Abstract
Most studies of behavioral/developmental effects of iron deficiency anemia (IDA) or iron supplementation in infancy have found social-emotional differences. Differences could relate to behavioral inhibition or lack of positive affect and altered response to reward. To determine long-term behavioral effects, the study was a follow-up of a randomized controlled trial of behavioral/developmental effects of preventing IDA in infancy. Healthy Chilean infants free of IDA at age 6 mo were randomly assigned to iron supplementation or no added iron (formula with iron/powdered cow milk, vitamins with/without iron) from ages 6 to 12 mo. At age 10 y, 59% (666 of 1123) and 68% (366 of 534) of iron-supplemented and no-added-iron groups were assessed. Social-emotional outcomes included maternal-reported behavior problems, self-reported behavior, examiner ratings, and video coding of a social stress task and gamelike paradigms. Examiners rated the iron-supplemented group as more cooperative, confident, persistent after failure, coordinated, and direct and reality-oriented in speech and working harder after praise compared with the no-added-iron group. In a task designed to elicit positive affect, supplemented children spent more time laughing and smiling together with their mothers and started smiling more quickly. In the social stress task they smiled and laughed more and needed less prompting to complete the task. All P values were <0.05; effect sizes were 0.14–0.36. There were no differences in behaviors related to behavioral inhibition, such as anxiety/depression or social problems. In sum, iron supplementation in infancy was associated with more adaptive behavior at age 10 y, especially in affect and response to reward, which may improve performance at school and work, mental health, and personal relationships.
Introduction
Social-emotional differences are among the most consistent findings in studies of the behavioral and developmental effects of iron deficiency in infancy. Infants with iron deficiency anemia (IDA) have been observed to be more wary, hesitant, fearful, withdrawn, shy, unhappy, or tense; stay closer to their mothers; be less playful; and show less pleasure and reaction to usual stimuli (1). Several randomized trials of supplemental iron showed affective benefits of iron (1).
Altered social-emotional behavior can influence how an infant reacts to the environment and how caregivers respond to that infant and consequently the infant’s experience of the physical and social environment (2–4). In the nutrition literature, the functional isolation hypothesis has provided a relevant framework (5, 6). The concept is that nutritional deficiencies can alter infants’ affect, attention, or activity, making them less able to seek or benefit from developmentally supportive experiences. Combined with the effects on caregivers, the behavioral alterations are postulated to contribute to the poorer developmental outcome observed in infants with generalized undernutrition or IDA (2). However, this useful framework is quite nonspecific. We have turned to the burgeoning behavioral neuroscience field for further ways to understand the behavioral effects of iron deficiency in infancy.
The behavior pattern seems to resemble the temperamental trait that has become known as behavioral inhibition in developmental psychology and psychiatry research. Behaviorally inhibited infants and toddlers respond to unfamiliar contexts with withdrawal, fear, and increased vigilance [see review (7)]. Individual differences in toddlers’ and preschoolers’ affect and behavior in reaction to novel situations have been the focus of numerous studies (7, 8). Since the mid-1980s, we have questioned whether IDA infants fit the pattern of behavioral inhibition (9, 10). Our subsequent observations of mother- and teacher-reported increases in anxiety/depression and social problems among young adolescents who had chronic, severe iron deficiency as infants (11) and feelings of detachment and disengagement in adulthood (12) also seem to parallel long-term outcome in studies in behaviorally inhibited children [see reviews (8, 13)]. However, the neurophysiologic profile of behavioral inhibition reported in the psychology and psychiatry literature (increased heart rate, altered cortisol response, etc.) did not map onto what was known from iron deficiency studies at the time. Furthermore, the amygdala, thought to be related to behavioral inhibition (14), has received little attention in the iron deficiency field.
In contrast, rodent models of iron deficiency pointed to impairments in striatal dopamine function [summarized in (15, 16)], and behavioral neuroscience research showed that dopamine is involved in response to the unfamiliar. Pharmacologically induced early dopamine terminal damage in the rat striatum led to a life-long hyperreactivity to novel objects and experiences in an unfamiliar environment (17). Some rat studies of early iron deficiency reported hesitant behavior or less exploration in experimental settings (18–20). Thus, the behavioral alterations reported in IDA infants could be compatible with dopamine system changes.
Dopamine is also implicated in other aspects of affect, such as laughter, positive affect, and response to reward (21). The first hint of this aspect in iron deficiency research was the report by Honig and Oski (22) of more solemnity in iron-deficient infants, suggesting lack of positive affect. Findings in our previous studies could also fit in the context of positive affect and reward, such as less reactivity to usual test stimuli, lack of social interaction or positive affect during testing, decreased orientation/engagement, and less ability to be soothed by words or objects when upset (23, 24). But some of these alterations could also fit with behavioral inhibition.
Thus, the specific nature of iron-related affective and social-emotional alterations in infancy is not entirely clear. Although the brain has complex interrelated systems, the relevant neural circuits, structures, and neurotransmitters may well differ depending on what best characterizes the behavior pattern. We have increasingly used a brain-behavior framework (25) to help clarify the nature of short- and long-term behavioral alterations with early iron deficiency.
This study assessed 10-y outcome in Chilean children who were randomly assigned to take supplemental iron or no added iron during infancy. We predicted that there would be more positive affect and social interaction and generally better social-emotional development in children who were administered supplemental iron in infancy. We probed positive affect and social inhibition by including gamelike paradigms that typically elicit smiling and laughter and a socially stressful paradigm that is widely used in research on anxiety and social problems.
At the conclusion of the infancy study, iron status in each group ranged from IDA to iron-sufficient. We therefore conducted secondary analyses at age 10 y on the basis of iron status, because converging results for iron supplementation and iron status would provide stronger evidence of iron’s role in early social-emotional development. We also pursued our unexpected findings of poorer cognitive and motor outcome at age 10 y in study children who were administered iron-fortified formula as infants, compared with low-iron formula, especially if they had high initial hemoglobin (26). The question was whether social-emotional outcome would show a similar pattern.
Participants and Methods
Summary of infancy preventive trial
The study was conducted in Chile between 1991 and 1996, a period in which infant iron deficiency was widespread and there was no national program of iron fortification. All infants were born at term of uncomplicated vaginal births, weighed >3.0 kg, and were free of acute or chronic health problems. Infants without IDA at 6 mo were randomly assigned to take study-provided supplements between ages 6 and 12 mo in a double-blind fashion (see Supplemental Fig. 1 for study diagram) (23). Initially, infants were randomly assigned to iron-fortified (12.7 mg/L) or low-iron (2.3 mg/L) formula. To avoid interference with breastfeeding, infants had to be consuming at least 250 mL/d of formula or cow milk. In the last years of enrollment (1994–1996), this requirement was dropped due to the success of breastfeeding campaigns in Chile. Exclusively breast-fed infants were no longer excluded, low-iron formula was no longer used, and a no-iron condition was included as originally planned (23). Infants consuming <250 mL/d of formula or cow milk at 6 mo were randomly assigned to vitamins without or with iron (10 mg/d). Those consuming more formula or cow milk were randomly assigned to be administered iron-fortified formula or cow milk formula without added iron. At 12 mo, infants underwent developmental testing and venipuncture for iron status. Hemoglobin <110 g/L was the cutoff for anemia. Iron deficiency was defined as ≥2 abnormal iron measures (mean corpuscular volume <70 fL (70 μm3), free erythrocyte protoporphyrin ≥1.77 μmol/L, serum ferritin <27 pmol/L) (23).
A total of 1657 infants completed the preventive trial with iron status measures at 12 mo. There were no differences between high- and low-iron groups in the prevalence of IDA (27) or behavioral/developmental outcome, and the groups were combined to form an iron-supplemented group (n = 1123) for comparison with the no-added-iron group (n = 534) (23). IDA was present in 3.1% and 22.6% of the supplemented and unsupplemented groups, respectively. The unsupplemented group showed poorer social-emotional behavior: more of these infants showed no positive affect, no social interaction, no social referencing, an inability to be soothed by words or objects, and no protest upon removal of attractive objects. The social-emotional domain showed the biggest magnitude of effects [effect size = 0.31 SD (28), which is substantial for a preventive trial].
Ten-year follow-up
The 10-y study was approved by the institutional review boards of the Universities of Michigan and Chile. Signed informed consent was obtained from the parents and assent was obtained from the children. The half-day assessment included anthropometry, measures of behavior and development, and a venous blood sample (10 mL) for iron status. We used cutoffs for age (29) to define anemia and iron deficiency (≥2 abnormal iron measures): hemoglobin <118 g/L, transferrin saturation <14%, free erythrocyte protoporphyrin >1.24 μmol/L RBCs, serum ferritin <27 pmol/L, and mean corpuscular volume <76 fL (30).
Social-emotional and behavioral outcomes at 10 y were based on parental report, child self-report, examiner observations, and quantitative coding of videotaped paradigms that were designed to assess positive affect and social inhibition. Because relevant measures have not been validated for this population, we selected measures on the basis of face validity. Our emphasis was within-sample group comparisons, rather than comparisons to reference norms. Some measures, such as the paradigms to elicit positive affect, were not standard but were chosen specifically to assess a salient construct related to iron deficiency. Examiners were experienced psychologists who were unaware of assignment to supplement group or iron status in infancy. Video coders were Spanish-speaking student research assistants at the University of Michigan; behavior was coded quantitatively by using Noldus Observer 5.0 (Noldus Information Technology). Examiners and coders achieved ≥80% agreement.
Behavior and Attitude Checklist.
Examiners rated the children’s behavior by using twenty-six 7-point Likert-like items anchored by descriptive terms at each extreme (31). We considered several items to be especially pertinent, such as tenseness with respect to behavioral inhibition and reactions to failure or praise with respect to reward. We used principal components factor analysis with varimax rotation for data reduction, retaining factors with an eigenvalue >1.0.
Child Behavior Checklist.
Caregivers, mostly mothers, reported their children’s behavior with the use of the Child Behavior Checklist, Spanish version (32). This 113-item measure was scored by a standard computerized scoring program, yielding T-scores for 8 subscales, internalizing problems, externalizing problems, and total problems. The most important outcome was the internalizing problem T-score, which captures anxiety, depression, and social problems.
Measures to elicit positive affect and social interaction.
We videotaped 2 gamelike paradigms to pursue our findings that infants who did not take iron were less likely to show positive affect, interact socially, refer to caregivers’ reactions or share pleasure or excitement with them. In the ball pass, mother and child were asked to pass a ball, held under the neck, back and forth without using their hands. In Jenga (Hasbro), a game involving a 30-cm tower of wooden pieces, mother and child took turns withdrawing pieces and stacking them on top, trying not to make the tower fall over (33). For the ball pass, the major outcome measure was shared positive affect, because the game is meant to be silly. The Jenga game was different, because withdrawing pieces without toppling the tower requires slow movements and concentration. Therefore, our main outcomes included pleasure or delight during the other person’s turn.
Trier Social Stress Test for Children.
We assessed response to a brief socially stressful situation (3, 4) to pursue previous findings of hesitance, withdrawal, anxiety/depression, and social problems associated with iron deficiency in infancy (1). After 5 min to prepare, the children were videotaped for 3 min while they finished a story in front of an impassive examiner. If they stopped talking, the psychologist calmly prompted them to continue. Child fidgeting, laughing, and smiling and psychologist prompts were coded, and coders made overall ratings of the child’s self-confidence and nervousness with the use of 4-point Likert-like scales. The major outcomes were indications of distress, such as nervousness and need for prompting.
Child Health and Illness Profile–Child Edition.
Parent and child report forms of the Child Health and Illness Profile—Child Edition (CHIP-CE) (35) include social-emotional questions on self-esteem, depressive symptoms, and social abilities. Summary T-scores do not capture positive affect/reward or behavioral inhibition; they cover satisfaction, discomfort, risks, resilience, and achievement. Therefore, we considered the CHIP-CE as an exploratory measure.
Data analysis
Statistical analyses were conducted by using SAS 9.2. (SAS Institute). To identify group differences in background characteristics that might warrant covariate control, we used t tests for continuous variables and chi-square tests for categorical ones. The statistical approach to test for differences in outcome followed the general linear model for continuous variables and used multiple regression, with consideration of background factors that showed group differences, and the backward elimination procedure. The primary analysis compared iron-supplemented and no-added-iron groups on 10-y social-emotional outcomes. Secondary analyses considered outcome depending on 1) iron status in infancy and 2) iron-fortified vs. low-iron formula. We used an α level of 0.05 (2-tailed) to determine statistical significance. Group summary statistics are presented as means ± SEs unless otherwise indicated.
Results
Sample at 10 y
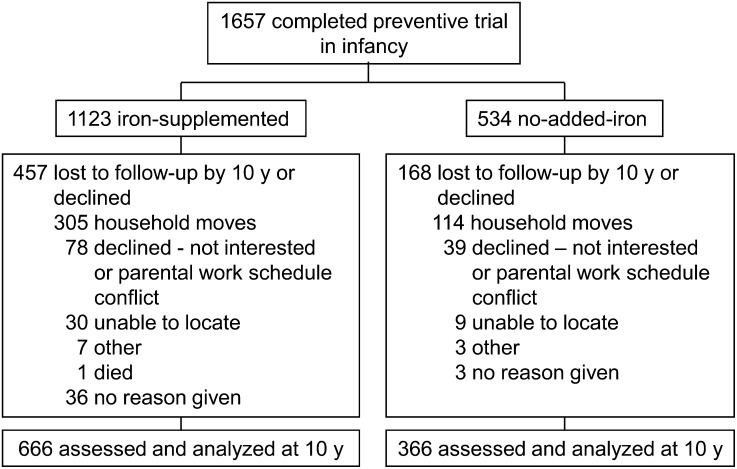
We assessed 1032 of 1657 children (62.3%) who completed the preventive trial at age 12 mo; 666 were administered iron supplementation in infancy and 366 did not (see Fig. 1 for a flowchart of participant involvement). For secondary analyses, there were 125 children with IDA in infancy and 895 without (12 unclassified); for iron-fortified vs. low-iron formula, there were 255 and 240 children, respectively.
FIGURE 1.
Flowchart of participants’ involvement from trial of supplemental iron in infancy to the 10-y follow-up.
Children who were or were not assessed at 10 y were similar in infancy background characteristics, such as gender, birth weight, breastfeeding, and most family characteristics. However, those with 10-y data had families who were more supportive of child development during the infancy study, experienced fewer life stressors, and had more household goods (P < 0.05). A higher proportion of the no-added-iron group participated at 10 y (68.5%; 366 of 534), compared with the iron-supplemented group (59.3%; 666 of 1123) (P = 0.00). Refusal was uncommon in both groups (6.9% and 7.3%). However, attrition due to family moves (the most common reason) was less problematic in the no-added-iron group (21.3% vs. 27.2% of the iron-supplemented group; P = 0.01).This may be due to improved tracking measures instituted later in the study, when the entire no-added-iron group was enrolled.
Iron-supplemented and no-added-iron groups were similar in background characteristics (Table 1), with a few noteworthy exceptions (P < 0.05). As in infancy, the no-added-iron group weighed more at birth and consumed less formula or cow milk than the iron-supplemented group. As discussed in reporting the infancy study (23), the difference in birth weight is likely due to the epidemiologic transition to overweight and obesity, which increased in Chile during the period of enrollment. The difference in formula/cow milk intake is due to the change in enrollment criteria to include exclusively breast-fed infants in the final years of the study. The iron-supplemented group was ∼4 d older at the 10-y testing than the no-added-iron group, and a lower proportion was male. The groups were comparable in BMI and iron status at 10 y. Only 3 children had IDA, and >91% were iron-sufficient. The groups were also similar in family background characteristics at 10 y. However, maternal intelligence quotient (IQ), which was measured in infancy, averaged 1.4 points lower in the iron-supplemented group.
TABLE 1.
Background characteristics of 10-y-old children who were or were not administered supplemental iron in infancy1
| Iron-supplemented(n = 666) | No added iron(n = 366) | P | |
| Child characteristics | |||
| Age at testing, y | 10.02 ± 0.10 | 10.03 ± 0.07 | 0.03 |
| Male, n (%) | 342 (51.4) | 211 (57.6) | 0.05 |
| BMI, kg/m2 | 19.3 ± 3.3 | 19.4 ± 3.4 | 0.49 |
| Birth weight, g | 3533 ± 355 | 3593 ± 381 | 0.01 |
| Formula/cow milk intake, mL/d | 477 ± 206 | 314 ± 178 | <0.001 |
| Iron sufficient at 10 y, n (%) | 618 (93.8) | 326 (91.3) | 0.14 |
| Family characteristics | |||
| Maternal age, y | 26.4 ± 6.0 | 26.1 ± 6.1 | 0.52 |
| Maternal IQ2 | 84.8 ± 10.2 | 86.2 ± 10.4 | 0.05 |
| Maternal depressive symptomatology score3 | 18.3 ± 13.1 | 17.6 ± 12.8 | 0.37 |
| Maternal education, y | 9.7 ± 2.8 | 9.8 ± 2.7 | 0.76 |
| Paternal education, y | 9.8 ± 3.0 | 9.5 ± 3.0 | 0.23 |
| Socioeconomic index4 | 34.4 ± 7.8 | 34.3 ± 7.0 | 0.74 |
| Father present, n (%) | 478 (72.0) | 280 (76.7) | 0.10 |
| Total HOME score5 | 36.7 ± 7.2 | 36.0 ± 7.0 | 0.11 |
| Number of life stressors6 | 4.9 ± 2.6 | 5.1 ± 2.7 | 0.37 |
Values are means ± SDs for continuous variables and n (%) for categorical variables. HOME, Home Observation for Measurement of the Environment; IQ, intelligence quotient.
Values reported from the infancy study. Maternal IQ was measured with a short form of the Wechsler Adult Intelligence Scale–Revised, and any missing data were imputed.
Measured by the Center for Epidemiologic Studies–Depression Scale. Scores range from 0–60 with a score ≥16 commonly used as a threshold for depressive symptomatology.
Measured by the Graffar scale, designed specifically to differentiate families at the lower end of the socioeconomic spectrum. A score of 36 falls in the medium range of the lower class spectrum.
Assessed by using the HOME-Revised.
Measured by a scale modified from the Social Readjustment Rating Scale.
Social-emotional outcome at 10 y
We present statistically significant results based on analyses with covariate control unless otherwise noted. Potential covariates were background factors with group differences.
Behavior and Attitude Checklist.
Six factors emerged from principal components factor analysis. The first factor seemed to reflect motivation and response to reward: persistence, behavior after failure (works hard vs. gives up easily), reaction to praise (accepts gracefully vs. accepts awkwardly), behavior after praise (works hard vs. retreats), cooperation, and confidence. The second factor seemed to reflect externalizing behaviors: aggression, impulsivity, thinking aloud, hyperactivity, and conversing spontaneously. The third factor reflected language: speech quality, articulateness, direct vs. vague responses, and reality-oriented vs. bizarre language. The fourth factor seemed to capture concern about performance: relaxed vs. tense, accepting vs. critical of own work, unaware vs. aware of failure, calm vs. agitated after failure, and not apologetic vs. apologetic after failure. The fifth factor reflected work habits and motor control: neat vs. careless, careful and systematic vs. trial and error, skillful vs. awkward movements, and good vs. defective motor coordination. The sixth factor related to speed of work habits and visual-motor reactions. (See Supplemental Table 1 for all factor loadings.)
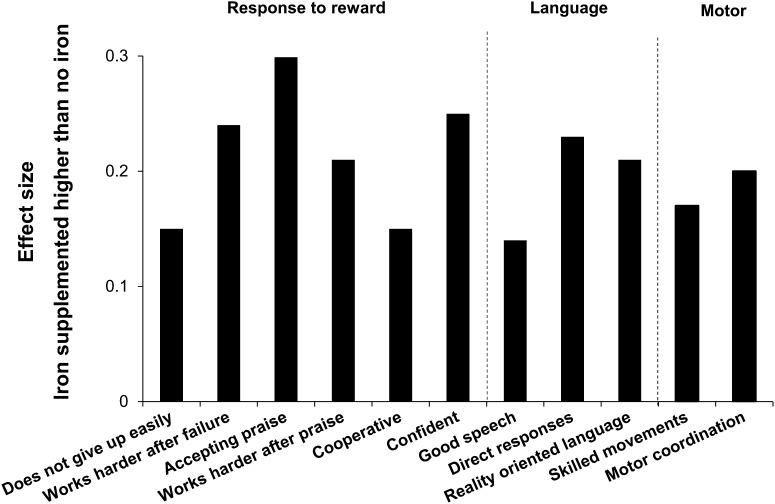
Psychologists rated the iron-supplemented group as showing more adaptive behavior on all but 1 factor (factor 4, performance concern) (Table 2). However, differences in the second factor (externalizing behavior) and the sixth factor (speed) became suggestive (P ≤ 0.10) after control for significant covariates (gender for both, mother’s IQ for speed). Effect sizes of significant group differences ranged from 0.14 to 0.35 on individual items on the 3 factors that remained significant after covariate control (Fig. 2). Compared with the no-added-iron group, the iron-supplemented group was reported to give up less easily, work harder after failure, be more accepting of praise, work harder after praise, and be more cooperative and confident. Their speech was rated as better, more direct, and more reality-oriented; and their movements were rated as more coordinated and less awkward. There were no differences in secondary analyses.
TABLE 2.
Behavior rating scale factors in 10-y-old children who were or were not administered supplemental iron in infancy1
| Factor | Iron-supplemented (n = 659) | No added iron (n = 363) | P | Significant covariates |
| Motivation and response to reward | ||||
| Unadjusted | 11.3 ± 0.1 | 12.3 ± 0.2 | <0.001 | |
| Adjusted | 11.2 ± 0.1 | 12.5 ± 0.2 | <0.001 | Gender, maternal IQ |
| Externalizing behavior | ||||
| Unadjusted | 9.6 ± 0.2 | 10.2 ± 0.2 | 0.04 | |
| Adjusted | 9.6 ± 0.2 | 10.1 ± 0.2 | 0.07 | Gender |
| Language | ||||
| Unadjusted | 5.8 ± 0.1 | 6.3 ± 0.2 | 0.01 | |
| Adjusted | 5.7 ± 0.1 | 6.4 ± 0.2 | 0.001 | Age at testing, gender, maternal IQ |
| Performance concern | ||||
| Unadjusted | 1.3 ± 0.1 | 1.6 ± 0.2 | 0.24 | |
| Adjusted | 1.3 ± 0.1 | 1.6 ± 0.2 | 0.24 | |
| Work habits and motor control | ||||
| Unadjusted | 8.3 ± 0.1 | 8.9 ± 0.2 | 0.002 | |
| Adjusted | 8.3 ± 0.1 | 8.8 ± 0.2 | 0.03 | Intensity of breastfeeding,2maternal IQ |
| Speed | ||||
| Unadjusted | 6.4 ± 0.1 | 6.1 ± 0.1 | 0.02 | |
| Adjusted | 6.5 ± 0.1 | 6.2 ± 0.1 | 0.10 | Gender, maternal IQ |
Values are means ± SEs for factor scores derived from the Behavior and Attitude Checklist, completed by project psychologists. Data were missing for n = 7 and n = 3 children from the iron-supplemented and no added iron groups, respectively. IQ, intelligence quotient.
As indexed by milliliters per day of formula or cow milk.
FIGURE 2.
Differences in psychologist-rated behavior between children who were or were not administered supplemental iron in infancy. Effect sizes are shown for statistically significant group differences on individual items for the 3 factors that remained significant after covariate control. Effect size is the difference between group means divided by the SD.
Child Behavior Checklist.
There were no differences between iron-supplemented and no-added-iron groups in internalizing problems or any other composite or subscale score (Supplemental Table 2). In secondary analyses regarding iron status at the preventive trial’s conclusion (IDA, iron deficiency without anemia, or iron sufficiency), there were significant linear effects for total problems (P = 0.02), internalizing problems (P = 0.008), and several subscales, including withdrawn (P = 0.00), anxious/depressed (P = 0.03), and aggression (P = 0.03). The threshold of effects was iron deficiency with or without anemia, except for the aggression subscale in which the threshold was IDA. However, differences were not statistically significant after covariate control. There were no group differences in secondary analyses comparing children who had been randomly assigned to take iron-fortified or low-iron formula in infancy.
Ball pass and Jenga.
During the ball pass, the iron-supplemented group spent a higher proportion of the time in shared positive affect (both mother and child smiling or laughing) or mother smiling/laughing while the child was not and a lower proportion during which neither was smiling or laughing, and the children were quicker to smile compared with those in the no-added-iron group (Table 3). During Jenga, the children in the iron-supplemented group spent a higher proportion of the time smiling/laughing while the mother was not, their mothers spent a higher proportion of the time smiling/laughing while the child was not, there was a lower proportion of time in which neither smiled or laughed, and the children smiled more quickly and monitored the examiner’s reaction less frequently. In secondary analyses regarding iron status in infancy, there were no differences for the ball pass. For Jenga, there were linear effects of infancy iron status for the proportion of time in which the mother was smiling/laughing while the child was not (P = 0.04; IDA the lowest) and how often the child checked the examiner’s reaction (P = 0.02; IDA the highest), but the results became nonsignificant after covariate control. There were no statistically significant differences for iron-fortified vs. low-iron formula in infancy.
TABLE 3.
Other affective outcomes at age 10 y in children who were or were not administered supplemental iron in infancy1
| Iron-supplemented (n = 574) | No added iron (n = 306) | P | Significant covariates | |
| Ball pass | ||||
| Mother and child smile/laugh, % of time | 36.2 ± 1.0 | 31.9 ± 1.4 | 0.01 | |
| Child smiles/laughs, mother does not, % of time | 10.1 ± 0.6 | 10.4 ± 0.8 | 0.75 | Gender |
| Mother smiles/laughs, child does not, % of time | 0.6 ± 0.09 | 0.2 ± 0.1 | 0.02 | Gender, age at testing |
| Neither mother or child smiles/laughs, % of time | 48.1 ± 1.0 | 52.6 ± 1.4 | 0.01 | |
| Latency to child’s first smile, s | 8.0 ± 0.5 | 9.7 ± 0.6 | 0.04 | Gender |
| Jenga | ||||
| Mother and child smile/laugh, % of time | 14.9 ± 0.8 | 14.1 ± 1.0 | 0.51 | Duration of task, age at testing |
| Child smiles/laughs, mother does not, % of time | 15.0 ± 0.7 | 11.7 ± 0.9 | 0.005 | Duration of task |
| Mother smiles/laughs, child does not, % of time | 4.1 ± 0.3 | 1.5 ± 0.4 | <0.001 | Duration of task, intensity of breastfeeding2 |
| Neither mother or child smiles/laughs, % of time | 54.3 ± 1.0 | 60.9 ± 1.4 | 0.001 | Duration of task, age at testing |
| Latency to child’s first smile, s | 19.3 ± 1.3 | 24.0 ± 1.8 | 0.03 | Duration of task |
| Child checks examiner, n | 1.8 ± 0.1 | 2.3 ± 0.1 | <0.001 | Gender, duration of task |
| Trier Social Stress Test for Children | ||||
| Child smiles, n | 2.3 ± 0.1 | 1.6 ± 0.1 | <0.001 | Duration of task, intensity of breastfeeding2 |
| Child laughs, n | 0.7 ± 0.1 | 0.4 ± 0.1 | 0.01 | Gender, duration of task, intensity of breastfeeding3 |
| Child fidgets, n | 0.9 ± 0.03 | 0.7 ± 0.04 | 0.0003 | |
| Prompts from examiner, n | 1.8 ± 0.1 | 2.4 ± 0.1 | <0.001 | Age at testing |
| Latency to child’s first smile, s | 45.4 ± 2.4 | 56.3 ± 3.7 | 0.01 | Duration of task |
| Rating of child self-confidence3 | 2.3 ± 0.04 | 2.1 ± 0.05 | 0.003 | |
| Rating of child nervousness4 | 2.8 ± 0.04 | 2.6 ± 0.05 | 0.12 |
Values are covariate adjusted means ± SEs for continuous variables and percentages. Because of technical problems with videotapes, the total n available for ball pass, Jenga, and Trier were 860, 919, and 861, respectively. The mean n per group is shown.
As indexed by milliliters per day of formula or cow milk.
Self-confidence was rated with a 4-point Likert-like scale where 4 = very confident.
Nervousness was rated with a 4-point Likert-like scale where 4 = always showing nervousness.
Trier Social Stress Test for Children.
Compared with the no-added-iron group, children in the iron-supplemented group smiled more quickly and more often and laughed and fidgeted more during storytelling. They were rated as more self-confident, and they required fewer prompts to continue telling their story (Table 3). There were no differences in nervousness ratings, which were unrelated to fidgeting (r = 0.038, P = 0.28). In secondary analyses for iron status in infancy, there were linear effects (IDA lowest) for frequency of smiling (P = 0.03) and fidgeting (P = 0.015). In secondary analyses for iron-fortified vs. low-iron formula, children who consumed the iron-fortified formula smiled more quickly (delay of 36.2 vs. 47.3 s, P = 0.02).
CHIP-CE.
There were no group differences for satisfaction, comfort, resilience, risk avoidance, and achievement summary scores based on child self-report (Supplemental Table 2). On the basis of parent report, the supplemented group scored lower than did the unsupplemented group on resilience (P = 0.011). Items on the resilience factor reflected physical activity and parental involvement. In secondary analyses regarding iron status in infancy, there were linear effects for parent-reported risk avoidance (P = 0.046) and parent-reported satisfaction (P = 0.03) (IDA the lowest), but differences became nonsignificant after covariate control.
Discussion
In this follow-up at age 10 y, children who were administered supplemental iron in infancy demonstrated more adaptive behavior in several areas. Compared with children who did not take iron, they were rated as showing more positive task orientation and better language and motor coordination. They shared more positive affect with their mothers and showed more pleasure in gamelike paradigms; and, in the socially stressful task, they were rated as more self-confident, smiled and laughed more, and needed less prompting to complete the task.
Although not entirely consistent, the pattern of results seems to be more related to positive affect and reward responsiveness than behavioral inhibition. The psychologists’ ratings are particularly relevant in this respect. The iron-supplemented group’s higher score on the factor capturing positive task orientation suggests more motivation and positive responsiveness to reward, inherent or otherwise. Specifically, a higher score indicates working harder after failure, not giving up easily, and working harder after praise, in addition to being more cooperative, confident, and accepting of praise. Children in the iron-supplemented group also seemed to show more pleasure in the games that we specifically included to elicit positive affect. In contrast, there were no group differences in parent report of internalizing problems, observer ratings of nervousness during the socially stressful situation, or psychologists’ ratings on a factor that might pertain to long-term outcome in behavioral inhibition (i.e., tenseness, awareness of failure, agitation and apologies after failure, and criticism of own work).
Nonspecific conditions, such as fatigue or illness, can reduce positive affect, but there were no group differences in health at age 10 y. Reduced positive affect has been observed among iron-deficient infants even in the absence of anemia (22, 24) and in the Chilean study among infants who did not take supplemental iron (23). Reduced response to the physical and social environment has also been observed in several studies (9, 23). These findings suggest that less positive affect and reward responsiveness might represent early behavioral changes when iron is inadequate. Less positive responsiveness to reward and reduced positive affect seem to be consistent with long-lasting dopaminergic effects of insufficient iron in infancy.
With regard to our secondary analyses of iron status in infancy, mothers reported more internalizing and total behavior problems for children who had iron deficiency with or without anemia in infancy—specifically, more withdrawal, anxiety, and depression. The findings are much like those reported in the Costa Rica follow-up at ages 11–14 y (11), but the Chilean study results were not statistically significant after control for background factors. There were several other linear effects of iron status in infancy that seemed to fit with the results for iron-supplemented vs. no-added-iron comparisons, which also lost significance after covariate control.
The robustness of the Costa Rican study findings of more wary, fearful behavior with IDA in infancy and more internalizing problems later on contrasts with the loss of statistical significance of such long-term differences after covariate control in the Chilean study. We suspect that the explanation lies in differences between the studies in the chronicity of iron deficiency or the timing of treatment. In the Chilean study, infants with IDA at 6 mo were excluded from the preventive trial, iron status was measured 6 mo later at the conclusion of the trial, and all IDA infants were treated with iron. Thus, the maximum period of IDA was 6 mo and probably shorter for most children, because it takes time for IDA to develop. In contrast, there was no such boundary on the duration of IDA in previous studies that showed behavioral effects of IDA in infancy, and the mean age of infants was considerably older in the Costa Rican study (17 mo). The implication is that more chronic IDA (or later treatment) may be associated with additional behavioral alterations in the short and long term that are more like behavioral inhibition, such as wariness, fearfulness, and hesitancy in infancy and internalizing problems later on. This seems plausible in light of findings in animal models that behavioral and neurotransmitter effects of iron deficiency vary with duration and severity of IDA and timing of treatment (15, 36, 37).
It should be emphasized that the lack of statistical significance of social-emotional differences with IDA in infancy in covariate-controlled analyses does not mean that there are no neurodevelopmental differences in this cohort. In fact, we found short- and long-term differences, controlling for covariates, between children with IDA in infancy and their nonanemic peers on neurophysiologic measures, such as sensory evoked potentials (38, 39), neurocognitive tests with event-related potentials (40, 41), polysomnographic recordings (42–44), and neuroendocrine responses (45). It is possible that differences on more global measures will become apparent over time, especially if there is a cumulative impact of neurophysiologic alterations.
With respect to secondary analyses related to iron-fortified vs. low-iron formula, we observed only a single statistically significant behavioral difference: children who consumed iron-fortified formula were quicker to smile in the socially stressful paradigm. Although smiling can indicate nervousness or anxiety, quicker smiling in the entire iron-supplemented group went along with less need for prompting, higher ratings of self-confidence, and no increase in ratings of nervousness, indicating more adaptive behavior. Therefore, we interpreted quicker smiling in those who consumed iron-fortified formula as a positive behavior, in contrast to our recently reported findings of lower cognitive and visual-motor scores at 10 y in the iron-fortified formula group, compared with the low-iron-formula group, especially among children with high hemoglobin at 6 mo (25). With only 26 such children, the results require replication before speculating about why social-emotional effects might differ from cognitive and motor ones.
The study has important limitations despite random assignment to a supplementation group in infancy, assessment by examiners who were unaware of treatment, and hypothesis-based behavioral paradigms. Changes in supplements and enrollment criteria during the infancy phase mean that the study is not a simple randomized clinical trial and, hence, is not the strongest basis for causal inference. Although we assessed >1000 children at 10 y, 38% of the participants in the infancy study were not assessed, primarily because we could not locate them. Even though the maximum period of IDA in infancy was 6 mo, the study cannot determine how long the children might have had iron deficiency without anemia or when it began, because we had no data on prenatal iron status. The restricted duration of IDA means that results may not apply to populations in whom IDA is more chronic or severe. Because virtually all infants were at least partially breast-fed, the results may not generalize to infants who consume only commercial or homemade formula. The findings may also not generalize to settings in which infants are not as well nourished and healthy.
In sum, we found evidence of more adaptive behavior in 10-y-old children who were randomly assigned to supplemental iron vs. no added iron in infancy. More adaptive behavior may affect performance at school and work, mental health, and personal relationships. Further follow-up will determine the functional significance of the differences observed.
Supplementary Material
Acknowledgments
B.L. and M.C. designed and conducted the research; B.L., M.C., J.B.S., and J.S. analyzed the data; B.L., M.C., K.M.C., J.B.S., and J.S. interpreted the data; B.L., K.M.C., and J.S. drafted the manuscript; B.L., M.C., K.M.C., J.B.S., and J.S. critically revised the manuscript; and B.L. had full access to all study data and takes responsibility for the integrity of the data and the accuracy of the data analysis. All authors read and approved the final manuscript.
Literature Cited
- 1.Lozoff B. Iron deficiency and child development. Food Nutr Bull. 2007;28:S560–71. [DOI] [PubMed] [Google Scholar]
- 2.Lozoff B, Klein NK, Nelson EC, McClish DK, Manuel M, Chacon ME. Behavior of infants with iron deficiency anemia. Child Dev. 1998;69:24–36. [PubMed] [Google Scholar]
- 3.Lozoff B, Wachs TD. Functional correlates of nutritional anemias in infancy and early childhood—child development and behavior. In: Ramakrishnan U, editor. Nutritional anemias. New York: CRC Press; 2001. p. 66–88.
- 4.Lozoff B, Black M. Impact of micronutrient deficiencies on behavior and development. In: Pettifor J, Zlotkin SH, editors. Nutrition-micronutrient deficiencies during the weaning period and the first years of life. Basel (Switzerland): Karger; 2003. p. 119–35.
- 5.Levitsky DA, Strupp BJ. Functional isolation in rats. In: Brozek J, Schürch B, editors. Malnutrition and behavior: critical assessment of key issues. Lausanne (Switzerland): Nestlé Foundation; 1984. p. 411–27.
- 6.Wachs TD. Nutritional deficiencies as a biological context for development. In: Hartup WW, Silbereisen RK, editors. Growing points in developmental science. New York: Psychology Press; 2002. p. 64–84.
- 7.Fox NA, Henderson HA, Marshall PJ, Nichols KE, Ghera MM. Behavioral inhibition: linking biology and behavior within a developmental framework. Annu Rev Psychol. 2005;56:235–62. [DOI] [PubMed] [Google Scholar]
- 8.Lahat A, Hong M, Fox NA. Behavioural inhibition: is it a risk factor for anxiety? Int Rev Psychiatry. 2011;23:248–57. [DOI] [PubMed] [Google Scholar]
- 9.Lozoff B, Wolf AW, Urrutia JJ, Viteri FE. Abnormal behavior and low developmental test scores in iron-deficient anemic infants. J Dev Behav Pediatr. 1985;6:69–75. [PubMed] [Google Scholar]
- 10.Lozoff B, Klein NK, Prabucki KM. Iron-deficient anemic infants at play. J Dev Behav Pediatr. 1986;7:152–8. [PubMed] [Google Scholar]
- 11.Lozoff B, Jimenez E, Hagen J, Mollen E, Wolf AW. Poorer behavioral and developmental outcome more than 10 years after treatment for iron deficiency in infancy. Pediatrics. 2000;105:E51. [DOI] [PubMed] [Google Scholar]
- 12.Lozoff B, Smith JB, Kaciroti N, Clark KM, Guevara S, Jimenez E. Functional significance of early iron deficiency: outcomes at 25 years. J Pediatr. 2013;163:1260–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rubin KH, Coplan RJ, Bowker JC. Social withdrawal in childhood. Annu Rev Psychol. 2009;60:141–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kagan J, Snidman N, McManis M, Woodward S. Temperamental contributions to the affect family of anxiety. Psychiatr Clin North Am. 2001;24:677–88. [DOI] [PubMed] [Google Scholar]
- 15.Beard J. Iron deficiency alters brain development and functioning. J Nutr. 2003;133 Suppl:1468S–72S. [DOI] [PubMed] [Google Scholar]
- 16.Youdim MB. Brain iron deficiency and excess: cognitive impairment and neurodegeneration with involvement of striatum and hippocampus. Neurotox Res. 2008;14:45–56. [DOI] [PubMed] [Google Scholar]
- 17.Schallert T, Petrie BF, Whishaw IQ. Neonatal dopamine depletion: spared and unspared sensorimotor and attentional disorders and effects of further depletion in adulthood. Psychobiology. 1989;17:386–96. [Google Scholar]
- 18.Weinberg J, Dallman PR, Levine S. Iron deficiency during early development in the rat: behavioral and physiological consequences. Pharmacol Biochem Behav. 1980;12:493–502. [DOI] [PubMed] [Google Scholar]
- 19.Weinberg J, Brett LP, Levine S, Dallman PR. Long-term effects of early iron deficiency on consummatory behavior in the rat. Pharmacol Biochem Behav. 1981;14:447–53. [DOI] [PubMed] [Google Scholar]
- 20.Felt BT, Beard JL, Schallert T, Shao J, Aldridge JW, Connor JR, Georgieff MK, Lozoff B. Persistent neurochemical and behavioral abnormalities in adulthood despite early iron supplementation for perinatal iron deficiency anemia in rats. Behav Brain Res. 2006;171:261–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Berridge KC, Robinson TE. Parsing reward. Trends Neurosci. 2003;26:507–13. [DOI] [PubMed] [Google Scholar]
- 22.Honig AS, Oski FA. Solemnity: a clinical risk index for iron deficient infants. Early Child Dev Care. 1984;16:69–84. [Google Scholar]
- 23.Lozoff B, De Andraca I, Castillo M, Smith J, Walter T, Pino P. Behavioral and developmental effects of preventing iron-deficiency anemia in healthy full-term infants. Pediatrics. 2003;112:846–54. [PubMed] [Google Scholar]
- 24.Lozoff B, Clark KM, Jing Y, Armony-Sivan R, Angelilli ML, Jacobson SW. Dose-response relationships between iron deficiency with or without anemia and infant social-emotional behavior. J Pediatr. 2008;152:696–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lozoff B, Georgieff MK. Iron deficiency and brain development. Semin Pediatr Neurol. 2006;13:158–65. [DOI] [PubMed] [Google Scholar]
- 26.Lozoff B, Castillo M, Clark KM, Smith JB. Iron-fortified vs low-iron infant formula: developmental outcome at 10 years. Arch Pediatr Adolesc Med. 2012;166:208–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Walter T, Pino P, Pizarro F, Lozoff B. Prevention of iron-deficiency anemia: comparison of high- and low-iron formulas in term healthy infants after six months of life. J Pediatr. 1998;132:635–40. [DOI] [PubMed] [Google Scholar]
- 28.Walker SP, Wachs TD, Gardner JM, Lozoff B, Wasserman GA, Pollitt E, Carter JA, Grantham-McGregor S, Engle P, Black M. Child development: risk factors for adverse outcomes in developing countries. Part of a 3-part Child Development series. Lancet. 2007;369:145–57. [DOI] [PubMed] [Google Scholar]
- 29.Looker AC, Dallman P, Carroll MD, Gunter EW, Johnson CL. Prevalence of iron deficiency in the United States. JAMA. 1997;277:973–6. [DOI] [PubMed] [Google Scholar]
- 30.Life Sciences Research Office. Assessment of the iron nutrition status of the U.S. population based on data collected in the Second National Health and Nutrition Survey, 1976–1980. Bethesda (MD): Federation of American Societies for Experimental Biology; 1984.
- 31.Sattler JM. Behavior and attitude checklist. In: Sattler J, editor. Assessment of children. San Diego: Sattler; 1988. p. 372–3.
- 32.Achenbach TM, Edelbrock C. Manual for the Child Behavior Checklist/4–18 and revised Child Behavior Profile. Burlington (VT): University of Vermont; 1991.
- 33.Johnson VK, Cowan PA, Cowan CP. Children's classroom behavior: the unique contribution of family organization. J Fam Psychol. 1999;13:355–71. [Google Scholar]
- 34.Buske-Kirschbaum A, Jobst SPD, Wustmans A, Kirschbaum C, Rauh W, Hellhammer D. Attenuated free cortisol response to psychosocial stress in children with atopic dermatitis. Psychosom Med. 1997;59:419–26. [DOI] [PubMed] [Google Scholar]
- 35.Riley AW, Forrest C, Green B, Rebok G, Starfield B, Robertson J, Keefer M. Manual for the Child Health and Illness Profile-Child Edition (CHIP-CE). Baltimore: Johns Hopkins University; 2001. [Google Scholar]
- 36.Unger EL, Hurst AR, Georgieff MK, Schallert T, Rao R, Connor JR, Kaciroti N, Lozoff B, Felt B. Behavior and monoamine deficits in pre/perinatal iron deficiency are not corrected by early postnatal moderate or high iron diet in the rat. J Nutr. 2012;142:2040–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Georgieff MK. Long-term brain and behavioral consequences of early iron deficiency. Nutr Rev. 2011;69:S43–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Roncagliolo M, Garrido M, Walter T, Peirano P, Lozoff B. Evidence of altered central nervous system development in infants with iron deficiency anemia at 6 mo: delayed maturation of auditory brain stem responses. Am J Clin Nutr. 1998;68:683–90. [DOI] [PubMed] [Google Scholar]
- 39.Algarín C, Peirano P, Garrido M, Pizarro F, Lozoff B. Iron deficiency anemia in infancy: long-lasting effects on auditory and visual systems functioning. Pediatr Res. 2003;53:217–23. [DOI] [PubMed] [Google Scholar]
- 40.Congdon EL, Westerlund A, Algarin CR, Peirano PD, Gregas M, Lozoff B, Nelson CA. Iron deficiency in infancy is associated with altered neural correlates of recognition memory at 10 years. J Pediatr. 2012;160:1027–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Algarín C, Nelson CA, Peirano PD, Westerlund A, Reyes SC, Lozoff B. Iron-deficiency anemia in infancy and poorer cognitive-motor inhibitory control at 10 years. Dev Med Child Neurol. 2013;55:453–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Peirano P, Algarin C, Garrido M, Algarin D, Lozoff B. Iron-deficiency anemia is associated with altered characteristics of sleep spindles in NREM sleep in infancy. Neurochem Res. 2007;32:1665–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Peirano P, Algarin C, Lozoff B, Manconi M, Ferri F. Iron deficiency anemia in infancy exerts long-term effects on the tibialis anterior motor activity during sleep in childhood. Sleep Med. 2012;13:1006–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Peirano PD, Algarin C, Garrido M, Lozoff B. Iron deficiency anemia in infancy is associated with altered temporal organization of sleep states in childhood. Pediatr Res. 2007;62:715–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Felt BT, Peirano P, Algarin C, Chamorro R, Sir T, Kaciroti N, Lozoff B. Long-term neuroendocrine effects of iron-deficiency anemia in infancy. Pediatr Res. 2012;71:707–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.