Abstract
Following brain injury, reactive glial cells can create scars which inhibit neural repair responses. In this issue of Cell Stem Cell, Guo et al. report that overexpression of NeuroD1 in vivo can directly reprogram reactive glial cells into glutamatergic and GABAergic neurons which integrate into the host’s neural circuitry.
Glial cells, including astrocytes and oligodendrocytes, support a wide range of brain functions such as synaptic formation/pruning and transmission and maintenance of the blood-brain barrier, as well as growth and myelination of axons. During injury and neurodegenerative diseases, astrocytes respond to damage by proliferating and undergoing hypertrophy, with increased expression of the intermediate filament glial fibrillary acidic protein (GFAP), resulting in formation of compact glial scars. This response is supplemented by NG2 cells, resident progenitors that give rise mostly to oligodendrocytes, which participate in gliosis in addition to regenerating cells lost to injury. Gliotic scars are generally inhibitory to neuronal survival and axonal regeneration. In this issue of Cell Stem Cell, Guo et al. exploit glial scars as a cellular source for direct reprogramming into neuronal populations.
“Melting” glial scars has been attempted for decades with little success. Alternative strategies include transforming inhibitory gliotic tissue into an environment conducive to neuronal regeneration, or perhaps directly converting this tissue into neurons. The latter idea has gained momentum following the transformative discovery that cells of one type could be directly converted into another lineage by introduction of cell fate-determining transcription factors (Takahashi et al., 2006). Fibroblasts have been reprogrammed to functional neurons in vitro by overexpressing the transcription factors Ascl1, Brn2a and
Myt1l, and the efficiency of this direct conversion was further enhanced by addition of the neuronal specific bHLH transcription factor NeuroD1 (Pang et al., 2011; Yang et al., 2011). Cultured astrocytes have been converted to neurons with even simpler combinations of transcription factors such as NeuroG2 and Ascl1, likely due to the common neuroectodermal origin of these cell types (Berninger et al., 2007). What remains to be determined is if reactive gliacan be converted to neurons in the environment of an injured or diseased brain and whether these reprogrammed neurons are functional in situ.
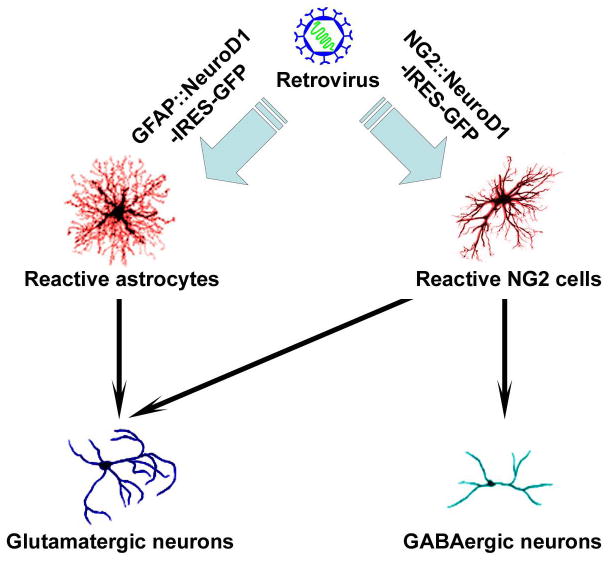
In this issue, Guo et al. show that they are able to convert reactive (proliferating) astrocytes and NG2 glia to functional neurons by retroviral expression of a single transcription factor, NeuroD1, in both injury and Alzheimer’s disease models (Guo et al., 2014). The authors first utilized a stab-wound mouse model of brain injury, which elicits an acute gliotic response. Following injection of retrovirus expressing NeuroD1 into cortical areas of the stab-wounded brain, without penetrating the hippocampus or sub ventricular zone (to avoid targeting neural progenitors), they show that infected cells convert into neurons as early as 3 days post-viral infection. Importantly, by whole cell patch clamping in brain slices, they demonstrate that the converted neurons are not only electrophysiologically active but that they also integrate into neural circuitry, as shown by spontaneous and evoked synaptic currents. This phenomenon was then replicated in a mouse model of Alzheimer disease (5XFAD mice), in which chronic and progressive gliosis takes place. Interestingly, in this model, the authors found that more new neurons were formed following infection with NeuroD1 retrovirus in later stages of disease, likely because of increasing numbers of proliferating reactive glia during disease progression. Together, the authors demonstrate convincingly that reactive glia in injured and diseased mouse brains can be converted to functional neurons by NeuroD1 overexpression (Fig. 1). Previously, in vivo reprogramming of astrocytes into neuroblasts via Sox2 overexpression in the adult brain has been reported (Niu et al., 2013). In contrast to the findings of Guo et al., cells infected with Sox2 could potentially give raise to many neural cell types, including converted neuroblasts, and further differentiation to functional neurons appears to require additional signals (Niu et al., 2013).
Figure 1. Direct Conversion of Reactive Glial Cells to Active Neurons Via NeuroD1 Expression.
Cortical reactive astrocytes and NG2 glial cells in stab-wound and Alzheimer’s (5XFAD) mouse models are directly converted to electrophysiologically active neurons by retroviral delivery of NeuroD1 invivo. Astrocytes are capable of becoming glutaminergic neurons while NG2 glial cells are capable of converting into both glutaminergic and GABAergic neurons.
Neurons converted directly from fibroblasts and astrocytes by pan-neural transcription factors, including NeuroD1, have been shown to be largely glutamatergic, though GABAergic neurons are also observed in vitro (Berninger et al., 2007; Pang et al; Vierbuchen et al.). In the current study, the authors observed that GABAergic neurons were formed even though the majority of converted neurons were glutamatergic. To determine whether these neurons with distinct neurotransmitter phenotypes arose from NeuroD1-mediated conversion of different populations of reactive glial cells, the authors employed cell type-specific promoters to drive NeuroD1 expression specifically in either astrocytes (using a GFAP promoter) or NG2 cells (using an NG2 promoter). In this set of experiments, they found that astrocytes are induced exclusively to glutaminergic neurons whereas NG2 cells are reprogrammed to either glutaminergic or GABAergic neurons by NeuroD1 (Fig. 1). This finding is quite interesting as it suggests that NeuroD1 may interact with other cell type-specific transcription factors to determine the transmitter phenotype of converted neurons. Neuronal phenotypes, including neurotransmitter identities, are often determined by region-specific expression of HLH transcription factors within their respective progenitor populations in the developing brain. Similarly, glial subtype specification is also impacted by homeodomain transcription factors. It would be of great interest, with significant value for future applications, to determine the roles that glial subtypes or their transcriptional codes play in shaping the functional phenotypes of reprogrammed neurons.
The authors then asked whether human glia could be similarly converted to functional neurons using this approach. To address this question, the authors used the same GFAP::NeuroD1 viral system to infect cultured fetal human cortical astrocytes and found that human astrocytes could likewise be efficiently reprogrammed into functional glutaminergic, but not GABAergic, neurons. This is similar to previous findings showing reprogramming of human non-neuronal cells to neural progenitors and functional neurons by similar approaches (Lu et al., 2013; Pang et al., 2011; Ring et al., 2012; Vierbuchen et al., 2010). Therefore, conversion of human glia directly to neurons is possible in vitro.
The ability to directly convert reactive glia into neurons in injured or disease brain tissue, as shown by Guo et al. (2014), raises the possibility of modifying inhibitory gliotic tissues for therapeutic gains. However, there are a number of issues that must be addressed before moving forward with potential clinical applications. Technically, a more efficient and safer method for introducing genetic material into patients’ cells will be necessary for clinical translation of these findings. More importantly, future work is needed to demonstrate integration of converted neurons into appropriate neural circuits and whether this contributes to functional improvement. Additionally, further work to elucidate the mechanisms of cell type-specific conversion into neurons with distinct phenotypes is required. Knowledge gained from these experiments could in turn allow for conversion of specific glial populations into neuronal subtypes for therapy in an injury- or neurodegenerative disease-specific manner. For instance, the ability to convert glial cells into motor or dopaminergic neurons in vivo could have profound implications for treatment of ALS and Parkinson’s disease, respectively. This study by Guo et al. lays the groundwork for further exploration of potential regenerative therapy for neurological injury and neurodegenerative diseases.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Berninger B, Costa MR, Koch U, Schroeder T, Sutor B, Grothe B, Götz M. Functional properties of neurons derived from in vitro reprogrammed postnatal astroglia. J Neurosci. 2007;27:8654–8664. doi: 10.1523/JNEUROSCI.1615-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Z, Zhang L, Wu Z, Chen Y, Wang F, Chen G. In Vivo Direct Reprogramming of Reactive Glial Cells into Functional Neurons after Brain Injury and in an Alzheimer’s Disease Model. Cell Stem Cell? 2014:1–15. doi: 10.1016/j.stem.2013.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu J, Liu H, Huang CT, Chen H, Du Z, Liu Y, Sherafat MA, Zhang SC. Generation of Integration-free and Region-Specific Neural Progenitors from Primate Fibroblasts. Cell Rep. 2013a;3:1580–1591. doi: 10.1016/j.celrep.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niu W, Zang T, Zou Y, Fang S, Smith DK, Bachoo R, Zhang C-L. In vivo reprogramming of astrocytes to neuroblasts in the adult brain. Nat Cell Biol. 2013;15:1164–1175. doi: 10.1038/ncb2843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang ZP, Yang N, Vierbuchen T, Ostermeier A, Fuentes DR, Yang TQ, Citri A, Sebastiano V, Marro S, Südhof TC, et al. Induction of human neuronal cells by defined transcription factors. Nature. 2011;476:220–223. doi: 10.1038/nature10202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ring KL, Tong LM, Balestra ME, Javier R, Andrews-Zwilling Y, Li G, Walker D, Zhang WR, Kreitzer AC, Huang Y. Direct Reprogramming of Mouse and Human Fibroblasts into Multipotent Neural Stem Cells with a Single Factor. Cell Stem Cell. 2012;11:100–109. doi: 10.1016/j.stem.2012.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K, Yamanaka S. Induction of Pluripotent Stem Cells from Mouse Embryonic and Adult Fibroblast Cultures by Defined Factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- Vierbuchen T, Ostermeier A, Pang ZP, Kokubu Y, Südhof TC, Wernig M. Direct conversion of fibroblasts to functional neurons by defined factors. Nature. 2010;463:1035–1041. doi: 10.1038/nature08797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang N, Ng YH, Pang ZP, Südhof TC, Wernig M. Induced Neuronal Cells: How to Make and Define a Neuron. Cell Stem Cell. 2011;9:517–525. doi: 10.1016/j.stem.2011.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]