Abstract
Recent incidents in the United States and abroad have heightened concerns about the use of ricin toxin as a bioterrorism agent. In this study, we produced, using a robust plant-based platform, four chimeric toxin-neutralizing monoclonal antibodies that were then evaluated for the ability to passively protect mice from a lethal-dose ricin challenge. The most effective antibody, c-PB10, was further evaluated in mice as a therapeutic following ricin exposure by injection and inhalation.
TEXT
Ricin toxin is a member of the medically important A-B family of plant and bacterial ribosome-inactivating proteins (RIPs) (1). In its mature form, ricin is a 65-kDa heterodimeric glycoprotein that is a natural constituent of the seeds of the castor plant (Ricinus communis). The 267-amino-acid A subunit (RTA) of ricin is an RNA N-glycosidase that depurinates a conserved adenosine residue within the so-called sarcin-ricin loop (SRL) of eukaryotic 28S rRNA, which is required for activation of the elongation factor EF-Tu (2, 3). RTA is linked via a single disulfide bond to ricin's B subunit (RTB), a 262-amino-acid lectin that is specific for glycoproteins and glycolipids terminating in galactose and N-acetylgalactosamine (Gal/GalNAc). In addition to its role in attachment, RTB also mediates the retrograde transport of ricin to the trans-Golgi network (TGN) and endoplasmic reticulum (ER), where RTA is ultimately delivered across the ER membrane and into the cytoplasm (4, 5). The extraordinary capacity of RTA to inactivate ribosomes (kcat, 1,500/min) makes ricin one of the most potent known RIPs (6, 7). For example, in rodents and nonhuman primates, the 50% lethal dose (LD50) of ricin by injection is approximately 5 μg/kg, while the LD50 of ricin by inhalation is estimated to be as low as 3 μg/kg (8). The respiratory mucosa is especially sensitive to ricin, as even trace amounts of toxin are known to elicit widespread necrosis in the airways and alveoli, peribronchovascular edema, mixed inflammatory cell infiltrates, and massive pulmonary alveolar flooding (9–15).
The U.S. Departments of Defense (DOD) and Health and Human Services (HHS) have ongoing initiatives to develop antibody-based products capable of providing passive protection against systemic and mucosal ricin exposure (16, 17). In addition to protective efficacy, issues related to platform technology, scalability, and speed of manufacturing will ultimately dictate which product(s) will be pursued for advanced development and are consistent with the unique needs for biodefense. In this regard, the Nicotiana benthamiana-based rapid antibody-manufacturing platform (RAMP) provides the potential for extremely fast and high-yield monoclonal antibody (MAb) production that has already proven to be applicable to biodefense (18–21). The technology entails mass infiltration of mature Nicotiana plants with an Agrobacterium tumefaciens suspension carrying T-DNA encoding viral replicons and results in high MAb recovery from original DNA constructs within days (22). The Nicotiana-based RAMP is being used for manufacturing recombinant MAbs or “plantibodies” for clinical studies under good manufacturing practices (GMP) (20, 23).
In this study, we sought to exploit the Nicotiana-based RAMP for the purpose of identifying and advancing potential immunotherapeutic agents for ricin toxin. In a proof-of-concept study, we recently characterized chimeric GD12 (c-GD12), a partially humanized murine monoclonal antibody (MAb) directed against an immunodominant, linear B cell epitope on RTA (24). c-GD12, produced in N. benthamiana, protected mice from the effects of a systemic ricin toxin challenge when administered before or up to 4 h after ricin injection. However, for as of yet underdetermined reasons, c-GD12 ultimately proved difficult to express in high quantities via traditional cell culture or the RAMP technologies. For that reason, we have now extended our studies to include four additional recently identified toxin-neutralizing MAbs (Table 1), two MAbs (SyH7 and PB10) directed against RTA (25, 26), and two MAbs (SylH3 and JB4) directed against RTB (27, 28).
TABLE 1.
Characteristics of murine and chimeric MAbs used in this study
| MAb | Target | KD(M)a | m-IC50 (μg/ml)b | c-IC50 (μg/ml)b | No. of mice survived/no. of mice challengedc | Reference |
|---|---|---|---|---|---|---|
| PB10 | RTA | 4 × 10−8 | 0.015 | 0.03 | 10/10 | 26 |
| SyH7 | RTA | 4 × 10−8 | 0.125 | 0.10 | 3/10 | 26 |
| SylH3 | RTB | 3.3 × 10−9 | 1.8 | 1.8 | 5/10 | 27 |
| JB4 | RTB | 2 × 10−10 | 1.1 | 1.1 | 10/10 | 28 |
Equilibrium dissociation constant as determined by BIAcore analysis and reported previously.
Inhibitory concentration (IC50) of murine (m) or chimeric (c) MAbs, as determined in Vero cytotoxicity assay.
BALB/c mice were administered 20 μg of each MAb and then challenged with ricin (10× LD50) 24 h later, as described in the text.
The murine VL and VH domains of SyH7, PB10, SylH3, and JB4 were amplified by PCR from cDNA derived from the respective murine B cell hybridomas (26–28). PCR amplicons were sequenced, and consensus contigs for each domain were generated based on the Kabat and International ImMunoGeneTics (IMGT) databases (29). The codon-optimized VL and VH regions of each MAb were then synthesized commercially (GeneArt; Life Technologies, Grand Island, NY) and fused to human IgG1 and κ constant regions as described previously (24). Mouse-human chimeric antibodies were produced using the N. benthamiana-based RAMP. For this study, production was done with a transgenic N. benthamiana line devoid of xylosyl transferase and fucosyl transferase activities, which results in immunoglobulins with N-glycans that are mammalian and generally more homogeneous than those produced in mammalian cell lines used for MAb production (e.g., CHO and NS0) (30).
All four of the chimeric antibodies were successfully expressed and purified from the N. benthamiana-based system. Briefly, 24- to 26-day-old plants were vacuum infiltrated with A. tumefaciens strains carrying viral vectors encoding the cognate VH and VL of SyH7, PB10, SylH3, and JB4. Seven days postinfiltration, leaf tissues were extracted, clarified using a plate-and-frame filter press (Ertel-Alsop), and then subjected to a MabSelect SuRe protein A column (GE Healthcare Biosciences, Pittsburgh, PA), Capto Q (GE Healthcare), and final polishing via a ceramic hydroxyapatite type II (CHT) column (80 μm) (Bio-Rad). Chimeric MAbs were fully assembled, as determined by SDS-PAGE, and had <1% aggregate, as determined by high-pressure liquid chromatography (HPLC)-size exclusion chromatography (SEC) (see Fig. S1 in the supplemental material). Yields were in the range of 100 to 200 mg per kg of fresh leaf biomass. Antibodies were placed into an appropriate formulation buffer and sterile filtered into crystal zenith vials (West Pharma, Exton, PA) and stored at −80°C.
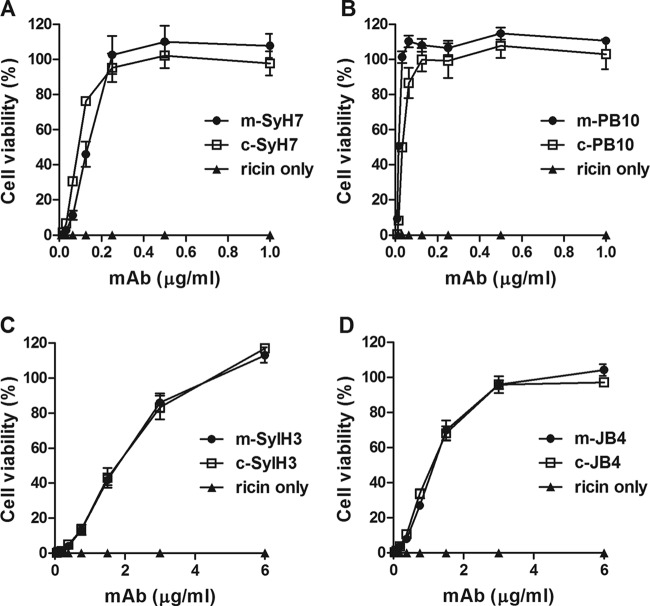
We found that, as determined by enzyme-linked immunosorbent assay (ELISA), the chimeric MAbs retained antigen specificity and apparent affinities (50% effective concentration [EC50]) compared to the parental murine MAbs (see Fig. S2 in the supplemental material). We next performed ricin toxin-neutralizing assays using Vero cells, as previously described (24). With respect to functional activity, all four chimeric MAbs (c-MAbs) had 50% inhibitory concentrations (IC50s) that were indistinguishable or, in the case of c-PB10 and c-SylH7, slightly better than their murine counterparts (Table 1 and Fig. 1). c-PB10 had the lowest IC50 of the four c-MAbs and its IC50 was >5-fold lower than that of c-GD12 (24).
FIG 1.
In vitro neutralizing capabilities of chimeric MAbs. Dilutions of indicated murine (m) or chimeric (c) MAbs were mixed with ricin (10 ng/ml), added to Vero cells for 2 h, washed, and then incubated with Dulbecco modified Eagle medium (DMEM) for an additional 48 h, after which cell viability was assessed. (A) SyH7, (B) PB10, (C) SylH3, (D) JB4. Treatments were performed in triplicate. Cells treated with media only were used as controls, with 100% viability. Variability of cell viability around 100% (±10%) is not uncommon in this assay.
We next compared c-PB10, c-SyH7, c-SylH3, and c-JB4 to determine which chimeric MAb was the most effective at ricin neutralization in vivo. BALB/c mice received 20 μg of chimeric MAb 24 h prior to being subjected to a 10× LD50 ricin challenge (∼2 μg per mouse) (Vector Laboratories, Burlingame, CA). The choice of 20 μg of MAb per mouse was based upon our previous work with c-GD12 and dozens of other anti-ricin MAbs that, as a rule of thumb, have demonstrated that 20 μg is a sufficient excess to detect in vivo toxin-neutralizing activity. Similarly, a ricin challenge of 10× LD50 versus 5× LD50 (used in many of our previous studies) was chosen because it provides a more stringent challenge model that results in more consistent and reproducible outcomes.
All studies involving animals were done in strict compliance with the Wadsworth Center's Institutional Animal Care and Use Committee (IACUC) guidelines.
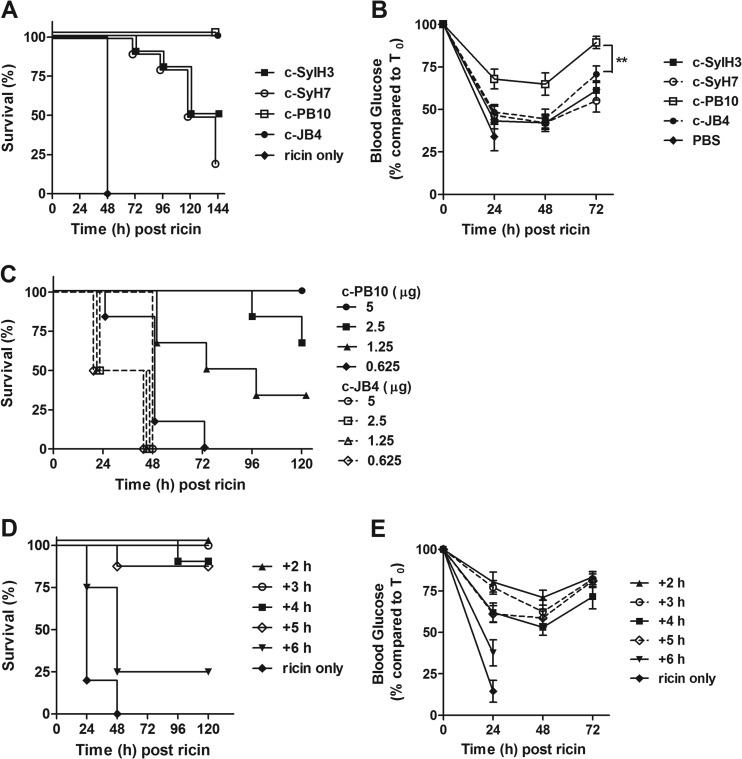
As expected, control mice that were challenged with ricin in the absence of antibody treatment succumbed to intoxication within 48 h (Fig. 2A). The effectiveness of the chimeric MAbs at the 20-μg dose was variable in that c-SylH3 and c-SyH7 conferred only partial protection against ricin challenge, while mice that received c-JB4 or c-PB10 were completely protected. Blood glucose levels (a well-established surrogate marker of ricin intoxication) measured from blood collected from the lateral tail vein during challenge were determined using an Accu-Chek Aviva system handheld meter (Roche Diagnostics, Indianapolis, IN). Blood glucose levels were recorded immediately before ricin challenge (t = 0) and every 24 h thereafter. Mice were euthanized when blood glucose levels fell below 20 mg/dl. The degree of hypoglycemia experienced by c-PB10-treated mice was statistically less severe than that of c-JB4-treated mice (Fig. 2B), demonstrating a quantitative difference between c-JB4 and c-PB10 in regard to toxin-neutralizing activity in vivo.
FIG 2.
Evaluation in mice of chimeric plantibodies in conferring passive immunity to ricin toxin. BALB/c mice (female, 6 to 8 weeks of age) were purchased from Taconic Labs (Hudson, NY) and housed under conventional, specific pathogen-free conditions. (A and B) Chimeric MAbs were administered to mice (n = 10 mice per group) by intraperitoneal (i.p.) injection 24 h prior to i.p. challenge with 10× LD50 ricin. Survival (A) was monitored over a period of 6 days. Morbidity (B) was assessed based on the onset of hypoglycemia, as done previously (36). (C) c-PB10 and c-JB4 were administered at indicated amounts to mice (n = 6 mice per group) by the i.p. route 24 h before the animals were challenged with 10× LD50 ricin, as described for panel A. Survival was monitored over a 5-day period. (D and E) c-PB10 (25 μg/mouse) was administered to mice (n = 8 mice per group) at indicated time points before or after the animals were challenged with 10× LD50 ricin. Survival (D) and blood glucose levels (E) were recorded as described above. Data are displayed as the mean ± SEM. Statistical significance was determined using the Student t test. **, P < 0.01.
To more fully compare the efficacy of c-PB10 and c-JB4, we performed a dose step-down experiment in which groups of BALB/c mice were administered the chimeric MAbs at 5 μg (∼0.2 mg/kg), 2.5 μg, 1.25 μg, or 0.6 μg per mouse 24 h prior to being challenged with 10× LD50 ricin (Fig. 2C). c-PB10 demonstrated a dose-dependent capacity to protect mice against ricin intoxication; 100% protection was achieved with 5 μg, 75% protection with 2.5 μg, and 40% protection was achieved with 1.25 μg, while the lowest dose of c-PB10 (0.6 μg) afforded no protection. c-JB4 proved to be considerably less effective than c-PB10 in that even the 5-μg amount of the chimeric MAb was insufficient to protect mice from ricin-induced death (Fig. 2C). Based on these data, c-PB10 was selected for further evaluation as a putative ricin immunotherapeutic.
To examine the therapeutic potential of c-PB10, groups of BALB/c mice were administered a single dose of c-PB10 (25 μg [∼1 mg/kg]) at hourly intervals following a 10× LD50 ricin toxin challenge (Fig. 2D and E). Control mice that did not receive c-PB10 succumbed to ricin intoxication within 48 h, whereas mice that received c-PB10 at 2 or 3 h after toxin challenge survived. Mice treated with c-PB10 at the 2- to 3-h time points experienced a transient reduction in blood glucose levels between 24 and 48 h, but those levels trended to baseline levels thereafter. Administration of c-PB10 4 or 5 h after ricin toxin challenge proved sufficient to protect 90% of the test animals, whereas antibody treatment at 6 h afforded only 30% protection. Reductions in blood glucose levels were correspondingly severe in the mice given c-PB10 at 4 to 6 h after ricin challenge. Overall, these data indicate that the therapeutic window in mice for antibody-mediated rescue from ricin intoxication is between 3 and 4 h, a result which is in accordance with what we observed previously with c-GD12 (24).
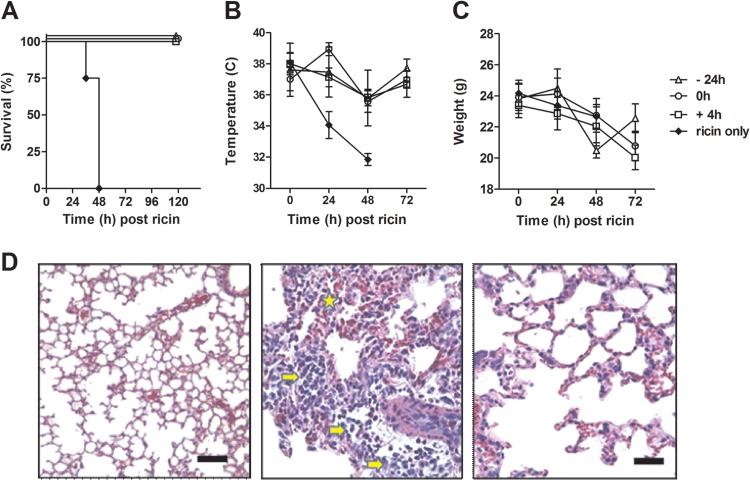
The respiratory tract is the compartment most vulnerable to ricin intoxication, and assessing the efficacy of potential therapeutic agents to protect against toxin exposure by this route is critical (8). To assess c-PB10 as a protectant against aerosolized ricin, BALB/c mice were administered a single intraperitoneal injection of ∼250 μg (10 mg/kg) of c-PB10 at one of three time points: 24 h before ricin exposure, at the time of ricin exposure, or 4 h after ricin exposure. As it is not known what concentrations of serum antibody are required to confer protective immunity to aerosol challenge, c-PB10 was administered to mice at a dose (i.e., 10 mg/kg) that would be considered toward the upper limit of what would be acceptable for a therapeutic antibody. Ricin exposure was performed in a whole-body inhalation exposure chamber (10). Aerosols were generated into the exposure system using a three-jet Collison nebulizer (BGI, Inc., Waltham, MA) operated at 20 pressure square inch gauge with an output flow of 7.5 l/min. Time-of-flight aerodynamic particle size distribution measured prior to the exposure using an aerodynamic particle sizer (APS) (model 3321; TSI, Inc., St. Paul, MN) indicated a highly respirable particle size distribution (mass median aerodynamic diameter [MMAD], 1 μm; geometric standard deviation (σg) = 1.4). Control mice that were exposed to ricin but not treated with c-PB10 experienced a rapid decrease in core body temperature and body weight, and then died within 48 h (Fig. 3A through C). Conversely, mice that received c-PB10 (irrespective of the time point of c-PB10 administration) experienced weight loss and a slight decrease in core body temperature but ultimately survived challenge. Histopathologic analysis of lung tissues revealed that systemic c-PB10 administration greatly damped neutrophil cell infiltration and toxin-induced tissue damage in ricin-challenged mice compared to control toxin-challenged mice (Fig. 3D). These experiments demonstrate that c-PB10 is sufficient in mice to serve as a protectant and therapeutic agent against aerosolized ricin challenge. However, we recognize that more comprehensive dose-response studies are necessary to determine the effectiveness of c-PB10 at lower concentrations. Such experiments are currently planned but are beyond the scope of this current article.
FIG 3.
c-PB10 protects mice against aerosolized ricin exposure. BALB/c mice (female, 18 to 20 g, 8 to 10 weeks of age) purchased from Harlan Laboratories (Indianapolis, IN) were administered a single i.p. injection of c-PB10 (10 mg/kg) 24 h prior (−24 h, open triangle), immediately after (0 h, open circles), or 4 h after (+4 h, open squares) aerosol exposure to ricin toxin (16.8 ± 3.8 μg/kg/animal [∼5× LD50]). Control animals (solid circles) were treated with sterile phosphate-buffered saline (PBS) immediately after ricin exposure. All animal studies conducted at the Tulane National Primate Research Center were done with approval from Tulane University's IACUC. (A) Survival of mice (n = 10 for each group) over a 5-day period. (B and C) Mean changes in core body temperatures and weight of groups of mice after ricin exposure. (D) Representative hematoxylin and eosin (H&E)-stained sections of lung tissues from unexposed control mice (left), a ricin-exposed mouse (middle), or a ricin-exposed mouse that received c-PB10 at time 0 h (right). Note inflammatory cells (e.g., neutrophils [arrow]) in alveolar spaces and evidence of hemorrhage (star) in ricin-challenged mouse but not c-PB10-treated mouse. Bar, 100 μm.
In summary, we generated mouse-human chimeric IgG1 derivatives of four ricin toxin-neutralizing MAbs and then expressed and purified them using a Nicotiana-based platform. The chimeric plantibodies, two directed against RTA (c-PB10 and c-SyH7) and two against RTB (c-JB4 and c-SylH3), were tested for their capacity to neutralize ricin in vitro and in vivo. We found that c-PB10 was the most effective of the four chimeric MAbs (and more effective than the previously described c-GD12) in that (i) it had the lowest IC50 in a Vero cell-based toxin-neutralizing assay, (ii) it was sufficient to passively protect mice against systemic and aerosol toxin challenge, and (iii) it had demonstrable therapeutic potential, in that it rescued mice from the effects of ricin when administered up to 3 to 4 h after toxin challenge. In addition, preliminary studies indicated that scale-up production of c-PB10 using the N. benthamiana-based RAMP is readily achievable (J. Morton and L. Zeitlin, unpublished observations). Although not investigated in this study, we have tested c-PB10 in combination with the other three chimeric MAbs; no enhanced toxin-neutralizing activity was observed in any of the combinations tested, indicating that c-PB10 alone affords maximal antiricin activity in vitro (E. Sully and N. Mantis, unpublished results). Collectively, these data make c-PB10 an ideal candidate for further development as a fully humanized immunoprotectant against ricin toxin, either as a stand-alone countermeasure or as part of a cocktail with other category B toxin-specific antibodies.
While active vaccination of mice and rabbits has been shown to elicit serum antibody titers that are sufficient to confer immunity against aerosolized ricin challenge, our study is the first to demonstrate that immunity in the respiratory tract can be achieved by MAb therapy (14, 31, 32). PB10 is known to recognize a conserved, immunodominant linear epitope (residues 98 to 106) within RTA's so-called α-helix B, which is a well-established target of potent toxin-neutralizing antibodies (26, 33, 34). Very recent studies have indicated that c-PB10 is a type II antibody in that it apparently neutralizes ricin not by blocking toxin attachment to cell surface receptors, but by interfering with toxin retrograde transport (A. Yermakova, J. O'Hara, T.-I. Klokk, K. Sandvig, and N. Mantis, manuscript in preparation). We postulate, therefore, that in the aerosol challenge model, c-PB10 likely neutralizes ricin on the epithelial surfaces of the respiratory tract, although it remains to be determined whether c-PB10 is found in mucosal secretions at the time of challenge and, if so, whether transudation or active transport is responsible for delivery of the antibody into this compartment (35). Therefore, moving forward it will also be important to resolve whether a secretory IgA (SIgA) derivative of c-PB10 affords any benefit over the IgG form of c-PB10 in mucosal protection.
Supplementary Material
ACKNOWLEDGMENTS
We thank Victor Klimyuk and Yuri Gleba (Icon Genetics, Halle, Germany) for providing access to magnICON and Herta Steinkellner (BOKU, Vienna, Austria) for access to the glycosylation-modified plants. We also gratefully acknowledge Joanne O'Hara (Wadsworth Center) for valuable feedback and technical assistance.
E.K.S. was supported by a Biodefense and Emerging Infectious Diseases (BD-EID) training grant from the National Institutes of Health (T32 AI055429; principal investigator, K. A. McDonough). This work was supported by NIH awards 5R43AI91078 and AI098774 (to L.Z.). This work was supported in part through the NIH/OD grant OD-011104-51 (Tulane National Primate Research Center Base grant).
Footnotes
Published ahead of print 26 February 2014
Supplemental material for this article may be found at http://dx.doi.org/10.1128/CVI.00003-14.
REFERENCES
- 1. Stirpe F, Battelli MG. 2006. Ribosome-inactivating proteins: progress and problems. Cell. Mol. Life Sci. 63:1850–1866. 10.1007/s00018-006-6078-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Endo Y, Mitsui K, Motizuki M, Tsurugi K. 1987. The mechanism of action of ricin and related toxins on eukaryotic ribosomes. The site and the characteristics of the modification in 28 S ribosomal RNA caused by the toxins. J. Biol. Chem. 262:5908–5912 [PubMed] [Google Scholar]
- 3. Voorhees RM, Schmeing TM, Kelley AC, Ramakrishnan V. 2010. The mechanism for activation of GTP hydrolysis on the ribosome. Science 330:835–838. 10.1126/science.1194460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sandvig K, Olsnes S, Pihl A. 1976. Kinetics of binding of the toxic lectins abrin and ricin to surface receptors of human cells. J. Biol. Chem. 251:3977–3984 [PubMed] [Google Scholar]
- 5. Sandvig K, Olsnes S. 1979. Effect of temperature on the uptake, excretion and degradation of abrin and ricin by HeLa cells. Exp. Cell Res. 121:15–25. 10.1016/0014-4827(79)90439-7 [DOI] [PubMed] [Google Scholar]
- 6. Endo Y, Tsurugi K. 1987. RNA N-glycosidase activity of ricin A-chain. Mechanism of action of the toxic lectin ricin on eukaryotic ribosomes. J. Biol. Chem. 262:8128–8130 [PubMed] [Google Scholar]
- 7. Olsnes S, Fernandez-Puentes C, Carrasco L, Vazquez D. 1975. Ribosome inactivation by the toxic lectins abrin and ricin. Kinetics of the enzymic activity of the toxin A-chains. Eur. J. Biochem. 60:281–288 [DOI] [PubMed] [Google Scholar]
- 8. Roy CJ, Song K, Sivasubramani SK, Gardner DJ, Pincus SH. 2012. Animal models of ricin toxicosis. Curr. Top. Microbiol. Immunol. 357:243–257. 10.1007/82_2011_173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Brown RF, White DE. 1997. Ultrastructure of rat lung following inhalation of ricin aerosol. Int. J. Exp. Path. 78:267–276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. DaSilva L, Cote D, Roy C, Martinez M, Duniho S, Pitt ML, Downey T, Dertzbaugh M. 2003. Pulmonary gene expression profiling of inhaled ricin. Toxicon. 41:813–822. 10.1016/S0041-0101(03)00035-7 [DOI] [PubMed] [Google Scholar]
- 11. Doebler JA, Wiltshire ND, Mayer TW, Estep JE, Moeller RB, Traub RK, Broomfield CA, Calamaio CA, Thompson WL, Pitt ML. 1995. The distribution of [125I]ricin in mice following aerosol inhalation exposure. Toxicology 98:137–149. 10.1016/0300-483X(94)02978-4 [DOI] [PubMed] [Google Scholar]
- 12. Griffiths GD, Phillips GJ, Bailey SC. 1999. Comparison of the quality of protection elicited by toxoid and peptide liposomal vaccine formulations against ricin as assessed by markers of inflammation. Vaccine 17:2562–2568. 10.1016/S0264-410X(99)00054-7 [DOI] [PubMed] [Google Scholar]
- 13. Lindauer ML, Wong J, Iwakura Y, Magun BE. 2009. Pulmonary inflammation triggered by ricin toxin requires macrophages and IL-1 signaling. J. Immunol. 183:1419–1426. 10.4049/jimmunol.0901119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. McLain DE, Lewis BS, Chapman JL, Wannemacher RW, Lindsey CY, Smith LA. 2011. Protective effect of two recombinant ricin subunit vaccines in the New Zealand white rabbit subjected to a lethal aerosolized ricin challenge: survival, immunological response and histopathological findings. Toxicol. Sci. 126:72–83. 10.1093/toxsci/kfr274 [DOI] [PubMed] [Google Scholar]
- 15. Wilhelmsen CL, Pitt ML. 1996. Lesions of acute inhaled lethal ricin intoxication in rhesus monkeys. Vet. Pathol. 33:296–302. 10.1177/030098589603300306 [DOI] [PubMed] [Google Scholar]
- 16. Reisler RB, Smith LA. 2012. The need for continued development of ricin countermeasures. Adv. Prev Med. 2012:149737. 10.1155/2012/149737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wolfe DN, Florence W, Bryant P. 2013. Current biodefense vaccine programs and challenges. Hum. Vaccin. Immunother. 9:1591–1597. 10.4161/hv.24063 [DOI] [PubMed] [Google Scholar]
- 18. Olinger GG, Jr, Pettitt J, Kim D, Working C, Bohorov O, Bratcher B, Hiatt E, Hume SD, Johnson AK, Morton J, Pauly M, Whaley KJ, Lear CM, Biggins JE, Scully C, Hensley L, Zeitlin L. 2012. Delayed treatment of Ebola virus infection with plant-derived monoclonal antibodies provides protection in rhesus macaques. Proc. Natl. Acad. Sci. U. S. A. 109:18030–18035. 10.1073/pnas.1213709109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Pettitt J, Zeitlin L, Kim do H, Working C, Johnson JC, Bohorov O, Bratcher B, Hiatt E, Hume SD, Johnson AK, Morton J, Pauly MH, Whaley KJ, Ingram MF, Zovanyi A, Heinrich M, Piper A, Zelko J, Olinger GG. 2013. Therapeutic intervention of Ebola virus infection in rhesus macaques with the MB-003 monoclonal antibody cocktail. Sci. Transl. Med. 5:199ra113. 10.1126/scitranslmed.3006608 [DOI] [PubMed] [Google Scholar]
- 20. Whaley KJ, Hiatt A, Zeitlin L. 2011. Emerging antibody products and Nicotiana manufacturing. Hum. Vaccin. 7:349–356. 10.4161/hv.7.3.14266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Whaley KJ, Morton J, Hume S, Hiatt E, Bratcher B, Klimyuk V, Hiatt A, Pauly M, Zeitlin L. 2014. Emerging antibody-based products. Curr. Top. Microbiol. Immunol. 375:107–126. 10.1007/82_2012_240 [DOI] [PubMed] [Google Scholar]
- 22. Giritch A, Marillonnet S, Engler C, van Eldik G, Botterman J, Klimyuk V, Gleba Y. 2006. Rapid high-yield expression of full-size IgG antibodies in plants coinfected with noncompeting viral vectors. Proc. Natl. Acad. Sci. U. S. A. 103:14701–14706. 10.1073/pnas.0606631103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Garabagi F, McLean MD, Hall JC. 2012. Transient and stable expression of antibodies in Nicotiana species. Methods Mol. Biol. 907:389–408. 10.1007/978-1-61779-974-7_23 [DOI] [PubMed] [Google Scholar]
- 24. O'Hara JM, Whaley K, Pauly M, Zeitlin L, Mantis NJ. 2012. Plant-based expression of a partially humanized neutralizing monoclonal IgG directed against an immunodominant epitope on the ricin toxin A subunit. Vaccine 30:1239–1243. 10.1016/j.vaccine.2011.12.058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. O'Hara JM, Kasten-Jolly JC, Reynolds CE, Mantis NJ. 2013. Localization of non-linear neutralizing B cell epitopes on ricin toxin's enzymatic subunit (RTA). Immunol. Lett. 158:7–13. 10.1016/j.imlet.2013.11.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. O'Hara JM, Neal LM, McCarthy EA, Kasten-Jolly JA, Brey RN, III, Mantis NJ. 2010. Folding domains within the ricin toxin A subunit as targets of protective antibodies. Vaccine 28:7035–7046. 10.1016/j.vaccine.2010.08.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Yermakova A, Mantis NJ. 2011. Protective immunity to ricin toxin conferred by antibodies against the toxin's binding subunit (RTB). Vaccine 29:7925–7935. 10.1016/j.vaccine.2011.08.075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Yermakova A, Vance DJ, Mantis NJ. 2012. Sub-domains of ricin's B subunit as targets of toxin neutralizing and non-neutralizing monoclonal antibodies. PLoS One 7:e44317. 10.1371/journal.pone.0044317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lefranc MP, Giudicelli V, Ginestoux C, Jabado-Michaloud J, Folch G, Bellahcene F, Wu Y, Gemrot E, Brochet X, Lane J, Regnier L, Ehrenmann F, Lefranc G, Duroux P. 2009. IMGT, the international ImMunoGeneTics information system. Nucleic Acids Res. 37:D1006–D1012. 10.1093/nar/gkn838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Schähs M, Strasser R, Stadlmann J, Kunert R, Rademacher T, Steinkellner H. 2007. Production of a monoclonal antibody in plants with a humanized N-glycosylation pattern. Plant Biotechnol. J. 5:657–663. 10.1111/j.1467-7652.2007.00273.x [DOI] [PubMed] [Google Scholar]
- 31. Pratt TS, Pincus SH, Hale ML, Moreira AL, Roy CJ, Tchou-Wong KM. 2007. Oropharyngeal aspiration of ricin as a lung challenge model for evaluation of the therapeutic index of antibodies against ricin A-chain for post-exposure treatment. Exp. Lung Res. 33:459–481. 10.1080/01902140701731805 [DOI] [PubMed] [Google Scholar]
- 32. Smallshaw JE, Richardson JA, Vitetta ES. 2007. RiVax, a recombinant ricin subunit vaccine, protects mice against ricin delivered by gavage or aerosol. Vaccine 25:7459–7469. 10.1016/j.vaccine.2007.08.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lemley PV, Amanatides P, Wright DC. 1994. Identification and characterization of a monoclonal antibody that neutralizes ricin toxicity in vitro and in vivo. Hybridoma. 13:417–421. 10.1089/hyb.1994.13.417 [DOI] [PubMed] [Google Scholar]
- 34. Vance DJ, Mantis NJ. 2012. Resolution of two overlapping neutralizing B cell epitopes within a solvent exposed, immunodominant alpha-helix in ricin toxin's enzymatic subunit. Toxicon 60:874–877. 10.1016/j.toxicon.2012.06.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Baker K, Qiao SW, Kuo T, Kobayashi K, Yoshida M, Lencer WI, Blumberg RS. 2009. Immune and non-immune functions of the (not so) neonatal Fc receptor, FcRn. Semin. Immunopathol. 31:223–236. 10.1007/s00281-009-0160-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Pincus SH, Eng Cooke LCL, Maddaloni M. 2002. Identification of hypoglycemia in mice as a surrogate marker of ricin toxicosis. Comp. Med. 52:530–533 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.