Abstract
Pertussis remains an important public health problem in many countries despite extensive immunization. Cultures and real-time PCR (RT-PCR) assays are the recommended pertussis diagnostic tests, but they lack sensitivity at the later stage of the disease. This study introduces the IgG anti-pertussis toxin enzyme-linked immunosorbent assay (PT ELISA) in our routine diagnosis to improve disease burden estimation. Serum samples and nasopharyngeal swabs (n = 503) were collected at the same time from patients presenting with cough illness suspected of being pertussis and tested by the PT ELISA and culture and/or RT-PCR, respectively. Patients were separated into three age groups: group 1, <1 year (n = 260; mean age, 3 months), group 2, 1 to 6 years (n = 81; mean age, 3 years), and group 3, ≥7 years (n = 162; mean age, 26 years). The times (means) from cough onset to specimen collection were 16, 24, and 26 days, respectively. In group 1, 83 (82.2%) of 101 positive cases were positive for pertussis by culture/RT-PCR, while 40 (39.6%) tested positive by PT ELISA. In group 2, 6 (19.4%) of 31 positive cases were culture/RT-PCR positive, and 29 (93.6%) were seropositive. In group 3, 13 (13.8%) of 94 positive cases were positive by culture/RT-PCR and 91 (96.8%) were positive by serology. Culture/RT-PCR detected more cases of pertussis in infants (P < 0.0001), whereas the PT ELISA detected more cases in adolescents and adults (P < 0.0001). The timing between cough onset and specimen collection or recent vaccination may have partially affected our results. Serology is a suitable, cost-effective, and complementary pertussis diagnostic tool, especially among older children, adolescents, and adults during the later disease phase.
INTRODUCTION
Pertussis remains an important public health problem in many countries, despite extensive immunization. Its reemergence has been reported in many parts of the world and is most predominant in the United States, South America, and Australia (1, 2). There are about 50 million pertussis-infected cases and almost 300,000 to 400,000 pertussis-related deaths per year (1, 3).
At the start of 1980, more than 40,000 cases of pertussis (incidence rate, >30/100,000 habitants-year) were reported annually in Brazil. The number of cases decreased substantially after 1983 as a result of increased childhood vaccination coverage, reaching the lowest level in 2010 with 606 reported cases (reference 4 and http://www.cve.saude.sp.gov.br). However, a progressive increase in pertussis cases was observed during 2011, and in 2012 the number of cases remained at epidemic levels up to week 33. It is noteworthy that the rate of incidence recorded in 2011 was similar to that for the late 1990s, reaching nearly four times the incidence rate observed in 2010 (1.12 versus 0.32/100,000 habitants) (4, 5). Brazil's notifiable diseases information system (SINAN) is the national public health surveillance system which is used to collect and monitor disease cases and data. According to the 2011 SINAN data, approximately 70% of pertussis cases were detected among children less than 1 year of age, of which 92% of these cases were children under the age of 7 months. The highest absolute number of these reported cases (815 cases) was from São Paulo State (reference 4 and http://www.cve.saude.sp.gov.br); however, many cases of pertussis may have been missed due to the lack of diagnosis and reporting in other regions.
In 1968, the diphtheria-tetanus-pertussis (DTP) vaccine was introduced, using the formulation composed of diphtheria (D), tetanus (T), and whole-cell pertussis (wP). The DTP vaccine is presently used in Brazil. Currently, the Ministry of Health's vaccine schedule recommends three doses of DTP, hepatitis B, and Haemophilus influenzae b (Hib) (pentavalent) vaccines at the ages of 2, 4, and 6 months and two boosters of DTP vaccine at 15 months and 4 to 6 years (6).
The usefulness of pertussis diagnostic methods depends on the phase of the disease when the specimen is collected. Cultures and real-time PCR (RT-PCR) assays are the most appropriate diagnostic tools at the earlier stage of disease, whereas the IgG anti-pertussis toxin (PT) enzyme-linked immunosorbent assay (PT ELISA) is useful in the late phase of illness, particularly among adults and adolescents who often have an atypical whooping cough symptom and do not usually seek immediate medical attention (7–9). The present study aimed to introduce a PT ELISA to the routine diagnosis of pertussis at Brazil's Public Health Laboratory in São Paulo.
(Part of this research was presented at the 112th General Meeting of the American Society for Microbiology, San Francisco, CA, 16 to 19 June 2012.)
MATERIALS AND METHODS
Study population and specimen collection.
From November 2009 to July 2012, 503 serum and nasopharyngeal swab specimens were collected at the same time from suspected pertussis cases and their respective household contacts presenting with cough illness suspected of being pertussis. The clinical definition of suspected pertussis in Brazil includes cough illness for 2 weeks (14 days) with one of the following symptoms: paroxysmal cough, inspiratory “whoop,” or posttussive vomiting. However, captured cases may be identified with shorter disease evolution. In any suspected case of pertussis, regardless of laboratory confirmation, specimens are collected from symptomatic household contacts. Symptomatic household contacts are individuals who have a cough, regardless of the time of cough duration.
The studied population comprised subjects who routinely attended eight sentinel surveillance hospitals in the city of São Paulo through the Surveillance Program of the Secretary of Health of São Paulo State. There are seven public and one private hospital, and of those eight, six are general and two are pediatric hospitals. The hospitals send clinical specimens from hospitalized persons with suspected pertussis and from outpatients to the Instituto Adolfo Lutz laboratories (Brazil's National Pertussis Reference Center) for pertussis laboratory diagnosis. Patients who received multiple or recent (within 3 months) blood transfusions and were immunosuppressed or were receiving immunosuppressive therapy were excluded from the study.
Subjects were separated into three age groups to reflect the ages affected by the primary vaccine series (group 1, <1 year, n = 260), the booster series (group 2, 1 to 6 years, n = 81), and the adolescent/adult population that should no longer exhibit postvaccination titers (group 3, ≥7 years, n = 162). Subjects were also grouped as such to maximize the equal distribution of subjects per group, since the largest number of patients were <1 year. Clinical and epidemiological data such as immunization histories, dates of the onset of symptoms, and dates of specimen collection were obtained from SINAN and registered in an electronic database.
The study was approved by the ethics committee of the Instituto Adolfo Lutz, and written informed consent was obtained from all subjects and parents or guardians (for underaged children) before specimens were collected.
Culture/RT-PCR assays and serology.
Specimens were cultured on charcoal agar with 10% sterile defibrinated sheep blood and cephalexin and incubated at 35 to 37°C for 10 days. Colonies suggestive of belonging to the genus Bordetella were confirmed by Gram stain and biochemical tests, as previously described (10). Detection of Bordetella pertussis O1 antigen and serotyping were performed by slide agglutination using O1, Fim 2, and Fim 3 antibodies, respectively. The O1 antiserum and Fim 2 and 3 antibodies were produced according to the methodologies described by Preston (11) and Ashworth et al. (12) at the National Reference Center for Pertussis, Adolfo Lutz Institute (São Paulo, Brazil).
DNA extraction from nasopharyngeal swabs was performed using a QIAamp DNA blood minikit (Qiagen, CA) according to the manufacturer's instructions. The RT-PCR assay was conducted in accordance with the method of Tatti et al. (13) with minor modifications. Two PCR targets, the ptxS1 gene encoding the pertussis toxin subunit 1 and the insertion sequence IS481, were used. The RT-PCRs were performed using an AB7500 thermocycler (Applied Biosystems, CA). Each reaction mixture contained 5 μl of bacterial DNA, 12.5 μl of 2× TaqMan Universal Master Mix (Applied Biosystems), 2 μl of each forward and reverse primer at 300 nmol/ml, 2 μl of probe (100 nmol/ml), and 1.5 μl of PCR-grade water (Roche Diagnostics, Indianapolis, IN) to bring the total reaction volume to 25 μl. The cycling conditions used were as follows: 2 min at 50°C and 10 min at 95°C, followed by 45 cycles of 95°C at 15 s and 1 min at 57°C. Positive (strain GL353) and nontemplate (PCR-grade water) controls were tested in duplicate in each run. Specimens were considered positive for the presence of B. pertussis if amplification occurred with both PCR targets. Culture and PCR results were combined for analysis, and no attempt was made to identify species other than B. pertussis.
IgG antibodies to PT in sera were detected by the PT ELISA using the Centers for Disease Control and Prevention (CDC) protocol as previously described (8, 14, 15). The concentrations of IgG antibodies were quantified using standard curves constructed with six ready-to-use standards (15 to 480 ELISA units [EU]/ml) provided by the CDC. Microplates were read at 450 nm using a microplate reader (Sunrise; Tecan, Switzerland), and the calibration curve was constructed using the software Magellan 6.6 (Tecan) employing a 4-parameter logistic regression curve. The quantitative cutoff IgG antibody values were as follows: seronegative, <49 EU/ml; indeterminate, 49 to 93 EU/ml); and seropositive (≥94 EU/ml) (8, 15). Indeterminate results were not included in the analysis because the numbers were too low to make a significant difference.
Statistical analysis.
Laboratory results were analyzed in Epi Info, version 6 (CDC, USA) and GraphPad Prism 5.0 (GraphPad Software, Inc., CA) software. Differences between age groups were evaluated using a chi-square test or a Kruskal-Wallis test with Dunn's posttest, as appropriate. A P value of <0.05 was considered statically significant.
RESULTS
Characteristics of the studied population according to age groups and laboratory results (positivity for at least one culture/RT-PCR or serology assay) are shown in Table 1. Overall, no differences between the pertussis-positive patients and the total number of patients analyzed were detected intragroup in relation to age and gender, but more females were detected in all three groups. Although there was no available information for 9%, 16%, and 23.5% of patients from groups 1, 2, and 3, respectively, differences in the mean days of cough from date of cough onset to specimen collection were observed, showing an increase in the number of days with the increase in age.
TABLE 1.
Characteristics of the studied population according to age and positivity to pertussis laboratory analysis
| Characteristic | Data for group no. (age range): |
|||||
|---|---|---|---|---|---|---|
| 1 (<1 yr) |
2 (1–6 yr) |
3 (≥7 yr) |
||||
| Total (n = 260) | Pertussis positive (n = 101)a | Total (n = 81) | Pertussis positive (n = 31)a | Total (n = 162) | Pertussis positive (n = 94)a | |
| Age (mean [range])b | 3 (1–11) | 2.7 (1–8) | 3 (1–6) | 3 (1–6) | 26 (7–88) | 24 (7–74) |
| Gender (n [%] female) | 153 (59) | 63 (62.4) | 48 (59.3) | 19 (61.3) | 105 (64.8) | 56 (59.6) |
| Time since cough onset | ||||||
| Mean (SD) (days) | 16 (10) | 17 (10.4) | 24 (17.8) | 26 (18) | 26 (19) | 30 (18.5) |
| Range (days) | 7–63 | 7–50 | 7–93 | 7–78 | 7–98 | 7–93 |
Positive by at least one test.
Ages given for group 1 are in months and for groups 2 and 3 are in years.
Table 2 presents the frequencies of pertussis-positive cases according to the age group when specimens were analyzed by culture, RT-PCR, and serology. Because all culture-positive specimens were also positive by RT-PCR but not all positive PCR results were culture positive, the results were combined.
TABLE 2.
Frequencies of positive pertussis cases in different age groups by culture and/or RT-PCR and serology (PT-ELISA)
| Characteristic | Group 1 (<1 yr) | Group 2 (1–6 yr) | Group 3 (≥7 yr) |
|---|---|---|---|
| No. of pertussis-positive cases/total no. of cases | 101/260 | 31/81 | 94/162 |
| No. (%) of positive cases by culture and/or RT-PCR | 61 (60.4)a | 2 (6.4) | 3 (3.2) |
| No. (%) of positive cases by PT-ELISA | 18 (17.8) | 25 (80.6) | 81 (86.2) |
| No. (%) of positive cases by combined assaysb | 22 (21.8) | 4 (13) | 10 (10.6) |
| No. of seronegative cases | 150 | 47 | 62 |
| Pc | <0.0001 | <0.0001 | <0.0001 |
Percentages were calculated with positive specimens by at least one test.
Combined assays: culture and/or RT-PCR and PT ELISA.
Culture and/or RT-PCR versus PT-ELISA.
Overall, culture and/or RT-PCR was more sensitive in detecting pertussis in patients of group 1, whereas the PT ELISA confirmed more cases in groups 2 and 3. All statistical analyses, including analysis of cases that were positive only by PT ELISA, showed significant differences with and between age groups. The highest positivity of culture and/or RT-PCR assays was detected in children less than 1 year of age (82.2%), while the highest positivity for the PT ELISA was detected in adolescents and adults (96.8%). Figure 1 stratifies the proportion of positive laboratory results by age, further demonstrating that while culture- and/or RT-PCR-positive specimens diminish with the patient's age, the PT ELISA positivity increases with age, corroborating the data presented in Table 1.
FIG 1.
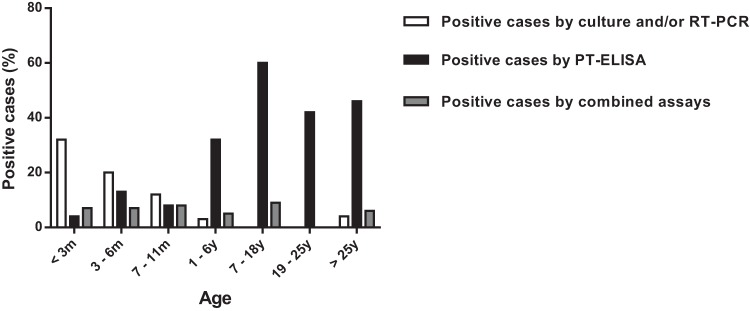
Proportion of positive pertussis cases diagnosed by culture and/or RT-PCR, PT ELISA, and combined assays, stratified by age. m, months; y, years.
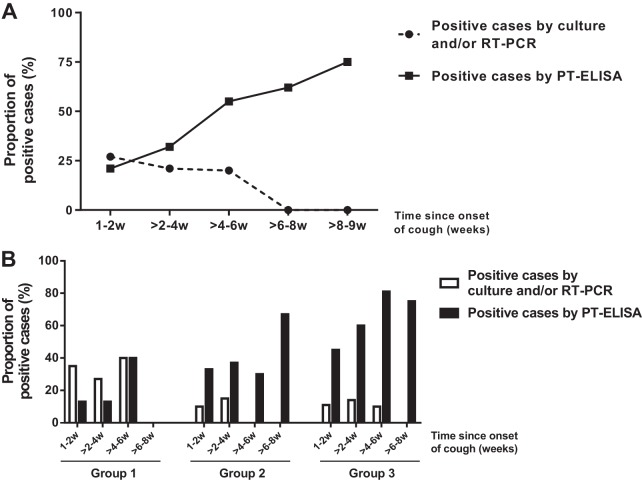
When the time of specimen collection (in weeks) after cough onset was analyzed in relation to the proportion of positive cases diagnosed by culture and/or RT-PCR and PT ELISAs, the timing of the sampling was statistically significant (P < 0.0001) and seemed to be the major determining factor (Fig. 2A). Figure 2A shows that the PT ELISA has a greater proportion of positive cases than culture and/or RT-PCR 2-4 weeks after cough onset. Conversely, during the initial 1- to 2-week period, culture and/or RT-PCR had a greater amount of positive cases versus the PT ELISA. It should be noted that this difference is due to the positivity profile observed in group 1 (higher positivity by culture and/or RT-PCR and low positivity by the PT ELISA).
FIG 2.
Proportion of positive cases diagnosed by culture and/or RT-PCR and PT ELISA stratified by time since cough onset in weeks (w). The total number of subjects per time point was used as the denominator. (A) Analysis based on time of symptoms for all reported cases; (B) cases separated by age groups: group 1 (<1 year), group 2 (1 to 6 years), and group 3 (≥7 years).
Culture and/or RT-PCR was the best test to detect cases of pertussis in children <1 year (group 1), mainly until 4 to 6 weeks of cough. In contrast, the PT ELISA was more efficient for detecting pertussis cases in groups 2 (1 to 6 years) and 3 (≥7 years) from 2 to 8 weeks of cough (Fig. 2B).
The geometric mean concentrations (GMC) and median (range) of IgG anti-PT antibodies among cases according to age groups were 216.5 and 233 EU/ml (96.3 to 533.0), 272.7 and 269.7 EU/ml (111.9 to 596), and 266 and 287.7 EU/ml (96.8 to 559) for groups 1, 2, and 3, respectively. GMC differences between groups 1 and 3 were statistically significant (P < 0.05).
DISCUSSION
Brazil's Ministry of Health currently recommends culture for pertussis diagnosis, which is performed in all state public health laboratories. RT-PCR testing is performed exclusively at the National Reference Laboratory. Using only culture and/or RT-PCR to diagnose pertussis could lead to failure in detecting truly infected individuals, because there are no sensitive tests to diagnose pertussis in the later phase of the disease (16).
Pertussis serology is currently used for diagnostic purposes in Australia and some countries in Europe (17, 18). In the United States, serology was useful for confirming the diagnosis during outbreaks (16, 19, 20). Validation and harmonization of serologic methods are still necessary before they can be widely applied as diagnostic tools (21–23).
In the present study, we tested the performance of the CDC PT ELISA (8, 15) for routine diagnosis of pertussis in São Paulo, Brazil. The next steps include analysis of normal controls from the Brazilian population to establish local cutoff values. The CDC ELISA cutoff of 94 EU/ml is similar to the majority of cutoff values of around 100 EU/ml proposed in other countries (8, 15, 24, 25).
Analysis of the performance of the PT ELISA in groups 1 (<1 year) and 2 (1 to 6 years) requires caution, because this assay was developed and validated for diagnosis in adolescents and adults (8). This fact could explain the results obtained in children of group 1. Sixty-one culture- and/or RT-PCR-positive patients from group 1 with negative serology results were younger than 4 months of age. It is known that during this period the immune system is immature, and, consequently, antibody production is reduced (26). Another explanation is that the majority of these serum specimens (61%) were collected suboptimally (before 2 weeks of cough onset), which is too early in the disease course and may not elicit a detectable antibody response.
Waiting periods of 6 months to 1 year for serodiagnosis after vaccination have been adopted in other countries (18, 27). In our study, 7 of 18 (39%) infant cases (group 1) that showed positive results by serology only received at least one of the three vaccine doses. Therefore, we cannot rule out the possibility that the high antibody levels detected were elicited by the recent DPT vaccination, albeit having less PT antigen than that of the acellular vaccine counterpart. Among the 25 positive cases detected by serology only in children aged 1 to 6 years old (group 2), the vaccination status was not known in 56% of the subjects. However, 7 of 10 cases with confirmed vaccination had received a booster dose more than 6 months before the specimen collection, suggesting that vaccination in those cases may not have confounded serodiagnosis. Further studies conducted in Brazil and abroad are necessary to verify whether vaccinated and naturally infected individuals of different ages have different antibody profiles and titer decays. These studies will provide information to better interpret the serological results as demonstrated by Dalby et al. (28).
In group 3 (≥7 years), despite incomplete clinical data, 61 of 81 positive cases (75.3%) detected by serology only were household contact cases, most of them being parents, relatives, or siblings. These data demonstrate that serology could improve the estimation of the disease burden in Brazil, particularly in this age group.
In summary, culture and/or RT-PCR assays confirmed more pertussis cases among young children, as expected. The PT ELISA confirmed cases mostly among adolescents, adults, and household contacts, who may not present typical pertussis symptoms and do not seek early diagnosis. To our knowledge, this is the first study conducted in Brazil that suggests the presence of pertussis infection in symptomatic adolescents and adults by serology. Because of its cost-effectiveness, rapidity, and ease of use, serology is a suitable pertussis diagnostic tool, especially among adolescents and adults during the later phases of the disease. In the future, we intend to propose an algorithm for pertussis diagnosis in Brazil which takes into consideration the age of the suspected case and the date/type of clinical specimen collected according to the duration of cough illness and vaccination status.
ACKNOWLEDGMENTS
We thank GlaxoSmithKline for providing the purified pertussis toxin antigens used in this study and the Secretary of Health of São Paulo and Instituto Adolfo Lutz for support. We also thank Adele Caterino de Araujo and Suely S. Kashino for comments on and review of the manuscript.
This work was supported in part by a Fogarty International Center Global Infectious Diseases Research Training Program grant, National Institutes of Health, University of Pittsburgh (D43TW006592).
Footnotes
Published ahead of print 5 March 2014
REFERENCES
- 1.Pan American Health Organization/World Health Organization. 2012. Epidemiological alert. Pertussis (whooping cough). Pan American Health Organization, Washington, DC [Google Scholar]
- 2. Clarke MF, Rasiah K, Copland J, Watson M, Koehler AP, Dowling K, Marshall HS. 2013. The pertussis epidemic: informing strategies for prevention of severe disease. Epidemiol. Infect. 141:463–471. 10.1017/S095026881200091X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bamberger ES, Srugo I. 2008. What is new in pertussis? Eur. J. Pediatr. 167:133–139. 10.1007/s00431-007-0548-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ministério Da Saúde. 2014. Ministério da Saúde alerta sobre a situação epidemiológica da coqueluche no Brasil. http://portalsaude.saude.gov.br/index.php/profissional-e-gestor/vigilancia/noticias-vigilancia/7569-
- 5.Ministério Da Saúde. 2012. Vacina adsorvida difteria, tétano e pertussis (acelular)—dTpa—para vacinação de gestantes. http://u.saude.gov.br/images/pdf/2014/janeiro/28/VacinadTPa-Gestantes-final.pdf
- 6.Secretaria de Estado da Saúde de São Paulo. 2006. Doenças de transmissão respiratória. Bol. Epidemiol. Paulista 27:20–26 [Google Scholar]
- 7. Meng CY, Zhang WH. 2006. Current status and perspective of worldwide disease burden of pertussis. Chin J. Vaccines Immunization 12:4 [Google Scholar]
- 8. Menzies SL, Kadwad V, Pawloski LC, Lin TL, Baughman AL, Martin M, Tondella ML, Meade BD. 2009. Development and analytical validation of an immunoassay for quantifying serum anti-pertussis toxin antibodies resulting from Bordetella pertussis infection. Clin. Vaccine Immunol. 16:1781–1788. 10.1128/CVI.00248-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tozzi AE, Celentano LP, Ciofi degli Atti ML, Salmaso S. 2005. Diagnosis and management of pertussis. CMAJ 172:509–515. 10.1503/cmaj.1040766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gonçalves CR, Vaz TM, Medeiros MI, Castro MT, Rocha MM, Melles CE, Irino K. 2007. Phenotypical and genotypical characterization of Bordetella pertussis strains isolated in Sao Paulo, Brazil, 1988-2002. Rev. Inst. Med. Trop. Sao Paulo 49:123–125. 10.1590/S0036-46652007000200012 [DOI] [PubMed] [Google Scholar]
- 11. Preston NW. 1966. Potency tests for pertussis vaccines: doubtful value of intracerebral challenge test in mice. J. Pathol. Bacteriol. 91:173–179. 10.1002/path.1700910121 [DOI] [PubMed] [Google Scholar]
- 12. Ashworth LA, Irons LI, Dowsett AB. 1982. Antigenic relationship between serotype-specific agglutinogen and fimbriae of Bordetella pertussis. Infect. Immun. 37:1278–1281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Tatti KM, Wu KH, Tondella ML, Cassiday PK, Cortese MM, Wilkins PP, Sanden GN. 2008. Development and evaluation of dual-target real-time polymerase chain reaction assays to detect Bordetella spp. Diagn. Microbiol. Infect. Dis. 61:264–272. 10.1016/j.diagmicrobio.2008.02.017 [DOI] [PubMed] [Google Scholar]
- 14. Kapasi A, Meade BD, Plikaytis B, Pawloski L, Martin MD, Yoder S, Rock MT, Coddens S, Haezebroeck V, Fievet-Groyne F, Bixler G, Jones C, Hildreth S, Edwards KM, Messonnier NE, Tondella ML. 2012. Comparative study of different sources of pertussis toxin (PT) as coating antigens in IgG anti-PT enzyme-linked immunosorbent assays. Clin. Vaccine Immunol. 19:64–72. 10.1128/CVI.05460-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Baughman AL, Bisgard KM, Edwards KM, Guris D, Decker MD, Holland K, Meade BD, Lynn F. 2004. Establishment of diagnostic cutoff points for levels of serum antibodies to pertussis toxin, filamentous hemagglutinin, and fimbriae in adolescents and adults in the United States. Clin. Diagn. Lab. Immunol. 11:1045–1053. 10.1128/CDLI.11.6.1045-1053.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wei SC, Tatti K, Cushing K, Rosen J, Brown K, Cassiday P, Clark T, Olans R, Pawloski L, Martin M, Tondella ML, Martin SW. 2010. Effectiveness of adolescent and adult tetanus, reduced-dose diphtheria, and acellular pertussis vaccine against pertussis. Clin. Infect. Dis. 51:315–321. 10.1086/653938 [DOI] [PubMed] [Google Scholar]
- 17. May ML, Doi SA, King D, Evans J, Robson JM. 2012. Prospective evaluation of an Australian pertussis toxin IgG and IgA enzyme immunoassay. Clin. Vaccine Immunol. 19:190-197. 10.1128/CVI.05430-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Guiso N, Berbers G, Fry NK, He Q, Riffelmann M, Wirsing von Konig CH. 2011. What to do and what not to do in serological diagnosis of pertussis: recommendations from EU reference laboratories. Eur. J. Clin. Microbiol. Infect. Dis. 30:307–312. 10.1007/s10096-010-1104-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mandal S, Tatti KM, Woods-Stout D, Cassiday PK, Faulkner AE, Griffith MM, Jackson ML, Pawloski LC, Wagner B, Barnes M, Cohn AC, Gershman KA, Messonnier NE, Clark TA, Tondella ML, Martin SW. 2012. Pertussis pseudo-outbreak linked to specimens contaminated by Bordetella pertussis DNA from clinic surfaces. Pediatrics 129:e424–e430. 10.1542/peds.2011-1710 [DOI] [PubMed] [Google Scholar]
- 20. Rodgers L, Martin SW, Cohn A, Budd J, Marcon M, Terranella A, Mandal S, Salamon D, Leber A, Tondella ML, Tatti K, Spicer K, Emanuel A, Koch E, McGlone L, Pawloski L, Lemaile-Williams M, Tucker N, Iyer R, Clark TA, Diorio M. 2013. Epidemiologic and laboratory features of a large outbreak of pertussis-like illnesses associated with cocirculating Bordetella holmesii and Bordetella pertussis—Ohio, 2010-2011. Clin. Infect. Dis. 56:322–331. 10.1093/cid/cis888 [DOI] [PubMed] [Google Scholar]
- 21. Lynn F, Reed GF, Meade BD. 1996. Collaborative study for the evaluation of enzyme-linked immunosorbent assays used to measure human antibodies to Bordetella pertussis antigens. Clin. Diagn. Lab. Immunol. 3:689–700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Tondella ML, Carlone GM, Messonnier N, Quinn CP, Meade BD, Burns DL, Cherry JD, Guiso N, Hewlett EL, Edwards KM, Xing D, Giammanco A, Wirsing von Konig CH, Han L, Hueston L, Robbins JB, Powell M, Mink CM, Poolman JT, Hildreth SW, Lynn F, Morris A. 2009. International Bordetella pertussis assay standardization and harmonization meeting report. Centers for Disease Control and Prevention, Atlanta, Georgia, United States, 19–20 July 2007. Vaccine 27:803–814. 10.1016/j.vaccine.2008.11.072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Xing D, Wirsing von Konig CH, Newland P, Riffelmann M, Meade BD, Corbel M, Gaines-Das R. 2009. Characterization of reference materials for human antiserum to pertussis antigens by an international collaborative study. Clin. Vaccine Immunol. 16:303–311. 10.1128/CVI.00372-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. de Melker HE, Versteegh FG, Conyn-Van Spaendonck MA, Elvers LH, Berbers GA, van Der Zee A, Schellekens JF. 2000. Specificity and sensitivity of high levels of immunoglobulin G antibodies against pertussis toxin in a single serum sample for diagnosis of infection with Bordetella pertussis. J. Clin. Microbiol. 38:800–806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Pebody RG, Gay NJ, Giammanco A, Baron S, Schellekens J, Tischer A, Olander RM, Andrews NJ, Edmunds WJ, Lecoeur H, Levy-Bruhl D, Maple PA, de Melker H, Nardone A, Rota MC, Salmaso S, Conyn-van Spaendonck MA, Swidsinski S, Miller E. 2005. The seroepidemiology of Bordetella pertussis infection in Western Europe. Epidemiol. Infect. 133:159–171. 10.1017/S0950268804003012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Cherry JD, Grimprel E, Guiso N, Heininger U, Mertsola J. 2005. Defining pertussis epidemiology: clinical, microbiologic and serologic perspectives. Pediatr. Infect. Dis. J. 24:S25–34. 10.1097/01.inf.0000160926.89577.3b [DOI] [PubMed] [Google Scholar]
- 27. Pawloski LC, Kirkland KB, Baughman AL, Martin MD, Talbot EA, Messonnier NE, Tondella ML. 2012. Does tetanus-diphtheria-acellular pertussis vaccination interfere with serodiagnosis of pertussis infection? Clin. Vaccine Immunol. 19:875–880. 10.1128/CVI.05686-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Dalby T, Petersen JW, Harboe ZB, Krogfelt KA. 2010. Antibody responses to pertussis toxin display different kinetics after clinical Bordetella pertussis infection than after vaccination with an acellular pertussis vaccine. J. Med. Microbiol. 59:1029–1036. 10.1099/jmm.0.020826-0 [DOI] [PubMed] [Google Scholar]