Abstract
More than a dozen epidemiological studies have reported that reduced levels or intake of omega-3 fatty acids or fish consumption is associated with increased risk for age-related cognitive decline or dementia such as Alzheimer's disease (AD). Increased dietary consumption or blood levels of docosahexaenoic acid (DHA) appear protective for AD and other dementia in multiple epidemiological studies; however, three studies suggest that the ApoE4 genotype limits protection. DHA is broadly neuroprotective via multiple mechanisms that include neuroprotective DHA metabolites, reduced arachidonic acid metabolites, and increased trophic factors or downstream trophic signal transduction. DHA is also protective against several risk factors for dementia including head trauma, diabetes, and cardiovascular disease. DHA is specifically protective against AD via additional mechanisms: It limits the production and accumulation of the amyloid β peptide toxin that is widely believed to drive the disease; and it also suppresses several signal transduction pathways induced by Aβ, including two major kinases that phosphorylate the microtubule associated protein tau and promote neurofibrillary tangle pathology. Based on the epidemiological and basic research data, expert panels have recommended the need for clinical trials with omega-3 fatty acids, notably DHA, for the prevention or treatment of age-related cognitive decline—with a focus on the most prevalent cause, AD. Clinical trials are underway to prevent and treat AD. Results to-date suggest that DHA may be more effective if it is begun early or used in conjunction with antioxidants.
Keywords: Alzheimer's disease, dementia, omega-3 fatty acid (n-3), docsahexaenoic acid (DHA), amyloid
Introduction
Alzheimer's Disease (AD) and other late life dementias double every five years after age 65. Some 80 million baby boomers are rapidly approaching this age in the U.S., and similar demographic shifts toward aging populations are occurring throughout the world. Until methods of preventing AD are found, it is not only the tens of millions of disease victims and their families that will suffer, but also our nation, our Medicare system, and our economy as whole. While genetics plays the major role in risk for familial AD with early onset, the majority of AD is late onset and appears to involve the interplay of multiple genetic and environmental risk factors (1). These interact to modulate the pathogenesis and risk of onset AD, but are not sufficiently powerful to act alone to determine outcomes. Among the environmental risk factors, increased consumption of fish/omega-3 fatty acids (this review), antioxidants (2), and non-steroidal anti-inflammatory drugs (3) are associated with reduced risk for AD. Based on this, our group and others have tested supplementation with the omega-3 fatty acid DHA, antioxidants, and NSAIDs in animal models for the treatment of AD. NSAIDs reduce amyloid accumulation in multiple models, but have toxicity issues, including GI bleeds and increased risk of cardiovascular events, that limit use for prevention (4). In contrast, omega-3 fatty acids are not only relatively inexpensive, but have an excellent safety profile and are proven to reduce cardiovascular disease mortality by 37% (5, 6). If omega-3 fatty acids can be proven to slow pathogenesis of AD and reduce AD risk, they would be an ideal intervention approach. Here we review the evidence for their efficacy.
Omega-3 Fatty Acids/DHA Reduce Risk for Alzheimer's Disease and Other Dementia
Omega- 3 fatty acids exert pleiotropic effects on the cardiovascular and central nervous systems (CNS) that may be protective against age-related cognitive decline, where the causes are either vascular or Alzheimer dementia or a mix of both. Low omega-3 fatty acid intake is one of many overlapping risk factors for both cardiovascular disease (CVD) and AD that correlate with diabetes, hypercholesterolemia, hypertension, hyperhomocysteinemia, dietary saturated fats, cholesterol, antioxidants, alcohol consumption, smoking, physical activity, the presence of atrial fibrillation, and atherosclerotic disease (7). Although low omega-3 fatty acid intake is only one of many risk factors, it matters. For example, recent meta-analysis indicates that omega-3 fatty acids from fish can provide a 36% reduction in an unambiguous, endpoint-death from coronary artery disease (8). The cardiovascular protective effects of omega-3 fatty acids are backed by repeated positive clinical trial results, which lead to practical recommendations for dietary supplementation (9), but clinical trials for dementia prevention have not been concluded. Nevertheless, a 2005 literature evidence-based meta-analysis on omega-3 fatty acids and dementia, requested by the U.S. Department of Health and Human Services, concluded that there was sufficient evidence to merit clinical trials for the treatment and prevention of AD (10). Since then, the results of a number of new studies have been reported. Nine epidemiological studies now support that increased dietary intake of fish is associated with reduced risk for cognitive decline or dementia (Table 1). Several of the studies show a 40–50% reduced risk of dementia. Curiously, two results found no effect in subjects with a major AD genetic risk factor, the ApoE4 allele of apolipoprotein E. Because ApoE4 is found in ∼40–50% of AD patients, a lack of response in ApoE4 cases would account for many of the apparent “non-responders” in epidemiological studies approaching 50% risk reduction. However, not all reports show the same ApoE genotype effect on omega-3 fatty acid response.
Table 1.
Dietary Fish Reduces AD Risk (9 positive studies).
| Study | Population | Methods | Findings | Other |
|---|---|---|---|---|
| Kalmijn et al. 1997 (70) | Zutphen N=476 men, 64-89 yrs | Diet history, MMSE | Fish intake reduced in 153 impaired men, but n-3 not associated with 3 yr cognitive decline | Fish intake inversely correlated with cognitive decline (adjusted OR = 0.45, p = 0.09); Linoleic Acid raised risk. |
| Kalmijn et al. 1997 (71) | Rotterdam, N=5,386, 55+ yrs | Diet history, dementia | High fish, RR (D)=0.4; RR (AD)=0.3 | Saturated fat and cholesterol increased risk |
| Morris et al., 2003 (72) | Chicago, N=815 unimpaired, 65-94 yrs | Diet history, 2.3 yr follow-up test for AD | 131/ 815 developed AD, 60% less risk of AD with fish | DHA but not EPA associated with low AD risk |
| Kalmijn et al., 2004 (73) | Zutphen N=1,613, 45-70 yrs, | Diet history, Cog testing | High n-3 reduces risk of cognitive impairment | Cholesterol and sat. fat increased risk |
| Morris et al., 2005 (74) | Chicago, N=3,718 65+, mean 74 yrs | Diet history, Cog testing | Fish consumption associated with reduced cognitive decline over 6 yrs | No clear association with n-3 intake |
| Huang et al., 2005 (75) | Boston | Diet history, Dementia/ AD | Fish consumption reduces AD risk by 41%, dementia by 28% | Risk reduction only in non-ApoE4 |
| Nurk et al., 2007 (76) | Norway, N=2031, 70-74 yrs | Diet history, Cog testing | Less than 10 g/ day fish intake predicts poor Cog performance | Most Cog function improved dose-dependently up to 75 g/ d fish |
| Barberger-Gateauet al. 2007 (77) | France, 3-City, n=8,085, Non-demented, 65+ | Diet history, 4 yr follow-up, Dementia/AD | 281 dementia (183 AD) Fish reduced dementia (HR=0.46) and AD (HR=0.65) | Fish only protective for AD in non-ApoE4 (HR=0.60) |
| van Gelder et al. 2007 (78) | Zutphen, (N=210, men 70-89 yrs) | Diet history, 5 yr follow-up, MMSE | ∼400 mg/day DHA+EPA associates with reduced decline | Dose-dependent effect |
Abbreviations: Cognitive, Cog; DHA, docosahexaenoic acid; eicosapentanoic acid, EPA; hazard ratio, HR; Mini-mental status examination, MMSE; OR, Odds Ratio. Sat., saturated.
One potential confounding factor in epidemiology study might be limited reliability in dietary survey data. However, there have been another eight studies in which high blood levels of omega-6 (relative to omega-3) fatty acids were associated with AD and/ or increased cognitive decline (Table 2). In general, these studies show protection from omega-3 fatty acids, confirming the results based on dietary intake estimates. The prospective Framingham study from Schaefer et al. 2006 (11) is notable in that blood levels taken at ∼10 years prior to assessment of cognitive status showed protection from dementia or AD (average age 76 years) in the group with the upper 25% quartile blood DHA levels. No other lipid was predictive of risk. The authors estimated a daily intake of 180 mg per day of DHA in the protected group and plasma DHA levels correlated with fish intake. However, estimated daily intakes of DHA from fish accounted for only half the variance suggesting that genetic factors or other n-3, notably alpha linolenic acid (ALA). In an atherosclerosis risk study from Minnesota that followed cognitive decline in younger patients with less AD risk (age 50–65 years), Beydoun et al. 2008 (12) found that higher plasma omega-3 fatty acid levels were associated with less decline in verbal fluency, particularly in hypertensive and dyslipidemic patients. They reported these patients as having more oxidative stress, which might deplete the highly peroxidizable long chain omega-3 fatty acids (omega-3 or n-3). Alternatively, because of the overlap between AD and CVD risk factors, protection against decline in midlife may involve reduction in both incipient vascular and Alzheimer dementia accelerated by hypertension and elevated blood lipids. In the same study and in others, high blood levels of omega-6 fatty acids (n-6) were associated with increased risk, consistent with the protective value of a low n-6/n-3 ratio. Supporting a possible association between omega-3 fatty acid levels and oxidative stress, Wang et al. 2008 (13) (Table 2) found an association between a high RBC n-3 index and levels of several lipid soluble antioxidants. The prospective blood level study of Whalley et al. 2008 (14) again reported less protection from high omega-3 fatty acid in ApoE4 carriers. ApoE4 carriers show evidence of increased oxidative stress, including lipid peroxidation products (15, 16), consistent with a large literature showing that ApoE4 increases susceptibility to oxidative stress in animal and cell culture experiments (reviewed in (17)). Thus, one logical explanation for a possible interaction between omega-3 fatty acids and ApoE4 genotype would be increased lipid peroxidation.
Table 2. Blood Omega-3 Fatty Acids and Cognitive Decline (8/10 Positive Studies).
| Study | Population | Methods | Findings | Other |
|---|---|---|---|---|
| Conquer et al. 2000 (79) | Canada, N=55 77-83 yrs | Plasma PL, Cog testing | Low plasma n-3 in AD and Cog-impaired aged | Higher n-6 and lower n-3/n-6 in AD or impaired |
| Heude et al., 2003 (80) | France-EVA, N=246 63-74 yrs baseline | RBC, 4 yrs follow-up, MMSE | High n-3 predicts reduced decline (OR=0.59) | High n-6, n-6/n-3 predicts more decline |
| Laurin et al., 2003 (81) | Canada, 65+ yrs | Plasma PL | Higher DHA in dementia cases | Unchanged n-3 in AD |
| Schaefer et al 2006 (11) | Framingham, N=899 unimpaired, Median 76 yrs, prospective | Plasma PL, 9.1 yr follow-up Dementia/AD | 99 /899 got dementia, 71 AD, high quartile DHA, RR=0.53 dementia 0.61 AD | 3 servings/ week in high DHA quartile |
| Dullemeijer et al., 2007 (82) | Holland FACIT (folic acid) trial, N=807, 50-70 yrs cross-section | Plasma PL, Cog testing | High n-3 predicts less decline in sensorimoter and complex speed over 3 yrs. | Plasma n-3 did not predict 3-yr changes in memory, information-processing speed, or word fluency. |
| Beydoun et al., 2007 (12, 83) | Minneapolis, (n=2251, 50-65 yrs) | Plasma PL/ chol. Esters. Cog testing, 6 yr follow-up | 140 decline, Higher n-3 reduces risk of decline in verbal fluency | Effects stronger w/ hypertension/ dyslipid-emia, important in middle age |
| Cherubini et al. 2007 (84) | Chianti, Italy, N=935, Mean age 75.6 | Plasma, MMSE | n-3 levels reduced with cognitive deficits | (low n-3/n-6 trend, p=0.09) |
| Tully et al., 2003 (85) | Dublin, N=148 AD (76.5 yrs), 45 (70 yrs) controls | Serum cholesterolester DHA | DHA significantly reduced in all AD | DHA levels reduced with dementia severity |
| Wang et al., 2008 (13) | Oregon, N=46, AD + controls | RBC DHA | MMSE directly correlates with RBC n-3 index | Lower DHA and antioxidants associate |
| Whalley et al., 2008 (14) | England, N=120, born in 1936, age 64-68 | RBC DHA | High RBC DHA associated w/ better cognitive function | Significant only in non-Apo E4 |
Abbreviations: cholesterol, chol; Cognitive, Cog; Etude sur le Vieillissement Arteriel, EVA; Folic Acid and Carotid Intima-Media Thickness, FACIT; phospholipids, PL; relative risk, RR; red blood cells, RBC.
In summary, in the three years since the 2005 evidence-based review of the omega-3 fatty acid and dementia-related literature, the results of eight new studies have added further support to the 2005 conclusions, which called for randomized clinical trials that are 1) sufficiently powered, 2) of an adequate length (e.g., three to five years of follow-up), 3) early intervention during “the lengthy presymptomatic latency period” as well as 4) in populations of cognitively-impaired adults, prior to a dementia diagnosis, such as individuals with various sub-types of mild cognitive impairment (MCI) (10).
The Western Diet is Omega-3 Deficient
The dietary intake and blood level studies in Tables 1 and 2 suggest a consistent reduction in risk for cognitive decline, AD, or other dementia for those in the upper quartiles or quintiles of omega-3 intake or levels. To be in this protected group based on epidemiology would require ∼200 mg DHA daily intake. Consistent with these studies, this is much higher than the average U.S. daily intake, which is generally estimated to be closer to ∼80 mg—or less than half that in the low-dementia risk groups. Because the risk factors are shared, these numbers are similar to those arising from the epidemiology for reducing CVD risk; however, the explanation for DHA-reduced dementia and AD risk likely involves more than that for reduced CVD and multiple effects, including neuroprotection and more specific effects on AD pathogenesis.
Alzheimer (AD) Pathogenesis
AD is believed to be caused by increased amyloid β (Aβ1-42) protein, which is derived from a larger β amyloid precursor protein, by sequential endoproteolysis catalyzed by enzymes called β- and γ- secretase. Aβ 1-42 aggregates to form neurotoxic oligomers and fibrils (1). These oligomers and fibrils of Aβ can cause oxidative damage, neuroinflammation, and synaptic dysfunction; as well as loss and activation of different kinases that phosphorylate tau protein and promote its aggregation into toxic species, including soluble tau oligomers and intraneuronal neurofibrillary tangles. While Aβ may initiate pathogenesis, tau pathology is a better correlate of neurodegeneration and progression, and synapse loss is the best correlate of cognitive decline (18).
DHA is Neuroprotective via Multiple Mechanisms
1. Reducing arachidonic acid and its metabolites
The omega-3 fatty acid DHA is preferentially taken up by the brain, where it is highly enriched in neurons and synapses, and esterified at the second position of phospholipids. While many effects relevant to CNS function have been reported (19), a subset of these shown in Table 3 would be predicted to be neuroprotective and relevant to Alzheimer and other dementia diseases. For example, because DHA and arachidonic acid (AA) compete for esterification into phospholipids at the most labile sites, higher ratios of n-3/n-6 intake reduce the flux of AA released by phospholipases, which are activated during glial or synaptic signaling, notably by calcium influx. In AD, there is both an overall pattern of chronic low level inflammation (20) and excitotoxic activity (21), resulting in elevated COX-2 (22, 23), increased AA products including cyclooxygenase products like PGE2 (24), and lipoxygenase products (25, 26). Increased DHA influx has the potential to reduce AA availability, particularly in compartments with higher lipid metabolism that contribute to both glial and neuronal hyperactivation. Reduced AA has been observed in brains of DHA-fed AD model mice with a positive response (27-29). Thus, DHA should share some neuroprotective mechanisms with NSAIDs, for example, COX-2 (30) and lipoxygenase (26) inhibitors. If a significant part of DHA's protective activity stems in part from NSAID-like activity, this is noteworthy because, while NSAIDs reduce Alzheimer risk in most epidemiology, the protective effects are not seen with use in the two to three years prior to assessment and are much clearer when there is five or more years duration of use (31). This “lagging” or delayed protection is obviously a problem for clinical trials, where duration is a major expense.
Table 3. Neuroprotective effects of DHA.
| Neuroprotective Effect | References |
|---|---|
| (1) Anti-inflammatory. Reducing AA and metabolites via COX and lipoxygenase (PG, HETES, etc) | Tassoni et al., 2008; Rao et al. and Cao et al., 2007 (86-88) |
| (2) Insulin/trophic factor potentiation via Akt | Akbar et al., 2005 (32) |
| (3) Increased brain-derived neurotrophic factor | Rao et al., 2007; Wu et al., 2004 (34, 89) |
| (4) Antioxidant. Direct (?). Increasing AO enzymes (catalase, GSH peroxidase) | Yavin et al., 2002; Hashimoto et al., 2005; Hossain et al., 1999 (90-92) |
| (5) Anti-apoptotic. Increasing NPD1, anti-apoptotic& reducing pro-apoptotic proteins | Lukiw et al., 2005; Bazan et al., 2005 (41, 93) |
| (6) Promotes Neurogenesis | Innis et al., 2007 (94) |
| (7) Increasing a glucose transporter | Tsukada et al., 2000; Pifferi et al., 2007 (95, 96) |
| (8) Coupling blood flow to glucose utilization | Tsukada et al., 2000; Pifferi et al., 2007 (95, 96) |
| (9) Improving synaptic membrane fluidity | Hashimoto et al., 2006 (97) |
| (10) Increasing G-protein coupling | Litman et al., 2001 (98) |
| (11) PPAR or RXR agonists | Gani et al., 2008; Calderon, et al., 2007 (42, 99) |
Abbreviations: antioxidant (AO); arachidonic acid, (AA); cyclooxygenase (COX); reduced glutathione (GSH); hydroxyeicosatetraenoic acids (HETE); prostaglandins (PG); neuroprotectin D1 (NPD1); peroxisome proliferators-activated receptor (PPAR); retinoid X receptor (RXR).
2. Trophic factor signaling
A second mechanism for neuroprotective activity for DHA involves potentiating the activation of one branch of classical neurotrophic factor (BDNF, NGF, bFGF) or insulin signaling via the phosphatidylinositol-3 kinase (PI3-K) > Akt pathway. DHA increases neuronal phosphatidylserine (PS) and the rate of a critical activation step: Akt membrane docking, which occurs through a plecstrin homology domain pocket that binds the PI3-K product PIP3 and PS (32). The net effect is to increase neurotrophic signaling to down-regulate pro-apoptotic regulators of caspases like Bad that control cell death. Some evidence for this activity was found with elevated inactivation of Bad and reduced caspase activation in AD model mice fed DHA (27, 33).
3. BDNF
A third reported neuroprotective effect of DHA is simply to increase the production of brain-derived neurotrophic factor (BDNF) (34), which is depleted in AD hippocampus and believed to be responsible for many of the positive effects of exercise on cognition (35).
4. Antioxidant defenses
A fourth class of neuroprotective effect comes from antioxidant activity, which may be direct as discussed by Yavin et al. 2002, or indirect by induction of antioxidant defense enzymes (Table 3). Because omega-3 fatty acids are highly unsaturated fatty acids and subject to oxidative attack via autocatalytic feed–forward lipid peroxidation reactions, they need additional protection and appear to induce antioxidant defenses enzymes. This may occur via transcriptional regulation by the Nrf2>Keap>antioxidant response element pathway that is known to be regulated by PI3-K>Akt signaling (36). Thus, DHA potentiation of Akt may promote antioxidant defenses. Whatever the mechanism, omega-3 fatty acids can reduce oxidative damage in AD model mice (33) and in humans (37). This is significant because oxidative damage is clearly elevated in AD, including oxidative damage products of DHA itself (38-40).
5. Neuroprotection D1 (NPD1)
A fifth type of neuroprotective effect of DHA is mediated by a lipoxygenase metabolite of DHA, neuroprotectin D1 (NPD1) (41). This metabolite has a variety of effects including up-regulation of anti-apoptotic and down-regulation of pro-apoptotic mediators that regulate caspases involved in cell death. It appears to be a very potent neuroprotective agent with multiple activities.
6–11. Other neuroprotective functions
Other potentially neuroprotective activities listed in Table 3 include 6) promoting neurogenesis, 7) increasing a glucose transporter, 8) coupling of blood flow to glucose utilization, 9) improving synaptic membrane fluidity, 10) G-protein coupling that is involved in many signal transduction pathways and 11) directly binding to lipid-related transcription factors including forms of LXR, RxR, and PPAR. It is unclear whether these direct transcriptional effects occur in vivo with DHA itself, because the EC50 for this type of mechanism may require higher levels of free DHA than other pathways (42); however, these are possible targets for DHA metabolites. It is interesting to note that the insulin sensitizing PPAR agonist rosaglitazone has been in clinical trials for AD and, reminiscent of fish epidemiology discussed above, showed protection in non-ApoE4 carriers (43).
These mechanistic studies in preclinical models suggest neuroprotective mechanisms. Some direct evidence for neuroprotection in people is suggested by a recent German study that showed improved survival and recovery from severe head injury when brain trauma patients received fish oil supplements through their parental feeding tubes (44).
Disease-Specific Mechanisms of DHA Protection in Alzheimer Disease
Most of the neuroprotective mechanisms discussed above are potentially relevant to AD and other neurodegenerative diseases or CNS damage. However, since AD is believed to be initiated by Aβ peptide, many potential AD treatments are aimed at inhibiting Aβ production, for example: secretase inhibitors and modulators. DHA's efficacy in reducing Aβ production/ amyloid accumulation in vitro and in animal models has now been observed by eight out of nine studies (Table 4). In the one study that did not see a reduction in Aβ, the supplemented diet also failed to change brain DHA or AA levels (45).
Table 4. DHA reduces production of the amyloid β protein that is believed to cause AD. (8/9 studies).
| Effect of Omega-3 on Aβ/ Amyloid | Reference |
|---|---|
| (1) Reduced Aβ production -human neurons | Lukiw et al., 2005 (93) |
| (2) Reduced Aβ pathway products | Sahlin et al., 2007 (100) |
| (3) Reduced Aβ production –cell lines | Oksman et al., 2006 (29) |
| (4) Reduced amyloid accumulation in vivo | Lim et al., 2005 (101) |
| (5) Reduced amyloid accumulation in vivo | Oksman et al., 2006 (29) |
| (6) Reduced amyloid accumulation in vivo | Hooijmans et al., 2007 (102) |
| (7) Reduced intraneuronal Aβ in vivo | Green et al., 2007 (28) |
| (8) Reduced amyloid in vivo | Berg et al., 2007 (103) |
| (9) No effect on amyloid (or brain DHA) | Arendash et al., 2007 (45) |
DHA appears to reduce Aβ production by several mechanisms, including the induction of increased neuronal expression of SorLa/LR11 in multiple model systems in vitro and in vivo (46). SorLa is an amyloid precursor sorting protein with diminished expression levels in surviving neurons at early stages of late onset AD, but not early onset familial AD (47). Because SorLa (also called LR11) is known to limit Aβ production by trafficking amyloid precursor proteins away from the secretases that make Aβ, reduced levels of LR11 would be predicted to play a causal role to increase AD risk. There is some genetic evidence to support this hypothesis (48-51)
Whether or not genetic variants that limit expression of LR11 contribute to late onset AD, the majority of patients have reductions in expression. Omega-3 fatty acids appear to be one known environmental risk factor that can up-regulate SorLA/LR11 expression in animal models. We have recently found that soluble forms of LR11 can be detected in human CSF, and that deficits occur in AD patients (52). It will be worth evaluating CSF LR11 levels in clinical trials with DHA treatment.
There are several other candidate mechanisms for DHA reducing amyloid. DHA may also be able to lower Aβ by reducing expression of presenilin 1 and γ-secretase activity (28). Omega-3 fatty acids in fish oil are also reported to increase expression of an Aβ-clearing transport protein, transthyretin (TTR) (53). Finally, because expression of a major Aβ-degrading enzyme, the insulin degrading enzyme (IDE), is regulated by the PI3-K> Akt pathway, DHA may increase IDE expression and Aβ clearance (54).
DHA Protects from Aβ oligomer Toxicity to Synapses
Consistent with DHA's many neuroprotective mechanisms, it can protect against Aβ oligomer toxicity and caspase activation in vitro (55). Lower doses of Aβ oligomers also cause loss of synapses without killing neurons. Recently, our group reported evidence for an Aβ oligomer-mediated defect in a pathway involving components implicated by different labs that were looking at Aβ oligomers in vitro. These included NMDA receptors, the tyrosine kinase fyn, Tiam1, rac, PAK, LIMK1, and, downstream, the actin-severing protein cofilin. This pathway is dysregulated in AD brain and regulates the oligomer-induced loss of dendritic spines in excitatory synapses in vitro, including the spine actin binding protein drebrin (56). Drebrin shows massive losses in AD temporal cortex and hippocampus, and DHA protects against this loss in a transgenic mouse model of AD (33). We now have evidence that DHA is remarkably effective in protecting against oligomer-induced synaptic marker loss in primary neurons in vitro, by blocking this synaptotoxic signal transduction pathway regulating dendritic spines and synapse formation (Q.L. Ma, F. Yang, E. Rosario, O.J. Ubeda, P.P. Chen, W. Beech, B. Hudspeth, S.A. Frautschy, G. M. Cole, manuscript in preparation). In our view, drebrin loss can be taken as an index of dysregulation of this pathway, and drebrin shows a precipitous loss in temporal cortex of AD brain as mild cognitive impairment emerges (MMSE >27) (57). After that, it shows no further reduction. Since drebrin loss was the most DHA-sensitive endpoint in our AD animal model studies, these results suggest that DHA intervention will be most effective at the earliest stages of cognitive decline.
DHA limits Tau Kinases that Promote Tau Pathology/Neurofibrillary Tangles
Neurofibrillary tangles are an AD pathology featuring intraneuronal accumulation of filamentous aggregates of the microtubule protein tau. This pathology is promoted by Aβ aggregate-induced induction of “tau kinases” that phosphorylate tau to keep it off microtubules, and to promote exposure of β conformation stable regions that bind to each other to make proteolytically stable tau amyloid fibrils. Two of the major tau kinases that are induced by Aβ are GSK3β and JNK. Since GSK3β is negatively regulated by the PI3-K> Akt pathway that DHA potentiates as discussed above. Thus, it is not surprising that in our amyloid plaque-forming AD model mice, on DHA-depleting high safflower oil diets, this inhibitory GSK3β phosphorylation is suppressed, and this is reversed by DHA treatment restoring inhibitory control of GSK3β (Ma et al., unpublished). While this effect would be predicted to limit tangle formation, those AD model mice lack a human tau transgene that is required for tangles to develop. Using triple transgenic AD model mice with two genes that increase Aβ and a mutant human tau, LaFerla's group has shown that DHA not only slows the accumulation of the intraneuronal Aβ implicated in the model, it also suppresses the activity of JNK and the accumulation of abnormally phosphorylated tau (28). Thus, DHA has multiple mechanisms for reducing both the amyloid peptide (Aβ) that initiates AD and the tau kinases and tau pathology that appear to perpetuate neurodegeneration. Coupled with more than 10 other neuroprotective effects listed in Table 3, DHA is very well-positioned to play a causal role in preventing AD that can explain the positive epidemiology studies.
DHA Protection is Incomplete and Should be Combined with Antioxidants
Nevertheless, despite profound anti-Alzheimer effects in vitro and in multiple AD animal models, DHA protection is incomplete. For example, in our late intervention model where it protected drebrin and CamKII alpha as it does with Aβ oligomer toxicity in vitro, DHA failed to protect NMDA receptor loss (27) and the ERK> CREB signal transduction pathway (Ma et al., unpublished). These results suggest that DHA treatment alone will likely have some benefits, but may work best in combination with other treatments. We have previously argued that oxidative damage in AD brains may be depleting DHA, suggesting a combination with an antioxidant treatment (33). Anyone who has worked with DHA in vitro, knows that it needs to be protected by antioxidants, particularly at higher doses. While this is much less clear in vivo, where antioxidant defenses are usually sufficient, oxidation of supplemented DHA may be a problem in pathological environments (58). DHA is better protected in vivo, but clearly not adequately protected in AD brain because, as reviewed above, its F-4 isoprostane products are elevated. DHA's lipid peroxidation products like bifunctional aldehydes can cross-link proteins, including tau proteins (59). Further, specific toxic oxidized forms of DHA have recently been identified (60). One might predict lipid peroxidation to be an issue in AD because Aβ aggregates are known to increase oxidative damage, and AD has increased mitochondrial defects and elevation of most known oxidative damage markers (reviewed in (33)). Since Aβ aggregates accumulate in synapses (61), and this is where peroxidizable DHA is concentrated in areas of high metabolism and even in the mitochondria (62), there is every reason to expect the observed increase in DHA oxidation products in AD brain and CSF. Given that DHA is so readily oxidized, there is a very strong rationale suggesting that it be combined with antioxidant interventions like the classical vitamin E plus C or the more brain permeable and mitochondrial-protective alpha lipoic acid. Our group has also advocated that it be combined with the polyphenolic antioxidant curcumin, because it has additional anti-amyloid and AD protective activities (63).
Clinical Trials
Collectively, the strong positive epidemiology and preclinical data have led to multiple clinical trials for the treatment or prevention of AD with omega-3 fatty acids, notably DHA (Table 5).
Table 5.
Omega-3 Fatty Acids in Clinical Trials For AD Treatment or Prevention.
| Clinical Trials-completed | Reference |
|---|---|
| 240 mg/ day DHA + AA (3 mos) improved memory and attention in amnestic MCI (n=12) (but not AD (N=8)) | Kotani et al., 2006 (64) |
| 4 g fish oil (1.7 g DHA for 6 months, n=174) ; no sig. effect for MMSE<27, but improved delayed recall, attention; MMSE >27 stabilized with w-3 (n=32) | Freund-Levi et al. 2006 (65) |
| Memo trial, normal or MMSE >21 (n=302, 65+ yrs, 1800 vs 400 mg EPA+DHA vs placebo for 26 wks)- increases in attention, especially in E4+ and men, but no change in cognition | Van de Rest et al., 2008 (104) |
| 6 months 1.8 g/day total n-3 g showed improvement in ADAS-cog compared to olive oil with MCI (p=0.03), No effect in AD; NCT00628017 | Chiu et al., 2008 (66) |
| OHSU pilot, MMSE 15-26 (n=39, Fish oil (750mg DHA, 1050 EPA +/- 600mg alpha lipoate vs placebo)- FO+ lipoate stabilized MMSE, ADLs (p=0.02), NCT00090402 | Shinto et al., 2008 (67) |
Abbreviations: activities of daily living (ADLs); arachidonic acid (AA); fish oil (FO); mild cognitive impairment (MCI); Oregon Health State University (OHSU).
Results to-date suggest that DHA alone may produce some benefits, but may not work well by itself in cases of established AD with MMSE <26; however, it may be effective with early intervention by itself (MCI), or even in established AD in combination with an antioxidant. For example, in a small Japanese trial from Kotani (64) as well as in the completed Swedish trial from Freund-Levi (65) and in a similar small 6 month Taiwanese trial from Chiu (66), DHA or fish oil appeared to stabilize MMSE in the MCI patients, but not in those with established AD. It is encouraging that the only example of a DHA/antioxidant combination treatment approach, a pilot alpha lipoate/ fish oil trial from Shinto and colleagues at OHSU (NCT000904029), MMSE was significantly stabilized over a 12-month period (67). If this level of efficacy is confirmed in larger trials, this will be a remarkable and cost-effective treatment. While these are the first trials to report, they are encouraging; additional trials are registered at clinical.trials.gov and ongoing (Table 6).
Table 6. Ongoing Clinical Trials (2008).
| Ongoing Trials | PI. |
|---|---|
| OPAL trial, cognitive decline, age 70-79 MMSE>24, 500 mg DHA + 200 mg EPA (n=800): UK identifier: ISRCTN72331636 | Dangour, et al., 2006 (UK) (105) |
| ADCS trial, mild to mod AD -2 g algal DHA/day for 18 months (n=400), NCT00440050 | J. Quinn, OHSU (NIA/ADCS/ Martek) |
| MIDAS Early memory deficits, 900 mg/d, 6 months (n=465); NCT00278135 | K. Yurko-Mauro (Martek Biosciences) |
| Primary Prevention of cognitive decline in frail elderly, MMSE>24, n=1200, omega 3 (VO137), 800 mg DHA /d 3 years BCT00672685 | B. Vellas, University Hospital, Toulouse |
Abbreviations: AD Cooperative studies (ADCS); Older People and n-3 Long-chain polyunsaturated fatty acids (OPAL). Mini-mental state examination (MMSE). Memory Improvement with Docosahexaenoic Acid study (MIDAS).
From the perspective of this review, it is unfortunate that none of the ongoing trials combine DHA with an antioxidant. The ADCS trial with DHA includes AD patients with MMSE 26 or below and is for an 18-month duration. It is larger and longer than the completed six-month Freund-Levi et al. treatment trial in Sweden, which failed to show clear cognitive benefit in similarly advanced AD patients, but the ADCS trial excludes MCI patients who are the most likely to benefit. Nevertheless, some 68% of the patients in the Freund-Levi trial were ApoE4 positive, and some of the epidemiology suggests that they may respond poorly. Therefore, there is hope for some positive results in the larger ADCS trial that may either take longer to manifest, or are ApoE genotype-dependent and require the larger patient pool. Further, since EPA can competitively reduce DHA incorporation, there may or may not be benefits from enhancing DHA delivery by using a slightly higher dose of algal DHA, rather than a fish oil product, and not having EPA in the formulation. We must await the trial result.
We would strongly advocate a combination approach in future planned trials with AD patients. We have argued for this on a theoretical basis and based on our preclinical results, but the positive data from the small OHSU pilot make this approach much more plausible. Despite these concerns, there is sufficient evidence to expect some significant protective effects, particularly with the early intervention efforts in three of the ongoing trials. Is DHA an essential nutrient? The counterargument is that dietary ALA provides sufficient DHA. As reviewed here, the epidemiology of fish and to a lesser extent DHA intake provide a strong case for protection from cognitive decline and dementia by higher intakes of marine omega 3 fatty acids than much of the population is achieving from other dietary n-3. The hypothesis that conversion from dietary ALA provides adequate DHA or EPA, does not explain the epidemiology showing protection from higher marine sources. Further, DHA rather than EPA is markedly enriched in the brain and the preclinical model data clearly implicate DHA as an effective protection against Alzheimer pathogenesis. That said, to date there are only a handful of small randomized clinical trials with fish oil showing protection and only in early stages of disease. More definitive, longer and larger, adequately powered trials with DHA are needed to examine DHA efficacy against cognitive decline due to Alzheimer. Fortunately, several trials are already currently underway. However, age-related cognitive decline is also caused by vascular disease and there, the clinical trial data are fairly compelling. Large clinical trials have shown that fish oil supplements reduce death from cardiovascular disease (CVD) in patients with substantial risk (6). Further, meta-analysis of the epidemiology of EPA +DHA intake shows similar protection from CVD death in healthy adults (68). In contrast, we lack similar evidence to conclude that merely lowering the n-6/n-3 ratio with ALA protects from CVD (69). This is very strong evidence that CVD protective levels of EPA/ DHA levels are not being derived from dietary ALA in the general population. While the relative roles for EPA and DHA in CVD and protection against stroke remain controversial (5), lowering the CVD death rate in our aging population is a compelling argument for an essential role for combined EPA + DHA, but neither n-3 fatty acid on its own.
Conclusions
The combination of a strong rationale from both positive epidemiology and preclinical trials have propelled omega-3 fatty acid supplements into multiple clinical trials for age-related cognitive decline and dementia, notably AD. Because dementia risk doubles with every five years over age 65, the caseload of AD and vascular dementia will more than double as the baby boomers age. Dementia is amongst the chronic age-related diseases that is the most disabling, demanding the most intensive and expensive care and frequent institutionalization. Dementia alone can be predicted to drive the cost of Medicare and private health insurance up to the point where it becomes an unsustainable economic burden. Compared with most new medications, omega-3 fatty acids would be an outstanding intervention from long-term safety, side-effect, and cost perspectives. The evidence to-date from the small completed trials suggests possible efficacy both with early intervention and, in combination with antioxidants, in more established AD. Because of their outstanding safety profile, low cost, and proven efficacy in reducing mortality from cardiovascular disease, omega-3 fatty acids, and DHA in particular, are particularly attractive for prevention. This is particularly true in populations with typical Western diets like the U.S., where the average dietary intake of DHA is less than half that required in the epidemiology to reduce risk for either CVD or AD. The evidence for CVD alone is sufficient to begin to take steps to get our population out of the low omega-3 fatty acid intake, high-risk groups for these major diseases. For established AD, we are just beginning to learn how to use DHA supplements for treatment. They may not work well alone at late-stage AD, particularly in groups with high oxidative stress like ApoE4, so we can expect to combine DHA with other treatments, including antioxidants.
Figure 1. Summary of mechanisms of Anti-Alzheimer Effects of DHA.
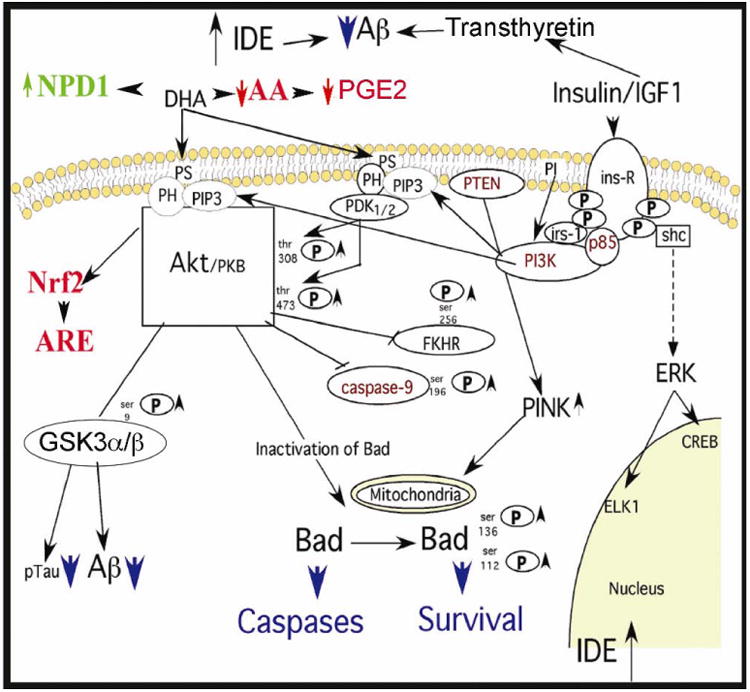
As reviewed in Tables 3 and 4, DHA has multiple-reported neuroprotective and anti-Aβ effects. These include reducing Aβ production by increasing SorLA/ LR11, and non-amyloidogenic processing of amyloid precursor and decreasing γ-secretase. Aβ clearance may be improved by induction of transthyretin or IDE. The DHA metabolite NPD1 can also be neuroprotective by reducing pro-apoptotic proteins such as Bad. Further, DHA increases in PS and PDK/AKT docking will potentiate neuroprotective trophic factor signaling through insulin, IGF1, BDNF, and other trophic factors. BDNF itself is induced by DHA. Downstream from AKT, there are anti-apoptotic effects on Bad and caspases, and up-regulation of the Nrf2 pathway to ARE and antioxidant defense enzymes. Further, the major tau kinase GSK3 will be inhibited, limiting tau/ tangles, which may also contribute to reduced Aβ production. Finally, one in vivo report found DHA limited activation of another tau kinase (JNK) and accumulation of tau pathology
Acknowledgments
Martek Biosciences provided travel expenses and an honorarium for attendance at the workshop and drafting of this paper for Dr. Cole. Author roles. The authors have no financial interests to declare. Dr. Cole wrote various drafts and helped to organize tables; Dr. Ma provided data; Dr. Frautschy assisted in the writing and editing of the manuscript and prepared tables and figures.
Sources of support: This work was supported by NCCAM R01AT3008 and NIA R01AG13471.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Greg M. Cole, Departments of Medicine and Neurology at University of California, Los Angeles, CA, USA; Geriatric Research, Education and Clinical Center, Greater Los Angeles Veterans Affairs Healthcare System, VA Medical Center, North Hills, CA, USA
Qiu-Lan Ma, Departments of Medicine, at University of California, Los Angeles, CA, USA; Geriatric Research, Education and Clinical Center, Greater Los Angeles Veterans Affairs Healthcare System, VA Medical Center, North Hills, CA, USA
Sally A. Frautschy, Departments of Medicine and Neurology at University of California, Los Angeles, CA, USA; Geriatric Research, Education and Clinical Center, Greater Los Angeles Veterans Affairs Healthcare System, VA Medical Center, North Hills, CA, USA
References
- 1.Cummings JL, Cole G. Alzheimer disease. JAMA. 2002;287:2335–2338. doi: 10.1001/jama.287.18.2335. [DOI] [PubMed] [Google Scholar]
- 2.Fotuhi M, Zandi PP, Hayden KM, Khachaturian AS, Szekely CA, Wengreen H, Munger RG, Norton MC, Tschanz JT, Lyketsos CG, Breitner JC, Welsh-Bohmer K. Better cognitive performance in elderly taking antioxidant vitamins E and C supplements in combination with nonsteroidal anti-inflammatory drugs: the Cache County Study. Alzheimers Dement. 2008;4:223–7. doi: 10.1016/j.jalz.2008.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Szekely CA, Green RC, Breitner JC, Ostbye T, Beiser AS, Corrada MM, Dodge HH, Ganguli M, Kawas CH, Kuller LH, Psaty BM, Resnick SM, Wolf PA, Zonderman AB, Welsh-Bohmer KA, Zandi PP. No advantage of A beta 42-lowering NSAIDs for prevention of Alzheimer dementia in six pooled cohort studies. Neurology. 2008;70:2291–8. doi: 10.1212/01.wnl.0000313933.17796.f6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cole GM, Morihara T, Lim GP, Yang F, Begum A, Frautschy SA. NSAID and Antioxidant Prevention of Alzheimer's Disease: Lessons from In Vitro and Animal Models. Ann N Y Acad Sci. 2004;1035:68–84. doi: 10.1196/annals.1332.005. [DOI] [PubMed] [Google Scholar]
- 5.Harris WS, Miller M, Tighe AP, Davidson MH, Schaefer EJ. Omega-3 fatty acids and coronary heart disease risk: clinical and mechanistic perspectives. Atherosclerosis. 2008;197:12–24. doi: 10.1016/j.atherosclerosis.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 6.Lee JH, O'Keefe JH, Lavie CJ, Marchioli R, Harris WS. Omega-3 fatty acids for cardioprotection. Mayo Clin Proc. 2008;83:324–32. doi: 10.4065/83.3.324. [DOI] [PubMed] [Google Scholar]
- 7.Rosendorff C, Beeri MS, Silverman JM. Cardiovascular risk factors for Alzheimer's disease. Am J Geriatr Cardiol. 2007;16:143–9. doi: 10.1111/j.1076-7460.2007.06696.x. [DOI] [PubMed] [Google Scholar]
- 8.Mozaffarian D, Rimm EB. Fish intake, contaminants, and human health: evaluating the risks and the benefits. JAMA. 2006;296:1885–99. doi: 10.1001/jama.296.15.1885. [DOI] [PubMed] [Google Scholar]
- 9.Lee JH, O'Keefe JH, Lavie CJ, Marchioli R, Harris WS. Omega3 fatty acids for cardioprotection. Mayo Clin Proc. 2008;83:324–32. doi: 10.4065/83.3.324. [DOI] [PubMed] [Google Scholar]
- 10.Maclean C, Issa A, Newberry S, Mojica W, Morton S, Garland R, Hilton L, Traina S, Shekelle P. Effects of omega-3 fatty acids on cognitive function with aging, dementia, and neurological diseases. Evid Rep Technol Assess (Summ) 2005:1–88. [PMC free article] [PubMed] [Google Scholar]
- 11.Schaefer EJ, Bongard V, Beiser AS, Lamon-Fava S, Robins SJ, Au R, Tucker KL, Kyle DJ, Wilson PW, Wolf PA. Plasma phosphatidylcholine docosahexaenoic acid content and risk of dementia and Alzheimer disease: the Framingham Heart Study. Arch Neurol. 2006;63:1545–50. doi: 10.1001/archneur.63.11.1545. [DOI] [PubMed] [Google Scholar]
- 12.Beydoun MA, Kaufman JS, Sloane PD, Heiss G, Ibrahim J. n-3 Fatty acids, hypertension and risk of cognitive decline among older adults in the Atherosclerosis Risk in Communities (ARIC) study. Public Health Nutr. 2008;11:17–29. doi: 10.1017/S1368980007000080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang W, Shinto L, Connor WE, Quinn JF. Nutritional biomarkers in Alzheimer's disease: the association between carotenoids, n-3 fatty acids, and dementia severity. J Alzheimers Dis. 2008;13:31–8. doi: 10.3233/jad-2008-13103. [DOI] [PubMed] [Google Scholar]
- 14.Whalley LJ, Deary IJ, Starr JM, Wahle KW, Rance KA, Bourne VJ, Fox HC. n-3 Fatty acid erythrocyte membrane content, APOE varepsilon4, and cognitive variation: an observational follow-up study in late adulthood. Am J Clin Nutr. 2008;87:449–54. doi: 10.1093/ajcn/87.2.449. [DOI] [PubMed] [Google Scholar]
- 15.Montine KS, Kim PJ, Olson SJ, Markesbery WR, Montine TJ. 4-hydroxy-2-nonenal pyrrole adducts in human neurodegenerative disease. J Neuropathol Exp Neurol. 1997;56:866–71. doi: 10.1097/00005072-199708000-00004. [DOI] [PubMed] [Google Scholar]
- 16.Ramassamy C, Averill D, Beffert U, Theroux L, Lussier-Cacan S, Cohn JS, Christen Y, Schoofs A, Davignon J, Poirier J. Oxidative insults are associated with apolipoprotein E genotype in Alzheimer's disease brain. Neurobiol Dis. 2000;7:23–37. doi: 10.1006/nbdi.1999.0273. [DOI] [PubMed] [Google Scholar]
- 17.Jofre-Monseny L, Minihane AM, Rimbach G. Impact of apoE genotype on oxidative stress, inflammation and disease risk. Mol Nutr Food Res. 2008;52:131–45. doi: 10.1002/mnfr.200700322. [DOI] [PubMed] [Google Scholar]
- 18.Masliah E, Crews L, Hansen L. Synaptic remodeling during aging and in Alzheimer's disease. J Alzheimers Dis. 2006;9:91–9. doi: 10.3233/jad-2006-9s311. [DOI] [PubMed] [Google Scholar]
- 19.Salem N, Jr, Litman B, Kim HY, Gawrisch K. Mechanisms of action docosahexaenoic acid in the nervous system. Lipids. 2001;36:945–959. doi: 10.1007/s11745-001-0805-6. [DOI] [PubMed] [Google Scholar]
- 20.Akiyama H, Barger S, Barnum S, Bradt B, Bauer J, Cole GM, Cooper NE, Eikelenboom P, Emmerling M, Fiebich BL, Finch CE, Frautschy S, Griffin WS, Hampel H, Hull M, Landreth G, Lue L, Mrak R, Mackenzie IR, McGeer PL, O'Banion MK, Pachter J, Pasinetti G, Plata-Salaman C, Rogers J, Rydel R, Shen Y, Streit W, Strohmeyer R, Tooyoma I, Van Muiswinkel FL, Veerhuis R, Walker D, Webster S, Wegrzyniak B, Wenk G, Wyss-Coray T. Inflammation and Alzheimer's disease. Neurobiol Aging. 2000;21:383–421. doi: 10.1016/s0197-4580(00)00124-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Palop JJ, Chin J, Roberson ED, Wang J, Thwin MT, Bien-Ly N, Yoo J, Ho KO, Yu GQ, Kreitzer A, Finkbeiner S, Noebels JL, Mucke L. Aberrant excitatory neuronal activity and compensatory remodeling of inhibitory hippocampal circuits in mouse models of Alzheimer's disease. Neuron. 2007;55:697–711. doi: 10.1016/j.neuron.2007.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ho L, Pieroni C, Winger D, Purohit DP, Aisen PS, Pasinetti GM. Regional distribution of cyclo-oxygenase-2 in the hippocampal formation in ALzheimer's disease. J Neurosci Res. 1999;57:295–303. doi: 10.1002/(SICI)1097-4547(19990801)57:3<295::AID-JNR1>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 23.Lukiw WJ, Bazan NG. Cycloxygenase 2 RNA message abundance, stability, and hypervariability in sporadic Alzheimer neocortex. J Neurosci Res. 1997;50:937–945. doi: 10.1002/(SICI)1097-4547(19971215)50:6<937::AID-JNR4>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 24.Montine TJ, Sidell KR, Crews BC, Markesbery WR, Marnett LJ, Roberts LJ, 2nd, Morrow JD. Elevated CSF prostaglandin E2 levels in patients with probable AD. Neurology. 1999;53:1495–8. doi: 10.1212/wnl.53.7.1495. [DOI] [PubMed] [Google Scholar]
- 25.Pratico D, Zhukareva V, Yao Y, Uryu K, Funk CD, Lawson JA, Trojanowski JQ, Lee VM. 12/15-lipoxygenase is increased in Alzheimer's disease: possible involvement in brain oxidative stress. Am J Pathol. 2004;164:1655–62. doi: 10.1016/S0002-9440(10)63724-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Firuzi O, Zhuo J, Chinnici CM, Wisniewski T, Pratico D. 5-Lipoxygenase gene disruption reduces amyloid-beta pathology in a mouse model of Alzheimer's disease. FASEB J. 2008;22:1169–78. doi: 10.1096/fj.07-9131.com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Calon F, Lim GP, Morihara T, Yang F, Ubeda O, Salem NJ, Frautschy SA, C GM. Dietary n-3 polyunsaturated fatty acid depletion activates caspases and decreases NMDA receptors in the brain of a transgenic mouse model of Alzheimer's disease. Eur J Neurosci. 2005;22:617–626. doi: 10.1111/j.1460-9568.2005.04253.x. [DOI] [PubMed] [Google Scholar]
- 28.Green KN, Martinez-Coria H, Khashwji H, Hall EB, Yurko-Mauro KA, Ellis L, LaFerla FM. Dietary docosahexaenoic acid and docosapentaenoic acid ameliorate amyloid-beta and tau pathology via a mechanism involving presenilin 1 levels. J Neurosci. 2007;27:4385–95. doi: 10.1523/JNEUROSCI.0055-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Oksman M, Iivonen H, Hogyes E, Amtul Z, Penke B, Leenders I, Broersen L, Lutjohann D, Hartmann T, Tanila H. Impact of different saturated fatty acid, polyunsaturated fatty acid and cholesterol containing diets on beta-amyloid accumulation in APP/PS1 transgenic mice. Neurobiol Dis. 2006;23:563–72. doi: 10.1016/j.nbd.2006.04.013. [DOI] [PubMed] [Google Scholar]
- 30.Kotilinek LA, Westerman MA, Wang Q, Panizzon K, Lim GP, Simonyi A, Lesne S, Falinska A, Younkin LH, Younkin SG, Rowan M, Cleary J, Wallis RA, Sun GY, Cole G, Frautschy S, Anwyl R, Ashe KH. Cyclooxygenase-2 inhibition improves amyloid-beta-mediated suppression of memory and synaptic plasticity. Brain. 2008;131:651–64. doi: 10.1093/brain/awn008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vlad SC, Miller DR, Kowall NW, Felson DT. Protective effects of NSAIDs on the development of Alzheimer disease. Neurology. 2008;70:1672–7. doi: 10.1212/01.wnl.0000311269.57716.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Akbar M, Calderon F, Wen Z, Kim HY. Docosahexaenoic acid: a positive modulator of Akt signaling in neuronal survival. Proc Natl Acad Sci U S A. 2005;102:10858–63. doi: 10.1073/pnas.0502903102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Calon F, Lim GP, Yang F, Morihara T, Teter B, Ubeda O, Rostaing P, Triller A, Salem N, Jr, Ashe KH, Frautschy SA, Cole GM. Docosahexaenoic acid protects from dendritic pathology in an Alzheimer's disease mouse model. Neuron. 2004;43:633–645. doi: 10.1016/j.neuron.2004.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rao JS, Ertley RN, Lee HJ, DeMar JC, Jr, Arnold JT, Rapoport SI, Bazinet RP. n-3 polyunsaturated fatty acid deprivation in rats decreases frontal cortex BDNF via a p38 MAPK-dependent mechanism. Mol Psychiatry. 2007;12:36–46. doi: 10.1038/sj.mp.4001888. [DOI] [PubMed] [Google Scholar]
- 35.Neeper SA, Gómez-Pinilla F, Choi J, Cotman CW. Exercise and brain neurotrophins. Nature. 1995;373:109. doi: 10.1038/373109a0. [DOI] [PubMed] [Google Scholar]
- 36.Lee JM, Johnson JA. An important role of Nrf2-ARE pathway in the cellular defense mechanism. J Biochem Mol Biol. 2004;37:139–43. doi: 10.5483/bmbrep.2004.37.2.139. [DOI] [PubMed] [Google Scholar]
- 37.Mori TA, Puddey IB, Burke V, Croft KD, Dunstan DW, Rivera JH, Beilin LJ. Effect of omega 3 fatty acids on oxidative stress in humans: GC-MS measurement of urinary F2-isoprostane excretion. Redox Rep. 2000;5:45–6. doi: 10.1179/rer.2000.5.1.45. [DOI] [PubMed] [Google Scholar]
- 38.Praticò D, Lee VMY, Trojanowski JQ, Rokach J, Fitzgerald GA. Increased F2-isoprostanes in Alzheimer's disease: evidence for enhanced lipid peroxidation in vivo. FASEB J. 1998;12:1777–1783. doi: 10.1096/fasebj.12.15.1777. [DOI] [PubMed] [Google Scholar]
- 39.Nourooz-Zadeh J, Liu EHC, Yhlen B, Anggard EE, Halliwell B. F4-Isoprostanes as specific marker of docosahexaenoic acid peroxidation in Alzheimer's disease. J Neurochem. 1999;72:734–740. doi: 10.1046/j.1471-4159.1999.0720734.x. [DOI] [PubMed] [Google Scholar]
- 40.Reich EE, Markesbery WR, Roberts LJ, 2nd, Swift LL, Morrow JD, Montine TJ. Brain regional quantification of F-ring and D-/E-ring isoprostanes and neuroprostanes in Alzheimer's disease. Am J Pathol. 2001;158:293–297. doi: 10.1016/S0002-9440(10)63968-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bazan NG. Neuroprotectin D1 (NPD1): a DHA-derived mediator that protects brain and retina against cell injury-induced oxidative stress. Brain Pathol. 2005;15:159–66. doi: 10.1111/j.1750-3639.2005.tb00513.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Calderon F, Kim HY. Role of RXR in neurite outgrowth induced by docosahexaenoic acid. Prostaglandins Leukot Essent Fatty Acids. 2007;77:227–32. doi: 10.1016/j.plefa.2007.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Risner ME, Saunders AM, Altman JF, Ormandy GC, Craft S, Foley IM, Zvartau-Hind ME, Hosford DA, Roses AD. Efficacy of rosiglitazone in a genetically defined population with mild-to-moderate Alzheimer's disease. Pharmacogenomics J. 2006 doi: 10.1038/sj.tpj.6500369. [DOI] [PubMed] [Google Scholar]
- 44.Heller AR, Rossler S, Litz RJ, Stehr SN, Heller SC, Koch R, Koch T. Omega-3 fatty acids improve the diagnosis-related clinical outcome*. Crit Care Med. 2006 doi: 10.1097/01.CCM.0000206309.83570.45. Publish Ahead of Print. [DOI] [PubMed] [Google Scholar]
- 45.Arendash GW, Jensen MT, Salem N, Jr, Hussein N, Cracchiolo J, Dickson A, Leighty R, Potter H. A diet high in omega-3 fatty acids does not improve or protect cognitive performance in Alzheimer's transgenic mice. Neuroscience. 2007;149:286–302. doi: 10.1016/j.neuroscience.2007.08.018. [DOI] [PubMed] [Google Scholar]
- 46.Ma QL, Teter B, Ubeda OJ, Morihara T, Dhoot D, Nyby MD, Tuck ML, Frautschy SA, Cole GM. Omega-3 fatty acid docosahexaenoic acid increases SorLA/LR11, a sorting protein with reduced expression in sporadic Alzheimer's disease (AD): relevance to AD prevention. J Neurosci. 2007;27:14299–307. doi: 10.1523/JNEUROSCI.3593-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sager KL, Wuu J, Leurgans SE, Rees HD, Gearing M, Mufson EJ, Levey AI, Lah JJ. Neuronal LR11/sorLA expression is reduced in mild cognitive impairment. Ann Neurol. 2007;62:640–7. doi: 10.1002/ana.21190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rogaeva E, Meng Y, Lee JH, Gu Y, Kawarai T, Zou F, Katayama T, Baldwin CT, Cheng R, Hasegawa H, Chen F, Shibata N, Lunetta KL, Pardossi-Piquard R, Bohm C, Wakutani Y, Cupples LA, Cuenco KT, Green RC, Pinessi L, Rainero I, Sorbi S, Bruni A, Duara R, Friedland RP, Inzelberg R, Hampe W, Bujo H, Song YQ, Andersen OM, Willnow TE, Graff-Radford N, Petersen RC, Dickson D, Der SD, Fraser PE, Schmitt-Ulms G, Younkin S, Mayeux R, Farrer LA, St George-Hyslop P. The neuronal sortilin-related receptor SORL1 is genetically associated with Alzheimer disease. Nat Genet. 2007;39:168–77. doi: 10.1038/ng1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Meng Y, Lee JH, Cheng R, St George-Hyslop P, Mayeux R, Farrer LA. Association between SORL1 and Alzheimer's disease in a genome-wide study. Neuroreport. 2007;18:1761–4. doi: 10.1097/WNR.0b013e3282f13e7a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lee JH, Cheng R, Schupf N, Manly J, Lantigua R, Stern Y, Rogaeva E, Wakutani Y, Farrer L, St George-Hyslop P, Mayeux R. The association between genetic variants in SORL1 and Alzheimer disease in an urban, multiethnic, community-based cohort. Arch Neurol. 2007;64:501–6. doi: 10.1001/archneur.64.4.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lee JH, Cheng R, Honig LS, Vonsattel JP, Clark L, Mayeux R. Association between genetic variants in SORL1 and autopsy-confirmed Alzheimer disease. Neurology. 2008;70:887–9. doi: 10.1212/01.wnl.0000280581.39755.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ma QL, Galasko DR, Ringman JM, Vinters HV, Edland SD, Pomakian J, Ubeda OJ, Rosario ER, Teter B, Frautschy SA, Cole GM. Reduction of SorLa/LR11, a sorting protein limiting beta-amyloid production, in Alzheimer Disease Cerebrospinal Fluid. Arch Neurol. 2009;66:1–10. doi: 10.1001/archneurol.2009.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Puskas LG, Kitajka K, Nyakas C, Barcelo-Coblijn G, Farkas T. Short-term administration of omega 3 fatty acids from fish oil results in increased transthyretin transcription in old rat hippocampus. Proc Natl Acad Sci U S A. 2003 doi: 10.1073/pnas.0337683100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhao L, Teter B, Morihara T, Lim GP, Ambegaokar SS, Ubeda OJ, Frautschy SA, Cole GM. Insulin-degrading enzyme as a downstream target of insulin receptor signaling cascade: implications for Alzheimer's disease intervention. J Neurosci. 2004;24:11120–6. doi: 10.1523/JNEUROSCI.2860-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Florent S, Malaplate-Armand C, Youssef I, Kriem B, Koziel V, Escanye MC, Fifre A, Sponne I, Leininger-Muller B, Olivier JL, Pillot T, Oster T. Docosahexaenoic acid prevents neuronal apoptosis induced by soluble amyloid-beta oligomers. J Neurochem. 2006;96:385–95. doi: 10.1111/j.1471-4159.2005.03541.x. [DOI] [PubMed] [Google Scholar]
- 56.Ma QL, Yang F, Calon F, Ubeda OJ, Hansen JE, Weisbart RH, Beech W, Frautschy SA, Cole GM. p21-activated kinase-aberrant activation and translocation in Alzheimer disease pathogenesis. J Biol Chem. 2008;283:14132–43. doi: 10.1074/jbc.M708034200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Counts SE, Nadeem M, Lad SP, Wuu J, Mufson EJ. Differential expression of synaptic proteins in the frontal and temporal cortex of elderly subjects with mild cognitive impairment. J Neuropathol Exp Neurol. 2006;65:592–601. doi: 10.1097/00005072-200606000-00007. [DOI] [PubMed] [Google Scholar]
- 58.Yang DY, Pan HC, Yen YJ, Wang CC, Chuang YH, Chen SY, Lin SY, Liao SL, Raung SL, Wu CW, Chou MC, Chiang AN, Chen CJ. Detrimental effects of post-treatment with fatty acids on brain injury in ischemic rats. Neurotoxicology. 2007;28:1220–9. doi: 10.1016/j.neuro.2007.08.003. [DOI] [PubMed] [Google Scholar]
- 59.Gamblin TC, King ME, Kuret J, Berry RW, Binder LI. Oxidative regulation of fatty acid-induced tau polymerization. Biochemistry. 2000;39:14203–14210. doi: 10.1021/bi001876l. [DOI] [PubMed] [Google Scholar]
- 60.Long EK, Murphy TC, Leiphon LJ, Watt J, Morrow JD, Milne GL, Howard JR, Picklo MJ., Sr Trans-4-hydroxy-2-hexenal is a neurotoxic product of docosahexaenoic (22:6; n-3) acid oxidation. J Neurochem. 2008;105:714–24. doi: 10.1111/j.1471-4159.2007.05175.x. [DOI] [PubMed] [Google Scholar]
- 61.Gylys KH, Fein JA, Yang F, Wiley DJ, Miller CA, Cole GM. Synaptic changes in Alzheimer's disease: increased amyloid-beta and gliosis in surviving terminals is accompanied by decreased PSD-95 fluorescence. Am J Pathol. 2004;165:1809–17. doi: 10.1016/s0002-9440(10)63436-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Brenna JT, Diau GY. The influence of dietary docosahexaenoic acid and arachidonic acid on central nervous system polyunsaturated fatty acid composition. Prostaglandins Leukot Essent Fatty Acids. 2007;77:247–50. doi: 10.1016/j.plefa.2007.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cole GM, Frautschy SA. Docosahexaenoic acid protects from amyloid and dendritic pathology in an Alzheimer's disease mouse model. Nutr Health. 2006;18:249–59. doi: 10.1177/026010600601800307. [DOI] [PubMed] [Google Scholar]
- 64.Kotani S, Sakaguchi E, Warashina S, Matsukawa N, Ishikura Y, Kiso Y, Sakakibara M, Yoshimoto T, Guo J, Yamashima T. Dietary supplementation of arachidonic and docosahexaenoic acids improves cognitive dysfunction. Neurosci Res. 2006;56:159–64. doi: 10.1016/j.neures.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 65.Freund-Levi Y, Eriksdotter-Jonhagen M, Cederholm T, Basun H, Faxen-Irving G, Garlind A, Vedin I, Vessby B, Wahlund LO, Palmblad J. Omega-3 fatty acid treatment in 174 patients with mild to moderate Alzheimer disease: OmegAD study: a randomized double-blind trial. Arch Neurol. 2006;63:1402–8. doi: 10.1001/archneur.63.10.1402. [DOI] [PubMed] [Google Scholar]
- 66.Chiu CC, Su KP, Cheng TC, Liu HC, Chang CJ, Dewey ME, Stewart R, Huang SY. The effects of omega-3 fatty acids monotherapy in Alzheimer's disease and mild cognitive impairment: a preliminary randomized double-blind placebo-controlled study. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:1538–44. doi: 10.1016/j.pnpbp.2008.05.015. [DOI] [PubMed] [Google Scholar]
- 67.Shinto L, Quinn J, Montine T, Baldauf-Wagner S, B Oken DB, Kaye J. Omega-3 Fatty Acids and Lipoic Acid in Alzheimer's Disease. Neurology. 2008;70:A393. doi: 10.3233/JAD-130722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Harris WS, Kris-Etherton PM, Harris KA. Intakes of long-chain omega-3 fatty acid associated with reduced risk for death from coronary heart disease in healthy adults. Curr Atheroscler Rep. 2008;10:503–9. doi: 10.1007/s11883-008-0078-z. [DOI] [PubMed] [Google Scholar]
- 69.Griffin BA. How relevant is the ratio of dietary n-6 to n-3 polyunsaturated fatty acids to cardiovascular disease risk? Evidence from the OPTILIP study. Curr Opin Lipidol. 2008;19:57–62. doi: 10.1097/MOL.0b013e3282f2e2a8. [DOI] [PubMed] [Google Scholar]
- 70.Kalmijn S, Feskens E, Launer L, Kromhout D. Polyunsaturated fatty acids, antioxidants and cognitive function in very old men. American Journal of Epidemiology. 1997;145:33–41. doi: 10.1093/oxfordjournals.aje.a009029. [DOI] [PubMed] [Google Scholar]
- 71.Kalmijn S, Launer LJ, Ott A, Witteman JC, Hofman A, Breteler MM. Dietary fat intake and the risk of incident dementia in the Rotterdam Study. Ann Neurol. 1997;42:776–82. doi: 10.1002/ana.410420514. [DOI] [PubMed] [Google Scholar]
- 72.Morris MC, Evans DA, Bienias JL, Tangney CC, Bennett DA, Wilson RS, Aggarwal N, Schneider J. Consumption of fish and n-3 fatty acids and risk of incident Alzheimer disease. Arch Neurol. 2003;60:940–6. doi: 10.1001/archneur.60.7.940. [DOI] [PubMed] [Google Scholar]
- 73.Kalmijn S, van Boxtel MP, Ocke M, Verschuren WM, Kromhout D, Launer LJ. Dietary intake of fatty acids and fish in relation to cognitive performance at middle age. Neurology. 2004;62:275–80. doi: 10.1212/01.wnl.0000103860.75218.a5. [DOI] [PubMed] [Google Scholar]
- 74.Morris MC, Evans DA, Tangney CC, Bienias JL, Wilson RS. Fish consumption and cognitive decline with age in a large community study. Arch Neurol. 2005;62:1849–53. doi: 10.1001/archneur.62.12.noc50161. [DOI] [PubMed] [Google Scholar]
- 75.Huang TL, Zandi PP, Tucker KL, Fitzpatrick AL, Kuller LH, Fried LP, Burke GL, Carlson MC. Benefits of fatty fish on dementia risk are stronger for those without APOE epsilon4. Neurology. 2005;65:1409–14. doi: 10.1212/01.wnl.0000183148.34197.2e. [DOI] [PubMed] [Google Scholar]
- 76.Nurk E, Drevon CA, Refsum H, Solvoll K, Vollset SE, Nygard O, Nygaard HA, Engedal K, Tell GS, Smith AD. Cognitive performance among the elderly and dietary fish intake: the Hordaland Health Study. Am J Clin Nutr. 2007;86:1470–8. doi: 10.1093/ajcn/86.5.1470. [DOI] [PubMed] [Google Scholar]
- 77.Barberger-Gateau P, Raffaitin C, Letenneur L, Berr C, Tzourio C, Dartigues JF, Alperovitch A. Dietary patterns and risk of dementia: the Three-City cohort study. Neurology. 2007;69:1921–30. doi: 10.1212/01.wnl.0000278116.37320.52. [DOI] [PubMed] [Google Scholar]
- 78.van Gelder BM, Tijhuis M, Kalmijn S, Kromhout D. Fish consumption, n-3 fatty acids, and subsequent 5-y cognitive decline in elderly men: the Zutphen Elderly Study. Am J Clin Nutr. 2007;85:1142–7. doi: 10.1093/ajcn/85.4.1142. [DOI] [PubMed] [Google Scholar]
- 79.Conquer JA, Tierney MC, Zecevic J, Bettger WJ, Fisher RH. Fatty acid analysis of blood plasma of patients with Alzheimer's disease, other types of dementia, and cognitive impairment. Lipids. 2000;35:1305–1312. doi: 10.1007/s11745-000-0646-3. [DOI] [PubMed] [Google Scholar]
- 80.Heude B, Ducimetiere P, Berr C. Cognitive decline and fatty acid composition of erythrocyte membranes--The EVA Study. Am J Clin Nutr. 2003;77:803–8. doi: 10.1093/ajcn/77.4.803. [DOI] [PubMed] [Google Scholar]
- 81.Laurin D, Verreault R, Lindsay J, Dewailly E, Holub BJ. Omega-3 fatty acids and risk of cognitive impairment and dementia. J Alzheimers Dis. 2003;5:315–22. doi: 10.3233/jad-2003-5407. [DOI] [PubMed] [Google Scholar]
- 82.Dullemeijer C, Durga J, Brouwer IA, van de Rest O, Kok FJ, Brummer RJ, van Boxtel MP, Verhoef P. n 3 fatty acid proportions in plasma and cognitive performance in older adults. Am J Clin Nutr. 2007;86:1479–85. doi: 10.1093/ajcn/86.5.1479. [DOI] [PubMed] [Google Scholar]
- 83.Beydoun MA, Kaufman JS, Satia JA, Rosamond W, Folsom AR. Plasma n-3 fatty acids and the risk of cognitive decline in older adults: the Atherosclerosis Risk in Communities Study. Am J Clin Nutr. 2007;85:1103–11. doi: 10.1093/ajcn/85.4.1103. [DOI] [PubMed] [Google Scholar]
- 84.Cherubini A, Andres-Lacueva C, Martin A, Lauretani F, Iorio AD, Bartali B, Corsi A, Bandinelli S, Mattson MP, Ferrucci L. Low plasma N-3 fatty acids and dementia in older persons: the InCHIANTI study. J Gerontol A Biol Sci Med Sci. 2007;62:1120–6. doi: 10.1093/gerona/62.10.1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Tully AM, Roche HM, Doyle R, Fallon C, Bruce I, Lawlor B, Coakley D, Gibney MJ. Low serum cholesteryl ester-docosahexaenoic acid levels in Alzheimer's disease: a case-control study. Br J Nutr. 2003;89:483–489. doi: 10.1079/BJN2002804. [DOI] [PubMed] [Google Scholar]
- 86.Tassoni D, Kaur G, Weisinger RS, Sinclair AJ. The role of eicosanoids in the brain. Asia Pac J Clin Nutr. 2008;17(1):220–8. [PubMed] [Google Scholar]
- 87.Rao JS, Ertley RN, DeMar JC, Jr, Rapoport SI, Bazinet RP, Lee HJ. Dietary n-3 PUFA deprivation alters expression of enzymes of the arachidonic and docosahexaenoic acid cascades in rat frontal cortex. Mol Psychiatry. 2007;12:151–7. doi: 10.1038/sj.mp.4001887. [DOI] [PubMed] [Google Scholar]
- 88.Cao D, Yang B, Hou L, Xu J, Xue R, Sun L, Zhou C, Liu Z. Chronic daily administration of ethyl docosahexaenoate protects against gerbil brain ischemic damage through reduction of arachidonic acid liberation and accumulation. J Nutr Biochem. 2007;18:297–304. doi: 10.1016/j.jnutbio.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 89.Wu A, Ying Z, Gomez-Pinilla F. Dietary omega-3 fatty acids normalize BDNF levels, reduce oxidative damage, and counteract learning disability after traumatic brain injury in rats. J Neurotrauma. 2004;21:1457–67. doi: 10.1089/neu.2004.21.1457. [DOI] [PubMed] [Google Scholar]
- 90.Yavin E, Brand A, Green P. Docosahexaenoic acid abundance in the brain: a biodevice to combat oxidative stress. Nutr Neurosci. 2002;5:149–57. doi: 10.1080/10284150290003159. [DOI] [PubMed] [Google Scholar]
- 91.Hashimoto M, Tanabe Y, Fujii Y, Kikuta T, Shibata H, Shido O. Chronic administration of docosahexaenoic acid ameliorates the impairment of spatial cognition learning ability in amyloid beta-infused rats. J Nutr. 2005;135:549–55. doi: 10.1093/jn/135.3.549. [DOI] [PubMed] [Google Scholar]
- 92.Hossain MS, Hashimoto M, Gamoh S, Masumura S. Antioxidative effects of docosahexaenoic acid in the cerebrum versus cerebellum and brainstem of aged hypercholesterolemic rats. J Neurochem. 1999;72:1133–1138. doi: 10.1046/j.1471-4159.1999.0721133.x. [DOI] [PubMed] [Google Scholar]
- 93.Lukiw WJ, Cui JG, Marcheselli VL, Bodker M, Botkjaer A, Gotlinger K, Serhan CN, Bazan NG. A role for docosahexaenoic acid-derived neuroprotectin D1 in neural cell survival and Alzheimer disease. J Clin Invest. 2005;115:2774–83. doi: 10.1172/JCI25420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Innis SM. Dietary (n-3) fatty acids and brain development. J Nutr. 2007;137:855–9. doi: 10.1093/jn/137.4.855. [DOI] [PubMed] [Google Scholar]
- 95.Tsukada H, Kakiuchi T, Fukumoto D, Nishiyama S, Koga K. Docosahexaenoic acid (DHA) improves the age-related impairment of the coupling mechanism between neuronal activation and functional cerebral blood flow response: a PET study in conscious monkeys. Brain Res. 2000;862:180–6. doi: 10.1016/s0006-8993(00)02115-6. [DOI] [PubMed] [Google Scholar]
- 96.Pifferi F, Jouin M, Alessandri JM, Haedke U, Roux F, Perriere N, Denis I, Lavialle M, Guesnet P. n-3 Fatty acids modulate brain glucose transport in endothelial cells of the blood-brain barrier. Prostaglandins Leukot Essent Fatty Acids. 2007;77:279–86. doi: 10.1016/j.plefa.2007.10.011. [DOI] [PubMed] [Google Scholar]
- 97.Hashimoto M, Hossain S, Shimada T, Shido O. Docosahexaenoic acid-induced protective effect against impaired learning in amyloid beta-infused rats is associated with increased synaptosomal membrane fluidity. Clin Exp Pharmacol Physiol. 2006;33:934–9. doi: 10.1111/j.1440-1681.2006.04467.x. [DOI] [PubMed] [Google Scholar]
- 98.Litman BJ, Niu SL, Polozova A, Mitchell DC. The role of docosahexaenoic acid containing phospholipids in modulating G protein-coupled signaling pathways: visual transduction. J Mol Neurosci. 2001;16:237–242. doi: 10.1385/JMN:16:2-3:237. discussion 279-84. [DOI] [PubMed] [Google Scholar]
- 99.Gani OA, Sylte I. Molecular recognition of Docosahexaenoic acid by peroxisome proliferator-activated receptors and retinoid-X receptor alpha. J Mol Graph Model. 2008 doi: 10.1016/j.jmgm.2008.04.008. [DOI] [PubMed] [Google Scholar]
- 100.Sahlin C, Pettersson FE, Nilsson LN, Lannfelt L, Johansson AS. Docosahexaenoic acid stimulates non-amyloidogenic APP processing resulting in reduced Abeta levels in cellular models of Alzheimer's disease. Eur J Neurosci. 2007;26:882–9. doi: 10.1111/j.1460-9568.2007.05719.x. [DOI] [PubMed] [Google Scholar]
- 101.Lim GP, Calon F, Morihara T, Yang F, Teter B, Ubeda O, Salem N, Jr, Frautschy SA, Cole GM. A diet enriched with the omega-3 fatty acid docosahexaenoic acid reduces amyloid burden in an aged Alzheimer mouse model. J Neurosci. 2005;25:3032–40. doi: 10.1523/JNEUROSCI.4225-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hooijmans CR, Rutters F, Dederen PJ, Gambarota G, Veltien A, van Groen T, Broersen LM, Lutjohann D, Heerschap A, Tanila H, Kiliaan AJ. Changes in cerebral blood volume and amyloid pathology in aged Alzheimer APP/PS1 mice on a docosahexaenoic acid (DHA) diet or cholesterol enriched Typical Western Diet (TWD) Neurobiol Dis. 2007;28:16–29. doi: 10.1016/j.nbd.2007.06.007. [DOI] [PubMed] [Google Scholar]
- 103.Berg B, Perez S, Lazarov O, Nadeem M, Sisodia S, Moore K, Mufson E. The dietary omega-3 fatty acid DHA reduces cortical and striatal β-amyloid load in APPswe/PS1ΔE9 transgenic mice. Society for Neuroscience. 2007;37 Abs. 857.6. [Google Scholar]
- 104.Van de Rest O, Geleijnse J, Kok F, van Staveren W, Dullemeijer C, OldeRikkert M, Beekman A, de Groot L. Effect of fish oil on cognitive performance in older subjects: a randomized controlled trial. Neurology. 2008;(71):430–8. doi: 10.1212/01.wnl.0000324268.45138.86. [DOI] [PubMed] [Google Scholar]
- 105.Dangour AD, Clemens F, Elbourne D, Fasey N, Fletcher AE, Hardy P, Holder GE, Huppert FA, Knight R, Letley L, Richards M, Truesdale A, Vickers M, Uauy R. A randomised controlled trial investigating the effect of n-3 long-chain polyunsaturated fatty acid supplementation on cognitive and retinal function in cognitively healthy older people: the Older People And n-3 Long-chain polyunsaturated fatty acids (OPAL) study protocol [ISRCTN72331636] Nutr J. 2006;5:20. doi: 10.1186/1475-2891-5-20. [DOI] [PMC free article] [PubMed] [Google Scholar]


