Summary
Aging contributes to physiological decline and vulnerability to disease. In the brain, even with minimal neuron loss, aging increases oxidative damage, inflammation, demyelination, impaired processing and metabolic deficits, particularly during pathological brain aging. We discuss the possible role of docosahexaenoic acid (DHA) in prevention of age related disruption of brain function. High-fat diabetogenic diets, cholesterol and the omega-6 fatty acid arachidonate and its prostaglandin metabolites have all been implicated in promoting AD pathogenesis. We argue that DHA acts to oppose this pathogenesis, exerting a plethora of pleiotropic activities to protect against AD pathogenesis.
Keywords: Alzheimer's disease, dementia, omega-3 fatty acid (n-3), docosahexaenoic acid (DHA), amyloid
INTRODUCTION
What's for dinner was one of the primeval questions that our brains evolved to answer. While researchers have learned a great deal about nutrients needed for optimum development, those needed for optimum aging remain elusive. The focus of this symposium, nutrition and the aging brain, addresses the difficult and important topic of what we should be eating for optimum brain aging. Health care costs to care for the aged represents an ever-increasing problem for developed nations with aging populations. In the United States, the government projects 52 trillion dollar Medicare deficits that will arise from an aging baby boomer population's chronic diseases of aging. This expense literally threatens to bankrupt the government and mandates healthcare “reform” legislation. The economic and medical problems of aging populations with exponentially rising health care costs are similar in many other developed and developing countries. There is no simple medical solution. A plethora of expensive new medications may provide better disease management and longer lives- but instead of reducing costs, would likely add to costs. Therefore, from the economic standpoint alone, we need to take a fresh approach to the diseases of aging, including those disabling diseases that emerge with the aging brain. We need to focus on cost-effective prevention measures.
NUTRITION, AS A CRITICAL APPROACH TO PREVENTION OF AGE-RELATED MEMORY DEFICITS
Unlike infectious diseases, the problem of the chronic diseases of aging, including brain aging seldom offer us foreign pathogens to target. Diseases of aging are primarily the consequence of chronic imbalances or dysregulation of normal pathways with important functional roles, and the targets are our normal gene products. Thus, the consequences of markedly stimulating or inhibiting single targets selected for important functional roles to control chronic diseases of aging in one or another tissue or cell type is likely to include adverse side-effects, for example, altering the target's normal level of function in another cell or tissue where it is not dysregulated. From this perspective, the standard pharmacologist's strategy of finding the most potent and specific drug from a high throughput search of compound libraries for single targets may not be the best approach. Nutritional interventions are inherently pleiotropic and key regulators of signal transduction pathways that have evolved to adjust and respond to the nutrient environment. One of the best examples of this is insulin signaling which is under intensive study in relationship to aging because it appears to play a key role in regulating lifespan1. A crude nutritional intervention, caloric restriction, is generally accepted as one of the few tools we have to modify rates of aging, including aging of the brain, which may be attenuated with caloric restriction in mice2. Thus, nutritional interventions are not only highly relevant to aging in general, they are also inherently more likely to involve low costs and to have a more favorable safety profile than novel drugs.
AGING OF THE BRAIN AND RELATED DISEASES
Like aging of the whole organism, aging of the brain is inherently complex, multi-faceted and poorly understood. It is normally accompanied by low levels of chronic inflammation, oxidative damage, lipofuscin and protein aggregate accumulation as well as myelin loss and very selective and modest neuron and synapse loss, for example in the pars compacta of substantia nigra. The most egregious phenotype with normal aging is that we slow down with reduced speeds of information processing and axon transmission rates, possibly due to the vulnerability of oligodendrocytes and myelination to oxidative damage3. An overview at the level of gene expression is consistent with inflammation, oxidative damage and reduced metabolism, possibly mitochondrial failure2.
In the presence of Alzheimer's disease (AD), a similar pattern of change is greatly exacerbated by accumulation of pathological protein aggregates in vulnerable regions. Pathological aggregates include those from β-amyloid protein, notably in extracellular plaques and vessels, but also within neurons, and others made from microtubule associated protein (MAP), tau, which is normally seen as predominantly axonal and microtubule associated, but which redistributes into paired helical filament (PHF) aggregates in neuronal perikarya and neurites, chiefly dendritic4. Similarly, in Parkinson's disease (PD) and the Lewy body variant of Alzheimer's there is a selected regional accumulation of alpha-synuclein aggregates in peryikarya (for example Lewy bodies in substantia nigra and cortex) and in neurites (Lewy neurites). Four age-related CNS diseases, AD, PD, Huntingtin Disease (HD) and frontal temporal dementia (FTD), exhibit regional protein aggregate pathology associated with selective neurodegeneration that explains their clinical phenotypes.
The other prevalent age-related brain pathologies, stroke and vascular or “multi-infarct” dementia (VAD or MID), involve primary vessel disease and are strongly associated with cardiovascular disease (CVD) risk factors that lead to large or small infarcts, respectively. The risk factors for CVD strongly overlap those for AD and perhaps a third or more dementia cases have mixed dementia with both types of pathology5, 6. Thus, solely on the basis of the need to control CVD risk factors, there is very little doubt that diet and exercise measures put in place to preserve cardiovascular health are likely to prove critical preventive measures for overall healthy brain aging. One of the CVD dietary interventions, the omega-3 fatty acids, are not only relatively inexpensive, but have an excellent safety profile and have already been shown to reduce cardiovascular disease mortality by 19-45%7. However, the focus of this review will be the potential role of omega-3 fatty acids in the prevention of the most prevalent and devastating form of pathological brain aging, AD.
ALZHEIMER's DISEASE AND LIPID METABOLISM
Early onset Alzheimer Disease (AD) is strongly familial and occurs because of rare genetic mutations, but the vast majority of AD cases develop after age 65 and are “late onset” and sporadic with aging as the dominant risk factor4. In fact, AD is so strongly age-related that incidence doubles every five years after age 65 resulting in a major public health problem in developed societies experiencing a demographic shift toward an aging population. While aging is the biggest single risk factor, AD is not an inevitable consequence of aging. Developing AD is subject to multiple genetic and environmental risk factors that determine age-dependent risk, presumably by modulating the pathogenesis. Because these genetic and environmental risk factors are not sufficiently powerful to act alone, the literature surrounding them is controversial and the evidence supporting them is frequently inconclusive.
Of the genetic risk factors, the E4 allele of the lipid transport protein apolipoprotein E is by far and away the single most potent and best established. Further, the clearest genetic protective factor for AD is the E2 allele of apolipoprotein E. The fact that ApoE, a lipid transport protein, is the single most potent known modifier of genetic AD risk raises the question of the role of brain lipids. The β-amyloid peptide (Aβ) that is implicated in initiating AD is hydrophobic and rides on ApoE and other lipid carriers so ApoE genotype can influence β-amyloid pathogenesis. But even without Aβ pathology, ApoE4 genotype modulates other AD risk factors. For example, ApoE4 influences the response to head injury and compensatory sprouting as well as three other pathogenic influences: cholesterol metabolism, oxidative damage and inflammation8.
Environmental factors that may impact AD risk include essential polyunsaturated fatty acids, which are substrates for pathways that are dysregulated in AD pathogenesis, such as lipid peroxidation and cyclooxygenase and lipoxygenase enzymes. The dysregulation of lipid metabolism pathways in the disease process is likely to interfere with learning and memory because lipid transport selectively involves regions with high synaptic plasticity that are dependent on lipid salvage pathways. Further, the best-studied protective environmental risk factors for AD include non-steroidal anti-inflammatory drug (NSAID) inhibitors of cyclooxygenase enzymes that act on an n-6 lipid substrate, arachidonic acid. Finally, increased consumption of statins, fish/omega -3 fatty acids, and lipid soluble antioxidants like vitamin E that prevent lipid peroxidation have all been reported to reduce AD risk. Thus, circumstantial but indirect evidence strongly implicates lipid metabolism in AD pathogenesis.
ALZHEIMER DISEASE: MOLECULAR PATHOGENESIS
AD is initiated by increased amyloid β (Aβ1-42) protein accumulation that is derived by proteolytic cleavage of a larger β amyloid precursor protein by two enzymes called β- and β-secretase. Aβ 1-42 is normally produced and cleared but has a marked propensity to aggregate and form neurotoxic oligomers, protofibrils and fibrils4. These oligomers and fibrils of Aβ interact with metals and glia to cause oxidative damage and neuroinflammation. They interact with neurons, notably at synapses to cause synaptic dysfunction and loss as well as activation of a least three kinases (JNK, GSK3β and CDK5) that hyperphosphorylate tau protein causing its release from microtubules (loss of normal function) and aggregation into possibly toxic soluble tau oligomers and intraneuronal neurofibrillary tangles. The precise pathways for tau toxicity remain unclear, but many reports argue that tau pathology appears to be a better correlate of neurodegeneration and progression than β-amyloid plaques. Synapse loss, not neuron loss, is the best structural correlate of cognitive decline and likely the proximate cause9, 10. While the common belief is that neuron loss drives dementia, it is encouraging to note that one can be cognitively intact with far fewer than the normal complement of neurons. This is nicely illustrated by the literature on hydrocephalus which shows many instances of individuals with normal or even above normal intelligence despite having grossly enlarged ventricles and very little brain11-13. These cases show that retardation or dementia need not be the result of substantially fewer neurons, provided that the remaining neurons are functionally normal and able to compensate. However, in AD many of the remaining neurons that should have increased compensatory sprouting and expanded dendritic arbor instead show a dying back of dendrites and a loss of dendritic spines14. Fixing this defect blocking compensatory synaptogenesis would be expected to produce major therapeutic benefits.
While β-amyloid and tau pathology develop over many decades in a prodromal period, it is the onset of synapse loss that drives cognitive decline. For example, in cross-sectional studies selected regional synapse loss in CA1-related stratum lacunosum15 and frontal cortex16 is reported in the earliest (MCI) stages of clinically significant AD. Total synaptic loss has been indexed by synaptophysin loss which continues throughout the clinical progression to severe dementia, but in some of the regions like superior temporal cortex and hippocampus that are affected very early, at the onset of mild cognitive impairment, for example, there is a much more dramatic, region-specific loss of drebrin, an actin-binding protein found in the dendritic spines of excitatory neurons with17.
Consistent with an initiating role in synapse loss, Aβ 1-42 oligomers bind and target excitatory synapses18 where they dysregulate the molecular Rac and Rho signaling pathways that control the dynamics of the actin cytoskeleton in dendritic spines. These pathways are implicated in synaptogenesis and many of the known genetic causes of developmental cognitive impairment, the X-linked mental retardation syndromes19. More specifically, Aβ oligomers engage and disrupt a synaptogenic pathway (NMDA/FYN/Tiam1/RAC/PAK /LIMK1/COFILIN) that is dysregulated in AD20, 21. Thus, the known genetic causes of developmental cognitive deficits and biochemical defects initiating AD converge at the nexus of an Aβ oligomer-mediated attack on plasticity at excitatory synapses involved in learning and memory. This raises the question of how best to safely intervene early (pre-clinically) before this destructive process takes place? Are safe and effective dietary interventions possible? There are several roles for dietary intervention.
HIGH SATURATED FAT AND HIGH CALORIE DIETS INCREASE AD RISK
High saturated fat/ high calorie diets and overeating are epidemic and obvious causes of obesity and metabolic syndrome which lead to insulin-resistant diabetes, a significant AD risk factor that doubles total dementia risk22. Diabetes also doubles specific AD risk23. Diabetes is an independent risk factor independent of the ApoE4 allele which alone doubles or triples AD risk24, but diabetes was reported to combine with ApoE4 to further raise risk to 4.5- fold over those lacking either factor25. Thus, a simple recommendation is to control those dietary risk factors involving overeating high fat/ high calorie diets that are established risk factors for type II diabetes, CVD and dementia.
A second and more complicated possibility is to address the role of neurotrophic insulin effects and insulin signaling in AD26. There is increasing evidence for a defect in insulin-like signaling that may limit glucose utilization, synaptic plasticity, and survival signaling. In fact, clinical trials with insulin sensitizing drugs (PPARg agonists) have already had some clinical trial success in ApoE3 but not ApoE4 carriers26, 27. As discussed below, the omega-3 (n-3) fatty acid DHA can ameliorate insulin-signaling defects in AD animal models.
OMEGA -6 (N-6) FATTY ACID METABOLITES AND AD PATHOGENESIS
Western diets are typically high in n-6 fatty acids, notably linoleic acid, which is an arachidonic acid precursor, and comparatively low in the omega-3 (n-3) fatty acids, alpha linolenic (ALA) and the long chain marine fatty acids, docosahexaenoic acid (DHA) and eicosopentaenoic acid (EPA). N-6 and n-3 fatty acids compete for incorporation into the labile second position of brain phospholipids, so that high n-6, low omega 3 intake ultimately leads to a preponderance of arachidonic acid (AA) in brain phospholipids. Since AA is the substrate for cyclooxygenase and lipoxygenase enzymes this will create a net pro-inflammatory environment that interacts directly with AD pathogenesis because Aβ aggregates directly activate glia28. Further, Aβ oligomers (Aβn) activate MAP kinases to phosphorylate and upregulate cytosolic phospholipase A2 (cPLA2) and arachidonic acid release (AA) resulting in elevated phospho-cPLA2 and AA metabolites from cyclooxygenases (COX) and lipoxygenases (LOX) in AD and in AD animal models29. This helps explain why markedly elevated dietary n-6 polyunsaturated fatty acid (PUFA) intake (safflower oil) exacerbated excitatory synaptic marker loss, notably NMDA receptor subumints, PSD-95 and drebrin loss in APP transgenic AD model mice30, 31. While there is no epidemiological data to support specifically lowering overall n-6 PUFA intake, Mediterranean diets appear to reduce AD and other dementia risk32, 33 and have lower intakes of added sugar, saturated fat, trans-fat, (n-6) fatty acids, and linoleic acid (LA), a lower glycemic index, and a lower ratio of (n-6):(n-3) fatty acids. A Mediterranean diet embodies most of the recipe for lowering dietary risk for CVD, type II diabetes and dementia. We argue that a key ingredient is higher n-3 intake in relation to n-6 fatty acids.
OMEGA-3 FATTY ACIDS/ DHA
As discussed above, omega- 3 fatty acids exert pleiotropic effects on cardiovascular and central nervous system (CNS) that may be protective against age-related cognitive decline caused by either vascular or Alzheimer dementia or a mix of both. Low omega-3 fatty acid intake is one of many overlapping risk factors for both cardiovascular disease (CVD) and AD that include type II diabetes, hypercholesterolemia, hypertension, hyperhomocysteinemia, dietary saturated fats, cholesterol, antioxidants, alcohol consumption, physical activity, the presence of atrial fibrillation and atherosclerotic disease5. Although low omega-3 fatty acid intake is only one of many risk factors, it is one that is easier to remedy and remarkably effective. For example, recent meta-analysis indicates omega-3 fatty acids from fish can provide a 36% reduction in an unambiguous endpoint, death from coronary artery disease34. The cardiovascular protective effects of omega-3 fatty acids are backed by repeated positive clinical trial results that lead to practical recommendations for dietary supplementation35, but unfortunately clinical trials for dementia prevention are more difficult to conduct than for CVD. Evidence for fish oil is compelling because it appears to prevent secondary cardiovascular events in high-risk patients with a first event.
Unlike cardiovascular events, the onset of dementia is erratic and insidious. Nevertheless, already by 2005, a US Department of Health and Human Services funded evidence-based meta-analysis of the literature on omega-3 fatty acids and dementia was able to conclude that there was sufficient evidence to warrant clinical trials for the treatment and prevention of AD36. Since this 2005 review, new studies have reported that often show a 40-50% reduced risk of dementia associated with high omega-3 intake. In reviewing these we noted 9 studies showing increased fish consumption associated with reduced AD risk while 8 out of 10 studies associated higher blood omega-3 levels with reduced cognitive decline or dementia37. Some but not all reports indicated a lack of protection in ApoE4 carriers, which likely represent nearly half of all AD cases and might explain why risk reduction from omega 3 has appeared to plateau around 50%. Plausible ad hoc explanations for an ApoE effect include differential effects of ApoE isoform on DHA trafficking or because ApoE4 carriers have increased oxidative stress including lipid peroxidation products38, 39, consistent with preclinical studies40.
Another nutrigenomic factor that may influence the response to supplements are recently discovered defective alleles of the delta-5 desaturase (FADS1) and delta-6 desaturases (FADS2) responsible for converting short chain dietary ALA into very long chain EPA and DHA41. Future omega 3 intervention studies need to consider likely “nutrigenomic” factors regulating the response to dietary PUFA into their “intent to treat” analyses or they may risk belying real efficacy with predictable non-responder populations.
The blood level analysis reports of protection from dementia include one from the prospective Framingham study (Schaefer et al 2006) that revealed that high (upper quartile) plasma DHA (and no other lipid) assessed ~10 years prior to ascertainment of cognitive status predicted reduced dementia and AD. The protected group had daily DHA intake estimated to average ~180 mg/day, which is more than double the average US daily intake. One can make a strong mathematical case that the average dietary intake of ALA and conversion to DHA is sufficient to meet normal needs in healthy animals and humans as measured by the balance of synthesis and consumption42. However, the apparent protective effects from exogenous very long chain omega-3, usually from marine sources, in epidemiology and animal models argue that there is something missing from the equation in the context of chronic age related diseases or trauma. This raises the question of DHA mechanisms against dementia.
MECHANISMS OF DHA PROTECTION AGAINST AGE-RELATED DEMENTIA
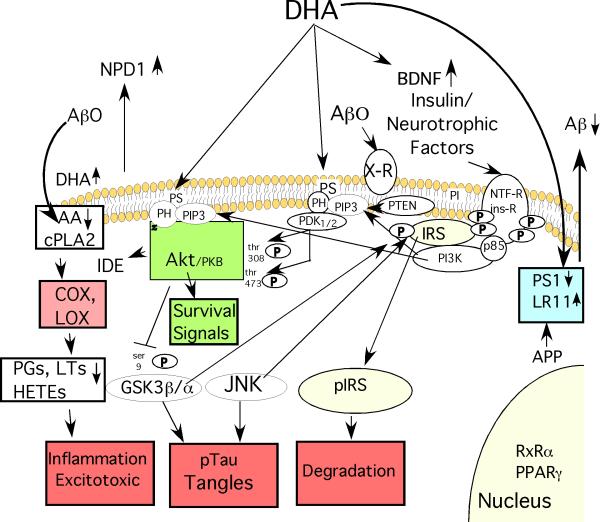
Our recent review referenced 12 neuroprotective or anti-AD effects of DHA reported in preclinical models for DHA37. Many of these are illustrated in Fig. 1. The neuroprotective effects are all from preclinical models but may be relevant because supplementing parental feeding with fish oil improved survival and recovery from severe head injury in patients with brain trauma43.
Fig. 1. Some of DHA's Protective Effects against Alzheimer Disease ( AD).
DHA reduces the production of the β-amyloid peptides (Aβ) from amyloid precursor protein (APP) by increasing expression of the anti-amyloidogenic chaperone LR11, a protein which prevents APP from reaching the proteolytic secretases that cut Aβ from APP. DHA can also reduce expression of presenilin 1 (PS1), a critical component of the gamma secretase that creates Aβ. Reducing Aβ production lowers levels of Aβ oligomers (Aβ0) that act on candidate membrane receptors (X-R) with multiple deleterious signal transduction effects. By incorporating into membrane phospholipids, DHA may also have multiple “fluidizing” effects on membrane structure, lipid raft and protein-protein coupling discussed in the text. DHA incorporation will also competitively reduce membrane levels of arachidonic acid (AA), whose release from membrane phospholipids by phospholipase A2 (PLA2) generates free intracellular AA that is a substrate for cyclooxygenase (COX) and lipoxygenase (LOX) enzymes that produce various prostaglandin (PG), Hete and leukotriene (LT) products known to promote inflammation and excitotoxicity that are elevated in AD pathogenesis. Aβ0 activate inflammation in glia and neuronal cPLA2 resulting in elevated AA and its COX and LOX products in AD and APP transgenic mice. DHA not only reduces these products but DHA itself is transformed by LOX to a potent neuroprotective mediator, neuroprotectin D1 (NPD1) and related mediators which have anti-inflammatory, neuroprotective and other anti-AD activities. DHA can activate PPAR and RxR transcription factors. DHA has also been reported to be rate limiting for phosphatidylserine on the inner membrane leaflet which promotes docking with the PI3-K product PIP-3 and resultant activation of both Akt and upstream PDK, the critical effectors of PI3-K /PDK/ Akt survival signaling pathway stemming from activation of insulin and neurotrophic factor receptors. Signaling through this PI3-K pathway upregulates an Aβ protease insulin degrading enzyme (IDE), has multiple pro-survival effects on apoptotic regulators (“survival signals”) that protect neurons and also inhibits a major tau kinase, GSK3β. Through this or other actions DHA also reduces activity of another important tau kinase, JNK. GSK3β and JNK are known to hyperphosphorylate tau resulting in neurofibrillary tangle formation, but they also target the critical insulin receptor substrates (IRS-1 and 2). This causes IRS to uncouple from both insulin and neurotrophic factor receptors and be rapidly degraded resulting in a state of insulin/neurotrophin resistance. This is likely to contribute to synaptic loss and a dying back of neuritic arbor. Finally, DHA can increase production of brain derived neurotrophic factor (BDNF), a factor, which plays an important positive role in activity-dependent synaptic plasticity, but is lost in AD. In summary, DHA has multiple pleiotropic activities predicted to slow AD pathogenesis at many levels.
DHA's neuroprotective effects in preclinical models include:
Anti-inflammatory. Reducing AA and metabolites via COX and lipoxygenase (PGs, HETES) that are increased by elevated cPLA2 activity as discussed above. Reduced AA was found in brains of DHA-fed AD model mice31, 44, 45.
Insulin/trophic factor potentiation via Akt due to increased phosphatidylserine required membrane docking46.
Increased brain-derived neurotrophic factor synthesis, a major neuroprotective factor47. This and other effects of DHA can be enhanced by exercise48.
Antioxidant. Possible direct membrane effects and increasing antioxidant enzymes (catalase, GSH peroxidase)49.
Anti-apoptotic/anti-inflammatory and other neuroprotective effects via metabolites. DHA is the precursor to neuroprotectin D1 (NPD1) which has multiple anti-apoptotic and other anti-AD activities50.
Promoting Neurogenesis and neurite outgrowth. Dietary DHA promotes neurogenesis, neurite outgrowth and improved cognition51 and increasing DHA selectively with the fat1 transgene confirms that these effects can be seen in adults and are due to increased DHA52. A mechanism for how DHA promotes stem cell differentiation to neurons has been recently proposed53.
DHA increases expression of a glucose transporter in brain endothelial cells,54.
Impaired coupling blood flow to glucose utilization in aged monkeys was ameliorated by DHA55.
As an integral membrane component esterified to phospholipids, DHA improves synaptic membrane fluidity56 and lipid raft function57.
DHA increases G-protein coupling, an effect best demonstrated in the retina58.
DHA activates peroxisome proliferator-activated receptors (PPARs) and rettinoid X receptor alpha (RXRα) receptors59, 60, which may explain some metabolic and anti-inflammatory effects. However, whether RxRα activation occurs with physiological DHA levels to promote neurogenesis is unclear61.
DHA is remarkably effective in protecting against oligomer-induced synaptic marker loss in primary neurons in vitro62. One mechanism is to prevent Aβ induced phosphorylation of insulin receptor substrate (IRS) by tau kinases, notably JNK. Elevated phosphorylation of IRS-1 occurs in AD and AD animal models, which should result in uncoupling of insulin and neurotrophic factor signaling to the neuroprotective PI3-K/ Akt pathway. This is one mechanism for insulin/ trophic factor resistance and failed synaptic plasticity and compensatory sprouting responses to trophic factors. We have also found that DHA blocks Aβ oligomer-induced defects in a synapse regulating pathway (NMDA/FYN/Tiam1/RAC/PAK /LIMK1/COFILIN) that is dysregulated in AD62.
DHA reduces Aβ production/ amyloid accumulation in vitro and in animal models in 8 out of 9 studies In contrast to other studies, the one study that did not see a reduction in Aβ, dietary DHA failed to increase DHA or reduce AA levels37. DHA appears to reduce Aβ production by several proposed mechanisms, including altering APP and secretase mobility, reducing expression of presenilin 1 and gamma secretase activity44 and the induction of increased neuronal expression of the anti-amyloidogenic chaperone, SorLa/LR1163. Omega-3 fatty acids may also increase expression of an Aβ-clearing transport protein, transthyretin (TTR)64 or increase IDE expression and Aβ clearance65.
DHA limits tau kinases that promote tau pathology/ neurofibrillary tangles notably JNK and GSK344, 62.
DHA is remarkably pleiotropic with many mechanisms for reducing amyloid (Aβ) production, tau kinases and tau pathology, and neurodegenerative pathways and neuron and synapse loss. However, DHA is very susceptible to lipid peroxidation to F4 isoprostanes and these are elevated in AD66, 67, indicating insufficient protection and providing a very strong rationale for combining DHA with protective antioxidants like alpha lipoic acid or vitamin E. Our group has also advocated that DHA be combined with the polyphenolic antioxidant curcumin because it has additional anti-aging, anti-amyloid, and AD protective activities68. Efficacy for this combination appears to extend beyond β-amyloid based models. Preliminary data from ongoing pre-clinical trials with the combination of curcumin and fish oil or curcumin and DHA in multiple AD models including triple transgenics expressing mutant human tau62 and pure wild type human tau transgenics (Frautschy et al, unpublished), suggest efficacy with this cocktail approach. The major obstacle for using curcumin (and related polyphenolics) as a supplement in humans is extremely poor bioavailability, chiefly from poor solubility, rapid glucuronidation and high first pass metabolism. But these obstacles to effective dosing in humans can be overcome with alternative lipidated formulations69. One such formulation is already in small clinical trials for Alzheimer's and other diseases of aging as well as trials in mice for longevity.
COMPLETED CLINICAL TRIALS
Based on the encouraging epidemiology and preclinical data, researchers have gone ahead with small clinical trials to test the utility of omega 3 preparations in subjects with mild cognitive impairment or established AD at sites in Japan, Sweden, Netherlands, the US and Taiwan as listed here:
Japan: 240 mg/ day DHA + ARA (3 months) improved memory and attention in amnestic MCI (n=12) (but not AD (n=8))70.
Sweden: A trial with 4 gm fish oil (1.7 gm DHA for 6 mos, n=174) found no significant effect for patients with MMSE<27. In this trial, 68% of the patients were ApoE4 positive, a genotype which some epidemiology suggests respond poorly. However, in a subgroup of patients with MMSE >27 there was improved delayed recall and attention and cognitive function appeared stablized with the fish oil (n=32)71.
Netherlands: The Memo trial tested 302 subjects (65+ yrs) who were said to be normal with “MMSE >21” (a low cutoff for “normal”) using three groups, 1800 vs 400 mg EPA+DHA vs placebo for 26 wks. They saw increases in attention. Surprisingly this was especially true in the E4 positive group and in the men, but they saw no change in overall cognition72.
Netherlands. P. Scheltens (Amsterdam) reported the 12 week Souvenaid trial with a proprietary “nutrient mix” (containing DHA, uridine monophosphate, B vitamins and antioxidants) in 212 mild AD (MMSE 20-26) at the International Conference on AD (Chicago, July 2008). Results showed improved delayed verbal recall, particularly in the mildest cases. Based on apparent success, a larger US based follow-up Souvenaid trial is planned.
Taiwan. Chiu and colleagues have reported on a 6 month trial with MCI and AD patients where 1.8g/day total n-3 appeared to result in significant improvement in ADAS-cog compared to olive oil treated controls, but only in the MCI with no effect in theAD (NCT00628017).
USA (Oregon). OHSU pilot, MMSE 15-26 (n=39, Fish oil (675 mg DHA, 975 EPA +/-600mg alpha lipoate vs placebo)- FO+ lipoate stabilized MMSE, ADLs (p=0.02)73.
USA (Martek, Columbia, MD, K. Yurko-Mauros and Martek colleagues have completed MIDAS, a shorter trial in 465 subjects with pre-existing memory complaints randomized to 900 mg/day algal DHA ) Martek for 6 months (n=465). The results reported in July 2009 at the ICAD meeting in Vienna show a significant improvement in the primary outcome measures Paired Associate Learning (PAL), a visuospatial episodic memory test and lowered heart rate74.
The results from these 6 clinical trials are not entirely consistent but generally suggest that fish oil or DHA alone may have little or no benefit by itself in cases of established AD with MMSE <26, but may be useful for early intervention in subjects with mild memory complaints or minimal cognitive impairment. In addition, as reviewed elsewhere in this symposium by Dr. R. Wurtman, there is some evidence that perhaps even in mild cases of established AD a combination with other agents such as UMP75, B vitamins and/ or antioxidants may also be especially protective. The observation that cognitive function appeared significantly stabilized or even improved in several trials encourages additional larger trials, particularly with those at the earliest stages of decline or even for primary prevention prior to any symptoms. We will learn more about any possible benefits of DHA when the ongoing ADCS DHA trial reports but the great advantage of nutritional or exercise intervention programs is that their potential for primary prevention.
Several suggested mechanisms argue for a prevention approach. For example, because NSAIDs have generally not worked in trials for established AD but may have worked at preclinical stages in the ADAPT primary prevention trial, any omega-3 NSAID-like activity in reducing arachidonic acid metabolites would likely be most useful for early intervention. Similarly, the most sensitive synaptic marker index of DHA depletion in our APP transgenic mouse studies was drebrin, a dendritic spine marker in excitatory synapses30. Severe drebrin loss in hippocampus and superior temporal cortex has been reported in AD, and superior cortical drebrin loss occurs with the transition to mild cognitive impairment17. Like Aβ42 accumulation that begins to plateau by the emergence of clinical decline, drebrin loss is another DHA-sensitive endpoint best suited to an early intervention.
ONGOING TRIALS
To look at disease progression in established AD (MMSE<26), J. Quinn (Portland, Oregon, USA) is coordinating a multi- site Alzheimer Disease Cooperative Studies Consortium Trial with 400 mild to moderate AD subjects randomized to 900 mg algal DHA (Martek) for 18 months. A. Dangour (London, UK) and R. Uauy (Santiago, Chile) are running the Opal trial (96) examining cognitive decline in subjects age 70-79 with MMSE>24 and randomizing to placebo or 500 mg DHA + 200 mg EPA (n=800) for 3 years. B. Vellas (Toulouse, France) is leading a large (n=1200) trial for primary prevention of cognitive decline in frail elderly with MMSE>24 using, omega 3 (VO137), 800 mg DHA /day, again over 3 years. When these trials report, we will learn more about the effects of DHA or fish oil alone, but the best results are likely to come from nutrition-based cocktails.
CONCLUSIONS
The evidence from preclinical models argues that brain aging and dementia can be influenced by nutritional factors. The argument remains most compelling for avoiding high saturated and trans-fat/ high calorie diets previously related to obesity, type II diabetes and increased cardiovascular disease risk- all also risk factors for age-related dementia. On the positive side, increasing intake of omega-3 fatty acids including marine long chain omega-3 and DHA in particular, appears to offer some protection against unhealthy brain aging leading to dementia. Based on strong positive epidemiology and a wealth of pleiotropic protective activities in preclinical models from fish oil and its principal neuroprotective component, DHA, the marine omega-3 as fish oil and algal DHA have already gone into pilot clinical trials. Results from 6 small clinical trials suggest possible protective effects in the earliest stages of cognitive impairment, but not with established AD. There is already some suggestion that nutritional cocktails including antioxidants, B vitamins and other synergistic components may prove to exert stronger protective effects. These initial results encourage hope that omega-3 may prove useful for prevention but additional results from larger trials, particularly those with early intervention will be required to prove efficacy. Some of these trials are already underway.
Acknowledgments
This work was supported by NCCAM AT3008 NIA R01AG13471
Abbreviations
- DHA
docosahexaenoic acid
- n-3
omega-3 polyunsaturated fatty acid
- n-6
omega-6 polyunsaturated fatty acid
- AD
Alzheimer's disease
Footnotes
Disclosure: The authors report no conflicts of interest.
REFERENCES
- 1.Cole GM, Frautschy SA. The role of insulin and neurotrophic factor signaling in brain aging and Alzheimer's Disease. Exp Gerontol. 2007;42:10–21. doi: 10.1016/j.exger.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 2.Lee CK, Weindruch R, Prolla TA. Gene-expression profile of the ageing brain in mice. Nat Genet. 2000;25:294–297. doi: 10.1038/77046. [DOI] [PubMed] [Google Scholar]
- 3.Bartzokis G, Tishler TA, Lu PH, Villablanca P, et al. Brain ferritin iron may influence age- and gender-related risks of neurodegeneration. Neurobiol Aging. 2007;28:414–423. doi: 10.1016/j.neurobiolaging.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 4.Cummings JL, Cole G. Alzheimer disease. JAMA. 2002;287:2335–2338. doi: 10.1001/jama.287.18.2335. [DOI] [PubMed] [Google Scholar]
- 5.Rosendorff C, Beeri MS, Silverman JM. Cardiovascular risk factors for Alzheimer's disease. Am J Geriatr Cardiol. 2007;16:143–149. doi: 10.1111/j.1076-7460.2007.06696.x. [DOI] [PubMed] [Google Scholar]
- 6.Viswanathan A, Rocca WA, Tzourio C. Vascular risk factors and dementia: how to move forward? Neurology. 2009;72:368–374. doi: 10.1212/01.wnl.0000341271.90478.8e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee JH, O'Keefe JH, Lavie CJ, Marchioli R, et al. Omega-3 fatty acids for cardioprotection. Mayo Clin Proc. 2008;83:324–332. doi: 10.4065/83.3.324. [DOI] [PubMed] [Google Scholar]
- 8.Mahley RW, Weisgraber KH, Huang Y. Apolipoprotein E4: a causative factor and therapeutic target in neuropathology, including Alzheimer's disease. Proc Natl Acad Sci U S A. 2006;103:5644–5651. doi: 10.1073/pnas.0600549103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Terry RD, Masliah E, Salmon DP, Butters N, et al. Physical basis of cognitive alterations in Alzheimer's disease: synapse loss is the major correlate of cognitive impairment. Annals Neurol. 1991;30:572–580. doi: 10.1002/ana.410300410. [DOI] [PubMed] [Google Scholar]
- 10.Terry RD. Cell death or synaptic loss in Alzheimer disease. J Neuropathol Exp Neurol. 2000;59:1118–1119. doi: 10.1093/jnen/59.12.1118. [DOI] [PubMed] [Google Scholar]
- 11.Lewin R. Is your brain really necessary? Science. 1980;210:1232–1234. doi: 10.1126/science.7434023. [DOI] [PubMed] [Google Scholar]
- 12.Jackson PH, Lorber J. Brain and ventricular volume in hydrocephalus. Z Kinderchir. 1984;39(Suppl 2):91–93. doi: 10.1055/s-2008-1044292. [DOI] [PubMed] [Google Scholar]
- 13.Berker E, Goldstein G, Lorber J, Priestley B, et al. Reciprocal neurological developments of twins discordant for hydrocephalus. Dev Med Child Neurol. 1992;34:623–632. doi: 10.1111/j.1469-8749.1992.tb11493.x. [DOI] [PubMed] [Google Scholar]
- 14.Coleman PD, Flood DG. Neuron numbers and dendritic extent in normal aging and Alzheimer's disease. Neurobiol Aging. 1987;8:521–545. doi: 10.1016/0197-4580(87)90127-8. [DOI] [PubMed] [Google Scholar]
- 15.Scheff SW, Price DA, Schmitt FA, DeKosky ST, et al. Synaptic alterations in CA1 in mild Alzheimer disease and mild cognitive impairment. Neurology. 2007;68:1501–1508. doi: 10.1212/01.wnl.0000260698.46517.8f. [DOI] [PubMed] [Google Scholar]
- 16.Masliah E, Mallory M, Alford M, DeTeresa R, et al. Altered expression of synaptic proteins occurs early during progression of Alzheimer's disease. Neurology. 2001;56:127–129. doi: 10.1212/wnl.56.1.127. [DOI] [PubMed] [Google Scholar]
- 17.Counts SE, Nadeem M, Lad SP, Wuu J, et al. Differential expression of synaptic proteins in the frontal and temporal cortex of elderly subjects with mild cognitive impairment. J Neuropathol Exp Neurol. 2006;65:592–601. doi: 10.1097/00005072-200606000-00007. [DOI] [PubMed] [Google Scholar]
- 18.Lacor PN, Buniel MC, Chang L, Fernandez SJ, et al. Synaptic targeting by Alzheimer's-related amyloid beta oligomers. J Neurosci. 2004;24:10191–10200. doi: 10.1523/JNEUROSCI.3432-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ramakers GJ. Rho proteins, mental retardation and the cellular basis of cognition. Trends Neurosci. 2002;25:191–199. doi: 10.1016/s0166-2236(00)02118-4. [DOI] [PubMed] [Google Scholar]
- 20.Zhao L, Ma QL, Calon F, Harris-White ME, et al. Role of p21-activated kinase pathway defects in the cognitive deficits of Alzheimer disease. Nat Neurosci. 2006;9:234–242. doi: 10.1038/nn1630. [DOI] [PubMed] [Google Scholar]
- 21.Ma QL, Yang F, Calon F, Ubeda OJ, et al. p21-activated kinase-aberrant activation and translocation in Alzheimer disease pathogenesis. J Biol Chem. 2008;283:14132–14143. doi: 10.1074/jbc.M708034200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Peila R, Rodriguez BL, Launer LJ. Type 2 diabetes, APOE gene, and the risk for dementia and related pathologies: The Honolulu-Asia Aging Study. Diabetes. 2002;51:1256–1262. doi: 10.2337/diabetes.51.4.1256. [DOI] [PubMed] [Google Scholar]
- 23.Ott A, Stolk RP, van Harskamp F, Pols HA, et al. Diabetes mellitus and the risk of dementia: The Rotterdam Study. Neurology. 1999;53:1937–1942. doi: 10.1212/wnl.53.9.1937. [DOI] [PubMed] [Google Scholar]
- 24.Profenno LA, Faraone SV. Diabetes and overweight associate with non-APOE4 genotype in an Alzheimer's disease population. Am J Med Genet B Neuropsychiatr Genet. 2008;147B:822–829. doi: 10.1002/ajmg.b.30694. [DOI] [PubMed] [Google Scholar]
- 25.Irie F, Fitzpatrick AL, Lopez OL, Kuller LH, et al. Enhanced risk for Alzheimer disease in persons with type 2 diabetes and APOE epsilon4: the Cardiovascular Health Study Cognition Study. Arch Neurol. 2008;65:89–93. doi: 10.1001/archneurol.2007.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Craft S. The role of metabolic disorders in Alzheimer disease and vascular dementia: two roads converged. Arch Neurol. 2009;66:300–305. doi: 10.1001/archneurol.2009.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Risner ME, Saunders AM, Altman JF, Ormandy GC, et al. Efficacy of rosiglitazone in a genetically defined population with mild-to-moderate Alzheimer's disease. Pharmacogenomics J. 2006 doi: 10.1038/sj.tpj.6500369. [DOI] [PubMed] [Google Scholar]
- 28.Akiyama H, Barger S, Barnum S, Bradt B, et al. Inflammation and Alzheimer's disease. Neurobiol Aging. 2000;21:383–421. doi: 10.1016/s0197-4580(00)00124-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sanchez-Mejia RO, Newman JW, Toh S, Yu GQ, et al. Phospholipase A2 reduction ameliorates cognitive deficits in a mouse model of Alzheimer's disease. Nat Neurosci. 2008;11:1311–1318. doi: 10.1038/nn.2213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Calon F, Lim GP, Yang F, Morihara T, et al. Docosahexaenoic acid protects from dendritic pathology in an Alzheimer's disease mouse model. Neuron. 2004;43:633–645. doi: 10.1016/j.neuron.2004.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Calon F, Lim GP, Morihara T, Yang F, et al. Dietary n-3 polyunsaturated fatty acid depletion activates caspases and decreases NMDA receptors in the brain of a transgenic mouse model of Alzheimer's disease. Eur J Neurosci. 2005;22:617–626. doi: 10.1111/j.1460-9568.2005.04253.x. [DOI] [PubMed] [Google Scholar]
- 32.Scarmeas N, Stern Y, Tang MX, Mayeux R, et al. Mediterranean diet and risk for Alzheimer's disease. Ann Neurol. 2006;59:912–921. doi: 10.1002/ana.20854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Scarmeas N, Stern Y, Mayeux R, Manly JJ, et al. Mediterranean diet and mild cognitive impairment. Arch Neurol. 2009;66:216–225. doi: 10.1001/archneurol.2008.536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mozaffarian D, Rimm EB. Fish intake, contaminants, and human health: evaluating the risks and the benefits. JAMA. 2006;296:1885–1899. doi: 10.1001/jama.296.15.1885. [DOI] [PubMed] [Google Scholar]
- 35.Lee JH, O'Keefe JH, Lavie CJ, Marchioli R, et al. Omega3 fatty acids for cardioprotection. Mayo Clin Proc. 2008;83:324–332. doi: 10.4065/83.3.324. [DOI] [PubMed] [Google Scholar]
- 36.MacLean C, Issa A, Newberry S, Mojica W, et al. US Department of Health and Human Services AfHRaQ, ed. AHRQ Publication: Evidence Report/Technology Assessment #114. 05-E011-2. Southern California/RAND evidence-based Practice Center; Los Angeles, CA: 2005. Effects of omega-3 fatty acids on cognitive function with aging, dementia, and neurological diseases. pp. 1–88. [PMC free article] [PubMed] [Google Scholar]
- 37.Cole GM, Ma QL, Frautschy SA. Omega-3 fatty acids and dementia. Prostaglandins Leukot Essent Fatty Acids. 2009 doi: 10.1016/j.plefa.2009.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Montine KS, Kim PJ, Olson SJ, Markesbery WR, et al. 4-hydroxy-2-nonenal pyrrole adducts in human neurodegenerative disease. J Neuropathol Exp Neurol. 1997;56:866–871. doi: 10.1097/00005072-199708000-00004. [DOI] [PubMed] [Google Scholar]
- 39.Ramassamy C, Averill D, Beffert U, Theroux L, et al. Oxidative insults are associated with apolipoprotein E genotype in Alzheimer's disease brain. Neurobiol Dis. 2000;7:23–37. doi: 10.1006/nbdi.1999.0273. [DOI] [PubMed] [Google Scholar]
- 40.Jofre-Monseny L, Minihane AM, Rimbach G. Impact of apoE genotype on oxidative stress, inflammation and disease risk. Mol Nutr Food Res. 2008;52:131–145. doi: 10.1002/mnfr.200700322. [DOI] [PubMed] [Google Scholar]
- 41.Rzehak P, Heinrich J, Klopp N, Schaeffer L, et al. Evidence for an association between genetic variants of the fatty acid desaturase 1 fatty acid desaturase 2 ( FADS1 FADS2) gene cluster and the fatty acid composition of erythrocyte membranes. Br J Nutr. 2009;101:20–26. doi: 10.1017/S0007114508992564. [DOI] [PubMed] [Google Scholar]
- 42.Rapoport SI, Igarashi M. Can the rat liver maintain normal brain DHA metabolism in the absence of dietary DHA? Prostaglandins Leukot Essent Fatty Acids. 2009 doi: 10.1016/j.plefa.2009.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Heller AR, Rossler S, Litz RJ, Stehr SN, et al. Omega-3 fatty acids improve the diagnosis-related clinical outcome*. Crit Care Med. 2006 doi: 10.1097/01.CCM.0000206309.83570.45. Publish Ahead of Print. [DOI] [PubMed] [Google Scholar]
- 44.Green KN, Martinez-Coria H, Khashwji H, Hall EB, et al. Dietary docosahexaenoic acid and docosapentaenoic acid ameliorate amyloid-beta and tau pathology via a mechanism involving presenilin 1 levels. J Neurosci. 2007;27:4385–4395. doi: 10.1523/JNEUROSCI.0055-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Oksman M, Iivonen H, Hogyes E, Amtul Z, et al. Impact of different saturated fatty acid, polyunsaturated fatty acid and cholesterol containing diets on beta-amyloid accumulation in APP/PS1 transgenic mice. Neurobiol Dis. 2006;23:563–572. doi: 10.1016/j.nbd.2006.04.013. [DOI] [PubMed] [Google Scholar]
- 46.Akbar M, Calderon F, Wen Z, Kim HY. Docosahexaenoic acid: a positive modulator of Akt signaling in neuronal survival. Proc Natl Acad Sci U S A. 2005;102:10858–10863. doi: 10.1073/pnas.0502903102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rao JS, Ertley RN, Lee HJ, DeMar JC, Jr., et al. n-3 polyunsaturated fatty acid deprivation in rats decreases frontal cortex BDNF via a p38 MAPK-dependent mechanism. Mol Psychiatry. 2007;12:36–46. doi: 10.1038/sj.mp.4001888. [DOI] [PubMed] [Google Scholar]
- 48.Wu A, Ying Z, Gomez-Pinilla F. Docosahexaenoic acid dietary supplementation enhances the effects of exercise on synaptic plasticity and cognition. Neuroscience. 2008;155:751–759. doi: 10.1016/j.neuroscience.2008.05.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hossain MS, Hashimoto M, Gamoh S, Masumura S. Antioxidative effects of docosahexaenoic acid in the cerebrum versus cerebellum and brainstem of aged hypercholesterolemic rats. J Neurochem. 1999;72:1133–1138. doi: 10.1046/j.1471-4159.1999.0721133.x. [DOI] [PubMed] [Google Scholar]
- 50.Lukiw WJ, Cui JG, Marcheselli VL, Bodker M, et al. A role for docosahexaenoic acid-derived neuroprotectin D1 in neural cell survival and Alzheimer disease. J Clin Invest. 2005;115:2774–2783. doi: 10.1172/JCI25420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Innis SM. Dietary (n-3) fatty acids and brain development. J Nutr. 2007;137:855–859. doi: 10.1093/jn/137.4.855. [DOI] [PubMed] [Google Scholar]
- 52.He C, Qu X, Cui L, Wang J, Kang JX. Improved spatial learning performance of fat-1 mice is associated with enhanced neurogenesis and neuritogenesis by docosahexaenoic acid. Proc Natl Acad Sci U S A. 2009 doi: 10.1073/pnas.0904835106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Katakura M, Hashimoto M, Shahdat HM, Gamoh S, et al. Docosahexaenoic acid promotes neuronal differentiation by regulating basic helix-loop-helix transcription factors and cell cycle in neural stem cells. Neuroscience. 2009;160:651–660. doi: 10.1016/j.neuroscience.2009.02.057. [DOI] [PubMed] [Google Scholar]
- 54.Pifferi F, Jouin M, Alessandri JM, Haedke U, et al. n-3 Fatty acids modulate brain glucose transport in endothelial cells of the blood-brain barrier. Prostaglandins Leukot Essent Fatty Acids. 2007;77:279–286. doi: 10.1016/j.plefa.2007.10.011. [DOI] [PubMed] [Google Scholar]
- 55.Tsukada H, Kakiuchi T, Fukumoto D, Nishiyama S, et al. Docosahexaenoic acid (DHA) improves the age-related impairment of the coupling mechanism between neuronal activation and functional cerebral blood flow response: a PET study in conscious monkeys. Brain Res. 2000;862:180–186. doi: 10.1016/s0006-8993(00)02115-6. [DOI] [PubMed] [Google Scholar]
- 56.Hashimoto M, Hossain S, Shimada T, Shido O. Docosahexaenoic acid-induced protective effect against impaired learning in amyloid beta-infused rats is associated with increased synaptosomal membrane fluidity. Clin Exp Pharmacol Physiol. 2006;33:934–939. doi: 10.1111/j.1440-1681.2006.04467.x. [DOI] [PubMed] [Google Scholar]
- 57.Wassall SR, Stillwell W. Docosahexaenoic acid domains: the ultimate non-raft membrane domain. Chem Phys Lipids. 2008;153:57–63. doi: 10.1016/j.chemphyslip.2008.02.010. [DOI] [PubMed] [Google Scholar]
- 58.Litman BJ, Niu SL, Polozova A, Mitchell DC. The role of docosahexaenoic acid containing phospholipids in modulating G protein-coupled signaling pathways: visual transduction. J Mol Neurosci. 2001;16:237–242. doi: 10.1385/JMN:16:2-3:237. discussion 279-284. [DOI] [PubMed] [Google Scholar]
- 59.de Urquiza AM, Liu S, Sjoberg M, Zetterstrom RH, et al. Docosahexaenoic acid, a ligand for the retinoid X receptor in mouse brain. Science. 2000;290:2140–2144. doi: 10.1126/science.290.5499.2140. [DOI] [PubMed] [Google Scholar]
- 60.Gani OA, Sylte I. Molecular recognition of Docosahexaenoic acid by peroxisome proliferator-activated receptors and retinoid-X receptor alpha. J Mol Graph Model. 2008 doi: 10.1016/j.jmgm.2008.04.008. [DOI] [PubMed] [Google Scholar]
- 61.Calderon F, Kim HY. Role of RXR in neurite outgrowth induced by docosahexaenoic acid. Prostaglandins Leukot Essent Fatty Acids. 2007;77:227–232. doi: 10.1016/j.plefa.2007.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ma QL, Yang F, Rosario ER, Ubeda OJ, Beech W, Gant DJ, Chen PP, Hudspeth B, Chen C, Zhao Y, Vinters HV, Frautschy SA, Cole GM. Abeta oligomers induce phosphorylation of tau and inactivation of insulin receptor substrate via JNK signaling: suppression by omega-3 fatty acids and curcumin. J Neuroscience. 2009 doi: 10.1523/JNEUROSCI.1071-09.2009. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ma QL, Teter B, Ubeda OJ, Morihara T, et al. Omega-3 fatty acid docosahexaenoic acid increases SorLA/LR11, a sorting protein with reduced expression in sporadic Alzheimer's disease (AD): relevance to AD prevention. J Neurosci. 2007;27:14299–14307. doi: 10.1523/JNEUROSCI.3593-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Puskas LG, Kitajka K, Nyakas C, Barcelo-Coblijn G, et al. Short-term administration of omega 3 fatty acids from fish oil results in increased transthyretin transcription in old rat hippocampus. Proc Natl Acad Sci U S A. 2003 doi: 10.1073/pnas.0337683100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhao L, Teter B, Morihara T, Lim GP, et al. Insulin-degrading enzyme as a downstream target of insulin receptor signaling cascade: implications for Alzheimer's disease intervention. J Neurosci. 2004;24:11120–11126. doi: 10.1523/JNEUROSCI.2860-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Nourooz-Zadeh J, Liu EHC, Yhlen B, Anggard EE, et al. F4-Isoprostanes as specific marker of docosahexaenoic acid peroxidation in Alzheimer's disease. J Neurochem. 1999;72:734–740. doi: 10.1046/j.1471-4159.1999.0720734.x. [DOI] [PubMed] [Google Scholar]
- 67.Montine KS, Quinn JF, Zhang J, Fessel JP, et al. Isoprostanes and related products of lipid peroxidation in neurodegenerative diseases. Chem Phys Lipids. 2004;128:117–124. doi: 10.1016/j.chemphyslip.2003.10.010. [DOI] [PubMed] [Google Scholar]
- 68.Cole GM, Frautschy SA. Docosahexaenoic acid protects from amyloid and dendritic pathology in an Alzheimer's disease mouse model. Nutr Health. 2006;18:249–259. doi: 10.1177/026010600601800307. [DOI] [PubMed] [Google Scholar]
- 69.Begum AN, Jones MR, Lim GP, Morihara T, et al. Curcumin Structure-Function, Bioavailability, and Efficacy in Models of Neuroinflammation and Alzheimer's Disease. JPET. 2008;326:196–208. doi: 10.1124/jpet.108.137455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kotani S, Sakaguchi E, Warashina S, Matsukawa N, et al. Dietary supplementation of arachidonic and docosahexaenoic acids improves cognitive dysfunction. Neurosci Res. 2006;56:159–164. doi: 10.1016/j.neures.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 71.Freund-Levi Y, Eriksdotter-Jonhagen M, Cederholm T, Basun H, et al. Omega-3 fatty acid treatment in 174 patients with mild to moderate Alzheimer disease: OmegAD study: a randomized double-blind trial. Arch Neurol. 2006;63:1402–1408. doi: 10.1001/archneur.63.10.1402. [DOI] [PubMed] [Google Scholar]
- 72.Van de Rest O, Geleijnse J, Kok F, van Staveren W, et al. Effect of fish oil on cognitive performance in older subjects: a randomized controlled trial. Neurology. 2008;71:430–438. doi: 10.1212/01.wnl.0000324268.45138.86. [DOI] [PubMed] [Google Scholar]
- 73.Shinto L, Quinn J, Montine T, Baldauf-Wagner S, et al. Omega-3 fatty acids and lipoic acid in Alzheimer's disease. Neurology. 2008;70:A393. doi: 10.3233/JAD-130722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.McCarthy D, Bailey-Hall E, Nelson E, Blackwell A. Results of the MIDAS Trial: Effects of Docosahexaenoic Acid on Physiological and Safety Parameters in Age-Related Cognitive Decline. Neurobiol Aging Supplement (ICAD) 2009 in press. [Google Scholar]
- 75.Holguin S, Martinez J, Chow C, Wurtman R. Dietary uridine enhances the improvement in learning and memory produced by administering DHA to gerbils. FASEB J. 2008;22:3938–3946. doi: 10.1096/fj.08-112425. [DOI] [PMC free article] [PubMed] [Google Scholar]