Abstract
Glycerol has become a desirable feedstock for the production of fuels and chemicals due to its availability and low price, but many barriers to commercialization remain. Previous investigators have made significant improvements in the yield of ethanol from glycerol. We have developed a fermentation process for the efficient microaerobic conversion of glycerol to ethanol by Escherichia coli that presents solutions to several other barriers to commercialization: rate, titer, specific productivity, use of inducers, use of antibiotics, and safety. To increase the rate, titer, and specific productivity to commercially relevant levels, we constructed a plasmid that overexpressed glycerol uptake genes dhaKLM, gldA, and glpK, as well as the ethanol pathway gene adhE. To eliminate the cost of inducers and antibiotics from the fermentation, we used the adhE and icd promoters from E. coli in our plasmid, and we implemented glycerol addiction to retain the plasmid. To address the safety issue of off-gas flammability, we optimized the fermentation process with reduced-oxygen sparge gas to ensure that the off-gas remained nonflammable. These advances represent significant progress toward the commercialization of an E. coli-based glycerol-to-ethanol process.
INTRODUCTION
Concerns about energy security, environmental damage, and sustainability have stimulated interest in developing non-petroleum-based sources of fuel. Biodiesel is one of the most common renewable transportation fuels, and its production has grown immensely in the last 2 decades (1). This has created a surplus of crude glycerol, because the transesterification process produces about 1 kg of crude glycerol for every 10 kg of biodiesel produced. This cheap, highly reduced carbon source can be used in microbial fermentations.
Considerable research effort has been focused on converting glycerol into useful chemicals (2). Although there are many organisms capable of metabolizing glycerol, we are most interested in industrial microbes that are amenable to genetic manipulations for the application of rational metabolic-engineering strategies. We chose to work with Escherichia coli, which has been shown to metabolize glycerol under anaerobic and microaerobic conditions (3–5). Previous studies have demonstrated the efficient conversion of glycerol into fuels and chemicals such as ethanol (6), 1,3-propanediol (7), 1,2-propanediol (8), poly(3-hydroxybutyrates) (PHBs) (9), succinic acid (10), and lactic acid (11, 12).
We chose ethanol as our product due to its potential as a biofuel and feedstock for the production of oxygenated fuels (13). Previous investigators have proven the concept of converting glycerol to ethanol in a microaerobic environment (6, 14, 15). With the deletion of specific by-product pathways, they have achieved ethanol yields from glycerol as high as 95% (6) and 97% (15) of the maximum theoretical yield. These yields are high enough for a commercial process, but we need to overcome many other barriers to commercialize the process. Yazdani and Gonzalez showed that simultaneous overexpression of dhaKLM and gldA increased glycerol utilization and ethanol synthesis (6). In the work presented here, we used the foundation created by the previous studies to engineer a strain and a process for the conversion of crude glycerol into ethanol. We implemented genetic and process manipulations to increase the rate, titer, and specific productivity, avoid the use of antibiotics and inducers, and avoid a flammable H2-O2 mixture in the reactor headspace. These advances remove several significant barriers to the commercialization of a glycerol-to-ethanol fermentation process.
MATERIALS AND METHODS
Bacterial strains, plasmids, and oligonucleotides.
All Escherichia coli strains, along with plasmids used in this work, are listed in Table 1. For oligonucleotides used in this work, see Table S1 in the supplemental material.
TABLE 1.
Strains and plasmids used in this study
| Strain or plasmid | Relevant characteristics | Reference or source |
|---|---|---|
| E. coli strains | ||
| ATCC 8739 | ATCC, Manassas, VA | |
| GB1 | ATCC 8739 ΔfrdA | This work |
| GB2 | ATCC 8739 ΔfrdA Δpta | This work |
| GB3 | ATCC 8739 ΔfrdA Δpta ΔldhA | This work |
| GB4 | ATCC 8739 ΔackA Δpta ΔldhA | This work |
| GB5 | ATCC 8739 ΔfrdABCD ΔackA Δpta ΔldhA ΔpoxB | This work |
| GB6 | ATCC 8739 ΔfrdABCD ΔackA Δpta ΔldhA ΔpoxB ΔgldA ΔglpK | This work |
| Plasmids | ||
| pZSKLMgldA | E. coli dhaKLM and gldA under the control of PLtetO-1 (oriR SC101; Cmr) | 6 |
| pZSadhEpKLMgldA | pZSKLMgldA derivative with the E. coli adhE promoter replacing the PLtetO-1 promoter (Cmr, oriR SC101) | This work |
| pGB1001 | pZSadhEpKLMgldA derivative with a ribosomal binding site inserted in front of gldA (Cmr, oriR SC101) | This work |
| pGB1001.1 | Intermediate construct putting the rrnB transcriptional terminator and E. coli adhE operon together in ptrcHisA (Life Technologies, Invitrogen) | This work |
| pGB1002 | pGB1001 derivative with an rrnB transcriptional terminator after the adhEp-dhaKLM-gldA operon and the E. coli adhE operon inserted after the rrnB terminator (Cmr, oriR SC101) | This work |
| pGB1003 | pGB1002 derivative expressing the glpK gene from E. coli under the control of the E. coli isocitrate dehydrogenase promoter (21) (Cmr, oriR SC101) | This work |
DNA manipulations and mutant construction.
Unless otherwise indicated, all DNA procedures followed standard protocols and specific recommendations from manufacturers. E. coli knockout mutants were constructed by the suicide vector method (16). E. coli genes were amplified from genomic DNA by using AccuPrime Pfx DNA polymerase (Life Technologies, Invitrogen) and cloned into vectors by using the Quick Ligation kit (New England BioLabs Inc., Ipswich, MA) or In-Fusion HD cloning kit (Clontech Laboratories Inc., Mountain View, CA).
Growth media and culture conditions.
The MOPS [3-(N-morpholino)propanesulfonic acid] minimal medium designed by Neidhardt et al. (17) was supplemented with 30 mM NH4Cl, 3.96 mM Na2HPO4 in place of 1.32 mM K2HPO4, 5 mM (NH4)2SO4, and 1 μM sodium selenite. This medium was used during the preliminary fermentations.
Unless otherwise specified, the minimal medium used for fermentations was MM29, which was made in the following manner. To 1 liter of water, we added 0.66 g (NH4)2SO4, 1.2 g Na2HPO4, 3 g NH4Cl, 0.25 g K2SO4, and 1 ml of micronutrient solution. The mixture was sterilized by autoclaving. After the mixture cooled to room temperature, 10 ml of divalent-cation solution was added to the medium to complete MM29. The micronutrient solution consisted of 0.173 g sodium selenite, 0.004 g (NH4)6Mo7O24 · H2O, 0.025 g H3BO3, 0.007 g CoCl2 · 6H2O, 0.003 g CuSO4 · 5H2O, 0.016 g MnCl2 · 4H2O, and 0.003 g ZnSO4 · 7H2O dissolved in 1 liter of water. The pH of the micronutrient solution was adjusted to 3.0 with 3 M HCl to fully dissolve the chemicals. The divalent-cation solution was made by adding the following chemicals to 1 liter of water: 40 g MgCl2 · 6H2O, 0.3 g FeSO4 · 7H2O, and 7 g CaCl2 · 2H2O. The divalent-cation solution was filter sterilized before use.
Chloramphenicol (40 mg/liter) was added when it was appropriate. Chemicals were obtained from Sigma-Aldrich Co. (St. Louis, MO). Technical-grade glycerol (99.7% purity) was obtained from KIC Chemicals (New Paltz, NY). Crude glycerol was obtained from REG (Ames, IA). The crude glycerin had the following reported composition (wt/vol): 83% glycerol, 11% water, and ∼6% ash (most of which was in the form of NaCl); minor constituents included free fatty acids (0.31%) and methanol (0.05%). No other MONG (matter organic nonglycerol) was detected in this sample. Experiments using crude glycerol directly substituted crude glycerol for technical-grade glycerol.
Bioreactor cultivation.
Bioreactor cultivations were carried out in one of three bioreactor systems. Preliminary fermentations used 0.75-liter glass jar reactors from Ward's Science (Rochester, NY). Batch cultures were grown in a 0.5-liter working volume with the agitation accomplished by a stir bar at the bottom of the vessel. The agitation was set to provide the desired oxygen transfer coefficient (kLa). The fermentor was sparged with air, and the pH was controlled at 6.3 by automatic addition of 5 M NaOH. Temperature was controlled at 37°C.
Bioreactor cultivations were also carried out in 7-liter stirred-tank reactors equipped with two Rushton impellers on the stirrer shaft (Biostat A Plus; Sartorius North America, Edgewood, NY). Batch cultures were grown in a 4-liter working volume with the agitation set to provide the desired kLa. The fermentor was sparged with air or a 5% O2-95% N2 gas mixture, and the pH was controlled at 6.3 by automatic addition of 9% NH4OH (wt/vol). Temperature was controlled at 37°C. Fed-batch cultures were fed by manual injections of a feed solution consisting of 1,000 g/liter glycerol in water.
The final bioreactor cultivations were carried out in 0.75-liter stirred-tank reactors equipped with two Rushton impellers on the stirrer shaft (Biostat Q Plus; Sartorius North America, Edgewood, NY). Batch cultures were grown in a 0.5-liter working volume with the agitation set to provide the desired kLa. The fermentor was sparged with a 5% O2-95% N2 gas mixture, and the pH was controlled at 6.3 by automatic addition of 9% NH4OH (wt/vol). Temperature was controlled at 37°C. Fed-batch cultures were fed by manual injections of a feed solution consisting of 1,000 g/liter glycerol in water. The cultures were fed so that the glycerol concentration remained above 0 g/liter. Each feed bolus raised the reactor glycerol concentration by only 10 to 15 g/liter to keep the osmotic shock to a minimum.
Analytical methods.
Optical density was measured at 600 nm (OD600). Fermentation broth samples were centrifuged, and the supernatants were analyzed by high-performance liquid chromatography (HPLC) for extracellular glycerol, lactate, acetate, succinate, and ethanol. The ion-exclusion HPLC technique used a Shimadzu Prominence SIL 20 system (Shimadzu Scientific Instruments, Inc., Columbia, MD) equipped with an HPX-87H organic acid column (Bio-Rad, Hercules, CA). Operating conditions consisted of a 2.5 mM H2SO4 mobile phase, a column temperature of 55°C, a flow rate of 0.6 ml/min, and an isocratic run mode. The transfer of oxygen in microaerobic cultures was characterized by the volumetric oxygen transfer coefficient, kLa (h−1). The rate of volumetric oxygen transfer (dC/dt) is estimated by the equation dC/dt = kLa(C* − C), where C is the actual dissolved oxygen concentration in the liquid (mg/liter), kL is the oxygen transport coefficient (cm/h), a is the gas-liquid interfacial area (cm2/cm3), and C* is the saturated dissolved oxygen concentration in the liquid (mg/liter) (18). C* was assumed to be 6.7 mg/liter in our calculations. We estimated the kLa by the static gassing-out method (18). Off-gas concentrations of N2, H2, O2, and CO2 were determined by a benchtop gas analysis system. We used an HPR-20 quartz inert capillary (QIC) from Hiden Analytical (Warrington, United Kingdom).
RESULTS
Metabolic engineering strategy to increase yield.
We first focused our efforts on reducing the accumulation of by-products in the glycerol-to-ethanol fermentation. The main fermentative pathways in E. coli are shown in Fig. 1. Possible modifications to the E. coli metabolism are also shown in Fig. 1. Intermediate strains contained subsets of these modifications, and the final production strain, GB6/pGB1003, contained all of the modifications. The genotypes of all strains in this study are listed in Table 1. We first constructed several deletion strains to determine which deletions or combinations of deletions were desirable. Figure 2 shows the results from running ATCC 8739 and GB1 through GB5 in the Ward's Science 0.75-liter reactors at a kLa of 2.4/h. The wild-type ATCC 8739 strain made ethanol as its main product but also accumulated significant amounts of succinate and acetate. The yield of ATCC 8739 was 0.36 g ethanol/g glycerol (the maximum theoretical yield is 0.5 g ethanol/g glycerol). In GB1, the deletion of frdA eliminated succinate production, but lactate was increased. In GB2, acetate and lactate remained as by-products after deletion of frdA and pta. In GB3, deletions of frdA, pta, and ldhA left acetate as the lone by-product. GB4 showed that the addition of the ackA deletion reduced acetate to its lowest levels. The final GB5 strain had all of the deletions and had a yield of 0.43 g ethanol/g glycerol, which was in line with results from previous studies (6, 14, 15). The deletions in GB5 increased the yield but disrupted metabolism such that the rate was reduced from 0.20 g/liter/h ethanol in the wild type to 0.14 g/liter/h ethanol in GB5. This led us to target selected genes for overexpression to increase the ethanol production rate.
FIG 1.

Main fermentative pathways involved in the microaerobic fermentation of glycerol in E. coli. Relevant genes and generation/consumption of redox equivalents are included. Glycerol dissimilation under microaerobic conditions proceeds through both the dhaKLM-gldA and glpK-glpD branches. Ethanol, lactate, succinate, and acetate are the main products of glycerol fermentation. Genetic modifications are indicated by a plus symbol (+) for plasmid overexpressions (dhaKLM, gldA, glpK, and adhE) and by a circle with a slash through it for chromosomal knockouts (KO) (frdABCD, ackA, pta, ldhA, poxB, gldA, and glpK). DHA, dihydroxyacetone; G3P, glyceraldehyde 3-phosphate; PEP, phosphoenolpyruvate; Pyr, pyruvate; Ac-CoA, acetyl coenzyme A.
FIG 2.

Fermentation product profiles of the wild-type strain and various mutants. Ethanol, succinate, lactate, and acetate titers are shown. Experiments were conducted in Ward's Science 0.75-liter reactors. The cells were grown in MOPS minimal medium at pH 6.3 and a kLa of 2.4/h for 72 h.
Metabolic engineering strategy to increase rate.
Our next goal was to increase the ethanol production rate as much as possible. The decreased specific glycerol consumption rate was a large factor in the decreased ethanol production rate. Cell growth was actually greater in GB5 (final OD600 = 6.7) than in ATCC 8739 (final OD600 = 4.3), possibly due to the minimization of by-product toxicity and the reduction in carbon loss to by-products. However, the specific glycerol consumption rate in GB5 (0.06 g/liter/h/OD) was much lower than the rate in ATCC 8739 (0.13 g/liter/h/OD). We constructed pGB1001 to overexpress dhaKLM and gldA (Table 1) and tested GB5/pGB1001 against GB5 at a kLa of 50/h (Table 2). The kLa was increased from previous experiments to allow for faster cell growth. As the baseline strain, GB5 grew well but had a low specific glycerol consumption rate and little ethanol production (Table 2). The addition of pGB1001 to GB5 led to large increases in final ethanol titer, ethanol yield, specific glycerol consumption rate, and specific ethanol production rate. To explore the possibility of adhE being a bottleneck, we constructed pGB1002, which overexpressed dhaKLM, gldA, and adhE. GB5/pGB1002 experienced a reduction in cell growth that was likely caused by a greater plasmid/protein burden (Table 2). The ethanol titer and yield were unchanged. However, the specific ethanol production rate showed a 70% increase due to the overexpression of adhE. We were satisfied at this point with the improvements in titer, yield, and specific ethanol production rate, and so we shifted our focus to the flammability problem caused by the H2 content of the off-gas.
TABLE 2.
Metabolic engineering to increase the rate of ethanol production in strain GB5a
| Strain | Final ethanol titer (g/liter) | Final OD600 | Yield (g ethanol/g glycerol) | Specific glycerol consumption rate (g/liter/h/OD) | Specific ethanol production rate (g/liter/h/OD) |
|---|---|---|---|---|---|
| GB5 | 1.0 | 16.3 | 0.02 | 0.04 | 0.001 |
| GB5/pGB1001 | 24.0 | 13.2 | 0.44 | 0.06 | 0.025 |
| GB5/pGB1002 | 25.7 | 8.3 | 0.47 | 0.10 | 0.043 |
Experiments were conducted in Ward's Science 0.75-liter reactors. The cells were grown in MOPS minimal medium at pH 6.3 and a kLa of 50/h for 72 h.
Elimination of off-gas flammability by reducing O2 in inlet gas.
According to published data, gas mixtures with hydrogen in the concentration range between 4% and 75% (vol/vol) are potentially flammable if oxygen is present at a concentration of approximately 5% (vol/vol) or more (19). In order to avoid this situation, we tested the use of inlet gas with 5% O2 and 95% N2. We cultivated GB5/pGB1002 in 7-liter bioreactors at a kLa of 16/h, which was the optimal kLa for this strain (data not shown). kLa values higher than 16/h resulted in excess acetate production. We also switched from using technical-grade glycerol to using crude REG glycerin for the remaining fermentations in this study, since this material more closely resembles the actual industrial feedstock for the fermentation. We had found that there was no performance difference between fermentations run with technical-grade glycerol and those employing crude REG glycerin (data not shown). The off-gas composition time course for GB5/pGB1002 sparged with air is shown in Fig. 3A, and the ethanol production time course is shown in Fig. 3C. The oxygen concentration stayed above 4% for the entire fermentation, and the hydrogen concentration rose very quickly after a short lag phase to reside in the flammable range for nearly the whole fermentation. When the 5% O2-95% N2 sparge gas was used, the off-gas stayed out of the flammability envelope throughout the whole fermentation (Fig. 3B). The off-gas oxygen concentration at 0 h was 3.4%, and the concentration dropped below 1% at 20 h. The hydrogen concentration rose as normal, but it was rendered safe by the low oxygen concentration. Figure 3C shows that the two types of sparge gas gave similar ethanol production rates. The fermentation produced 0.52 g/liter/h ethanol with air as the inlet gas and 0.57 g/liter/h ethanol with 5% O2 as the inlet gas.
FIG 3.
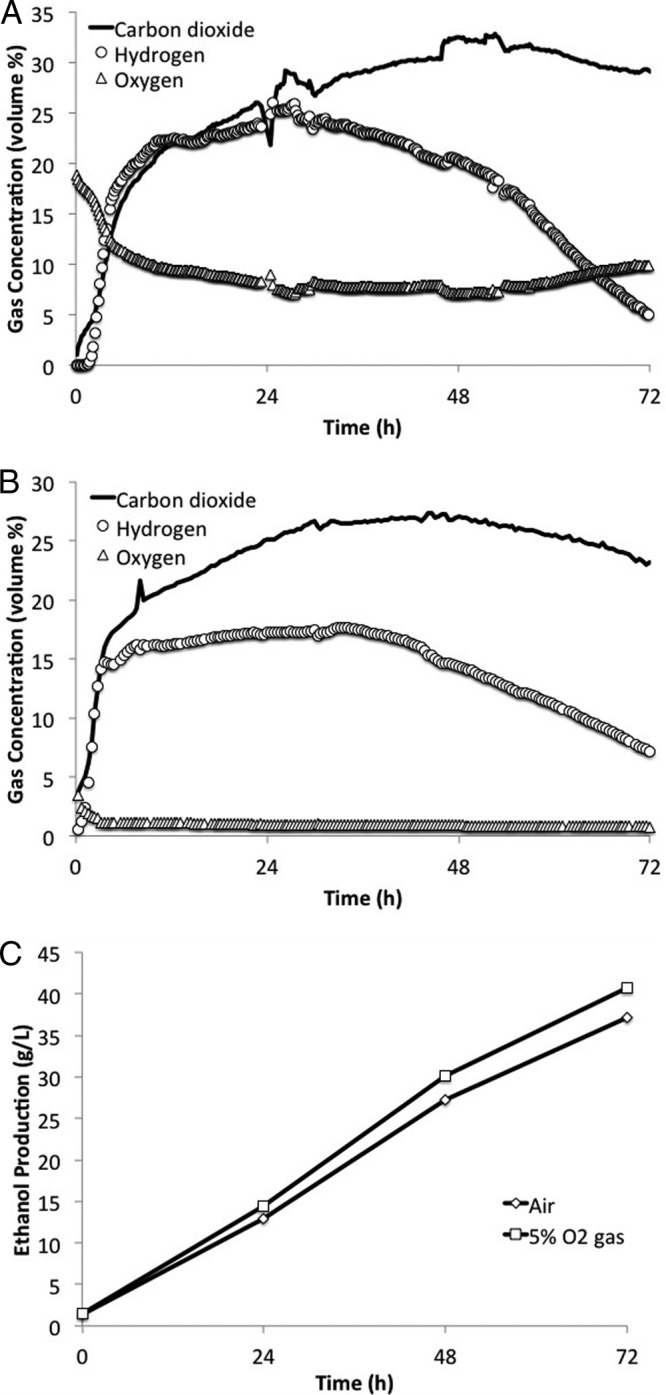
Changing the sparge gas composition to avoid off-gas flammability. (A) Off-gas composition profile time course with air as sparge gas. Nitrogen and trace components are not shown. (B) Off-gas composition profile time course with the 5% O2 gas mixture as sparge gas. Nitrogen and trace components are not shown. (C) Comparison of ethanol production profiles for fermentations using sparged air and those using sparged 5% O2 gas. Experiments were conducted in Sartorius Biostat A Plus 7-liter reactors. GB5/pGB1002 was grown in MM29 minimal medium at pH 6.3 and a kLa of 16/h for 72 h.
Implementation of plasmid addiction.
The use of antibiotics to reduce contamination and select for bacteria with plasmids is not economically feasible in a commercial-scale process (20). To address this issue, we implemented a plasmid addiction system based on glycerol addiction. Our goal was to construct an addicted strain to match the ethanol time course (Fig. 4) and fermentation performance parameters (Table 3) of GB5/pGB1002. Note that the fermentation process had undergone some optimization compared to the GB5/pGB1002 fermentation described in Table 2. We constructed GB6, which has the gldA and glpK genes deleted, rendering GB6 unable to grow on glycerol as its sole carbon source under both aerobic and anaerobic conditions (data not shown). The results for the combination of GB6 and pGB1002 show that ethanol production was nearly eliminated (Fig. 4) and that every performance parameter was significantly reduced (Table 3). The overexpression of dhaKLM and gldA was not enough to fully complement the deletions of gldA and glpK. We hypothesized that under the microaerobic fermentation conditions, both gldA and glpK needed to be expressed to fully complement the chromosomal deletions in GB6. We proceeded to construct plasmid pGB1003, which expressed glpK under the control of the Picd promoter in addition to dhaKLM and gldA. The combination of GB6 and pGB1003 recovered the ethanol production time course that was seen in GB5/pGB1002 (Fig. 4) after we increased the kLa to 60/h from 16/h. However, the specific glycerol consumption rate and specific ethanol production rate of GB6/pGB1003 were inferior to those of GB5/pGB1002 (Table 3). This forced us to grow the fermentation to a higher OD600, which in turn decreased the yield. Our next step was to optimize the process to improve the performance of the glycerol-addicted strain.
FIG 4.

Implementation of plasmid addiction. Data for GB5/pGB1002, GB6/pGB1002, and GB6/pGB1003 are shown. Experiments for GB5/pGB1002 and GB6/pGB1002 were conducted in Sartorius Biostat A Plus 7-liter reactors. GB5/pGB1002 and GB6/pGB1002 were grown in MM29 minimal medium at pH 6.3 and a kLa of 16/h for 72 h. The experiment for GB6/pGB1003 was conducted in Sartorius Biostat Q Plus 0.75-liter reactors. GB6/pGB1003 was grown in MM29 minimal medium at pH 6.3 and a kLa of 60/h for 72 h.
TABLE 3.
Implementation of plasmid addictiona
| Strain | Final ethanol titer (g/liter) | Final OD600 | Yield (g ethanol/g glycerol) | Specific glycerol consumption rate (g/liter/h/OD) | Specific ethanol production rate (g/liter/h/OD) |
|---|---|---|---|---|---|
| GB5/pGB1002 | 40.8 | 8.5 | 0.44 | 0.15 | 0.067 |
| GB6/pGB1002 | 5.4 | 3.7 | 0.28 | 0.07 | 0.020 |
| GB6/pGB1003 | 41.9 | 16.9 | 0.37 | 0.09 | 0.034 |
Experiments for GB5/pGB1002 and GB6/pGB1002 were conducted in Sartorius Biostat A Plus 7-liter reactors. GB5/pGB1002 and GB6/pGB1002 were grown in MM29 minimal medium at pH 6.3 and a kLa of 16/h for 72 h. The experiment for GB6/pGB1003 was conducted in Sartorius Biostat Q Plus 0.75-liter reactors. GB6/pGB1003 was grown in MM29 minimal medium at pH 6.3 and a kLa of 60/h for 72 h.
Process optimization for maximum performance.
We tested several different aeration conditions and found that a kLa of 95/h was the optimal oxygen transfer rate. We ran the fermentation in fed-batch mode (the feeding schedule is described in Materials and Methods). The glycerol concentration, time course, and growth curve (final OD600 = 19.3) are shown in Fig. 5A. This process resulted in an improved ethanol titer (46.5 g/liter) and small amounts of by-products (Fig. 5B). The process optimization also improved the other performance parameters for GB6/pGB1003 shown in Table 3. The ethanol yield was 0.44 g ethanol/g glycerol, the specific glycerol consumption rate was 0.12 g/liter/h/OD, and the specific ethanol production rate was 0.053 g/liter/h/OD. The dissolved-oxygen profile showed that microaerobic conditions were reached within about 5 min of the inoculation (see Fig. S1 in the supplemental material). The culture stayed microaerobic for the remainder of the 45-h fermentation. The redox potential leveled off about 10 h into the fermentation at ∼−390 mV and then slowly rose for the rest of the fermentation (see Fig. S1). The off-gas composition remained outside the H2-O2 flammability envelope for the entire fermentation (Fig. 5C). This experiment showed that we were able to increase yield, rate, and titer of GB6/pGB1003 while maintaining an inert off-gas and a lack of inducer/antibiotics.
FIG 5.

Process data from optimized fermentation. Data for GB6/pGB1003 are shown. (A) Glycerol time course profile for optimized fermentation. (B) Metabolite time course profiles for optimized fermentation. The time course profiles for ethanol, acetate, succinate, and lactate are shown. (C) Off-gas composition profile time course with 5% O2 gas mixture as the inlet gas. Nitrogen and trace components are not shown. The experiment for GB6/pGB1003 was conducted in Sartorius Biostat Q Plus 0.75-liter reactors. GB6/pGB1003 was grown in MM29 minimal medium at pH 6.3 and a kLa of 95/h for 45 h.
DISCUSSION
Several recent publications have explored the microaerobic fermentation of glycerol to ethanol (3, 6, 14, 15). They provided the proof of concept and data showing many avenues for greatly improving rate and yield. Although the yields were commercially relevant, many other aspects of the strains and fermentation processes were not suited for commercial application. We undertook the current work to extend their results for commercial application.
The first step toward increasing the ethanol production rate was to increase the rate of glycerol dissimilation. Yazdani and Gonzalez (6) showed that overexpression of dhaKLM and gldA under the control of the PLtetO-1 promoter increased the specific glycerol consumption rate by 1.4-fold. We found that overexpression of dhaKLM and gldA (with an additional ribosomal binding site in front of gldA) under the control of the adhE promoter increased the specific glycerol consumption rate by 1.5-fold before accounting for plasmid burden. The metabolic profiling of gene deletion mutants by Durnin et al. indicated that both respiratory (GlpK-GlpD) and fermentative (GldA-DHAK) pathways play a significant role in the conversion of glycerol to the glycolytic intermediate dihydroxyacetone (3). We confirmed the importance of the role of GlpK-GlpD in microaerobic glycerol metabolism. During the implementation of plasmid addiction, we deleted both gldA and glpK from GB5 to create GB6. When only dhaKLM and gldA were complemented in GB6/pGB1002, the specific glycerol consumption decreased approximately 2-fold relative to that of GB5/pGB1002. Through overexpression of both dhaKLM-gldA and glpK in GB6/pGB1003, we nearly recovered the original specific glycerol consumption rate seen in GB5/pGB1002. We did not expect to fully recover the original specific glycerol consumption rate, due to the chromosomal deletions in GB6. We hypothesize that overexpression of both glycerol dissimilation branches instead of just dhaKLM-gldA had a 3-fold effect: (i) the absolute amount of glycerol dissimilation enzymes was increased and therefore increased flux from glycerol to dihydroxyacetone phosphate (DHAP), (ii) the chromosomal gldA and glpK deletions in GB6 were fully complemented, and (iii) the generation of reducing equivalents, the use of cofactors, and the production of intermediates such as dihydroxyacetone and glycerol 3-phosphate were more like the wild-type situation. Since NADH was being created faster due to dhaKLM-gldA and glpK overexpression, we overexpressed adhE to help recycle the NADH and maintain redox balance. Overexpressing adhE improved the rate and yield by pulling more carbon toward ethanol instead of by-products.
The amount of oxygen provided to the culture has a profound effect on substrate consumption, growth rate, and product mix due to its role as the terminal electron acceptor for the recycling of reducing equivalents. Yazdani and Gonzalez found the use of microaerobic conditions (kLa = 0.5/h) instead of anaerobic conditions greatly improved specific and volumetric glycerol utilization (6). Trinh and Srienc found that the maximum ethanol yield (97% of the theoretical maximum) for their system occurred at a kLa of 9/h (15). More oxygen transfer led to more carbon flux toward biomass. We found that a kLa of 95/h with the 5% O2 gas was the optimum oxygen transfer rate with our system. The dissolved oxygen dropped to microaerobic levels within 5 min, but we were able to track the redox potential to observe further changes in the fermentation broth. The most productive portion of the fermentation occurred when the redox potential was near −350 to −390 mV. We hypothesize that the reduced nature of the broth coupled with oxygen limitation encouraged a high rate of ethanol production. This situation was termed “growth-associated ethanol production” by Trinh and Srienc (15), because the only way to consume more glycerol was to recycle NADH through ethanol production, as shown in Fig. 1. As the ethanol toxicity became a factor later in the fermentation, the dissimilation of glycerol slowed down and less NADH was produced. This is reflected in the slow rise of the broth's redox potential. In future work, we would like to run fermentations with variable oxygen transfer so that we can control the redox potential at a certain set point at which ethanol production is favored.
In the development of a commercial process, economics must be seriously considered. The profit margins for commodity chemicals such as ethanol are thin, making the use of expensive inducers infeasible. The blank pZS vector contains a PLtetO-1 promoter that is induced with anhydrotetracycline. To eliminate the use of anhydrotetracycline, we replaced the PLtetO-1 promoter with the adhE promoter for the expression of dhaKLM-gldA. We hypothesized that the adhE promoter would be strongly activated under microaerobic conditions. This choice of promoter would allow the expression of dhaKLM-gldA to be in sync with the expression of adhE and dhaKLM-gldA's normal role as the fermentative branch of the glycerol dissimilation pathway. We used the native adhE promoter in front of the adhE gene on the plasmid. We used the isocitrate dehydrogenase promoter (Picd) to control the expression of glpK. This promoter would express glpK strongly during the aerobic preculture stages and then reduce glpK expression during the microaerobic fermentation through the arcA and fnr gene products (21). The use of these promoters gave us the expression profiles that we wanted without the need for inducers.
Also in the interest of economics, antibiotics cannot be used in a commercial process to stabilize plasmids. Plasmid-based expression systems are often used to produce chemicals, polymers, fuels, and proteins. In these bioprocesses, plasmids have a huge impact on the productivity of the process. Plasmid-free cells lead to losses in decreased productivity and profitability. The use of antibiotics in industrial fermentations is not feasible due to the cost of the antibiotic itself and the cost of inactivating and removing the antibiotic from the spent broth. Furthermore, the addition of antibiotics does not even guarantee the stability of the plasmid. Chromosomal integration of foreign genes is applicable in some cases, but important factors such as polar effects and limited gene copy number are disadvantages (20). Kroll et al. described, as a possible solution to these problems, several plasmid addiction systems (20). We chose to implement a catabolism-based addiction system. It was necessary to delete gldA and glpK for complete plasmid addiction under microaerobic conditions. During the proof-of-concept experiments, deletion of only one branch of glycerol dissimilation resulted in approximately 50% plasmid retention (data not shown). The plasmid retention of the double-deletion addiction system was >90% (data not shown). Relative to the antibiotic-stabilized system, the plasmid addiction system had similar ethanol production after some process parameters were adjusted (Fig. 5). Therefore, we were able to eliminate the use of antibiotics without any decrease in process performance.
The glycerol-to-ethanol process creates a significant amount of hydrogen under microaerobic and acidic conditions through the enzymes pyruvate formate lyase (encoded by pflB) and formate-hydrogen lyase. Due to that production of hydrogen, the off-gas may be flammable and the prevention of accidental explosions during the process must be a safety consideration. As shown by Schröder and Molnarne, the lower explosion limit (LEL) for H2 in air is 3.8% by volume (19). When we used conventional aeration, our ethanol process routinely surpassed this threshold within a few hours of starting the fermentation and reached over 25% by volume at its peak. A common solution for a flammable atmosphere would be to pump an overlay of air into the fermentor headspace to dilute the H2 to 0.95% by volume or below, to comply with the 4:1 margin of safety below the LEL recommended by the National Fire Protection Association standard NFPA 86. The inlet air sparge rate of 0.01 volume/volume/min (vvm) used in the fermentation shown in Fig. 3A would require a 0.24 vvm air overlay, which would incur capital and utility expenses. We would also incur additional capital expenses to make the fermentor and other equipment explosion-proof in case of overlay equipment failure. Furthermore, the effect of a large air overlay on our fermentation is unknown. These findings led us to explore the possibility of limiting the inlet gas oxygen concentration to 5%, which is below the amount of oxygen needed to ignite any amount of hydrogen. This strategy was inherently safe, and we were able to maintain the same process performance that we had achieved with air as the inlet gas. A gas separation system would be required to generate the 5% O2 inlet gas stream, and this expense was included in our economic model.
Glycerol offers the advantages of low price and a high degree of reduction, making it an ideal substrate from which to produce reduced products. Building on previous demonstrations of microaerobic glycerol fermentations by other investigators, we have advanced our glycerol-to-ethanol process toward commercialization. Our process has commercially relevant productivity, does not require inducers or antibiotics, and results in an O2-limited off-gas that is inherently safe. Other issues remain, such as the cost of the ammonia for pH control and the cost of the gas separation skid. We will continue to work on several fronts to eliminate or absorb these costs, including (i) further genetic manipulation or process optimization to increase ethanol productivity and decrease by-product accumulation, (ii) medium optimization to reduce nutrient costs, and (iii) optimization of the glycerol feeding schedule.
Supplementary Material
ACKNOWLEDGMENTS
All the authors are employees of Glycos Biotechnologies, Inc., which supported this work.
We thank Ramon Gonzalez at Rice University for his support and for supplying plasmid pZSKLMgldA.
Footnotes
Published ahead of print 28 February 2014
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AEM.03863-13.
REFERENCES
- 1.McCoy M. 2006. Glycerin surplus. Chem. Eng. News 84:7–8. 10.1021/cen-v084n006.p007a [DOI] [Google Scholar]
- 2.Yazdani SS, Gonzalez R. 2007. Anaerobic fermentation of glycerol: a path to economic viability for the biofuels industry. Curr. Opin. Biotechnol. 18:213–219. 10.1016/j.copbio.2007.05.002 [DOI] [PubMed] [Google Scholar]
- 3.Durnin G, Clomburg J, Yeates Z, Alvarez PJ, Zygourakis K, Campbell P, Gonzalez R. 2009. Understanding and harnessing the microaerobic metabolism of glycerol in Escherichia coli. Biotechnol. Bioeng. 103:148–161. 10.1002/bit.22246 [DOI] [PubMed] [Google Scholar]
- 4.Gonzalez R, Murarka A, Dharmadi Y, Yazdani SS. 2008. A new model for the anaerobic fermentation of glycerol in enteric bacteria: trunk and auxiliary pathways in Escherichia coli. Metab. Eng. 10:234–245. 10.1016/j.ymben.2008.05.001 [DOI] [PubMed] [Google Scholar]
- 5.Murarka A, Dharmadi Y, Yazdani SS, Gonzalez R. 2008. Fermentative utilization of glycerol by Escherichia coli and its implications for the production of fuels and chemicals. Appl. Environ. Microbiol. 74:1124–1135. 10.1128/AEM.02192-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yazdani SS, Gonzalez R. 2008. Engineering Escherichia coli for the efficient conversion of glycerol to ethanol and co-products. Metab. Eng. 10:340–351. 10.1016/j.ymben.2008.08.005 [DOI] [PubMed] [Google Scholar]
- 7.Tang X, Tan Y, Zhu H, Zhao K, Shen W. 2009. Microbial conversion of glycerol to 1,3-propanediol by an engineered strain of Escherichia coli. Appl. Environ. Microbiol. 75:1628–1634. 10.1128/AEM.02376-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clomburg JM, Gonzalez R. 2011. Metabolic engineering of Escherichia coli for the production of 1,2-propanediol from glycerol. Biotechnol. Bioeng. 108:867–879. 10.1002/bit.22993 [DOI] [PubMed] [Google Scholar]
- 9.Nikel PI, Pettinari MJ, Galvagno MA, Mendez BS. 2008. Poly(3-hydroxybutyrate) synthesis from glycerol by a recombinant Escherichia coli arcA mutant in fed-batch microaerobic cultures. Appl. Microbiol. Biotechnol. 77:1337–1343. 10.1007/s00253-007-1255-7 [DOI] [PubMed] [Google Scholar]
- 10.Blankschien MD, Clomburg JM, Gonzalez R. 2010. Metabolic engineering of Escherichia coli for the production of succinate from glycerol. Metab. Eng. 12:409–419. 10.1016/j.ymben.2010.06.002 [DOI] [PubMed] [Google Scholar]
- 11.Mazumdar S, Clomburg JM, Gonzalez R. 2010. Escherichia coli strains engineered for homofermentative production of d-lactic acid from glycerol. Appl. Environ. Microbiol. 76:4327–4336. 10.1128/AEM.00664-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mazumdar S, Blankschien MD, Clomburg JM, Gonzalez R. 2013. Efficient synthesis of L-lactic acid from glycerol by metabolically engineered Escherichia coli. Microb. Cell Fact. 12:7. 10.1186/1475-2859-12-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stephanopoulos G, Aristos AA, Nielsen J. 1998. Metabolic engineering: principles and methodologies. Academic Press, San Diego, CA [Google Scholar]
- 14.Nikel PI, Ramirez MC, Pettinari MJ, Mendez BS, Galvagno MA. 2010. Ethanol synthesis from glycerol by Escherichia coli redox mutants expressing adhE from Leuconostoc mesenteroides. J. Appl. Microbiol. 109:492–504. 10.1111/j.1365-2672.2010.04668.x [DOI] [PubMed] [Google Scholar]
- 15.Trinh CT, Srienc F. 2009. Metabolic engineering of Escherichia coli for efficient conversion of glycerol to ethanol. Appl. Environ. Microbiol. 75:6696–6705. 10.1128/AEM.00670-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Orchard SS, Goodrich-Blair H. 2005. Pyrimidine nucleoside salvage confers an advantage to Xenorhabdus nematophila in its host interactions. Appl. Environ. Microbiol. 71:6254–6259. 10.1128/AEM.71.10.6254-6259.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Neidhardt FC, Bloch PL, Smith DF. 1974. Culture medium for enterobacteria. J. Bacteriol. 119:736–747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shuler ML, Kargi F. 1992. Bioprocess engineering: basic concepts, 2nd ed. Prentice Hall, Upper Saddle River, NJ [Google Scholar]
- 19.Schröder V, Molnarne M. 2005. Flammability of gas mixtures. Part 1: fire potential. J. Hazard. Mater. 121:37–44. 10.1016/j.jhazmat.2005.01.032 [DOI] [PubMed] [Google Scholar]
- 20.Kroll J, Klinter S, Schneider C, Voss I, Steinbuchel A. 2010. Plasmid addiction systems: perspectives and applications in biotechnology. Microb. Biotechnol. 3:634–657. 10.1111/j.1751-7915.2010.00170.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chao G, Shen J, Tseng CP, Park SJ, Gunsalus RP. 1997. Aerobic regulation of isocitrate dehydrogenase gene (icd) expression in Escherichia coli by the arcA and fnr gene products. J. Bacteriol. 179:4299–4304 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


