Abstract
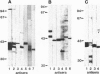
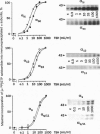
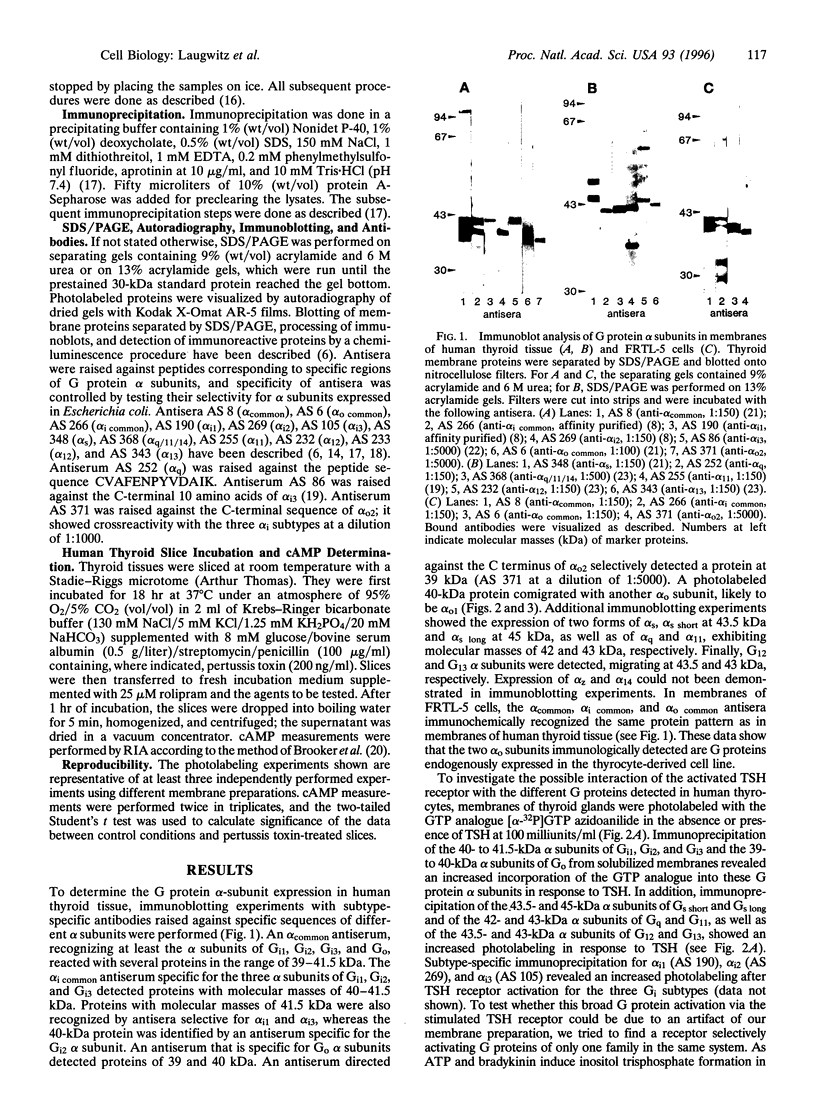
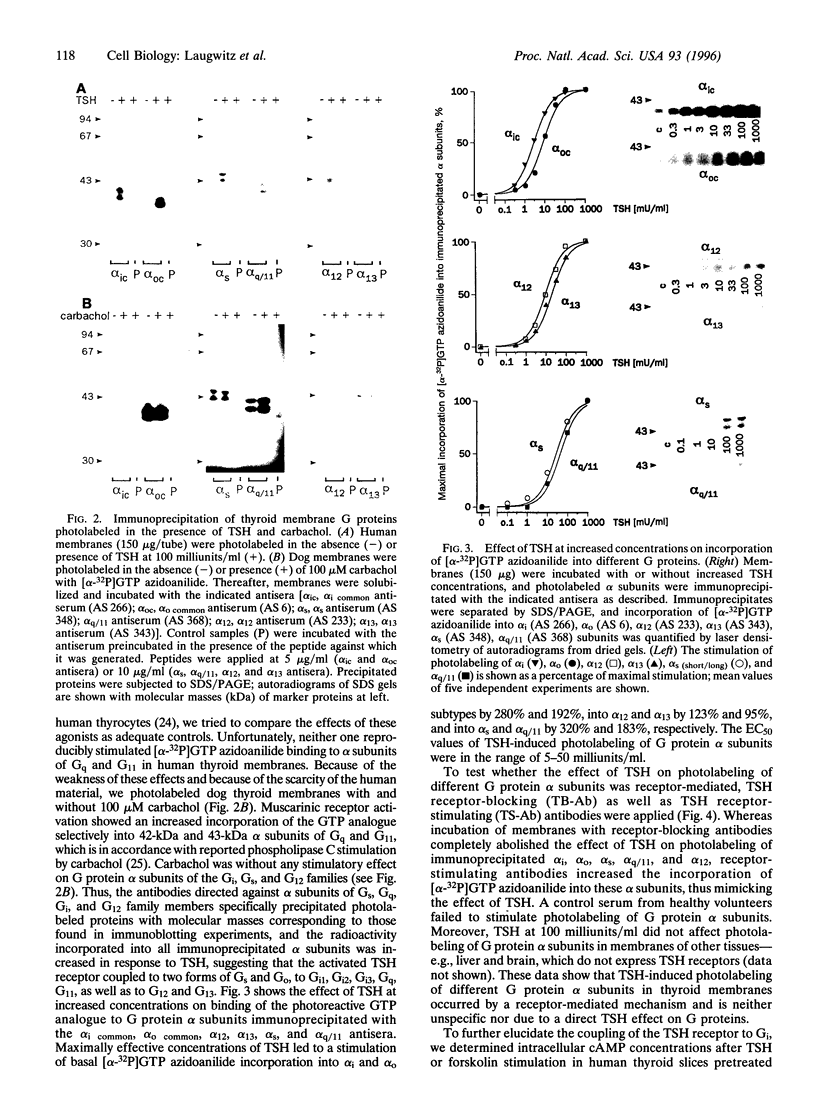
Thyrotropin is the primary hormone that, via one heptahelical receptor, regulates thyroid cell functions such as secretion, specific gene expression, and growth. In human thyroid, thyrotropin receptor activation leads to stimulation of the adenylyl cyclase and phospholipase C cascades. However, the G proteins involved in thyrotropin receptor action have been only partially defined. In membranes of human thyroid gland, we immunologically identified alpha subunits of the G proteins Gs short, Gs long, Gi1, Gi2, Gi3, G(o) (Go2 and another form of Go, presumably Go1), Gq, G11, G12, and G13. Activation of the thyrotropin (TSH) receptor by bovine TSH led to increased incorporation of the photoreactive GTP analogue [alpha-32P]GTP azidoanilide into immunoprecipitated alpha subunits of all G proteins detected in thyroid membranes. This effect was receptor-dependent and not due to direct G protein stimulation because it was mimicked by TSH receptor-stimulating antibodies of patients suffering from Grave disease and was abolished by a receptor-blocking antiserum from a patient with autoimmune hypothyroidism. The TSH-induced activation of individual G proteins occurred with EC50 values of 5-50 milliunits/ml, indicating that the activated TSH receptor coupled with similar potency to different G proteins. When human thyroid slices were pretreated with pertussis toxin, the TSH receptor-mediated accumulation of cAMP increased by approximately 35% with TSH at 1 milliunits/ml, indicating that the TSH receptor coupled to Gs and G(i). Taken together, these findings show that, at least in human thyroid membranes, in which the protein is expressed at its physiological levels, the TSH receptor resembles a naturally occurring example of a general G protein-activating receptor.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allgeier A., Offermanns S., Van Sande J., Spicher K., Schultz G., Dumont J. E. The human thyrotropin receptor activates G-proteins Gs and Gq/11. J Biol Chem. 1994 May 13;269(19):13733–13735. [PubMed] [Google Scholar]
- Aramori I., Nakanishi S. Signal transduction and pharmacological characteristics of a metabotropic glutamate receptor, mGluR1, in transfected CHO cells. Neuron. 1992 Apr;8(4):757–765. doi: 10.1016/0896-6273(92)90096-v. [DOI] [PubMed] [Google Scholar]
- Bourne H. R., Sanders D. A., McCormick F. The GTPase superfamily: conserved structure and molecular mechanism. Nature. 1991 Jan 10;349(6305):117–127. doi: 10.1038/349117a0. [DOI] [PubMed] [Google Scholar]
- Brooker G., Harper J. F., Terasaki W. L., Moylan R. D. Radioimmunoassay of cyclic AMP and cyclic GMP. Adv Cyclic Nucleotide Res. 1979;10:1–33. [PubMed] [Google Scholar]
- Chabre O., Conklin B. R., Brandon S., Bourne H. R., Limbird L. E. Coupling of the alpha 2A-adrenergic receptor to multiple G-proteins. A simple approach for estimating receptor-G-protein coupling efficiency in a transient expression system. J Biol Chem. 1994 Feb 25;269(8):5730–5734. [PubMed] [Google Scholar]
- Clapham D. E., Neer E. J. New roles for G-protein beta gamma-dimers in transmembrane signalling. Nature. 1993 Sep 30;365(6445):403–406. doi: 10.1038/365403a0. [DOI] [PubMed] [Google Scholar]
- Dhanasekaran N., Prasad M. V., Wadsworth S. J., Dermott J. M., van Rossum G. Protein kinase C-dependent and -independent activation of Na+/H+ exchanger by G alpha 12 class of G proteins. J Biol Chem. 1994 Apr 22;269(16):11802–11806. [PubMed] [Google Scholar]
- Eason M. G., Kurose H., Holt B. D., Raymond J. R., Liggett S. B. Simultaneous coupling of alpha 2-adrenergic receptors to two G-proteins with opposing effects. Subtype-selective coupling of alpha 2C10, alpha 2C4, and alpha 2C2 adrenergic receptors to Gi and Gs. J Biol Chem. 1992 Aug 5;267(22):15795–15801. [PubMed] [Google Scholar]
- Faure M., Voyno-Yasenetskaya T. A., Bourne H. R. cAMP and beta gamma subunits of heterotrimeric G proteins stimulate the mitogen-activated protein kinase pathway in COS-7 cells. J Biol Chem. 1994 Mar 18;269(11):7851–7854. [PubMed] [Google Scholar]
- Gross B., Misrahi M., Sar S., Milgrom E. Composite structure of the human thyrotropin receptor gene. Biochem Biophys Res Commun. 1991 Jun 14;177(2):679–687. doi: 10.1016/0006-291x(91)91842-z. [DOI] [PubMed] [Google Scholar]
- Hepler J. R., Gilman A. G. G proteins. Trends Biochem Sci. 1992 Oct;17(10):383–387. doi: 10.1016/0968-0004(92)90005-t. [DOI] [PubMed] [Google Scholar]
- Hescheler J., Schultz G. G-proteins involved in the calcium channel signalling system. Curr Opin Neurobiol. 1993 Jun;3(3):360–367. doi: 10.1016/0959-4388(93)90129-m. [DOI] [PubMed] [Google Scholar]
- Iida-Klein A., Guo J., Xie L. Y., Jüppner H., Potts J. T., Jr, Kronenberg H. M., Bringhurst F. R., Abou-Samra A. B., Segre G. V. Truncation of the carboxyl-terminal region of the rat parathyroid hormone (PTH)/PTH-related peptide receptor enhances PTH stimulation of adenylyl cyclase but not phospholipase C. J Biol Chem. 1995 Apr 14;270(15):8458–8465. doi: 10.1074/jbc.270.15.8458. [DOI] [PubMed] [Google Scholar]
- Laugwitz K. L., Offermanns S., Spicher K., Schultz G. mu and delta opioid receptors differentially couple to G protein subtypes in membranes of human neuroblastoma SH-SY5Y cells. Neuron. 1993 Feb;10(2):233–242. doi: 10.1016/0896-6273(93)90314-h. [DOI] [PubMed] [Google Scholar]
- Laugwitz K. L., Spicher K., Schultz G., Offermanns S. Identification of receptor-activated G proteins: selective immunoprecipitation of photolabeled G-protein alpha subunits. Methods Enzymol. 1994;237:283–294. doi: 10.1016/s0076-6879(94)37069-9. [DOI] [PubMed] [Google Scholar]
- Laurent E., Mockel J., Takazawa K., Erneux C., Dumont J. E. Stimulation of generation of inositol phosphates by carbamoylcholine and its inhibition by phorbol esters and iodide in dog thyroid cells. Biochem J. 1989 Nov 1;263(3):795–801. doi: 10.1042/bj2630795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law S. F., Yasuda K., Bell G. I., Reisine T. Gi alpha 3 and G(o) alpha selectively associate with the cloned somatostatin receptor subtype SSTR2. J Biol Chem. 1993 May 25;268(15):10721–10727. [PubMed] [Google Scholar]
- Milligan G. Mechanisms of multifunctional signalling by G protein-linked receptors. Trends Pharmacol Sci. 1993 Jun;14(6):239–244. doi: 10.1016/0165-6147(93)90019-g. [DOI] [PubMed] [Google Scholar]
- Nürnberg B., Spicher K., Harhammer R., Bosserhoff A., Frank R., Hilz H., Schultz G. Purification of a novel G-protein alpha 0-subtype from mammalian brain. Biochem J. 1994 Jun 1;300(Pt 2):387–394. doi: 10.1042/bj3000387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Offermanns S., Laugwitz K. L., Spicher K., Schultz G. G proteins of the G12 family are activated via thromboxane A2 and thrombin receptors in human platelets. Proc Natl Acad Sci U S A. 1994 Jan 18;91(2):504–508. doi: 10.1073/pnas.91.2.504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Offermanns S., Schultz G. Complex information processing by the transmembrane signaling system involving G proteins. Naunyn Schmiedebergs Arch Pharmacol. 1994 Oct;350(4):329–338. doi: 10.1007/BF00178947. [DOI] [PubMed] [Google Scholar]
- Offermanns S., Schultz G., Rosenthal W. Identification of receptor-activated G proteins with photoreactive GTP analog, [alpha-32P]GTP azidoanilide. Methods Enzymol. 1991;195:286–301. doi: 10.1016/0076-6879(91)95174-i. [DOI] [PubMed] [Google Scholar]
- Offermanns S., Wieland T., Homann D., Sandmann J., Bombien E., Spicher K., Schultz G., Jakobs K. H. Transfected muscarinic acetylcholine receptors selectively couple to Gi-type G proteins and Gq/11. Mol Pharmacol. 1994 May;45(5):890–898. [PubMed] [Google Scholar]
- Parks S., Wieschaus E. The Drosophila gastrulation gene concertina encodes a G alpha-like protein. Cell. 1991 Jan 25;64(2):447–458. doi: 10.1016/0092-8674(91)90652-f. [DOI] [PubMed] [Google Scholar]
- Prather P. L., McGinn T. M., Erickson L. J., Evans C. J., Loh H. H., Law P. Y. Ability of delta-opioid receptors to interact with multiple G-proteins is independent of receptor density. J Biol Chem. 1994 Aug 19;269(33):21293–21302. [PubMed] [Google Scholar]
- Raspé E., Laurent E., Andry G., Dumont J. E. ATP, bradykinin, TRH and TSH activate the Ca(2+)-phosphatidylinositol cascade of human thyrocytes in primary culture. Mol Cell Endocrinol. 1991 Oct;81(1-3):175–183. doi: 10.1016/0303-7207(91)90216-f. [DOI] [PubMed] [Google Scholar]
- Saunier B., Tournier C., Jacquemin C., Pierre M. Stimulation of mitogen-activated protein kinase by thyrotropin in primary cultured human thyroid follicles. J Biol Chem. 1995 Feb 24;270(8):3693–3697. doi: 10.1074/jbc.270.8.3693. [DOI] [PubMed] [Google Scholar]
- Selzer E., Wilfing A., Schiferer A., Hermann M., Grubeck-Loebenstein B., Freissmuth M. Stimulation of human thyroid growth via the inhibitory guanine nucleotide binding (G) protein Gi: constitutive expression of the G-protein alpha subunit Gi alpha-1 in autonomous adenoma. Proc Natl Acad Sci U S A. 1993 Feb 15;90(4):1609–1613. doi: 10.1073/pnas.90.4.1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon M. I., Strathmann M. P., Gautam N. Diversity of G proteins in signal transduction. Science. 1991 May 10;252(5007):802–808. doi: 10.1126/science.1902986. [DOI] [PubMed] [Google Scholar]
- Spengler D., Waeber C., Pantaloni C., Holsboer F., Bockaert J., Seeburg P. H., Journot L. Differential signal transduction by five splice variants of the PACAP receptor. Nature. 1993 Sep 9;365(6442):170–175. doi: 10.1038/365170a0. [DOI] [PubMed] [Google Scholar]
- Spicher K., Kalkbrenner F., Zobel A., Harhammer R., Nürnberg B., Söling A., Schultz G. G12 and G13 alpha-subunits are immunochemically detectable in most membranes of various mammalian cells and tissues. Biochem Biophys Res Commun. 1994 Feb 15;198(3):906–914. doi: 10.1006/bbrc.1994.1129. [DOI] [PubMed] [Google Scholar]
- Stephens L., Smrcka A., Cooke F. T., Jackson T. R., Sternweis P. C., Hawkins P. T. A novel phosphoinositide 3 kinase activity in myeloid-derived cells is activated by G protein beta gamma subunits. Cell. 1994 Apr 8;77(1):83–93. doi: 10.1016/0092-8674(94)90237-2. [DOI] [PubMed] [Google Scholar]
- Sternweis P. C. The active role of beta gamma in signal transduction. Curr Opin Cell Biol. 1994 Apr;6(2):198–203. doi: 10.1016/0955-0674(94)90136-8. [DOI] [PubMed] [Google Scholar]
- Vassart G., Dumont J. E. The thyrotropin receptor and the regulation of thyrocyte function and growth. Endocr Rev. 1992 Aug;13(3):596–611. doi: 10.1210/edrv-13-3-596. [DOI] [PubMed] [Google Scholar]
- Wong S. K., Ross E. M. Chimeric muscarinic cholinergic:beta-adrenergic receptors that are functionally promiscuous among G proteins. J Biol Chem. 1994 Jul 22;269(29):18968–18976. [PubMed] [Google Scholar]
- Wu D., LaRosa G. J., Simon M. I. G protein-coupled signal transduction pathways for interleukin-8. Science. 1993 Jul 2;261(5117):101–103. doi: 10.1126/science.8316840. [DOI] [PubMed] [Google Scholar]
- Xu N., Bradley L., Ambdukar I., Gutkind J. S. A mutant alpha subunit of G12 potentiates the eicosanoid pathway and is highly oncogenic in NIH 3T3 cells. Proc Natl Acad Sci U S A. 1993 Jul 15;90(14):6741–6745. doi: 10.1073/pnas.90.14.6741. [DOI] [PMC free article] [PubMed] [Google Scholar]