Abstract
During fungal rock phosphate (RP) solubilization, a significant quantity of fluoride (F−) is released together with phosphorus (P), strongly inhibiting the process. In the present study, the effect of two F− adsorbents [activated alumina (Al2O3) and biochar] on RP solubilization by Aspergillus niger was examined. Al2O3 adsorbed part of the F− released but also adsorbed soluble P, which makes it inappropriate for microbial RP solubilization systems. In contrast, biochar adsorbed only F− while enhancing phosphate solubilization 3-fold, leading to the accumulation of up to 160 mg of P per liter. By comparing the values of F− measured in solution at the end of incubation and those from a predictive model, it was estimated that up to 19 mg of F− per liter can be removed from solution by biochar when added at 3 g liter−1 to the culture medium. Thus, biochar acted as an F− sink during RP solubilization and led to an F− concentration in solution that was less inhibitory to the process. In the presence of biochar, A. niger produced larger amounts of citric, gluconic, and oxalic acids, whether RP was present or not. Our results show that biochar enhances RP solubilization through two interrelated processes: partial removal of the released F− and increased organic acid production. Given the importance of organic acids for P solubilization and that most of the RPs contain high concentrations of F−, the proposed solubilization system offers an important technological improvement for the microbial production of soluble P fertilizers from RP.
INTRODUCTION
In recent years, phosphorus (P) scarcity has been identified as a bottleneck in the sustainability of agricultural systems (1). P is an essential and irreplaceable element for life. Most soils are P deficient, which makes P fertilizer application to soils obligatory to improve crop productivity. The primary sources of P fertilizers are rock phosphates (RPs) that are chemically solubilized with inorganic acids. However, the reserves of high-grade RPs that are economically exploitable with this technology are being depleted, increasing the price of fertilizers and endangering agricultural systems that are highly dependent on P inputs (1, 2). This scenario requires the development of new techniques that enable the use of low-grade RPs or alternative P sources (3).
Phosphate-solubilizing microorganisms (PSMs) are recognized as a promising alternative for P fertilization management because of their ability to mobilize P from sparingly soluble sources, including low-grade RPs. These microorganisms have been used in liquid and solid fermentation systems aimed at solubilizing RPs (4–6). However, it was recently demonstrated that during the solubilization process, PSMs become exposed to various chemical elements released from the RP (7). Released fluoride (F−) was observed to cause a strong decrease in P solubilization, suggesting that most of the microbial RP solubilization systems may operate at suboptimal conditions (7), given that fluorine is a ubiquitous element in RPs (8). Thus, it is expected that strategies to remove F− while it is released from RPs could increase the overall efficiency of RP solubilization.
The common strategy used for F− removal from aqueous solution is its adsorption on various types of materials (9). Selective adsorption can be achieved using, for instance, materials containing aluminum, such as activated alumina. Complex materials, such as biochar (9) and bone char (10), have also been used for efficient F− removal. These chars are a low-cost and environmentally friendly option because they are obtained by pyrolysis of biomass wastes. Thus, in the present study, the effects of two F− adsorbents, namely, activated alumina and biochar, on the solubilization of RP by A. niger was investigated.
MATERIALS AND METHODS
Microorganism.
The isolate A. niger FS1 was obtained from the Collection of Phosphate Solubilizing Fungi, Microbiology Department, Institute of Biotechnology Applied to Agriculture (BIOAGRO), Federal University of Viçosa, Viçosa, Brazil. The fungus was maintained at 30°C in petri dishes containing potato dextrose agar (PDA).
Rock phosphate and adsorbents.
RP from Araxá, Brazil, was used as an insoluble P source in the experiments. This RP (particle size < 75 μm in diameter) was previously characterized as a mixture of fluorapatite and hydroxyapatite [Ca10(PO4)6(F, OH)] (7) and contains 13.97% P and 1.59% F. The F− adsorbents were activated alumina (Al2O3; particle size, 0.05 to 0.2 mm) and biochar. The biochar was produced by pyrolysis of biomass wastes of holm oak (Quercus ilex) at 480°C and supplied by Piroeco Bioenergy (Málaga, Spain). The biochar had a particle size less than 2 mm, a fixed carbon content of 85.56%, a content of volatiles of 12.24%, an ash content of 2.2%, and a pH in H2O of 8.7. Its elemental composition (mg kg−1) was as follows: N, 5,000; P, 2,400; K, 11,700; Mg, 5,600; Mn, 781; Zn, 22; Cu, 13; Ni, 11; Pb, 1.4; Cr, 0.7; and Cd, 0.07. Soluble P content in 2% citric acid (1:100) was 899.9 mg P per kg of biochar.
Experimental conditions.
Experiments were conducted in 250-ml Erlenmeyer flasks containing 100 ml of the National Botanical Research Institute's phosphate growth medium (NBRIP) (11) [10 g of glucose, 5 g of MgCl2·6H2O, 0.25 g of MgSO4·7H2O, 0.2 g of KCl, 0.1 g of (NH4)2SO4, 1 liter of deionized water]. After adjustment of the pH to 7, RP and adsorbents were added at 3 g liter−1 singly or in a 1:1 ratio to separate flasks as follows: RP plus biochar, RP plus Al2O3, RP, biochar, and Al2O3. The medium was autoclaved and inoculated with 106 fungal conidia from a conidial suspension prepared in 0.1% (vol/vol) Tween 80. All flasks were incubated for 5 days on an orbital shaker at 160 rpm and 30°C. Experiments were conducted in triplicate by following a completely randomized design, and the treatment means were compared by the Tukey's test (P < 0.05).
Analytical methods.
Because of the precipitation of oxalic acid by calcium ions (12) during phosphate solubilization, a special sampling procedure was adopted. At the end of incubation, culture flasks were removed from the shaker and the suspended matter in the culture medium was allowed to settle for 2 h. Then, 40 ml of the supernatant was filtered through quantitative filter paper (P free, 15- to 17-μm pore size) for the analysis of soluble P, soluble F−, and pH. The remainder of the medium in the fermentation flask was acidified with concentrated H2SO4 (95%) to pH 0.5 to 1.0 to solubilize the precipitated calcium oxalate. The acidified sample was filtered through 0.2-μm membranes and used for the determination of organic acids by ultraperformance liquid chromatography-tandem MS (UPLC/MS/MS) as described previously (7). The measured oxalic acid concentrations were converted to the quantity of oxalic acid in the total volume of medium.
Soluble P was determined spectrophotometrically using the vanadate-molybdate reagent (Fluka; catalog no. 94685). F− was determined spectrophotometrically using SPADNS reagent (Fluka catalog no. 70081). Before F− determination, samples were distilled to remove interfering components (13).
Adsorption assay.
Because the adsorption of F− or P on both adsorbents cannot be determined directly in the fermentation medium, an assay for measuring this process without the presence of RP or fungus was done. Erlenmeyer flasks containing 50 ml of NBRIP medium (without any P source) and containing 3 g liter−1 of either biochar or Al2O3 were incubated under the same conditions as described above. Combinations of different doses of F− (0, 7.5, 15, 22.5, and 30 mg liter−1, prepared from NaF) and soluble P (0, 50, 100, 150, and 200 mg liter−1, prepared from KH2PO4) were added to the flasks according to a composite central design (Table 1). This experimental design was chosen because it allows the analysis of the interaction effects between the variables F− and soluble P. The experimental design and the regression analyses were done using the option design of experiments (DOE) of the statistical software Minitab 16.1. At the end of incubation, the medium was filtered through quantitative filter paper (P free, 15- to 17-μm pore size) and the concentration of P was determined as described above. The concentration of F− was determined with an ion-specific electrode (ISE). The quantity of F− and P adsorbed was calculated by subtracting the element content measured in solution at the end of incubation from that added at the beginning.
TABLE 1.
Combinations of fluoride and phosphorus doses used in the adsorption assay as defined by the composite central designa
| Component | Concn (mg liter−1) used in indicated combination |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | |
| F− | 7.5 | 7.5 | 22.5 | 22.5 | 15 | 15 | 15 | 15 | 15 | 0 | 30 | 15 | 15 | 15 |
| P | 50 | 150 | 50 | 150 | 100 | 100 | 100 | 0 | 200 | 100 | 100 | 100 | 100 | 100 |
The whole experiment was performed separately for each adsorbent, namely, biochar and Al2O3.
Prediction of the release of F− from RP.
A predictive model was used to estimate the total F− released in the presence of the adsorbents. In a previous work (7), we verified that the concentrations of F− and P released during fungal RP solubilization were correlated (0.93; P < 0.01). The data shown in reference 7 were used to fit a regression equation that estimates the concentration of F− released as a function of solubilized P (mg liter−1) from RP (Fig. 1):
| (1) |
FIG 1.
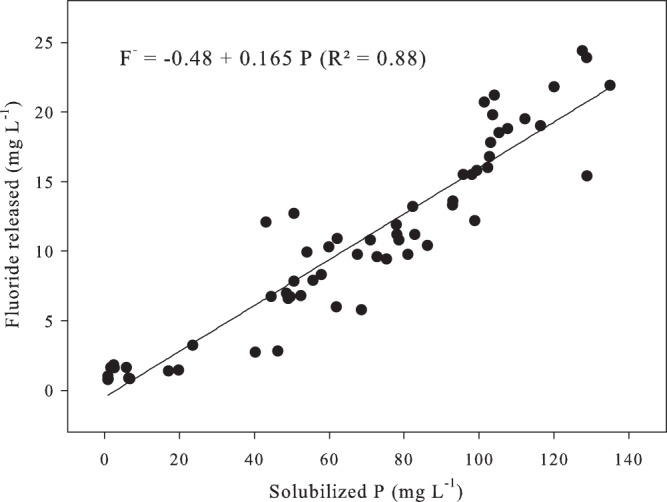
Fluoride release as a function of solubilized P during Araxá RP solubilization by Aspergillus niger.
Solubilized P and F− released were obtained from a 10-day batch fermentation performed using the same fungus, RP, fermentation medium, and incubation conditions as adopted in the present work, except that no adsorbents were added to the medium (7). The concentrations of F− and solubilized P were determined every 12 h, in triplicate. The pairs of data (solubilized P and F−) were plotted in a scatter plot and linear regression analysis was done (Fig. 1).
RESULTS
Biochar led to solubilized P levels from Araxá RP that were three times higher than those observed in the presence of Al2O3 or in the absence of any of the adsorbents tested (Fig. 2). As expected, no P was detected in the controls with biochar or Al2O3, indicating that the element was derived only from Araxá RP.
FIG 2.
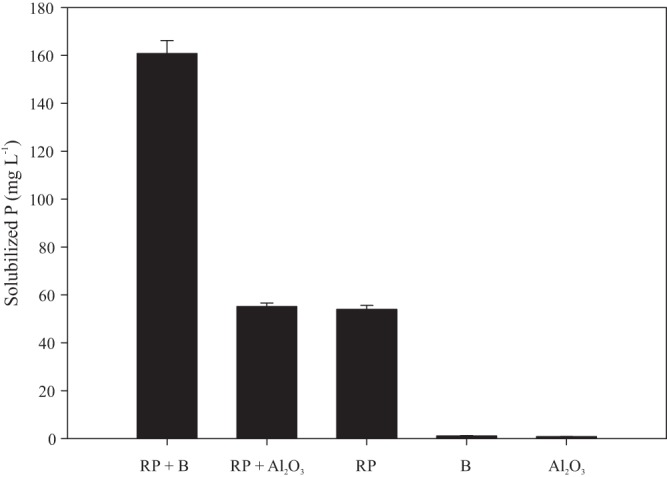
Effect of biochar (B) and Al2O3 on the solubilization of RP by Aspergillus niger in liquid medium. Error bars denote standard deviations.
The concentrations of F− measured in the media with adsorbents were lower than predicted by equation 1, indicating that F− adsorption took place (Fig. 3). Applying the values of solubilized P in the equation, the predicted F− concentrations in the treatments RP plus biochar and RP plus Al2O3 were 26 and 8.4 mg of F− per liter, respectively (Fig. 3).
FIG 3.
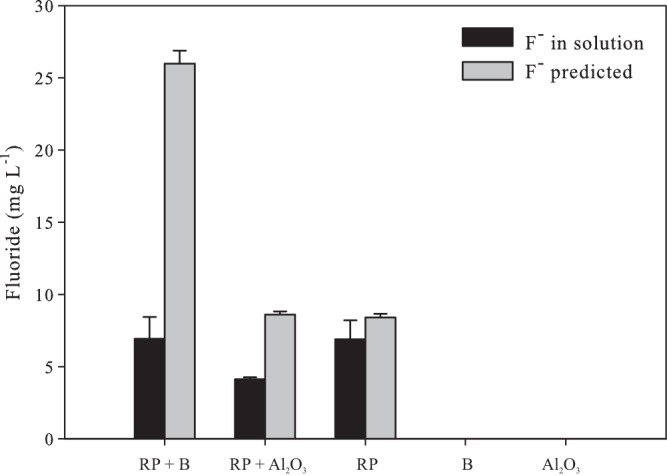
Effect of biochar (B) and Al2O3 on the concentration of fluoride (F−) after RP solubilization by Aspergillus niger. The predicted release of F− during RP solubilization was calculated with equation 1. Error bars denote standard deviations.
In the absence of either RP or A. niger, Al2O3 adsorbed significant quantities of F− and soluble P, reaching 20 and 55 mg liter−1, respectively (Fig. 4). In contrast, biochar adsorbed only F−, with a maximum of 5.3 mg liter−1. Since the F− concentration in the adsorption assay was determined using an ISE, which measures ion activity, the quadratic relationship between F− adsorption by biochar and F− concentration resulted probably from the effect of the increasing molality on the ionic activity coefficient through ion-ion (ion-pair association) and ion-solvent (solvation) interactions (14). Finally, since biochar did not adsorb P, solubilized P was not underestimated in the treatment RP plus biochar, allowing the correct estimate of released F− by equation 1. Also, for both adsorbents, no significant interaction effect was observed between P and F−.
FIG 4.
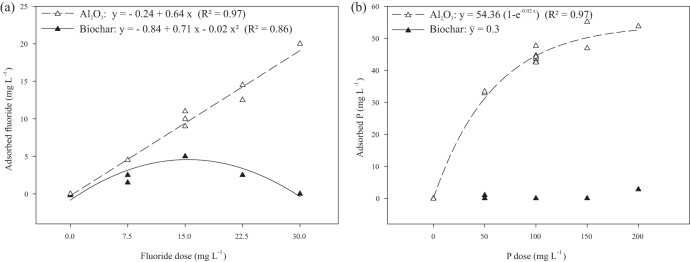
Adsorption of fluoride (left) and phosphorus (right) on biochar and Al2O3. Assays were performed using the NBRIP medium (without any P source) with 3 g liter−1 of biochar or Al2O3 and incubated for 5 days at 160 rpm and 30°C.
The production of organic acids and the medium pH were also affected by the addition of the adsorbents (Table 2). Biochar increased the production of citric, gluconic, and oxalic acids compared with the control with only RP. Similar concentrations of the acids were found in both treatments with biochar. The lowest pH values were observed when biochar was added to the medium, regardless of the addition of RP. The production of citric and gluconic acids was, respectively, 2.7 and 2.6 times higher in the treatment with RP plus Al2O3 than those in the control with only RP.
TABLE 2.
Effect of biochar and activated alumina on production of organic acids and medium pH during solubilization of RP by Aspergillus niger in liquid mediuma
| Treatment | Concn of acid produced (mg liter−1) |
pH | ||
|---|---|---|---|---|
| Citric | Gluconic | Oxalic | ||
| RP + B | 272.0b ± 28.6 | 128.7ab ± 4.8 | 106.1a ± 8.7 | 3.07c ± 0.03 |
| RP + Al2O3 | 378.1a ± 11.2 | 143.7a ± 16.4 | 37.0b ± 1.5 | 3.40b ± 0.03 |
| RP | 137.5c ± 17.7 | 56.3c ± 13.4 | 32.1b ± 4.1 | 3.39b ± 0.03 |
| B | 284.3b ± 41.4 | 103.5b ± 3.7 | 113.5a ± 5.4 | 2.15d ± 0.05 |
| Al2O3 | 0.7d ± 0.2 | 20.0d ± 5.4 | 24.9b ± 1.2 | 3.91a ± 0.12 |
Values are means of three replicates ± standard deviations. In each column, means followed by the same letter are not significantly different by Tukey's test (P < 0.05). B, biochar.
DISCUSSION
The addition of biochar to the solubilization medium containing RP caused a significant increase in the concentration of solubilized P. Our data suggest that this could be based on two interrelated processes: (i) partial removal from solution of the F− released from RP and (ii) enhancement of the production of organic acids, which are capable of solubilizing P by forming stable complexes with cations that form poorly soluble compounds with P (15, 16).
As observed previously (7), a small increase in F− concentration results in a large decrease in the level of solubilized P. The decrease in RP solubilization is related to inhibitory effects of F− on A. niger metabolism, especially on citric acid production and medium acidification (7). Both of these processes were increased under the application of biochar as F− adsorbent (Table 2). Medium acidification is one of the P solubilization mechanisms and results from the release of H+ by the fungus during metabolic processes such as NH4+ assimilation and respiration (17). Thus, by alleviating F− toxicity, biochar can increase medium acidification and hence P solubilization by acidic attack on RP (18, 19). Based on the data from the adsorption assay, biochar can adsorb an amount of up to 5.3 mg of F− per liter (Fig. 4). However, in the presence of the fungus, this value was probably higher. A total of 26 mg of F− per liter was predicted to be released together with P in the treatment RP plus biochar (Fig. 3). By subtracting the measured concentration of F− in solution from that predicted, it is estimated that up to 19 mg liter−1 was adsorbed by the biochar. The difference between the predicted and observed adsorption of F− can be an effect of the medium pH. Mohan et al. (9) showed that maximum F− adsorption on two distinct biochars occurs at pH 2 because more sites for F− binding are created by protonation of basic functions. They demonstrated that the highest percentages of adsorption occurred at pHs lower than 4. Thus, the acidification of the fermentation medium caused by A. niger (Table 2) may have improved the adsorption of F− by biochar.
In addition to the removal of F−, biochar promoted a greater production of organic acids by A. niger than that of the control with only RP. This effect was independent of the presence of RP (Table 2), and therefore of F−, which suggests that the increase in the production of organic acids was not exclusively related to the alleviation of F− toxicity by biochar. Greater microbial activity and biomass production are commonly observed after biochar addition to the soil (20, 21). Biochar can also increase the abundance of P-solubilizing bacteria (22). However, little is known about the mechanisms that might explain the positive effect of biochar on microorganisms. Although biochar has been used as a soil amendment, elucidation of the effect of biochar on microbial activity in soil by in vitro experiments has not been published. No report on the effect of biochar on the production of organic acids is available. Possibly, its positive effect is due to its ability to adsorb ions (21, 23) and thus control the concentrations of inhibitors of organic acid production. The cultivation medium used in this work is widely used in P solubilization studies (11). Despite allowing the comparison of results from different studies, this medium was not optimized for organic acid production (24). For example, it contains a significant amount of Mg, which can be inhibitory to citrate synthase (EC 2.3.3.1) (25). Biochar could alleviate such inhibition by controlling Mg2+ concentration. Another possible effect of biochar is related to its alkalinity. After its addition to the medium, the pH increased to 8.2. The production of oxalic (26) and gluconic (27) acids is favored by near neutral pH. Thus, at least initially, this alkaline condition could favor the production of these compounds.
Al2O3 addition to the fermentation medium did not result in any increase in P solubilization, even though the levels of F− were lower in this treatment (Fig. 3). Since Al2O3 also adsorbed P (Fig. 4), solubilized P and, consequently, the amount of fluoride released from RP was probably underestimated. As P adsorption by Al2O3 is increased near pH 4 (28, 29), it was not possible to determine the exact amount of P adsorbed under the acidic conditions prevalent at the end of the experiment. Though Al2O3 showed the highest F− adsorption, it is not advantageous for microbial P solubilization systems because of the nonspecific P adsorption, which would make necessary an additional desorption step for total P recovery.
The production of organic acids in the treatment with Al2O3 presented a pattern different from that obtained with biochar. Interestingly, the highest concentration of citric acid was detected in the treatment with RP plus Al2O3. In higher plants, the exudation of citric acid is associated with Al3+ detoxification (30), and some authors suggest that some fungi could also detoxify Al3+ in this manner (31, 32). Under the conditions tested, some Al3+ released from Al2O3 could have stimulated citric acid production by A. niger. An increased P solubilization was expected as a consequence of a higher concentration of citric acid in the medium (15), but this was not observed. Citric acid may have formed complexes with Al3+, and thus, less acid was available to form complexes with calcium from RP. Further studies are necessary to confirm this hypothesis.
The data obtained in this work indicate that biochar increases A. niger RP solubilization by alleviating F− toxicity and thereby stimulating the production of organic acids. A 3-fold increase in P solubilization was achieved using this cheap and abundant product obtained from agricultural wastes. Given the importance of organic acids in P solubilization (15, 16) and that most RPs contains high concentrations of F− (8), the proposed strategy offers an important improvement for microbial RP solubilization systems.
Conclusions.
Biochar enhances RP solubilization by A. niger in liquid fermentation through two interrelated processes: partial removal of F− released from RP and increased production of organic acids. These results suggest that biochar can be used to enhance microbial RP solubilization, especially in the case of batch cultures in which toxic compounds such as F− can accumulate.
ACKNOWLEDGMENTS
We are grateful to Piroeco Bioenergy (Málaga, Spain) for generously supplying the biochar used in the experiments.
We are also thankful to the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) for financing this work and providing scholarships to Gilberto de Oliveira Mendes, Ivo Ribeiro Silva, and Maurício Dutra Costa. Financial support for this study was also provided by Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG) project CAG-APQ-00712-12 and the Spanish projects CTM2011-027797 and P09RNM-5196.
Footnotes
Published ahead of print 7 March 2014
REFERENCES
- 1.Cordell D, White S. 2011. Peak phosphorus: clarifying the key issues of a vigorous debate about long-term phosphorus security. Sustainability 3:2027–2049. 10.3390/su3102027 [DOI] [Google Scholar]
- 2.Vaccari DA, Strigul N. 2011. Extrapolating phosphorus production to estimate resource reserves. Chemosphere 84:792–797. 10.1016/j.chemosphere.2011.01.052 [DOI] [PubMed] [Google Scholar]
- 3.Vassilev N, Martos E, Mendes G, Martos V, Vassileva M. 2013. Biochar of animal origin: a sustainable solution to the global problem of high-grade rock phosphate scarcity? J. Sci. Food Agric. 93:1799–1804. 10.1002/jsfa.6130 [DOI] [PubMed] [Google Scholar]
- 4.Vassilev N, Medina A, Azcon R, Vassileva M. 2006. Microbial solubilization of rock phosphate on media containing agro-industrial wastes and effect of the resulting products on plant growth and P uptake. Plant Soil 287:77–84. 10.1007/s11104-006-9054-y [DOI] [Google Scholar]
- 5.Mendes GO, Dias CS, Silva IR, Junior JI, Pereira OL, Costa MD. 2013. Fungal rock phosphate solubilization using sugarcane bagasse. World J. Microbiol. Biotechnol. 29:43–50. 10.1007/s11274-012-1156-5 [DOI] [PubMed] [Google Scholar]
- 6.Goldstein AH, Rogers RD, Mead G. 1993. Mining by microbe. Nat. Biotechnol. 11:1250–1254. 10.1038/nbt1193-1250 [DOI] [Google Scholar]
- 7.Mendes GO, Vassilev NB, Bonduki VHA, Silva IR, Ribeiro JI, Costa MD. 2013. Inhibition of Aspergillus niger phosphate solubilization by fluoride released from rock phosphate. Appl. Environ. Microbiol. 79:4906–4913. 10.1128/AEM.01487-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aigueperse J, Mollard P, Devilliers D, Chemla M, Faron R, Romano R, Cuer JP. 2000. Fluorine compounds, inorganic, p 397–441 In Ullmann's encyclopedia of industrial chemistry, vol 15 Wiley-VCH Verlag, Weinheim, Germany [Google Scholar]
- 9.Mohan D, Sharma R, Singh VK, Steele P, Pittman CU. 2012. Fluoride removal from water using bio-char, a green waste, low-cost adsorbent: equilibrium uptake and sorption dynamics modeling. Ind. Eng. Chem. Res. 51:900–914. 10.1021/ie202189v [DOI] [Google Scholar]
- 10.Medellin-Castillo NA, Leyva-Ramos R, Ocampo-Perez R, Garcia de la Cruz RF, Aragon-Piña A, Martinez-Rosales JM, Guerrero-Coronado RM, Fuentes-Rubio L. 2007. Adsorption of fluoride from water solution on bone char. Ind. Eng. Chem. Res. 46:9205–9212. 10.1021/ie070023n [DOI] [Google Scholar]
- 11.Nautiyal CS. 1999. An efficient microbiological growth medium for screening phosphate solubilizing microorganisms. FEMS Microbiol. Lett. 170:265–270. 10.1111/j.1574-6968.1999.tb13383.x [DOI] [PubMed] [Google Scholar]
- 12.Schneider KD, van Straaten P, de Orduna RM, Glasauer S, Trevors J, Fallow D, Smith PS. 2010. Comparing phosphorus mobilization strategies using Aspergillus niger for the mineral dissolution of three phosphate rocks. J. Appl. Microbiol. 108:366–374. 10.1111/j.1365-2672.2009.04489.x [DOI] [PubMed] [Google Scholar]
- 13.Clesceri LS, Greenberg AE, Eaton AD. (ed). 1999. Standard methods for the examination of water and wastewater, 20th ed. APHA, Washington, DC [Google Scholar]
- 14.Hernández-Luis F, Vazquez MV, Esteso MA. 2004. Activity coefficients of NaF in aqueous mixtures with ε-increasing co-solvent: ethylene carbonate-water mixtures at 298.15 K. Fluid Phase Equilib. 218:295–304. 10.1016/j.fluid.2004.01.029 [DOI] [Google Scholar]
- 15.Kpomblekou-A K, Tabatabai MA. 1994. Effect of organic acids on release of phosphorus from phosphate rocks. Soil Sci. 158:442–453. 10.1097/00010694-199415860-00006 [DOI] [Google Scholar]
- 16.Fox TR, Comerford NB, McFee WW. 1990. Phosphorus and aluminum release from a spodic horizon mediated by organic acids. Soil Sci. Soc. Am. J. 54:1763–1767. 10.2136/sssaj1990.03615995005400060043x [DOI] [Google Scholar]
- 17.Illmer P, Schinner F. 1995. Solubilization of inorganic calcium phosphates—solubilization mechanisms. Soil Biol. Biochem. 27:257–263. 10.1016/0038-0717(94)00190-C [DOI] [Google Scholar]
- 18.Arcand MM, Schneider KD. 2006. Plant- and microbial-based mechanisms to improve the agronomic effectiveness of phosphate rock: a review. An. Acad. Bras. Cien. 78:791–807. 10.1590/S0001-37652006000400013 [DOI] [PubMed] [Google Scholar]
- 19.Mendes GO, Freitas ALM, Pereira OL, Silva IR, Vassilev NB, Costa MD. 2014. Mechanisms of phosphate solubilization by fungal isolates when exposed to different P sources. Ann. Microbiol. 10.1007/s13213-013-0656-3 [DOI] [Google Scholar]
- 20.Steiner C, Das KC, Garcia M, Förster B, Zech W. 2008. Charcoal and smoke extract stimulate the soil microbial community in a highly weathered xanthic Ferralsol. Pedobiologia 51:359–366. 10.1016/j.pedobi.2007.08.002 [DOI] [Google Scholar]
- 21.Lehmann J, Rillig MC, Thies J, Masiello CA, Hockaday WC, Crowley D. 2011. Biochar effects on soil biota—a review. Soil Biol. Biochem. 43:1812–1836. 10.1016/j.soilbio.2011.04.022 [DOI] [Google Scholar]
- 22.Anderson CR, Condron LM, Clough TJ, Fiers M, Stewart A, Hill RA, Sherlock RR. 2011. Biochar induced soil microbial community change: implications for biogeochemical cycling of carbon, nitrogen and phosphorus. Pedobiologia 54:309–320. 10.1016/j.pedobi.2011.07.005 [DOI] [Google Scholar]
- 23.Liang B, Lehmann J, Solomon D, Kinyangi J, Grossman J, O'Neill B, Skjemstad JO, Thies J, Luizao FJ, Petersen J, Neves EG. 2006. Black carbon increases cation exchange capacity in soils. Soil Sci. Soc. Am. J. 70:1719–1730. 10.2136/sssaj2005.0383 [DOI] [Google Scholar]
- 24.Vassilev N, Mendes G, Costa M, Vassileva M. 2013. Biotechnological tools for enhancing microbial solubilization of insoluble inorganic phosphates. Geomicrobiol. J. 10.1080/01490451.2013.822615 [DOI] [Google Scholar]
- 25.Kubicek CP, Röhr M. 1980. Regulation of citrate synthase from the citric acid-accumulating fungus, Aspergillus niger. Biochim. Biophys. Acta 615:449–457 [PubMed] [Google Scholar]
- 26.Kubicek CP, Schreferl-Kunar G, Wohrer W, Rohr M. 1988. Evidence for a cytoplasmic pathway of oxalate biosynthesis in Aspergillus niger. Appl. Environ. Microbiol. 54:633–637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mischak H, Kubicek CP, Röhr M. 1985. Formation and location of glucose oxidase in citric acid producing mycelia of Aspergillus niger. Appl. Microbiol. Biotechnol. 21:27–31 [Google Scholar]
- 28.Chen Y-SR, Butler JN, Stumm W. 1973. Adsorption of phosphate on alumina and kaolinite from dilute aqueous solutions. J. Colloid Interface Sci. 43:421–436. 10.1016/0021-9797(73)90388-3 [DOI] [Google Scholar]
- 29.Zheng T-T, Sun Z-X, Yang X-F, Holmgren A. 2012. Sorption of phosphate onto mesoporous γ-alumina studied with in-situ ATR-FTIR spectroscopy. Chem. Cent. J. 6:26. 10.1186/1752-153X-6-26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jones DL. 1998. Organic acids in the rhizosphere—a critical review. Plant Soil 205:25–44. 10.1023/A:1004356007312 [DOI] [Google Scholar]
- 31.Jentschke G, Godbold DL. 2000. Metal toxicity and ectomycorrhizas. Physiol. Plant. 109:107–116. 10.1034/j.1399-3054.2000.100201.x [DOI] [Google Scholar]
- 32.Gadd GM. 1999. Fungal production of citric and oxalic acid: importance in metal speciation, physiology and biogeochemical processes. Adv. Microb. Physiol. 41:47–92. 10.1016/S0065-2911(08)60165-4 [DOI] [PubMed] [Google Scholar]


