Abstract
Among modifiable lifestyle factors, diet may affect cognitive health. Cross-sectional and longitudinal associations may exist between dietary exposures [e.g., caffeine (mg/d), alcohol (g/d), and nutrient adequacy] and cognitive performance and change over time. This was a prospective cohort study, the Baltimore Longitudinal Study of Aging (n = 628–1305 persons depending on the cognitive outcome; ∼2 visits/person). Outcomes included 10 cognitive scores, spanning various domains of cognition. Caffeine and alcohol intakes and a nutrient adequacy score (NAS) were estimated from 7-d food diaries. Among key findings, caffeine intake was associated with better baseline global cognition among participants with a baseline age (Agebase) of ≥70 y. A higher NAS was associated with better baseline global cognition performance (overall, women, Agebase <70 y), better baseline verbal memory (immediate and delayed recall, Agebase ≥70 y), and slower rate of decline or faster improvement in the attention domain (women). For an Agebase of <70 y, alcohol consumption was associated with slower improvement on letter fluency and global cognition over time. Conversely, for an Agebase of ≥70 y and among women, alcohol intake was related to better baseline attention and working memory. In sum, patterns of diet and cognition associations indicate stratum-specific associations by sex and baseline age. The general observed trend was that of putative beneficial effects of caffeine intake and nutrient adequacy on domains of global cognition, verbal memory, and attention, and mixed effects of alcohol on domains of letter fluency, attention, and working memory. Further longitudinal studies conducted on larger samples of adults are needed to determine whether dietary factors individually or in combination are modifiers of cognitive trajectories among adults.
Introduction
Preventing age-related cognitive decline can help maintain quality of life (1). Dietary factors, such as the neuroactive compounds caffeine and alcohol, may affect cognitive health (2–4). Cognitive health benefits were also ascribed to healthy patterns of dietary intake and dietary quality (5). However, few studies with a prospective cohort design have examined all 3 predictors (i.e., caffeine and alcohol intakes and dietary quality) simultaneously, covarying for the others. In fact, to our knowledge, research has to date restricted its aim to a single cognitive test score, thus failing to incorporate multiple domains of cognition. Consequently, well-designed cohort studies are needed to clarify independent associations of dietary quality and caffeine and alcohol intakes with cognition. Such studies would ascertain temporality, include multiple cognitive domains, and would account for potential confounding effects within the diet.
Caffeine, primarily obtained from coffee, is the most widely used neuroactive compound worldwide (6). Acting as a brain stimulant, it causes heightened alertness and arousal (6) and can improve perceptual speed, vigilance, and even memory (7, 8). As a methylxanthine, caffeine blocks brain adenosine receptors, triggering cholinergic stimulation and potentially improving cognitive performance and slowing age-related cognitive decline (8). Caffeine’s putative beneficial effects on cognition are domain-specific (9–17). However, several studies found no association (18–20), whereas others noted differential associations by gender (9, 21).
Alcohol, on the other hand, is a well-known depressant drug widely consumed in Western diets. Although many large epidemiologic studies have examined cognition in relation to alcohol consumption, the direction of the association remains uncertain (22–51).
Importantly, caffeine- and alcohol-containing drinks are often consumed with other foods and with each other at different meals. Thus, intakes of caffeine and alcohol may be correlated with each other and with dietary quality or nutrient adequacy (i.e., an index of dietary quality based solely on nutrients). Furthermore, poor dietary quality has been associated with adverse cognitive outcomes, including decline and poor function (2, 52–58). Thus, dietary quality may confound relations of caffeine and alcohol intakes with cognition. Previous studies failed to account for this potential confounding effect. In our present study, we evaluated the independent associations of caffeine and alcohol intake and nutrient adequacy with cross-sectional and longitudinal cognitive performance in a U.S. population of older adults. We hypothesized caffeine to have putative beneficial and sex-specific effects for certain domains (e.g., attention, perceptual speed, and memory), and alcohol to have both beneficial and deleterious effects, depending on the population subgroup (age group or gender) and the cognitive domain. In contrast, nutrient adequacy was hypothesized to affect a wide range of domains in a positive manner.
Based on previous evidence, particularly in the case of caffeine and alcohol intake (9, 14, 21, 36, 40), we presented sex-specific findings. In addition, variations in nutrient requirements by age at cut-points of 50 y and 70 y had been noted [e.g., fiber (lower at ≥50 y vs. <50 y), sodium (lower at ≥70 y vs. <70 y), calcium (different ranges for ≥50 y vs. <50 y), iron (reduced with age), phosphorus [upper limit (UL)9 reduced at 70 y], vitamin B-6 (increases with age, including ≥50 y), and vitamin D (increases at age ≥70 y)] (59–62). Moreover, studies have commonly stratified by age associations of dietary components with cognition (15, 17, 40, 57). Therefore, we stratified our results by sex and age accordingly, similar to previous studies (9, 14, 15, 17, 21, 36, 40, 57).
Materials and Methods
Database and study population
The Baltimore Longitudinal Study of Aging (BLSA) is an ongoing prospective open cohort study of community-dwelling adults that was initiated in 1958 by the National Institute on Aging. BLSA participants were generally highly educated adults with a first-visit age of 17 to 97 y (median = 60.7; means ± SDs = 58.9 ± 18.0), and around 60% were men. Total enrollment included n1 = 3047 participants (n′1 = 20,385 visits, 1958–2009) (63). Exclusionary criteria are summarized elsewhere (64). Examinations were conducted at ∼2-y intervals, and the protocol was approved by the Medstar Research Institute’s Institutional Review Board. Examinations included physical, neurocognitive, medical history, dietary assessment, laboratory, and radiologic tests and measurements. Participants completed a written informed consent form per visit (63).
Eligible participants (n), observations or visits (n′), and visits/participants (n″) were as follows: 1) had complete dietary intake data from 1961 to 2007 (n2 = 1821 participants; n′2 = 4537 visits; n″2 range = 1–12 visits/participant, mean = 2.5 visits/participant); 2) had complete cognitive data from 1962 to 2008 (n3a-3j = 1199–2704 participants; n′3a-3j = 5111–10,704 visits depending on the cognitive test score; n″3 range = 1–22 visits/participant; mean = 3.4–4.4 visits/participant); and 3) had ≥1 visits that were concurrent (i.e., during the same visit/year) between dietary and cognitive data (n4a-4j = 628–1305 participants; n′4a-4j = 1218–2528 visits; n″4 range = 1–10 visits/participant; mean = 1.9–2.0 visits/participant). Moreover, the mean (range) first-visit age for final samples with cognitive and dietary data (4a-4j) was 62 to 69 y (17–99 y), depending on the cognitive test. However, a distinction was made between first-visit characteristics (including age) and baseline characteristics. The “baseline” (base) visit was the earliest visit/year (Yearbase) with concurrent data on diet and cognition. Timing was similar for most tests, except for the Benton Visual Retention Test (BVRT), historically initiated earlier in the BLSA. In samples 4a-4j, Yearbase ranged from 1961 to 2007 for BVRT and 1985 to 2007 for other cognitive tests. The baseline age (Agebase) range for samples 4a-4j was 18 to 93 y (mean: 62 y) for BVRT and 27 to 96 y (mean: 68–72 y) for other cognitive tests.
Dietary assessment: caffeine and alcohol intake and nutrient adequacy score
Dietary intake was assessed with 7-d dietary records. BLSA participants were instructed by trained dietitians to estimate portion size, weigh foods, and complete the records (65–67). Intake was assessed in a noncontinuous fashion: 1961 to 1965 (1.01% of n′2 = 4537), 1968 to 1975 (31.10% of n′2), 1984 to 1992 (23.46% of n′2), and 1994 to 2007 (44.3% of n′2). Overall (n′2 = 4537, 1961–2007), the means ± SDs of completed dietary records was 6.04 ± 1.73 (IQR: 6–7). Food codes and amounts were recorded for each diary, with nutrient intakes [absolute and relative amounts (i.e., per 1000 kcal or % energy)] estimated using a revised and up-to-date nutrient database (68) and averaged over available diaries per individual visit. Specifically, caffeine (per 100 mg/d) and alcohol (g/d) intakes and the nutrient adequacy score (NAS) were of primary interest. Moderate alcohol consumption was defined as 14 to 28 g/d and was compared with lower and higher amounts of intake as a sensitivity analysis, based on previous studies (22–35, 37–39).
To estimate the NAS, age/sex-specific DRIs for U.S. adults were used among others to categorize individuals according to adequacy of dietary intake for macronutrients (e.g., protein, carbohydrates, fat) and micronutrients (vitamins and minerals). Among DRI, adequate intake (AI) was used to reference an amount of vitamins and minerals above which a participant’s intake was adequate without exceeding the UL. For carbohydrates, protein, and total fat (% energy), the acceptable macronutrient distribution range was used instead. Saturated fat (% energy) intake in moderation was estimated using the 2005 Healthy Eating Index complete score. Similarly, cholesterol (mg) intake was determined adequate based on the 1995 Healthy Eating Index complete score (60–62).
For all NAS components, AI or AI and UL in combination were used as follows: 1) AI only: total fiber, potassium, thiamin, riboflavin, and vitamin E; and 2) AI and UL: sodium, calcium, iron, magnesium, phosphorus, zinc, retinol, niacin, vitamin B-6, folate (UL applied only to synthetic folic acid), and vitamins C and D (59–62). Nutrient adequacy was determined for each nutrient in relation to its age/sex-specific recommended intake (0: inadequate; 1: adequate). The NAS (range: 0–22) was computed as the sum of 22 nutrient components, with a higher score reflecting better overall nutrient adequacy, similar to a previous study (69).
Cognitive assessment
A battery of 6 cognitive tests was used.
Mini Mental State Examination.
Administered in the BLSA since the mid 1980s, the Mini Mental State Examination (MMSE) is a brief mental status test measuring orientation, concentration, immediate and delayed memory, language, and constructional praxis (70). Scores range from 0 to 30, with higher scores indicating better cognitive performance.
BVRT.
The BVRT is a test of short-term visual memory and constructional abilities (71). Administration A has been used in the BLSA since 1960, with a modified error scoring system, based on the BVRT manual scoring, such that higher scores indicate poorer visual memory.
California Verbal Learning Test.
Administered in the BLSA since 1993, the California Verbal Learning Test (CVLT) is a 16-item shopping list measuring verbal learning and memory. The variables of interest in this study were List A sum across 5 learning trials and long-delay free recall. Scores ranged from 0 to 80 for List A sum and 0 to 16 for long-delay free recall. Higher scores indicate better verbal memory (72).
Verbal Fluency Tests.
Administered in the BLSA since the mid 1980s, the Verbal Fluency Test includes the letter (F, A, S) assessment measuring phonemic fluency (VFT-L) (73, 74) and the categorical (fruits, animals, vegetables) assessment measuring semantic fluency (VFT-C) (75). Participants were required to generate as many words as possible for 60 s, starting with either a specific letter or category. Higher scores indicate better verbal fluency, with the total number of words, minus intrusions and perseverations, analyzed for each test.
Trail Making Tests.
Trail Making Tests A and B are tests of attention (Trails A) and executive functioning (Trails B), specifically cognitive control and visuo-motor scanning (76). Participants corrected incident errors by returning to their last correct response and continuing from there. The stopwatch recorded the time while corrections were made. Scores reflected time to completion (in seconds) separately for Trails A and B. Higher scores indicate poorer performance.
Digits Span Forward and Backward.
The Wechsler Adult Intelligence Scale-Revised, digits span-forward (DS-F) and digits span-backward (DS-B) (77), assesses attention and working memory, respectively. DS-F involves orally presenting a series of single-digit numbers at increasing digit span lengths for participants to repeat in the same order. The numbers’ span length ranges from 3 to 9 digits. Two trials at each span are presented. The test is discontinued when participants incorrectly repeat both trials at a specified span. DS-B is similar to DS-F, except that participants repeat a series of increasingly longer spans of single digit numbers in reverse order. The numbers’ span length ranges from 2 to 8 digits. The total score for both DS-F and DS-B is 14.
In sum, most cognitive test scores’ direction was “better performance with a higher score” the reverse was true for the BVRT and Trails A and B.
Covariates
Potentially confounding covariates were Agebase, Yearbase, sex, race/ethnicity (non-Hispanic white, non-Hispanic black, and other ethnicity), education (y), smokingbase (“never,” “former”, or “current smoker”), and measured baseline BMI [BMIbase, weight/(height)2 in kg/m2]. First-visit measures (i.e., visit 1 of the BLSA) were also considered for the descriptive part of the analysis.
Statistical methods
Analyses were performed using Stata version 11.0 (78). Participant characteristics (fixed and at first-visit) were described and compared by data availability and by sex using a 1-factor ANOVA, t test, and χ2 test.
Mixed-effects linear regression models were used to examine associations of baseline caffeine and alcohol intakes and NAS with baseline cognitive performance (cross-sectional effect) and their relations with cognitive change over time (longitudinal effect), controlling for Agebase, sex, and other “baseline” or fixed covariates, including race/ethnicity, education, baseline smoking status, and baseline BMI. These models, which we term time-interval, mixed-effects regression models, were adapted from a previously published study that described the methodology in detail (79). Time elapsed (y) was measured from Agebase (per cognitive test).
Equations 1.1–1.4
Multilevel models vs. composite models
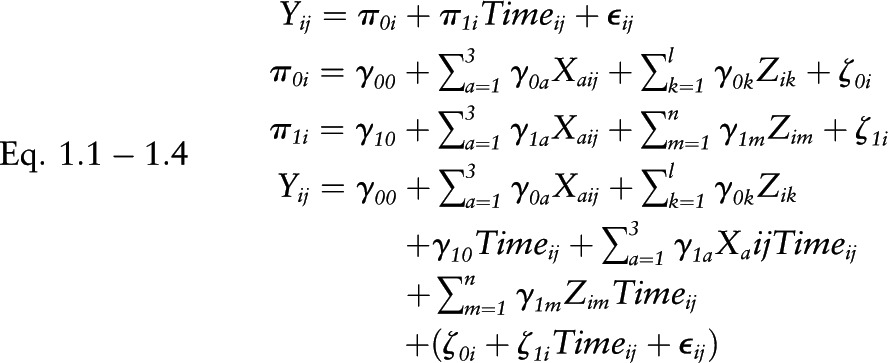 |
where Yij represents cognitive test scores for individual “i” and visit “j” 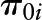 , level-1 intercept for individual i;
, level-1 intercept for individual i; 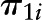 , level-1 slope for individual i;
, level-1 slope for individual i; 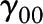 , level-2 intercept of the random intercept
, level-2 intercept of the random intercept 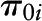 ;
; 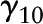 , level-2 intercept of the slope
, level-2 intercept of the slope 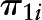 ; and
; and 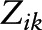 , a vector of individual-level fixed baseline covariates (including Agebase) predicting level-1 intercepts (
, a vector of individual-level fixed baseline covariates (including Agebase) predicting level-1 intercepts (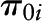 ) and level-1 slopes (
) and level-1 slopes (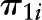 ). Among
). Among 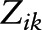 covariates, education (y) was centered at 16y (approximate mean for BLSA), BMI at 25 kg/m2, total energy intake at 2000 kcal/d, Agebase at 50 y, and Yearbase at 2000. Xija are the main predictor variables: “caffeine centered at 0 mg/d,” “NAS centered at 10,” and “alcohol centered at 10 g/d”
covariates, education (y) was centered at 16y (approximate mean for BLSA), BMI at 25 kg/m2, total energy intake at 2000 kcal/d, Agebase at 50 y, and Yearbase at 2000. Xija are the main predictor variables: “caffeine centered at 0 mg/d,” “NAS centered at 10,” and “alcohol centered at 10 g/d” 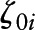 and
and 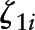 are level-2 disturbances; and
are level-2 disturbances; and 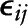 is the within-person level-1 disturbance. In a sensitivity analysis, alcohol was entered as categorical variable and interacted as such with time elapsed: 0 = 14 to 28 g/d, 1 = <14 g/d, 2 = >28 g/d. Thus, having lower and higher than moderate consumption was compared with moderate consumption.
is the within-person level-1 disturbance. In a sensitivity analysis, alcohol was entered as categorical variable and interacted as such with time elapsed: 0 = 14 to 28 g/d, 1 = <14 g/d, 2 = >28 g/d. Thus, having lower and higher than moderate consumption was compared with moderate consumption.
Estimated parameters with SE and P values reflected rate of cognitive change over time (γ10), the effects of caffeine and alcohol intakes and NAS on baseline cognitive performance (time = 0) (γ01, γ02, and γ03; γ031 and γ032 for categorical alcohol), and effects of caffeine and alcohol intakes and NAS on annual rate of cognitive change over time (γ11, γ12, and γ13; γ131 and γ132 for categorical alcohol). Analysis was presented for the overall sample and was further stratified by sex and Agebase (<70 y vs. ≥70 y).
The effect modification by sex and Agebase was tested by including additional interaction terms in the “overall population” model (e.g., Agebase× caffeine and Agebase× caffeine × time, separately). We used a 2-stage Heckman selection model adjusting for bias because of nonrandom participant selection for final analyses (80, 81).
We further estimated cognitive test scores and plotted their predicted means against time, with Agebase set alternatively at 50 y and 70 y. Each exposure was examined separately controlling for the other covariates. Caffeine intake was alternatively set at 0 mg/d vs. 300 mg/d; alcohol intake at 10 g/d vs. 50 g/d; and NAS at 5 vs. 15. Thus, cognitive performance trajectories for the hypothetical population with set covariate distribution was examined over time and compared by exposure level to illustrate direction and magnitude of fixed effects γ0a and γ1a.
Type I error was set at 0.05 for each of the 3 exposure variable-related hypotheses. Adjustment for multiple testing reduced the type I error to 0.05/3 = 0.017, and thus only P values <0.017 were considered statistically significant. However, type I error for 3-way interaction terms was set to 0.10 because of reduced power to detect significant associations (82).
Results
As shown in Table 1, compared with men, women were generally older and had more ethnic diversity, performed better on the MMSE, and had lower prevalence of current smoking. The distribution of study characteristics by sex and data completeness is presented in Supplemental Table 1 and Supplemental Figure 1.
TABLE 1.
Baseline characteristics of participants included in the final analysis with the MMSE (global cognitive function) and dietary data available, stratified by sex, BLSA, 1962–20081
| n2 | n′3 | Values | |
| Men, n | 415 | ||
| First-visit age, y | 407 | 66.8 ± 13.9* | |
| Race/ethnicity | 407 | ||
| Non-Hispanic white | 347 | 85.3* | |
| Non-Hispanic black | 50 | 12.3 | |
| Other | 10 | 2.5 | |
| First-visit education, y | 378 | 16.9 ± 2.7* | |
| First-visit smoking | 366 | ||
| Never smoker | 120 | 32.8* | |
| Former smoker | 184 | 50.3 | |
| Current smoker | 62 | 16.9 | |
| First-visit BMI, kg/m2 | 393 | 26.3 ± 3.7 | |
| Energy intake, kcal/d | 1458 | 2156 ± 565* | |
| NAS | 1458 | 11.10 ± 3.02* | |
| >10 (above median), % | 1458 | 56.5* | |
| Caffeine, mg/d | 1458 | 127.4 ± 211.0 | |
| 100–300 mg/d (1–3 cups of coffee), % | 1458 | 29.8* | |
| Alcohol, g/d | 1458 | 11.5 ± 0.4* | |
| 14–28 g/d (1–2 drinks), % | 1458 | 18.2 | |
| MMSE total score | 753 | 28.4 ± 2.2* | |
| Women, n | 312 | ||
| First-visit age, y | 309 | 69.8 ± 12.1 | |
| Race/ethnicity | 311 | ||
| Non-Hispanic white | 234 | 75.2 | |
| Non-Hispanic black | 62 | 19.9 | |
| Other | 15 | 4.8 | |
| First-visit education, y | 282 | 15.8 ± 2.6 | |
| First-visit smoking | 251 | ||
| Never smoker | 102 | 40.6 | |
| Former smoker | 124 | 49.4 | |
| Current smoker | 25 | 10.0 | |
| First-visit BMI, kg/m2 | 301 | 25.9 ± 4.6 | |
| Energy intake, kcal/d | 978 | 1697 ± 427 | |
| NAS | 978 | 12.87 ± 3.16 | |
| >10 (above median), % | 978 | 77.4 | |
| Caffeine, mg/d | 978 | 138.7 ± 194.0 | |
| 100–300 mg/d (1–3 cups of coffee), % | 978 | 40.8 | |
| Alcohol, g/d | 978 | 5.8 ± 0.3 | |
| 14–28 g/d (1–2 drinks), % | 978 | 9.8 | |
| MMSE total score | 680 | 28.8 ± 2.1 |
Values are means ± SDs or percentages. *P < 0.05 for null hypothesis of no sex difference between means or proportions using the t test and χ2 test, respectively, within each of the samples. BLSA, Baltimore Longitudinal Study of Aging; MMSE, Mini Mental State Examination; NAS, nutrient adequacy score.
Number of participants in the analysis.
Total number of visits included in the analysis.
Several key findings emerged from the time-interval, mixed-effects regression models. For most cognitive tests, younger participants at baseline performed better than older participants (γAge in the direction of poorer performance with higher age, Table 2). For a few tests, there was also an appreciable decline over time (γ10 in the direction of decline), controlling for Agebase, whereas for others there was a learning effect over time that was reduced or tapered off with increasing age (γ10 in the direction of improvement over time, P < 0.05, but with a γAge×Time in the direction of tapered-off learning and an eventual time-related decline at higher Agebase values).
TABLE 2.
Analysis of baseline caffeine intake (continuous, 100 mg/d), alcohol intake (g/d), and the NAS, and longitudinal change in cognitive performance (total and sex-stratified), time-interval mixed-effects linear regression analysis, BLSA, 1962–20081
| Total: model 1 |
Men: model 2 |
Women: model 3 |
||||
| γ ± SEE2 | P3 | γ ± SEE2 | P3 | γ ± SEE2 | P3 | |
| MMSE, total score4 | n = 555 | n′ = 1102 | n = 328 | n′ = 595 | n = 227 | n′ = 507 |
| Fixed effects | ||||||
| Intercept (γ00 for π0i) | +29.48 ± 0.27 | <0.001 | +29.76 ± 0.39 | <0.001 | +29.22 ± 0.36 | <0.001 |
| Time (γ10 for π1i) | +0.135 ± 0.106 | 0.201 | +0.086 ± 0.148 | 0.559 | +0.191 ± 0.182 | 0.294 |
| Agebase | −0.052 ± 0.009 | <0.001 | −0.067 ± 0.013 | <0.001 | −0.032 ± 0.011 | 0.004 |
| Agebase × time | −0.009 ± 0.004 | 0.014 | −0.005 ± 0.006 | 0.386 | −0.011 ± 0.006 | 0.044 |
| Gender (women vs. men) | +0.182 ± 0.159 | 0.253 | — | — | — | — |
| Gender × time | +0.020 ± 0.059 | 0.733 | — | — | — | — |
| Caffeine (γ01 for π0i) | +0.094 ± 0.047 | 0.043 | +0.001 ± 0.001 | 0.099 | +0.060 ± 0.072 | 0.407 |
| Caffeine × time (γ11 for π1i) | −0.004 ± 0.016 | 0.795 | −0.000 ± 0.000 | 0.322 | +0.008 ± 0.032 | 0.813 |
| NAS (γ02 for π0i) | +0.072 ± 0.025 | 0.004 | +0.058 ± 0.034 | 0.094 | +0.104 ± 0.036 | 0.003 |
| NAS × time (γ12 for π1i) | −0.004 ± 0.009 | 0.624 | +0.003 ± 0.012 | 0.838 | −0.013 ± 0.016 | 0.405 |
| Alcohol (γ03 for π0i) | +0.006 ± 0.006 | 0.295 | +0.008 ± 0.006 | 0.198* | −0.008 ± 0.011 | 0.432 |
| Alcohol × time (γ13 for π1i) | −0.003 ± 0.002 | 0.095 | −0.001 ± 0.002 | 0.692* | −0.008 ± 0.005 | 0.132 |
| Random effects | ||||||
| Level 1 residuals (Rij) | +0.79 ± 0.03 | <0.001 | +0.93 ± 0.05 | <0.001 | +0.622 ± 0.034 | <0.001 |
| Level 2 residuals | ||||||
| Intercept (ξ0i) | +1.38 ± 0.05 | <0.001 | +1.36 ± 0.08 | <0.001 | +1.305 ± 0.034 | <0.001 |
| Linear slope (ξ1i) | +0.32 ± 0.02 | <0.001 | +0.30 ± 0.03 | <0.001 | +0.389 ± 0.037 | <0.001 |
| CVLT-List A, total score | n = 568 | n’ = 1111 | n = 296 | n’ = 559 | n = 272 | n’ = 552 |
| Intercept (γ00 for π0i) | +58.32 ± 1.29 | <0.001 | +59.57 ± 1.85 | <0.001 | +63.18 ± 1.71 | <0.001 |
| Time (γ10 for π1i) | +0.060 ± 0.393 | 0.878 | +0.191 ± 0.535 | 0.720 | −0.175 ± 0.52 | 0.751 |
| Agebase | −0.424 ± 0.036 | <0.001 | −0.472 ± 0.058 | <0.001 | −0.380 ± 0.046 | <0.001 |
| Agebase × time | −0.010 ± 0.010 | 0.317 | −0.004 ± 0.015 | 0.789 | −0.008 ± 0.014 | 0.554 |
| Gender (women vs. men) | +6.547 ± 0.957 | <0.001 | — | — | — | — |
| Gender × time | −0.269 ± 0.249 | 0.280 | — | — | — | — |
| Caffeine (γ01 for π0i) | +0.090 ± 0.276 | 0.745 | −0.204 ± 0.348 | 0.559 | +0.587 ± 0.480 | 0.222 |
| Caffeine × time (γ11 for π1i) | +0.004 ± 0.064 | 0.944 | −0.063 ± 0.078 | 0.419 | +0.076 ± 0.109 | 0.484 |
| NAS (γ02 for π0i) | +0.291 ± 0.152 | 0.055 | +0.365 ± 0.231 | 0.114 | +0.282 ± 0.203 | 0.164 |
| NAS × time (γ12 for π1i) | +0.086 ± 0.040 | 0.031 | +0.116 ± 0.056 | 0.040 | +0.081 ± 0.059 | 0.169 |
| Alcohol (γ03 for π0i) | +0.053 ± 0.031 | 0.088 | +0.050 ± 0.038 | 0.191 | +0.059 ± 0.057 | 0.295 |
| Alcohol × time (γ13 for π1i) | −0.008 ± 0.007 | 0.208 | −0.007 ± 0.007 | 0.298* | +0.016 ± 0.017 | 0.351 |
| CVLT-delayed recall, total score | n = 568 | n’ = 1111 | n = 296 | n’ = 559 | n = 272 | n’ = 552 |
| Intercept (γ00 for π0i) | +12.52 ± 0.40 | <0.001 | +12.94 ± 0.58 | <0.001 | +13.47 ± 0.52 | <0.001 |
| Time (γ10 for π1i) | −0.021 ± 0.113 | 0.850 | +0.008 ± 0.159 | 0.959 | −0.072 ± 0.151 | 0.631 |
| Agebase | −0.112 ± 0.011 | <0.001 | −0.139 ± 0.018 | <0.001 | −0.089 ± 0.014 | <0.001 |
| Agebase × time | −0.004 ± 0.003 | 0.157 | −0.004 ± 0.004 | 0.376 | −0.003 ± 0.004 | 0.502 |
| Gender (women vs. men) | +1.281 ± 0.299 | <0.001 | — | — | — | — |
| Gender × time | −0.024 ± 0.071 | 0.738 | — | — | — | — |
| Caffeine (γ01 for π0i) | +0.015 ± 0.086 | 0.858 | +0.006 ± 0.110 | 0.953 | −0.017 ± 0.146 | 0.909 |
| Caffeine × time (γ11 for π1i) | −0.083 ± 0.018 | 0.964 | −0.033 ± 0.023 | 0.148* | +0.042 ± 0.030 | 0.157 |
| NAS (γ02 for π0i) | +0.102 ± 0.047 | 0.030 | +0.131 ± 0.073 | 0.071 | +0.092 ± 0.062 | 0.140 |
| NAS × time (γ12 for π1i) | +0.017 ± 0.011 | 0.131 | +0.029 ± 0.017 | 0.081 | +0.014 ± 0.016 | 0.373 |
| Alcohol (γ03 for π0i) | +0.013 ± 0.010 | 0.174 | +0.013 ± 0.012 | 0.550 | +0.012 ± 0.017 | 0.477 |
| Alcohol × time (γ13 for π1i) | −0.001 ± 0.002 | 0.498 | −0.001 ± 0.002 | 0.550 | +0.004 ± 0.005 | 0.366 |
| BVRT, total errors | n = 1005 | n’ = 1975 | n = 620 | n’ = 1152 | n = 385 | n’ = 822 |
| Intercept (γ00 for π0i) | +3.68 ± 0.31 | <0.001 | +4.03 ± 0.39 | <0.001 | +3.49 ± 0.43 | <0.001 |
| Time (γ10 for π1i) | +0.051 ± 0.048 | 0.288 | +0.051 ± 0.058 | 0.384 | −0.072 ± 0.082 | 0.377 |
| Agebase | +0.112 ± 0.006 | <0.001 | +0.110 ± 0.008 | <0.001 | +0.111 ± 0.010 | <0.001 |
| Agebase × time | +0.005 ± 0.001 | <0.001 | +0.004 ± 0.001 | <0.001 | +0.005 ± 0.002 | 0.004 |
| Gender (women vs. men) | −0.005 ± 0.244 | 0.985 | — | — | — | — |
| Gender × time | −0.054 ± 0.035 | 0.128 | — | — | — | — |
| Caffeine (γ01 for π0i) | −0.157 ± 0.070 | 0.024 | −0.181 ± 0.092 | 0.050 | −0.164 ± 0.112 | 0.144 |
| Caffeine × time (γ11 for π1i) | +0.012 ± 0.005 | 0.031 | +0.008 ± 0.012 | 0.507 | +0.011 ± 0.007 | 0.138 |
| NAS (γ02 for π0i) | −0.065 ± 0.037 | 0.076 | −0.052 ± 0.051 | 0.311 | −0.090 ± 0.054 | 0.099 |
| NAS × time (γ12 for π1i) | −0.001 ± 0.005 | 0.878 | +0.002 ± 0.007 | 0.795 | −0.002 ± 0.008 | 0.776 |
| Alcohol (γ03 for π0i) | −0.007 ± 0.007 | 0.307 | −0.001 ± 0.007 | 0.933 | −0.020 ± 0.016 | 0.220 |
| Alcohol × time (γ13 for π1i) | −0.000 ± 0.001 | 0.851 | −0.000 ± 0.001 | 0.539 | +0.001 ± 0.002 | 0.718 |
| VFT-C, total score | n = 602 | n’ = 1236 | n = 346 | n’ = 655 | n = 256 | n’ = 581 |
| Intercept (γ00 for π0i) | +18.29 ± 0.47 | <0.001 | +18.21 ± 0.65 | <0.001 | +20.07 ± 0.70 | <0.001 |
| Time (γ10 for π1i) | +0.167 ± 0.093 | 0.073 | +0.128 ± 0.140 | 0.362 | +0.314 ± 0.138 | 0.022 |
| Agebase | −0.169 ± 0.015 | <0.001 | −0.173 ± 0.022 | <0.001 | −0.165 ± 0.023 | <0.001 |
| Agebase × time | −0.014 ± 0.003 | <0.001 | −0.012 ± 0.005 | 0.030 | −0.017 ± 0.004 | <0.001 |
| Gender (women vs. men) | −0.341 ± 0.460 | 0.458 | — | — | — | — |
| Gender × time | −0.084 ± 0.139 | 0.545 | — | — | — | — |
| Caffeine (γ01 for π0i) | −0.036 ± 0.009 | 0.680 | +0.002 ± 0.108 | 0.985 | −0.001 ± 0.002 | 0.481 |
| Caffeine × time (γ11 for π1i) | +0.006 ± 0.014 | 0.633 | −0.003 ± 0.018 | 0.871 | +0.000 ± 0.000 | 0.231 |
| NAS (γ02 for π0i) | +0.051 ± 0.047 | 0.279 | +0.052 ± 0.062 | 0.410 | +0.056 ± 0.074 | 0.443 |
| NAS × time (γ12 for π1i) | +0.003 ± 0.008 | 0.741 | +0.001 ± 0.012 | 0.907 | +0.004 ± 0.012 | 0.739 |
| Alcohol (γ03 for π0i) | +0.012 ± 0.010 | 0.257 | +0.006 ± 0.011 | 0.576 | +0.025 ± 0.023 | 0.263 |
| Alcohol × time (γ13 for π1i) | −0.000 ± 0.001 | 0.975 | −0.000 ± 0.002 | 0.967 | +0.003 ± 0.003 | 0.352 |
| VFT-L, total score | n = 601 | n’ = 1233 | n = 346 | n′ = 645 | n = 255 | n’ = 577 |
| Intercept (γ00 for π0i) | +15.13 ± 0.60 | <0.001 | +14.43 ± 0.87 | <0.001 | +16.25 ± 0.84 | <0.001 |
| Time (γ10 for π1i) | +0.239 ± 0.102 | 0.020 | +0.273 ± 0.140 | 0.052 | +0.190 ± 0.174 | 0.277 |
| Agebase | −0.040 ± 0.019 | 0.041 | −0.019 ± 0.029 | 0.515 | −0.057 ± 0.027 | 0.036 |
| Agebase × time | −0.012 ± 0.003 | 0.001 | −0.013 ± 0.005 | 0.012 | −0.012 ± 0.005 | 0.026 |
| Gender (women vs. men) | +0.729 ± 0.387 | 0.059 | — | — | — | — |
| Gender × time | −0.001 ± 0.055 | 0.987 | — | — | — | — |
| Caffeine (γ01 for π0i) | +0.028 ± 0.113 | 0.980 | +0.097 ± 0.145 | 0.502* | −0.116 ± 0.187 | 0.533 |
| Caffeine × time (γ11 for π1i) | −0.023 ± 0.015 | 0.121 | −0.038 ± 0.018 | 0.039 | −0.000 ± 0.000 | 0.994 |
| NAS (γ02 for π0i) | −0.051 ± 0.060 | 0.393 | +0.033 ± 0.084 | 0.687 | −0.139 ± 0.088 | 0.113 |
| NAS × time (γ12 for π1i) | +0.001 ± 0.009 | 0.891 | +0.006 ± 0.012 | 0.586 | +0.001 ± 0.015 | 0.941 |
| Alcohol (γ03 for π0i) | +0.021 ± 0.013 | 0.107 | +0.028 ± 0.015 | 0.066 | −0.006 ± 0.027 | 0.827 |
| Alcohol × time (γ13 for π1i) | −0.002 ± 0.002 | 0.141 | −0.002 ± 0.002 | 0.294 | −0.002 ± 0.004 | 0.652 |
| DS-F, total score | n = 541 | n’ = 1067 | n = 285 | n’ = 544 | n = 256 | n’ = 523 |
| Intercept (γ00 for π0i) | +9.50 ± 0.30 | <0.001 | +10.04 ± 0.43 | <0.001 | +8.74 ± 0.40 | <0.001 |
| Time (γ10 for π1i) | −0.002 ± 0.060 | 0.978 | +0.015 ± 0.093 | 0.869 | +0.013 ± 0.077 | 0.862 |
| Agebase | −0.036 ± 0.008 | <0.001 | −0.053 ± 0.013 | <0.001 | −0.026 ± 0.010 | 0.011 |
| Agebase × time | −0.002 ± 0.001 | 0.237 | −0.002 ± 0.002 | 0.293 | −0.001 ± 0.002 | 0.588 |
| Gender (women vs. men) | −0.493 ± 0.215 | 0.022 | — | — | — | — |
| Gender × time | +0.012 ± 0.034 | 0.729 | — | — | — | — |
| Caffeine (γ01 for π0i) | −0.039 ± 0.061 | 0.519 | −0.077 ± 0.076 | 0.312 | +0.031 ± 0.106 | 0.773 |
| Caffeine × time (γ11 for π1i) | −0.010 ± 0.009 | 0.286 | −0.005 ± 0.013 | 0.706 | −0.022 ± 0.014 | 0.111 |
| NAS (γ02 for π0i) | −0.003 ± 0.035 | 0.934 | −0.002 ± 0.053 | 0.975 | −0.010 ± 0.047 | 0.835 |
| NAS × time (γ12 for π1i) | +0.010 ± 0.006 | 0.085 | +0.001 ± 0.009 | 0.988 | +0.022 ± 0.008 | 0.005 |
| Alcohol (γ03 for π0i) | +0.015 ± 0.007 | 0.036 | +0.022 ± 0.009 | 0.012 | +0.003 ± 0.013 | 0.788 |
| Alcohol × time (γ13 for π1i) | +0.000 ± 0.001 | 0.850 | −0.001 ± 0.001 | 0.406 | +0.003 ± 0.002 | 0.156 |
| DS-B, total score | n = 545 | n’ = 1066 | n = 284 | n’ = 540 | n = 261 | n’ = 526 |
| Intercept (γ00 for π0i) | +8.90 ± 0.32 | <0.001 | +9.08 ± 0.48 | <0.001 | +8.57 ± 0.40 | <0.001 |
| Time (γ10 for π1i) | −0.367 ± 0.098 | <0.001 | −0.383 ± 0.143 | 0.008 | −0.298 ± 0.132 | 0.024 |
| Agebase | −0.036 ± 0.009 | <0.001 | −0.044 ± 0.015 | 0.003 | −0.032 ± 0.011 | 0.002 |
| Agebase × time | +0.006 ± 0.002 | 0.008 | +0.008 ± 0.004 | 0.041 | +0.006 ± 0.003 | 0.050 |
| Gender (women vs. men) | −0.257 ± 0.224 | 0.251 | — | — | — | — |
| Gender × time | +0.103 ± 0.057 | 0.073 | — | — | — | — |
| Caffeine (γ01 for π0i) | −0.040 ± 0.064 | 0.533 | −0.068 ± 0.084 | 0.418 | −0.034 ± 0.111 | 0.759 |
| Caffeine × time (γ11 for π1i) | −0.008 ± 0.015 | 0.576 | −0.022 ± 0.020 | 0.263 | +0.014 ± 0.024 | 0.558 |
| NAS (γ02 for π0i) | −0.051 ± 0.036 | 0.160 | −0.052 ± 0.058 | 0.373 | −0.035 ± 0.047 | 0.462 |
| NAS × time (γ12 for π1i) | +0.003 ± 0.010 | 0.742 | −0.003 ± 0.015 | 0.849 | +0.012 ± 0.013 | 0.378 |
| Alcohol (γ03 for π0i) | +0.021 ± 0.007 | 0.004 | +0.024 ± 0.009 | 0.013 | +0.018 ± 0.013 | 0.171 |
| Alcohol × time (γ13 for π1i) | −0.002 ± 0.002 | 0.106 | −0.003 ± 0.002 | 0.078 | +0.001 ± 0.004 | 0.788 |
Models were further adjusted for baseline year of intake, race/ethnicity, education (y), baseline smoking status, and baseline BMI. See Materials and Methods for more details on covariate coding and model specifications. Random effects are presented only for the MMSE, for simplicity. *P < 0.10 for interaction with gender to test effect modification by gender for each of the 3 predictors’ effects (i.e., caffeine intake, alcohol intake, and NAS) on cognitive performance at baseline and cognitive change over time. BLSA, Baltimore Longitudinal Study of Aging; BVRT, Benton Visual Retention Test; CVLT, California Verbal Learning Test; DS-B, digits span-backward; DS-F, digits span-forward; MMSE, Mini Mental State Examination; NAS, nutrient adequacy score; Trails A, Trail Making Test, part A; Trails B, Trail Making Test, part B; VFT-C, Verbal Fluency Test-Categorical; VFT-L, Verbal Fluency Test-Letter.
n = number of participants in the analysis.
n′ = total number of visits included in the analysis.
Cognitive scores were in the direction of higher score indicated better performance with the exception of the BVRT and Trails A and B.
Importantly, when examining associations of caffeine intake, NAS, and alcohol intake with cognitive performance at baseline (Tables 2 and 3; γ01, γ02, and γ03), and change over time (γ11, γ12, and γ13), there was apparent effect modification by Agebase (<70 y vs. ≥70 y) and, occasionally, by sex. When testing interaction terms, sex and Agebase differentials in diet’s association with cognition (cross-sectional and longitudinal) were significant for some but not all associations (P < 0.10).
TABLE 3.
Analysis of baseline caffeine intake (continuous, 100 mg/d), alcohol intake (g/d), and the NAS, and longitudinal change in cognitive performance (Agebase-stratified), time-interval, mixed-effects linear regression analysis, BLSA, 1962–20081
| Baseline age <70 y: model 4 |
Baseline age ≥70 y: model 5 |
|||
| γ ± SEE2 | P3 | γ ± SEE2 | P3 | |
| MMSE, total score4 | n = 243 | n′ = 514 | n = 312 | n′ = 588 |
| Fixed effects | ||||
| Intercept (γ00 for π0i) | +29.07 ± 0.40 | <0.001 | +29.49 ± 0.62 | <0.001 |
| Time (γ10 for π1i) | +0.164 ± 0.099 | 0.097 | +0.056 ± 0.363 | 0.878 |
| Agebase | +0.001 ± 0.024 | 0.979 | −0.064 ± 0.019 | 0.001 |
| Agebase × time | −0.009 ± 0.005 | 0.087 | −0.007 ± 0.011 | 0.532 |
| Gender (women vs. men) | −0.149 ± 0.182 | 0.412 | +0.512 ± 0.247 | 0.032 |
| Gender × time | +0.050 ± 0.037 | 0.178 | +0.017 ± 0.127 | 0.894 |
| Caffeine (γ01 for π0i) | −0.004 ± 0.005 | 0.922* | +0.218 ± 0.083 | 0.008 |
| Caffeine × time (γ11 for π1i) | −0.008 ± 0.010 | 0.377* | +0.016 ± 0.040 | 0.700 |
| NAS (γ02 for π0i) | +0.084 ± 0.027 | 0.002 | +0.077 ± 0.040 | 0.055 |
| NAS × time (γ12 for π1i) | −0.014 ± 0.006 | 0.017 | −0.007 ± 0.020 | 0.723 |
| Alcohol (γ03 for π0i) | +0.005 ± 0.006 | 0.325 | +0.010 ± 0.010 | 0.288 |
| Alcohol × time (γ13 for π1i) | −0.002 ± 0.001 | 0.008 | −0.008 ± 0.005 | 0.139 |
| Random effects | ||||
| Level 1 residuals (Rij) | +0.80 ± 0.05 | <0.001 | +0.78 ± 0.04 | <0.001 |
| Level 2 residuals | ||||
| Intercept (ξ0i) | +0.91 ± 0.07 | <0.001 | +1.61 ± 0.08 | <0.001 |
| Linear slope (ξ1i) | +0.10 ± 0.02 | <0.001 | +0.49 ± 0.04 | <0.001 |
| CVLT-List A, total score | n = 321 | n’ = 579 | n = 247 | n’ = 532 |
| Intercept (γ00 for π0i) | +57.20 ± 1.49 | <0.001 | +68.51 ± 4.02 | <0.001 |
| Time (γ10 for π1i) | +0.215 ± 0.559 | 0.701 | −0.672 ± 1.061 | 0.526 |
| Agebase | −0.264 ± 0.070 | <0.001 | −0.747 ± 0.121 | <0.001 |
| Agebase × time | −0.010 ± 0.020 | 0.628 | +0.006 ± 0.035 | 0.852 |
| Gender (women vs. men) | +6.768 ± 1.230 | <0.001 | +7.449 ± 1.552 | <0.001 |
| Gender × time | −0.297 ± 0.347 | 0.392 | −0.218 ± 0.457 | 0.633 |
| Caffeine (γ01 for π0i) | +0.479 ± 0.323 | 0.138* | −0.796 ± 0.503 | 0.113 |
| Caffeine × time (γ11 for π1i) | +0.005 ± 0.074 | 0.944 | −0.043 ± 0.141 | 0.763 |
| NAS (γ02 for π0i) | +0.018 ± 0.183 | 0.919 | +0.630 ± 0.260 | 0.015 |
| NAS × time (γ12 for π1i) | +0.128 ± 0.054 | 0.019 | +0.030 ± 0.068 | 0.654 |
| Alcohol (γ03 for π0i) | +0.035 ± 0.035 | 0.311 | +0.098 ± 0.061 | 0.110 |
| Alcohol × time (γ13 for π1i) | −0.009 ± 0.008 | 0.221 | −0.010 ± 0.017 | 0.563 |
| CVLT-delayed recall, total score | n = 321 | n’ = 579 | n = 247 | n’ = 532 |
| Intercept (γ00 for π0i) | +12.73 ± 0.45 | <0.001 | +14.97 ± 1.29 | <0.001 |
| Time (γ10 for π1i) | +0.060 ± 0.146 | 0.682 | −0.344 ± 0.331 | 0.299 |
| Agebase | −0.053 ± 0.021 | 0.011 | −0.217 ± 0.039 | <0.001 |
| Agebase × time | −0.003 ± 0.005 | 0.521 | +0.001 ± 0.011 | 0.899 |
| Gender (women vs. men) | +1.091 ± 0.369 | 0.003 | +1.921 ± 0.498 | <0.001 |
| Gender × time | −0.075 ± 0.090 | 0.407 | −0.004 ± 0.498 | 0.975 |
| Caffeine (γ01 for π0i) | +0.053 ± 0.097 | 0.580 | −0.111 ± 0.161 | 0.490 |
| Caffeine × time (γ11 for π1i) | +0.009 ± 0.019 | 0.656* | −0.051 ± 0.044 | 0.247 |
| NAS (γ02 for π0i) | +0.005 ± 0.055 | 0.921* | +0.230 ± 0.083 | 0.006 |
| NAS × time (γ12 for π1i) | +0.025 ± 0.014 | 0.077 | +0.014 ± 0.021 | 0.489 |
| Alcohol (γ03 for π0i) | +0.010 ± 0.010 | 0.336 | +0.016 ± 0.019 | 0.410 |
| Alcohol × time (γ13 for π1i) | −0.002 ± 0.002 | 0.242 | −0.000 ± 0.005 | 0.967 |
| BVRT, total errors | n = 667 | n′ = 1354 | n = 338 | n’ = 620 |
| Intercept (γ00 for π0i) | +3.13 ± 0.34 | <0.001 | +2.09 ± 1.25 | <0.001 |
| Time (γ10 for π1i) | +0.002 ± 0.041 | 0.956 | +0.745 ± 0.408 | 0.068 |
| Agebase | +0.073 ± 0.008 | <0.001 | 0.183 ± 0.038 | <0.001 |
| Agebase × time | +0.003 ± 0.001 | <0.001 | −0.013 ± 0.013 | 0.292 |
| Gender (women vs. men) | +0.263 ± 0.272 | 0.336 | −0.341 ± 0.460 | 0.458 |
| Gender × time | −0.043 ± 0.032 | 0.173 | −0.084 ± 0.139 | 0.545 |
| Caffeine (γ01 for π0i) | −0.096 ± 0.071 | 0.179 | −0.082 ± 0.161 | 0.611 |
| Caffeine × time (γ11 for π1i) | +0.005 ± 0.005 | 0.278 | +0.073 ± 0.046 | 0.116 |
| NAS (γ02 for π0i) | −0.073 ± 0.039 | 0.060 | −0.013 ± 0.077 | 0.866 |
| NAS × time (γ12 for π1i) | +0.009 ± 0.004 | 0.044 | −0.037 ± 0.021 | 0.075 |
| Alcohol (γ03 for π0i) | −0.008 ± 0.007 | 0.226 | −0.004 ± 0.017 | 0.814 |
| Alcohol × time (γ13 for π1i) | −0.000 ± 0.000 | 0.620 | +0.003 ± 0.005 | 0.533 |
| VFT-C, total score | n = 275 | n’ = 561 | n = 327 | n’ = 675 |
| Intercept (γ00 for π0i) | +17.32 ± 0.68 | <0.001 | +20.08 ± 1.06 | <0.001 |
| Time (γ10 for π1i) | +0.532 ± 0.177 | 0.003 | +0.152 ± 0.242 | 0.528 |
| Agebase | −0.149 ± 0.038 | <0.001 | −0.219 ± 0.032 | <0.001 |
| Agebase × time | −0.034 ± 0.010 | <0.001 | −0.018 ± 0.008 | 0.027 |
| Gender (women vs. men) | +1.771 ± 0.458 | <0.001 | +1.780 ± 0.411 | <0.001 |
| Gender × time | +0.087 ± 0.072 | 0.230 | +0.011 ± 0.081 | 0.896 |
| Caffeine (γ01 for π0i) | +0.021 ± 0.116 | 0.853 | −0.001 ± 0.001 | 0.553 |
| Caffeine × time (γ11 for π1i) | −0.012 ± 0.017 | 0.462 | +0.000 ± 0.000 | 0.213 |
| NAS (γ02 for π0i) | +0.086 ± 0.066 | 0.193 | +0.012 ± 0.067 | 0.857 |
| NAS × time (γ12 for π1i) | −0.012 ± 0.011 | 0.260* | +0.019 ± 0.013 | 0.139 |
| Alcohol (γ03 for π0i) | +0.002 ± 0.013 | 0.890 | +0.030 ± 0.016 | 0.059 |
| Alcohol × time (γ13 for π1i) | −0.001 ± 0.002 | 0.658 | +0.002 ± 0.003 | 0.627 |
| VFT-L, total score | n = 275 | n’ = 561 | n = 326 | n’ = 672 |
| Intercept (γ00 for π0i) | +14.74 ± 0.84 | <0.001 | +16.95 ± 1.44 | <0.001 |
| Time (γ10 for π1i) | +0.700 ± 0.185 | <0.001 | +0.111 ± 0.259 | 0.667 |
| Agebase | −0.002 ± 0.047 | 0.973 | −0.113 ± 0.044 | 0.008 |
| Agebase × time | −0.036 ± 0.010 | <0.001 | −0.011 ± 0.009 | 0.212 |
| Gender (women vs. men) | +0.792 ± 0.555 | 0.153 | +0.896 ± 0.558 | 0.108 |
| Gender × time | −0.018 ± 0.070 | 0.792 | −0.077 ± 0.086 | 0.371 |
| Caffeine (γ01 for π0i) | −0.147 ± 0.142 | 0.300 | +0.241 ± 0.187 | 0.198 |
| Caffeine × time (γ11 for π1i) | −0.026 ± 0.018 | 0.136 | −0.016 ± 0.028 | 0.552 |
| NAS (γ02 for π0i) | −0.084 ± 0.081 | 0.298 | +0.006 ± 0.090 | 0.951 |
| NAS × time (γ12 for π1i) | −0.012 ± 0.011 | 0.273 | +0.013 ± 0.014 | 0.334 |
| Alcohol (γ03 for π0i) | +0.027 ± 0.016 | 0.096 | +0.018 ± 0.022 | 0.420 |
| Alcohol × time (γ13 for π1i) | −0.006 ± 0.002 | 0.001 | +0.003 ± 0.004 | 0.348 |
| DS-F, total score | n = 287 | n’ = 507 | n = 254 | n’ = 560 |
| Intercept (γ00 for π0i) | +9.80 ± 0.39 | <0.001 | +10.00 ± 0.82 | <0.001 |
| Time (γ10 for π1i) | −0.021 ± 0.098 | 0.826 | −0.095 ± 0.136 | 0.481 |
| Agebase | −0.024 ± 0.018 | 0.181 | −0.068 ± 0.024 | 0.006 |
| Agebase × time | −0.001 ± 0.003 | 0.708 | +0.004 ± 0.004 | 0.371 |
| Gender (women vs. men) | −0.652 ± 0.313 | 0.037 | −0.227 ± 0.316 | 0.472 |
| Gender × time | −0.035 ± 0.053 | 0.514 | +0.051 ± 0.048 | 0.283 |
| Caffeine (γ01 for π0i) | −0.099 ± 0.080 | 0.994 | +0.053 ± 0.101 | 0.600 |
| Caffeine × time (γ11 for π1i) | −0.001 ± 0.001 | 0.994 | −0.030 ± 0.016 | 0.062 |
| NAS (γ02 for π0i) | −0.044 ± 0.048 | 0.357 | +0.059 ± 0.053 | 0.264 |
| NAS × time (γ12 for π1i) | +0.018 ± 0.009 | 0.040 | −0.001 ± 0.008 | 0.930 |
| Alcohol (γ03 for π0i) | +0.009 ± 0.009 | 0.291 | +0.024 ± 0.012 | 0.045 |
| Alcohol × time (γ13 for π1i) | −0.000 ± 0.001 | 0.694 | −0.000 ± 0.002 | 0.962 |
| DS-B, total score | n = 289 | n’ = 511 | n = 256 | n’ = 555 |
| Intercept (γ00 for π0i) | +8.93 ± 0.42 | <0.001 | +9.38 ± 0.86 | <0.001 |
| Time (γ10 for π1i) | −0.478 ± 0.148 | 0.001 | −0.186 ± 0.242 | 0.444 |
| Agebase | −0.036 ± 0.020 | 0.068 | −0.054 ± 0.026 | 0.037 |
| Agebase × time | +0.011 ± 0.004 | 0.018 | +0.001 ± 0.008 | 0.849 |
| Gender (women vs. men) | −0.251 ± 0.336 | 0.455 | −0.213 ± 0.330 | 0.518 |
| Gender × time | +0.141 ± 0.084 | 0.093 | +0.126 ± 0.089 | 0.154 |
| Caffeine (γ01 for π0i) | −0.027 ± 0.086 | 0.756 | −0.052 ± 0.108 | 0.626 |
| Caffeine × time (γ11 for π1i) | −0.004 ± 0.017 | 0.980 | −0.030 ± 0.029 | 0.296 |
| NAS (γ02 for π0i) | −0.075 ± 0.051 | 0.138 | −0.024 ± 0.056 | 0.659 |
| NAS × time (γ12 for π1i) | −0.062 ± 0.013 | 0.963 | +0.06 ± 0.016 | 0.700 |
| Alcohol (γ03 for π0i) | +0.015 ± 0.010 | 0.116 | +0.030 ± 0.012 | 0.015 |
| Alcohol × time (γ13 for π1i) | −0.002 ± 0.002 | 0.303 | −0.006 ± 0.004 | 0.121 |
Models were further adjusted for baseline year of intake, race/ethnicity, education (y), baseline smoking status and baseline BMI. See Materials and Methods for more details on covariate coding and model specifications. Random effects are presented only for the MMSE, for simplicity. *P < 0.10 for interaction with baseline age to test effect modification by age for each of the 3 predictors’ effects (i.e., caffeine intake, alcohol intake, and NAS) on cognitive performance at baseline and cognitive change over time. BLSA, Baltimore Longitudinal Study of Aging; BVRT, Benton Visual Retention Test; CVLT, California Verbal Learning Test; DS-B, digits span-backward; DS-F, digits span-forward; MMSE, Mini Mental State Examination; NAS, nutrient adequacy score; Trails A, Trail Making Test, part A; Trails B, Trail Making Test, part B; VFT-C, Verbal Fluency Test-Categorical; VFT-L, Verbal Fluency Test-Letter.
n = number of participants in the analysis.
n′ = total number of visits included in the analysis.
Cognitive scores were in the direction of higher score indicated better performance with the exception of the BVRT and Trails A and B.
Among key findings, caffeine intake was associated with better global cognitive function (MMSE) at baseline for those ≥70 y (P = 0.008), independently of potential confounding covariates. Second, the NAS was associated with better baseline performance on the MMSE overall (P = 0.004), in women (P = 0.003), and in those <70 y (P = 0.003). When Agebase was ≥70 y, the NAS was associated with better baseline performance on the CVLT-List A and CVLT-Delayed Recall. Similarly, the NAS was associated with slower decline or improvement over time on the DS-F test among women. Finally, alcohol intake was associated with faster decline or slower improvement on the MMSE (P = 0.008) and on the VFT-L test (P = 0.001) when Agebase was <70 y. However, when Agebase was ≥70 y, alcohol was related to better baseline performance on the DS-F and DS-B (Tables 2 and 3). There were no significant associations between any of the continuous dietary exposures and Trails A or B (Supplemental Table 2).
Our key findings are illustrated visually in Supplemental Figure 2 if a population with fixed characteristics (listed in the footnote of the supplemental figures) was followed up from ages 50 y and 70 y for a period of ∼9y and was alternatively exposed to 2 different levels of each dietary exposure, keeping the other exposures and covariates constant.
In a sensitivity analysis (Supplemental Table 3), categorical alcohol intake was entered into time-interval, mixed-effects regression models. Compared with 14 to 28 g/d consumption, individuals with >28 g/d of alcohol intake had faster decline or slower improvement on the MMSE, particularly among women and in the older group (Agebase ≥70 y, γ132 = −0.59 ± 0.22, P = 0.009). This finding is at slight odds from our previous results with continuous alcohol intake where we found this relation in the younger group. Moreover, consuming <14 g/d was associated with slower decline or faster improvement in the VFT-L compared with a moderate intake of 14 to 28 g/d (Agebase <70 y, γ131 = +0.24 ± 0.07, P < 0.001). A similar pattern was noted also in the younger group for both Trails A (Agebase <70 y, γ131 = −1.40 ± 0.53, P = 0.009) and Trails B (<70 y at baseline, γ131 = −3.41 ± 1.23, P = 0.006). Overall, among men, and for Agebase ≥70 y, lower alcohol intake compared with moderate consumption was associated with poorer performance on the DS-B (overall, γ031 = −0.76 ± 0.28, P = 0.008). However, and particularly among men, lower alcohol intake compared with moderate alcohol consumption was linked to slower decline on that test over time (men, γ131 = +0.19 ± 0.08, P = 0.014).
Discussion
We examined cross-sectional and longitudinal relations of caffeine and alcohol intakes and nutrient adequacy with cognitive performance in the BLSA. Outcomes included 10 cognitive test scores spanning the domains of global cognition, verbal memory, visual memory/visuo-constructive ability, verbal fluency, attention, working memory, and executive function. Caffeine and alcohol intakes and the NAS were estimated from 7-d food diaries. Using time-interval, mixed-effect regression models, with baseline defined as the earliest available visit with dietary and cognitive data, caffeine intake was associated with better baseline global cognition (MMSE), particularly when baseline age was ≥70 y, independently of key potential confounders. A higher NAS was associated with slower decline or faster improvement on a test of attention (DS-F, for women), and with better baseline performance on immediate and delayed recall for verbal memory (CVLT-List A and DR for participants aged ≥70 y at baseline). A higher NAS was also associated with better baseline performance on global cognition overall among women and among participants aged <70 y at baseline. Alcohol intake, on the other hand, was associated with slower improvement on letter fluency (VFT-L) and global cognition among those aged <70 y at baseline. Conversely, alcohol intake was associated with better attention (DS-F) and working memory (DS-B) performance, particularly among men and individuals ≥70 y at baseline. Some nonlinear associations were found, with moderate alcohol consumption only showing a beneficial effect on baseline DS-B (a measure of working memory), specifically when compared with lower intakes. However, longitudinal associations indicated that alcohol has potentially deleterious effects over time with lower intake being a better choice than moderate intake.
Caffeine and alcohol consumption and the NAS have been associated with cognition in some studies, with mixed findings with respect to the associations’ directionality. Although 2 cross-sectional studies found habitual caffeine intake to be linked with better cognitive or long-term memory performance (12, 17), 2 others failed to detect an association (19, 20). However, using data from the same cohort as in a previous study (12), after a 6-y follow-up no association was found (15). The Longitudinal Lothian Birth Cohort 1936 Study found a potential neuroprotective effect of caffeine intake, but only for coffee (83). Two other longitudinal studies reported such effects of caffeine intake in older women, but not men (9, 21). In contrast, inverse J-shaped associations between coffee consumption and 10-y cognitive declines were seen in elderly men, with the least decline occurring for men consuming 3 cups of coffee per day in the Finland, Italy, and the Netherlands Elderly (FINE) cohort (16). However, among cohort studies, the Finnish Twin Cohort Study failed to detect an association after a 28-y follow-up (18). Protective associations between tea consumption, another source of caffeine, and cognitive decline have been demonstrated in Chinese adults (10, 11). The results of our study suggest that there might be potential acute beneficial effects of caffeine on global cognition, but not other domains. Despite no sex differences, age differentials were significant whereby the putative beneficial effect on global cognition was more noticeable among older adults aged ≥70 y.
Among studies on alcohol intake and cognition (22–51), the Rotterdam Study (36) found that past alcohol consumption was predictive of speed and flexibility in a U-shaped manner, with the best performance among those drinking 1 to 4 glasses per day, particularly women, as was found in other studies (22–35, 37–39). However, a linear dose-response relation has also been shown, sometimes with differences by sex (26). One cohort study found that overall, moderate consumption was protective against poor cognitive function, but that the reverse was true among ApoE4+ individuals (22). This effect modification was not found in another study (43). Slower memory decline with increased alcohol consumption in men was found in one study, although the opposite relation was found in the case of psychomotor speed among women (44). A cross-sectional positive relation between alcohol intake and memory was noted in one study with both men and women (45). However, heavy alcohol use has also been linked to poorer cognitive outcomes (34, 46–48). Finally, few studies found no association between alcohol consumption and cognitive outcomes (49–51). Our study detected an association between alcohol intake and faster decline in global cognition and letter fluency, as well as attention and executive function, when comparing moderate consumption with lower intake—findings that were not previously replicated. In contrast, an acutely beneficial effect of alcohol in domains of attention and working memory was found in our study, which shows that alcohol can potentially alter cognitive trajectories and cross-sectional performance differently across different domains.
Individual nutrients were shown to affect cognition with the most widely studied ones being n–3 fatty acids (84–86), some B vitamins (87–91), and antioxidants (92–94), all potentially protective against cognitive impairment. Recent studies are beginning to explore how complete dietary patterns may improve cognitive performance (52, 54, 58) and slow age-related cognitive decline (2, 55, 57), particularly diets high in fruits, vegetables, nuts, unsaturated fats from fish or olive oil, and whole grain breads/cereals, and low in red and processed meats, high fat dairy, and desserts. Consistent with previous studies, we observed that a nutrient-adequate diet was associated with better performance on global cognition, particularly among those aged <70 y at baseline.
Among studies examining dietary quality or patterns in relation to cognition (2, 52–58), limited research has explored specific cognitive domains (i.e., memory, executive function, attention). Nevertheless, some studies suggest that diets with adequate nutrients are associated with better verbal memory (58) and selective attention (52), which is consistent with our findings of better baseline performance on verbal memory and slower rates of decline on verbal memory and attention. In fact, rodent models suggest that higher intakes of saturated fats and simple carbohydrates are associated with neurophysiologic changes (i.e., insulin signaling, synaptic plasticity, and neurogenesis) in the hippocampus and hippocampal-dependent learning and memory (95).
Our investigation has many strengths, which include the use of a large and long-term prospective cohort study with repeated measurements on dietary intake and a comprehensive battery of cognitive performance, allowing us to assess effects of baseline diet on baseline cognitive performance and on cognitive change over time. Specifically, associations of caffeine and alcohol intake and NAS with cognitive performance over time were examined while controlling for key potential confounders, including each of those 3 exposures and socio-demographic and lifestyle factors. Moreover, use of advanced statistical techniques such as time-interval, mixed-effects linear regression models is a major study strength.
Our findings, however, should be interpreted with caution in light of several limitations. First, the BLSA is an open-cohort study of participants selected as a convenience sample, with continuous recruitment and dropout throughout the follow-up. Second, sample selectivity was noted whereby the final analytic sample differed from the original eligible BLSA cohort. To reduce selection biases, we used a 2-stage Heckman selection model (80). Third, although observation frequency for dietary intakes and cognitive function was adequate, data structure was largely unbalanced, given that first-visit age and duration between visits varied across participants. Consequently, we used time-interval, mixed-effects linear regression models, assuming missingness at random (79). Fourth, other covariates such as cardiovascular risk factors were not considered given their potential mediating effects between diet and cognition. Additionally, chance findings may be caused by the number of hypotheses being tested and the subgroup. However, for the most part, we considered those hypotheses to be independent, given that cognitive domains included in each test were distinctive. Our analyses adjusted for multiple testing accounting for multiplicity of exposures. Finally, residual confounding and selection bias could explain some positive findings and low power may underlie some negative findings.
In conclusion, associations of caffeine and alcohol intake and nutrient adequacy with longitudinal cognitive performance are mixed in this sample of older adults. Consistent with prior studies, potential beneficial effects were found for some measures, but not others, with moderation by baseline age and sex. Although findings for alcohol consumption were mixed, the findings were generally supportive of the idea that a high-quality diet and higher caffeine intake may benefit cognition acutely and even prevent age-related declines in certain cognitive domains, including global cognition, verbal memory, and attention. Further longitudinal studies conducted on larger samples of adults are needed to determine whether dietary factors individually or in combination are modifiers of cognitive trajectories among adults.
Supplementary Material
Acknowledgments
The authors thank Dr. Melissa Kitner-Triolo and Dr. Lori L. Beason-Held (NIA/NIH/IRP) for their internal review and comments on the manuscript. M.A.B. wrote and revised the manuscript, planned analysis, performed data management and statistical analysis, and had primary responsibility for the final content; A.A.G. wrote and revised parts of the manuscript, and participated in the literature review and plan of analysis; H.A.B. participated in the literature search and review and in the revision of the manuscript; T.T. wrote and revised parts of the manuscript and participated in the literature search and plan of analysis; K.L.T. participated in data acquisition, wrote and revised parts of the manuscript, and participated in the literature search and plan of analysis; S.A.T. wrote and revised parts of the manuscript and participated in the literature search and plan of analysis; L.F. participated in data acquisition and plan of analysis, and revised the manuscript; and A.B.Z. participated in data acquisition, plan of analysis, and write-up and revision of the manuscript. All authors read and approved the final manuscript.
Footnotes
Abbreviations used: AI, adequate intake; BLSA, Baltimore Longitudinal Study of Aging; BVRT, Benton Visual Retention Test; CVLT, California Verbal Learning Test; DS-B, digits span-backward; DS-F, digits span-forward; MMSE, Mini Mental State Examination; NAS, nutrient adequacy score; Trails A, Trail Making Test, part A; Trails B, Trail Making Test, part B; UL, upper limit; VFT-C, Verbal Fluency Test-Categorical; VFT-L, Verbal Fluency Test-Letter.
Literature Cited
- 1.Moritz DJ, Kasl SV, Berkman LF. Cognitive functioning and the incidence of limitations in activities of daily living in an elderly community sample. Am J Epidemiol. 1995;141:41–9. [DOI] [PubMed] [Google Scholar]
- 2.Del Parigi A, Panza F, Capurso C, Solfrizzi V. Nutritional factors, cognitive decline, and dementia. Brain Res Bull. 2006;69:1–19. [DOI] [PubMed] [Google Scholar]
- 3.Arab L, Khan F, Lam H. Epidemiologic evidence of a relationship between tea, coffee, or caffeine consumption and cognitive decline. Adv Nutr. 2013;4:115–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Peters R, Peters J, Warner J, Beckett N, Bulpitt C. Alcohol, dementia and cognitive decline in the elderly: a systematic review. Age Ageing. 2008;37:505–12. [DOI] [PubMed] [Google Scholar]
- 5.Lourida I, Soni M, Thompson-Coon J, Purandare N, Lang IA, Ukoumunne OC, Llewellyn DJ. Mediterranean diet, cognitive function, and dementia: a systematic review. Epidemiology. 2013;24:479–89. [DOI] [PubMed] [Google Scholar]
- 6.James JE. Caffeine & health. London: Academic Press; 1991. [Google Scholar]
- 7.Bättig K, Buzzi R. Effect of coffee on the speed of subject-paced information processing. Neuropsychobiology. 1986;16:126–30. [DOI] [PubMed] [Google Scholar]
- 8.Riedel W, Hogervorst E, Leboux R, Verhey F, van Praag H, Jolles J. Caffeine attenuates scopolamine-induced memory impairment in humans. Psychopharmacology (Berl). 1995;122:158–68. [DOI] [PubMed] [Google Scholar]
- 9.Arab L, Biggs ML, O'Meara ES, Longstreth WT, Crane PK, Fitzpatrick AL. Gender differences in tea, coffee, and cognitive decline in the elderly: the Cardiovascular Health Study. J Alzheimers Dis. 2011;27:553–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Feng L, Gwee X, Kua EH, Ng TP. Cognitive function and tea consumption in community dwelling older Chinese in Singapore. J Nutr Health Aging. 2010;14:433–8. [DOI] [PubMed] [Google Scholar]
- 11.Feng L, Li J, Ng TP, Lee TS, Kua EH, Zeng Y. Tea drinking and cognitive function in oldest-old Chinese. J Nutr Health Aging. 2012;16:754–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hameleers PA, Van Boxtel MP, Hogervorst E, Riedel WJ, Houx PJ, Buntinx F, Jolles J. Habitual caffeine consumption and its relation to memory, attention, planning capacity and psychomotor performance across multiple age groups. Hum Psychopharmacol. 2000;15:573–81. [DOI] [PubMed] [Google Scholar]
- 13.Ng TP, Feng L, Niti M, Kua EH, Yap KB. Tea consumption and cognitive impairment and decline in older Chinese adults. Am J Clin Nutr. 2008;88:224–31. [DOI] [PubMed] [Google Scholar]
- 14.Ritchie K, Carriere I, de Mendonca A, Portet F, Dartigues JF, Rouaud O, Barberger-Gateau P, Ancelin ML. The neuroprotective effects of caffeine: a prospective population study (the Three City Study). Neurology. 2007;69:536–45. [DOI] [PubMed] [Google Scholar]
- 15.van Boxtel MP, Schmitt JA, Bosma H, Jolles J. The effects of habitual caffeine use on cognitive change: a longitudinal perspective. Pharmacol Biochem Behav. 2003;75:921–7. [DOI] [PubMed] [Google Scholar]
- 16.van Gelder BM, Buijsse B, Tijhuis M, Kalmijn S, Giampaoli S, Nissinen A, Kromhout D. Coffee consumption is inversely associated with cognitive decline in elderly European men: the FINE Study. Eur J Clin Nutr. 2007;61:226–32. [DOI] [PubMed] [Google Scholar]
- 17.Jarvis MJ. Does caffeine intake enhance absolute levels of cognitive performance? Psychopharmacology (Berl). 1993;110:45–52. [DOI] [PubMed] [Google Scholar]
- 18.Laitala VS, Kaprio J, Koskenvuo M, Raiha I, Rinne JO, Silventoinen K. Coffee drinking in middle age is not associated with cognitive performance in old age. Am J Clin Nutr. 2009;90:640–6. [DOI] [PubMed] [Google Scholar]
- 19.Johnson-Kozlow M, Kritz-Silverstein D, Barrett-Connor E, Morton D. Coffee consumption and cognitive function among older adults. Am J Epidemiol. 2002;156:842–50. [DOI] [PubMed] [Google Scholar]
- 20.Smith AP. Caffeine, cognitive failures and health in a non-working community sample. Hum Psychopharmacol. 2009;24:29–34. [DOI] [PubMed] [Google Scholar]
- 21.Ritchie K, Artero S, Portet F, Brickman A, Muraskin J, Beanino E, Ancelin ML, Carriere I. Caffeine, cognitive functioning, and white matter lesions in the elderly: establishing causality from epidemiological evidence. J Alzheimers Dis. 2010;20(Suppl 1):S161–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Anttila T, Helkala EL, Viitanen M, Kareholt I, Fratiglioni L, Winblad B, Soininen H, Tuomilehto J, Nissinen A, Kivipelto M. Alcohol drinking in middle age and subsequent risk of mild cognitive impairment and dementia in old age: a prospective population based study. BMJ. 2004;329:539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stampfer MJ, Kang JH, Chen J, Cherry R, Grodstein F. Effects of moderate alcohol consumption on cognitive function in women. N Engl J Med. 2005;352:245–53. [DOI] [PubMed] [Google Scholar]
- 24.Ganguli M, Vander Bilt J, Saxton JA, Shen C, Dodge HH. Alcohol consumption and cognitive function in late life: a longitudinal community study. Neurology. 2005;65:1210–7. [DOI] [PubMed] [Google Scholar]
- 25.Elias PK, Elias MF, D'Agostino RB, Silbershatz H, Wolf PA. Alcohol consumption and cognitive performance in the Framingham Heart Study. Am J Epidemiol. 1999;150:580–9. [DOI] [PubMed] [Google Scholar]
- 26.Bond GE, Burr R, McCurry SM, Graves AB, Larson EB. Alcohol, aging, and cognitive performance in a cohort of Japanese Americans aged 65 and older: the Kame Project. Int Psychogeriatr. 2001;13:207–23. [DOI] [PubMed] [Google Scholar]
- 27.Zuccalà G, Onder G, Pedone C, Cesari M, Landi F, Bernabei R, Cocchi A. Dose-related impact of alcohol consumption on cognitive function in advanced age: results of a multicenter survey. Alcohol Clin Exp Res. 2001;25:1743–8. [PubMed] [Google Scholar]
- 28.Rodgers B, Windsor TD, Anstey KJ, Dear KB, Jorm A, Christensen H. Non-linear relationships between cognitive function and alcohol consumption in young, middle-aged and older adults: the PATH Through Life Project. Addiction. 2005;100:1280–90. [DOI] [PubMed] [Google Scholar]
- 29.Reid MC, Van Ness PH, Hawkins KA, Towle V, Concato J, Guo Z. Light to moderate alcohol consumption is associated with better cognitive function among older male veterans receiving primary care. J Geriatr Psychiatry Neurol. 2006;19:98–105. [DOI] [PubMed] [Google Scholar]
- 30.Solfrizzi V, D'Introno A, Colacicco AM, Capurso C, Del Parigi A, Baldassarre G, Scapicchio P, Scafato E, Amodio M, Capurso A, et al. Alcohol consumption, mild cognitive impairment, and progression to dementia. Neurology. 2007;68:1790–9. [DOI] [PubMed] [Google Scholar]
- 31.Xu G, Liu X, Yin Q, Zhu W, Zhang R, Fan X. Alcohol consumption and transition of mild cognitive impairment to dementia. Psychiatry Clin Neurosci. 2009;63:43–9. [DOI] [PubMed] [Google Scholar]
- 32.Au Yeung SL, Jiang C, Zhang W, Lam TH, Cheng KK, Leung GM, Schooling CM. Moderate alcohol use and cognitive function in the Guangzhou Biobank Cohort Study. Ann Epidemiol. 2010;20:873–82. [DOI] [PubMed] [Google Scholar]
- 33.Bond GE, Burr R, McCurry SM, Rice MM, Borenstein AR, Kukull WA, Teri L, Bowen JD, McCormick WC, Larson EB. Alcohol, gender, and cognitive performance: a longitudinal study comparing older Japanese and non-Hispanic white Americans. J Aging Health. 2004;16:615–40. [DOI] [PubMed] [Google Scholar]
- 34.Chan KK, Chiu KC, Chu LW. Association between alcohol consumption and cognitive impairment in Southern Chinese older adults. Int J Geriatr Psychiatry. 2010;25:1272–9. [DOI] [PubMed] [Google Scholar]
- 35.Bond GE, Burr RL, McCurry SM, Rice MM, Borenstein AR, Larson EB. Alcohol and cognitive performance: a longitudinal study of older Japanese Americans. The Kame Project. Int Psychogeriatr. 2005;17:653–68. [DOI] [PubMed] [Google Scholar]
- 36.Kalmijn S, van Boxtel MP, Verschuren MW, Jolles J, Launer LJ. Cigarette smoking and alcohol consumption in relation to cognitive performance in middle age. Am J Epidemiol. 2002;156:936–44. [DOI] [PubMed] [Google Scholar]
- 37.Hendrie HC, Gao S, Hall KS, Hui SL, Unverzagt FW. The relationship between alcohol consumption, cognitive performance, and daily functioning in an urban sample of older black Americans. J Am Geriatr Soc. 1996;44:1158–65. [DOI] [PubMed] [Google Scholar]
- 38.Dufouil C, Ducimetiere P, Alperovitch A. Sex differences in the association between alcohol consumption and cognitive performance. EVA Study Group. Epidemiology of Vascular Aging. Am J Epidemiol. 1997;146:405–12. [DOI] [PubMed] [Google Scholar]
- 39.Leroi I, Sheppard JM, Lyketsos CG. Cognitive function after 11.5 years of alcohol use: relation to alcohol use. Am J Epidemiol. 2002;156:747–52. [DOI] [PubMed] [Google Scholar]
- 40.Zanjani F, Downer BG, Kruger TM, Willis SL, Schaie KW. Alcohol effects on cognitive change in middle-aged and older adults. Aging Ment Health. 2013;17:12–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wright CB, Elkind MS, Luo X, Paik MC, Sacco RL. Reported alcohol consumption and cognitive decline: the Northern Manhattan Study. Neuroepidemiology. 2006;27:201–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Britton A, Singh-Manoux A, Marmot M. Alcohol consumption and cognitive function in the Whitehall II Study. Am J Epidemiol. 2004;160:240–7. [DOI] [PubMed] [Google Scholar]
- 43.Ngandu T, Helkala EL, Soininen H, Winblad B, Tuomilehto J, Nissinen A, Kivipelto M. Alcohol drinking and cognitive functions: findings from the Cardiovascular Risk Factors Aging and Dementia (CAIDE) Study. Dement Geriatr Cogn Disord. 2007;23:140–9. [DOI] [PubMed] [Google Scholar]
- 44.Richards M, Hardy R, Wadsworth ME. Alcohol consumption and midlife cognitive change in the British 1946 Birth Cohort Study. Alcohol Alcohol. 2005;40:112–7. [DOI] [PubMed] [Google Scholar]
- 45.Corley J, Jia X, Brett CE, Gow AJ, Starr JM, Kyle JA, McNeill G, Deary IJ. Alcohol intake and cognitive abilities in old age: the Lothian Birth Cohort 1936 Study. Neuropsychology. 2011;25:166–75. [DOI] [PubMed] [Google Scholar]
- 46.Gross AL, Rebok GW, Ford DE, Chu AY, Gallo JJ, Liang KY, Meoni LA, Shihab HM, Wang NY, Klag MJ. Alcohol consumption and domain-specific cognitive function in older adults: longitudinal data from the Johns Hopkins Precursors Study. J Gerontol B Psychol Sci Soc Sci. 2011;66:39–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sabia S, Gueguen A, Berr C, Berkman L, Ankri J, Goldberg M, Zins M, Singh-Manoux A. High alcohol consumption in middle-aged adults is associated with poorer cognitive performance only in the low socio-economic group. Results from the GAZEL Cohort Study. Addiction. 2011;106:93–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhou H, Deng J, Li J, Wang Y, Zhang M, He H. Study of the relationship between cigarette smoking, alcohol drinking and cognitive impairment among elderly people in China. Age Ageing. 2003;32:205–10. [DOI] [PubMed] [Google Scholar]
- 49.Broe GA, Creasey H, Jorm AF, Bennett HP, Casey B, Waite LM, Grayson DA, Cullen J. Health habits and risk of cognitive impairment and dementia in old age: a prospective study on the effects of exercise, smoking and alcohol consumption. Aust N Z J Public Health. 1998;22:621–3. [DOI] [PubMed] [Google Scholar]
- 50.Edelstein SL, Kritz-Silverstein D, Barrett-Connor E. Prospective association of smoking and alcohol use with cognitive function in an elderly cohort. J Womens Health. 1998;7:1271–81. [DOI] [PubMed] [Google Scholar]
- 51.Krahn D, Freese J, Hauser R, Barry K, Goodman B. Alcohol use and cognition at mid-life: the importance of adjusting for baseline cognitive ability and educational attainment. Alcohol Clin Exp Res. 2003;27:1162–6. [DOI] [PubMed] [Google Scholar]
- 52.Capurso A, Solfrizzi V, Panza F, Torres F, Mastroianni F, Grassi A, Del Parigi A, Capurso C, Pirozzi MR, Centonze S, et al. Dietary patterns and cognitive functions in elderly subjects. Aging (Milano). 1997;9:45–7. [DOI] [PubMed] [Google Scholar]
- 53.Huijbregts PP, Feskens EJ, Rasanen L, Fidanza F, Alberti-Fidanza A, Nissinen A, Giampaoli S, Kromhout D. Dietary patterns and cognitive function in elderly men in Finland, Italy and The Netherlands. Eur J Clin Nutr. 1998;52:826–31. [DOI] [PubMed] [Google Scholar]
- 54.Velho S, Marques-Vidal P, Baptista F, Camilo ME. Dietary intake adequacy and cognitive function in free-living active elderly: a cross-sectional and short-term prospective study. Clin Nutr. 2008;27:77–86. [DOI] [PubMed] [Google Scholar]
- 55.Féart C, Samieri C, Barberger-Gateau P. Mediterranean diet and cognitive function in older adults. Curr Opin Clin Nutr Metab Care. 2010;13:14–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tangney CC, Kwasny MJ, Li H, Wilson RS, Evans DA, Morris MC. Adherence to a Mediterranean-type dietary pattern and cognitive decline in a community population. Am J Clin Nutr. 2011;93:601–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Vercambre MN, Grodstein F, Berr C, Kang JH. Mediterranean diet and cognitive decline in women with cardiovascular disease or risk factors. J Acad Nutr Diet. 2012;112:816–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kesse-Guyot E, Andreeva VA, Jeandel C, Ferry M, Hercberg S, Galan P. A healthy dietary pattern at midlife is associated with subsequent cognitive performance. J Nutr. 2012;142:909–15. [DOI] [PubMed] [Google Scholar]
- 59.IOM. Dietary reference intakes. The essential guide to nutrient requirements. Washington: National Academy of Science; 2006.
- 60.Guenther PM, Reedy J, Krebs-Smith SM. Development of the Healthy Eating Index-2005. J Am Diet Assoc. 2008;108:1896–901. [DOI] [PubMed] [Google Scholar]
- 61.U.S. Department of Agriculture (USDA). Healthy Eating Index 2005 [cited 2007 March 20]. Available from: http://www.cnpp.usda.gov/Publications/HEI/healthyeatingindex2005factsheet.pdf.
- 62.McCullough ML, Feskanich D, Rimm EB, Giovannucci EL, Ascherio A, Variyam JN, Spiegelman D, Stampfer MJ, Willett WC. Adherence to the Dietary Guidelines for Americans and risk of major chronic disease in men. Am J Clin Nutr. 2000;72:1223–31. [DOI] [PubMed] [Google Scholar]
- 63.Shock N, Greulich RC, Andres R, Arenberg D, Costa PT, Lakatta EG, Tobin JD. Normal human aging: the Baltimore Longitudinal Study of Aging. Washington: U.S: Government Printing Office; 1984. [Google Scholar]
- 64.Zonderman AB, Giambra LM, Arenberg D, Resnick SM, Costa PT, Jr, Kawas CH. Changes in immediate visual memory predict cognitive impairment. Arch Clin Neuropsychol. 1995;10:111–23. [PubMed] [Google Scholar]
- 65.McGandy RB, Barrows CH, Jr, Spanias A, Meredith A, Stone JL, Norris AH. Nutrient intakes and energy expenditure in men of different ages. J Gerontol. 1966;21:581–7. [DOI] [PubMed] [Google Scholar]
- 66.Elahi VK, Elahi D, Andres R, Tobin JD, Butler MG, Norris AH. A longitudinal study of nutritional intake in men. J Gerontol. 1983;38:162–80. [DOI] [PubMed] [Google Scholar]
- 67.Hallfrisch J, Muller D, Drinkwater D, Tobin J, Andres R. Continuing diet trends in men: the Baltimore Longitudinal Study of Aging (1961–1987). J Gerontol. 1990;45:M186–91. [DOI] [PubMed] [Google Scholar]
- 68.U.S. Department of Agriculture (USDA), Agriculture Research Service FSRG. Food and Nutrient Database for Dietary Studies, 3.0 [database on the Internet]. Available from: http://www.ars.usda.gov/Services/docs.htm?docid=17031.
- 69.Schröder H, Vila J, Marrugat J, Covas MI. Low energy density diets are associated with favorable nutrient intake profile and adequacy in free-living elderly men and women. J Nutr. 2008;138:1476–81. [DOI] [PubMed] [Google Scholar]
- 70.Folstein MF, Folstein SE, McHugh PR. "Mini-mental state". A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–98. [DOI] [PubMed] [Google Scholar]
- 71.Benton AL. editor. Revised visual retention test. 5th ed. New York: The Psychological Corporation; 1974. [Google Scholar]
- 72.Delis DC, Freeland J, Kramer JH, Kaplan E. Integrating clinical assessment with cognitive neuroscience: construct validation of the California Verbal Learning Test. J Consult Clin Psychol. 1988;56:123–30. [DOI] [PubMed] [Google Scholar]
- 73.Lezak MD. Neuropsychological assessment. 2nd ed. New York: Oxford University Press; 1983. [Google Scholar]
- 74.Lezak MD. Neuropsychological assessment. 3rd ed. New York: Oxford University Press; 1995. [Google Scholar]
- 75.Rosen W. Verbal fluency in aging and dementia. J Clin Neuropsychol. 1980;2:135–46. [Google Scholar]
- 76.Reitan R. Trail Making Test: manual for administration and scoring. Tucson (AZ): Reitan Neuropsychological Laboratory; 1992. [Google Scholar]
- 77.Wechsler D. WAIS-R manual. Cleveland (OH): The Psychological Corporation; 1981. [Google Scholar]
- 78.STATA. Statistics/data analysis: release 11.0. College Station (TX): Stata Corporation; 2009. [Google Scholar]
- 79.Blackwell E, de Leon CF, Miller GE. Applying mixed regression models to the analysis of repeated-measures data in psychosomatic medicine. Psychosom Med. 2006;68:870–8. [DOI] [PubMed] [Google Scholar]
- 80.Heckman JJ. Sample selection bias as a specification error. Econometrica. 1979;47:153–61. [Google Scholar]
- 81.Beydoun MA, Boueiz A, Abougergi MS, Kitner-Triolo MH, Beydoun HA, Resnick SM, O'Brien R, Zonderman AB. Sex differences in the association of the apolipoprotein E epsilon 4 allele with incidence of dementia, cognitive impairment, and decline. Neurobiol Aging. 2012;33:720–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Selvin S. Statistical analysis of epidemiologic data. 3rd ed. Oxford (United Kingdom): Oxford University Press; 2004. [Google Scholar]
- 83.Corley J, Jia X, Kyle JA, Gow AJ, Brett CE, Starr JM, McNeill G, Deary IJ. Caffeine consumption and cognitive function at age 70: the Lothian Birth Cohort 1936 Study. Psychosom Med. 2010;72:206–14. [DOI] [PubMed] [Google Scholar]
- 84.Beydoun MA, Kaufman JS, Sloane PD, Heiss G, Ibrahim J. n-3 fatty acids, hypertension and risk of cognitive decline among older adults in the Atherosclerosis Risk in Communities (ARIC) Study. Public Health Nutr. 2008;11:17-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Solfrizzi V, Colacicco AM, D'Introno A, Capurso C, Torres F, Rizzo C, Capurso A, Panza F. Dietary intake of unsaturated fatty acids and age-related cognitive decline: a 8.5-year follow-up of the Italian Longitudinal Study on Aging. Neurobiol Aging. 2006;27:1694–704. [DOI] [PubMed] [Google Scholar]
- 86.Morris MC, Evans DA, Tangney CC, Bienias JL, Schneider JA, Wilson RS, Scherr PA. Dietary copper and high saturated and trans fat intakes associated with cognitive decline. Arch Neurol. 2006;63:1085–8. [DOI] [PubMed] [Google Scholar]
- 87.Kado DM, Karlamangla AS, Huang MH, Troen A, Rowe JW, Selhub J, Seeman TE. Homocysteine versus the vitamins folate, B6, and B12 as predictors of cognitive function and decline in older high-functioning adults: MacArthur Studies of Successful Aging. Am J Med. 2005;118:161–7. [DOI] [PubMed] [Google Scholar]
- 88.Tucker KL, Qiao N, Scott T, Rosenberg I, Spiro A III. High homocysteine and low B vitamins predict cognitive decline in aging men: the Veterans Affairs Normative Aging Study. Am J Clin Nutr. 2005;82:627–35. [DOI] [PubMed] [Google Scholar]
- 89.Ravaglia G, Forti P, Maioli F, Martelli M, Servadei L, Brunetti N, Porcellini E, Licastro F. Homocysteine and folate as risk factors for dementia and Alzheimer disease. Am J Clin Nutr. 2005;82:636–43. [DOI] [PubMed] [Google Scholar]
- 90.Mooijaart SP, Gussekloo J, Frolich M, Jolles J, Stott DJ, Westendorp RG, de Craen AJ. Homocysteine, vitamin B-12, and folic acid and the risk of cognitive decline in old age: the Leiden 85-Plus Study. Am J Clin Nutr. 2005;82:866–71. [DOI] [PubMed] [Google Scholar]
- 91.Feng L, Ng TP, Chuah L, Niti M, Kua EH. Homocysteine, folate, and vitamin B-12 and cognitive performance in older Chinese adults: findings from the Singapore Longitudinal Ageing Study. Am J Clin Nutr. 2006;84:1506–12. [DOI] [PubMed] [Google Scholar]
- 92.Morris MC. Diet and Alzheimer's disease: what the evidence shows. MedGenMed. 2004;6:48. [PMC free article] [PubMed] [Google Scholar]
- 93.Luchsinger JA, Tang MX, Shea S, Mayeux R. Antioxidant vitamin intake and risk of Alzheimer disease. Arch Neurol. 2003;60:203–8. [DOI] [PubMed] [Google Scholar]
- 94.Kalmijn S, Feskens EJ, Launer LJ, Kromhout D. Polyunsaturated fatty acids, antioxidants, and cognitive function in very old men. Am J Epidemiol. 1997;145:33–41. [DOI] [PubMed] [Google Scholar]
- 95.Kanoski SE, Davidson TL. . Western diet consumption and cognitive impairment: links to hippocampal dysfunction and obesity. Physiol Behav. 2011;103:59–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


