Abstract
Objective
Lung contusion injury produces a vulnerable window within the inflammatory defenses of the lung that predisposes the patient to pneumonia. IL-10 is a known anti-inflammatory mediator produced by macrophages and capable of down-regulating acute lung inflammation. We investigated the impact of increased levels of IL-10 within the lung on survival and the host response to trauma in the setting of lung contusion and Gram-negative pneumonia.
Design
A bi-transgenic, tetracycline inducible, lung specific human IL-10 overexpression (IL-10 OE) mouse model and single transgenic (TG-) control mice were used. Mice underwent lung contusion injury (LC) or sham injury (Sham) at time -6 hrs. At time 0 animals were inoculated intratracheally with 500 CFU of Klebsiella pneumoniae (Pneu). Bronchoalveolar lavage fluid (BAL), lung tissue specimens, or purified macrophages were collected. Lung tissue and blood bacteria levels were quantified. Cytokine levels were assayed by ELISA and gene expression levels were evaluated by real time PCR. Cell type identification and quantification was done using real time PCR and flow cytometry.
Main Results
IL-10 OE mice demonstrated decreased 5 day survival compared to TG-mice following LC+Pneu (0 vs. 30%, p<0.0001). IL-10 OE mice had significantly higher lung bacteria counts (p=0.02) and levels of bacteremia (p=0.001) at 24 hrs. The IL-10 OE mice recruited more neutrophils into the alveoli as measured in BAL fluid compared to TG- mice. Alveolar macrophages from IL-10 OE mice displayed increased alternative activation (M2 macrophages, p=0.046) whereas macrophages from TG- mice exhibited classical activation (M1 macrophages) and much higher intracellular bacterial killing potential (p=0.03). IL-6, KC, and MIP-2 levels were significantly elevated in IL-10 OE LC+Pneu animals (p<0.05).
Conclusions
Lung specific IL-10 over expression induces alternative activation of alveolar macrophages. This shift in macrophage phenotype decreases intracellular bacterial killing, resulting in a more pronounced bacteremia and accelerated mortality in a model of lung contusion and pneumonia.
Keywords: Chest trauma, lung contusion, inflammation, pneumonia
INTRODUCTION
Thoracic trauma, including lung contusion, places patients at risk for pneumonia, acute respiratory distress syndrome, and multiple organ system dysfunction (1, 2). Trauma patients who develop a respiratory complication have a substantial increase in their hospital length of stay and hospital costs (3) Thus, there is an imperative to better understand the mechanism(s) by which thoracic trauma impacts the host immune response. It is known that, in the setting of sepsis, alterations in the pro- and anti-inflammatory equilibrium can affect the subsequent acute reaction to pathogens within the lung. Alveolar macrophages became deactivated in a cecal ligation and puncture model of sepsis and IL-10 was determined to play a key role in this mechanism (4). Administration of a monoclonal antibody to IL-10 just prior to pulmonary Pseudomonas aeruginosa challenge in these septic mice significantly improved both survival and clearance of bacteria from the lungs (4).
The relationship between IL-10 and macrophage phenotype in the context of lung contusion injury and bacterial infection has not been fully investigated. IL-10 is known to suppress macrophage activity by inhibiting the production of interferon gamma, IL-2, IL-12, and IL-18 (5). Modulation of the inflammatory response is essential to preserve balance within the immune system. IL-10 can attenuate the exuberant production of proinflammatory cytokines in the septic state of systemic immune activation (6). However, IL-10 mediated suppression of acute pro-inflammatory cytokines may be unfavorable to the host’s ability to clear bacterial pneumonia. For example, in the setting of Klebsiella sp. pneumonia, systemic blockade of IL-10 using a monoclonal antibody attenuated mortality and resulted in a significant reduction in bacteria presence in the lung and plasma (7). This suggests that endogenous IL-10 is an important mediator of the pro-inflammatory response and acts to balance bacterial clearance with downregulation of the immune system to prevent iatrogenic damage to the host.
Utilizing an in-vivo model of lung specific over expression of IL-10, we were able to investigate the precise role of IL-10 in a model of lung contusion and pneumonia. Lung specific IL-10 over expression was achieved using a transgenic mouse capable of regulatable IL-10 expression in the presence of doxycycline. These mice were created in an FVB/n genetic background and have been characterized previously (8). The bi-transgenic mice (IL10 OE) possess a reverse tetracycline transactivator under the control of a Clara cell secretory protein promoter and a tetracycline-dependent, CMV promoter-driven IL-10 human transgene (tet-O-CMV-IL-10) that allows drug regulated and lung specific overexpression of human IL-10 (hIL-10). Access to mice chow containing 0.0625% doxycycline results in rapid (within 24 hours) hIL-10 over-expression that peaks by day 5 and is limited to the lungs of mice possessing both transgenes. A single transgene mouse (TG-), containing only one of the two active transgenes (tet-O-CMV-IL-10), served as the control. A TG- mouse given access to doxycycline chow does not express hIL-10. IL-OE mice provided standard rather than doxycycline chow also do not express hIL-10 (i.e. no transgene leak is present).
Human IL-10 shares 73% homology with the amino acid sequence of murine IL-10 (mIL-10). hIL-10 is recognized by mouse IL-10 receptors (9). An advantage of using hIL-10 in our model is that it allows for differentiation/identification of the over expressed lung specific hIL-10 from endogenous mIL-10 while preserving the local and systemic effects of both proteins. We employed the bi-transgene model to study how IL-10 influences the ratio of pulmonary M1/M2 macrophages, alters bacterial clearance, and affects mice survival following lung contusion injury and subsequent Gram-negative pneumonia.
MATERIALS AND METHODS
Animals
Age and gender matched mice were used in all experiments. Tetracycline-inducible and lung specific human IL-10 overexpression-transgenic (IL-10 OE) mice were obtained and maintained in a breeding colony. These bi-transgenic mice were bred in an FVB/n genetic background and have been characterized previously (8). The mice possess a reverse tetracycline transactivator under the control of a Clara cell secretory protein promoter and a tetracycline-dependent, CMV promoter-driven IL-10 human transgene (tet-O-CMV-IL-10) that allows drug regulated and lung specific overexpression of human IL-10 (hIL-10). Access to mice chow containing 0.0625% doxycycline (TestDiet, Purina, Richmond, IN) results in rapid (within 24 hours) hIL-10 overexpression limited to the lungs of mice possessing both transgenes. A single transgene mouse (TG-), containing only one of the two active transgenes (tet-O-CMV-IL-10), served as the control. A TG- mouse given access to doxycycline chow does not express hIL-10. IL-OE mice provided standard rather than doxycycline chow also do not express hIL-10 (i.e. no transgene leak is present). To achieve maximal expression of hIL-10 IL-10 OE mice were fed doxycycline chow for 5 days prior to use in experiments [9]. TG- mice were also fed doxycycline chow for 5 days before use. An additional control was utilized in some experiments by maintaining the IL-10 OE mice and TG- mice on standard chow. Mice were housed under specific pathogen-free conditions and were age matched prior to being used in experiments. All experiments were performed in accordance with National Institutes of Health guidelines for care and use of animals. Approval for the experimental protocol was obtained from the University of Michigan Committee on Use and Care of Animals.
Lung Contusion Injury
At time -6 hours a blunt lung contusion injury (LC) was created using a cortical contusion impactor apparatus (10, 11). Under ketamine anesthesia fur was clipped from the planned contusion area and mice were fixed onto an adjustable height table. After correct positioning, the right chest of the mouse is struck by the cortical contusion impactor along the posterior axillary line 1 cm above the costal margin using a velocity of 5.8 m/sec and a depth of 10 mm. Immediately after contusion all animals received buprenorphine to avoid post-treatment discomfort. Mice were then allowed to recover spontaneously. Sham lung contusion (Sham) animals underwent all of the interventions outlined above except actually being stuck by the contusion apparatus.
Pneumonia Model
K. pneumoniae, strain 43816, serotype 2 was purchased from American Type Culture Collection, Manassas, VA. This microbial strain was cultured in trypticase soy broth (Becton Dickinson, Franklin Lakes, NJ) with reinoculation the following morning into fresh medium to bring the bacteria into log growth phase. The bacteria were centrifuged at 600 g at 4°C for 10 minutes, washed with sterile 0.9% saline, centrifuged again, and then resuspended in sterile saline. Optical density was read on a spectrophotometer (Milton Roy, Rochester, N.Y.) at a wavelength of 620 nm. Once an optical density of 1.4 was obtained, appropriate serial dilutions were subsequently made to achieve a concentration of 500 CFU’s of bacteria per 30 µL of inoculum, the LD50 for TG- mice using this strain of Klebsiella. At time 0 (6 hours after lung contusion injury or sham lung contusion injury) mice were inoculated with 30 µL of the bacterial suspension (Pneu) via sterile cutdown and intratracheal injection under isoflurane anesthesia.
Bronchoalveolar Lavage
At selected times post lung contusion or post inoculation, mice were euthanized and a tracheal cutdown performed. The lungs were lavaged with 1.5 mL of sterile saline using a syringe, angiocatheter, and three way stopcock apparatus. For cytospin cell preparation, 0.1 mL of the bronchoalveolar lavage (BAL) samples were centrifuged at 400 g for 5 minutes at room temperature using Cytospin II (Shandon Scientific, Pittsburg, PA) and stained with Diff-Quick (Dade Behring Inc., Newark, DE). The remainder of the each BAL sample was centrifuged at 400 g for 8 minutes. Following centrifugation the supernatant was separated from the cells and these samples were stored frozen at −80°C until use. Spun down cells were resuspended in appropriate buffer and used in the flow cytometry or neutrophil sequestration assay (Myeloperoxidase assay, MPO). BAL samples were also collected from mice that underwent the same handling but were not subjected to lung contusion or intratracheal injection of bacteria. These samples were used to obtain baseline values.
Lung Homogenate and Quantification of Lung Bacteria
The thoracic cavity was opened, and lungs flushed of blood with 2 mL of 0.9% sterile saline. Both lungs were removed and homogenized in 1 mL of 0.9% sterile saline. Serial 10-fold dilutions were made in 0.9% sterile saline and 100 µL volumes of homogenate were plated on 5% blood agar plates (Thermo Fisher Scientific, Remel Products, Lenexa, KS). Plates were incubated overnight at 37°C and colony forming units (CFU’s) counted after 16 hours. The remaining volumes of lung homogenate were centrifuged at 3000 g for 15 minutes at 4°C. Supernatants were collected and diluted 1:1 in lysis buffer (1× PBS, 1% Triton X-100, 1 tablet Complete X protease inhibitor [Roche, Indianapolis, IN], pH 7.4). Samples were stored at −80°C until use.
Quantification of Blood Bacteria
Blood was obtained via cardiac puncture using a sterile 18-gauge needle. To measure bacteria titers in blood, 100 uL of undiluted blood was plated onto 5% sheep blood agar plates. Plates were incubated overnight at 37°C and colony forming units (CFU’s) counted after 16 hours.
Detection of Neutrophil Myeloperoxidase activity
100 mg of lung tissue was mechanically homogenized in 1 mL ice cold potassium phosphate buffer consisting of 115 mM monobasic potassium phosphate (Sigma Aldrich, Milwaukee, WI). Homogenates were centrifuged at 3000g for 10 min at 4°C, the supernatants were removed and the pellets were re-suspended in 1 mL C-TAB buffer consisting of dibasic potassium phosphate, cetyltrimethylammonium bromide, and acetic acid (Sigma Aldrich, Milwaukee, WI). The suspensions were sonicated (Branson Sonifier 250, Danbury, CT) on ice for 40 seconds. Homogenates were centrifuged at 3000g for 10 min at 4°C and the supernatant collected. Supernatants were incubated in 60°C water bath for 2 hours (Shaker Bath, 2568; Forma Scientific, Marietta, OH). Samples were stored at −80°C until needed or assayed immediately.
20 µL standards (Calbiochem, Gibbstown, NJ) or samples were added to a 96-well immunosorbent micro-plates (NUNC, Rochester, NY), followed by the addition of 155 µL of 20mM TMB/DMF consisting of 3,3`,5,5`-tetramethylbenzidine/N,N-dimethylformamide in 115 mM potassium phosphate buffer (Fischer Scientific, Pittsburgh, PA) to each well. The samples were mixed well, after which 20 µL of 3 mM H2O2 was rapidly added to each well. The reaction was stopped immediately by adding 50 µL/well of 0.061 mg/mL Catalase (Roche, Indianapolis, IN). The plates were read using a microplate reader at 620 nm. Myeloperoxidase (MPO) concentrations were calculated using a linear standard curve and adjusted for previous dilution. The final concentrations were expressed as µg/mL.
Isolation of BAL and Lung Macrophages for Real Time PCR
Mouse alveolar macrophages were purified from individual BAL or lung tissue samples and integrity determined by nuclear and cytoplasmic DiffQuick staining and verified by flow cytometry. 1) BAL: macrophages were purified by plastic adherence for 40 minutes at 37°C. Free floating cells were removed by washing and adherent cells were used for the study. 2) Lung tissue: whole tissue were minced into a fine slurry and digested using 1mg/mL of collagenase A (Roche Applied Science, Indianapolis, IN) in alpha-MEM media containing 7.5% fetal calf serum (Gibco /Invitrogen, Grand Island, NY) and 2 units/mL of DNAse (Roche Applied Science, Indianapolis, IN) for 40 minutes at 37°C with constant agitation (85 rpm). The resultant digest was further processed by 10 passes through an18-gauge needle and filtered using 40 µm cell-strainer. This single cell suspension of lung tissue was plated and macrophages isolated by plastic adherence for 40 minutes at 37°C. Free floating cells were removed by washing and adherent cells were used for the study. RNA was isolated from the upper right lobes of lung using TRIzol (Ambion, Invitrogen, Carlsbad, CA). Levels of mRNA for Arginase 1, nitric oxide synthase 2 (inducible) (NOS2), interferon gamma (IFN-γ), interleukin 1 beta (IL-1β), and interleukin 10 (IL-10) were assessed using quantitative PCR analysis (TaqMan®) with predeveloped primers and probe sets (Applied Biosystems, Carlsbad, CA). Quantification of the genes of interest were normalized to GAPDH and expressed as fold increases over the negative control for each treatment at each time point.
Quantification of Lung Macrophage Intracellular Bacteria
Lung macrophages were purified as described above. Cells were plated for 40 minutes, washed three times with PBS and one set of samples lysed with 0.5% Triton X-100 (12). Serial 10-fold dilutions were made and the cell lysates samples plated on 5% blood agar plates. Plates were incubated for 16 hours at 37°C and colony forming units (CFU’s) counted. A second set of purified macrophages were used to assess expression of the Klebsiella pneumoniae specific hemolysin gene, khe (Accession: AF293352.1). This gene encodes a unique 20 kDa peptide that is present in all strains of K. Pneumoniae (13). Lung macrophage mRNA was isolated and processed as described above. Primers for khe were obtained (Integrated DNA Technologies, Inc, San Diego, CA) and levels of mRNA quantitated using TaqMan PCR analysis. Quantification of khe gene expression was normalized to GAPDH levels and expressed as fold increase over the negative control.
Cytokine, Chemokine, and Albumin Analysis (ELISA)
Cytokine, chemokine, and albumin levels were measured in BAL supernatant using prefabricated ELISA kits (murine IL-6, IL-10, keratinocyte-derived chemokine (KC) and macrophage inflammatory protein (MIP-2)) according to manufacturer’s protocol (R&D Systems, Inc, Minneapolis, MN). A separate kit was obtained to specifically measure levels of hIL-10 in samples exclusive of murine IL-10. Levels of albumin in the BAL sample were assayed using an ELISA kit (Bethyl Laboratories, Inc., Montgomery, TX). Plates were read using a microplate reader (Biotek Instruments, Winooski, VT) at 450 and 540 nm. Concentrations were calculated using an 8-point standard curve and are expressed as pg/mL (cytokines) or µg/mL (albumin).
Flow Cytometry
BAL samples were collected, red blood cells lysed, and cells counted using a hemacytometer. Lung tissue was digested for 1 hour at 37°C with 1 mg/mL of collagenase A (Roche). The resulting single cell suspension was filtered and washed, red blood cells lysed, and cells counted. A LIVE/DEAD (Invitrogen, Carlsbad, CA) fixable stain was added and the cells incubated for 20 minutes at room temperature while removed from exposure to light. After appropriate washing in flow buffer (PBS+1%FCS) and blocking with Fc block (CD16/32) cells were divided into 2 sets and 1×106 cells were surface-stained with the following fluorochrome conjugated mouse antibodies: Gr-1-PE, CD11c-APC-Cy7, F4/80-AF488, CD11b-PE-Cy7, and CD206-APC (BioLegend and BD Biosciences, San Jose, CA). Stained cells were then washed and fixed with 1% formalin for 20 minutes at room temperature. Following two final washes, flow-cytometric analysis was performed using a BD LSR II flow cytometer (BD Biosciences). Obtained data were analyzed using FlowJo software (Tree Star, Inc., Ashland, OR).
Histology
Fresh lung tissue samples were fixed in 10% buffered formalin and embedded in paraffin. Sections 4-µm thick were sliced and affixed to slides, deparaffinized, and stained with hematoxylin and eosin to assess morphological changes. Lung histology samples were scored using the following system: distribution of cellular infiltrate (0=none, 1=focal, 2= multifocal, 3=locally extensive, 4=multifocal and locally extensive, 5=diffuse), inflammation severity (0=none, 1=mild, 2= moderate, 3=severe), infiltrate type (0=none, 1=acute, 2= subacute, 3=chronic), necrosis (0=none, 1=minimal, 2=moderate, 3= severe). A final score was computed by summing the scores in each subdivision.
Statistical Methods
All statistical analysis and graphs were performed using GraphPad Prism 5.0 software (GraphPad Software, La Jolla, CA). Results are presented as mean values ± the 95% confidence interval unless otherwise noted. Continuous variables were analyzed using an unpaired two-tailed Student’s t-test. Multiple groups were compared by 2-way ANOVA Bonferroni or Sidak’s post-tests. Survival curves were generated using the Kaplan-Meier method. The Log-rank (Mantel-Cox) Test was used to compare the survival data between experimental groups. Statistical significance was defined as a p-value < 0.05.
RESULTS
1. Overexpression of lung-associated hIL-10 results in accelerated mortality and diminished bacterial clearance from pneumonia following lung contusion injury
IL-10 OE and TG- mice were fed doxycycline-containing chow for 5 days prior to experimental use to ensure maximal hIL-10 expression within the lung (previously published data) [9]. Doxycycline chow was continued during the entire experiment. Mice underwent lung contusion injury at time -6 hours and then intratracheal inoculation of 500 CFU’s of Klebsiella Pneumoniae at time 0 hours. They were monitored twice daily for 5 days and survival recorded. IL-10 OE mice demonstrated significantly accelerated mortality, diminished bacterial clearance from the lung, and elevated evidence of bacteremia compared to the TG- control mice following lung contusion and exposure to pneumonia (Figure 1). There was also a difference in bacterial clearance from the lung and bacteremia between IL-10 OE and TG- mice in the absence of lung contusion injury (Sham+Pneu) and both groups of mice had reduced levels of bacteria in the lung at 24 hours when compared to the lung contused animals. The IL-10 OE mice experienced more rapid mortality if they were exposed to pneumonia after lung contusion compared to being subjected to pneumonia alone (p=0.02, Log-rank Test).
Figure 1.
Effect of lung-specific IL-10 overexpression on survival and bacterial clearance following lung contusion injury and pneumonia. (A) IL-10 OE mice experienced accelerated mortality compared to TG- mice from pneumonia following lung contusion (p<0.0001, Log-rank test). Mice were administered doxycycline containing chow starting 5 days prior to experimental use to induce hIL-10 expression. Lung contusion or sham injury was created at time 0 hours. Mice were exposed to 500 CFU’s Klebsiella pneumoniae 6 hours post lung contusion. (B,C) IL-10 OE mice had decreased levels of bacterial clearance in the lung and increased evidence of systemic bacteremia compared to TG- controls 24 hours after pneumonia inoculation and 30 hours after lung contusion injury (*p<0.05, t-test). Results are expressed as mean CFU’s per whole lung or 100uL of blood. (D) Pneumonia challenged TG- mice had greater 5-day survival than IL-10 OE mice following sham lung contusion (50 vs. 0%, p=0.02, Log-rank test). (E,F) There was also a difference in bacterial clearance from the lung between the IL-10 OE and TG- mice in the absence of lung contusion injury (*p<0.05, t-test), andr the IL-10 OE mice did have evidence of increased bacteremia compared to the TG- mice (*p<0.05, t-test). N=10-12 mice per group. IL-10 OE, lung-specific hIL-10 overexpression mice; TG-, single transgene control mice.
2. Secondary bacterial pneumonia following lung contusion results in increased lung injury and inflammation in animals with overexpression of IL-10
Histologic examination of right side lung tissue obtained at the time of euthanasia from Sham LC + pneumonia challenged IL-10 OE and TG- mice showed similar amounts of leukocyte infiltration in response to bacterial pneumonia (Figure 2A, Sham+Pneu). However, IL-10 OE mice with a traumatic lung contusion which were then challenged with pneumonia demonstrated a much higher degree of tissue injury and acute inflammation compared to TG- animals (Figure 2A, LC+Pneu). IL-10 OE mice showed a focal lung tissue response to LC + pneumonia with robust infiltration of leukocytes into areas in a patchy distribution. TG- mice exposed to LC + pneumonia demonstrated a more uniform leukocyte infiltration response over the contused lobe(s). The area of contused lung in IL-10 OE animals was also visually a deep purple color consistent with acute hemorrhage and pulmonary consolidation. At 30 hours post contusion and 24 hours post pneumonia, alveoli in IL-10 OE mice remained collapsed and had evidence of a dense infiltration of inflammatory cells. The IL-10 OE mice also had evidence of bacterial organisms within the alveoli (arrow). Lung histology scores are shown for the four experimental groups (Figure 2B).
Figure 2.
(A) Hematoxylin and eosin stained lung tissue (×60 magnification). Lung tissue obtained from the right lung 30 hours following lung contusion and 24 hours following pneumonia. There is dense infiltration of inflammatory cells into the lung parenchyma and collapse of the alveoli in IL-10 OE mice following LC + pneumonia (LC+Pneu). Arrows indicate bacteria. Representative microphotographs shown. (B) Lung histology scores. Analysis of results for the Sham + Pneu and LC + Pneu groups was performed using 1-way ANOVA (p=0.0003) and Dunn’s multiple comparison test. *p<0.05 post-hoc test. N=5–6 mice per group.
a. Permeability injury
Alteration of lung tissue alveolar-capillary integrity was quantified by assay of albumin levels in BAL samples. Leakage of albumin into the alveoli reflects the degree of permeability caused by acute injury. BAL levels of albumin were significantly elevated in the LC + pneumonia group compared to the Sham LC + pneumonia group for both IL-10 OE and TG- mice (Figure 3A). The albumin level in BAL fluid of IL-10 OE mice following LC + pneumonia was significantly higher than in the TG- mice, suggesting that increased IL-10 and failure to effective clear the pathogen exacerbates the loss of alveolar-capillary integrity in the setting of acute lung injury resulting from LC + pneumonia.
Figure 3.
Levels of albumin (A) and myeloperoxidase (B) present in BAL fluid as a measure of lung injury 24 hours after induction of Klebsiella pneumonia and 30 hours following lung contusion. Analysis of results for the Sham + Pneu and LC + Pneu groups was performed using 2-way ANOVA (p<0.0001) and Sidak’s multiple comparison test. *p<0.05 post-hoc test. N=7 mice per group.
b. Neutrophil myeloperoxidase activity
We examined neutrophil sequestration in BAL and homogenized lung tissue using an MPO assay. IL-10 OE mice displayed significantly enhanced MPO levels in the BAL fluid of LC + pneumonia challenged mice compared to Sham LC + pneumonia group (Figure 3B). Comparison of LC + pneumonia or Sham LC+ pneumonia TG- mice did not reveal significant difference in MPO levels. The pattern of MPO activity in lung parenchyma (homogenate assay) was dissimilar to what was seen in BAL fluid: no statistically significant differences were obtained in any of groups compared (data not shown).
c. Cytokine and cellular responses
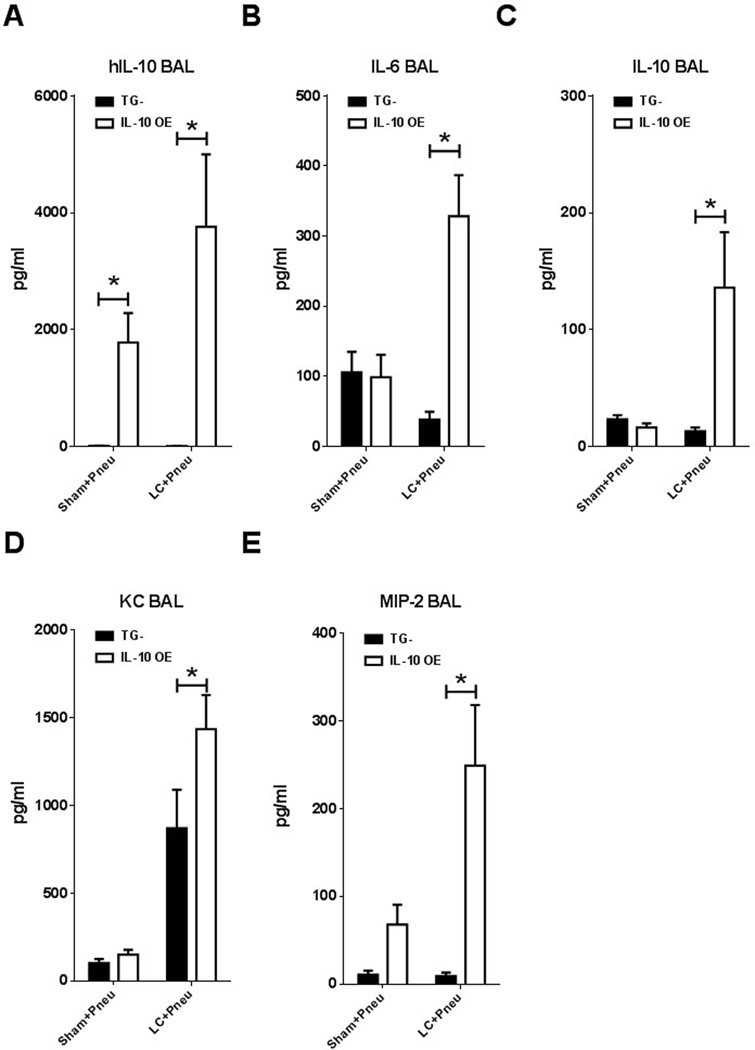
The presence of soluble inflammatory mediators in alveoli following lung contusion and pneumonia was investigated by collecting BAL fluid from IL-10 OE and TG- mice under four different experimental conditions: naïve, 5 days of doxycycline chow, 5 days doxycycline chow and Sham LC + pneumonia, or 5 days doxycycline chow and LC + pneumonia. The IL-10 OE mice receiving doxycycline chow for five days expressed significantly elevated levels of hIL-10 when compared to TG- mice fed the same chow (Figure 4A). There were no differences detected in BAL fluid for expression of IL-6, murine IL-10, KC and MIP-2 between TG- and IL-10 OE mice in the naïve and 5 day doxycycline chow groups (data not shown). We did find that lung contusion injury followed by pneumonia challenge resulted in increased levels of IL-6, murine IL-10, KC, and MIP-2 in the BAL fluid obtained 24 hours post infection of IL-10 OE mice and this was coupled with a significant difference between the LC+ pneumonia exposed IL-10 OE and TG- animals (Figure 4B2013;E). This suggests that in the presence of elevated levels of lung specific IL-10 there is a more robust pro-inflammatory cytokine response to LC injury followed by exposure to pneumonia. In prior experiments involving just pneumonia, a similar finding was obtained with elevated levels of KC and TNFα present in BAL fluid of IL-10 OE mice exposed to Pseudomonas aeruginosa pneumonia for 24 hours (14). This intense and sustained pro-inflammatory cytokine generation may be related to failure of the IL-10 OE mice to effectively clear bacteria, leading to exuberant activation of the innate immune system due to the presence of high levels of bacteria in the lung.
Figure 4.
Effects of lung-specific IL-10 overexpression on alveolar cytokine/chemokine levels following lung contusion injury and pneumonia. Levels of IL-6, IL-10, hIL-10, KC and MIP-2 were assayed in BAL fluid collected 24 hours after pneumonia inoculation and 30 hours after lung contusion injury. All the groups except the naïve (data not shown) received 5 days of doxycycline chow prior to the experiment. Pneumonia challenged mice were administered 500 CFU’s intratracheal Klebsiella pneumoniae 6 hours after lung contusion (A–E). Mice were subjected to sham or lung contusion injury at 0 hours (A–E). BAL samples were collected 24 hours after pneumonia inoculation and 30 hours after lung contusion injury. *p<0.05 2-way ANOVA with Bonferroni post-tests. N=6-11 mice per group. IL-10 OE, lung-specific hIL-10 overexpression mice; TG-, single transgene control mice.
Microscopic examination of BAL cytospins from both IL-10 OE and TG- mice revealed enhanced recruitment of immune cells into the alveoli in the setting of LC + pneumonia compared to Sham + pneumonia challenged mice (Figure 5). We analyzed these BAL cells by flow cytometry according to their forward light scatter and side light scatter as well as expression of Gr-1, CD11b, CD11c, F4/80 and CD206. Lung contusion enhanced the recruitment of neutrophils into the alveoli of IL-10 OE and TG- mice compared to sham injury (Figure 6). Similar to the myeloperoxidase assay results, the IL-10 OE mice displayed significantly higher levels of neutrophils in the BAL fluid of LC + pneumonia challenged mice compared to the TG- mice. No difference was evident in the total levels of macrophages present among the IL-10 OE and TG- mice or in the experimental setting of pneumonia alone vs. LC + pneumonia. After focusing on the appropriate cell population based on size and granularity, M1 macrophages were identified by characterizing macrophage mannose receptor expression as CD11c+CD206- cells and M2 macrophages as CD11c+CD206+ cells. IL-10 OE mice did demonstrate a reversal in the ratio of M1 to M2 macrophages present within the alveoli 24 hours after pneumonia and 30 hours after lung contusion injury. TG- mice had higher levels of classic M1 macrophages and IL-10 OE mice had predominantly alternatively activated M2 macrophages (Figure 6B). This difference in the ratio of M1 to M2 cells was dependent on the phenotype of the mouse (TG- vs. IL-10 OE) in the setting of pneumonia but was independent of the presence or absence of lung contusion injury.
Figure 5.
Cytospin analysis of cells in BAL samples after Sham + pneumonia or LC + pneumonia challenge. BAL samples were collected 24 hours after pneumonia and 30 hours after LC, centrifuged onto glass slides, stained with Diff-Quick and visualized by light microscopy at ×60 magnification. Representative microphotographs shown.
Figure 6.
Distribution of inflammatory cells in BAL fluid and lung parenchyma following pneumonia and/or lung contusion in IL-10 OE mice. All mice received 5 days of doxycycline chow prior to experimental use. Pneumonia challenged mice were administered 500 CFU’s intratracheal Klebsiella pneumoniae 6 hours after lung contusion. Mice were subjected to sham or lung contusion injury at 0 hours. BAL and lung tissue samples were collected 24 hours after pneumonia inoculation and 30 hours after lung contusion injury. (A) Neutrophil and macrophage distribution in BAL fluid determined using flow cytometry. (B) Gating strategy for determination of M1 (CD11c+CD206-) or M2 (CD11c+CD206+) macrophage populations. (C) Distribution of lung parenchyma inflammatory cells in IL-10 OE and TG- mice. *p<0.05 2-way ANOVA with Sidak’s multiple comparison post-tests. N=5 mice per group. IL-10 OE, lung-specific hIL-10 overexpression mice; TG-, single transgene control mice.
In the lung parenchyma, lung contusion prior to pneumonia increased the recruitment of neutrophils into the tissue for the TG- control mice, however, in the IL-10 OE mice the level of neutrophil recruitment was reduced in the LC + pneumonia mice and was similar to the TG- controls with pneumonia alone (Figure 6C). Lung parenchyma macrophages had an activation shift similar to what was observed in macrophage cells collected in BAL samples, however the effect was less pronounced. IL-10 overexpression did result in a decreased M1/M2 ratio (1.1 IL-10 OE vs. 4.5 TG-).
3. Lung contusion induces change in macrophage polarization to a M2 phenotype in IL-10 OE mice
Alternative macrophage activation was also investigated by assaying for arginase 1 expression in purified macrophages isolated from BAL fluid and lung tissue after lung contusion along without induction of pneumonia. Arginase 1 is a gene marker that defines M2 macrophages (15). Lung contusion injury produced significant elevations in the level of arginase 1 expression in macrophages isolated from BAL fluid and lung tissue 6 hours and 24 hours after chest trauma in the IL-10 OE mice compared to the TG- controls (Figure 7).
Figure 7.
Effect of lung contusion on expression of Arginase 1 in BAL and lung parenchyma macrophages isolated from IL-10 overexpression and control mice. All mice received 5 days of doxycycline chow prior to experimental use. (A) Macrophages were isolated from BAL samples by plastic adherence and harvested for quantitative real time PCR gene expression analysis. Experimental conditions consisted of naïve mice, 6 hours post-lung contusion injury and 24 hours after lung contusion injury. (B) Macrophages isolated from lung parenchyma by Collagenase A digestion and plastic adherence for real time PCR gene expression. Results are expressed as mean fold increase in gene expression relative to GAPDH. *p<0.05 2-way ANOVA with Bonferroni post-tests. N=5 mice per group. IL-10 OE, lung-specific hIL-10 overexpression mice; TG-, single transgene control mice.
4. IL-10 overexpression is associated with reduced intracellular bactericidal activity
Macrophages were isolated from tissue of lung contusion and pneumonia challenged mice. Analysis of gene expression in the lung tissue macrophages revealed the following pattern: IL-10 OE mice had much higher levels of the M2 or alternative macrophage marker arginase 1 (Figure 8A), whereas macrophages from TG- mice had increased IL-1β, NOS2, and IFN-γ expression which is consistent with an M1 or classical macrophage phenotype. Potent arginase 1 expression was coupled with enhanced production of IL-6, IL-10, KC and MIP-2 in IL-10 OE BAL samples (Figure 4).
Figure 8.
Evaluation of lung parenchyma macrophages isolated from IL-10 OE and TG- mice 30 hours after lung contusion and 24 hours after pneumonia for macrophage phenotype and evidence of intracellular bacterial killing. (A) Macrophages were isolated by plastic adherence following lung tissue digestion. Macrophages were used for mRNA isolation and M1/M2 markers of quantified by real time PCR gene expression. M1 phenotype is represented by IL-1β, NOS2, and IFN-γ. The M2 phenotype is characterized by Arginase 1 and IL-10 expression. (B) The Klebsiella hemolysin gene is a protein expressed by Klebsiella pneumoniae. Gene expression of khe reflects the presence of live bacteria within isolated pulmonary macrophages. This finding was confirmed by plating isolated and lysed macrophages on 5% blood agar and assessing for colony forming unit growth. Results are expressed as mean fold increase in gene expression relative to GAPDH. *p<0.05 unpaired two-tailed t-test. N=5 mice per group. IL-10 OE, lung-specific hIL-10 overexpression mice; TG-, single transgene control mice.
The Klebsiella hemolysin gene is a protein expressed by Klebsiella pneumoniae (13). Gene expression of khe reflects the presence of live bacteria within isolated pulmonary macrophages. Khe hemolysin expression was significantly elevated in macrophages isolated from IL-10 OE mice following lung contusion and pneumonia compared to macrophages from TG- mice (Figure 8B). This finding was confirmed by plating isolated and lysed macrophages on 5% blood agar and assessing for colony forming unit growth which represents quantification of the amount of viable intracellular bacteria.
DISCUSSION
Lung contusion as a result of trauma to the chest alters the innate immune response and its ability to clear bacteria (16). Our data demonstrate that high pulmonary levels of the anti-inflammatory cytokine IL-10 in the setting of lung contusion followed by pneumonia results in accelerated mortality, increased lung bacterial counts, and intensified progression to systemic bacteremia. Lung contusion may be an essential factor resulting in accelerated mortality among IL-10 OE mice due to increased physiologic injury as reflected by enhanced albumin leakage into the alveoli compared to the sham injured animals. Pneumonia induced lung injury (Sham+Pneu) did not differ between IL-10 OE and TG- animals as measured by BAL albumin extravasation data. However, IL-10 OE mice did experience increased mortality in both the LC + pneumonia and Sham LC + pneumonia experiments consistent with prior observations. Pneumonia following lung contusion enhanced the presence of soluble inflammatory mediators such as IL-6, KC, and MIP-2 in BAL fluid. Alveolar macrophages isolated from IL-10 OE mice displayed an increased shift to the alternative M2 macrophage phenotype and compromised bacterial killing capacity, whereas macrophages from TG- mice demonstrated classical M1 activation and a much higher intracellular bacterial killing potential. Given the high live bacteria counts in IL-10 OE mice circulation following pneumonia, the accelerated mortality among these animals may be a consequence of systemic bacteremia resulting from failure to clear the bacteria from the lungs and the result of severe sepsis.
Traumatic injury, and specifically lung contusion, creates an inflammatory state resulting in excess priming and deployment of the innate immune system (17). Lung contusion injury alters the innate immune system by triggering reduced alveolar macrophage HLA-DR expression, a parameter that correlates inversely with development of infectious complications and mortality in trauma patients (18). As the severity of injury increases, the number of apoptotic T cells ingested by macrophages becomes elevated (19). Macrophages are deactivated, MHC II is down-regulated and increased production of the anti-inflammatory mediators TGF-β and IL-10 occurs (4). This change in macrophage activation can lead to immune suppression, decreased inflammation and increased bacterial load. In our previous work, we found that the altered inflammatory state induced by lung contusion leads to a temporary vulnerability from secondary bacterial infection (pneumonia) (16). For lung contusion followed by pneumonia challenge 6 hours after injury, we found high levels of recruited neutrophils and macrophages present in the lungs 8 hrs after injury and 2 hrs after induction of pneumonia. However, the majority of these macrophages were in the process of undergoing apoptosis within the alveoli or were already necrotic (16). We also observed increased levels of mouse IL-10 in the lungs of lung contusion + pneumonia animals suggesting that this anti-inflammatory cytokine may play a role in modulating the innate immune response in this setting. Conversely, in the late period (48 hrs) following lung contusion the reverse has been found by Southard, et al (20). For animals exposed to a Pseudomonas aeruginosa bacterial insult 48 hrs after lung contusion there was decreased mortality, enhanced cytokine response, and increased toll-like receptor 4 expression on alveolar macrophages. The investigators did not study any changes in pulmonary levels of IL-10.
It is known that hIL-10 specifically binds to both mouse and human cells and mouse IL-10 can block hIL-10 binding to mouse but not human cells (9). The unique phenotype of our IL-10 OE mice was outlined in previous studies. Human IL-10 over expression resulted in up-regulation of the Th-2 cytokines IL-13 and IL-5 along with induction of Th-2 related chemokines MCP-1 (macrophage chemoattractant protein 1) and CCL-3 (C-C motif ligand 3) in non-infected mice. A subset of CD11b+CD11c+CD49+F4/80-Gr-1- cells was identified as the principal source of IL-10-induced IL-13 production (21). In a lung injury model, both IL-10 OE and TG- strains demonstrated a significant increase in alveolar WBC and neutrophil count following intra-tracheal LPS challenge (5,000 ng per mice) compared to negative controls (fed doxycycline and treated with PBS). Long-term IL-10 over expression correlated with induction of lung fibrosis via CCL2/CCR2 axis and was mediated in part by fibrocytes and M2-macrophage recruitment (22). IL-10 over expression appears to recruit macrophages into the lung and induce the alternatively activated (M2) phenotype when compared to TG- mice.
IL-10 has been documented to drive alternative activation of macrophages and also appears to affect iNOS function since IL-10 deficient macrophages are more efficient at killing K. pneumoniae than wild type macrophages (23, 24). The iNOS activation is crucial in producing bacterial killing since iNOS deficient macrophages demonstrate a significantly enhanced intracellular bacterial burden (24). At the same time, M1 macrophages are understood to be involved in macrophage-mediated tissue injury (25). The activity of M1 macrophages is balanced by a population of alternatively activated M2 macrophages which have considerably less microbicidal activity and considered to be anti-inflammatory cells, playing a role in tissue repair.
The results of the experiments presented here for the trauma setting, consisting of lung contusion + pneumonia, coupled with those previously obtained using the IL-10 OE model for pneumonia alone, suggests that the observed accelerated mortality is due to onset of bacteremia and failure to clear the intrapulmonary bacteria as opposed to local neutrophil induced tissue damage alone (14). In our experiments we found that lung contusion in the presence of high levels of IL-10 shifted the innate immune system of the lung towards inducing the alternative macrophage phenotype focused on tissue repair rather than bacterial clearance.
CONCLUSION
In conclusion, elevated levels of pulmonary IL-10 were found to drive macrophages towards the M2 or alternative phenotype. In the setting of lung contusion and pneumonia we demonstrated that a predominant M2 phenotype has a detrimental impact by decreasing intracellular bacterial killing, resulting in a more pronounced bacteremia and accelerating mortality.
Acknowledgments
Source of Funding: Krishnan Raghavendran was supported by National Institutes of Health grant R01-HL102013. Mark R. Hemmila was supported by National Institutes of Health grant K08-GM078610 with joint support from the American College of Surgeons and the American Association for the Surgery of Trauma. For the remaining authors none were declared.
Footnotes
Conflict of Interest Disclosure: None.
AUTHOR CONTRIBUTIONS
Vladislav A. Dolgachev - Study design, data collection, data analysis, data interpretation, writing, figures.
Bi Yu - Study design, data collection.
Lei Sun - Study design, critical revision.
Thomas Shanley - Study design, critical revision.
Krishnan Raghavendran - Study design, data analysis, data interpretation, writing, critical revision.
Mark R. Hemmila - Study design, data analysis, data interpretation, writing, figures, critical revision
Contributor Information
Vladislav A. Dolgachev, Department of Surgery, University of Michigan, Ann Arbor, MI.
Bi Yu, Department of Surgery, University of Michigan, Ann Arbor, MI.
Lei Sun, Department of Pediatrics and Communicable Diseases, University of Michigan, Ann Arbor, MI.
Thomas P. Shanley, Department of Pediatrics and Communicable Diseases, University of Michigan, Ann Arbor, MI.
Krishnan Raghavendran, Department of Surgery, University of Michigan, Ann Arbor, MI.
Mark R. Hemmila, Department of Surgery, University of Michigan, Ann Arbor, MI.
REFERENCES
- 1.Michelet P, Couret D, Bregeon F, Perrin G, D'Journo XB, Pequignot V, Vig V, Auffray JP. Early onset pneumonia in severe chest trauma: a risk factor analysis. J Trauma. 2010;68(2):395–400. doi: 10.1097/TA.0b013e3181a601cb. [DOI] [PubMed] [Google Scholar]
- 2.Moore EE, Moore FA, Harken AH, Johnson JL, Ciesla D, Banerjee A. The two-event construct of postinjury multiple organ failure. Shock. 2005;24(Suppl 1):71–74. doi: 10.1097/01.shk.0000191336.01036.fe. [DOI] [PubMed] [Google Scholar]
- 3.Hemmila MR, Jakubus JL, Maggio PM, Wahl WL, Dimick JB, Campbell DA, Jr, Taheri PA. Real money: complications and hospital costs in trauma patients. Surgery. 2008;144(2):307–316. doi: 10.1016/j.surg.2008.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reddy RC, Chen GH, Newstead MW, Moore T, Zeng X, Tateda K, Standiford TJ. Alveolar macrophage deactivation in murine septic peritonitis: role of interleukin 10. Infect Immun. 2001;69(3):1394–1401. doi: 10.1128/IAI.69.3.1394-1401.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Duell BL, Tan CK, Carey AJ, Wu F, Cripps AW, Ulett GC. Recent insights into microbial triggers of interleukin-10 production in the host and the impact on infectious disease pathogenesis. FEMS Immunol Med Microbiol. 2012;64(3):295–313. doi: 10.1111/j.1574-695X.2012.00931.x. [DOI] [PubMed] [Google Scholar]
- 6.Mulligan MS, Jones ML, Vaporciyan AA, Howard MC, Ward PA. Protective effects of IL-4 and IL-10 against immune complex-induced lung injury. J Immunol. 1993;151(10):5666–5674. [PubMed] [Google Scholar]
- 7.Greenberger MJ, Strieter RM, Kunkel SL, Danforth JM, Goodman RE, Standiford TJ. Neutralization of IL-10 increases survival in a murine model of Klebsiella pneumonia. J Immunol. 1995;155(2):722–729. [PubMed] [Google Scholar]
- 8.Spight D, Zhao B, Haas M, Wert S, Denenberg A, Shanley TP. Immunoregulatory effects of regulated, lung-targeted expression of IL-10 in vivo. Am J Physiol Lung Cell Mol Physiol. 2005;288(2):L251–L265. doi: 10.1152/ajplung.00122.2004. [DOI] [PubMed] [Google Scholar]
- 9.Tan JC, Indelicato SR, Narula SK, Zavodny PJ, Chou CC. Characterization of interleukin-10 receptors on human and mouse cells. J Biol Chem. 1993;268(28):21053–21059. [PubMed] [Google Scholar]
- 10.Machado-Aranda DA, Suresh MV, Yu B, Raghavendran K. Electroporation-mediated in vivo gene delivery of the Na(+)/K(+)-ATPase pump reduced lung injury in a mouse model of lung contusion. J Trauma Acute Care Surg. 2012;72(1):32–39. doi: 10.1097/TA.0b013e31823f0606. discussion 39–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Suresh MV, Yu B, Machado-Aranda D, Bender MD, Ochoa-Frongia L, Helinski JD, Davidson BA, Knight PR, Hogaboam CM, Moore BB, Raghavendran K. Role of Macrophage Chemoattractant Protein 1 in Acute Inflammation Following Lung Contusion. Am J Respir Cell Mol Biol. 2012 doi: 10.1165/rcmb.2011-0358OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Regueiro V, Campos MA, Pons J, Alberti S, Bengoechea JA. The uptake of a Klebsiella pneumoniae capsule polysaccharide mutant triggers an inflammatory response by human airway epithelial cells. Microbiology. 2006;152(Pt 2):555–566. doi: 10.1099/mic.0.28285-0. [DOI] [PubMed] [Google Scholar]
- 13.Hartman LJ, Selby EB, Whitehouse CA, Coyne SR, Jaissle JG, Twenhafel NA, Burke RL, Kulesh DA. Rapid real-time PCR assays for detection of Klebsiella pneumoniae with the rmpA or magA genes associated with the hypermucoviscosity phenotype: screening of nonhuman primates. J Mol Diagn. 2009;11(5):464–471. doi: 10.2353/jmoldx.2009.080136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sun L, Guo RF, Newstead MW, Standiford TJ, Macariola DR, Shanley TP. Effect of IL-10 on neutrophil recruitment and survival after Pseudomonas aeruginosa challenge. Am J Respir Cell Mol Biol. 2009;41(1):76–84. doi: 10.1165/rcmb.2008-0202OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Byers DE, Holtzman MJ. Alternatively activated macrophages and airway disease. Chest. 2011;140(3):768–774. doi: 10.1378/chest.10-2132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dolgachev VA, Yu B, Reinke JM, Raghavendran K, Hemmila MR. Host susceptibility to gram-negative pneumonia after lung contusion. J Trauma Acute Care Surg. 2012;72(3):614–622. doi: 10.1097/TA.0b013e318243d9b1. discussion 622-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hoth JJ, Martin RS, Yoza BK, Wells JD, Meredith JW, McCall CE. Pulmonary contusion primes systemic innate immunity responses. J Trauma. 2009;67(1):14–21. doi: 10.1097/TA.0b013e31819ea600. discussion 21-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hoth JJ, Scott MJ, Owens RK, Stassen NA, Franklin GA, Cheadle WG, Rodriguez JL. Trauma alters alveolar effector cell apoptosis. Surgery. 2003;134(4):631–637. doi: 10.1016/s0039-6060(03)00310-6. discussion 637-8. [DOI] [PubMed] [Google Scholar]
- 19.Kasten KR, Tschop J, Adediran SG, Hildeman DA, Caldwell CC. T cells are potent early mediators of the host response to sepsis. Shock. 2010;34(4):327–336. doi: 10.1097/SHK.0b013e3181e14c2e. [DOI] [PubMed] [Google Scholar]
- 20.Southard R, Ghosh S, Hilliard J, Davis C, Mazuski C, Walton A, Hotchkiss R. Pulmonary contusion is associated with toll-like receptor 4 upregulation and decreased susceptibility to pseudomonas pneumonia in a mouse model. Shock. 2012;37(6):629–633. doi: 10.1097/SHK.0b013e31824ee551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sun L, Cornell TT, Levine A, Berlin AA, Hinkovska-Galcheva V, Fleszar AJ, Lukacs NW, Shanley TP. Dual role of interleukin-10 in the regulation of respiratory syncitial virus (RSV)-induced lung inflammation. Clin Exp Immunol. 2013;172(2):263–279. doi: 10.1111/cei.12059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sun L, Louie MC, Vannella KM, Wilke CA, LeVine AM, Moore BB, Shanley TP. New concepts of IL-10-induced lung fibrosis: fibrocyte recruitment and M2 activation in a CCL2/CCR2 axis. Am J Physiol Lung Cell Mol Physiol. 2011;300(3):L341–L353. doi: 10.1152/ajplung.00122.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Park-Min KH, Antoniv TT, Ivashkiv LB. Regulation of macrophage phenotype by long-term exposure to IL-10. Immunobiology. 2005;210(2–4):77–86. doi: 10.1016/j.imbio.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 24.Rothfuchs AG, Gigliotti D, Palmblad K, Andersson U, Wigzell H, Rottenberg ME. IFN-alpha beta-dependent, IFN-gamma secretion by bone marrow-derived macrophages controls an intracellular bacterial infection. J Immunol. 2001;167(11):6453–6461. doi: 10.4049/jimmunol.167.11.6453. [DOI] [PubMed] [Google Scholar]
- 25.Laskin DL, Sunil VR, Gardner CR, Laskin JD. Macrophages and tissue injury: agents of defense or destruction? Annu Rev Pharmacol Toxicol. 2011;51:267–288. doi: 10.1146/annurev.pharmtox.010909.105812. [DOI] [PMC free article] [PubMed] [Google Scholar]