Abstract
Subclinical micronutrient deficiencies remain a hidden aspect of malnutrition for which comprehensive data are lacking in school-aged children. We assessed the micronutrient status of Nepalese children, aged 6 to 8 y, born to mothers who participated in a community-based antenatal micronutrient supplementation trial from 1999 to 2001. Of 3305 participants, plasma indicators were assessed in a random sample of 1000 children. Results revealed deficiencies of vitamins A (retinol <0.70 μmol/L, 8.5%), D (25-hydroxyvitamin D <50 nmol/L, 17.2%), E (α-tocopherol <9.3 μmol/L, 17.9%), K (decarboxy prothombin >2 μg/L, 20%), B-12 (cobalamin <150 pmol/L, 18.1%), B-6 [pyridoxal-5′-phosphate (PLP) <20 nmol/L, 43.1%], and β-carotene (41.5% <0.09 μmol/L), with little folate deficiency (6.2% <13.6 nmol/L). Deficiencies of iron [ferritin <15 μg/L, 10.7%; transferrin receptor (TfR) >8.3 mg/L, 40.1%; TfR:ferritin >500 μg/μg, 14.3%], iodine (thyroglobulin >40 μg/L, 11.4%), and selenium (plasma selenium <0.89 μmol/L, 59.0%) were observed, whereas copper deficiency was nearly absent (plasma copper <11.8 μmol/L, 0.7%). Hemoglobin was not assessed. Among all children, 91.7% experienced at least 1 micronutrient deficiency, and 64.7% experienced multiple deficiencies. Inflammation (α-1 acid glycoprotein >1 g/L, C-reactive protein >5 mg/L, or both) was present in 31.6% of children, affecting the prevalence of deficiency as assessed by retinol, β-carotene, PLP, ferritin, TfR, selenium, copper, or having any or multiple deficiencies. For any nutrient, population deficiency prevalence estimates were altered by ≤5.4% by the presence of inflammation, suggesting that the majority of deficiencies exist regardless of inflammation. Multiple micronutrient deficiencies coexist in school-aged children in rural Nepal, meriting more comprehensive strategies for their assessment and prevention.
Introduction
Micronutrient status in young school-aged children in developing countries is generally not well characterized. Resources for nutritional assessment have largely been focused on the preschool years when nutrient requirements are exacerbated by demands of rapid growth and deficiencies may have long-term developmental consequences (1). However, poor nutritional status likely persists into preadolescent years, where it can affect school performance and reproductive health during puberty, particularly where child-bearing occurs early in life (2). A better understanding of nutritional status throughout childhood may motivate strategies to alleviate malnutrition in this age group, particularly where micronutrient deficiencies, or “hidden hunger,” exist (3).
Where data on micronutrient status of children is available, it is often confined to vitamin A (4), iron, or iodine status (2), whose roles in child survival, anemia prevention, and cognition have been studied for decades. Other nutrients are assessed less frequently, although attention has been paid to their roles during childhood development: vitamin D functions in bone health (5) and immunity (6), vitamin E in modulating oxidative stress (7), vitamin K in bone health (8), and B vitamins such as folate, vitamin B-12, and vitamin B-6 contribute to reduced risk of metabolic disease (9) and improved cognition (10). Selenium functions as an antioxidant and is required for immunity and thyroid function (11, 12), and copper also functions as an antioxidant and in bone health, among other roles (13). Moreover, assessments are often conducted in schools (2) rather than community settings, which does not allow for regional representation if school attendance is not high. Micronutrient deficiencies likely coexist where diet is poor because of expense or limited or seasonal availability of food (14). Exploration of multiple deficiencies may provide a comprehensive view of nutritional need and options for prevention.
Micronutrient deficiencies may increase vulnerability to infection (15), and the resulting inflammatory response also affects the interpretation of several micronutrient status indicators. Elevated circulating markers of inflammation, including C-reactive protein (CRP)6 and α-1 acid glycoprotein (AGP) have most notably been associated with lower circulating retinol, the major indictor of vitamin A status, and higher ferritin, an indicator of iron stores (16–18). This means that, by these indicators, the prevalence of vitamin A deficiency attributable to dietary insufficiency could be overestimated and that of iron deficiency underestimated when inflammation is not considered. Other nutrients are known or likely to be affected by inflammation. Yet, the prevalence of inflammation and implications for interpretation of micronutrient deficiencies are not frequently described in school-aged children, who may be less prone to illness than children <5 y of age, despite residing in the same impoverished and unhygienic environments.
We provide a comprehensive profile of micronutrient status in a cohort of 6- to 8-y-old children in the east central terai of Nepal, and its association with inflammation. This work provides novel data on micronutrient deficiencies of school-aged children in this region while demonstrating the influence of inflammation on micronutrient status indicators and population prevalence estimates of micronutrient deficiencies.
Methods
Study design, population, and sample collection.
Children 6 to 8 y of age, born to mothers who participated in a cluster, randomized, controlled, 5-arm trial of antenatal micronutrient supplementation on pregnancy outcomes in rural Nepal from 1999 to 2001 (19), were reassessed from mid-October, 2006 to mid-February, 2008. The trial screened all nonpregnant, nonlactating, nonsterilized, and nonmenopausal married women of reproductive age in the study area for over 2 y for missed periods and enrolled women who became pregnant (19). Details of the follow-up survey have also been reported (20, 21). Among other data, information on ethnicity, household socioeconomic characteristics, child educational status, diet based on the frequency of consumption of key foods in the past week, and 7-d morbidity as the number of days with particular symptoms (fever, diarrhea, productive cough, rapid breathing), was obtained during home visits. Height using a portable stadiometer (Harpenden) and weight using an electronic scale (Model 881; Seca) were measured. Blood was collected and processed to plasma in a field laboratory, where cholesterol was measured (Cholestech LDX analyzer; Cholestech) and up to 4 aliquots of plasma were transferred to cryovials. Frozen plasma was shipped to Johns Hopkins University in liquid nitrogen vapor shippers and stored at −80°C.
Of 4130 children born into the 2000 to 2001 trial (19), representing nearly all children born into the community over the 2-y period, 3524 were recontacted, a 93% follow-up rate for children known to be alive and residing in the area (20), with blood obtained from 3305 children. Selection for micronutrient status assessment was based on having multiple aliquots of plasma and complete data from the original trial and the follow-up, with 2726 children contributing 4 aliquots of plasma, 2130 of whom had complete data and formed the sampling frame for the random selection of 1000 samples, balanced across maternal intervention groups. Of these, 493 children had participated in cognitive assessments prior to this activity, and 80 were administered either iron and folic acid or zinc supplements from 12 to 36 mo of age (22, 23). No other nutritional interventions occurred from the time of the maternal trial to the follow-up.
Micronutrient status assessments were performed in archived samples as part of a study to examine the plasma proteome for biomarkers of nutritional status (24). A sample size of 1000 for micronutrient status assessments was chosen as sufficient to characterize the micronutrient status of the broader population.
Activities related to the data collection and laboratory assessments received ethical approval from Institutional Review Boards at Johns Hopkins Bloomberg School of Public Health and the Institute of Medicine in Kathmandu, Nepal.
Micronutrient and inflammation marker assessment.
Micronutrient assessments were performed in the laboratory at the Center for Human Nutrition at Johns Hopkins University. For vitamins A, E, D, copper, and selenium, methods and status relative to the plasma proteome were reported previously in a subset of 500 children (24). Plasma retinol, α- and γ-tocopherols, β-carotene, and other carotenoids were assessed by HPLC (Waters 2795 with autosampler, photodiode array detector, and Empower 2 software) with a method modified from Yamini et al. (25). Vitamin B-6 was assessed as pyridoxal-5′-phosphate (PLP) by HPLC with fluorescence detection (Waters 2475) (26), calibrated against controls provided by the CDC (inter-assay CV = 18.2%, ∼70 runs). The spectrofluorometer was set at 300 nm for excitation and 400 nm for emission, and an Allsphere ODS-2, 5-μm, 4.6-mm column (Alltech) and a Supelguard Discovery C18 2-cm × 4.0-mm guard column were used with a mobile phase of 0.1-mol/L potassium dihydrogen phosphate buffer, 0.1-mol/L sodium perchlorate (NaClO4), and 0.5-g/L sodium bisulfite (NaHSO3) adjusted to pH 3.0 with 8.5% phosphoric acid following sample preparation in magnesium acetate. Total 25-hydroxyvitamin D [25(OH)D] (IDS, Inc.; CV = 6.5%), decarboxy prothombin, or protein induced in vitamin K absence (PIVKA-II) (Asserachrom; Diagnostica Stago; CV = 15.0%), and transferrin receptor (TfR) (Ramco Labs; CV = 10.1%) were assessed using commercial immunoassays. Radial immunodiffusion (Kent Laboratories; CV = 10.0%) was used for assessing AGP. Folic acid (CV = 6.8%) and cobalamin (vitamin B-12; CV = 10.4%) were run together, as were ferritin (CV = 7.5%) and thyroglobulin (CV = 8.8%) on a benchtop clinical chemistry analyzer (Immulite 1000; Siemens Diagnostics), as was CRP (CV = 5.8%). Copper (CV = 4.7%) and selenium (CV = 9.7%) (27) were assessed by graphite furnace atomic absorption spectroscopy (AAnalyst 800 with WinLab software; Perkin Elmer).
Interpretive cutoffs for deficiency.
Weight and height data were converted to Z-scores against the WHO Reference 2007 growth charts for children aged 5 to 19 y using AnthroPlus software (28). Cutoffs of −2 Z-scores for weight-for-age (underweight), height-for-age (stunted), and BMI-for-age (thin) were used, as defined by the WHO for children >5 y of age (28). Conventional cutoffs for micronutrient deficiencies and acute phase proteins were used where available, although not all micronutrients have been well-studied in children to guide the selection of cutoffs. Vitamin A deficiency was defined as retinol <0.70 μmol/L, whereas <1.05 μmol/L was considered marginal or deficient (29). Vitamin A deficiency >10% indicates a public health problem (29). For β-carotene, <0.09 μmol/L was considered deficient (25). Plasma α-tocopherol concentrations <9.3 μmol/L were considered indicative of vitamin E deficiency to be consistent with data reported for mothers of these children in early pregnancy (14), although consensus is lacking for cutoffs in children, with recommendations ranging from 7 to 12 μmol/L (7). A cutoff of <12 μmol/L and a ratio with cholesterol of <2.2 μmol/mmol α-tocopherol:cholesterol were also examined (7). Values <50 nmol/L for 25(OH)D were considered indicative of vitamin D deficiency (30), and PIVKA-II >2 μg/L was evidence of vitamin K deficiency (31). When no children were below the conventional cutoff of <7 nmol/L for plasma folate (32), a cutoff of <13.6 nmol/L was used to indicate low status (33). Cobalamin <150 pmol/L indicated vitamin B-12 deficiency (32), and PLP <20 nmol/L indicated vitamin B-6 deficiency (34). Iron stores were considered depleted when ferritin was <15 μg/L (35), and tissue iron deficiency was defined as TfR >8.3 mg/L (36). The TfR:ferritin index was calculated with TfR expressed as μg/L, and a cutoff of >500 μg/μg was considered indicative of iron deficiency, as reported by Grant et al. (36). Thyroglobulin >40 μg/L indicated iodine deficiency (37). Copper and selenium cutoffs were set at <11.8 μmol/L and <0.89 μmol/L, respectively (38).
Cutoffs of >5.0 mg/L for CRP and >1.0 g/L for AGP were used to indicate inflammation (16), where “any inflammation” was defined by elevated CRP, AGP, or both.
Statistical analysis.
Data on household and child-level characteristics, including anthropometry, diet and morbidity, or population micronutrient concentrations, are presented as means ± SDs or medians (IQRs) when distributions were skewed, for continuous variables. The prevalence of categorical baseline characteristics, inflammation, and micronutrient deficiencies is presented as percentage (95% CI). The number of concurrent deficiencies was obtained by summarizing the total number of nutritional deficiencies per child out of 11 possible nutrients (vitamins A, E, D, K, B-6, B-12, folate, iron, iodine, selenium, and copper), using an elevated TfR:ferritin index to define iron deficiency, and excluding β-carotene given that it is a vitamin precursor. Pearson’s correlation analysis was used to examine associations among indicators of nutritional status and with inflammation. For correlation analysis and subsequent statistical tests, continuous data with skewed distributions were log10-transformed but are presented as geometric means in the tables. Data for β-carotene and PIVKA-II that were undetectable were assigned values of 0.001 before log10-transformation.
The association of inflammation with micronutrient status indicators was assessed as follows. For continuous data, t tests were used to determine whether micronutrient indicators differed by the presence of inflammation, with means (95% CIs) presented. The prevalence of deficiency was also stratified by inflammatory status, with data presented as the percentages (95% CIs) of affected children by categories of children unaffected by and those with inflammation. Corresponding ORs were reported as an indication of the strength of the association of inflammation with micronutrient deficiency prevalence that could be compared across micronutrients. Finally, for retinol and iron status indicators, adjustments suggested by Thurnham et al. (16–18) were performed to ascertain whether prevalence estimates changed by this approach, and adjusted values are denoted with subscripted “adj.”
Where inflammation had a significant effect on deficiency prevalence, the proportion of deficiency in the sample attributable to the presence of inflammation was determined as the population attributable risk (PAR), with 95% CIs estimated using the method of Fleiss for cross-sectional studies (39, 40). The PAR takes into account both the magnitude of the association of inflammation with micronutrient status and the prevalence of inflammation, and provides an estimate of the proportion of cases of apparent deficiency that would be reduced by eliminating inflammation. The extent to which the presence of inflammation contributed to over- or underestimates of the population prevalence of deficiency relative to the absence of inflammation was calculated as the PAR times the population prevalence of deficiency, equivalent to the prevalence of deficiency in the entire population minus the prevalence in those without concurrent inflammation.
Potential confounders of associations of inflammation with micronutrient status were initially examined. Although important for future exploration of risk factors for micronutrient deficiencies, these variables were either unrelated to deficiencies or inflammation (maternal intervention, child age and sex) or did not affect associations of inflammation with nutrient indicators (season of sample collection, ethnicity), so adjustments were not performed for the final presentation. Differences in statistical tests were considered significant at P < 0.05. All statistical tests were performed using Stata, version 12 (Stata Corp).
Results
Sociodemographic, anthropometric, diet, and health characteristics of participating children are shown in Table 1. Children ranged from 6.2 to 8.5 y of age, and both sexes were equally represented. Nearly a third of children had not been enrolled in school prior to the survey. The majority of participants were Hindu and of the Vaishya caste, and the majority were Madheshi (originating from the northern plains of India) vs. Pahadi (from the hills of Nepal). Half of the households had electricity, and although land ownership was common, most homes were made of thatch with 20% of wood and cement. Distributions of these characteristics are similar to those reported for the larger cohort of 3524 children from which this sample was derived (20), although there was somewhat greater representation of the higher castes (3.6% more Brahmin and Chettri), 7.2% lower prevalence of stunting, and 3.1% higher prevalence in recent meat consumption than in nonsampled children (all significant, P < 0.05; not shown), despite comparable recent intakes of other food groups and morbidity patterns. Of the 2130 children with sufficient aliquots of plasma, there were no significant differences in any of these characteristics between selected (n = 1000) and unselected (n = 1130) children (not shown).
TABLE 1.
Sociodemographic, dietary, and morbidity characteristics of children in rural Nepal (n = 1000)1
| Category | Values |
| Demographic | |
| Age, y | 7.5 ± 0.4 |
| Sex: male | 51.1 (48.0, 54.2) |
| Education | |
| Ever in school | 69.7 (66.9, 72.5) |
| Literate | 16.9 (14.6, 19.2) |
| Religion/caste | |
| Hindu-Brahmin | 8.0 (6.3, 9.7) |
| Hindu-Chettri | 6.6 (5.1, 8.3) |
| Hindu-Vaishya | 66.4 (63.4, 69.3) |
| Hindu-Shudra | 11.9 (10.0, 14.1) |
| Muslim | 6.4 (5.1, 8.1) |
| Other (Christian/Buddhist) | 0.7 (0.2, 1.2) |
| Ethnicity | |
| Pahadi | 33.0 (30.1, 35.9) |
| Madheshi | 67.0 (64.1, 69.9) |
| Economic | |
| Electricity in home | 50.2 (47.1, 53.3) |
| Land ownership | 79.1 (76.6, 81.5) |
| Wood or cement walls in homes | 20.0 (17.5, 22.5) |
| Anthropometry | |
| Weight, kg | 18.3 ± 2.4 |
| Height, cm | 113.9 ± 5.6 |
| BMI, kg/m2 | 14.0 ± 1.0 |
| Weight-for-age Z-score | −2.0 ± 0.9 |
| Height-for-age Z-score | −1.8 ± 0.9 |
| BMI-for-age Z-score | −1.2 ± 0.8 |
| Underweight2 | 48.2 (45.1, 51.3) |
| Stunted2 | 42.0 (38.9, 45.1) |
| Thin2 | 16.1 (13.8, 18.4) |
| Dietary intake, reported as consumed ≥2 times in the last 7 d | |
| Dairy | 67.9 (65.0, 70.8) |
| Meat | 19.3 (16.9, 21.7) |
| Fish | 18.4 (16.0, 20.8) |
| Eggs | 6.1 (4.6, 7.6) |
| Dark green leafy vegetables | 57.1 (54.0, 60.2) |
| Morbidity, symptoms reported in the last 7 d | |
| Fever | 8.3 (6.6, 10.0) |
| Diarrhea | 2.7 (1.7, 3.7) |
| Productive cough | 4.0 (2.8, 5.2) |
| Rapid breathing | 3.2 (2.1, 4.3) |
| Any of the above symptoms | 14.6 (12.4, 16.8) |
| Infection/inflammation | |
| CRP >5 mg/L only | 0.6 (0.1, 1.1) |
| CRP >5 mg/L and AGP >1.0 g/L | 5.8 (4.4, 7.2) |
| AGP >1.0 g/L only | 25.2 (22.5, 27.9) |
| Any inflammation | 31.6 (28.7, 34.5) |
Values are means ± SDs or percentages (95% CIs). AGP, α-1 acid glycoprotein; CRP, C-reactive protein.
Underweight, weight-for-age Z-score: <−2; stunted, height-for-age Z-score: <−2; thin, BMI-for-age Z-score: <−2 (28).
Nearly half the children were underweight and stunted (Table 1), with a high prevalence of thinness (low BMI). Although a majority had consumed ≥2 servings of dairy (mostly local yogurt) and dark green leafy vegetables in the previous week, fewer than 20% had done so with meat, fish, or eggs. Morbidity symptoms were reported in ∼15% of children based on the 7-d recall, but elevated acute phase proteins, especially AGP, were found in twice this percentage of children. Among 854 reportedly asymptomatic children, 235 (27.5%) had biochemical evidence of inflammation, whereas 81 of 146 (55.5%) symptomatic children did. More specifically, 47 of 854 (5.5%) asymptomatic and 17 of 146 (11.6%) symptomatic children had elevated CRP values, and 229 of 854 (26.8%) asymptomatic and 81 of 146 (55.5%) symptomatic children had elevated AGP values. The vast majority of inflammation was associated with elevated AGP alone or in combination with elevated CRP, with elevated CRP alone in only 6 children.
Micronutrient status indicators (means ± SDs) and the prevalence of deficiency, irrespective of inflammation, are shown in Table 2. Fewer than 10% of children were vitamin A deficient, although >50% had marginal to deficient status. Estimates of vitamin E (by plasma α-tocopherol <9.3 μmol/L), D, K, and B-12 deficiencies were nearly 20%. However, when α-tocopherol was expressed per unit cholesterol, deficiency was nearly nonexistent. Nearly half of the children had low circulating β-carotene and PLP (vitamin B-6) values, whereas only 6.2% had low folate concentrations. For iron, elevated TfR values occurred in 3 times as many children as low ferritin, with an elevated TfR:ferritin index found in ∼14% of children. Iodine deficiency was present in 11% and selenium deficiency in over half the children, with copper deficiency nearly absent. Over 90% of children were affected by at least 1 deficiency, with 8.3% absent of any deficiency. Although 27% had just 1 deficiency, 48.9% had 2 to 3 concurrent deficiencies, and 15.7% had 4 to 8 concurrent deficiencies.
TABLE 2.
Distributions of plasma vitamin and mineral indicators and prevalence of deficiency among children in rural Nepal (n = 1000)1
| Nutrient/indicator | Values2 | Prevalence of deficiency (95% CI)3 |
| % | ||
| Vitamin A | ||
| Retinol, μmol/L | 1.04 ± 0.27 | 8.5 (6.8, 10.2) |
| 55.3 (52.2, 58.4) | ||
| β-Carotene, μmol/L | 0.104 (0.059, 0.202) | 41.5 (38.4, 44.6) |
| Vitamin E | ||
| α-Tocopherol, μmol/L | 12.2 ± 3.3 | 17.9 (15.5, 20.3) |
| 52.1 (49.0, 55.2) | ||
| α-Tocopherol:cholesterol, μmol/mmol | 4.05 ± 0.85 | 0.6 (0.1, 1.1) |
| Vitamin D | ||
| 25(OH)D, nmol/L | 66.3 ± 18.5 | 17.2 (14.9, 19.5) |
| Vitamin K | ||
| PIVKA-II, μg/L | 1.34 (0.81, 1.88) | 20.4 (17.9, 22.9) |
| Vitamin B-6 | ||
| PLP, nmol/L | 23.9 ± 13.0 | 43.1 (40.0, 46.2) |
| Vitamin B-12 | ||
| Cobalamin, pmol/L | 225 (166, 304) | 18.1 (15.7, 20.5) |
| Folate | ||
| Folic acid, nmol/L | 23.3 (18.3, 29.9) | 6.2 (4.7, 7.7) |
| Iron | ||
| Ferritin, μg/L | 39.4 (24.1, 55.3) | 10.7 (8.7, 12.6) |
| TfR, mg/L | 7.8 (6.4, 9.4) | 40.1 (37.1, 43.1) |
| TfR:ferritin, μg/μg | 196 (124, 332) | 14.3 (12.1, 16.4) |
| Selenium | ||
| Total circulating selenium, μmol/L | 0.86 ± 0.27 | 59.0 (55.9, 62.0) |
| Copper | ||
| Total circulating copper, μmol/L | 23.5 ± 5.3 | 0.7 (0.2, 1.2) |
| Iodine | ||
| Thyroglobulin, μg/L | 16.9 (11.7, 25.4) | 11.4 (9.4, 13.3) |
| Presence of any deficiency (≥1) | — | 91.7 (90.0, 93.4) |
| Presence of multiple deficiencies (≥2) | — | 64.7 (61.7, 67.7) |
Missing values: n = 1 for PIVKA-II and cobalamin, n = 2 for folic acid, n = 5 for ferritin and thyroglobulin, n = 1 for selenium and copper, n = 9 for any and multiple deficiencies. PIVKA-II, protein induced in vitamin K absence; PLP, pyridoxal-5′-phosphate; TfR, transferrin receptor; 25(OH)D, 25-hydroxyvitamin D.
Values are means ± SDs or medians (IQRs) if distributions were skewed.
Cut-offs for deficiency: retinol <0.70 and <1.05 μmol/L, respectively; β-carotene <0.09 μmol/L; α-tocopherol <9.3 μmol/L and <12 μmol/L, respectively; α-tocopherol:cholesterol <2.2 μmol/mmol; 25(OH)D <50 nmol/L; PIVKA-II >2 μg/L; PLP <20 nmol/L; cobalamin <150 pmol/L; folic acid <13.6 nmol/L; ferritin <15 μg/L; TfR >8.3 mg/L; TfR:ferritin >500 μg/μg; selenium <0.89 μmol/L; copper <11.8 μmol/L; thyroglobulin >40 μg/L.
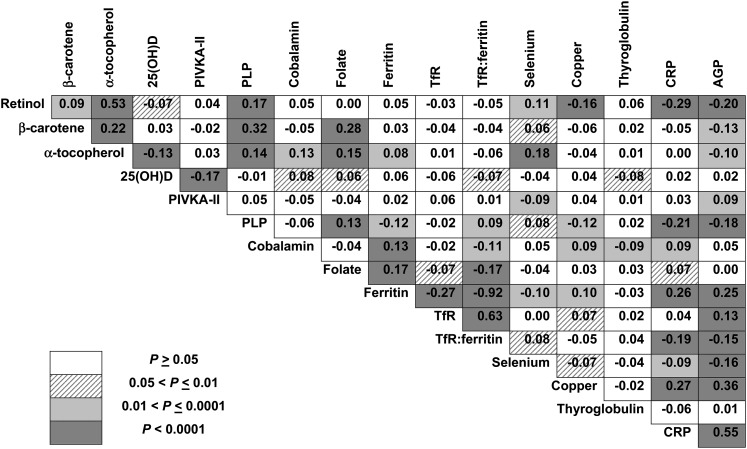
Correlations among micronutrient status indicators and inflammatory markers are shown in Figure 1. Retinol, β-carotene, α-tocopherol, and PLP were positively correlated. Although not associated with retinol, folate was also positively associated with β-carotene, α-tocopherol, PLP, and ferritin, and was inversely associated with TfR:ferritin. α-Tocopherol was positively associated with cobalamin and selenium. Cobalamin was positively associated with ferritin, which was inversely associated with TfR. Inverse associations of retinol and PLP with copper existed, perhaps via opposite associations of inflammatory markers on those indicators. Inflammatory markers (CRP and AGP) were inversely associated with retinol, β-carotene, PLP, TfR:ferritin, and selenium, and less so with α-tocopherol, whereas ferritin was positively associated with AGP and CRP.
FIGURE 1.
Correlations among micronutrient status indicators and markers of inflammation in Nepalese children (n = 1000). Numbers reflect Pearson’s correlation coefficient (r); corresponding ranges of P values to denote the strength of the associations are noted by the intensity of the shading of the cells in the figure. Micronutrient status indicator values for β-carotene, PIVKA-II, cobalamin, folate, ferritin, TfR, TfR:ferritin, CRP, and AGP are log10-transformed to normalize skewed distributions. AGP, α-1 acid glycoprotein; CRP, C-reactive protein; PIVKA-II, protein induced in vitamin K absence; PLP, pyridoxal 5′phosphate; TfR, transferrin receptor; 25(OH)D, 25-hydroxyvitamin D.
The influence of inflammation on mean micronutrient indicators (Table 3) is consistent with the correlation data, in that lower mean retinol, β-carotene, α-tocopherol, PLP, cobalamin, and selenium values but higher ferritin, TfR, TfR:ferritin, and copper values occurred with inflammation. Changes in mean concentrations ranged from a 24% decline in β-carotene to a 33% increase in ferritin in the inflamed relative to uninflamed children (data not shown). Inflammation-related adjustments resulted in mean ± SD retinoladj of 1.08 ± 0.27, geometric mean ± SD ferritinadj of 32.9 ± 1.93, TfRadj of 7.7 ± 1.4, and TfR:ferritinadj of 233 ± 2.29, all nearly identical to mean values observed among children without inflammation. Correction factors used based on elevated CRP, both CRP and AGP, and AGP were 1.24, 1.36, and 1.09 for retinol; 0.96, 0.56, and 0.80 for ferritin; 1.04, 0.86, and 0.92 for TfR; and 1.08, 1.55, and 1.15 for TfR:ferritin, respectively.
TABLE 3.
Influence of inflammation on mean plasma micronutrient status indicator concentrations in rural Nepalese children (n = 1000)1
| Indicator | No inflammation (n = 684) | Inflammation (n = 316) | P |
| Retinol, μmol/L | 1.07 (1.05, 1.09) | 0.97 (0.93, 0.99) | <0.0001 |
| β-Carotene,2 μmol/L | 0.12 (0.11, 0.13) | 0.09 (0.08, 0.10) | 0.0001 |
| α-Tocopherol, μmol/L | 12.4 (12.1, 12.6) | 11.8 (11.5, 12.1) | 0.008 |
| α-Tocopherol:cholesterol, μmol/mmol | 4.07 (4.00, 4.14) | 3.99 (3.90, 4.09) | 0.2 |
| 25(OH)D, nmol/L | 66.4 (65.0, 68.1) | 66.1 (64.2, 68.1) | 0.8 |
| PIVKA-II,2 μg/L | 0.92 (0.83, 1.01) | 1.05 (0.91, 1.20) | 0.1 |
| PLP, nmol/L | 25.2 (24.2, 26.2) | 20.9 (19.6, 22.3) | <0.0001 |
| Cobalamin,2 pmol/L | 225 (218, 238) | 238 (226, 251) | 0.05 |
| Folic acid,2 nmol/L | 23.7 (23.1, 24.4) | 23.1 (22.1, 24.1) | 0.3 |
| Ferritin,2 μg/L | 32.9 (31.4, 34.6) | 43.7 (40.5, 47.3) | <0.0001 |
| TfR,2 mg/L | 7.7 (7.5, 7.9) | 8.4 (8.1, 8.8) | <0.0001 |
| TfR:ferritin,2 μg/μg | 233 (219, 248) | 193 (175, 212) | 0.0009 |
| Total circulating selenium, μmol/L | 0.89 (0.87, 0.91) | 0.81 (0.85, 0.88) | <0.0001 |
| Total circulating copper, μmol/L | 22.4 (22.0, 22.8) | 25.7 (25.1, 23.8) | <0.0001 |
| Thyroglobulin,2 μg/L | 17.5 (16.6, 18.4) | 17.0 (15.7, 18.5) | 0.6 |
Values are means (95% CIs). Missing values: n = 1 for PIVKA-II and cobalamin, n = 2 for folic acid, n = 5 for ferritin and thyroglobulin, n = 1 for selenium and copper. PIVKA-II, protein induced in vitamin K absence; PLP, pyridoxal-5′-phosphate; TfR, transferrin receptor; 25(OH)D, 25-hydroxyvitamin D.
Geometric means and 95% CIs derived from the log10 scale are shown; t tests were performed on the log10-transformed data.
The prevalence of deficiency stratified by inflammatory status is shown in Table 4. Inflammation increased the likelihood of being classified as deficient in vitamin A, β-carotene, vitamin B-6, folic acid, and selenium, and any micronutrient deficiency or multiple micronutrient deficiencies. Retinoladj resulted in a prevalence of deficiency of 5.4% and 49.1% for retinol <1.05 μmol/L. The proportion of children considered iron deficient by ferritin and TfR:ferritin did not differ by inflammatory status, although a higher proportion of children with inflammation was considered iron deficient by TfR. Prevalence of deficiency by ferritinadj, TfRadj, and TfR:ferritinadj was 11.9%, 36.0%, and 14.7%, respectively. ORs of the likelihood of being considered deficient with inflammation, where significant, ranged from 1.5 (for β-carotene) to 4.6 (for retinol <0.70 μmol/L). Deficiencies of vitamin E and copper did not differ by inflammatory status, despite mean values being associated with inflammation. Vitamins D, K, B-12, and thyroglobulin were unaffected by inflammation.
TABLE 4.
Prevalence of micronutrient deficiencies by inflammatory status and the contribution of concurrent inflammation to the likelihood of being characterized as being deficient by micronutrient status indicators in plasma of children in rural Nepal (n = 1000)1
| Indicator | Cut-off | No inflammation (95% CI) (n = 684) | Inflammation (95% CI) (n = 316) | OR (95% CI)2 | P3 |
| % | % | ||||
| Retinol, μmol/L | <0.7 | 4.4 (2.8, 5.9) | 17.4 (13.2, 21.6) | 4.6 (2.9, 7.3) | <0.0001 |
| <1.05 | 49.9 (46.1, 53.6) | 67.1 (61.9, 72.3) | 2.1 (1.6, 2.7) | <0.0001 | |
| β-Carotene, μmol/L | <0.09 | 38.2 (34.5, 41.8) | 48.7 (43.2, 54.3) | 1.5 (1.2, 2.0) | 0.002 |
| α-Tocopherol, μmol/L | <9.3 | 17.0 (14.1, 19.8) | 19.9 (15.5, 24.4) | 1.2 (0.9, 1.7) | 0.3 |
| <12.0 | 50.4 (46.7, 54.2) | 55.7 (50.2, 61.2) | 1.2 (0.9, 1.6) | 0.1 | |
| α-Tocopherol:cholesterol, μmol/mmol | <2.2 | 0.4 (0.0, 0.9) | 0.9 (-0.1, 2.0) | 2.2 (0.4, 10.8) | 0.3 |
| 25(OH)D, nmol/L | <50 | 17.7 (14.8, 20.6) | 16.1 (12.1, 20.2) | 0.9 (0.6, 1.28) | 0.5 |
| PIVKA-II, μg/L | >2 | 19.6 (16.6, 22.6) | 22.2 (17.5, 26.8) | 1.2 (0.8, 1.6) | 0.4 |
| PLP, nmol/L | <20 | 38.3 (34.7, 42.0) | 53.5 (48.0, 59.0) | 1.9 (1.4, 2.4) | <0.0001 |
| Cobalamin, pmol/L | <150 | 18.6 (15.6, 21.5) | 17.1 (13.0, 21.3) | 0.9 (0.6, 1.3) | 0.6 |
| Folic acid, nmol/L | <13.6 | 4.4 (2.8, 5.9) | 10.2 (6.8, 13.6) | 2.5 (1.5, 4.1) | 0.0007 |
| Ferritin, μg/L | <15 | 11.9 (9.5, 14.4) | 7.9 (4.9, 10.9) | 0.6 (0.4, 1.0) | 0.05 |
| TfR, mg/L | >8.3 | 35.8 (32.2, 39.4) | 49.4 (43.8, 54.9) | 1.7 (1.3, 2.3) | 0.0001 |
| TfR:ferritin, μg/μg | >500 | 15.1 (32.2, 39.4) | 12.7 (9.0, 16.3) | 0.8 (0.6, 1.2) | 0.3 |
| Total circulating selenium, μmol/L | <0.89 | 54.8 (51.1, 58.6) | 67.9 (62.8, 73.1) | 1.7 (1.3, 2.3) | 0.0001 |
| Total circulating copper, μmol/L | <11.8 | 0.9 (0.2, 1.6) | 0.3 (0.0, 0.9) | 0.3 (0.04, 3.0) | 0.3 |
| Thyroglobulin, μg/L | >40 | 11.8 (9.3, 14.2) | 10.5 (7.1, 13.9) | 0.9 (0.6, 1.3) | 0.2 |
| Any deficiency | ≥1 | 90.0 (87.7, 92.2) | 95.5 (93.3, 97.8) | 2.4 (1.3, 4.3) | 0.002 |
| Multiple deficiencies | ≥2 | 60.2 (56.5, 63.9) | 74.4 (69.5, 79.2) | 1.9 (1.4, 2.6) | <0.0001 |
Missing values: n = 1 for PIVKA-II and cobalamin, n = 2 for folic acid, n = 5 for ferritin and thyroglobulin, n = 1 for selenium and copper, n = 9 for any deficiency and multiple deficiencies. PIVKA-II, protein induced in vitamin K absence; PLP, pyridoxal-5′-phosphate; TfR, transferrin receptor; 25(OH)D, 25-hydroxyvitamin D.
Determined by logistic regression for the likelihood of being considered micronutrient deficient in children with inflammation relative to those without.
P value for the OR of being classified with a micronutrient deficiency in children with inflammation relative to those without.
The contribution of inflammation to estimates of the total population prevalence of deficiency is shown in Table 5 among inflammation-affected nutrients. The proportion of micronutrient deficiencies (PAR, 95% CI) explained by inflammation ranged from 0.019 for any deficiency (i.e., explaining 1.9% of the 91.7% prevalence of any deficiency) to 0.484 (0.319, 0.609) for retinol <0.07 μmol/L (i.e., explaining 48.4% of the population prevalence of 8.5% for vitamin A deficiency). Inclusion of all children in population prevalence estimates of deficiency resulted in a potential 1.2% underestimate in iron deficiency by ferritin <15 μg/L to a 5.4% increase in retinol <1.05 μmol/L.
TABLE 5.
Contribution of inflammation to population estimates of micronutrient deficiency among children in rural Nepal (n = 1000)1
| Indicator | Cutoff | PRR2 | PAR (95% CI)3 | Population prevalence explained by presence of inflammation4 |
| Retinol, μmol/L | <0.7 | 3.95 | 0.484 (0.319, 0.609) | +4.1% |
| <1.05 | 1.34 | 0.098 (0.060, 0.136) | +5.4% | |
| β-Carotene, μmol/L | <0.09 | 1.27 | 0.081 (0.028, 0.130) | +3.3% |
| PLP, nmol/L | <20 | 1.40 | 0.111 (0.060, 0.160) | +4.8% |
| Folic acid, nmol/L | <13.6 | 2.32 | 0.294 (0.094, 0.450) | +1.8% |
| Ferritin, μg/L | <15 | 0.66 | −0.118 (−0.238, −0.010) | −1.2% |
| TfR, mg/L | >8.3 | 1.38 | 0.107 (0.052, 0.158) | +4.3% |
| Total circulating selenium, μmol/L | <0.89 | 1.24 | 0.070 (0.035, 0.104) | +4.2% |
| Any deficiency | ≥1 | 1.06 | 0.019 (0.008, 0.030) | +1.7% |
| Multiple deficiencies | ≥2 | 1.24 | 0.070 (0.038, 0.099) | +4.6% |
Limited to micronutrient indicators in plasma where significant differences in prevalence of deficiency by inflammatory status occurred, from Table 4. PAR, population attributable risk; PLP, pyridoxal-5′-phosphate; PRR, prevalence rate ratio; TfR, transferrin receptor.
Calculated as the prevalence of deficiency among children with inflammation divided by the prevalence in children without inflammation, from Table 4.
Calculated as Pe(PRR−1)/(Pe(PRR−1)+1), where Pe is the proportion of the population exposed to inflammation (0.316) (39, 40). 95% CIs were calculated as lower limit = 1−exp(ln(1−PAR)+1.96s); upper limit = 1−exp(ln(1−PAR)−1.96s), where “s” is calculated as the square root of [b+(a+d)PAR]/nc, and b = number with inflammation but no deficiency, a = number with inflammation and deficiency, d = number with no inflammation or deficiency, n = total number of participants, c = children with deficiency but no inflammation, according to Fleiss (40).
Discussion
Evidence of undernutrition, morbidity, poor diet, and concurrent micronutrient deficiencies are present in these children of the rural terai of Nepal. Given the high rates of participation in the initial maternal trial and follow-up, community-based data collection, similar dietary and morbidity patterns among sampled and unsampled children, and random selection of biospecimens, our results likely represent the micronutrient status of children throughout the terai of Nepal. Fewer than one-fifth of the children were free of any deficiencies, and about half had multiple deficiencies. Evidence of inflammation, primarily elevated AGP, occurred in about one-third of children. Although some micronutrient deficiencies were associated with inflammation, its presence had a limited impact on prevalence estimates of micronutrient deficiencies at the population level.
Associations between illness and micronutrient status are complex and bi-directional. Micronutrients contribute to host immune defenses (15), and illness may be associated with poor appetite or increased sequestration, losses, or utilization of nutrients, leading to actual or functional deficiencies (41), or excesses of markers associated with the acute phase response, such as ferritin and plasma copper (42). In this study, inflammation affected twice as many children as reported being ill in the previous week, supporting the use of biochemical indicators as measures of inflammation. Elsewhere in Asia and Africa, inflammation was observed in over half of preschool children surveyed, with more concurrently elevated CRP and AGP (18, 36), suggesting more persistent inflammation among these Nepalese children. Thus, the prevalence and pattern of inflammation were less apt to influence population estimates of micronutrient deficiencies in this setting than elsewhere. Although inflammation did significantly affect some nutrient indicators, and adjustments produced prevalence estimates similar to those observed in the noninflamed children, hypothetically the complete elimination of inflammation would result in prevalence estimates of deficiency within ∼5% of those observed among all children.
Knowledge of the underlying associations between micronutrient indicators and illness, dynamics of inflammation across population contexts, and intended use of data may inform how best to account for coexisting inflammation with nutritional assessment. Adjustment for, or elimination of, cases with inflammation assumes a transient influence of inflammation on micronutrient indicators and resolution to unaffected values following convalescence. Caution should be used when micronutrient deficiencies may predispose to illness, or nutrients used or lost during inflammation, to ensure that actual deficiencies in the population are not underestimated by correcting or eliminating inflamed cases. Moreover, reporting adjusted data alone may render findings incomparable to historical data and result in a loss of information on both micronutrient and inflammatory status, when knowledge of both entities may be useful for targeting or evaluating interventions, particularly as attention is turned to improving nutritional status with efforts to control infectious diseases (43).
Vitamin A deficiency prevalence was <10%. Children could have been administered semiannual vitamin A supplements through national distribution programs in their preschool years, following receipt in utero and via breast milk to 3 mo of age in the maternal trial (19), and were also consuming dairy and green leafy foods. However, about half the children still had marginal to deficient status, and risk of vitamin A deficiency can be inferred from low circulating β-carotene, indicating insufficient consumption of bioavailable provitamin A–rich foods (i.e., yellow fruits or vegetables) or conversion of available carotenoids to retinol (44). Singh and West (4) estimated ∼14% vitamin A deficiency in Nepal a decade ago based primarily on data from adolescent girls from this study site, but >20% regionally in school-aged children. Recent biochemical data from school children in northwest India showed a higher (15.8%) prevalence of deficiency but similar prevalence of retinol <1.05 mmol/L (56.1%), and no effect of elevated CRP on retinol (33). Nepalese children with inflammation were about four times more likely to be classified as vitamin A deficient (17.4%) than those without (4.4%). Complete elimination of inflammation would hypothetically reduce the prevalence of deficiency by half (8.5% to 4.4%, or 5.4% by adjustment), but would have little relative impact on marginal to deficient status (55.3% to 49.9%, or 49.1% by adjustment). Whether low-circulating retinol is associated with poor diet, the functional deficiency of concurrent inflammation, or both, it reflects inadequate availability of retinol to the tissues, potentially putting children at higher risk of illness, xerophthalmia, or mortality (45).
Cutoffs for vitamin E deficiency have not been firmly established for children. Although it is thought ideal to maintain circulating status at concentrations >12 μmol/L, by which half of the children were considered deficient, children may have lower concentrations commensurate with circulating lipids (7). Thus, lower cutoffs for circulating α-tocopherol may be appropriate in childhood. Mean α-tocopherol was similar to that in children in Korea, Vietnam, and Thailand, as reviewed by Dror and Allen (7), although regionally representative data are lacking. The prevalence of α-tocopherol <9.3 μmol/L was also similar to that observed in the mothers of these children during their early pregnancies (25%) (14), indicating that vitamin E deficiency may be pervasive throughout life in this area of Nepal.
Deficiencies of both vitamins D and K also occurred in nearly 20% of children. These nutrients were unrelated to inflammation and were poorly correlated with other indicators. Vitamin D deficiency in childhood may affect bone development and immunity (5, 6). Estimates of deficiency in the region are limited and wide-ranging (7–70%) (46), likely driven by season and sun exposure. Less is known of the impact of vitamin K deficiency, with most studies available in children with cystic fibrosis, among whom half had elevated PIVKA-II in one study (31). It is likely that bone health is compromised by undercarboxylated osteocalcin at a less severe degree of vitamin K deficiency than is clotting function (31), for which PIVKA-II is an indicator; thus, the prevalence of vitamin K deficiency as reflected by PIVKA-II concentrations may underestimate the risk of vitamin K deficiency on bone status in these children.
Of the water-soluble vitamins involved in one-carbon metabolism, there was a high prevalence of deficiency of vitamins B-12 (18.1%) and B-6 (43.1%), but not folate. These patterns were also observed among the mothers of these children during their pregnancies, with vitamin B-6 (40%) and B-12 (28%) deficiencies far more prevalent than folate (12%) (14), consistent with infrequent consumption of meat but more regular consumption of dark green vegetables. Cobalamin is thought to be at its highest concentration in mid-childhood in healthy children (∼600 pmol/L), and the Nepalese children had lower mean concentrations than those observed in nonbreastfed preschoolers (47) and schoolchildren (33) in the region (260–460 pmol/L). There may be long-term associations of these patterns with metabolic health mediated through elevated homocysteine (9); this is currently being explored. An association of low folate with inflammation was observed, as seen previously (33), and low folate may predispose to respiratory illness or diarrhea based on its function in epithelial cell integrity and innate and adaptive immunity (15, 47, 48). The consequences of vitamin B-6 deficiency are not well defined, and even in the United States, 25% of children have been classified as deficient (34). Speculation exists as to a role for PLP as a cofactor in enzymes required to mount an inflammatory response; thus, inflammation may alter the distribution and increase the utilization of, and requirement for, vitamin B-6 (49).
Estimates of iron deficiency differed using ferritin (10.7%), TfR (40.1%), or TfR:ferritin, which provides a reasonable composite estimate (14.3%). Although hemoglobin data were not available, a recent evaluation in the area found anemia in ∼25% of children (23), and regionally ∼20% iron deficiency is estimated (2). TfR was increased with inflammation to a higher extent than ferritin, suggesting a tendency toward erythropoietic activity during inflammation (36) or more iron deficiency among less healthy children. The TfR:ferritin ratio was not significantly associated with inflammation, unlike observations elsewhere (36). Iron status remains perhaps the most challenging micronutrient deficiency to assess under conditions of inflammation when elevated ferritin may underestimate iron deficiency. Hepcidin, a hormonal regulator of iron absorption during deficiency (low hepcidin) and/or sequestration in stores during sufficiency and inflammation (elevated hepcidin), may ultimately be a useful indicator of the body’s iron need under both conditions (50).
Low plasma selenium was observed in over half the children and varied inversely with inflammatory markers, whereas there was no evidence of copper deficiency. The tendency of these plasma markers to change in opposite directions during inflammation is likely mediated through changes in production and distribution of carrier proteins selenoprotein P (51) and ceruloplasmin (52). Data on these indicators is uncommon in children, but nearly identical prevalences were observed in preschool-aged children in Vietnam (38). Iodine deficiency was modest, with elevated thyroglobulin occurring in just over 10% of children. Iodine adequacy is dependent on iodized salt in the region, which was recently found in ∼65% of homes (23).
Correlations among nutritional status indicators may be explained by dietary patterns or intersecting metabolic pathways, areas of future exploration. Retinol, β-carotene, and α-tocopherol may be related via fat in the diet, as a source of vitamin A and E and promoting the absorption of β-carotene (44). Folate and β-carotene may be related by common consumption of plant-source foods. Correlations of vitamin B-6 with other vitamins may be related to the diversity of food consumed and mediated by associations with acute phase proteins. Positive correlations between cobalamin, copper, and ferritin may be associated with meat intakes, and a positive correlation between antioxidants α-tocopherol and selenium suggests the persistence of oxidative stress in some children (11).
Although our data add substantially to information on micronutrient status in school-aged children in this region of south Asia, there are limitations to this study. Cross-sectional data preclude assertions about causal relations among micronutrient status and inflammation. Given the scope of this paper, we were unable to fully explore associations of diet, seasonality, and socioeconomic factors with micronutrient status, which we intend for future analyses. We do not, however, have data on other factors that could affect micronutrient status, such as geohelminths, malaria, or other specific pathogens. Moreover, data on hemoglobin, urinary iodine, and zinc status would greatly enhance the panel of micronutrients.
Our data do, however, show that deficiencies were common, particularly for nutrients that often go unexplored. Causes and consequences of multiple micronutrient deficiencies in this age group deserve further exploration to improve understanding of nutritional risks faced throughout the childhood years in underprivileged areas where diets are poor and morbidity persistent, and to motivate prevention. This will require more comprehensive strategies for micronutrient status assessment.
Acknowledgments
The authors thank Joanne Katz, James Tielsch, and Luke Mullany of the Nepal Nutrition Intervention Project, Sarlahi, for their contributions. The authors also thank Brittany Kmush and Veena Singh for contributions in the laboratory, and Michael Rybak of the CDC for assistance with the vitamin B-6 assay. K.J.S. designed the research, analyzed the data, wrote the manuscript, and had primary responsibility for the final content; P.C. and K.P.W. designed the research and wrote the manuscript; L.S.-F.W. analyzed the data; and M.A., H.C., A.N.-B., C.P.S., S.K.K., and S.L. conducted the research. All authors read and approved the final manuscript.
Footnotes
Abbreviations used: AGP, α-1 acid glycoprotein; CRP, C-reactive protein; PAR, population attributable risk; PIVKA-II, protein induced in vitamin K absence; PLP, pyridoxal-5′-phosphate; TfR, transferrin receptor; 25(OH)D, 25-hydroxyvitamin D.
Literature Cited
- 1.Caulfield LE, Richard SA, Rivera JA, Musgrove P, Black RE. Stunting, wasting, and micronutrient deficiency disorders. In: Disease control priorities in developing countries. 2nd ed. New York: Oxford University Press; 2006. p.551–68. [Google Scholar]
- 2.Best C, Neufingerl N, van Geel L, van den Briel T, Osendarp S. The nutritional status of school-aged children: why should we care? Food Nutr Bull. 2010;31:400–17. [DOI] [PubMed] [Google Scholar]
- 3.UNICEF. First call for children. World declaration and 1990–2000 plan of action on the survival, protection, and development of children. New York: UNICEF; 1990.
- 4.Singh V, West KP., Jr Vitamin A deficiency and xerophthalmia among school-aged children in Southeastern Asia. Eur J Clin Nutr. 2004;58:1342–9. [DOI] [PubMed] [Google Scholar]
- 5.Pettifor JM, Prentice A. The role of vitamin D in paediatric bone health. Best Pract Res Clin Endocrinol Metab. 2011;25:573–84. [DOI] [PubMed] [Google Scholar]
- 6.Walker VP, Modlin RL. The vitamin D connection to pediatric infections and immune function. Pediatr Res. 2009;65:106R–13R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dror DK, Allen LH. Vitamin E deficiency in developing countries. Food Nutr Bull. 2011;32:124–43. [DOI] [PubMed] [Google Scholar]
- 8.Cashman KD. Vitamin K status may be an important determinant of childhood bone health. Nutr Rev. 2005;63:284–9. [DOI] [PubMed] [Google Scholar]
- 9.Kerr MA, Livingstone B, Bates CJ, Bradbury I, Scott JM, Ward M, Pentieva K, Mansoor MA, McNulty H. Folate, related B vitamins, and homocysteine in childhood and adolescence: potential implications for disease risk in later life. Pediatrics. 2009;123:627–35. [DOI] [PubMed] [Google Scholar]
- 10.Bjørke-Monsen AL, Ueland PM. Cobalamin status in children. J Inherit Metab Dis. 2011;34:111–9. [DOI] [PubMed] [Google Scholar]
- 11.Beck MA. Selenium and vitamin E status: impact on viral pathogenicity. J Nutr. 2007;137:1338–40. [DOI] [PubMed] [Google Scholar]
- 12.Zimmermann MB, Kohrle J. The impact of iron and selenium deficiencies on iodine and thyroid metabolism: biochemistry and relevance to public health. Thyroid. 2002;12:867–78. [DOI] [PubMed] [Google Scholar]
- 13.de Romaña DL, Olivares M, Uauy R, Araya M. Risks and benefits of copper in light of new insights of copper homeostasis. J Trace Elem Med Biol. 2011;25:3–13. [DOI] [PubMed] [Google Scholar]
- 14.Jiang T, Christian P, Khatry SK, Wu L, West KP., Jr Micronutrient deficiencies in early pregnancy are common, concurrent, and vary by season among rural Nepali pregnant women. J Nutr. 2005;135:1106–12. [DOI] [PubMed] [Google Scholar]
- 15.Wintergerst ES, Maggini S, Hornig DH. Contribution of selected vitamins and trace elements to immune function. Ann Nutr Metab. 2007;51:301–23. [DOI] [PubMed] [Google Scholar]
- 16.Thurnham DI, Mburu AS, Mwaniki DL, deWagt AD. Micronutrients in childhood and the influence of subclinical inflammation. Proc Nutr Soc. 2005;64:502–9. [DOI] [PubMed] [Google Scholar]
- 17.Thurnham DI, McCabe LD, Haldar S, Wieringa FT, Northrop-Clewes CA, McCabe GP. Adjusting plasma ferritin concentrations to remove the effects of subclinical inflammation in the assessment of iron deficiency: a meta-analysis. Am J Clin Nutr. 2010;92:546–55. [DOI] [PubMed] [Google Scholar]
- 18.Thurnham DI, McCabe GP, Northrop-Clewes CA, Nestel P. Effects of subclinical infection on plasma retinol concentrations and assessment of prevalence of vitamin A deficiency: meta-analysis. Lancet. 2003;362:2052–8. [DOI] [PubMed] [Google Scholar]
- 19.Christian P, Khatry SK, Katz J, Pradhan EK, LeClerq SC, Shrestha SR, Adhikari RK, Sommer A, West KP., Jr Effects of alternative maternal micronutrient supplements on low birth weight in rural Nepal: double blind randomised community trial. BMJ. 2003;326:571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stewart CP, Christian P, Schulze KJ, LeClerq SC, West KP, Jr, Khatry SK. Antenatal micronutrient supplementation reduces metabolic syndrome in 6- to 8-year-old children in rural Nepal. J Nutr. 2009;139:1575–81. [DOI] [PubMed] [Google Scholar]
- 21.Stewart CP, Christian P, LeClerq SC, West KP, Jr, Khatry SK. Antenatal supplementation with folic acid + iron + zinc improves linear growth and reduces peripheral adiposity in school-age children in rural Nepal. Am J Clin Nutr. 2009;90:132–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Christian P, Morgan ME, Murray-Kolb L, LeClerq SC, Khatry SK, Schaefer B, Cole PM, Katz J, Tielsch JM. Preschool iron-folic acid and zinc supplementation in children exposed to iron-folic acid in utero confers no added cognitive benefit in early school-age. J Nutr. 2011;141:2042–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Christian P, Murray-Kolb LE, Khatry SK, Katz J, Schaefer BA, Cole PM, LeClerq SC, Tielsch JM. Prenatal micronutrient supplementation and intellectual and motor function in early school-aged children in Nepal. JAMA. 2010;304:2716–23. [DOI] [PubMed] [Google Scholar]
- 24.Cole RN, Ruczinski I, Schulze K, Christian P, Herbrich S, Wu L, DeVine LR, O'Meally RN, Shrestha S, Boronina TN, et al. The plasma proteome identifies expected and novel proteins correlated with micronutrient status in undernourished Nepalese children. J Nutr. 2013;143:1540–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yamini S, West KP, Jr, Wu L, Dreyfuss ML, Yang DX, Khatry SK. Circulating levels of retinol, tocopherol and carotenoid in Nepali pregnant and postpartum women following long-term beta-carotene and vitamin A supplementation. Eur J Clin Nutr. 2001;55:252–9. [DOI] [PubMed] [Google Scholar]
- 26.Kimura M, Kanehira K, Yokoi K. Highly sensitive and simple liquid chromatographic determination in plasma of B6 vitamers, especially pyridoxal 5′-phosphate. J Chromatogr A. 1996;722:295–301. [DOI] [PubMed] [Google Scholar]
- 27.Jacobson BE, Lockitch G. Direct determination of selenium in serum by graphite-furnace atomic absorption spectrometry with deuterium background correction and a reduced palladium modifier: age-specific reference ranges. Clin Chem. 1988;34:709–14. [PubMed] [Google Scholar]
- 28.WHO. Growth reference 5–19 years. Geneva, Switzerland: WHO; 2007 [cited 2011 Mar 1]. Available from: http://www.who.int/growthref/en/.
- 29.WHO. Global prevalence of vitamin A deficiency in populations at risk, 1995–2005. WHO global database on vitamin A deficiency. Geneva, Switzerland: WHO; 2009. [Google Scholar]
- 30.Institute of Medicine. Dietary reference intakes for calcium and vitamin D. Washington: National Academy Press; 2011. [Google Scholar]
- 31.Dougherty KA, Schall JI, Stallings VA. Suboptimal vitamin K status despite supplementation in children and young adults with cystic fibrosis. Am J Clin Nutr. 2010;92:660–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Green R. Indicators for assessing folate and vitamin B12 status and for monitoring the efficacy of intervention strategies. Food Nutr Bull. 2008;29:S52–63. [DOI] [PubMed] [Google Scholar]
- 33.Osei A, Houser R, Bulusu S, Joshi T, Hamer D. Nutritional status of primary schoolchildren in Garhwali Himalayan villages of India. Food Nutr Bull. 2010;31:221–33. [DOI] [PubMed] [Google Scholar]
- 34.Morris MS, Picciano MF, Jacques PF, Selhub J. Plasma pyridoxal 5′-phosphate in the US population: the National Health and Nutrition Examination Survey, 2003–2004. Am J Clin Nutr. 2008;87:1446–54. [DOI] [PubMed] [Google Scholar]
- 35.WHO/CDC. Assessing the iron status of populations: report of a joint WHO/CDC technical consultation on the assessment of iron status at the population level. Geneva, Switzerland: WHO; 2005. [Google Scholar]
- 36.Grant FK, Suchdev PS, Flores-Ayala R, Cole CR, Ramakrishnan U, Ruth LJ, Martorell R. Correcting for inflammation changes estimates of iron deficiency among rural Kenyan preschool children. J Nutr. 2012;142:105–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.WHO/UNICEF/ICCIDD. Assessment of iodine deficiency disorders and monitoring their elimination: a guide for programme managers. Geneva, Switzerland: WHO; 2007. [Google Scholar]
- 38.Van Nhien N, Khan NC, Ninh NX, Van Huan P. Hop le T, Lam NT, Ota F, Yabutani T, Hoa VQ, Motonaka J, et al. Micronutrient deficiencies and anemia among preschool children in rural Vietnam. Asia Pac J Clin Nutr. 2008;17:48–55. [PubMed] [Google Scholar]
- 39.Lui KJ. Notes on interval estimation of the attributable risk in cross-sectional sampling. Stat Med. 2001;20:1797–809. [DOI] [PubMed] [Google Scholar]
- 40.Fleiss JL. Inference about population attributable risk from cross-sectional studies. Am J Epidemiol. 1979;110:103–4. [DOI] [PubMed] [Google Scholar]
- 41.Calder PC, Jackson AA. Undernutrition, infection and immune function. Nutr Res Rev. 2000;13:3–29. [DOI] [PubMed] [Google Scholar]
- 42.Brown KH, Lanata CF, Yuen ML, Peerson JM, Butron B, Lonnerdal B. Potential magnitude of the misclassification of a population's trace element status due to infection: example from a survey of young Peruvian children. Am J Clin Nutr. 1993;58:549–54. [DOI] [PubMed] [Google Scholar]
- 43.Ahmed T, Hossain M, Sanin KI. Global burden of maternal and child undernutrition and micronutrient deficiencies. Ann Nutr Metab. 2012;61:8–17. [DOI] [PubMed] [Google Scholar]
- 44.West CE, Eilander A, van Lieshout M. Consequences of revised estimates of carotenoid bioefficacy for dietary control of vitamin A deficiency in developing countries. J Nutr. 2002;132:2920S–6S. [DOI] [PubMed] [Google Scholar]
- 45.Sommer A. Vitamin A deficiency and clinical disease: an historical overview. J Nutr. 2008;138:1835–9. [DOI] [PubMed] [Google Scholar]
- 46.Yang Z, Laillou A, Smith G, Schofield D, Moench-Pfanner R. A review of vitamin D fortification: implications for nutrition programming in Southeast Asia. Food Nutr Bull. 2013;34:S81–9. [DOI] [PubMed] [Google Scholar]
- 47.Manger MS, Taneja S, Strand TA, Ueland PM, Refsum H, Schneede J, Nygard O, Sommerfelt H, Bhandari N. Poor folate status predicts persistent diarrhea in 6- to 30-month-old north Indian children. J Nutr. 2011;141:2226–32. [DOI] [PubMed] [Google Scholar]
- 48.Strand TA, Taneja S, Bhandari N, Refsum H, Ueland PM, Gjessing HK, Bahl R, Schneede J, Bhan MK, Sommerfelt H. Folate, but not vitamin B-12 status, predicts respiratory morbidity in north Indian children. Am J Clin Nutr. 2007;86:139–44. [DOI] [PubMed] [Google Scholar]
- 49.Morris MS, Sakakeeny L, Jacques PF, Picciano MF, Selhub J. Vitamin B-6 intake is inversely related to, and the requirement is affected by, inflammation status. J Nutr. 2010;140:103–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Prentice AM, Doherty CP, Abrams SA, Cox SE, Atkinson SH, Verhoef H, Armitage AE, Drakesmith H. Hepcidin is the major predictor of erythrocyte iron incorporation in anemic African children. Blood. 2012;119:1922–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hollenbach B, Morgenthaler NG, Struck J, Alonso C, Bergmann A, Kohrle J, Schomburg L. New assay for the measurement of selenoprotein P as a sepsis biomarker from serum. J Trace Elem Med Biol. 2008;22:24–32. [DOI] [PubMed] [Google Scholar]
- 52.Shenkin A. Basics in clinical nutrition: physiological function and deficiency states of trace elements. e-SPEN, The European e-Journal of Clinical Nutrition and Metabolism. 2008;3:e255–58. [Google Scholar]