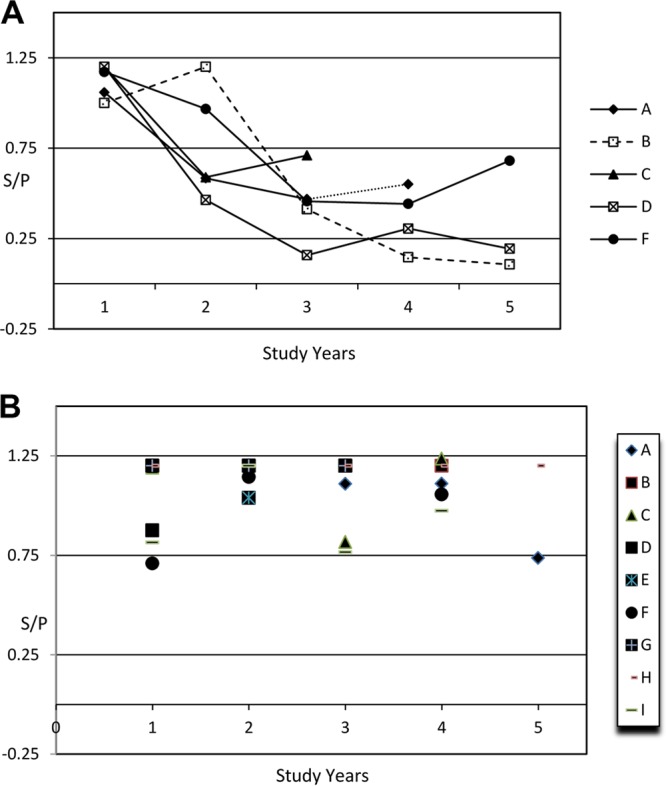
Serological responses to Johne's disease vaccination. (A) Line plot showing the trend of serum S/P ratios (y axis) tested annually during the 5-year study period (years 1 to 5), from >2 years to 7 years of age (x axis), for selected animals (n = 5; animals A, B, C, D, and F). (B) Scatter plot showing the trend of serum S/P ratios (y axis, with maximum capped values of 1.2) tested during the 5-year study period (years 1 to 5), from >2 years to 7 years of age (x axis), for selected animals (n = 9; animals A to I).
The second herd, herd B, initially showed considerable rates of infection (>20% within-herd prevalence) in both the vaccinated and unvaccinated groups. The fecal shedding rate was 23.2% in 2004, at the start of the study, and remained above 20% until 2006, with several high-level shedders being identified within the herd (Table 1). ELISA positivity rates ranged between 51 and 100% among vaccinated animals in any given study year. Overall, 36.2% of vaccinated animals showed no ELISA reactivity. Fecal shedding dropped from an initial rate of greater than 20% to less than 10% in subsequent years. Vaccinated animals were found not to be fully protected in herd B, as was the case in herd A. Several animals (range, 4.0 to 50%) were found to be shedding M. avium subsp. paratuberculosis and were identified as high shedders even after being vaccinated. Overall, the numbers of M. avium subsp. Paratuberculosis-shedding animals decreased with time. In herd B, similar to herd A, some animals were fecal culture positive for M. avium subsp. paratuberculosis but were serologically negative in both the vaccinated and infected groups.
Serum and milk antibody responses.
Positive correlations between the serum and milk antibody responses were noticed for both vaccinated herds (herd A, r = 0.69; herd B, r = 0.63). Many vaccinated animals showed measurable M. avium subsp. Paratuberculosis-specific antibody responses in serum but showed no specific antibodies for M. avium subsp. paratuberculosis in milk. In herd A, 440 sample pairs collected from lactating vaccinated animals were tested with milk and serum ELISAs; 173 (39.3%) were found to be positive for M. avium subsp. paratuberculosis-specific antibody responses by serum testing but were negative by milk testing. In herd B, among the 141 vaccinated animals sampled, 51 (49.0%) tested positive in serum testing but negative when milk was analyzed for M. avium subsp. paratuberculosis-specific responses. There was no major difference in serum and milk antibody responses among the unvaccinated animals (n = 104), including the animals that were shedding M. avium subsp. paratuberculosis. For 5/104 animals (4.8%) in herd A and 7/125 animals (5.6%) in herd B, although the animals were shedding M. avium subsp. paratuberculosis, both milk and serum ELISAs failed to detect infected animals among the unvaccinated animals. In the same unvaccinated category, 93% of animals sampled for all 3 tests (fecal, serum, and milk testing) were negative.
Serological cross-reactivity to Mycobacterium bovis.
Sera that were strongly positive for M. avium subsp. paratuberculosis antibody responses (S/P ratios) due to infection or vaccination were tested with a M. bovis ELISA. Our results showed no reactivity with the M. bovis ELISA, although the animal sera were positive for M. avium subsp. paratuberculosis antibodies. Twenty serum samples with S/P ratios of >0.25, 10 serum samples with S/P ratios of <0.25 from animals vaccinated against M. avium subsp. paratuberculosis, and 16 serum samples with S/P ratios of >0.25 from M. avium subsp. paratuberculosis-infected animals that were serologically positive were included in this comparison. None of the sera showed any reactivity to M. bovis.
DISCUSSION
Vaccination of calves with a killed vaccine has not been shown to completely prevent Mycobacterium avium subsp. paratuberculosis infection but is considered to be an effective tool for controlling the spread of disease (8). Another study showed reductions in herd infection rates but claimed that similar reductions were possible without vaccination with changes in management practices (3). Our study describes in detail observational findings for the two M. avium subsp. paratuberculosis-vaccinated herds. The infection levels in both of these study herds dropped, albeit slowly, when extensive management changes were not incorporated along with the vaccination strategy. However, due to multiple confounding factors during the study period, including management changes on the two farms, culling, herd replacements, and the inability of currently available immunological tests to distinguish vaccinated animals from infected animals, the observed protection could not be attributed only to vaccination, although it appears that vaccination has some protective role. The role of vaccination in protection against Johne's disease would be strengthened with data comparisons between vaccinated and unvaccinated herds. A control herd was not included in the study. However, a Johne's disease-infected herd (543 animals) that was participating in a demonstration herd project but did not receive vaccination that was followed during the same study period did not show any drop in infection levels during the study period (2006 to 2011). This herd maintained within-herd infection rates of approximately 10% for the entire study period (E. Hovingh, unpublished data). Another important observation for both study herds was the overall reduction in herd infection levels with the passage of time, not just in the vaccinated group. Among other factors, this was probably due to the risk of organism introduction being reduced in the unvaccinated group because of decreased infection rates in the vaccinated group (15). At least within the time frame of the current study, it was evident that Johne's disease infection could not be completely eliminated by vaccination alone, with or without management changes in the study herds.
Immunologically, M. avium subsp. paratuberculosis vaccination of cattle has been shown to result in M. avium subsp. paratuberculosis-specific gamma interferon (IFN-γ) responses in vaccinated calves by 7 days and specific antibody responses in 80% of calves by 3 to 6 months (16, 17). The strong antibody levels reported by those authors were sustained throughout the 12-month study period (16, 17). Because the infection is mainly intracellular, antibodies may not be able to confer direct protection, but the antibodies have been shown to confound detection of bovine TB (6, 7, 11). In the study groups, the antibody responses against M. avium subsp. paratuberculosis among vaccinated animals were maintained for several years, particularly if the study subjects started with stronger antibody responses. The antibody responses did not always correlate with protection. One of the issues in fully assessing the protective effects of antibodies is the current inability to distinguish vaccine immunity from immunity resulting from infection. Using the S/P ratio as an indicator of antibody immunity, at least 70% of all vaccinated cattle, if not clearly positive (above the 0.25 cutoff value), showed some level of measurable antibody response. The remainder of the vaccinated animals failed to show any measurable antibody response. Some of the ELISA-negative sera were also found to be nonreactive when tested with M. avium subsp. paratuberculosis agar gel immunodiffusion (AGID) testing. In fact, the AGID test results were negative even when M. avium subsp. paratuberculosis IDEXX ELISA results were positive, indicating either that the AGID test may be detecting a different class of M. avium subsp. paratuberculosis antibodies or that the two tests use different antigens. It is possible that some vaccinated animals in our study became seronegative by the time they were first tested (>2 years) but this appears to be less likely, as other authors have also reported failed antibody responses or antibody anergy in calves, measured even soon after vaccination (16, 18). Antibody reactivity in vaccinated animals did decline over the study period, but failed antibody responses to vaccination point to the involvement of some other immune mechanism. The reason why some animals show anergic antibody responses, which has also been reported earlier, is not fully understood and has not received much attention (16, 18). The anergic responses could result either from fetal exposure to M. avium subsp. paratuberculosis resulting in immunotolerance to the M. avium subsp. paratuberculosis antigens or the maternal antibody responses in calves inhibiting antibody responses in the vaccinated animals. The development of fetal regulatory T cells responsible for immunotolerance resulting from exposure to maternal alloantigens has been demonstrated earlier (19). Similar findings have also been reported for other infections upon fetal exposure (20), with T-cell responses reported to be normal but B-cell or antibody responses being affected.
In the vaccinated animals, milk and serum antibody responses were found to be positively correlated but milk frequently tested negative even when serum results were positive. The difference between milk and serum reactivity does not appear to be because of the two different ELISA kits used to test the two different specimen types. The two kits have been reported to have comparable sensitivities and only a slight difference in specificities (21). Differences in serum and milk M. avium subsp. paratuberculosis antibody reactivities have also been reported for infected cattle, and differences in infected cattle have been explained based on parity, stage of lactation, and dilution effects, with a likelihood of cattle testing M. avium subsp. paratuberculosis antibody positive when tested between 4 and 12 weeks of parturition (22). In infected animals, antibody production from local lymphoid systems adds to the total M. avium subsp. paratuberculosis antibody response, as these tissues have been shown to be infected (23); in Johne's disease-vaccinated cattle, the contribution of local immunity to milk antibodies against M. avium subsp. paratuberculosis using inactivated vaccine is likely negligible.
Antibody responses resulting from Johne's disease vaccination or infection are said to increase the risk of nonspecificity for TB testing in cattle, sheep, and deer herds (6–8, 10, 11, 24). Widespread use of Johne's disease vaccination can pose a problem for TB surveillance. Cattle and deer vaccinated with killed M. avium subsp. paratuberculosis vaccine have been reported to demonstrate antibody responses to M. avium subsp. paratuberculosis and also to M bovis (6, 12). Using the new IDEXX M. bovis assay (using MPB70 and 83 antigens), we did not see any cross-reactive antibody responses against bovine TB in vaccinated cattle in M. avium subsp. paratuberculosis-vaccinated or -infected animals. Our findings are supported by another study, in which testing of deer herds with modified ELISA tools did not show any cross-reactions in chromatographically based enzyme immunoassays (6). The loss of cross-reactivity appears to be tied to use of the antigens MPB70 and MPB83 in the new ELISAs. These antigens have shown promise for improved diagnosis of bovine TB (13). MPB70 antigen, in particular, can identify TB-infected cattle late in the infection cycle, and MPB83 detects early antibody responses. Detection of antibodies to these antigens is boosted by skin tuberculin testing when cattle are infected with bovine TB (25). Although animals were not tested in our study soon after tuberculin skin testing, animals that received both tuberculin testing and vaccination against Johne's disease did not show any seroreactivity to Mycobacterium tuberculosis despite showing positive responses to M. avium subsp. paratuberculosis. In a previous study in which vaccination with M. avium subsp. paratuberculosis was followed by tuberculin skin testing, the specificity of M. tuberculosis antigens in serological assays was not compromised by M. avium subsp. paratuberculosis vaccination (7).
Vaccination can be a useful strategy for the management of Johne's disease but is not used frequently due to the associated risks (4). Concerns about M. avium subsp. paratuberculosis vaccination interfering with the interpretation of diagnostic tests for M. avium subsp. paratuberculosis or bovine TB are valid. Current M. avium subsp. paratuberculosis vaccines do not afford full protection but do offer economic promise while better vaccines are being researched (26, 27). The newer serological bovine TB ELISAs may help address some of the concerns about M. avium subsp. paratuberculosis vaccines interfering with TB testing, but this needs to be further evaluated in geographically diverse settings. Until vaccines that afford better protection are developed, using good management practices on farms and reducing the numbers of high-level shedders, controlling vertical transmission, and making sure that only testing-negative animals enter the herd are some approaches to decrease infection levels on farms.
ACKNOWLEDGMENTS
We thank the USDA-APHIS Veterinary Services and the Johne's Disease Integrated Program, funded by the USDA National Institute of Food and Agriculture (Washington, DC), for providing partial funding for this study.
We also thank Carol Burns, Louise Byler, Hannah Lysczek, Eric Hue, Ken Risner, Nancy Carpenter, and Willard Feria for their technical assistance.
Footnotes
Published ahead of print 12 March 2014
REFERENCES
- 1. Cho J, Tauer LW, Schukken YH, Gomez MI, Smith RL, Lu Z, Grohn YT. 2012. Economic analysis of Mycobacterium avium subspecies paratuberculosis vaccines in dairy herds. J. Dairy Sci. 95:1855–1872. 10.3168/jds.2011-4787 [DOI] [PubMed] [Google Scholar]
- 2. Harris NB, Barletta RG. 2001. Mycobacterium avium subsp. paratuberculosis in veterinary medicine. Clin. Microbiol. Rev. 14:489–512. 10.1128/CMR.14.3.489-512.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kalis CH, Hesselink JW, Barkema HW, Collins MT. 2001. Use of long-term vaccination with a killed vaccine to prevent fecal shedding of Mycobacterium avium subsp paratuberculosis in dairy herds. Am. J. Vet. Res. 62:270–274. 10.2460/ajvr.2001.62.270 [DOI] [PubMed] [Google Scholar]
- 4. Patton EA. 2011. Paratuberculosis vaccination. Vet. Clin. North Am. Food Anim. Pract. 27:573–580. 10.1016/j.cvfa.2011.07.004 [DOI] [PubMed] [Google Scholar]
- 5. van Schaik G, Kalis CH, Benedictus G, Dijkhuizen AA, Huirne RB. 1996. Cost-benefit analysis of vaccination against paratuberculosis in dairy cattle. Vet. Rec. 139:624–627 [PubMed] [Google Scholar]
- 6. Buddle BM, Wilson T, Denis M, Greenwald R, Esfandiari J, Lyashchenko KP, Liggett S, Mackintosh CG. 2010. Sensitivity, specificity, and confounding factors of novel serological tests used for the rapid diagnosis of bovine tuberculosis in farmed red deer (Cervus elaphus). Clin. Vaccine Immunol. 17:626–630. 10.1128/CVI.00010-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Coad M, Clifford DJ, Vordermeier HM, Whelan AO. 2013. The consequences of vaccination with the Johne's disease vaccine, Gudair, on diagnosis of bovine tuberculosis. Vet. Rec. 172:266. 10.1136/vr.101201 [DOI] [PubMed] [Google Scholar]
- 8. Kohler H, Gyra H, Zimmer K, Drager KG, Burkert B, Lemser B, Hausleithner D, Cubler K, Klawonn W, Hess RG. 2001. Immune reactions in cattle after immunization with a Mycobacterium paratuberculosis vaccine and implications for the diagnosis of M. paratuberculosis and M. bovis infections. J. Vet. Med. B Infect. Dis. Vet. Public Health 48:185–195. 10.1046/j.1439-0450.2001.00443.x [DOI] [PubMed] [Google Scholar]
- 9. Mackintosh CG, Labes RE, Thompson BR, Clark RG, de Lisle GW, Johnstone PD, Griffin JF. 2008. Efficacy, immune responses and side-effects of vaccines against Johne's disease in young red deer (Cervus elaphus) experimentally challenged with Mycobacterium avium subsp paratuberculosis. N. Z. Vet. J. 56:1–9. 10.1080/00480169.2008.36797 [DOI] [PubMed] [Google Scholar]
- 10. Nedrow AJ, Gavalchin J, Smith MC, Stehman SM, Maul JK, McDonough SP, Thonney ML. 2007. Antibody and skin-test responses of sheep vaccinated against Johne's disease. Vet. Immunol. Immunopathol. 116:109–112. 10.1016/j.vetimm.2006.12.007 [DOI] [PubMed] [Google Scholar]
- 11. Stringer LA, Wilson PR, Heuer C, Hunnam JC, Mackintosh CG. 2011. Effect of vaccination and natural infection with Mycobacterium avium subsp. paratuberculosis on specificity of diagnostic tests for bovine tuberculosis in farmed red deer (Cervus elaphus). N. Z. Vet. J. 59:218–224. 10.1080/00480169.2011.596182 [DOI] [PubMed] [Google Scholar]
- 12. Thomsen VT, Nielsen SS, Thakur A, Jungersen G. 2012. Characterization of the long-term immune response to vaccination against Mycobacterium avium subsp. paratuberculosis in Danish dairy cows. Vet. Immunol. Immunopathol. 145:316–322. 10.1016/j.vetimm.2011.11.021 [DOI] [PubMed] [Google Scholar]
- 13. Waters WR, Buddle BM, Vordermeier HM, Gormley E, Palmer MV, Thacker TC, Bannantine JP, Stabel JR, Linscott R, Martel E, Milian F, Foshaug W, Lawrence JC. 2011. Development and evaluation of an enzyme-linked immunosorbent assay for use in the detection of bovine tuberculosis in cattle. Clin. Vaccine Immunol. 18:1882–1888. 10.1128/CVI.05343-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Secott TE, Ohme AM, Barton KS, Wu CC, Rommel FA. 1999. Mycobacterium paratuberculosis detection in bovine feces is improved by coupling agar culture enrichment to an IS900-specific polymerase chain reaction assay. J. Vet. Diagn. Invest. 11:441–447. 10.1177/104063879901100509 [DOI] [PubMed] [Google Scholar]
- 15. Knust B, Patton E, Ribeiro-Lima J, Bohn JJ, Wells SJ. 2013. Evaluation of the effects of a killed whole-cell vaccine against Mycobacterium avium subsp paratuberculosis in 3 herds of dairy cattle with natural exposure to the organism. J. Am. Vet. Med. Assoc. 242:663–669. 10.2460/javma.242.5.663 [DOI] [PubMed] [Google Scholar]
- 16. Spangler E, Heider LE, Bech-Nielsen S, Dorn CR. 1991. Serologic enzyme-linked immunosorbent assay responses of calves vaccinated with a killed Mycobacterium paratuberculosis vaccine. Am. J. Vet. Res. 52:1197–1200 [PubMed] [Google Scholar]
- 17. Stabel JR, Robbe-Austerman S. 2011. Early immune markers associated with Mycobacterium avium subsp. paratuberculosis infection in a neonatal calf model. Clin. Vaccine Immunol. 18:393–405. 10.1128/CVI.00359-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Stabel JR, Waters WR, Bannantine JP, Lyashchenko K. 2011. Mediation of host immune responses after immunization of neonatal calves with a heat-killed Mycobacterium avium subsp. paratuberculosis vaccine. Clin. Vaccine Immunol. 18:2079–2089. 10.1128/CVI.05421-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mold JE, Michaelsson J, Burt TD, Muench MO, Beckerman KP, Busch MP, Lee TH, Nixon DF, McCune JM. 2008. Maternal alloantigens promote the development of tolerogenic fetal regulatory T cells in utero. Science 322:1562–1565. 10.1126/science.1164511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Endsley JJ, Roth JA, Ridpath J, Neill J. 2003. Maternal antibody blocks humoral but not T cell responses to BVDV. Biologicals 31:123–125. 10.1016/S1045-1056(03)00027-7 [DOI] [PubMed] [Google Scholar]
- 21. Collins MT, Wells SJ, Petrini KR, Collins JE, Schultz RD, Whitlock RH. 2005. Evaluation of five antibody tests for diagnosis of bovine paratuberculosis. Clin. Diagn. Lab. Immunol. 12:685–692. 10.1128/CDLI.12.6.685-692.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lombard JE, Byrem TM, Wagner BA, McCluskey BJ. 2006. Comparison of milk and serum enzyme-linked immunosorbent assays for diagnosis of Mycobacterium avium subspecies paratuberculosis infection in dairy cattle. J. Vet. Diagn. Invest. 18:448–458. 10.1177/104063870601800504 [DOI] [PubMed] [Google Scholar]
- 23. Sweeney RW, Whitlock RH, Rosenberger AE. 1992. Mycobacterium paratuberculosis cultured from milk and supramammary lymph nodes of infected asymptomatic cows. J. Clin. Microbiol. 30:166–171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Muskens J, van Zijderveld F, Eger A, Bakker D. 2002. Evaluation of the long-term immune response in cattle after vaccination against paratuberculosis in two Dutch dairy herds. Vet. Microbiol. 86:269–278. 10.1016/S0378-1135(02)00006-8 [DOI] [PubMed] [Google Scholar]
- 25. Wiker HG. 2009. MPB70 and MPB83: major antigens of Mycobacterium bovis. Scand. J. Immunol. 69:492–499. 10.1111/j.1365-3083.2009.02256.x [DOI] [PubMed] [Google Scholar]
- 26. Bannantine JP, Paulson AL, Chacon O, Fenton RJ, Zinniel DK, McVey DS, Smith DR, Czuprynski CJ, Barletta RG. 2011. Immunogenicity and reactivity of novel Mycobacterium avium subsp. paratuberculosis PPE MAP1152 and conserved MAP1156 proteins with sera from experimentally and naturally infected animals. Clin. Vaccine Immunol. 18:105–112. 10.1128/CVI.00297-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lu Z, Schukken YH, Smith RL, Grohn YT. 2013. Using vaccination to prevent the invasion of Mycobacterium avium subsp. paratuberculosis in dairy herds: a stochastic simulation study. Prev. Vet. Med. 110:335–345. 10.1016/j.prevetmed.2013.01.006 [DOI] [PubMed] [Google Scholar]


