Abstract
Although there are currently no approved treatments for cancer cachexia, there is an intensified interest in developing therapies because of the high mortality index associated with muscle wasting diseases. Successful treatment of the cachectic patient focuses on improving or maintaining body weight and musculoskeletal function. Nutraceutical compounds, including the natural phytochemical quercetin, are being examined as potential treatments because of their anti-inflammatory, antioxidant, and anticarcinogenic properties. The purpose of this study was to determine the effect of quercetin supplementation on the progression of cachexia in the adenomatous polyposis coli (Apc)Min/+ mouse model of colorectal cancer. At 15 wk of age, C57BL/6 and male ApcMin/+ mice were supplemented with 25 mg/kg of quercetin or vehicle solution mix of Tang juice and water (V) daily for 3 wk. Body weight, strength, neuromuscular performance, and fatigue were assessed before and after quercetin or V interventions. Indicators of metabolic dysfunction and inflammatory signaling were also assessed. During the treatment period, the relative decrease in body weight in the ApcMin/+ mice gavaged with V (ApcMin/+V; −14% ± 2.3) was higher than in control mice gavaged with V (+0.6% ± 1.0), control mice gavaged with quercetin (−2% ± 1.0), and ApcMin/+ mice gavaged with quercetin (ApcMin/+Q; −9% ± 1.3). At 18 wk of age, the loss of grip strength and muscle mass shown in ApcMin/+V mice was significantly attenuated (P < 0.05) in ApcMin/+Q mice. Furthermore, ApcMin/+V mice had an induction of plasma interleukin-6 and muscle signal transducer and activator of transcription 3 phosphorylation, which were significantly (P < 0.05) mitigated in ApcMin/+Q mice, despite having a similar tumor burden. Quercetin treatment did not improve treadmill run-time-to-fatigue, hyperglycemia, or hyperlipidemia in cachectic ApcMin/+ mice. Overall, quercetin supplementation positively affected several aspects of cachexia progression in mice and warrants further exploration as a potential anticachectic therapeutic.
Introduction
Cachexia has been defined as the progressive loss of skeletal muscle mass and fat tissue, which leads to progressive functional impairment of the skeletal system (1). In general, the detriments in muscle mass and strength that occur with the progression of cachexia lead to difficulties in the performance of everyday tasks and, ultimately, a decrease in quality of life. Cachexia incidence with cancer varies between 20 and 80% of patients depending on the cancer type (2). With the rising cost of health insurance and healthcare costs, the development of economically feasible and effective therapeutic interventions has become increasingly important. These treatments could improve quality of life, decrease cancer-linked mortality rates, and minimize the economic burden created by cancer cachexia.
Cancer cachexia (CC)6 can be classified by the degree of body weight loss, metabolic dysfunction, and inflammation present (1). There are 3 distinct stages of CC, known as precachexia, cachexia, and refractory cachexia (1). For cachexia research to achieve useable therapeutic outcomes, it is critically important to distinguish these stages during treatment of CC, and it is also imperative that research studies accurately account for these cachectic stages. Additionally, research examining the therapeutic benefits of cachexia treatments needs to focus on improvements in the wasting condition that are independent of the underlying disease. Unfortunately, a confounder for many research designs examining nutrition and pharmaceutical cachexia treatments is that the treatments are administered at an early time point, which influences the disease development and progression for the induction of cachexia, such as tumor growth.
ApcMin/+ mice, commonly used as a model of CC, have a nonsense mutation in the adenomatous polyposis coli (Apc) gene (3). In fact, the molecular root of the majority of sporadic colon cancer cases in humans is a mutation in this gene, which increases the risk of developing colon cancer because of the spontaneous growth and burden of the polyps in similar form to ApcMin/+ mice (4, 5). ApcMin/+ mice are characterized by the formation of spontaneous intestinal adenomas through 12 wk of age, development of anemia between 10 and 12 wk of age, and systemic inflammation. The slow progression of muscle and fat wasting begins at ~14 wk of age and continues throughout the life span (6, 7). Extensive studies from our laboratory showed that elevated concentrations of IL-6 are associated with the progression of CC leading to reduced muscle oxidative capacity of both slow and fast muscle fibers, and alterations in mitochondrial biogenesis (8–11).
In the war against cancer, substantial attention has been recently given to naturally found nutritional compounds, such as quercetin, because of their anticarcinogenic properties (12–14). Quercetin is a flavanol found mostly in fruits and vegetables. Besides their anticarcinogenic properties, flavanols possess antioxidant, anti-inflammatory, antiviral, psychostimulant, cardio-protective, and neuro-protective properties (15–20). Quercetin was previously shown to be an effective therapeutic treatment for conditions in which the immune system is compromised (21). However, its anticachectic effects have yet to be examined.
The purpose of this study was to determine the effect of quercetin supplementation on the progression of cachexia in the ApcMin/+ mouse model of colon cancer. We initiated quercetin treatment at 15 wk of age after the normal onset of cachexia and after intestinal adenoma formation had peaked. ApcMin/+ mice begin to show body weight loss and increased circulating markers of inflammation (plasma IL-6 and monocyte chemotactic protein-1 concentrations) after 14 wk of age, which are characteristics of a precachectic stage as defined by Fearon et al. (1). We hypothesized that 3 wk of quercetin supplementation after tumor development, and the initiation of cachexia, would attenuate the loss of body weight, fat mass, lean mass, muscular strength, neuromuscular performance, inflammation, and involuntary fatigue—all of which have been associated with the progression of cachexia.
Methods
Mice
C57BL/6 and ApcMin/+ mice were originally obtained from the Jackson Laboratory and were bred at the Animal Resources Facilities at the University of South Carolina. Mice were genotyped for heterozygous expression of the Apc gene by using RT-PCR and then housed 3 to 5 per cage. At 15 wk of age, male C57BL/6 (n = 12) and male ApcMin/+ (n = 10) mice randomly were administered either quercetin (25 mg/kg; Nutravail) mixed with orange-flavored Tang (Kraft Foods) (C57BL/6-Q and ApcMin/+Q, respectively) or an equal volume of vehicle solution mix of Tang juice and water (V) (C57BL/6-V and ApcMin/+V, respectively) alone via gavage daily for a duration of 3 wk. The quercetin dose was selected according to daily consumption of quercetin in Western countries (22) and based on previous doses used in rodent studies (21, 23, 24). The gavage regimen was chosen over a grain-based diet with quercetin to avoid a possible reduction in food consumption in mice with cancer. The duration of quercetin treatment (15–18 wk of age) was limited to 3 wk because of the normal progression of weight loss in ApcMin/+ mice, becoming severe between 18 and 20 wk of age, and to minimize alterations in the overall tumor burden, which were shown previously in this mouse model (25). The number of mice was limited to match age, genotype, and precachectic status. Mice were maintained on a 12:12-h dark:light cycle in 24°C, were given a standard rodent unpurified diet (HarlanTeklad Rodent Diet, no. 8604: 32% protein, 14% fat, and 54% carbohydrate) and consumed water ad libitum. Body weight and food consumption were monitored weekly for the duration of the experiment. Functional tests were evaluated at pretreatment (15 wk of age) and post-treatment (18 wk of age). Metabolic measurements (glucose, insulin, and HOMA index) were assessed in C57BL/6-V, ApcMin/+V, and ApcMin/+Q mice post-treatment. The Institutional Animal Care and Use Committee of the University of South Carolina approved all mouse experimentation.
HPLC analysis of quercetin bioavailability
A subset of C57BL/6 mice (n = 3) were gavaged with 25 mg/kg of quercetin, and blood samples were drawn at various intervals for a total period of 12 h (0.25, 0.5, 1, 2, 4, and 12 h). Blood was centrifuged (3000 × g; 10 min, 4°C) and plasma was processed for quercetin detection. Total plasma quercetin (quercetin and its primary conjugates) was measured following solid-phase extraction via reverse-phase HPLC with UV detection. Quercetin conjugates were hydrolyzed by incubating 500-μL plasma aliquots with 10-μL 10% dl-DTT solution, 50-μL 0.58 mol/L acetic acid, and 50 μL of a mixture of β-glucuronidase and arylsulfatase (Roche Diagnostics) for 18 h at 37°C (pH 5.2). After incubation, 500 μL of 0.01 M oxalic acid was added, and each sample was mixed on a vortex then centrifuged for 5 min at 10,000 × g. Supernatants (1 mL) were then applied to solid-phase extraction cartridges (Oasis HLB 1cc, 30 mg, SPE cartridge; Waters Corp) that were preconditioned with 1-mL methanol (MeOH), 0.5-mL 0.01 M oxalic acid, and 1-mL dH2O, and drawn through at a rate of 0.5 mL/min with the use of a vacuum manifold (Waters Corp). Cartridges were washed twice with 0.5-mL MeOH. Eluant was collected into 1.5-mL microcentrifuge tubes. DTT (10% solution, 10 μL) was added to the combined eluant to prevent oxidation, and the samples were mixed on a vortex for 1 min and placed in a vacuum concentrator (Savant Speed Vac SC 110) until MeOH was completely evaporated. The residue was reconstituted with 150-μL MeOH/dH2O (1:1) and then transferred to 1-mL glass vials with tri-spring inserts. Chromatographic analysis was performed on a Waters Breeze system (Waters Corp) consisting of a Waters 1525 Binary HPLC pump, 717 Plus Autosampler, 2487 UV detector, and Symmetry C18, 5-μm 4.6 × 150-mm column. MeOH and dH2O (80:20, v:v) were used as the mobile phase and flow rate was 0.5 mL/min. Column and sample temperature were maintained at 30°C. Data were acquired and processed with the use of Breeze software (v3.02). Quantification of the quercetin peak was based on the standard addition method.
Functional tests
Grip strength.
Forelimb and hindlimb grip strength testing was conducted on a force (Newtons) transducer (Columbus Instruments). Mice gripped the wire-grated transducer with their front and back limbs and were gently pulled by the tail until they released their hold on the grid. Three sets of 5 consecutive measurements (15 repetitions total) were taken, with a 3- to 5-min rest between sets. The average of all 15 repetitions provided a mean force measurement for each mouse.
Rotorod.
The mice were acclimated to the rotorod (Columbus Instruments) testing procedures for 3 d before the start of testing. Neuromuscular function was examined with the use of a rotorod ramping protocol from 0 to 25 rpm (0.02 × g) over a period of 90 s followed by 25 rpm from 90 to 120 s (26). Each mouse performed the protocol 3 times, and each trial was separated by a 1- to 2-min rest period. The longest time of the 3 tests was recorded for each mouse.
Run-time-to-fatigue.
Mice ran on a motorized treadmill at 20 m/min at a 5% grade with a 15-min warm-up (5 min at 5 m/min, 5 min at 10 m/min, and 5 min at 15 m/min). Fatigue was defined as the time at which mice were no longer able or willing to keep up with the treadmill despite gentle hand prodding for a period of 1 min.
Glucose, insulin, TGs, and IL-6.
Blood samples were collected from the tip of the tail as previously described (26). Blood glucose concentrations were determined in whole blood by using a glucometer (Bayer). Collected blood was centrifuged at 9279 × g for 10 min at 4°C. Plasma was divided and stored at −80°C until analysis. Plasma insulin and IL-6 concentrations were determined by a commercially available ELISA kit (Ultra Sensitive Mouse Insulin Kit, Crystal Chem; and IL-6 Mouse ELISA Kit, Invitrogen, respectively), and a colorimetric kit was used to measure plasma TGs (Infinity Triglycerides Reagent; Thermo Scientific). All assays were performed according to the manufacturer’s instructions. Insulin resistance was estimated by the HOMA index according to the following formula: insulin resistance index = insulin (μU/mL) × glucose (mmol/L)/22.5.
Tissue collection.
At 18 wk of age, mice were given a subcutaneous injection of ketamine-xylazine-acepromazine cocktail (1.4 mL/kg body weight). The hindlimb skeletal muscles (soleus, gastrocnemius, quadriceps), epididymal fat pad, and spleen were excised and weighed. The tibia was removed as a measurement of normal development. The small intestine was dissected, rinsed with PBS, and the most distal portion was fixed in 10% buffered formalin (Fisher) for tumor count (22).
Western blot analysis.
Quadriceps muscles were homogenized in mueller buffer (8) containing a cocktail protease inhibitor (Sigma), and total protein concentrations were determined by the Bradford method. Crude-muscle homogenate (50 g) was fractioned on 8 to 15% SDS-polyacrylamide gels. Gels were transferred (70 V) to a polyvinylidene difluoride membrane overnight. Membranes were stained with Ponceau to verify equal protein loading to each gel. Membranes were blocked for 1 h in a 5% milk, tris-buffer saline solution with 0.1% Tween-20 (TBS-T). Primary antibodies for NF-κB (phospho NF-κB p65, total NF-κB p65; Cell Signaling, Cat #3039 and #4764) and signal transducer and activator of transcription 3 (STAT3) (phospho STAT3, total STAT3; Cell Signaling, Cat #9138 and #9139) were diluted 1:1000 in a 5% milk, TBS-T solution followed by an overnight incubation at 4°C. Anti-rabbit or anti-mouse IgG horseradish peroxidase conjugated secondary antibody (Cell Signaling, Cat #7047, 7076) was incubated with the membranes at 1:2000 dilutions for 1 h in a 5% milk, TBS-T solution. Enhance chemiluminescence (GE Healthcare Life Sciences) was used to visualize the antibody-antigen interaction. The images were scanned, and blots were quantified by densitometry using scientific imaging software (Image J) and normalized to C57BL/6-V.
Statistical analysis
Data were analyzed by using commercially available statistical software (SigmaStat; SPSS). Multiple tests were used to analyze the data because of the study design. A 2-factor ANOVA (genotype × treatment) was used to determine differences in the percentage change of body weight, fat mass, muscle mass, organ mass, tibia length, polyp counts, and protein concentration. A repeated-measure 2-factor ANOVA (time × treatment) was used to determine changes in grip strength, neuromuscular function, and the run-time-to-fatigue test pre- and post-treatment. A 1-factor ANOVA was used to determine differences in metabolic measurements. Because of limitations encountered during the study, the following metabolic measurements were only collected in C57BL/6-V, ApcMin/+V, and ApcMin/+Q mice: glucose, insulin, HOMA index, and TGs. A Student-Newman-Keuls test was used for posthoc analyses. Any statistical test that did not pass the equal-variance test (Levene’s test) was subject to a Kruskal-Wallis test (insulin and HOMA measurements), or transformed (spleen, tibia length, and polyps). Analysis for phospho STAT3 showed unequal homogeneity even after transformation, and because differences in variance was between genotypes, we looked at the simple effect of treatment (t test) (27). Data are presented as means ± SEMs and the level of significance was set at P < 0.05. Effect sizes (ESs) were reported for statistically significant findings.
Results
Quercetin bioavailability.
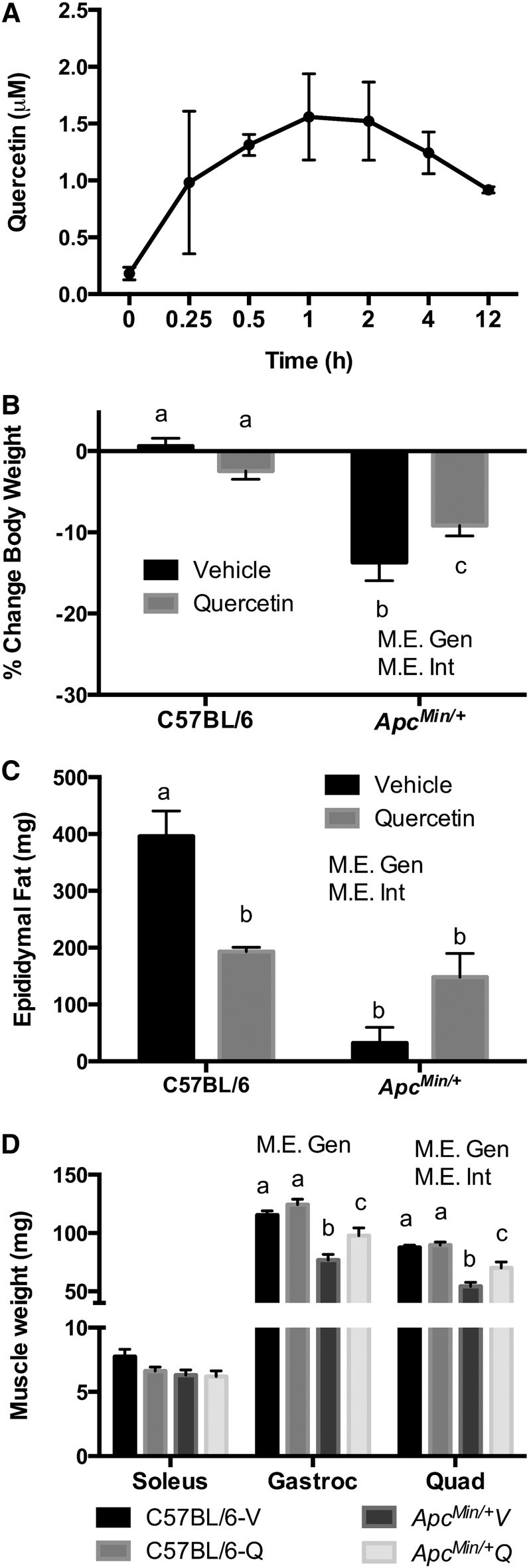
To determine the bioavailability of quercetin in plasma, quercetin was given to C57BL/6 mice via gavage (Fig. 1A). Quercetin appeared in systemic circulation at 15 min, reached its highest plasma concentration at 1 h, and was detected in plasma 12 h after administration.
FIGURE 1.
Quercetin bioavailability in plasma in C57BL/6 mice and percentage change in body weight, fat, and muscle mass in C57BL/6 and ApcMin/+ mice supplemented with vehicle or quercetin via oral gavage. Plasma quercetin concentrations (n = 3/time point) (A). Percentage change in body weight pre- and post-intervention (B), epididymal fat (C), and muscle mass (D) at 18 wk of age. Values are means ± SEMs, n = 4–8/group. Means without a common letter differ, P < 0.05. Apc, adenomatous polyposis coli; ApcMin/+Q, ApcMin/+ mice gavaged with quercetin; ApcMin/+V, ApcMin/+ mice gavaged with vehicle solution mix of Tang juice and water; C57BL/6-Q, control mice gavaged with quercetin; C57BL/6-V, control mice gavaged with vehicle solution mix of Tang juice and water; Gastroc, gastrocnemeous; Gen, genotype; Int, interaction; M.E., main effect; Quad, quadriceps; vehicle, vehicle solution mix of Tang juice and water.
Body composition and cachexia-related symptoms in ApcMin/+ mice supplemented with quercetin.
At baseline (age 15 wk), ApcMin/+ mice had initiated body weight loss; body weights were 1 to 4% less than their measured peak weight (data not shown). This loss categorized them in the precachectic stage according to the most recent definition of cachexia (1). The percentage change in body weight was calculated pre- and post-intervention (15 and 18 wk of age). The percentage change in body weight for each mouse was used instead of absolute body weight changes because C57BL/6 mice weighed more than ApcMin/+ mice at the start of the study. Absolute group mean body weight data, however, are presented in Supplemental Table 1. C57BL/6 mice did not exhibit any significant change in percentage of body weight during 3 wk of V or quercetin supplementation (Fig. 1B). In contrast, ApcMin/+V mice exhibited a 14% ± 2.3 loss in body weight, corresponding to the development of CC. Although quercetin supplementation in ApcMin/+Q mice significantly attenuated body weight loss (−9% ± 1.3) in comparison with ApcMin/+V mice (P < 0.05) (ES: 2.2), it was unable to block cachexia (Fig. 1B). We were unable to calculate individual food intake because mice were not housed individually. However, in general, we did not observe any differences in food intake (food consumed by mice in each cage/number of mice in cage) among the groups over the course of the study (Supplemental Table 2).
Regarding epididymal fat weight, C57BL/6-Q mice had a significant reduction in fat mass compared with C57BL/6-V mice (P < 0.05) (ES: 4.5). As expected, with the development of cachexia, ApcMin/+V mice had significantly less epididymal fat than C57BL/6-V mice (P < 0.05) (ES: 8.2). Quercetin supplementation did not attenuate the loss of epididymal fat in ApcMin/+Q mice (Fig. 1C).
We also examined the weight of several hindlimb muscles at the end of the study. There was no difference in the weight of the oxidative soleus muscle between the C57BL/6 and ApcMin/+ mice (Fig. 1D). Conversely, the gastrocnemius and quadriceps muscle weights were significantly decreased in the ApcMin/+ mice when compared with the C57BL/6 mice (P < 0.05) (ES: 8.0 and 4.7, respectively). However, quercetin supplementation significantly attenuated gastrocnemius and quadriceps muscle mass loss in ApcMin/+Q mice (P < 0.05) (ES: 4.3 and 2.0, respectively). The changes observed in the muscle mass of ApcMin/+ mice were independent of body size, as determined by tibia length (Table 1).
TABLE 1.
The effect of quercetin on cachexia-related symptoms in C57BL/6 and Min/+ mice1
| C57BL/6 |
ApcMin/+ |
P values for 2-factor ANOVA |
|||||
| Vehicle (n = 8) | Quercetin (n = 4) | Vehicle (n = 5) | Quercetin (n = 5) | G | T | G × T | |
| Spleen, mg | 81 ± 4.2a | 88 ± 1.6a | 435 ± 45b | 378 ± 18b | <0.01 | 0.23 | 0.23 |
| Heart, mg | 119 ± 12.7 | 113 ± 4.9 | 113 ± 9.6 | 100 ± 2.1 | 0.16 | 0.13 | 0.97 |
| Testes, mg | 196 ± 8.0a | 193 ± 7.2a | 142 ± 14b | 183 ± 11a | 0.01 | 0.11 | 0.02 |
| Tibia length, mm | 17.2 ± 0.1 | 17.3 ± 0.1 | 17.1 ± 0.1 | 17.1 ± 0.3 | 0.31 | 0.88 | 0.99 |
| Polyps, n | 1 ± 1.0a | 1 ± 0.7a | 28 ± 14b | 14 ± 10b | 0.01 | 0.20 | 0.20 |
Values are means ± SEs. Labeled means in a row without a common letter differ, P ≤ 0.05. Apc, adenomatous polyposis coli; G, genotype; T, treatment; vehicle, vehicle solution mix of Tang juice and water.
Mass of the spleen, heart, and testes was measured at the end of the study as a sign of inflammation, heart hypertrophy, and hypogonadism, respectively (Table 1). No differences were observed in the weight of the spleen, heart, and testes of C57BL/6-V and C57BL/6-Q mice. In contrast, ApcMin/+V and ApcMin/+Q mice had a significant increase in spleen weight at 18 wk of age when compared with C57BL/6-V and C57BL/6-Q mice (P < 0.05) (ES: 7.9). Surprisingly, quercetin supplementation prevented hypogonadism in ApcMin/+Q mice (P < 0.05) (ES: 2.9). On the other hand, heart mass and tibia length were not different between C57BL/6 and ApcMin/+ mice. Additionally, no significant difference was observed in tumor number between ApcMin/+V and ApcMin/+Q mice.
Functional status of ApcMin/+ mice supplemented with quercetin.
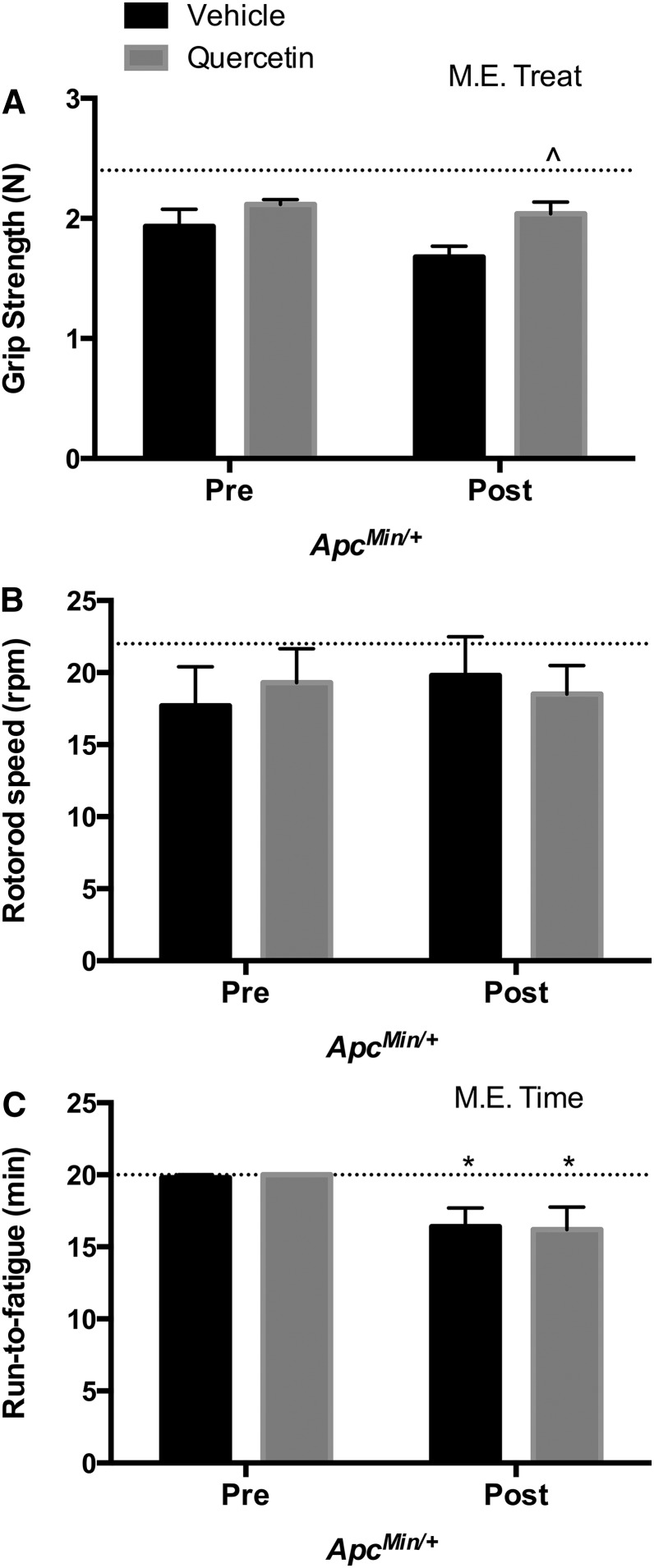
We examined the effects of quercetin on functional measurements related to volitional muscular strength, neuromuscular function, and fatigue. These variables were examined pre- and post-intervention in both C57BL/6 and ApcMin/+ mice. C57BL/6 mice treated with V or quercetin supplementation demonstrated no changes during the study in grip strength, rotorod, or run-time-to-fatigue (data not shown). Regarding ApcMin/+ mice, at baseline (pre-intervention), all ApcMin/+ mice had similar responses to grip strength, rotorod, and run-time-to-fatigue tests. Corresponding with the development of cachexia, ApcMin/+V mice had significant decrements in muscular strength at 18 wk of age (post-intervention) when compared with ApcMin/+Q mice (P < 0.05) (ES: 5.0) (Fig. 2A). Neither V nor quercetin supplementation affected neuromuscular function in ApcMin/+ mice (Fig. 2B). Post-intervention, both ApcMin/+V and ApcMin/+Q mice demonstrated a significant decrease in run-time-to-fatigue over time (P < 0.05) (ES: 2.9 and 2.5, respectively) (Fig. 2C).
FIGURE 2.
Functional tests in ApcMin/+ mice supplemented with vehicle or quercetin via oral gavage at 15 and 18 wk of age. Grip strength (A), rotorod (B), and run-time-to-fatigue tests (C). Values are means ± SEMs, n = 5/group. Dotted line represents the mean of C57BL/6 mice. *P < 0.05, significant difference from ApcMin/+V and ApcMin/+Q pre-intervention. ^P < 0.05, significant difference from ApcMin/+V post-intervention. Apc, adenomatous polyposis coli; ApcMin/+Q, ApcMin/+ mice gavaged with quercetin; ApcMin/+V, ApcMin/+ mice gavaged with vehicle solution mix of Tang juice and water; M.E., main effect; Post, 18 wk of age; Pre, 15 wk of age; Treat, treatment; vehicle, vehicle solution mix of Tang juice and water.
Metabolic profile of ApcMin/+ mice supplemented with quercetin.
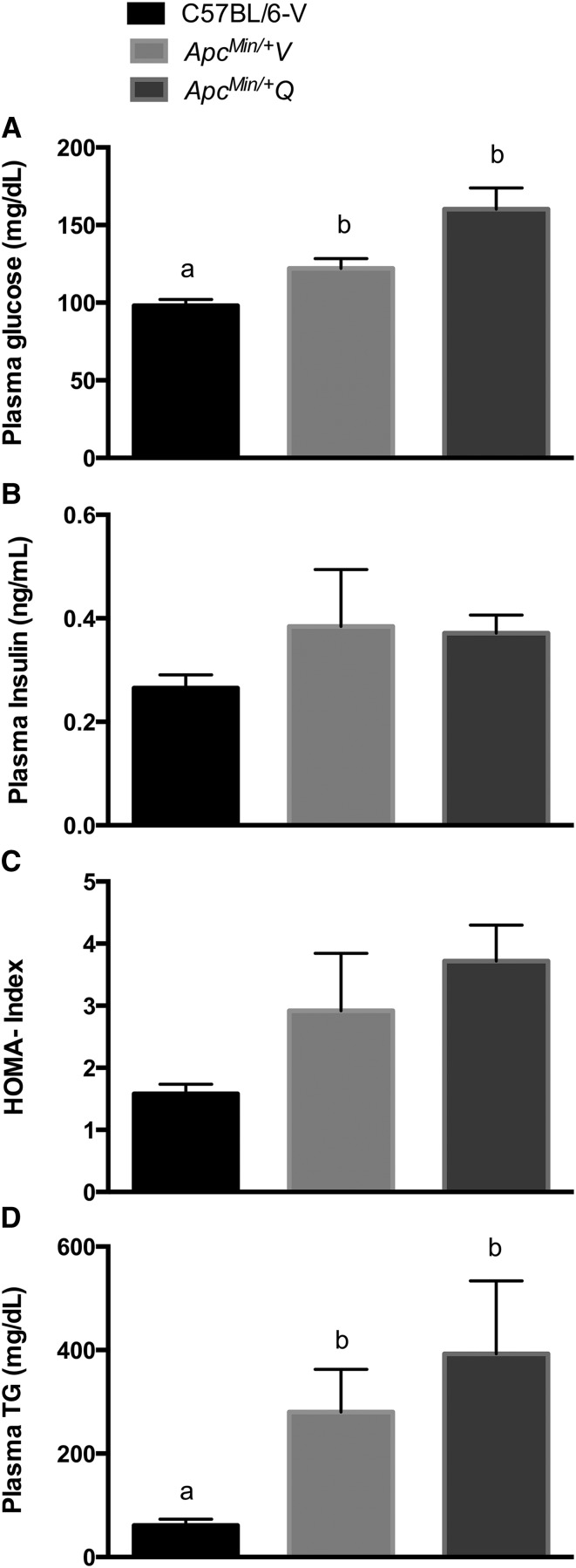
ApcMin/+V and ApcMin/+Q mice had significantly elevated concentrations of plasma glucose and TGs post-intervention when compared with C57BL/6-V mice (P < 0.05) (ES: ApcMin/+V = 1.7; ApcMin/+Q = 2.3; C57BL/6-V = 1.1; C57BL/6-Q = 1.7) (Fig. 3A-D). No differences were observed between C57BL/6-V, ApcMin/+V, and ApcMin/+Q mice with regard to plasma insulin concentration and HOMA index (Fig. 3B–C).
FIGURE 3.
Metabolic measurements in C57BL/6-V and ApcMin/+ mice supplemented with vehicle or quercetin via oral gavage at 18 wk of age. Blood glucose (A), plasma insulin (B), HOMA index (C), and plasma TGs (D). Values are means ± SEMs, n = 4–7/group. Means without a common letter differ, P < 0.05. Apc, adenomatous polyposis coli; ApcMin/+Q, ApcMin/+ mice gavaged with quercetin; ApcMin/+V, ApcMin/+ mice gavaged with vehicle solution mix of Tang juice and water; C57BL/6-V, control mice gavaged with vehicle solution mix of Tang juice and water; vehicle, vehicle solution mix of Tang juice and water.
Inflammatory status of ApcMin/+ mice supplemented with quercetin.
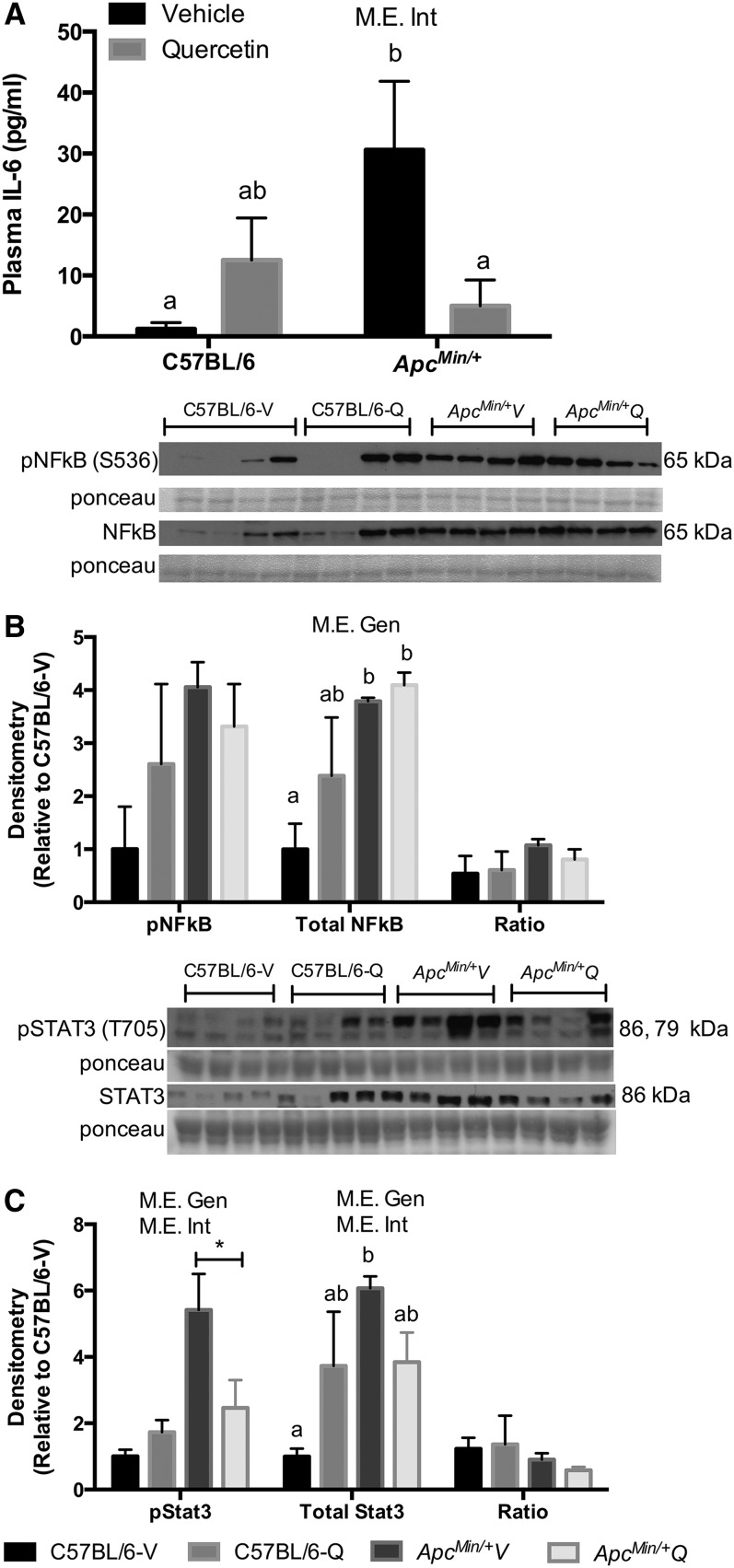
We determined the effects of quercetin on indices of systemic and muscle inflammation. Plasma concentration of the cytokine IL-6 and quadriceps muscle total and phosphorylated NF-κB and STAT3 expression were examined. These inflammation markers are induced with the development of cachexia in ApcMin/+ mice (8, 26). As previously reported, the concentration of plasma IL-6 and the phosphorylation of STAT3 were lower in C57BL/6 mice, when compared with ApcMin/+ mice (Fig. 4A–C). We did not observe any changes in NF-κB activation with quercetin supplementation. Interestingly, quercetin supplementation significantly reduced plasma IL-6 concentration and STAT3 activation (P < 0.05) (ES: 1.0 and 1.4, respectively).
FIGURE 4.
Systemic and local inflammatory signaling of C57BL/6 and ApcMin/+ mice supplemented with vehicle or quercetin via oral gavage at 18 wk of age. Plasma concentration of IL-6, values are means ± SEMs, n = 4–8/group (A). Activation of NF-κB, values are means ± SEMs, n = 4/group (B). STAT3, values are means ± SEMs, n = 4/group (C) in the quadriceps muscle. Means without a common letter differ, P < 0.05. *P < 0.05, significant differences between ApcMin/+V and ApcMin/+Q. Apc, adenomatous polyposis coli; ApcMin/+Q, ApcMin/+ mice gavaged with quercetin; ApcMin/+V, ApcMin/+ mice gavaged with vehicle solution mix of Tang juice and water; C57BL/6-Q, control mice gavaged with quercetin; C57BL/6-V, control mice gavaged with vehicle solution of Tang juice and water; Gen, genotype; Int, interaction; M.E., main effect; pNF-κB, phosphorylated NF-κB; pSTAT3, phosphorylated signal transducer and activator of transcription 3; STAT3, signal transducer and activator of transcription 3; vehicle, vehicle solution mix of Tang juice and water.
Discussion
Over the last decade, the flavonoid quercetin has garnered substantial attention from researchers interested in its potent anti-inflammatory properties. The NF-κB pathway has been linked to quercetin’s anti-inflammatory characteristics. Quercetin also has the capacity to alter proinflammatory cytokine production, including IL-6 (28, 29). Most recently, quercetin was found to block IL-6 activation on glioblastoma cells by inhibiting glycoprotein 130, janus kinase (JAK) 1, and STAT3 phosphorylation (30). Although the effect that quercetin has on tumor burden and cancer progression has been examined (21, 25), there is a paucity of research on the influence of quercetin administration on the development of cachexia. Thus, our study provides novel and important information on the potential therapeutic impact of quercetin supplementation for the treatment of CC through the modulation of inflammation in the ApcMin/+ mouse model of CC. Quercetin supplementation in mice improved body weight, muscular strength, and muscle mass retention, prevented hypogonadism, blunted systemic IL-6 concentrations, and prevented muscle STAT3 phosphorylation independent of tumor burden and anorexia. However, we did not observe any diminution in spleen mass. Previous studies in ApcMin/+ mice have shown substantial splenomegaly starting as early as 12 wk of age (31). Our treatment with quercetin started at 15 wk of age, which may explain why quercetin was not able to prevent spleen enlargement measured at 18 wk of age. Although the sample size in the current study was not large related to the complexity of the model, our investigative team’s prior studies examining either anti-inflammatory or nutraceutical treatments in mouse disease models suggest that the sample size provides sufficient power for the main outcomes analyzed in the study. Additionally, we observed large effect sizes, which may mitigate any problem related to sample size for most questions.
The bioavailability of polyphenols, including quercetin, has been studied in detail because of their potential health benefits (32, 33). In this study we were able to measure quercetin in plasma 12 h after oral gavage. Other studies in humans and rodents have demonstrated that quercetin is absorbed in plasma and can accumulate in tissues such as the liver, kidney, muscle, heart, and brain (33–36). The accumulation of quercetin may be a result of the slow removal of quercetin metabolites, which allows quercetin to be in plasma as long as 28 h (34, 35). Quercetin bioavailability in tissue was not measured in this experiment, therefore, we cannot eliminate the possibility that other organs such as the liver may be involved in driving cachexia in this mouse model as seen in C-26 tumor-bearing mice (37).
Although the molecular mechanisms responsible for the onset and progression of cachexia still need to be defined, it is clear that increased inflammation, through the activation of the NF-κB and JAK/STAT3 signaling pathways, plays a key role in cachexia’s detrimental influence on bodily tissues (38–41). In fact, cachectic ApcMin/+ mice exhibit an induction of the muscle NF-κB and JAK/STAT3 signaling pathways, which coincides with an increase in systemic inflammation (26, 38, 39). For instance, IL-6–initiated signaling is associated with metabolic changes that impede protein synthesis and exacerbate protein degradation pathways (8, 42, 43). We have shown in mice that a global knockout of the interleukin-6 (IL6) gene can block the initiation of cachexia, and treatment with an IL-6 receptor antibody can result in the inhibition of further muscle loss in later stages of cachexia (8, 11). On the other hand, IL-6 overexpression was shown to accelerate cachexia development (8, 11). Based on our current results, we speculate that quercetin supplementation was involved in the decrease of plasma IL-6 concentrations and muscle phospho STAT3 in ApcMin/+Q mice. Further work is needed to determine whether the preservation of muscle mass and strength was made possible through the blocking of the IL-6/STAT3 signaling. Likely quercetin targets for further investigation include IL-6 receptor expression, and glycoprotein 130 expression, in cachectic tissues including skeletal muscle. There is the possibility that other inflammatory molecules, including TNFα and monocyte chemotactic protein-1, may play a role in the development of cachexia (44–48). Further experiments are therefore required to establish the regulation of these signal mediators by quercetin.
CC has been known to preferentially catabolize glycolytic muscle in contrast to oxidative muscle (9, 26, 49), which can be interpreted as low or high tolerance, respectively, for systemic inflammation and metabolic alterations (50). Our results exhibit similar patterns; the mass of the oxidative soleus muscle did not change, however, substantial reductions in the mass of gastrocnemius and quadriceps muscles were evident in ApcMin/+V mice. Quercetin supplementation, however, prevented wasting of these glycolytic muscles. IL-6–dependent CC shows evidence of a decrease in oxidative capacity, disruption of the mitochondrial fission and fusion machinery, and a hindering of mitochondrial biogenesis in the skeletal muscle of severely cachectic ApcMin/+ mice (9). It is plausible that quercetin preserved skeletal muscle oxidative capacity through its ability to increase mitochondrial biogenesis and increase oxidative capacity, as previously shown in both humans and rodents (51). However, a recent study showed that increased muscle mitochondrial biogenesis was not sufficient to prevent CC in the Lewis lung carcinoma model (52). Although not in the scope of the current study, further investigation is warranted to understand whether the preservation of muscle mass and strength by quercetin administration are related to improved muscle oxidative capacity.
Hypogonadism is a characteristic of many advanced cancer patients. This condition has been associated with fatigue, weakness, high concentrations of plasma IL-6, and low concentrations of testosterone (53–55). However, scientists have not agreed on the causation of testis atrophy; some hypotheses include cancer treatment and inflammation as possible culprits responsible for hypogonadism (55, 56). In this study, testis atrophy was completely blocked by quercetin supplementation. This result may be due to the fact that quercetin significantly reduced plasma IL-6 concentrations in ApcMin/+ mice. However, more studies are necessary to identify if quercetin can maintain endogenous concentrations of testosterone in cachectic mice as was shown with the hypogonadism model of streptozotocin-induced diabetic rats (57).
Metabolic disturbances including high plasma concentrations of glucose and TGs, and insulin resistance, are a hallmark of cachexia. Although quercetin previously was shown to attenuate insulin resistance in human adipocytes (58), rats (57), and mice (59), in this study the benefits of quercetin administration on muscle wasting were independent of any effect on alterations in insulin resistance, glucose, or TG metabolism. Additionally, the quercetin treatment did not prevent adipose tissue loss, despite the low concentration of IL-6 in plasma. This suggests that fat loss can occur independent of systemic concentration of IL-6 in this cachectic model. These variations on quercetin’s effect on metabolism are most likely results of differences in model, dose, availability, and means of quercetin administration when comparing across studies.
In summary, quercetin supplementation blunted the IL-6/STAT3 inflammatory signaling pathway in the ApcMin/+ mouse model of CC, which was associated with improvements in body weight, muscle mass, and strength. We speculate that quercetin hindered CC given its ability to dampen the inflammatory response that was linked to cachexia in this mouse model. This study provides support for the further development of quercetin as an anticachectic treatment. Future research should focus on dose-response, efficacy studies, and specific mechanisms responsible for the anticachectic benefits of quercetin.
Supplementary Material
Acknowledgments
The authors thank Dr. Jack Ginsberg for his statistical expertise. K.T.V., J.A.C., E.A.M., and J.M.D. designed the research; K.T.V. and R.T.E. conducted the research; K.T.V., R.T.E., A.A.N., and M.J.P. analyzed the data; K.T.V., R.T.E., and J.A.C. wrote the paper; and J.A.C. was responsible for the final content. All authors read and approved the final manuscript.
Footnotes
Abbreviations used: Apc, adenomatous polyposis coli; ApcMin/+Q, ApcMin/+ mice gavaged with quercetin; ApcMin/+V, ApcMin/+ mice gavaged with vehicle solution mix of Tang juice and water; CC, cancer cachexia; C57BL/6-Q, control mice gavaged with quercetin; C57BL/6-V, control mice gavaged with vehicle solution mix of Tang juice and water; ES, effect size; IL6, interleukin-6; JAK, janus kinase; STAT3, signal transducer and activator of transcription 3; TBS-T, tris-buffer saline solution with 0.1% Tween-20; V, vehicle solution mix of Tang juice and water.
Literature Cited
- 1.Fearon K, Strasser F, Anker SD, Bosaeus I, Bruera E, Fainsinger RL, Jatoi A, Loprinzi C, MacDonald N, Mantovani G, http://www.ncbi.nlm.nih.gov/pubmed?term=Davis%20M%5BAuthor%5D&cauthor=true&cauthor_uid=21296615et al. Definition and classification of cancer cachexia: an international consensus. Lancet Oncol. 2011;12:489–95. [DOI] [PubMed]
- 2.Dewys WD, Begg C, Lavin PT, Band PR, Bennett JM, Bertino JR, Cohen MH, Douglass HO Jr., Engstrom PF, Ezdinli EZ, http://www.ncbi.nlm.nih.gov/pubmed?term=Horton%20J%5BAuthor%5D&cauthor=true&cauthor_uid=7424938et al. Prognostic effect of weight loss prior to chemotherapy in cancer patients. Eastern Cooperative Oncology Group. Am J Med. 1980;69:491–7. [DOI] [PubMed]
- 3.Moser AR, Pitot HC, Dove WF. A dominant mutation that predisposes to multiple intestinal neoplasia in the mouse. Science. 1990;247:322–4. [DOI] [PubMed] [Google Scholar]
- 4.Powell SM, Zilz N, Beazer-Barclay Y, Bryan TM, Hamilton SR, Thibodeau SN, Vogelstein B, Kinzler KW. APC mutations occur early during colorectal tumorigenesis. Nature. 1992;359:235–7. [DOI] [PubMed] [Google Scholar]
- 5.McCart AE, Vickaryous NK, Silver A. Apc mice: models, modifiers and mutants. Pathol Res Pract. 2008;204:479–90. [DOI] [PubMed] [Google Scholar]
- 6.Su LK, Kinzler KW, Vogelstein B, Preisinger AC, Moser AR, Luongo C, Gould KA, Dove WF. Multiple intestinal neoplasia caused by a mutation in the murine homolog of the APC gene. Science. 1992;256:668–70. [DOI] [PubMed] [Google Scholar]
- 7.Corpet DE, Pierre F. Point: from animal models to prevention of colon cancer. Systematic review of chemoprevention in min mice and choice of the model system. Cancer Epidemiol Biomarkers Prev. 2003;12:391–400. [PMC free article] [PubMed] [Google Scholar]
- 8.White JP, Baynes JW, Welle SL, Kostek MC, Matesic LE, Sato S, Carson JA. The regulation of skeletal muscle protein turnover during the progression of cancer cachexia in the Apc(Min/+) mouse. PLoS ONE. 2011;6:e24650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.White JP, Baltgalvis KA, Puppa MJ, Sato S, Baynes JW, Carson JA. Muscle oxidative capacity during IL-6-dependent cancer cachexia. Am J Physiol Regul Integr Comp Physiol. 2011;300:R201–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baltgalvis KA, Berger FG, Pena MM, Davis JM, Carson JA. Effect of exercise on biological pathways in ApcMin/+ mouse intestinal polyps. J Appl Physiol. 2008;104:1137–43. [DOI] [PubMed] [Google Scholar]
- 11.Baltgalvis KA, Berger FG, Pena MM, Davis JM, Muga SJ, Carson JA. Interleukin-6 and cachexia in ApcMin/+ mice. Am J Physiol Regul Integr Comp Physiol. 2008;294:R393–401. [DOI] [PubMed] [Google Scholar]
- 12.Sharmila G, Bhat FA, Arunkumar R, Elumalai P, Raja Singh P, Senthilkumar K, Arunakaran J. Chemopreventive effect of quercetin, a natural dietary flavonoid on prostate cancer in in vivo model. Clin Nutr. 2014;66:138–46. [DOI] [PubMed] [Google Scholar]
- 13.Tabaczar S, Pieniazek A, Czepas J, Piasecka-Zelga J, Gwozdzinski K, Koceva-Chyla A. Quercetin attenuates oxidative stress in the blood plasma of rats bearing DMBA-induced mammary cancer and treated with a combination of doxorubicin and docetaxel. Gen Physiol Biophys. 2013;32:535–43. [DOI] [PubMed] [Google Scholar]
- 14.Russo GL, Russo M, Spagnuolo C, Tedesco I, Bilotto S, Iannitti R, Palumbo R. Quercetin: a pleiotropic kinase inhibitor against cancer. Cancer Treat Res. 2014;159:185–205. [DOI] [PubMed] [Google Scholar]
- 15.Zern TL, Wood RJ, Greene C, West KL, Liu Y, Aggarwal D, Shachter NS, Fernandez ML. Grape polyphenols exert a cardioprotective effect in pre- and postmenopausal women by lowering plasma lipids and reducing oxidative stress. J Nutr. 2005;135:1911–7. [DOI] [PubMed] [Google Scholar]
- 16.Alexander SP. Flavonoids as antagonists at A1 adenosine receptors. Phytother Res. 2006;20:1009–12. [DOI] [PubMed] [Google Scholar]
- 17.Mattivi F, Guzzon R, Vrhovsek U, Stefanini M, Velasco R. Metabolite profiling of grape: flavonols and anthocyanins. J Agric Food Chem. 2006;54:7692–702. [DOI] [PubMed] [Google Scholar]
- 18.Harwood M, Danielewska-Nikiel B, Borzelleca JF, Flamm GW, Williams GM, Lines TC. A critical review of the data related to the safety of quercetin and lack of evidence of in vivo toxicity, including lack of genotoxic/carcinogenic properties. Food Chem Toxicol. 2007;45:2179–205. [DOI] [PubMed] [Google Scholar]
- 19.Utesch D, Feige K, Dasenbrock J, Broschard TH, Harwood M, Danielewska-Nikiel B, Lines TC. Evaluation of the potential in vivo genotoxicity of quercetin. Mutat Res. 2008;654:38–44. [DOI] [PubMed] [Google Scholar]
- 20.Davis JM, Murphy EA, Carmichael MD, Davis B. Quercetin increases brain and muscle mitochondrial biogenesis and exercise tolerance. Am J Physiol Regul Integr Comp Physiol. 2009;296:R1071–7. [DOI] [PubMed] [Google Scholar]
- 21.Camargo CA, da Silva ME, da Silva RA, Justo GZ, Gomes-Marcondes MC, Aoyama H. Inhibition of tumor growth by quercetin with increase of survival and prevention of cachexia in Walker 256 tumor-bearing rats. Biochem Biophys Res Commun. 2011;406:638–42. [DOI] [PubMed] [Google Scholar]
- 22.Russo GL. Ins and outs of dietary phytochemicals in cancer chemoprevention. Biochem Pharmacol. 2007;74:533–44. [DOI] [PubMed] [Google Scholar]
- 23.Gupta C, Tripathi DN, Vikram A, Ramarao P, Jena GB. Quercetin inhibits diethylnitrosamine-induced hepatic preneoplastic lesions in rats. Nutr Cancer. 2011;63:234–41. [DOI] [PubMed] [Google Scholar]
- 24.Monteiro MM, Franca-Silva MS, Alves NF, Porpino SK, Braga VA. Quercetin improves baroreflex sensitivity in spontaneously hypertensive rats. Molecules. 2012;17:12997–3008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Murphy EA, Davis JM, McClellan JL, Carmichael MD. Quercetin's effects on intestinal polyp multiplicity and macrophage number in the Apc(Min/+) mouse. Nutr Cancer. 2011;63:421–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Puppa MJ, White JP, Velazquez KT, Baltgalvis KA, Sato S, Baynes JW, Carson JA. The effect of exercise on IL-6-induced cachexia in the Apc (Min/+) mouse. J Cachexia Sarcopenia Muscle. 2012;3:117–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Keppel G, Wickens TD. Design and analysis: a researcher’s handbook. 4th ed. Upper Saddle River (NJ): Pearson; 2004.
- 28.Gupta SC, Sundaram C, Reuter S, Aggarwal BB. Inhibiting NF-kappaB activation by small molecules as a therapeutic strategy. Biochim Biophys Acta. 2011;1799:775–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Min YD, Choi CH, Bark H, Son HY, Park HH, Lee S, Park JW, Park EK, Shin HI, Kim SH. Quercetin inhibits expression of inflammatory cytokines through attenuation of NF-kappaB and p38 MAPK in HMC-1 human mast cell line. Inflamm Res. 2007;56:210–5. [DOI] [PubMed] [Google Scholar]
- 30.Michaud-Levesque J, Bousquet-Gagnon N, Beliveau R. Quercetin abrogates IL-6/STAT3 signaling and inhibits glioblastoma cell line growth and migration. Exp Cell Res. 2012;318:925–35. [DOI] [PubMed] [Google Scholar]
- 31.You S, Ohmori M, Pena MM, Nassri B, Quiton J, Al-Assad ZA, Liu L, Wood PA, Berger SH, Liu Z, et al. Developmental abnormalities in multiple proliferative tissues of Apc(Min/+) mice. Int J Exp Pathol. 2006;87:227–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Manach C, Williamson G, Morand C, Scalbert A, Remesy C. Bioavailability and bioefficacy of polyphenols in humans. I. Review of 97 bioavailability studies. Am J Clin Nutr. 2005;81:230S–42S. [DOI] [PubMed] [Google Scholar]
- 33.Mukai R, Fujikura Y, Murota K, Uehara M, Minekawa S, Matsui N, Kawamura T, Nemoto H, Terao J. Prenylation enhances quercetin uptake and reduces efflux in Caco-2 cells and enhances tissue accumulation in mice fed long-term. J Nutr. 2013;143:1558–64. [DOI] [PubMed] [Google Scholar]
- 34.Conquer JA, Maiani G, Azzini E, Raguzzini A, Holub BJ. Supplementation with quercetin markedly increases plasma quercetin concentration without effect on selected risk factors for heart disease in healthy subjects. J Nutr. 1998;128:593–7. [DOI] [PubMed] [Google Scholar]
- 35.Moon JH, Nakata R, Oshima S, Inakuma T, Terao J. Accumulation of quercetin conjugates in blood plasma after the short-term ingestion of onion by women. Am J Physiol Regul Integr Comp Physiol. 2000;279:R461–7. [DOI] [PubMed] [Google Scholar]
- 36.Hollman PC, de Vries JH, van Leeuwen SD, Mengelers MJ, Katan MB. Absorption of dietary quercetin glycosides and quercetin in healthy ileostomy volunteers. Am J Clin Nutr. 1995;62:1276–82. [DOI] [PubMed] [Google Scholar]
- 37.Jones A, Friedrich K, Rohm M, Schafer M, Algire C, Kulozik P, Seibert O, Muller-Decker K, Sijmonsma T, Strzoda D, http://www.ncbi.nlm.nih.gov/pubmed?term=Sticht%20C%5BAuthor%5D&cauthor=true&cauthor_uid=23307490et al. TSC22D4 is a molecular output of hepatic wasting metabolism. EMBO Mol Med. 2013;5:294–308. [DOI] [PMC free article] [PubMed]
- 38.Zhou W, Jiang ZW, Tian J, Jiang J, Li N, Li JS. Role of NF-kappaB and cytokine in experimental cancer cachexia. World J Gastroenterol. 2003;9:1567–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Watchorn TM, Dowidar N, Dejong CH, Waddell ID, Garden OJ, Ross JA. The cachectic mediator proteolysis inducing factor activates NF-kappaB and STAT3 in human Kupffer cells and monocytes. Int J Oncol. 2005;27:1105–11. [PubMed] [Google Scholar]
- 40.Bonetto A, Aydogdu T, Kunzevitzky N, Guttridge DC, Khuri S, Koniaris LG, Zimmers TA. STAT3 activation in skeletal muscle links muscle wasting and the acute phase response in cancer cachexia. PLoS ONE. 2011;6:e22538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bonetto A, Aydogdu T, Jin X, Zhang Z, Zhan R, Puzis L, Koniaris LG, Zimmers TA. JAK/STAT3 pathway inhibition blocks skeletal muscle wasting downstream of IL-6 and in experimental cancer cachexia. Am J Physiol Endocrinol Metab. 2012;303:E410–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Patra SK, Arora S. Integrative role of neuropeptides and cytokines in cancer anorexia-cachexia syndrome. Clin Chim Acta. 2012;413:1025–34. [DOI] [PubMed] [Google Scholar]
- 43.Kim HJ, Yun J, Kim KH, Kim SH, Lee SC, Bae SB, Kim CK, Lee NS, Lee KT, Park SK, et al. Pathophysiological role of hormones and cytokines in cancer cachexia. J Korean Med Sci. 2012;27:128–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tisdale MJ. Cancer cachexia: metabolic alterations and clinical manifestations. Nutrition. 1997;13:1–7. [DOI] [PubMed] [Google Scholar]
- 45.Argilés JM, Lopez-Soriano FJ. The role of cytokines in cancer cachexia. Med Res Rev. 1999;19:223–48. [DOI] [PubMed] [Google Scholar]
- 46.Deans C, Wigmore SJ. Systemic inflammation, cachexia and prognosis in patients with cancer. Curr Opin Clin Nutr Metab Care. 2005;8:265–9. [DOI] [PubMed] [Google Scholar]
- 47.DeJong CH, Busquets S, Moses AG, Schrauwen P, Ross JA, Argiles JM, Fearon KC. Systemic inflammation correlates with increased expression of skeletal muscle ubiquitin but not uncoupling proteins in cancer cachexia. Oncol Rep. 2005;14:257–63. [PubMed] [Google Scholar]
- 48.Wang H, Lai YJ, Chan YL, Li TL, Wu CJ. Epigallocatechin-3-gallate effectively attenuates skeletal muscle atrophy caused by cancer cachexia. Cancer Lett. 2011;305:40–9. [DOI] [PubMed] [Google Scholar]
- 49.Acharyya S, Ladner KJ, Nelsen LL, Damrauer J, Reiser PJ, Swoap S, Guttridge DC. Cancer cachexia is regulated by selective targeting of skeletal muscle gene products. J Clin Invest. 2004;114:370–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tisdale MJ. Reversing cachexia. Cell. 2010;142:511–2. [DOI] [PubMed] [Google Scholar]
- 51.Davis JM, Murphy EA, Carmichael MD. Effects of the dietary flavonoid quercetin upon performance and health. Curr Sports Med Rep. 2009;8:206–13. [DOI] [PubMed] [Google Scholar]
- 52.Wang X, Pickrell AM, Zimmers TA, Moraes CT. Increase in muscle mitochondrial biogenesis does not prevent muscle loss but increased tumor size in a mouse model of acute cancer-induced cachexia. PLoS ONE. 2012;7:e33426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Baracos VE. Cancer-associated cachexia and underlying biological mechanisms. Annu Rev Nutr. 2006;26:435–61. [DOI] [PubMed] [Google Scholar]
- 54.Teunissen SC, Wesker W, Kruitwagen C, de Haes HC, Voest EE, de Graeff A. Symptom prevalence in patients with incurable cancer: a systematic review. J Pain Symptom Manage. 2007;34:94–104. [DOI] [PubMed] [Google Scholar]
- 55.Vigano A, Piccioni M, Trutschnigg B, Hornby L, Chaudhury P, Kilgour R. Male hypogonadism associated with advanced cancer: a systematic review. Lancet Oncol. 2010;11:679–84. [DOI] [PubMed] [Google Scholar]
- 56.Burney BO, Hayes TG, Smiechowska J, Cardwell G, Papusha V, Bhargava P, Konda B, Auchus RJ, Garcia JM. Low testosterone levels and increased inflammatory markers in patients with cancer and relationship with cachexia. J Clin Endocrinol Metab. 2012;97:E700–9. [DOI] [PubMed] [Google Scholar]
- 57.Khaki A, Fathiazad F, Nouri M, Maleki NA, Khamnei HJ, Ahmadi P. Beneficial effects of quercetin on sperm parameters in streptozotocin-induced diabetic male rats. Phytother Res. 2010;24:1285–91. [DOI] [PubMed] [Google Scholar]
- 58.Chuang CC, Martinez K, Xie G, Kennedy A, Bumrungpert A, Overman A, Jia W, McIntosh MK. Quercetin is equally or more effective than resveratrol in attenuating tumor necrosis factor-{alpha}-mediated inflammation and insulin resistance in primary human adipocytes. Am J Clin Nutr. 2010;92:1511–21. [DOI] [PubMed] [Google Scholar]
- 59.Jeong SM, Kang MJ, Choi HN, Kim JH, Kim JI. Quercetin ameliorates hyperglycemia and dyslipidemia and improves antioxidant status in type 2 diabetic db/db mice. Nutr Res Pract. 2012;6:201–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.