Abstract
The environmental Gram-negative encapsulated bacillus Burkholderia pseudomallei is the causative agent of melioidosis, a disease associated with high morbidity and mortality rates in areas of Southeast Asia and northern Australia in which the disease is endemic. B. pseudomallei is also classified as a tier I select agent due to the high level of lethality of the bacterium and its innate resistance to antibiotics, as well as the lack of an effective vaccine. Gram-negative bacteria, including B. pseudomallei, secrete outer membrane vesicles (OMVs) which are enriched with multiple protein, lipid, and polysaccharide antigens. Previously, we demonstrated that immunization with multivalent B. pseudomallei-derived OMVs protects highly susceptible BALB/c mice against an otherwise lethal aerosol challenge. In this work, we evaluated the protective efficacy of OMV immunization against intraperitoneal challenge with a heterologous strain because systemic infection with phenotypically diverse environmental B. pseudomallei strains poses another hazard and a challenge to vaccine development. We demonstrated that B. pseudomallei OMVs derived from strain 1026b afforded significant protection against septicemic infection with B. pseudomallei strain K96243. OMV immunization induced robust OMV-, lipopolysaccharide-, and capsular polysaccharide-specific serum IgG (IgG1, IgG2a, and IgG3) and IgM antibody responses. OMV-immune serum promoted bacterial killing in vitro, and passive transfer of B. pseudomallei OMV immune sera protected naive mice against a subsequent challenge. These results indicate that OMV immunization provides antibody-mediated protection against acute, rapidly lethal sepsis in mice. B. pseudomallei-derived OMVs may represent an efficacious multivalent vaccine strategy against melioidosis.
INTRODUCTION
Burkholderia pseudomallei is a Gram-negative, encapsulated, facultative, intracellular bacillus and the causative agent of melioidosis, a major public health concern in the regions of Southeast Asia and northern Australia in which the disease is endemic (1). Recent reports have expanded the zone of endemicity to include the Indian subcontinent, southern China, Hong Kong, and Taiwan (2). Sporadic cases occur in Brazil, elsewhere in the Americas, and in the islands of the Pacific Ocean and the Indian Ocean (1, 2). In northern Thailand, the incidence increased from 8.0 cases per 100,000 persons in 2000 to 21.3 cases per 100,000 persons in 2006, with a mortality rate of 42.6%, making melioidosis the third leading cause of death from infection in that region (after HIV/AIDS and tuberculosis) (3). Infection with B. pseudomallei can occur through inhalation of contaminated soil or aerosols, ingestion of contaminated food or water, or percutaneous inoculation via penetrating injuries or preexisting abrasions in the skin (1). The clinical symptoms of melioidosis are nonspecific and can range from asymptomatic disease to acute, rapidly progressive pneumonia, sepsis, and death (1). Chronic infection with B. pseudomallei also occurs, and reactivation of latent infection several decades after exposure has been documented (4). Treatment of melioidosis is challenging, as B. pseudomallei is naturally resistant to multiple antibiotics and establishes an intracellular niche within host cells (5). There is no commercially available vaccine for human use, although numerous vaccine candidates are currently in preclinical stages of investigation (6–8). Beyond its public health significance, B. pseudomallei has bioweapon potential and is listed as a tier 1 select agent, further emphasizing the urgent need for a protective vaccine.
The protean clinical manifestations observed in human melioidosis cases may result from differences in bacterial strains, virulence, or doses, routes of infection, and host immune status (1), each of which complicates vaccine development. A 20-year study conducted in Australia determined that the principal case presentation was pneumonia, which occurred in 51% of melioidosis cases, with 49% case fatality. Bacteremia was present in 55% of melioidosis cases, and septic shock developed in 21% of cases (9). Death due to sepsis has been observed in 30 to 50% of melioidosis cases occurring in areas in which the disease is endemic, as well as those in the Western Hemisphere (10). Therefore, an ideal vaccine against B. pseudomallei would be one capable of providing long-term protection against both pneumonic and septicemic melioidosis. An additional barrier to vaccine development is the presence of virulent coendemic strains, such as B. pseudomallei strains K96243, 1026b, 1710b, and 1106a, all of which were isolated from human clinical samples in Thailand (11). B. pseudomallei isolates demonstrate genotypic and phenotypic heterogeneity (12), so it is imperative that a B. pseudomallei vaccine provide broad-spectrum protection against multiple strains.
In preclinical studies, immunization with live attenuated B. pseudomallei strains has generated some of the best protection observed to date (6–8); however, the ability of B. pseudomallei to establish latent infections poses safety concerns regarding the use of live vaccines, particularly in immunocompromised individuals who are predisposed to infections (13). A number of purified subunit antigen preparations, including lipopolysaccharide (LPS), capsular polysaccharide (CPS), and native or recombinant proteins, have been evaluated and provide variable degrees of protection against B. pseudomallei in small-animal models (6–8). While these preparations offer increased safety over the use of live vaccines, it is unclear whether immunization with a single antigen would be capable of providing complete protection against diverse B. pseudomallei strains and against more than one route of infection.
We previously demonstrated that immunization with multivalent outer membrane vesicles (OMVs) derived from B. pseudomallei strain 1026b provided significant protection against pneumonic melioidosis in mice (14). OMVs are noninfectious particles that are naturally secreted from the Gram-negative bacterial cell membrane (15). The process of extracellular membrane vesicle secretion is conserved among Gram-negative and Gram-positive bacteria, mycobacteria, and eukaryotic cells, although the mechanisms of secretion may differ (15, 16). Gram-negative bacteria release outer membrane, periplasmic, and cytoplasmic components within OMVs that may serve functions in pathogenesis, immunomodulation, communication, and genetic exchange (15). OMVs contain an assortment of virulence factors and Toll-like receptor agonists within the vesicle lumen and on the surface, most of which retain their native orientations and functions (15). For these reasons, OMVs represent a practical acellular multivalent vaccine platform. The multiantigenic nature of an OMV vaccine may enhance the potential to provide broad-spectrum protection against diverse B. pseudomallei strains and multiple routes of infection with a single vaccine preparation.
In this study, we evaluated the protective efficacy of a multivalent OMV vaccine derived from B. pseudomallei strain 1026b against septicemic infection with a heterologous clinical isolate, B. pseudomallei strain K96243. We demonstrate that immunization of mice with a nonadjuvanted OMV vaccine provides significant protection against rapidly lethal sepsis caused by high-dose intraperitoneal (i.p.) challenge with a heterologous strain. Furthermore, we show that protection against acute B. pseudomallei infection (up to 14 days) is mediated by serum antibodies composed of protein- and polysaccharide-specific IgM and IgG.
MATERIALS AND METHODS
Ethics statement.
This study was performed in strict accordance with the National Institutes of Health (NIH) guide for the care and use of laboratory animals. The protocols were approved by the Tulane University institutional animal care and use committee (protocols 4042 and 4048) and the animal care and use committee of the University of Texas Medical Branch (protocol 0503014). For survival studies, death was not used as an endpoint. Animals were humanely euthanized once they displayed >20% weight loss, exhibited paralysis, or were unresponsive to handling. Animals were observed at least three times per day, including weekends. Euthanasia was performed by CO2 overdose and was confirmed by cervical dislocation.
OMV purification.
OMVs were prepared as previously described (14, 17), with minor modifications. B. pseudomallei strain 1026b (BEI Resources) was freshly streaked from a glycerol stock onto Pseudomonas isolation agar (PIA) and incubated for 48 to 72 h at 37°C. An individual colony of B. pseudomallei was inoculated into Luria broth (LB) and incubated for 16 to 18 h at 37°C. The overnight culture was diluted 1:100 into fresh LB and incubated at 37°C until late log phase (optical density at 600 nm [OD600] of 4.5 to 5.0) was reached, between 16 and 18 h. Intact bacteria were pelleted by centrifugation (6,000 × g for 10 min at 4°C) using an SLA-1500 fixed-angle rotor. Following centrifugation, the supernatant was filtered through a 0.22-μm polyethersulfone (PES) membrane (Millipore) in order to remove any remaining bacteria or large bacterial fragments. The absence of bacterial contamination was verified by incubating two 0.5-ml aliquots of supernatant on LB agar for 48 to 72 h at 37°C. OMVs were precipitated by overnight incubation with 1.5 M ammonium sulfate (Fisher Scientific) and then were harvested by centrifugation (11,000 × g for 20 min at 4°C) using an SLA-1500 rotor. Crude vesicles were resuspended in 60% sucrose (Sigma) in 10 mM Tris-HCl (pH 7.4), layered at the bottom of a 35 to 60% density gradient, and subjected to ultracentrifugation (200,000 × g for 3 h at 4°C) using a 50.2Ti rotor. Fractions of equal volume were removed from the top, individually subjected to trichloroacetic acid (TCA) precipitation, and then evaluated by SDS-PAGE for visualization of protein profiles by Coomassie blue staining, as previously described (see Fig. S1 in the supplemental material) (14, 17). Fractions containing identical protein profiles were pooled (see Fig. S1 in the supplemental material) and subjected to ultracentrifugation (200,000 × g for 1.5 h at 4°C) to obtain purified vesicles. Purified vesicles were resuspended in LPS-free water, visually confirmed by transmission electron microscopy (see Fig. S2 in the supplemental material), and quantitated by the Bradford assay, as previously described (14, 17).
Active immunization and challenge.
Female BALB/c mice (8 to 10 weeks of age) were purchased from Charles River Laboratories (Wilmington, MA) and were maintained at 5 per cage in polystyrene microisolator units, under pathogen-free conditions. Animals were fed sterile rodent chow and water ad libitum and were allowed to acclimate for 1 week prior to use.
In total, three independent active immunization and challenge experiments were performed, using three individually extracted batches of OMVs, to confirm reproducibility (see Fig. S3 in the supplemental material). On day 0, BALB/c mice were immunized subcutaneously (s.c.) with 5 μg of B. pseudomallei strain 1026b-derived OMVs diluted in 100 μl of sterile saline. Control (naive) animals received vehicle only (saline). OMV-immunized groups received boosts on days 21 and 42. A subset of immunized mice (n = 5 per group) was utilized to evaluate antibody responses to OMV vaccination, and the mice were not challenged. Five weeks after the last immunization, immunized and control mice were challenged intraperitoneally (i.p.) with target doses of 5× the 50% lethal dose (LD50) (∼2 × 104 CFU) and 50× LD50 (∼2 × 105 CFU) of B. pseudomallei strain K96243 (BEI Resources). Actual infectious doses delivered to the mice were determined by plating the inocula. Survival was monitored up to 21 days postinfection. A subset of mice (n = 4) was euthanized weekly, and spleens were harvested in order to assess persistent infection. Tissues were aseptically removed from euthanized animals, individually placed in 1 ml 0.9% NaCl, and homogenized with sterile disposable tissue grinders (Fisher Scientific). Tenfold serial dilutions of spleen homogenates were plated on PIA. Colonies were counted after incubation for 3 days at 37°C, and results are reported as CFU per organ.
Passive immunization and challenge.
For passive transfer experiments, BALB/c mice (n = 5 per group) were given 300 μl of pooled OMV-immune sera obtained from mice that had been actively immunized with 2.5 μg of B. pseudomallei-derived OMVs or control sera from nonimmunized mice. Burkholderia OMV-immune sera contained 750 μg/ml of B. pseudomallei OMV-specific IgG, whereas control sera contained less than 0.3 μg/ml of B. pseudomallei OMV-specific IgG, as determined by an enzyme-linked immunosorbent assay (ELISA). Sera were administered by i.p. injection 1 h prior to challenge. Naive mice (n = 5 per group) received no treatment. Mice were challenged i.p. with approximately 50× LD50 of B. pseudomallei strain K96243. Two independent experiments were performed; survival was monitored up to day 14.
Characterization of antigen-specific antibody responses.
Serum samples were collected 4 weeks after the final immunization, to evaluate antigen-specific antibody responses. OMV-, LPS-, and CPS-specific IgG, IgG1, and IgG2a serum antibody titers were measured by ELISAs using microtiter plates coated with 500 ng of OMV, LPS, or CPS, as we previously described (14). Purified LPS was provided by Kate Brown (University of Texas at Austin) and was prepared from acapsular Burkholderia thailandensis strain E264 using a modified hot phenol extraction method, as previously described (18). Purified CPS was provided by Don Woods (University of Calgary) and was prepared from an O-antigen-deficient B. pseudomallei mutant strain by using methods described by Perry et al. (19). Measurements of antigen-specific IgM and IgG3 were performed as described above, with minor modifications. Alkaline phosphatase-conjugated goat anti-mouse IgG3 (1:20,000 dilution; Abcam) and alkaline phosphatase-conjugated goat anti-mouse IgM (1:300 dilution; Sigma) secondary antibodies were incubated for 1 h at room temperature prior to development, as previously described (14). Results obtained were expressed as the geometric mean reciprocal endpoint titers for total serum immunoglobulin. The endpoint titer was defined as the greatest dilution yielding an optical density at 405 nm (OD405) greater than 3 standard deviations above the mean OD405 value for preimmune sera. The limit of detection for the assays was the inverse of the initial serum dilution, which was 1:10.
Serum bactericidal assay.
An overnight culture of B. pseudomallei strain K96243 was diluted 1:100 in fresh LB and grown to the log phase (OD600 of 0.64). The bacteria were adjusted to 1 × 106 CFU and incubated with 30% heat-inactivated (56°C for 30 min), pooled, OMV-immune serum or 6 μg/ml anti-CPS monoclonal antibody (MAb) 3C5 (20) (provided by David Aucoin, University of Nevada School of Medicine) in LB broth containing 5% fetal bovine serum (FBS) (Sigma), in a 24-well plate (135 rpm at 37°C). Bacteria incubated in 5% FBS-LB alone were used as negative controls. Four hours after incubation, 10-fold serial dilutions were plated on LB agar and incubated for 24 to 48 h at 37°C. The bacterial counts were reported as CFU/ml. Each experimental group was assayed in triplicate, and two independent experiments were performed.
Statistical analyses.
Statistical analyses were performed using GraphPad Prism version 5.0 (GraphPad Software, San Diego, CA). Statistical analyses of antibody responses were performed using the Mann-Whitney test. Analyses of bacterial survival in serum bactericidal assays were performed using one-way analysis of variance (ANOVA) with the Bonferroni post hoc test. Survival curves were analyzed using a log-rank Mantel-Cox test. P values of <0.05 were considered statistically significant.
RESULTS
Immunization with B. pseudomallei OMVs provides significant protection against systemic challenge with B. pseudomallei strain K96243.
Our previous work demonstrated that mice immunized with OMVs were significantly protected against pneumonic melioidosis following inhalational challenge with B. pseudomallei (14). Percutaneous inoculation with diverse environmental strains of B. pseudomallei also represents a primary route of infection. We therefore tested the capacity of the OMV vaccine to prevent systemic infection in a murine model of sepsis. BALB/c mice were immunized with 5 μg of purified OMVs and challenged intraperitoneally (i.p.) with B. pseudomallei strain K96243. Strain K96243 was selected because of its demonstrated lethality in this challenge model (LD50 of ∼4 × 103 CFU for BALB/c mice) (21) and because i.p. administration of B. pseudomallei strain 1026b (NR-4074) did not cause any deaths in naive animals within 21 days at doses up to 2 × 106 CFU (data not shown). Naive mice rapidly succumbed to i.p. infection with 8 × 105 CFU of strain K96243 and demonstrated 100% mortality rates within 24 h (Fig. 1). In contrast, OMV-immunized mice were significantly protected against lethal septicemic infection (P < 0.001) and displayed 94%, 83%, and 67% survival at days 7, 14, and 21, respectively (Fig. 1; pooled data from two independent experiments). Given the extreme lethality in naive mice, we lowered the challenge dose and repeated the study. OMV-immunized mice challenged with 2 × 104 CFU of B. pseudomallei K96243 were significantly protected in comparison with naive mice (P < 0.05) and demonstrated 100% survival at 21 d postinfection, compared with 40% survival in naive mice.
FIG 1.
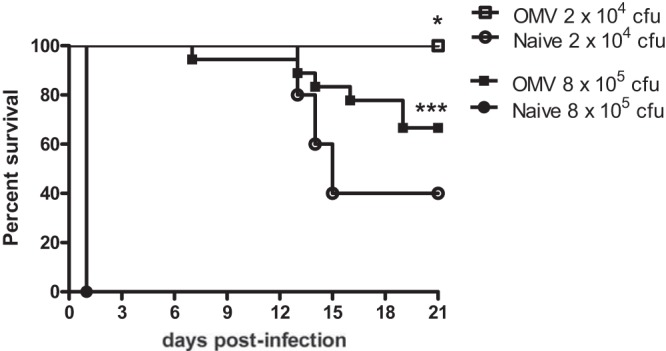
Immunization with B. pseudomallei strain 1026b-derived OMVs protects mice against septicemic infection with a heterologous strain. Naive mice or mice immunized with OMVs were challenged with 2 × 104 CFU (n = 5 mice per group) or 8 × 105 CFU (n = 10 mice per group) of B. pseudomallei strain K96243 by i.p. inoculation. Pooled data from two independent experiments are shown for animals challenged with 8 × 105 CFU. *, P < 0.05; ***, P < 0.001, using a log-rank Mantel-Cox survival analysis.
It was evident that some bacteria evaded immune clearance, leading to colonization and persistence in the spleen and potentially other tissues that were not examined. Bacteria (up to 108 CFU per organ) (see Fig. S4 in the supplemental material) were detected in the spleens of all survivors, indicating that the mice would eventually succumb to the infection. This was not unexpected, because all B. pseudomallei vaccine candidates tested to date, including live attenuated vaccine strains, have failed to provide sterilizing immunity in the BALB/c mouse model (6–8).
Immunization with B. pseudomallei OMVs induces OMV-, LPS-, and CPS-specific antibody responses.
We previously demonstrated that native OMVs derived from broth-grown B. pseudomallei are composed of the protective polysaccharide antigens LPS and CPS, as well as numerous immunogenic proteins (14). However, we did not determine the contribution of LPS or CPS to the antibody response induced by OMV immunization. We postulated that protection against rapidly fatal sepsis in OMV-immunized mice may be due to the presence of OMV-specific as well as LPS- and/or CPS-specific antibodies. We therefore measured the OMV-, LPS-, and CPS-specific serum antibody levels by ELISAs using microtiter plates coated with purified preparations of each antigen. Antibody responses were assessed 4 weeks after immunization by using separate groups of mice that were immunized but not challenged.
Subcutaneous immunization with B. pseudomallei OMVs in the absence of exogenous adjuvant induced significant increases in OMV-specific serum IgM, IgG, IgG1, IgG2a, and IgG3 levels versus naive mice (P < 0.05 for all) (Fig. 2; Table 1). The geometric mean reciprocal endpoint titers for OMV-specific IgM and IgG were 5,572 and 89,248, respectively. Typically, OMV vaccines induce robust type 2 antibody responses (22, 23) that are characterized by IgG1/IgG2a ratios greater than 1 (24). Immunization with B. pseudomallei OMVs produced a type 2 antibody response with an IgG1/IgG2a ratio of 1.8 and with IgG1 > IgG2a > IgG3 (Table 1).
FIG 2.
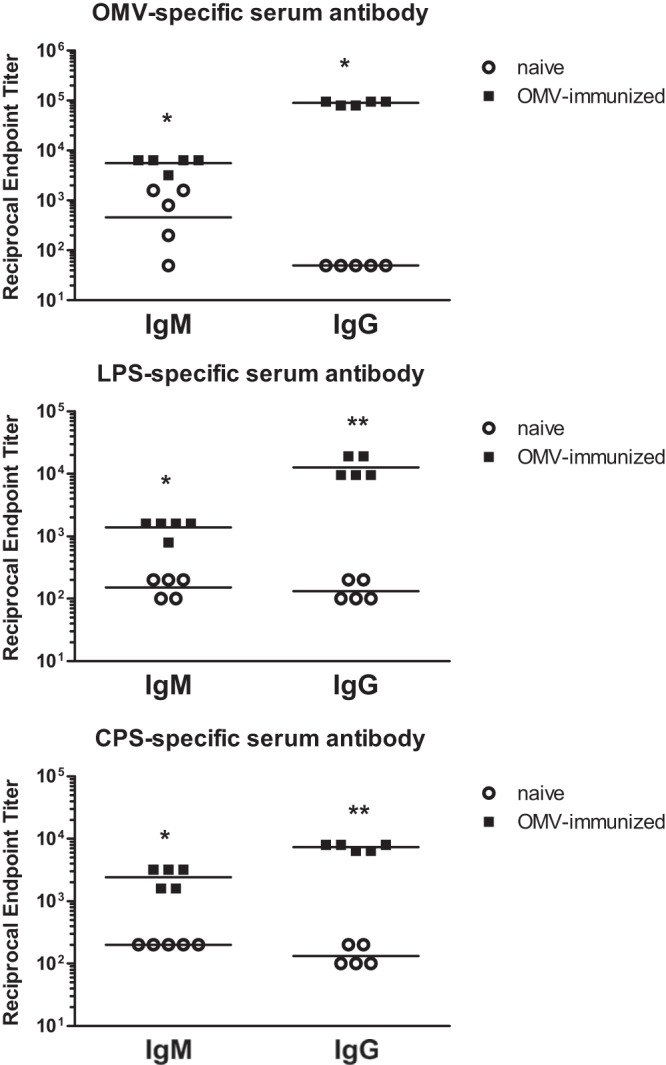
OMV immunization induces OMV-, LPS-, and CPS-specific serum antibodies. OMV-, LPS-, and CPS-specific IgM and IgG titers were measured in the sera of OMV-immunized and naive mice (n = 5) by ELISAs. Horizontal lines, geometric mean values. Statistical significance was determined by comparing the OMV-immunized group with the naive group using the nonparametric Mann-Whitney test. *, P < 0.05; **, P < 0.01.
TABLE 1.
OMV immunization induces OMV-, LPS-, and CPS-specific serum IgG1, IgG2a, and IgG3
| Antigen | Group | Geometric mean titera |
IgG1/IgG2a ratio | ||
|---|---|---|---|---|---|
| IgG1 | IgG2a | IgG3 | |||
| OMV | Naive | 50 | 50 | 50 | 1 |
| OMV | 31,037b | 17,269b | 12,679b | 1.8 | |
| LPS | Naive | 115 | 100 | 50 | 1.2 |
| OMV | 4,800c | 909b | 3,200c | 5.3 | |
| CPS | Naive | 115 | 100 | 50 | 1.2 |
| OMV | 3,482b | 1,056b | 348c | 3.3 | |
The geometric mean of the reciprocal endpoint titer is indicated. Statistical significance was determined by comparing the OMV-immunized group with the naive group using the Mann-Whitney test. P < 0.05 was considered significant.
P = 0.01.
P = 0.009.
Immunization with B. pseudomallei OMVs also induced significant increases in LPS-specific serum IgM (P < 0.05), IgG (P < 0.01), IgG1 (P = 0.009), IgG2a (P = 0.01), and IgG3 (P = 0.009) levels versus naive mice (Fig. 2; Table 1). The IgG1/IgG2a ratio for LPS was 5.3, which indicates a predominant type 2 antibody response to LPS. Similarly, immunization with B. pseudomallei OMVs induced significant increases in CPS-specific serum IgM (P < 0.05), IgG (P < 0.01), IgG1 (P = 0.01), IgG2a (P = 0.01), and IgG3 (P = 0.009) levels (Fig. 2; Table 1). The CPS-specific IgG1/IgG2a ratio was 3.3, again indicative of a type 2 response. For both LPS and CPS, IgG1 was the predominant IgG subtype induced by OMV immunization. Collectively, these data demonstrate that OMV immunization induces high titers of OMV-, LPS-, and CPS-specific serum antibodies.
OMV-immune serum promotes killing of B. pseudomallei.
The ability of OMVs to induce high titers of OMV-, LPS-, and CPS-specific antibodies suggests that the protection observed in OMV-immunized mice may be partially attributable to antibodies. We therefore employed a serum bactericidal assay in order to determine whether OMV-immune serum conferred antibacterial activity. B. pseudomallei strain K96243 was incubated in LB containing 5% FBS only or with the addition of heat-inactivated OMV-immune serum or a monoclonal antibody specific for B. pseudomallei CPS (anti-CPS MAb) that has been shown to provide protection against experimental challenge (20). The numbers of viable bacteria were then determined after 4 h of incubation. B. pseudomallei incubated with FBS alone displayed an increase in bacterial numbers after 4 h (Fig. 3), consistent with previous observations that B. pseudomallei can resist the effects of serum complement (25). In contrast, bacterial cultures incubated in FBS with OMV-immune serum or the anti-CPS MAb contained significantly fewer organisms than did the control cultures after 4 h (P < 0.01) (Fig. 3). These results indicate that OMV-immune serum, obtained from immunized mice, displays antibacterial activity in vitro.
FIG 3.
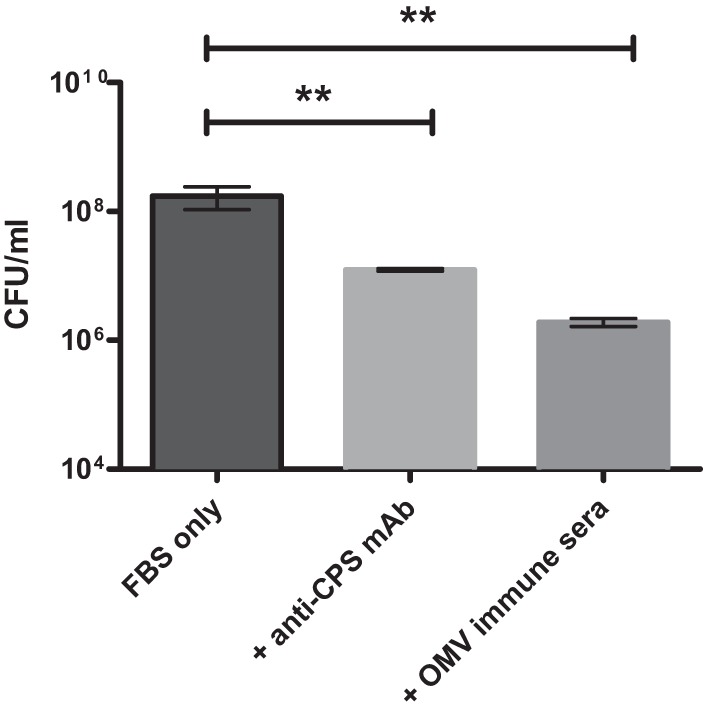
OMV-immune serum promotes killing of B. pseudomallei in vitro. Serum bactericidal assays were performed by incubating 1 × 106 bacteria with FBS alone or with FBS plus heat-inactivated OMV-immune sera or anti-CPS monoclonal antibody at 37°C, with gentle agitation. At 4 h after incubation, samples were serially diluted and plated on LB agar to determine viable CFU/ml. Error bars, mean ± standard error. Experiments were performed in triplicate. **, P < 0.01, using one-way ANOVA.
Passive transfer of OMV-immune sera provides significant protection against B. pseudomallei septicemic infection.
The ability of OMV-immune sera to promote the killing of B. pseudomallei in vitro suggested that antibodies may account for the protection observed in actively immunized mice. In order to further examine antibody-mediated protection, mice were given single doses of OMV-immune sera or control sera and then challenged with 5 × 105 CFU of B. pseudomallei strain K96243 administered i.p. 1 h later. Mice that received OMV-immune sera were significantly protected against infection and displayed 80% survival at day 14, at which point the study was terminated (Fig. 4). The median survival times (MSTs) for the control and naive groups were 1 and 3 days, respectively, compared with >14 days (MST undefined) for the OMV-immune group. Overall survival rates for the naive (20%) and control (0%) groups were not significantly different (log-rank test, P = 0.1), while mice that received OMV-immune sera were significantly protected versus naive (P = 0.01) and control mice (P = 0.002). These results demonstrate that OMV-immune serum, which contains OMV-, LPS-, and CPS-specific antibodies, is sufficient to confer protection against otherwise rapidly lethal septicemic infection with B. pseudomallei.
FIG 4.
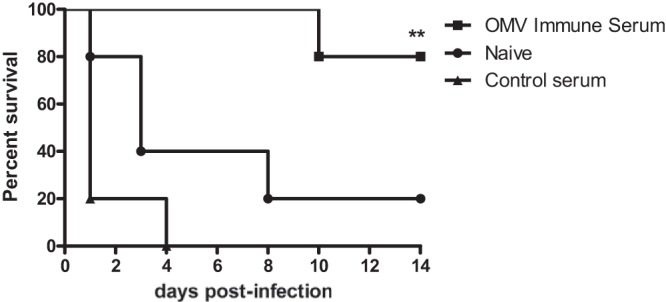
Passive transfer of OMV-immune sera protects mice against septicemic melioidosis. Naive BALB/c mice (n = 5 per group) were treated with OMV-immune sera, were treated with control sera, or were left untreated and were challenged 1 h later with 5 × 105 CFU of B. pseudomallei strain K96243 by i.p. inoculation. Two independent experiments were performed, and survival was monitored for 14 days. **, P < 0.01, compared with control mice using a log-rank Mantel-Cox survival analysis.
DISCUSSION
The increasing prevalence of B. pseudomallei infections, together with the classification of B. pseudomallei as a tier 1 biological threat agent, substantiates the urgent need for a protective vaccine against this organism. Barriers to vaccine development include the presence of phenotypically diverse B. pseudomallei strains, multiple routes of infectivity, and multifaceted disease presentations, including acute and chronic manifestations. While a number of vaccine candidates have demonstrated short-term protection in rodent models of melioidosis, none has provided complete protection against inhalational melioidosis or sterilizing immunity (6–8). We previously demonstrated that active immunization with B. pseudomallei-derived OMVs provided significant protection against lethal aerosol challenge with B. pseudomallei (14). The encouraging results obtained with the multivalent OMV vaccine against inhalational B. pseudomallei challenge warranted further study against other manifestations of the disease. The present study shows that the OMV vaccine provides significant protection against rapidly lethal sepsis produced by systemic challenge with a heterologous B. pseudomallei strain. This finding is significant because it demonstrates the ability of the OMV vaccine to protect against more than one route of challenge and because sepsis caused by B. pseudomallei infection is the most frequent and most lethal form of the disease (3, 9). It is difficult to compare the OMV vaccine study results with other published work, due to differences in challenge strains, doses, and infection routes. However, the protection achieved with the acellular multivalent OMV vaccine is consistent with that provided by cellular multicomponent vaccines such as inactivated whole-cell or live attenuated B. pseudomallei vaccines. For example, immunization with OMVs provided 83% protection up to day 14 after i.p. challenge with 8 × 105 CFU, while immunization with heat-killed B. pseudomallei strain K96243 provided 80% protection at day 14 after challenge with 3.5 × 105 CFU of K96243 (26). Immunization of mice with a live attenuated B. pseudomallei serC-mutant strain provided 80% protection at day 21 after i.p. challenge with 1 × 104 CFU, while OMV immunization provided 100% protection at day 21 after i.p. challenge with 2 × 104 CFU of K96243 (27). Thus, vaccination with OMVs may represent a promising alternative to the use of cell-based B. pseudomallei vaccines.
This study demonstrates that OMV immunization can mediate protection against a B. pseudomallei strain (strain K96243) different from the strain used to derive the OMV vaccine (strain 1026b). The variety of coendemic B. pseudomallei strains capable of causing human infections indicates that a vaccine effective against heterologous B. pseudomallei strains is essential. In this regard, a successful vaccine strategy against melioidosis may require the incorporation of multiple and/or highly conserved antigens that can direct protective immune responses against multiple strains and can minimize the potential for escape variants. Passive transfer of LPS- and CPS-specific monoclonal antibodies, alone or in combination, protected BALB/c mice from intranasal challenge with homologous and heterologous strains, confirming that antibodies directed toward conserved epitopes within these polysaccharide antigens are cross-protective (20). Our studies indicate that B. pseudomallei strain 1026b-derived OMVs provide cross-protection against challenge with the heterologous B. pseudomallei strain K96243. This is associated with induction of an antibody response against multiple OMV antigens, including a subset directed against the LPS and CPS components. The core genome of B. pseudomallei strain K96243 shares 86% sequence identity with those of other sequenced B. pseudomallei strains (28). However, B. pseudomallei strains K96243 and 1026b both possess LPS genotype A, the most frequent genotype (97%) found among Australian and Southeast Asian strains (29). In addition, the O-antigen biosynthesis genes are considered identical or very similar (30). This suggests that protective antibody responses induced by OMV LPS derived from strain 1026b may provide cross-protection to strain K96243 and other strains with genotype A. Similarly, clinical and environmental isolates of B. pseudomallei produce antigenically similar but structurally diverse CPS forms (31–33). Thus, a protective antibody response against OMV CPS may also confer cross-protection to multiple strains. It is important to note that human OMV vaccines against Neisseria meningitidis serogroup B, such as those derived from the Cuban epidemic strain (CU385, B:4:P1.15) or the Norwegian epidemic strain (44/76, B:15:P1.7,16), have typically conferred little cross-protection during epidemics caused by heterologous strains (34, 35). The lack of cross-protection among Neisseria strains is likely due to variation in the immunodominant surface antigen PorA, which largely accounts for the OMV vaccine-mediated antibody protection (36). It is therefore essential to identify and to characterize the protective determinants of any OMV-based vaccine, in order to assess its potential for broad-spectrum protection.
Active immunization with B. pseudomallei OMVs induced a robust OMV-, LPS-, and CPS–specific antibody response, including total IgG, IgG1, IgG2a, IgG3, and IgM. Sera from melioidosis patients contain predominantly IgG1 and IgG2, which react with culture filtrate antigens (CFAs) (37), LPS (38), or crude extracts (38, 39) from B. pseudomallei. Similarly, CFA-reactive serum IgM was detected in 66% of septicemic melioidosis patients (37). Mean CFA- and LPS-specific IgM titers were highest in patients with localized or superficial soft-tissue infections (37, 38), indicating that IgM may help limit systemic infection with B. pseudomallei. Interestingly, only sera from individuals surviving septicemic melioidosis demonstrated a robust LPS-specific IgG3 response (38), indicating that this antibody response may be important for protection against lethal systemic infection. Passive transfer of a LPS- or CPS-specific IgG3 monoclonal antibody protected mice against intranasal challenge and eliminated splenic bacterial colonization in survivors (20). Murine LPS- and CPS-specific IgM and IgG (IgG1, IgG2b, and IgG3) monoclonal antibodies can promote complement-mediated killing and opsonophagocytosis of B. pseudomallei in vitro (40). Thus, circulating OMV-, LPS-, and/or CPS-specific serum IgM and IgG (IgG1, IgG2a, and IgG3) in OMV-immunized mice may account for the significant protection observed against lethal septicemic infection. Although B. pseudomallei can resist complement deposition in naive immune serum (25), OMV-immune serum promoted antibacterial activity against B. pseudomallei, as demonstrated by the serum bactericidal assay. Furthermore, passive transfer of B. pseudomallei OMV-immune sera protected naive BALB/c mice from acute septicemia (up to 14 days postinfection), whereas nonspecific control sera did not confer protection against B. pseudomallei. These results attest to the protective efficacy of the antibody response induced by immunization with B. pseudomallei-derived OMVs. Our results are in agreement with recent work showing that the protection afforded by immunization with a live B. pseudomallei ΔpurM (Bp82) vaccine is dependent primarily on humoral immunity (41). It will be informative to determine the relative contributions of OMV proteins and polysaccharides to OMV vaccine-mediated protection and to evaluate the breadth of cross-protection against additional B. pseudomallei strains in future studies.
Our results emphasize the need to stimulate both humoral and cell-mediated immune responses against B. pseudomallei in order to provide sterile immunity. Survivors demonstrated persistent B. pseudomallei infection, indicating that the antibody response induced by OMV immunization was not sufficient to completely eradicate the bacteria at the challenge doses used. It is possible that the antibody response was suboptimal; however, as the infection progresses and B. pseudomallei establishes intracellular residence, cell-mediated immunity is likely required to facilitate bacterial clearance (42). All B. pseudomallei vaccine candidates, including live attenuated strains, have failed to elicit sterilizing immunity in the BALB/c mouse model (6–8). Moving forward, it may be prudent to evaluate vaccines with Th1-promoting adjuvants or to use mouse strains that are better capable of eliciting Th1 immune responses, such as C57BL/6 (43). It may also be necessary to enrich the OMV cargo with virulence determinants specific to the intracellular stage of infection (44, 45) or with antigens that induce strong T cell responses (46–48). A number of studies have shown that expression and delivery of homologous and heterologous antigens with OMVs are feasible (45, 49, 50). We previously showed that immunization with B. pseudomallei OMVs stimulates memory T cell responses and gamma interferon (IFN-γ) production (14). T cell responses directed against essential antigens expressed strictly by intracellular B. pseudomallei may enhance the ability of the OMV vaccine to eradicate persistent bacteria. Nonetheless, the significant protection against acute sepsis and the delay in disease progression noted for OMV-immunized animals establish a greater window of opportunity for initiation of antibiotic treatment or supportive therapy (10). In conclusion, the protective efficacy achieved with the acellular B. pseudomallei OMV vaccine against septicemic melioidosis is promising and provides further incentive to identify, to incorporate, and to deliver critical protective antigens using the multivalent OMV platform.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by grant U54 AI057156 from the National Institute of Allergy and Infectious Diseases (NIAID) and in part by grant OD-011104-51 from the NIH Office of the Director (Tulane National Primate Research Center Base grant).
The contents are solely the responsibility of the authors and do not necessarily represent the official views of the Regional Centers of Excellence Program, the NIAID, or the NIH.
We are sincerely grateful to David Aucoin for providing monoclonal antibody 3C5, Don Woods and Richard Moore for providing purified CPS, and Kate Brown for providing purified LPS. We thank Saja Asakrah, Nicole Kikendall, and Carrie Griffith for their technical assistance.
Footnotes
Published ahead of print 26 March 2014
Supplemental material for this article may be found at http://dx.doi.org/10.1128/CVI.00119-14.
REFERENCES
- 1. Wiersinga WJ, Currie BJ, Peacock SJ. 2012. Melioidosis. N. Engl. J. Med. 367:1035–1044. 10.1056/NEJMra1204699 [DOI] [PubMed] [Google Scholar]
- 2. Currie BJ, Dance DA, Cheng AC. 2008. The global distribution of Burkholderia pseudomallei and melioidosis: an update. Trans. R. Soc. Trop. Med. Hyg. 102(Suppl 1):S1–S4. 10.1016/S0035-9203(08)70002-6 [DOI] [PubMed] [Google Scholar]
- 3. Limmathurotsakul D, Wongratanacheewin S, Teerawattanasook N, Wongsuvan G, Chaisuksant S, Chetchotisakd P, Chaowagul W, Day NP, Peacock SJ. 2010. Increasing incidence of human melioidosis in Northeast Thailand. Am. J. Trop. Med. Hyg. 82:1113–1117. 10.4269/ajtmh.2010.10-0038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ngauy V, Lemeshev Y, Sadkowski L, Crawford G. 2005. Cutaneous melioidosis in a man who was taken as a prisoner of war by the Japanese during World War II. J. Clin. Microbiol. 43:970–972. 10.1128/JCM.43.2.970-972.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Currie BJ, Fisher DA, Anstey NM, Jacups SP. 2000. Melioidosis: acute and chronic disease, relapse and re-activation. Trans. R. Soc. Trop. Med. Hyg. 94:301–304. 10.1016/S0035-9203(00)90333-X [DOI] [PubMed] [Google Scholar]
- 6. Silva EB, Dow SW. 2013. Development of Burkholderia mallei and pseudomallei vaccines. Front. Cell. Infect. Microbiol. 3:10. 10.3389/fcimb.2013.00010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Peacock SJ, Limmathurotsakul D, Lubell Y, Koh GC, White LJ, Day NP, Titball RW. 2012. Melioidosis vaccines: a systematic review and appraisal of the potential to exploit biodefense vaccines for public health purposes. PLoS Negl. Trop. Dis. 6:e1488. 10.1371/journal.pntd.0001488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Patel N, Conejero L, De Reynal M, Easton A, Bancroft GJ, Titball RW. 2011. Development of vaccines against Burkholderia pseudomallei. Front. Microbiol. 2:198. 10.3389/fmicb.2011.00198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Currie BJ, Ward L, Cheng AC. 2010. The epidemiology and clinical spectrum of melioidosis: 540 cases from the 20 year Darwin prospective study. PLoS Negl. Trop. Dis. 4:e900. 10.1371/journal.pntd.0000900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cheng AC. 2010. Melioidosis: advances in diagnosis and treatment. Curr. Opin. Infect. Dis. 23:554–559. 10.1097/QCO.0b013e32833fb88c [DOI] [PubMed] [Google Scholar]
- 11. Van Zandt KE, Tuanyok A, Keim PS, Warren RL, Gelhaus HC. 2012. An objective approach for Burkholderia pseudomallei strain selection as challenge material for medical countermeasures efficacy testing. Front. Cell. Infect. Microbiol. 2:120. 10.3389/fcimb.2012.00120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Holden MT, Titball RW, Peacock SJ, Cerdeno-Tarraga AM, Atkins T, Crossman LC, Pitt T, Churcher C, Mungall K, Bentley SD, Sebaihia M, Thomson NR, Bason N, Beacham IR, Brooks K, Brown KA, Brown NF, Challis GL, Cherevach I, Chillingworth T, Cronin A, Crossett B, Davis P, DeShazer D, Feltwell T, Fraser A, Hance Z, Hauser H, Holroyd S, Jagels K, Keith KE, Maddison M, Moule S, Price C, Quail MA, Rabbinowitsch E, Rutherford K, Sanders M, Simmonds M, Songsivilai S, Stevens K, Tumapa S, Vesaratchavest M, Whitehead S, Yeats C, Barrell BG, Oyston PC, Parkhill J. 2004. Genomic plasticity of the causative agent of melioidosis, Burkholderia pseudomallei. Proc. Natl. Acad. Sci. U. S. A. 101:14240–14245. 10.1073/pnas.0403302101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Limmathurotsakul D, Chaowagul W, Chierakul W, Stepniewska K, Maharjan B, Wuthiekanun V, White NJ, Day NP, Peacock SJ. 2006. Risk factors for recurrent melioidosis in northeast Thailand. Clin. Infect. Dis. 43:979–986. 10.1086/507632 [DOI] [PubMed] [Google Scholar]
- 14. Nieves W, Asakrah S, Qazi O, Brown KA, Kurtz J, Aucoin DP, McLachlan JB, Roy CJ, Morici LA. 2011. A naturally derived outer-membrane vesicle vaccine protects against lethal pulmonary Burkholderia pseudomallei infection. Vaccine 29:8381–8389. 10.1016/j.vaccine.2011.08.058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kulp A, Kuehn MJ. 2010. Biological functions and biogenesis of secreted bacterial outer membrane vesicles. Annu. Rev. Microbiol. 64:163–184. 10.1146/annurev.micro.091208.073413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Deatherage BL, Cookson BT. 2012. Membrane vesicle release in bacteria, eukaryotes, and archaea: a conserved yet underappreciated aspect of microbial life. Infect. Immun. 80:1948–1957. 10.1128/IAI.06014-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Nieves W, Heang J, Asakrah S, Honer zu Bentrup K, Roy CJ, Morici LA. 2010. Immunospecific responses to bacterial elongation factor Tu during Burkholderia infection and immunization. PLoS One 5:e14361. 10.1371/journal.pone.0014361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Qazi O, Rani M, Gnanam AJ, Cullen TW, Stead CM, Kensing H, McCaul K, Ngugi S, Prior JL, Lipka A, Nagy JM, Gregory CW, Judy BM, Harding SV, Titball RW, Sidhu SS, Trent MS, Kitto GB, Torres A, Estes DM, Iverson B, Georgiou G, Brown KA. 2011. Development of reagents and assays for the detection of pathogenic Burkholderia species. Faraday Discuss. 149:23–36. 10.1039/c005422b [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Perry MB, MacLean LL, Schollaardt T, Bryan LE, Ho M. 1995. Structural characterization of the lipopolysaccharide O antigens of Burkholderia pseudomallei. Infect. Immun. 63:3348–3352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. AuCoin DP, Reed DE, Marlenee NL, Bowen RA, Thorkildson P, Judy BM, Torres AG, Kozel TR. 2012. Polysaccharide specific monoclonal antibodies provide passive protection against intranasal challenge with Burkholderia pseudomallei. PLoS One 7:e35386. 10.1371/journal.pone.0035386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Warawa JM. 2010. Evaluation of surrogate animal models of melioidosis. Front. Microbiol. 1:141. 10.3389/fmicb.2010.00141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bishop AL, Schild S, Patimalla B, Klein B, Camilli A. 2010. Mucosal immunization with Vibrio cholerae outer membrane vesicles provides maternal protection mediated by antilipopolysaccharide antibodies that inhibit bacterial motility. Infect. Immun. 78:4402–4420. 10.1128/IAI.00398-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Holst J, Martin D, Arnold R, Huergo CC, Oster P, O'Hallahan J, Rosenqvist E. 2009. Properties and clinical performance of vaccines containing outer membrane vesicles from Neisseria meningitidis. Vaccine 27(Suppl 2):B3–B12. 10.1016/j.vaccine.2009.04.071 [DOI] [PubMed] [Google Scholar]
- 24. DuBois AB, Freytag LC, Clements JD. 2007. Evaluation of combinatorial vaccines against anthrax and plague in a murine model. Vaccine 25:4747–4754. 10.1016/j.vaccine.2007.03.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Reckseidler-Zenteno SL, DeVinney R, Woods DE. 2005. The capsular polysaccharide of Burkholderia pseudomallei contributes to survival in serum by reducing complement factor C3b deposition. Infect. Immun. 73:1106–1115. 10.1128/IAI.73.2.1106-1115.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sarkar-Tyson M, Smither SJ, Harding SV, Atkins TP, Titball RW. 2009. Protective efficacy of heat-inactivated B. thailandensis, B. mallei or B. pseudomallei against experimental melioidosis and glanders. Vaccine 27:4447–4451. 10.1016/j.vaccine.2009.05.040 [DOI] [PubMed] [Google Scholar]
- 27. Rodrigues F, Sarkar-Tyson M, Harding SV, Sim SH, Chua HH, Lin CH, Han X, Karuturi RK, Sung K, Yu K, Chen W, Atkins TP, Titball RW, Tan P. 2006. Global map of growth-regulated gene expression in Burkholderia pseudomallei, the causative agent of melioidosis. J. Bacteriol. 188:8178–8188. 10.1128/JB.01006-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sim SH, Yu Y, Lin CH, Karuturi RK, Wuthiekanun V, Tuanyok A, Chua HH, Ong C, Paramalingam SS, Tan G, Tang L, Lau G, Ooi EE, Woods D, Feil E, Peacock SJ, Tan P. 2008. The core and accessory genomes of Burkholderia pseudomallei: implications for human melioidosis. PLoS Pathog. 4:e1000178. 10.1371/journal.ppat.1000178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Anuntagool N, Wuthiekanun V, White NJ, Currie BJ, Sermswan RW, Wongratanacheewin S, Taweechaisupapong S, Chaiyaroj SC, Sirisinha S. 2006. Lipopolysaccharide heterogeneity among Burkholderia pseudomallei from different geographic and clinical origins. Am. J. Trop. Med. Hyg. 74:348–352 [PubMed] [Google Scholar]
- 30. Tuanyok A, Stone JK, Mayo M, Kaestli M, Gruendike J, Georgia S, Warrington S, Mullins T, Allender CJ, Wagner DM, Chantratita N, Peacock SJ, Currie BJ, Keim P. 2012. The genetic and molecular basis of O-antigenic diversity in Burkholderia pseudomallei lipopolysaccharide. PLoS Negl. Trop. Dis. 6:e1453. 10.1371/journal.pntd.0001453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Burtnick MN, Heiss C, Roberts RA, Schweizer HP, Azadi P, Brett PJ. 2012. Development of capsular polysaccharide-based glycoconjugates for immunization against melioidosis and glanders. Front. Cell. Infect. Microbiol. 2:108. 10.3389/fcimb.2012.00108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sim BM, Chantratita N, Ooi WF, Nandi T, Tewhey R, Wuthiekanun V, Thaipadungpanit J, Tumapa S, Ariyaratne P, Sung WK, Sem XH, Chua HH, Ramnarayanan K, Lin CH, Liu Y, Feil EJ, Glass MB, Tan G, Peacock SJ, Tan P. 2010. Genomic acquisition of a capsular polysaccharide virulence cluster by non-pathogenic Burkholderia isolates. Genome Biol. 11:R89. 10.1186/gb-2010-11-8-r89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Zou N, Tsai S, Feng SH, Newsome T, Kim HY, Li B, Zhang S, Lo SC. 2008. Relationship between antigenicity and pathogenicity for Burkholderia pseudomallei and Burkholderia mallei revealed by a large panel of mouse MAbs. Hybridoma (Larchmt.) 27:231–240. 10.1089/hyb.2008.0012 [DOI] [PubMed] [Google Scholar]
- 34. Tappero JW, Lagos R, Ballesteros AM, Plikaytis B, Williams D, Dykes J, Gheesling LL, Carlone GM, Hoiby EA, Holst J, Nokleby H, Rosenqvist E, Sierra G, Campa C, Sotolongo F, Vega J, Garcia J, Herrera P, Poolman JT, Perkins BA. 1999. Immunogenicity of 2 serogroup B outer-membrane protein meningococcal vaccines: a randomized controlled trial in Chile. JAMA 281:1520–1527 [DOI] [PubMed] [Google Scholar]
- 35. Oster P, O'Hallahan J, Aaberge I, Tilman S, Ypma E, Martin D. 2007. Immunogenicity and safety of a strain-specific MenB OMV vaccine delivered to under 5-year olds in New Zealand. Vaccine 25:3075–3079. 10.1016/j.vaccine.2007.01.023 [DOI] [PubMed] [Google Scholar]
- 36. van der Voort ER, van der Ley P, van der Biezen J, George S, Tunnela O, van Dijken H, Kuipers B, Poolman J. 1996. Specificity of human bactericidal antibodies against PorA P1.7,16 induced with a hexavalent meningococcal outer membrane vesicle vaccine. Infect. Immun. 64:2745–2751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Chenthamarakshan V, Kumutha MV, Vadivelu J, Puthucheary SD. 2001. Distribution of immunoglobulin classes and IgG subclasses against a culture filtrate antigen of Burkholderia pseudomallei in melioidosis patients. J. Med. Microbiol. 50:55–61 [DOI] [PubMed] [Google Scholar]
- 38. Ho M, Schollaardt T, Smith MD, Perry MB, Brett PJ, Chaowagul W, Bryan LE. 1997. Specificity and functional activity of anti-Burkholderia pseudomallei polysaccharide antibodies. Infect. Immun. 65:3648–3653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Rolim DB, Vilar DC, de Goes Cavalcanti LP, Freitas LB, Inglis TJ, Nobre Rodrigues JL, Nagao-Dias AT. 2011. Burkholderia pseudomallei antibodies in individuals living in endemic regions in northeastern Brazil. Am. J. Trop. Med. Hyg. 84:302–305. 10.4269/ajtmh.2011.10-0220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Zhang S, Feng SH, Li B, Kim HY, Rodriguez J, Tsai S, Lo SC. 2011. In vitro and in vivo studies of monoclonal antibodies with prominent bactericidal activity against Burkholderia pseudomallei and Burkholderia mallei. Clin. Vaccine Immunol. 18:825–834. 10.1128/CVI.00533-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Silva EB, Goodyear A, Sutherland MD, Podnecky NL, Gonzalez-Juarrero M, Schweizer HP, Dow SW. 2013. Correlates of immune protection following cutaneous immunization with an attenuated Burkholderia pseudomallei vaccine. Infect. Immun. 81:4626–4634. 10.1128/IAI.00915-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Healey GD, Elvin SJ, Morton M, Williamson ED. 2005. Humoral and cell-mediated adaptive immune responses are required for protection against Burkholderia pseudomallei challenge and bacterial clearance postinfection. Infect. Immun. 73:5945–5951. 10.1128/IAI.73.9.5945-5951.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Hsieh CS, Macatonia SE, O'Garra A, Murphy KM. 1995. T cell genetic background determines default T helper phenotype development in vitro. J. Exp. Med. 181:713–721. 10.1084/jem.181.2.713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Allwood EM, Devenish RJ, Prescott M, Adler B, Boyce JD. 2011. Strategies for intracellular survival of Burkholderia pseudomallei. Front. Microbiol. 2:170. 10.3389/fmicb.2011.00170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Chen DJ, Osterrieder N, Metzger SM, Buckles E, Doody AM, DeLisa MP, Putnam D. 2010. Delivery of foreign antigens by engineered outer membrane vesicle vaccines. Proc. Natl. Acad. Sci. U. S. A. 107:3099–3104. 10.1073/pnas.0805532107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Harland DN, Chu K, Haque A, Nelson M, Walker NJ, Sarkar-Tyson M, Atkins TP, Moore B, Brown KA, Bancroft G, Titball RW, Atkins HS. 2007. Identification of a LolC homologue in Burkholderia pseudomallei, a novel protective antigen for melioidosis. Infect. Immun. 75:4173–4180. 10.1128/IAI.00404-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Stevens MP, Haque A, Atkins T, Hill J, Wood MW, Easton A, Nelson M, Underwood-Fowler C, Titball RW, Bancroft GJ, Galyov EE. 2004. Attenuated virulence and protective efficacy of a Burkholderia pseudomallei bsa type III secretion mutant in murine models of melioidosis. Microbiology 150:2669–2676. 10.1099/mic.0.27146-0 [DOI] [PubMed] [Google Scholar]
- 48. Tippayawat P, Pinsiri M, Rinchai D, Riyapa D, Romphruk A, Gan YH, Houghton RL, Felgner PL, Titball RW, Stevens MP, Galyov EE, Bancroft GJ, Lertmemongkolchai G. 2011. Burkholderia pseudomallei proteins presented by monocyte-derived dendritic cells stimulate human memory T cells in vitro. Infect. Immun. 79:305–313. 10.1128/IAI.00803-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Muralinath M, Kuehn MJ, Roland KL, Curtiss R., III 2011. Immunization with Salmonella enterica serovar Typhimurium-derived outer membrane vesicles delivering the pneumococcal protein PspA confers protection against challenge with Streptococcus pneumoniae. Infect. Immun. 79:887–894. 10.1128/IAI.00950-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Koeberling O, Delany I, Granoff DM. 2011. A critical threshold of meningococcal factor H binding protein expression is required for increased breadth of protective antibodies elicited by native outer membrane vesicle vaccines. Clin. Vaccine Immunol. 18:736–742. 10.1128/CVI.00542-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


