Abstract
Malolactic fermentation in wine is often carried out by Oenococcus oeni. Wine is a stressful environment for bacteria because ethanol is a toxic compound that impairs the integrity of bacterial membranes. The small heat shock protein (sHsp) Lo18 is an essential actor of the stress response in O. oeni. Lo18 prevents the thermal aggregation of proteins and plays a crucial role in membrane quality control. Here, we investigated the interaction between Lo18 and four types of liposomes: one was prepared from O. oeni grown under optimal growth conditions (here, control liposomes), one was prepared from O. oeni grown in the presence of 8% ethanol (here, ethanol liposomes), one was prepared from synthetic phospholipids, and one was prepared from phospholipids from Bacillus subtilis or Lactococcus lactis. We observed the strongest interaction between Lo18 and control liposomes. The lipid binding activity of Lo18 required the dissociation of oligomeric structures into dimers. Protein protection experiments carried out in the presence of the liposomes from O. oeni suggested that Lo18 had a higher affinity for control liposomes than for a model protein. In anisotropy experiments, we mimicked ethanol action by temperature-dependent fluidization of the liposomes. Results suggest that the principal determinant of Lo18-membrane interaction is lipid bilayer phase behavior rather than phospholipid composition. We suggest a model to describe the ethanol adaptation of O. oeni. This model highlights the dual role of Lo18 in the protection of proteins from aggregation and membrane stabilization and suggests how modifications of phospholipid content may be a key factor determining the balance between these two functions.
INTRODUCTION
Oenococcus oeni is a lactic acid bacterium that grows in wine and carries out malolactic fermentation (MLF). MLF occurs following alcoholic fermentation by yeasts and increases the quality of wine (1). During the wine-making process, it is current practice to add O. oeni to wine to start MLF (1). However, wine is a stressful medium for bacterial growth, and bacteria must adapt to high ethanol concentrations, acidic pH, and low temperatures to survive. Many of the molecular and physiological bases of the stress response in O. oeni have been worked out (2–6). One major adaptation involves mechanisms that counteract the increase in membrane fluidity at high ethanol concentrations. For example, O. oeni is able to modify its membrane phospholipid content (7, 8). This is also true for Lactobacillus plantarum. In response to ethanol, this bacterium synthesizes large amounts of saturated fatty acids, resulting in a decrease in the relative proportion of unsaturated fatty acids, which helps to counteract the deleterious effects of ethanol on membrane fluidity (9).
In addition to lipid modifications, heat shock proteins (Hsp) are produced when the bacterial envelope detects changes to the cellular environment. For example, the cyanobacterium Synechocystis synthesizes Hsp70 (also known as the molecular chaperone DnaK) in response to the membrane fluidizer bimoclomol, a hydroxylamine derivative (10). Several Hsp, such as DnaK and GroEL, can bind membranes (11). Torok et al. also showed that during heat shock in vitro, Synechocystis GroEL interacts with model lipid membranes, resulting in the increase of the molecular order of lipid bilayers and stabilization of the membrane (12).
Similarly, a particular subclass of Hsp called small heat shock proteins (sHsp) also bind membranes to regulate bilayer fluidity (13–15). These sHsp have a molecular mass ranging from 12 to 43 kDa and can form large oligomers with molecular chaperone activity that protects polypeptides from aggregation (16–19). The association of sHsp with lipid substrates is linked to conformational changes (20–22). The affinity of sHsp for lipids is sensitive to phospholipid type. For example, mammalian Hsp22 binds anionic lipid vesicles but not liposomes with a neutral charge (20), and the Synechocystis Hsp17 binds preferentially to the polar lipid monoglucosyldiacylglycerol (MGlcDG) (23). The binding of Hsp17 or human alpha-crystallin to membranes leads to the preservation of membrane structure and integrity during thermal adaptation (24). However, little is known about the role of sHsp in the preservation of membrane integrity during adaptation of lactic acid bacteria to technological processes.
Under stress conditions O. oeni produces large amounts of the sHsp Lo18 (2–4, 25), particularly during ethanol shock (3, 26). As it is not yet possible to construct the appropriate mutants in O. oeni or to overexpress the proteins to verify their in vivo function, many studies have been performed with heterologous models (27, 28) or in vitro with purified proteins (17). In vitro experiments with purified Lo18 have characterized both its molecular chaperone (protection of proteins from aggregation) and lipochaperone (stabilization of liposome fluidity) activities (17, 26, 29). Recently, we provided strong evidence for a relationship between the dynamic oligomerization of Lo18 and its chaperone and lipochaperone activities (17). The optimal pH for the liposome stabilization activity of Lo18 is pH 7, where the protein appears as a mixture of species, predominantly containing a 12-subunit oligomer formed by the association of dimeric building blocks (17).
Here, we studied the interaction between Lo18 and liposomes prepared from phospholipids extracted from the cytoplasmic membrane of O. oeni. These bacteria were grown either under optimal growth conditions as a control condition or under conditions of 8% ethanol to mimic conditions in wine. We also examined the interaction between Lo18 and other bacterial or synthetic liposomes. These experiments allowed us to assess the conditions that determine the binding of Lo18 to the membrane. We propose a model for ethanol tolerance in O. oeni which describes the role of Lo18 and phospholipid content in membrane stabilization.
MATERIALS AND METHODS
Bacterial strains, growth conditions, and Lo18 purification.
O. oeni ATCC BAA-1163 cells were grown at 30°C in modified FT80 (mFT80) medium (30). Precultures were performed in the same medium and were used to inoculate larger cultures at an initial absorbance at 600 nm (A600) of 0.1 (1 absorbance unit corresponds to 2 × 108 CFU · ml−1). For ethanol conditions, mFT80 medium was supplemented with 8% ethanol (vol/vol). O. oeni cells were harvested in the exponential growth phase when the A600 reached 0.4.
Bacillus subtilis subsp. subtilis CIP 52.65 was cultured aerobically (at 130 rpm) in LB broth at 37°C and harvested in the exponential growth phase (A600 of 0.6; 1 absorbance unit corresponds to 4 × 108 CFU · ml−1).
Lactococcus lactis subsp. cremoris strain MG1363 was grown under anaerobic conditions at 30°C in GM17 medium (medium corresponding to M17 [31] containing glucose at 0.5%). Cells were harvested in the exponential growth phase (A600 of 1.5; 1 absorbance unit corresponds to 6 × 108 CFU · ml−1).
Lo18 was produced in Escherichia coli BL21 Star(DE3) containing the plasmid pET28a-hsp18 (28). E. coli cells were grown under aerobic conditions at 37°C in LB broth supplemented with kanamycin (50 μg · ml−1). Lo18 expression and purification were performed according to Maitre et al. (17). The purified protein was stored at 3 mg · ml−1 and at −20°C in 50 mM sodium phosphate buffer, pH 7. All experiments with purified Lo18 were performed at pH 7 because Lo18 activities are optimal at this pH (17).
Preparation of liposome samples from bacterial cells.
Liposomes were prepared from total lipids from 50 absorbance units of O. oeni harvested in the exponential phase after growth either under optimal culture conditions (here, control liposomes) or under conditions of 8% ethanol (here, ethanol liposomes). For B. subtilis and L. lactis, cells were harvested in the exponential growth phase after growth under optimal culture conditions, and 50 absorbance units were used to prepare liposomes. Lipid extractions were carried out using the Bligh and Dyer method (32), and liposomes were prepared as previously described (17, 26). Liposomes were kept for a maximum of 1 week at 4°C.
Preparation of synthetic liposomes.
The synthetic phospholipids dipalmitoylphosphatidylglycerol (DPPG), dipalmitoylphosphatidylethanolamine (DPPE), dioleoylphosphatidylglycerol (DOPG), and dioleoylphosphatidylethanolamine (DOPE) (Table 1) were purchased from Sigma (France) and dissolved in chloroform at a final concentration of 30 mM. Phospholipids were dried at 65°C under nitrogen-reduced pressure and were hydrated by shaking with prewarmed 50 mM phosphate buffer at pH 7. Small unilamellar liposome suspensions with a lipid concentration of 0.25 mg · ml−1 were obtained by sonication (two times for 30 s) (ELMA D-78224; VWR, France).
TABLE 1.
Characteristics of commercial phospholipids
| Phospholipid name | Abbreviation | Head group | Fatty acid | Transition temp (°C)a |
|---|---|---|---|---|
| Dipalmitoylphosphatidylglycerol | DPPG | Glycerol | Palmitic acid | 41 |
| Dipalmitoylphosphatidylethanolamine | DPPE | Ethanolamine | Palmitic acid | 63 |
| Dioleoylphosphatidylglycerol | DOPG | Glycerol | Oleic acid | −18 |
| Dioleoylphosphatidylethanolamine | DOPE | Ethanolamine | Oleic acid | −16 (10)b |
Temperature of transition from the lamellar gel phase (Lβ) to the lamellar liquid-crystalline phase (Lα).
The value in parentheses represents the temperature of transition from the lamellar liquid-crystalline phase (Lα) to the inverted hexagonal type II phase (HII).
Fatty acid analysis.
One milliliter of control or ethanol liposomes prepared as described above or total phospholipids from 50 absorbance units of O. oeni cells cultured in the presence or absence of 8% of ethanol were used for lipid analysis. Extraction and methylation of fatty acids were performed according to Méchin et al. (33). Fatty acid methyl esters (FAME) from liposomes were concentrated under nitrogen flow. Analytical gas chromatography (GC) of FAME was carried out by a 6890HP system (Agilent Technologies, Germany) equipped with a capillary column (25 m; 0.22-mm inside diameter [i.d.]) coated with 0.25-μm BPX70 film (SGE, Ring Wood, Australia) and a flame detector. Column temperature was set at 100°C for 1 min and then increased to 170°C at the rate of 2°C per min. The data were acquired with an HPCORE Chemstation system (Agilent Technologies, Germany) and are expressed as a percentage of the area of the main peaks. Individual fatty acids were identified using commercial FAME standards. For each condition, the results are the average of six GC profiles (two injections of three extractions prepared from independent liposomes).
Measurement of liposome fluidity stabilization.
Liposome fluidity measurements were performed by the fluorescence anisotropy method with 1,6-diphenyl-1,3,5-hexatriene (DPH) as a probe (Sigma, France). Experiments were done in the absence or presence of 10 μM purified Lo18, and a mass ratio of 1:2 (m/m) was used between Lo18 and liposomes. Ten minutes after the addition of 3 μM DPH, the fluorescence intensity was recorded at 402 nm with excitation at 352 nm using a Fluorolog-3 spectrofluorimeter (Jobin Yvon, France). Temperature ramping with a rate of 5°C per min was controlled by a Peltier system (Wavelength Electronics, USA). Each experiment was performed in triplicate. Lysozyme was used as negative control (data not shown).
Size exclusion chromatography and cross-linking experiments.
Lo18 was incubated in the absence or presence of control or ethanol liposomes (mass ratio of Lo18/liposomes of 1:1, 1:2, 1:5, and 1:10) for 30 min at 42°C before being loaded onto a Superdex 200 5/150 GL column (GE Healthcare, France) previously equilibrated with 50 mM phosphate buffer at pH 7. Absorbance was recorded at 280 nm. Each experiment was performed in triplicate. For cross-linking, 1.5 μM Lo18 was incubated with control liposomes (Lo18/liposome ratio of 1:2 or 1:10) for 30 min at 42°C. Cross-linking was carried out by adding a freshly prepared solution of bis(sulfosuccinimidyl) suberate (BS3) in cross-linking buffer (25 mM morpholineethanesulfonic acid [MES], pH 7.5, 25 mM NaCl, 0.5 mM dithiothreitol [DTT]) such that the final concentration of BS3 was 2 mM. The mixture was incubated for 30 min at room temperature, after which 2.6 μg of cross-linked samples was loaded onto a gel. Immunoblotting procedures were performed as previously described (3).
Chaperone activity in the presence of liposomes.
Citrate synthase (CS) was prepared as described previously (17). CS at a concentration of 300 nM was thermally denatured at 45°C in the presence or absence of 9.6 μM Lo18 in 50 mM sodium phosphate buffer at pH 7 containing different Lo18/liposome ratios (1:0.04, 1:0.3, 1:2, and 1:25 [m/m], corresponding to 12.5, 92.5, 925, and 9,250 μg of lipids, respectively). Aggregation was followed by light-scattering measurement at 360 nm (Uvicon XS70 spectrophotometer equipped with a Peltier thermosystem; Secoman, France). Three independent experiments were carried out for each condition. Lysozyme was used as negative control, and we checked that the presence of liposomes had no effect on CS aggregation (data not shown).
RNA extraction, reverse transcription, and real-time PCR experiments.
Total RNA was extracted by using an RNeasy kit (Qiagen, France) according to the manufacturer's instructions and dissolved in 30 μl of ultrapure water. RNA concentration was determined by measuring the absorbance at 260 nm. Contaminating DNA was removed by DNase treatment (Fermentas, France) prior to reverse transcription. Removal of DNA was checked by qPCR. cDNA was obtained by using an iScript cDNA Synthesis Kit (Bio-Rad, France). Real-time PCR (primers listed in Table 2) was performed according to (34) using a CFX96 real-time system (Bio-Rad, France) with Go Taq qPCR Master Mix (Promega, France) in 96-well plates. Results were expressed using the 2−ΔΔCT method (where CT is threshold cycle) (35). Normalization was performed relative to a calibrator condition (exponential-phase cells growing under optimal culture conditions), and two reference genes (ldhD and gyrA) were used. For each condition, experiments were carried out with cDNA obtained from RNA extracted from three independent cultures.
TABLE 2.
Primers used for qRT-PCR
| Gene | Product | Forward primer (5′→3′) | Reverse primer (5′→3′) | Amplicon length (bp) |
|---|---|---|---|---|
| ldhD | d-Lactate dehydrogenase | GCCGCAGTAAAGAACTTGATG | TGCCGACAACACCAACTGTTT | 102 |
| gyrA | DNA gyrase subunit A | CGCCCGACAAACCGCATAAA | CAAGGACTCATAGATTGCCGAA | 95 |
| hsp18 | Heat shock protein Lo18 | TATGATGGGCAACTTGAT | AACTCCTGATACCGTTAG | 199 |
Statistical analysis.
A one way analysis of variance (ANOVA) and a Holm-Sidak test (n = 3; P < 0.05) were used to investigate significant differences. SigmaStat, version 3.0.1, software (SPSS, Inc., USA) was used for statistical analysis.
RESULTS
Interaction of Lo18 with liposomes obtained from O. oeni cells.
Two types of liposomes were prepared: control liposomes, obtained from O. oeni cultivated under optimal culture conditions, and ethanol liposomes, obtained from O. oeni cultivated under conditions of 8% ethanol. The liposomes obtained were representative of the O. oeni membrane in terms of fatty acid composition and ratio (Fig. 1). O. oeni grown in 8% ethanol showed a distinct fatty acid profile characterized by high saturated and cyclic fatty acid contents.
FIG 1.
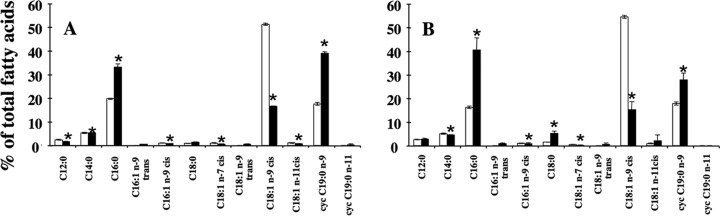
Fatty acid content of O. oeni whole membrane and liposomes derived from the membrane. The fatty acid content (percentage of the total fatty acids) of O. oeni (A) and of liposomes prepared from lipids extracted from O. oeni membranes (B) is shown. In each panel, white columns correspond to optimal growth conditions and black columns correspond to cultures grown in 8% ethanol. The mean of six GC profiles is presented; vertical bars indicate standard deviations; asterisks show statistically significant differences between the fatty acid content of control and ethanol liposomes (P < 0.05). cyc, cyclic.
The fluidity of control and ethanol liposomes was assayed, in the presence or absence of Lo18, by steady-state fluorescence anisotropy of DPH during temperature ramping (15 to 70°C). In control liposomes, the addition of Lo18 significantly reduced the temperature-dependent fluidization: in the presence of Lo18, anisotropy was 70% of the initial value, whereas anisotropy was 37% of the initial value in the absence of Lo18 (Fig. 2A). This control experiment agreed with our previous data (17). For ethanol liposomes (Fig. 2B), the initial anisotropy value (R = 0.202 units) was significantly higher than that for control liposomes (R = 0.163 units). This reflects a higher rigidity, consistent with the phospholipid content of ethanol-adapted cells. The effect of temperature in liposome fluidity was less pronounced for ethanol liposomes than for control liposomes (anisotropy was 60% of the initial value for ethanol liposomes and 37% for control liposomes). Lo18 reduced this fluidizing effect in ethanol liposomes by only 10%. Thus, Lo18 protected ethanol liposomes from the fluidizing effects of temperature but much less efficiently than control liposomes.
FIG 2.
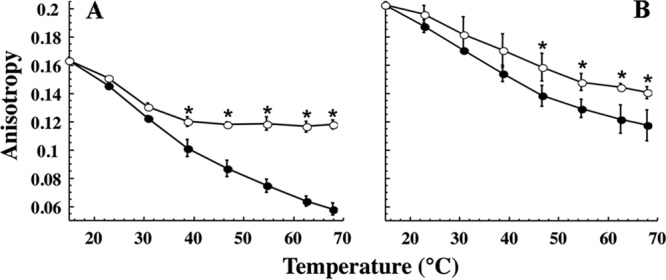
Influence of Lo18 and ethanol growth on the fluidity of liposomes derived from O. oeni. Liposome fluidity is expressed in anisotropy R values as a function of temperature in the absence (filled circles) or presence (open circles) of 10 μM Lo18. Liposomes were prepared from membrane lipids extracted from O. oeni grown under optimal growth conditions (A) or grown in 8% ethanol (B). The mass ratio between protein and liposomes was 1:2. The experiments were done in triplicate; vertical bars indicate standard deviations. Asterisks show statistically significant differences (P < 0.05).
Interaction of Lo18 with synthetic liposomes and liposomes obtained from other bacteria.
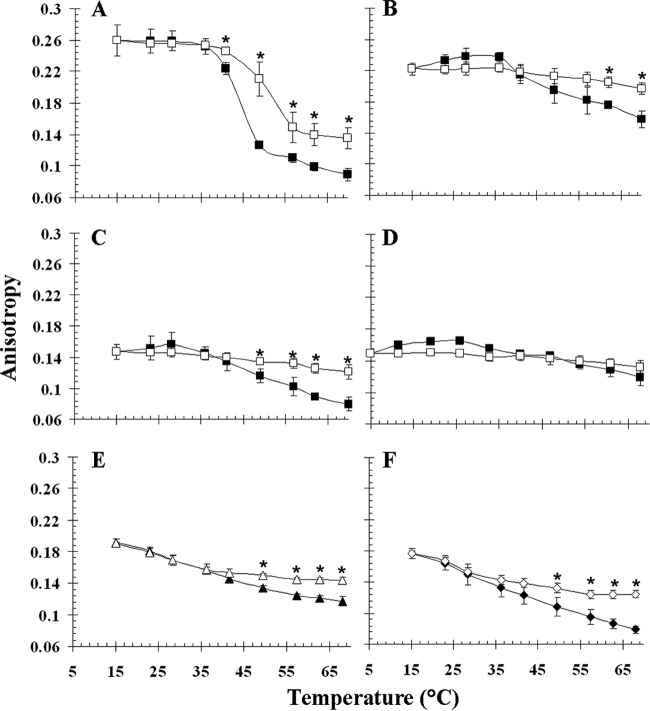
We used the most abundant phospholipids of the O. oeni membrane to generate synthetic liposomes. Synthetic phospholipids with different polar head groups or fatty acid chains were chosen. Liposome fluidity as a function of temperature (as assessed by steady-state fluorescence anisotropy) is shown for DPPG, DPPE, DOPG, and DOPE liposomes in Fig. 3A to D. In the absence of Lo18, anisotropy values decreased for the four types of synthetic liposomes during temperature ramping. However, the kinetics depended on the liposomes considered. A phase transition (at around 40°C) between the gel phase and the liquid-crystalline phase was observed only for liposomes composed of DPPG (Fig. 3A). Liposomes formed by DPPE were in the gel phase, and liposomes composed of DOPG or DOPE were in the crystalline phase for all temperatures tested (Fig. 3B, C, and D) (Table 1 gives transition phase temperatures). Lo18 stabilized the fluidity of liposomes composed of DPPG, DPPE, or DOPG when the temperature reached a value between 40 and 50°C. Lo18 did not stabilize the fluidity of liposomes composed of DOPE (Fig. 3D). This may be explained by the formation of a nonlamellar bilayer because DOPE is a cone-shaped lipid that forms inverted vesicles in hexagonal type II phase (HII) for temperatures above 10°C (36).
FIG 3.
Influence of Lo18 on the fluidity of liposomes derived from B. subtilis or L. lactis or of synthetic lipids. Liposome fluidity is expressed in anisotropy R values as a function of the temperature in the absence (filled symbols) or presence (open symbols) of 10 μM Lo18. Liposomes were composed of DPPG (A), DPPE (B), DOPG (C), or DOPE (D) or lipids extracted from B. subtilis (E) or L. lactis (F) cytoplasmic membrane. The mass ratio between protein and liposomes was 1:2. The experiments were done in triplicate; vertical bars indicate standard deviations. Asterisks show statistically significant differences (P < 0.05).
The stabilizing effect of Lo18 on other natural membranes was checked by using liposomes made from phospholipids extracted from B. subtilis or L. lactis. These two bacteria have a fatty acid content distinct from that of O. oeni (11, 37). B. subtilis contains predominantly branched saturated fatty acids, leading to a membrane of higher rigidity (R = 0.191 units) than O. oeni (R = 0.163) and L. lactis (R = 0.177). Lo18 stabilized B. subtilis- and L. lactis-derived liposomes at temperatures higher than 50°C.
Changes to Lo18 structure in the presence of liposomes.
We previously showed that the role of Lo18 in both membrane stabilization and protection of proteins from aggregation is dependent on its oligomer plasticity (17, 28, 29). However, the identity of the oligomeric form active in membrane stabilization remains elusive. Analytical gel filtration was used to determine the oligomeric status of Lo18 in the presence of liposomes. The elution profile of purified Lo18 at pH 7 gave a single peak (Fig. 4, dotted line), corresponding to the 12- to 16-subunit species previously identified at this pH (17). Preincubation of Lo18 with control liposomes at 42°C, however, resulted in a second peak of lower molecular mass (Fig. 4A). The amplitude of this peak increased with increases in the Lo18/control liposome ratio. Both calibration of the column and trapping of a predominant 36-kDa species cross-linked by BS3 suggested that this species was likely a dimer. In contrast, the elution profiles of purified Lo18 and Lo18 preincubated with ethanol liposomes at 42°C were similar (Fig. 4B). Notably, the 36-kDa species observed in the elution profile of Lo18 preincubated with control liposomes was present in very small amounts in Lo18 preincubated with ethanol liposomes. The amount of the high-molecular-weight species remained unchanged, even in the presence of liposomes, when the incubations were performed at room temperature (data not shown).
FIG 4.
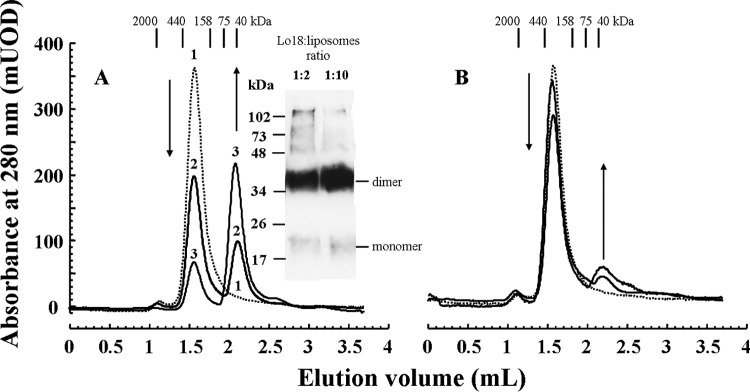
Influence of liposomes on the oligomerization of Lo18. Elution profiles of Lo18 loaded onto a Superdex 200 column. Prior to analysis, samples were incubated at 42°C for 30 min with or without liposomes. (A) Lo18 oligomerization in the presence of control liposomes. Profile 1 (dotted line), Lo18 alone; profile 2, Lo18/liposome ratio (m/m) of 1:2; profile 3, Lo18/liposome ratio of 1:10. Results of the column calibration are displayed. The inset shows the results of cross-linked Lo18 in the presence of control liposomes (Lo18/liposome ratios of 1:2 and 1:10). (B) Elution profile of Lo18 alone (dotted line) and Lo18 incubated with ethanol liposomes (Lo18/liposome ratios [m/m] of 1:2 and 1:10; black lines, similar profiles). In each panel, arrows indicate changes to the elution profiles upon the addition of liposomes. UOD, units of optical density.
Chaperone activity of Lo18 is altered in the presence of liposomes.
The ability of Lo18 to protect citrate synthase (CS) from thermal aggregation was checked in the presence of either control or ethanol liposomes at different concentrations or in liposomes formed by phospholipids extracted from B. subtilis or L. lactis membranes (Fig. 5). When heated at 45°C, CS forms insoluble aggregates that scatter light at 360 nm (Fig. 5, filled circles). As we previously described (17), Lo18 has chaperone activity and protects CS from thermal aggregation at pH 7 (Fig. 5, open circles). Increasing amounts of control liposomes caused a dose-dependent decrease of the chaperone activity of Lo18 (Fig. 5A). Control liposomes more efficiently impaired the chaperone activity of Lo18 than liposomes obtained with O. oeni cells grown with ethanol or those derived from B. subtilis and L. lactis (Fig. 5).
FIG 5.
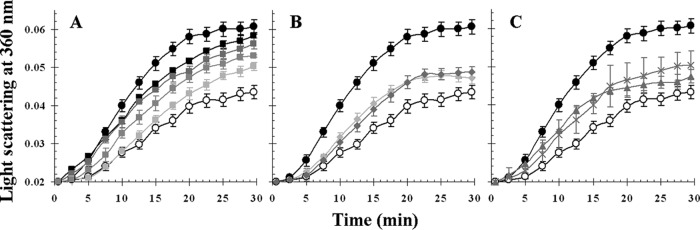
Chaperone activity of Lo18 in the presence of liposomes. Light scattering at 360 nm, which is a measure of the thermal aggregation of 300 nM citrate synthase (CS) at 45°C, was recorded as a function of time in the absence (filled circles) or presence of 9.6 μM Lo18 (open circles). (A) The dose-dependent inhibition of chaperone activity caused by increasing amounts of control liposomes (light gray squares, 12.5 μg; medium gray squares, 92.5 μg; dark gray squares, 925 μg; black squares, 9,250 μg). (B) The dose-dependent inhibition of chaperone activity caused by increasing amounts of ethanol liposomes (light gray diamonds, 925 μg; dark gray diamonds, 9,250 μg). (C) The inhibition of chaperone activity caused by liposomes composed of phospholipids extracted from membranes of B. subtilis (gray crosses, 9,250 μg) or L. lactis (gray triangles, 9,250 μg). The mean of three experiments is represented; vertical bars indicate standard deviations.
Abundance of hsp18 mRNA in O. oeni in the presence of ethanol.
The expression of the hsp18 gene in O. oeni under optimal growth conditions (mFT80 medium) and at various time points during shock or growth under conditions of 8% ethanol was measured by quantitative reverse transcription-PCR (qRT-PCR) and the comparative critical threshold (ΔΔCT) method in three independent experiments. The abundance of hsp18 mRNA was (26.73 ± 0.28)-fold higher after an ethanol shock of 30 min than under optimal growth conditions. The expression of O. oeni hsp18 may also be induced during the direct inoculation of wine in the wine-making process. We also measured hsp18 gene expression during long-term ethanol growth, corresponding to conditions where changes to membrane fatty acid content have occurred (Fig. 1A). The relative abundance of hsp18 mRNA decreased during the course of ethanol growth but nonetheless remained significantly higher than under optimal growth conditions: at 16 h hsp18 mRNA was (16.37 ± 0.92)-fold higher than under optimal growth conditions, and at 36 h hsp18 mRNA was (8.93 ± 0.74)-fold higher than under optimal growth conditions. For growth at 30 min, 16 h, and 36 h, the difference between mRNA levels with ethanol and mRNA level under control conditions was significant (P < 0.05).
DISCUSSION
In this study, we investigated the role of the sHsp Lo18 and phospholipid content in membrane stabilization that leads to ethanol tolerance in O. oeni. Thus far, the description of interactions between bacterial sHsp and phospholipid membranes was mainly restricted to Hsp17 from the cyanobacterium Synechocystis sp. strain PCC 6803. Hsp17 interacts with thylakoid membranes during heat and light stresses, and its synthesis is induced by changes to the membrane physical state (38, 39). In particular, Hsp17 can stabilize the membrane bilayer in the liquid-crystalline phase (Lα) and inhibits the formation of the inverted hexagonal structure (HII) of membranes composed of dielaidoylphosphatidylethanolamine (DEPE) (24). Moreover, the lipid binding activity of Hsp17 requires the dissociation of Hsp17 oligomers into dimers (21). The insertion of Hsp17 into the lipid bilayer of thylakoids may also occur (15, 40).
O. oeni has evolved adaptation strategies to ethanol stress which allows this bacterium to grow in wine. One strategy involves the induction of the expression of the hsp18 gene, encoding the sHsp Lo18. Lo18 was previously shown to participate in the modulation of membrane and liposome fluidity in response to stress (17, 26, 28), thus protecting membranes from the deleterious effects of ethanol. To characterize further the interaction between Lo18 and the O. oeni membrane, we used two types of liposomes prepared from O. oeni grown under optimal growth conditions or grown in 8% ethanol. We checked that the fatty acid content of the liposome preparations was representative of the composition of O. oeni membrane under optimal or ethanol conditions. We also used liposomes prepared with lipids extracted from other bacteria or with synthetic phospholipids.
The chaperone activity of Lo18, as assessed by its ability to protect a model protein, citrate synthase (CS), from thermal aggregation, was inhibited in the presence of liposomes, regardless of their lipid composition. In vivo, Lo18 localizes to the membrane after heat shock (5, 26, 29), and experiments showed that Lo18 has lipochaperone activity (17, 26). This suggests that liposomes compete with denatured CS for Lo18 binding. This liposome-induced inhibition of Lo18 protein chaperone activity was dose dependent for control liposomes and reached a plateau at around 9,250 μg of liposomes. Control liposomes more efficiently impaired the chaperone activity of Lo18 than ethanol liposomes or liposomes derived from B. subtilis and L. lactis. This suggests that the phospholipid composition is a key determinant of Lo18 membrane association. Accordingly, liposomes derived from L. lactis were more efficiently stabilized by Lo18 during temperature ramping than liposomes derived from B. subtilis. This may be due to the phospholipid composition of the L. lactis membrane which is composed of saturated, unsaturated, and cyclic fatty acids (37), similar to the O. oeni membrane. In contrast, the B. subtilis membrane has a high saturated fatty acid content (90%) (11). Phospholipid composition also influences the degree to which temperature affects the lipid organization (termed “lipid bilayer phase behavior”). Liposomes composed of DPPG, DDE, DOPG, or DOPE, all of which have different transition temperatures, were differentially stabilized by Lo18. This suggests that lipid bilayer phase behavior is a factor that determines Lo18 interaction with membranes. Interestingly, changes to the oligomeric structure of Lo18 in the presence of control liposomes were observed only if the liposomes were preincubated at 42°C. Thus, the fluidizing effect of the temperature on lipid membranes may act as a catalyst for the dissociation of Lo18 into dimers that can bind lipids.
The liposomes composed of DOPE organize as nonlamellar structures, with the fatty acids exposed at the exterior and the polar heads organized interiorly (36). Lo18 did not stabilize liposomes composed of DOPE on temperature ramping. This suggests that the protein does not interact directly with the fatty acid chains but may instead associate with the surface of the membrane when the polar heads of the phospholipids are exposed. This interpretation is consistent with previous reports showing the peripheral association of Lo18 with the O. oeni membrane after heat shock (5), where Lo18 was observed at the cytoplasmic side of the membrane by immunoelectron microscopy (26), and the release of Lo18 from membranes treated with urea and Na2CO3 (5).
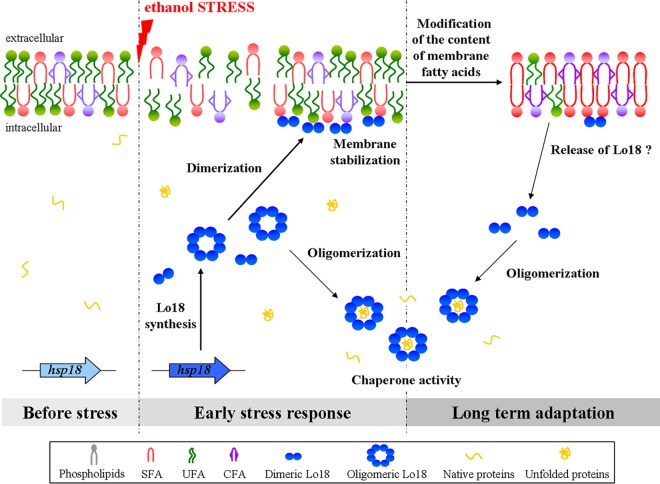
These observations led us to propose a model of how Lo18 contributes to ethanol tolerance in O. oeni (Fig. 6). Membrane fluidity abruptly increases in response the ethanol (41–43), and the envelope stress signal leads to an increase in hsp18 gene expression. Lo18 forms oligomers composed of dimeric building blocks (17). Here, oligomers of Lo18 dissociate to dimers, which can interact with the phospholipid membrane, where they participate in membrane stabilization by an unknown mechanism. The distribution of the protein between the membrane and the cytoplasm (5, 26) suggests that Lo18 can participate both in membrane stabilization and in the protection of cytoplasmic proteins from aggregation. During the adaptation process, enzymes involved in fatty acid biosynthesis (44–46) or modification, such as cyclopropane fatty acid (CFA) synthase (46) or isomerases (47), promote transitions in the content of the phospholipid membrane, which helps to counteract the fluidizing effect of ethanol. These lipid transitions are crucial for long-term adaptation of O. oeni to ethanol and are detected only after long periods of growth under ethanol conditions. Lo18 has a low affinity for the ethanol-adapted membrane, which may allow the release of this sHsp to the cytoplasm, where it exerts its chaperone activity on ethanol-aggregated proteins. We found that the abundance of hsp18 mRNA was much higher in O. oeni 30 min after ethanol shock than in bacteria grown under optimal growth conditions. The abundance of hsp18 transcripts decreased under ethanol growth conditions as a function of time. Nonetheless, hsp18 transcripts were more abundant in O. oeni grown under ethanol conditions for 36 h than in bacteria grown under optimal conditions. These findings suggest that Lo18 is involved in both the early stages and later stages of the ethanol stress response. Our model provides a basis for understanding the role of Lo18 in the adaptation process of O. oeni to ethanol that can be extended to bacterial cultures during the wine-making process.
FIG 6.
Model of Lo18 activities in response to ethanol stress in O. oeni cells. Ethanol stress increases membrane fluidity. To maintain membrane integrity, O. oeni produces the sHsp Lo18 that has the dual role of both membrane stabilization and protection of proteins from aggregation. The dissociation of Lo18 oligomers may be crucial for these activities. Dimers are preferentially found at the membrane surface. During stress, the fatty acid content of the bacterial membrane is modified. This leads to the release of dimeric Lo18 from the membrane, making it available for oligomerization and chaperone activity for cytoplasmic proteins. SFA, saturated fatty acids; UFA, unsaturated fatty acids.
ACKNOWLEDGMENTS
We gratefully acknowledge Aurélie Rieu for stimulating discussions and suggestions over the course of this study.
This work was supported by the Ministère de l'Education Nationale, de la Recherche et de la Technologie and the Conseil Régional de Bourgogne (grant number 2009 B2R4 018).
Footnotes
Published ahead of print 28 February 2014
REFERENCES
- 1.Lonvaud-Funel A. 1999. Lactic acid bacteria in the quality improvement and depreciation of wine. Antonie Van Leeuwenhoek 76:317–331. 10.1023/A:1002088931106 [DOI] [PubMed] [Google Scholar]
- 2.Guzzo J, Cavin J, Divies C. 1994. Induction of stress proteins in Leuconostoc oenos to perform direct inoculation of wine. Biotechnol. Lett. 16:1189–1194. 10.1007/BF01020849 [DOI] [Google Scholar]
- 3.Guzzo J, Delmas F, Pierre F, Jobin M, Samyn B, Van Beeumen J, Cavin J, Divies C. 1997. A small heat shock protein from Leuconostoc oenos induced by multiple stresses and during stationary growth phase. Lett. Appl. Microbiol. 24:393–396. 10.1046/j.1472-765X.1997.00042.x [DOI] [PubMed] [Google Scholar]
- 4.Guzzo J, Jobin M, Diviès C. 1998. Increase of sulfite tolerance in Oenococcus oeni by means of acidic adaptation. FEMS Microbiol. Lett. 160:43–47. 10.1111/j.1574-6968.1998.tb12888.x [DOI] [Google Scholar]
- 5.Jobin M, Delmas F, Garmyn D, Divies C, Guzzo J. 1997. Molecular characterization of the gene encoding an 18-kilodalton small heat shock protein associated with the membrane of Leuconostoc oenos. Appl. Environ. Microbiol. 63:609–614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jobin M, Garmyn D, Diviès C, Guzzo J. 1999. The Oenococcus oeni clpX homologue is a heat shock gene preferentially expressed in exponential growth phase. J. Bacteriol. 181:6634–6641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Teixeira H, Goncalves M, Rozes N, Ramos A, San Romao M. 2002. Lactobacillic acid accumulation in the plasma membrane of Oenococcus oeni: a response to ethanol stress? Microb. Ecol. 43:146–153. 10.1007/s00248-001-0036-6 [DOI] [PubMed] [Google Scholar]
- 8.Grandvalet C, Assad-Garcia J, Chu-Ky S, Tollot M, Guzzo J, Gresti J, Tourdot-Maréchal R. 2008. Changes in membrane lipid composition in ethanol-and acid-adapted Oenococcus oeni cells: characterization of the cfa gene by heterologous complementation. Microbiology 154:2611–2619. 10.1099/mic.0.2007/016238-0 [DOI] [PubMed] [Google Scholar]
- 9.van Bokhorst-van de Veen H, Abee T, Tempelaars M, Bron P, Kleerebezem M, Marco M. 2011. Short-and long-term adaptation to ethanol stress and its cross-protective consequences in Lactobacillus plantarum. Appl. Environ. Microbiol. 77:5247–5256. 10.1128/AEM.00515-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Torok Z, Tsvetkova N, Balogh G, Horvath I, Nagy E, Penzes Z, Hargitai J, Bensaude O, Csermely P, Crowe J, Maresca B, Vigh L. 2003. Heat shock protein coinducers with no effect on protein denaturation specifically modulate the membrane lipid phase. Proc. Natl. Acad. Sci. U. S. A. 100:3131–3136. 10.1073/pnas.0438003100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Seydlova G, Halada P, Fiser R, Toman O, Ulrych A, Svobodova J. 2012. DnaK and GroEL chaperones are recruited to the Bacillus subtilis membrane after short-term ethanol stress. J. Appl. Microbiol. 112:765–774. 10.1111/j.1365-2672.2012.05238.x [DOI] [PubMed] [Google Scholar]
- 12.Torok Z, Horvath I, Goloubinoff P, Kovacs E, Glatz A, Balogh G, Vigh L. 1997. Evidence for a lipochaperonin: association of active protein folding GroESL oligomers with lipids can stabilize membranes under heat shock conditions. Proc. Natl. Acad. Sci. U. S. A. 94:2192–2197. 10.1073/pnas.94.6.2192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Horvath I, Multhoff G, Sonnleitner A, Vigh L. 2008. Membrane-associated stress proteins: more than simply chaperones. Biochim. Biophys. Acta 1778:1653–1664. 10.1016/j.bbamem.2008.02.012 [DOI] [PubMed] [Google Scholar]
- 14.Nakamoto H, Vigh L. 2007. The small heat shock proteins and their clients. Cell Mol. Life Sci. 64:294–306. 10.1007/s00018-006-6321-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Horvath I, Glatz A, Nakamoto H, Mishkind M, Munnik T, Saidi Y, Goloubinoff P, Harwood J, Vigh L. 2012. Heat shock response in photosynthetic organisms: membrane and lipid connections. Prog. Lipid Res. 51:208–220. 10.1016/j.plipres.2012.02.002 [DOI] [PubMed] [Google Scholar]
- 16.Sun Y, MacRae T. 2005. Small heat shock proteins: molecular structure and chaperone function. Cell Mol. Life Sci. 62:2460–2476. 10.1007/s00018-005-5190-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maitre M, Weidmann S, Rieu A, Fenel D, Schoehn G, Ebel C, Covès J, Guzzo J. 2012. The oligomer plasticity of the small heat shock protein Lo18 from Oenococcus oeni influences its role in both membrane stabilization and protein protection. Biochem. J. 444:97–104. 10.1042/BJ20120066 [DOI] [PubMed] [Google Scholar]
- 18.Narberhaus F. 2002. Alpha-crystallin-type heat shock proteins: socializing minichaperones in the context of a multichaperone network. Microbiol. Mol. Biol. Rev. 66:64–93. 10.1128/MMBR.66.1.64-93.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lentze N, Studer S, Narberhaus F. 2003. Structural and functional defects caused by point mutations in the alpha-crystallin domain of a bacterial alpha-heat shock protein. J. Mol. Biol. 328:927–937. 10.1016/S0022-2836(03)00356-5 [DOI] [PubMed] [Google Scholar]
- 20.Chowdary T, Raman B, Ramakrishna T, Rao C. 2007. Interaction of mammalian Hsp22 with lipid membranes. Biochem. J. 401:437–445. 10.1042/BJ20061046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Balogi Z, Cheregi O, Giese K, Juhasz K, Vierling E, Vass I, Vigh L, Horvath I. 2008. A mutant small heat shock protein with increased thylakoid association provides an elevated resistance against UV-B damage in Synechocystis 6803. J. Biol. Chem. 283:22983–22991. 10.1074/jbc.M710400200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang H, Fu X, Jiao W, Zhang X, Liu C, Chang Z. 2005. The association of small heat shock protein Hsp16.3 with the plasma membrane of Mycobacterium tuberculosis: dissociation of oligomers is a prerequisite. Biochem. Biophys. Res. Commun. 330:1055–1061. 10.1016/j.bbrc.2005.03.092 [DOI] [PubMed] [Google Scholar]
- 23.Balogi Z, Torok Z, Balogh G, Josvay K, Shigapova N, Vierling E, Vigh L, Horvath I. 2005. “Heat shock lipid” in cyanobacteria during heat/light-acclimation. Arch. Biochem. Biophys. 436:346–354. 10.1016/j.abb.2005.02.018 [DOI] [PubMed] [Google Scholar]
- 24.Tsvetkova N, Horvath I, Torok Z, Wolkers W, Balogi Z, Shigapova N, Crowe L, Tablin F, Vierling E, Crowe J, Vigh L. 2002. Small heat-shock proteins regulate membrane lipid polymorphism. Proc. Natl. Acad. Sci. U. S. A. 99:13504–13509. 10.1073/pnas.192468399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guzzo J, Jobin M, Delmas F, Fortier L, Garmyn D, Tourdot-Maréchal R, Lee B, Diviès C. 2000. Regulation of stress response in Oenococcus oeni as a function of environmental changes and growth phase. Int. J. Food Microbiol. 55:27–31. 10.1016/S0168-1605(00)00209-9 [DOI] [PubMed] [Google Scholar]
- 26.Coucheney F, Gal L, Beney L, Lherminier J, Gervais P, Guzzo J. 2005. A small HSP, Lo18, interacts with the cell membrane and modulates lipid physical state under heat shock conditions in a lactic acid bacterium. Biochim. Biophys. Acta 1720:92–98. 10.1016/j.bbamem.2005.11.017 [DOI] [PubMed] [Google Scholar]
- 27.Grandvalet C, Coucheney F, Beltramo C, Guzzo J. 2005. CtsR is the master regulator of stress response gene expression in Oenococcus oeni. J. Bacteriol. 187:5614–5623. 10.1128/JB.187.16.5614-5623.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Weidmann S, Rieu A, Rega M, Coucheney F, Guzzo J. 2010. Distinct amino acids of the Oenococcus oeni small heat shock protein Lo18 are essential for damaged protein protection and membrane stabilization. FEMS Microbiol. Lett. 309:8–15. 10.1111/j.1574-6968.2010.01999.x [DOI] [PubMed] [Google Scholar]
- 29.Delmas F, Pierre F, Coucheney F, Divies C, Guzzo J. 2001. Biochemical and physiological studies of the small heat shock protein Lo18 from the lactic acid bacterium Oenococcus oeni. J. Mol. Microbiol. Biotechnol. 3:601–610 http://www.horizonpress.com/backlist/jmmb/v/v3/v3n4/13.pdf [PubMed] [Google Scholar]
- 30.Cavin J, Prevost H, Lin J, Schmitt P, Divies C. 1989. Medium for screening Leuconostoc oenos strains defective in malolactic fermentation. Appl. Environ. Microbiol. 55:751–753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Terzaghi B, Sandine W. 1975. Improved medium for lactic streptococci and their bacteriophages. Appl. Microbiol. 29:807–813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bligh E, Dyer W. 1959. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 37:911–917. 10.1139/o59-099 [DOI] [PubMed] [Google Scholar]
- 33.Méchin L, Dubois-Brissonnet F, Heyd B, Leveau J. 1999. Adaptation of Pseudomonas aeruginosa ATCC 15442 to didecyldimethylammonium bromide induces changes in membrane fatty acid composition and in resistance of cells. J. Appl. Microbiol. 86:859–866. 10.1046/j.1365-2672.1999.00770.x [DOI] [PubMed] [Google Scholar]
- 34.Desroche N, Beltramo C, Guzzo J. 2005. Determination of an internal control to apply reverse transcription quantitative PCR to study stress response in the lactic acid bacterium Oenococcus oeni. J. Microbiol. Methods 60:325–333. 10.1016/j.mimet.2004.10.010 [DOI] [PubMed] [Google Scholar]
- 35.Livak K, Schmittgen T. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25:402–408. 10.1006/meth.2001.1262 [DOI] [PubMed] [Google Scholar]
- 36.Denich T, Beaudette L, Lee H, Trevors J. 2003. Effect of selected environmental and physico-chemical factors on bacterial cytoplasmic membranes. J. Microbiol. Methods 52:149–182. 10.1016/S0167-7012(02)00155-0 [DOI] [PubMed] [Google Scholar]
- 37.To T, Grandvalet C, Tourdot-Maréchal R. 2011. Cyclopropanation of membrane unsaturated fatty acids is not essential to the acid stress response of Lactococcus lactis subsp. cremoris. Appl. Environ. Microbiol. 77:3327–3334. 10.1128/AEM.02518-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Horvath I, Glatz A, Varvasovszki V, Torok Z, Pali T, Balogh G, Kovacs E, Nadasdi L, Benko S, Joo F, Vigh L. 1998. Membrane physical state controls the signaling mechanism of the heat shock response in Synechocystis PCC 6803: identification of hsp17 as a “fluidity gene.” Proc. Natl. Acad. Sci. U. S. A. 95:3513–3518. 10.1073/pnas.95.7.3513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Torok Z, Goloubinoff P, Horvath I, Tsvetkova NM, Glatz A, Balogh G, Varvasovszki V, Los DA, Vierling E, Crowe JH. 2001. Synechocystis HSP17 is an amphitropic protein that stabilizes heat-stressed membranes and binds denatured proteins for subsequent chaperone-mediated refolding. Proc. Natl. Acad. Sci. U. S. A. 98:3098–3103. 10.1073/pnas.051619498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vigh L, Horvath I, Maresca B, Harwood J. 2007. Can. the stress protein response be controlled by “membrane-lipid therapy”? Trends Biochem. Sci. 32:357–363. 10.1016/j.tibs.2007.06.009 [DOI] [PubMed] [Google Scholar]
- 41.Dombek K, Ingram L. 1984. Effects of ethanol on the Escherichia coli plasma membrane. J. Bacteriol. 157:233–239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sikkema J, De Bont J, Poolman B. 1995. Mechanisms of membrane toxicity of hydrocarbons. Microbiol. Rev. 59:201–222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Weber F, De Bont J. 1996. Adaptation mechanisms of microorganisms to the toxic effects of organic solvents on membranes. Biochim. Biophys. Acta 1286:225–245. 10.1016/S0304-4157(96)00010-X [DOI] [PubMed] [Google Scholar]
- 44.Magnuson K, Jackowski S, Rock C, Cronan J., Jr 1993. Regulation of fatty acid biosynthesis in Escherichia coli. Microbiol. Rev. 57:522–542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lu Y, Rock C. 2006. Transcriptional regulation of fatty acid biosynthesis in Streptococcus pneumoniae. Mol. Microbiol. 59:551–566. 10.1111/j.1365-2958.2005.04951.x [DOI] [PubMed] [Google Scholar]
- 46.Grogan D, Cronan J., Jr 1997. Cyclopropane ring formation in membrane lipids of bacteria. Microbiol. Mol. Biol. Rev. 61:429–441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Diefenbach R, Heipieper H, Keweloh H. 1992. The conversion of cis into trans unsaturated fatty acids in Pseudomonas putida P8: evidence for a role in the regulation of membrane fluidity. Appl. Microbiol. Biotechnol. 38:382–387. 10.1007/BF00170090 [DOI] [Google Scholar]