Abstract
Diarrhea and linear growth faltering continue to burden low-income countries and are among the most important contributors to poor health during early childhood. Diarrhea is thought to adversely affect linear growth, but catch-up growth can occur if no additional insults are experienced. We sought to characterize catch-up growth in relation to diarrhea burden in a multisite dataset of 1007 children. Using longitudinal anthropometry and diarrheal surveillance data from 7 cohort studies in 4 countries, we examined the relation between diarrhea prevalence and growth in 3- to 6-mo periods using linear mixed-effect models. Growth during each period was calculated as a function of age using linear splines. We incorporated the longitudinal prevalence of diarrhea in both current and previous periods into the model. Diarrhea during the current period was associated with slower linear and ponderal growth. Faster (catch-up) growth in length was observed in children with no diarrhea in age groups immediately after an age group in which diarrhea was experienced [age group >6–12 mo: 0.03 mm/mo for each percentage diarrhea prevalence in the previous period (95% CI: 0.007, 0.06) relative to 11.3 mm/mo mean growth rate; age group >12–18 mo: 0.04 mm/mo (95% CI: 0.02, 0.06) relative to 8.9 mm/mo mean growth rate; age group >18–24 mo: 0.04 mm/mo (95% CI: 0.003, 0.09) relative to 7.9 mm/mo mean growth rate]. The associations were stronger in boys than in girls when separate models were run. Similar results were observed when weight was the outcome variable. When diarrheal episodes are followed by diarrhea-free periods in the first 2 y of life, catch-up growth is observed that may allow children to regain their original trajectories. The finding of a greater effect of diarrhea on linear growth in boys than in girls was unexpected and requires additional study. Diarrhea burdens are high throughout the first 2 y of life in these study sites, therefore reducing the likelihood of catch-up growth. Extending diarrhea-free periods may increase the likelihood of catch-up growth and decrease the prevalence of stunting.
Introduction
Although improvements in child growth on a global scale have been observed over the past 20 y, 26% of children worldwide are stunted [height-for-age Z-score (HAZ) < −2] (1). Childhood stunting is indicative of inadequate dietary intake and high infectious disease burdens and has been associated with both short-term (increased child mortality) and long-term (decreased educational attainment and economic productivity throughout adulthood) consequences (1). The association between diarrhea and linear growth during early childhood has been studied intensely in the literature, with some inconsistent findings (2–5). Many studies found a long-term association between overall diarrhea burden and height (5–15), although not all observed this association (16–19). Diarrhea represents a syndrome with a variety of etiologies and associated severity (20), and site-specific etiologies and treatment practices may modify the association, potentially resulting in inconsistent results.
Growth in early childhood is thought by some to be a saltatory process, alternating between periods of slower and faster growth (21). Among acutely malnourished children, it is thought that a child needs to recover in weight before resuming linear growth (22, 23). Linear growth may falter if a child experiences recurrent weight faltering associated with diarrhea episodes (3, 24), but catch-up growth is possible given adequate diet and time between infections (25), as was demonstrated very successfully in international adoption studies (26). Nutritional supplementation was observed to offset the effect of diarrhea on growth faltering, likely through contributing to catch-up growth (8). However, the opportunity for catch-up growth may be missed if diarrhea-free periods are abbreviated, resulting in failure to achieve full biologic growth potential.
Previous studies primarily considered length (or HAZ) at a certain age as the outcome and summarized diarrhea burden over the entire period of follow-up. Although this method provides an estimate of the overall association between diarrhea and growth, it lacks precision with respect to timing and the potential for catch-up growth. A previous analysis performed using these data considered the lagged effects of diarrhea on growth over time and found that cumulative months with diarrhea had a small but significant effect on linear growth at age 2 y (5). Few studies have had the sample sizes available to quantify catch-up growth after a diarrheal episode. This study will consider the association between age-specific diarrhea burden and growth velocity in both length and weight, with the hypothesis that gaps between episodes of diarrhea allow for faster, catch-up growth to occur.
Methods
Study design.
We used growth and disease surveillance data from 7 cohort studies performed in four countries [Peru (11, 13, 27), Brazil (12), Guinea-Bissau (28, 29), and Bangladesh (30)]. The studies are longitudinal cohorts that were conducted over a period of 2 decades and were performed in Africa, Asia, and Latin America (Supplemental Table 1). We searched for longitudinal cohort studies with diarrhea surveillance and anthropometry and obtained the original data whenever possible (31). Children were retained in the dataset if they had their first anthropometric measurement before age 2 mo and if they had at least 4 anthropometric measures before age 24 mo. We included 1007 children from 4 countries in this analysis (Table 1). Many of the studies collected anthropometry data on a monthly basis, whereas others collected anthropometry less frequently. HAZ and weight-for-age Z-score (WAZ) were calculated using the WHO Multicenter Growth Reference Study program (32). At baseline, 14% of the children were stunted, whereas at the final anthropometric measure (ages 12–24 mo), 37% of the children were stunted (Table 1).
TABLE 1.
Description of studies included in the combined dataset1
| Stunted (HAZ < −2) |
Mean diarrhea prevalence per year2 |
|||||||||||
| Location, year | Reference | Participants | Male | Mean length measures | Baseline | Final | Overall | Girls | Boys | Acute episodes | Prolonged episodes | Persistent episodes |
| n | n (%) | n | % | d/y | n (%) | n (%) | n (%) | |||||
| Peru, 1985 | Lanata et al. (27) | 111 | 56 (50) | 19 | 5 | 32 | 33 | 33 | 32 | 1289 (89) | 124 (9) | 34 (2) |
| Peru, 1989 | Checkley et al. (11) | 83 | 44 (53) | 17 | 1 | 19 | 32 | 34 | 30 | 384 (74) | 72 (14) | 60 (12) |
| Peru, 1995 | Checkley et al. (13) | 157 | 87 (55) | 20 | 5 | 25 | 9 | 9 | 9 | 894 (96) | 31 (3) | 2 (0.2) |
| Brazil, 1989 | Moore et al. (12) | 118 | 56 (47) | 7 | 4 | 23 | 23 | 22 | 25 | 788 (81) | 126 (13) | 55 (6) |
| Guinea-Bissau, 1987 | Mølbak et al. (28) | 79 | 37 (47) | 6 | 9 | 41 | 44 | 38 | 50 | 910 (84) | 124 (11) | 47 (4) |
| Guinea-Bissau, 1996 | Valentiner-Branth et al. (29) | 209 | 96 (46) | 10 | 10 | 23 | 24 | 22 | 26 | 1564 (87) | 177 (10) | 55 (3) |
| Bangladesh, 1993 | Pathela et al. (30) | 250 | 142 (57) | 21 | 38 | 69 | 24 | 23 | 25 | 1507 (76) | 393 (20) | 82 (4) |
| Total | 1007 | 518 (51) | 15 | 14 | 37 | 25 | 24 | 25 | 7336 (84) | 1047 (12) | 335 (4) | |
HAZ, height-for-age Z-score.
Mean diarrhea prevalence = n diarrhea days/n surveillance days × 365.
Field workers visited families at least twice a week to collect daily diarrhea data in all of the studies except for Guinea-Bissau, which collected daily diarrhea data on a weekly basis. The definition of diarrhea for the studies was ≥3 liquid or semiliquid stools in a 24-h period, and diarrhea episodes were separated by 2 diarrhea-free days. Mean diarrhea prevalence ranged from 9 d/y in Peru 1995 to 44 d/y in Guinea-Bissau 1987. Diarrhea burden was similar in girls and boys in most of the studies (Table 1). The majority of diarrhea episodes were acute (duration: <8 d), 12% were prolonged (duration: 8–14 d), and 4% were persistent (duration: >14 d) (Table 1).
Biostatistical methods.
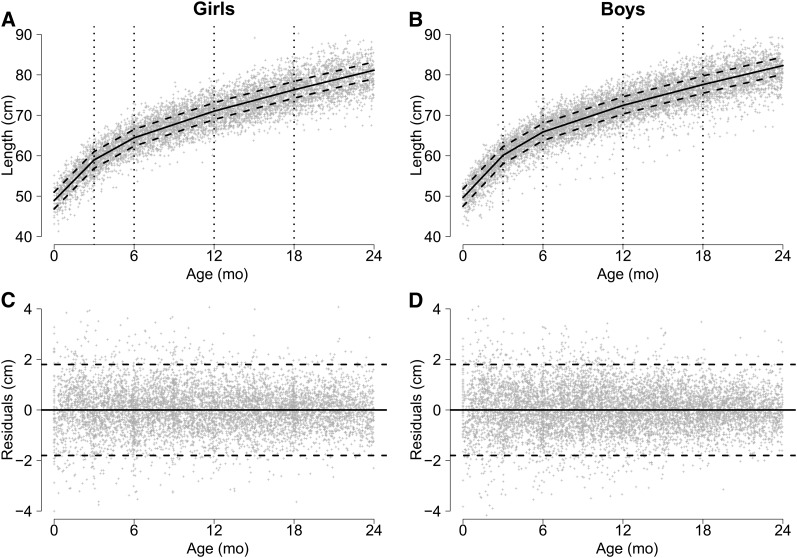
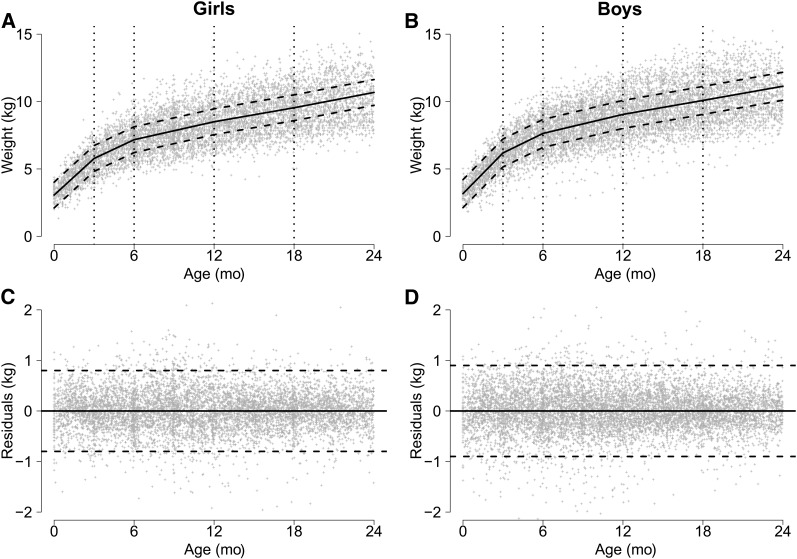
To quantify the association between diarrhea and linear and ponderal (weight) growth velocity, we modeled growth as a piecewise linear function of age with knots at 3, 6, 12, and 18 mo (Figs. 1 and 2). Also included in the model is a coefficient for sex, indicator variables for each study, and whether the child was moderately underweight (WAZ < −1) at their first visit, which occurred before age 2 mo. We accounted for heterogeneity in growth among children using random effects for the intercept (b0) and age (b1) by child. Diarrhea burden was added to the model as an interaction term with age for each of the current and previous time periods. Model details can be found in the Supplemental Text. Lengths and weights at age 24 mo were estimated using 3 categories of diarrhea burden: 0%, the mean percentage diarrhea burden in each time period (5%, 7%, 8%, 7%, and 5% in the age groups 0–3, >3–6, >6–12, >12–18, and >18–24 mo), and twice the mean percentage diarrhea burden in each period (10%, 14%, 16%, 14%, and 10% diarrhea prevalence in the age groups 0–3, >3–6, >6–12, >12–18, and >18–24 mo). Wald tests were used to test for interactions between age, diarrhea burden, and sex. We used R version 2.14.2 (33) for statistical analyses, specifically, the nlme package for the random-effects models, the gmodels package to derive the predicted effects at age 24 mo, and the graphics package for the figures.
FIGURE 1.
Relation between age and length (A, B) and residuals (C, D), by sex, in a combined dataset including anthropometry data collected in children aged <24 mo from 7 cohort studies. Spline model (solid line) has knots at ages 3, 6, 12, and 18 mo. The dashed lines represent ±2 SDs.
FIGURE 2.
Relation between age and weight (A, B) and residuals (C, D), by sex, in a combined dataset including anthropometry data collected in children aged <24 mo from 7 cohort studies. Spline model (solid line) has knots at ages 3, 6, 12, and 18 mo. The dashed lines represent ±2 SDs.
Results
Association between diarrhea and linear growth.
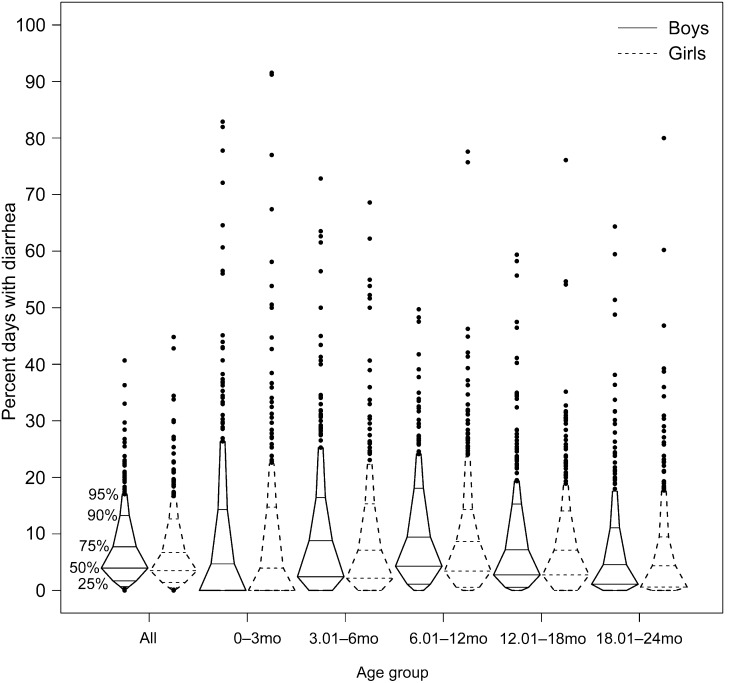
In Figure 3, we display the percentage of days ill with diarrhea in children by age group. Specifically, we show that diarrhea burden was greatest between 6 and 18 mo and that there appeared to be little difference in diarrhea burden with sex. We calculated the association between diarrhea and linear growth during each period, overall and by sex (Supplemental Table 2). Diarrhea during the current period was associated with lower length velocity during all time periods (Fig. 4A). When stratified by sex, the confidence interval for the association between concurrent diarrhea and length velocity did not overlap 0 in boys at >3–6, >6–12, and >18–24 mo. Although all of the point estimates for girls were negative, all of the confidence intervals overlapped 0.
FIGURE 3.
Percentage of days with diarrhea overall and during 3- to 6-mo time periods, by sex.
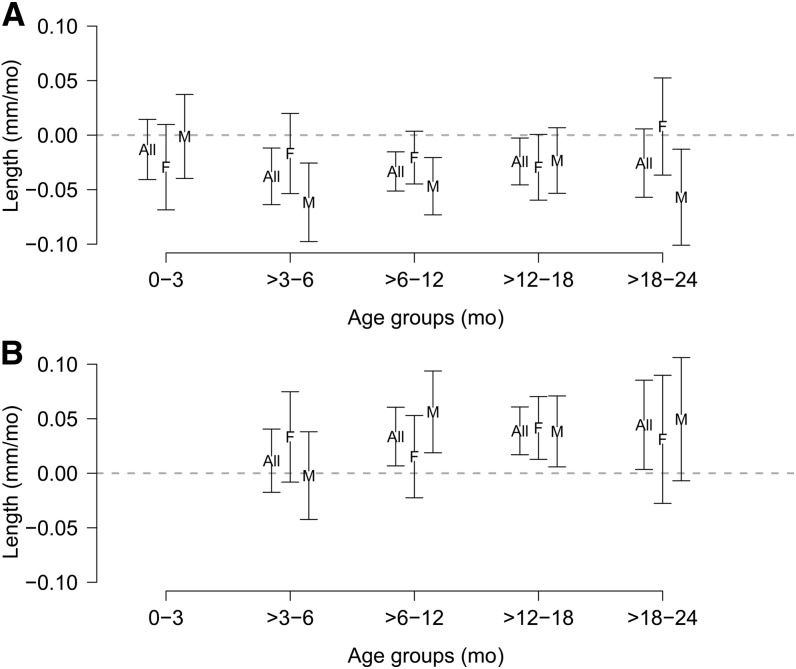
FIGURE 4.
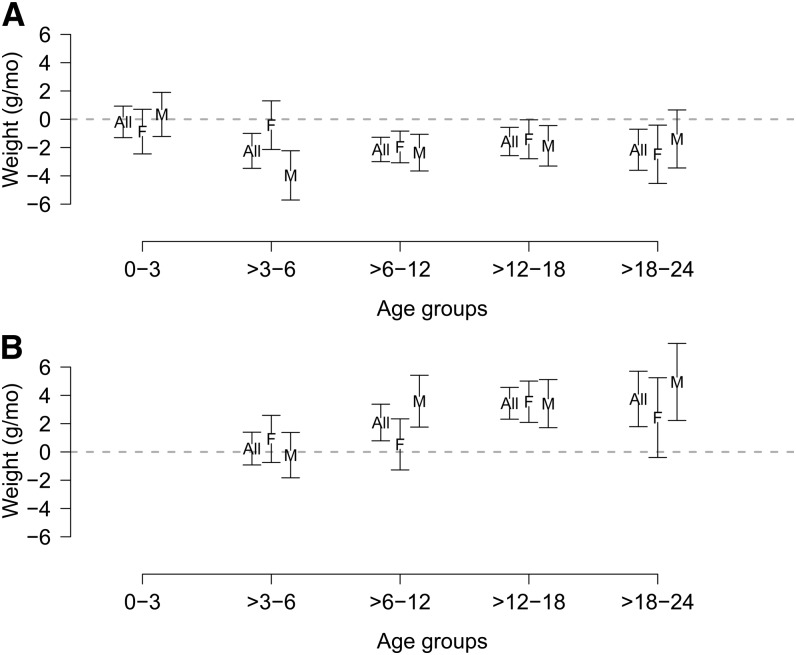
Difference in length slopes at 0–3, >3–6, >6–12, >12–18, and >18–24 mo contributed by current period diarrhea prevalence (A) and previous period diarrhea prevalence (B), overall and by sex. Values are coefficients produced by the models and represent difference in slope measured in millimeters per month of age associated with percentage of days with diarrhea during that period (A) or the previous period (B). All, overall; F, female; M, male.
Interaction terms considering age, diarrhea burden, and sex were important (age × sex interaction, P < 0.001; 3-way interaction of age × diarrhea × sex, P = 0.01); therefore, we present the sex-specific analyses and the overall model results in some cases for comparison. Individual Wald tests were important only for the interactions age × sex in the age groups 0–3 mo (P = 0.02) and >6–12 mo (P = 0.01) and age × diarrhea × sex during the age group >6–12 mo (P = 0.04).
Catch-up linear growth.
Catch-up growth in the absence of current diarrhea was indicated by the positive coefficients associated with diarrhea during the previous period (Supplemental Fig. 1). If the child did not have additional diarrhea during the current period, linear growth would speed up, potentially returning the child to his or her original trajectory, and this trend toward catch-up growth was observed in all age groups (Fig. 4B). Diarrhea in the previous time period followed by no diarrhea in the current time period was associated with higher length velocity overall, with some minor differences by sex.
Length at age 24 mo.
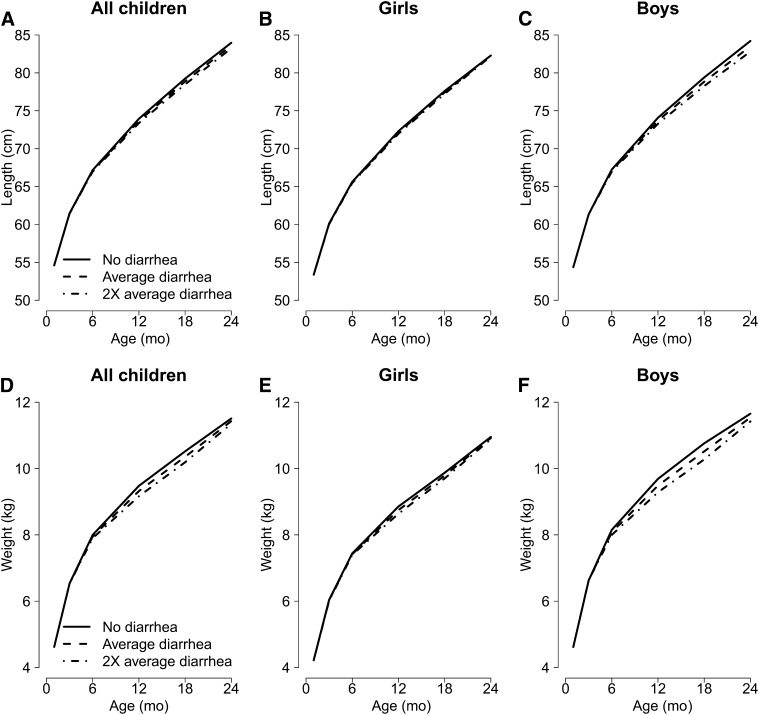
We can estimate the length of children at age 24 mo based on the model results according to different diarrhea scenarios. No diarrhea during the first 2 y of life was associated with greater length at age 2 y when compared with the mean or twice the mean diarrhea prevalence (Fig. 5A; Supplemental Table 3). This association appeared to be stronger in boys than in girls when the models are run separately (Fig. 5B, C; Table 2). Considering the sex-specific model estimates, the cumulative association between the mean diarrhea prevalence and length at age 24 mo was equivalent to 0.7-cm shorter length in boys (HAZ = −0.2) at age 24 mo compared with children with no diarrhea. Twice the mean burden of diarrhea was associated with 1.4-cm shorter length in boys (HAZ = −0.4) at age 24 mo. No relation between diarrhea and linear growth was observed in girls. Subgroup analysis by study demonstrates qualitatively similar but not statistically significant results in most studies (Supplemental Fig. 2).
FIGURE 5.
Growth trajectories (length in A–C, weight in D–F) of children with different burdens of diarrhea [no diarrhea, the mean burden of diarrhea (5%, 7%, 8%, 7%, and 5% of days with diarrhea in the age groups 0–3, >3–6, >6–12, >12–18, and >18–24 mo, respectively), and twice the mean burden of diarrhea during the first 2 y of life], overall and by sex, from a combined dataset including data from 7 cohort studies.
TABLE 2.
Estimates of length, HAZ, weight, and WAZ at age 24 mo1
| Boys |
Girls |
|||||||
| Diarrhea burden | Length | HAZ | Weight | WAZ | Length | HAZ | Weight | WAZ |
| cm | kg | cm | kg | |||||
| No diarrhea | 84.2 (83.7, 84.7) | −1.0 (−1.1, −0.8) | 11.7 (11.5, 11.9) | −0.3 (−0.5, −0.2) | 82.3 (81.8, 82.8) | −1.1 (−1.2, −0.9) | 11.0 (10.8, 11.2) | −0.4 (−0.5, −0.2) |
| Mean burden2 | 83.5 (83.0, 84.0) | −1.2 (−1.4, −1.0) | 11.5 (11.3, 11.7) | −0.5 (−0.6, −0.3) | 82.2 (81.7, 82.7) | −1.1 (−1.3, −0.9) | 10.9 (10.7, 11.1) | −0.4 (−0.6, −0.3) |
| 2× Mean burden | 82.8 (82.2, 83.4) | −1.4 (−1.6, −1.2) | 11.4 (11.2, 11.6) | −0.6 (−0.7, −0.4) | 82.2 (81.7, 82.7) | −1.1 (−1.3, −0.9) | 10.9 (10.7, 11.1) | −0.4 (−0.6, −0.3) |
Data are presented as estimates (95% CIs). The models were run separately for boys and girls. HAZ, height-for-age Z-score; WAZ, weight-for-age Z-score.
The mean burden of diarrhea overall is 5%, 7%, 8%, 7%, and 5% of days with diarrhea in the age groups 0–3, >3–6, >6–12, >12–18, and >18–24 mo, respectively.
Association between diarrhea and weight gain.
We observed similar trends with models using weight as the primary outcome (Supplemental Table 4). Diarrhea during the current period was negatively associated with weight gain in most age groups, and associations were somewhat similar for boys and girls (Fig. 6A). Boys lost more weight than girls did, on average, as a result of diarrhea in the age group >3–6 mo.
FIGURE 6.
Difference in weight slopes at 0–3, >3–6, >6–12, >12–18, and >18–24 mo contributed by current period diarrhea prevalence (A) and previous period diarrhea prevalence (B), overall and by sex. Values are coefficients produced by the models, which represent difference in slope measured in grams per month of age associated with percentage of days with diarrhea during that period (A) or the previous period (B). All, overall; F, female; M, male.
Catch-up ponderal growth.
Catch-up ponderal growth was found in the overall model and ranged from 1 to 4 g/mo per percentage diarrhea prevalence in the previous age group (Fig. 6B). The confidence intervals for catch-up ponderal growth did not overlap 0 in boys in the age groups >6–12, >12–18, and >18–24 mo and for girls in the age group >12–18 mo.
Weight at age 24 mo.
No diarrhea in the first 2 y of life was associated with greater weight when compared with the mean diarrhea burden (Fig. 5D), and, as with length, this relation appeared to be greater in boys (Fig. 5E, F; Table 2). Overall, mean diarrhea burden was associated with 0.1 lower WAZ compared with children with no diarrhea (Supplemental Table 3). Mean diarrhea prevalence was associated with 0.2-kg lower weight in boys (WAZ = −0.2), and twice the mean diarrhea prevalence was associated with 0.3-kg lower weight in boys (WAZ = −0.3) (Table 2). No association between diarrhea and ponderal growth was observed in girls.
Discussion
Diarrhea was associated with decreased linear and ponderal growth in early childhood, but catch-up growth was observed if diarrhea prevalence decreased from one period to the next. Persistent growth deficits may be associated with repeated diarrheal insults that serve to prevent catch-up growth in the first 2 y of life. Subgroup analysis indicates that boys may experience more linear growth faltering after diarrhea than girls do, but more research is needed to explore these findings.
Catch-up growth has been documented in a number of previous studies. Catch-up growth in weight was documented by Ashworth (22) who demonstrated increased rates of weight acquisition in severely malnourished children who consumed food ad libitum and catch-up growth in length was observed in subgroups of children at a feeding center in the West Indies (34). Nutritional supplementation was found to either decrease growth faltering after diarrhea or increase chances of catch-up growth in length to occur, therefore eliminating the effect of diarrhea on linear growth in a study in Colombia (8). The results from this study in which some children were administered a protein-rich nutritional supplementation vs. none suggests the importance of diet in reducing the risk of growth faltering or increasing the chance of catch-up growth after diarrhea. In a study in Egypt, the relation between diarrhea and growth was decreased when there was sufficient time (3–6 mo) since the last diarrhea had occurred, allowing for catch-up growth (17). Similarly, children in Brazil with high diarrhea prevalence during multiple periods were found to grow less than children without recurrent high amounts of diarrhea (3). Finally, catch-up growth was observed in a study that considered the effect of Cryptosporidium parvum on linear growth faltering, but the extent of catch-up growth differed depending on the age of infection and presence of malnutrition at baseline, with some age groups recovering fully and others experiencing persistent deficits (11, 24). The model we used allowed us to observe catch-up growth in the absence of diarrhea, and we quantified the negative association between repeated diarrheal episodes and catch-up growth during the first 2 y of life. Simpler models that consider only the relation between overall diarrhea burden and growth may miss the more nuanced relation described here. In addition, summary models will be less likely to observe an association between diarrhea and growth, because the timing of episodes will affect the relation with growth faltering.
The suggestion of differential associations between diarrhea prevalence and growth by sex may be partially due to somewhat lower diarrhea burdens in girls than in boys. Another possible biologic mechanism for differential association between diarrhea and linear growth by sex has to do with zinc deficiency, which is associated with growth faltering, poor immune function, and increased incidence and severity of diarrhea, and is thought to be widespread in developing countries (1). In general, boys are at greater risk of zinc deficiency than girls (35). Zinc can be lost during diarrhea; therefore, if diarrhea burden is higher in boys, they will be at greater risk of zinc deficiency. Another potential contributing factor may be different underlying biologic responses to diarrhea in boys and girls, which is supported by a similar finding in research into the effect of inflammatory bowel diseases and Crohn disease on growth (36–39). Girls were found to have more severe symptoms of Crohn disease than boys; however, boys were more likely to develop growth failure than girls (38) and were observed to have impaired catch-up growth when compared with girls (36).
There are a number of unmeasured variables that potentially play a role in these overall and sex-specific relations, including etiology of infection, treatment factors, socioeconomic status, and feeding practices, among others; these variables were not available in the combined dataset used for this analysis. The use of multiple cohorts in a combined analysis allowed for a more detailed analysis than has been implemented previously by single-cohort studies, but our findings highlight the need for a comprehensive, standardized multicenter study similar to the MAL-ED (The Etiology, Risk Factors and Interactions of Enteric Infections and Malnutrition and the Consequences for Child Health and Development) cohort (40). In an initial publication about the MAL-ED cohort, measures of intestinal inflammation were associated with linear growth faltering, even after controlling for overt diarrheal illness (40). This finding supports previous assertions that tropical enteropathy, resulting from exposure to enteric pathogens and characterized by impairment of the absorptive and barrier functions of the gut (41), is an undervalued contributor to stunting. Considering the conceptual framework of Humphrey (41), high amounts of exposure to enteric pathogens results in tropical enteropathy, which plays a large role in growth faltering, even in the absence of overt diarrhea. Water and sanitation activities could have large impacts on stunting prevalence through decreasing the burden of enteric infection (41, 42). Future work in this area should include additional exploration of the relation between enteric infection (with or without diarrhea, as identified by fecal markers of infection or pathogen identification) and linear growth, as well as additional variables, such as diet, etiology, treatment, and socioeconomic status.
In addition to finding a negative association between diarrhea and linear growth velocity in early childhood, we were able to quantify the faster, catch-up growth that occurred in subsequent diarrhea-free periods (Fig. 4). Unfortunately, in many low-resource settings, diarrhea and other infectious insults in conjunction with nutritional deficiencies are ubiquitous, and catch-up growth does not occur. Long-term linear growth faltering can result from these continued insults during childhood; therefore, prevention of diarrhea and extension of diarrhea-free periods in early childhood may improve linear growth. The suggestion of a greater detrimental effect of diarrhea on growth in boys than girls is somewhat novel and requires additional study. Decreasing the burden of diarrhea in populations will also decrease mortality associated with diarrhea in the population; therefore, diarrhea prevention programs (e.g., rotavirus vaccine, breastfeeding, zinc supplementation, and improved water and sanitation) should be prioritized to improve child health.
Supplementary Material
Acknowledgments
The authors thank other members of the Childhood Malnutrition and Infection Network, including the following: Dr. Sean R. Moore (University of Cincinnati, Cincinnati, OH), Dr. Aldo A. M. Lima (Federal University of Ceará, Fortaleza, Brazil), Dr. Relana C. Pinkerton (University of Virginia, Charlottesville, VA), Dr. Peter Aaby (Statens Serum Institute, Copenhagen, Denmark, and Bandim Health Project, Bissau, Guinea-Bissau), Lilia Z. Cabrera (A.B. PRISMA, Lima, Peru), Dr. Caryn Bern (CDC, Atlanta, GA), Dr. Charles R. Sterling (University of Arizona, Tucson, AZ), Dr. Leonardo D. Epstein (Catholic University of Chile, Santiago, Chile), Dr. Lawrence Moulton (The Johns Hopkins University, Baltimore, MD), Dr. Michael Perch (Statens Serum Institute), Dr. Thea K. Fischer (Statens Serum Institute), Dr. Halvor Sommerfelt (Center of International Health, University of Bergen, Bergen, Norway), Dr. Hans Steinsland (Center of International Health, University of Bergen), and Hector Verastegui (Nutrition Research Institute, Lima, Peru). S.A.R. and W.C. were responsible for the development of the concept and methods; S.A.R. conducted the analysis and wrote the first draft of the manuscript; R.E.B., R.H.G., R.L.G., C.F.L., K.M., R.B.S., and P.V.-B. contributed data to the combined dataset; R.E.B., R.H.G., R.L.G., G.K., C.F.L., K.M., Z.A.R., R.B.S., and P.V.-B. contributed edits to the manuscript and assisted in interpretation of the results; S.A.R. confirms that references have been checked for accuracy and completeness; and W.C. had ultimate oversight over study design, administration, analysis, and writing of the manuscript. All authors read and approved the final manuscript.
Literature Cited
- 1.Black RE, Victora CG, Walker SP, Bhutta ZA, Christian P, de Onis M, Ezzati M, Grantham-McGregor S, Katz J, Martorell R, et al. Maternal and child undernutrition and overweight in low-income and middle-income countries. Lancet 2013;382:427–51. Erratum in: Lancet 2013;382:396. [DOI] [PubMed] [Google Scholar]
- 2.Black RE. Would control of childhood infectious diseases reduce malnutrition? Acta Paediatr Scand Suppl. 1991;374:133–40. [DOI] [PubMed] [Google Scholar]
- 3.Schorling JB, Guerrant RL. Diarrhoea and catch-up growth. Lancet. 1990;335:599–600. [DOI] [PubMed] [Google Scholar]
- 4.Briend A. Is diarrhoea a major cause of malnutrition among the under-fives in developing countries? A review of available evidence. Eur J Clin Nutr. 1990;44:611–28. [PubMed] [Google Scholar]
- 5.Richard SA, Black RE, Gilman RH, Guerrant RL, Kang G, Lanata CF, Mølbak K, Rasmussen ZA, Sack RB, Valentiner-Branth P, et al. Diarrhea in early childhood: short-term association with weight and long-term association with length. Am J Epidemiol. 2013;178:1129–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Black RE, Brown KH, Becker S. Effects of diarrhea associated with specific enteropathogens on the growth of children in rural Bangladesh. Pediatrics. 1984;73:799–805. [PubMed] [Google Scholar]
- 7.Bairagi R, Chowdhury MK, Kim YJ, Curlin GT, Gray RH. The association between malnutrition and diarrhoea in rural Bangladesh. Int J Epidemiol. 1987;16:477–81. [DOI] [PubMed] [Google Scholar]
- 8.Lutter CK, Mora JO, Habicht JP, Rasmussen KM, Robson DS, Sellers SG, Super CM, Herrera MG. Nutritional supplementation: effects on child stunting because of diarrhea. Am J Clin Nutr. 1989;50:1–8. [DOI] [PubMed] [Google Scholar]
- 9.Baumgartner RN, Pollitt E. The Bacon Chow Study: analyses of the effects of infectious illness on growth of infants. Nutr Res. 1983;3:9–21. [Google Scholar]
- 10.Walker SP, Grantham-McGregor SM, Powell CA, Himes JH, Simeon DT. Morbidity and the growth of stunted and nonstunted children, and the effect of supplementation. Am J Clin Nutr. 1992;56:504–10. [DOI] [PubMed] [Google Scholar]
- 11.Checkley W, Epstein LD, Gilman RH, Black RE, Cabrera L, Sterling CR. Effects of Cryptosporidium parvum infection in Peruvian children: growth faltering and subsequent catch-up growth. Am J Epidemiol. 1998;148:497–506. [DOI] [PubMed] [Google Scholar]
- 12.Moore SR, Lima AA, Conaway MR, Schorling JB, Soares AM, Guerrant RL. Early childhood diarrhoea and helminthiases associate with long-term linear growth faltering. Int J Epidemiol. 2001;30:1457–64. [DOI] [PubMed] [Google Scholar]
- 13.Checkley W, Epstein LD, Gilman RH, Cabrera L, Black RE. Effects of acute diarrhea on linear growth in Peruvian children. Am J Epidemiol. 2003;157:166–75. [DOI] [PubMed] [Google Scholar]
- 14.Mondal D, Petri WA, Jr, Sack RB, Kirkpatrick BD, Haque R. Entamoeba histolytica-associated diarrheal illness is negatively associated with the growth of preschool children: evidence from a prospective study. Trans R Soc Trop Med Hyg. 2006;100:1032–8. [DOI] [PubMed] [Google Scholar]
- 15.Lee G, Yori P, Olortegui MP, Pan W, Caulfield L, Gilman RH, Sanders JW, Delgado HS, Kosek M. Comparative effects of vivax malaria, fever and diarrhoea on child growth. Int J Epidemiol. 2012:41:531–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Condon-Paoloni D, Cravioto J, Johnston FE, De Licardie ER, Scholl TO. Morbidity and growth of infants and young children in a rural Mexican village. Am J Public Health. 1977;67:651–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wierzba TF, El-Yazeed RA, Savarino SJ, Mourad AS, Rao M, Baddour M, El-Deen AN, Naficy AB, Clemens JD. The interrelationship of malnutrition and diarrhea in a periurban area outside Alexandria, Egypt. J Pediatr Gastroenterol Nutr. 2001;32:189–96. [DOI] [PubMed] [Google Scholar]
- 18.Briend A, Hasan KZ, Aziz KM, Hoque BA. Are diarrhoea control programmes likely to reduce childhood malnutrition? Observations from rural Bangladesh. Lancet. 1989;2:319–22. [DOI] [PubMed] [Google Scholar]
- 19.Alvarado BE, Zunzunegui MV, Delisle H, Osorno J. Growth trajectories are influenced by breast-feeding and infant health in an Afro-Colombian community. J Nutr. 2005;135:2171–8. [DOI] [PubMed] [Google Scholar]
- 20.Keusch GT, Fontaine O, Bhargava A, Boschi-Pinto C, Bhutta ZA, Gotuzzo E, Rivera JA, Chow J, Shahid-Salles SA, Laxminarayan R. Diarrheal diseases. Disease control priorities in developing countries, 2nd ed. New York: Oxford University Press; 2006:371–88. [Google Scholar]
- 21.Lampl M, Veldhuis JD, Johnson ML. Saltation and stasis: a model of human growth. Science. 1992;258:801–3. [DOI] [PubMed] [Google Scholar]
- 22.Ashworth A. Regulation of weight and height during recovery from severe malnutrition. Proceedings of the 9th International Congress of Nutrition; 1972 Sep 4–9; Mexico City, Mexico. Basel, Switzerland: Karger; 1975;280–5.
- 23.Richard SA, Black RE, Checkley W. Revisiting the relationship of weight and height in early childhood. Adv Nutr. 2012;3:250–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mølbak K, Andersen M, Aaby P, Hojlyng N, Jakobsen M, Sodemann M, da Silva AP. Cryptosporidium infection in infancy as a cause of malnutrition: a community study from Guinea-Bissau, west Africa. Am J Clin Nutr. 1997;65:149–52. [DOI] [PubMed] [Google Scholar]
- 25.Golden MH. Is complete catch-up possible for stunted malnourished children? Eur J Clin Nutr 1994;48(Suppl 1):S58–70; discussion S71. [PubMed] [Google Scholar]
- 26.Van Ijzendoorn MH, Bakermans-Kranenburg MJ, Juffer F. Plasticity of growth in height, weight, and head circumference: meta-analytic evidence of massive catch-up after international adoption. J Dev Behav Pediatr. 2007;28:334–43. [DOI] [PubMed] [Google Scholar]
- 27.Lanata CF, Black RE, Maurtua D, Gil A, Gabilondo A, Yi A, Miranda E, Gilman RH, Leon-Barua R, Sack RB. Etiologic agents in acute vs persistent diarrhea in children under three years of age in peri-urban Lima, Peru. Acta Paediatr Suppl. 1992;381:32–8. [DOI] [PubMed] [Google Scholar]
- 28.Mølbak K, Jensen H, Ingholt L, Aaby P. Risk factors for diarrheal disease incidence in early childhood: a community cohort study from Guinea-Bissau. Am J Epidemiol. 1997;146:273–82. [DOI] [PubMed] [Google Scholar]
- 29.Valentiner-Branth P, Steinsland H, Santos G, Perch M, Begtrup K, Bhan MK, Dias F, Aaby P, Sommerfelt H, Mølbak K. Community-based controlled trial of dietary management of children with persistent diarrhea: sustained beneficial effect on ponderal and linear growth. Am J Clin Nutr. 2001;73:968–74. [DOI] [PubMed] [Google Scholar]
- 30.Pathela P, Zahid Hasan K, Roy E, Huq F, Kasem Siddique A, Bradley Sack R. Diarrheal illness in a cohort of children 0–2 years of age in rural Bangladesh: I. Incidence and risk factors. Acta Paediatr. 2006;95:430–7. [DOI] [PubMed] [Google Scholar]
- 31.Checkley W, Buckley G, Gilman RH, Assis AM, Guerrant RL, Morris SS, Mølbak K, Valentiner-Branth P, Lanata CF, Black RE. Multi-country analysis of the effects of diarrhoea on childhood stunting. Int J Epidemiol. 2008;37:816–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.WHO Multicentre Growth Reference Study Group. WHO Child Growth Standards. Acta Paediatr Suppl. 2006;450:76–85. [DOI] [PubMed] [Google Scholar]
- 33.R Core Development Team. A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2008. [Google Scholar]
- 34.Walker SP, Golden MHN. Growth in length of children recovering from severe malnutrition. Eur J Clin Nutr. 1988;42:395–404. [PubMed] [Google Scholar]
- 35.Black MM. Zinc deficiency and child development. Am J Clin Nutr. 1998;68:464S–9S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Griffiths AM, Nguyen P, Smith C, MacMillan JH, Sherman PM. Growth and clinical course of children with Crohn's disease. Gut. 1993;34:939–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Langholz E, Munkholm P, Krasilnikoff PA, Binder V. Inflammatory bowel diseases with onset in childhood. Clinical features, morbidity, and mortality in a regional cohort. Scand J Gastroenterol. 1997;32:139–47. [DOI] [PubMed] [Google Scholar]
- 38.Gupta N, Bostrom AG, Kirschner BS, Ferry GD, Winter HS, Baldassano RN, Gold BD, Abramson O, Smith T, Cohen SA, et al. Gender differences in presentation and course of disease in pediatric patients with Crohn disease. Pediatrics. 2007;120:e1418–25. [DOI] [PubMed] [Google Scholar]
- 39.Gupta N, Lustig RH, Kohn MA, McCracken M, Vittinghoff E. Sex differences in statural growth impairment in Crohn’s disease: role of IGF-1. Inflamm Bowel Dis. 2011;17:2318–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kosek M, Haque R, Lima A, Babji S, Shrestha S, Qureshi S, Amidou S, Mduma E, Lee G, Yori PP, et al. Fecal markers of intestinal inflammation and permeability associated with the subsequent acquisition of linear growth deficits in infants. Am J Trop Med Hyg. 2013;88:390–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Humphrey JH. Child undernutrition, tropical enteropathy, toilets, and handwashing. Lancet. 2009;374:1032–5. [DOI] [PubMed] [Google Scholar]
- 42.Guerrant RL, DeBoer MD, Moore SR, Scharf RJ, Lima AA. The impoverished gut–a triple burden of diarrhoea, stunting and chronic disease. Nat Rev Gastroenterol Hepatol. 2013;10:220–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.