SUMMARY
Serotonin (5-HT) plays important roles in the maintenance and modulation of neural systems throughout the animal kingdom. The actions of 5-HT have been well characterized for several crustacean model circuits; however, a dissection of the serotonergic transduction cascades operating in these models has been hampered by the lack of pharmacological tools for invertebrate receptors. Here we provide pharmacological profiles for two 5-HT receptors from the swamp crayfish, Procambarus clarkii: 5-HT2β and 5-HT1α. In so doing, we also report the first functional expression of a crustacean 5-HT1 receptor, and show that it inhibits accumulation of cAMP. The drugs mCPP and quipazine are 5-HT1α agonists and are ineffective at 5-HT2β. Conversely, methiothepin and cinanserin are antagonists of 5-HT2β but do not block 5-HT1α. A comparison of these two receptors with their orthologs from the California spiny lobster, Panulirus interruptus, indicates conservation of protein structure, signaling and pharmacology. This conservation extends beyond crustacean infraorders. The signature residues that form the ligand-binding pocket in mammalian 5-HT receptors are found in the crustacean receptors. Similarly, the protein domains involved in G protein coupling are conserved between the two crustacean receptors and other characterized arthropod and mammalian 5-HT receptors. Considering the apparent conservation of pharmacological properties between crustacean 5-HT receptors, these tools could be applicable to related crustacean physiological preparations.
Keywords: agonist, antagonist, neuromodulation, G protein-coupled receptor, amine, cloning
INTRODUCTION
Serotonin (5-HT) is an important neurotransmitter and neurohormone in most animal species. Diverse processes, including social behaviors and a variety of systemic physiological functions are regulated by this biogenic amine. Animals have evolved a suite of 5-HT receptor types; thus, a single neurotransmitter can have multiple and complex effects on different tissues. To date, seven classes of serotonin receptors have been identified in mammals. These receptor classes are categorized with respect to their signal transduction mechanisms and pharmacological properties (Gerhardt and van Heerikhuizen, 1997; Hoyer et al., 2002; Kroeze et al., 2002).
In invertebrate nervous systems 5-HT can modulate motor pattern generation (Hooper and DiCaprio, 2004), escape and social status (Edwards et al., 1999), aggression (Kravitz, 2000) and learning (Barbas et al., 2003; Bicker, 1999; Kandel and Schwartz, 1982). Serotonin has multiple and complex roles in crustacean models. 5-HT application to the stomatogastric nervous system of decapod crustaceans elicits distinct responses from individual identified neurons (Flamm and Harris-Warrick, 1986), and these responses can differ for the same identified neuron in different species (Katz and Tazaki, 1992). In the crayfish, the social status of an individual determines the directionality of 5-HT modulation of the lateral giant escape circuit response to sensory stimulation (Yeh et al., 1997; Yeh et al., 1996). The rate and concentration of 5-HT application to the circuit also affect this response (Teshiba et al., 2001). These studies suggest that crustaceans express several different 5-HT receptor types and that the expression or signaling of these receptors might change in response to social or environmental stimuli.
Crustacean hormonal circuits with small numbers of identifiable cells are ideal preparations for investigating the mechanistic basis of modulation and plasticity. Such studies have, however, been limited by the lack of pharmacological tools. Although the protein sequences and second-messenger couplings of 5-HT receptors are relatively well conserved across all species, their pharmacological profiles can vary significantly between vertebrate and invertebrate preparations, and even within invertebrate preparations (Tierney, 2001). This is not surprising as one would not expect selection for or against amino acids involved in binding synthetic ligands. We have recently identified and cloned two crustacean 5-HT receptors from the spiny lobster, Panulirus interruptus, infraorder Achelata: 5-HT1αPan and 5-HT2βPan (Clark et al., 2004; Sosa et al., 2004). We were interested to know whether the actions of pharmacological agents were conserved across crustacean species. We therefore obtained full-length clones for the same two receptors from a second distantly related decapod crustacean, the swamp crayfish, Procambarus clarkii (infraorder Astacidea), and expressed these receptors in a heterologous system in order to determine their second messenger couplings and pharmacological profiles. These parameters were then compared across the two species.
MATERIALS AND METHODS
Animals
Crayfish, Procambarus clarkii Girard 1852, were obtained from Atchafalaya Biological Supply (Raceland, LA, USA) and kept communally at 20°C in 40 l tanks with continual filtration and aeration.
Chemicals and cell lines
HEK293, NIH3T3, COS-7 and MDCK cells, Earle’s minimal essential medium (EMEM), horse serum, trypsin, penicillin and streptomycin were obtained from the American Type Culture Collection (Mannassas, VA, USA). Dulbecco’s modified Eagle’s medium (DMEM) was from Mediatech Inc. (Herndon, VA, USA). Dialyzed fetal bovine serum (FBS), TRex cell line (293-TR), pDNA4/TO plasmid, blasticidin and zeocin were from Invitrogen (Carlsbad, CA, USA). Cinanserin was obtained from Tocris (Ballwin, MO, USA). All other chemicals were from Sigma (St Louis, MO, USA). For pharmacology experiments, amine and agonist stock solutions (10−1 mol l−1) were made fresh for every experiment in medium or 50% ethanol, respectively. Two exceptions were tyramine (Tyr), which was made fresh as a 10−2 mol l−1 stock in medium, and methysergide, which was made as a 10−2 mol l−1 stock in DMSO and stored at −20°C. Antagonist drugs were made as 10−2 mol l−1 stock solutions in DMSO and stored at −20°C.
Cloning of full-length 5-HT1α and 5-HT2β from Procambarus clarkii and generation of expression constructs
Complete cloning and sequencing of 5-HT2βPan and 5-HT1αPan from Panulirus interruptus have been previously described (Clark et al., 2004; Sosa et al., 2004).
We also previously cloned a large segment of 5-HT1αPro spanning transmembrane domains III–VII from Procambarus clarkii (Sosa et al., 2004). We have now completed the sequencing of the 5-HT1αPro cDNA using rapid amplification of DNA ends (SMART RACE cDNA Amplification kit; BD Biosciences, Clontech, Cambridge, UK) as previously described (Clark et al., 2004). Constructs containing the complete ORF were assembled using standard procedures (Ausubel et al., 1990). Both strands of the construct were sequenced and errors that had been introduced in the cloning process were corrected using QuikChange site directed mutagenesis (Stratagene, La Jolla, CA, USA). The construct was then cloned into the pDNA4/TO (Invitrogen) expression plasmid.
5-HT2βPro was cloned from crayfish cDNA using degenerate RT-PCR and RACE. Previously, 5-HT2βPan had been identified in the Drosophila genome database and the ortholog from Panulirus was fully cloned and characterized for signal transduction properties (Clark et al., 2004). Degenerate primers were designed based on conserved regions of these Panulirus and Drosophila orthologs of 5-HT2βPro (written 5′-3′): 5-5-1, GAYGTIYTITTYTGY-ACIGCIWSIATHATG; 5-5-2, ATGCAYYTITGYACIYTIWSI-GTIGAYMGI TT; 5-3, CATDATDATIARIGGDATRTARA-ARCA; 3-5-1, CAYGGIMGIAAYATHMGIATGGARCA; 3-5-2, WUIGARCARAARGCNACNAARGU; 3-3, YUURUURAAIA-WIGURUARRA.
Multiple cDNA preparations, each from a separate crayfish nervous tissue mRNA preparation, were used as templates for nested PCR experiments with these degenerate primers to amplify fragments of the crayfish ortholog, as described previously (Baro et al., 1994; Sosa et al., 2004). Primers specific to 5-HT2βPro were designed to generate a large clone of 5-HT2βPro. The N- and C-terminals of 5-HT2βPro were then cloned using SMART RACE as described above. A construct containing the complete ORF was assembled, sequenced and inserted into the pIRESneo (Clontech, Mountain View, CA, USA) expression plasmid as previously described for 5-HT2βPan (Clark et al., 2004).
Sequence data were analyzed using Sequencher 4.1 (Gene Codes Corp., Ann Arbor, MI, USA). Sequences for other arthropod species were obtained from GenBank and alignments were created to determine sequence identities using the ClustalW algorithm with default settings in Lasergene MegAlign (DNASTAR Inc., Madison, WI, USA). Full Procambarus sequences have been deposited in GenBank under accession numbers EU131666 (5-HT2βPro) and EU131667 (5-HT1αPro).
Generation of cell cultures expressing crustacean 5-HT receptors
We previously characterized the signaling and pharmacological properties of the 5-HT2βPan receptor transiently expressed in HEK293 cells (Clark et al., 2004). We used the same techniques to characterize the Procambarus ortholog, 5-HT2βPro. Briefly, cells were maintained in EMEM supplemented with 10% FBS, 50 i.u. ml−1 penicillin and 50 μg ml−1 streptomycin (normal medium). Cells were plated on 60 mm dishes in EMEM without antibiotics, allowed to grow to 95–100% confluency and then transfected with 2 μg of DNA using lipofectamine (Invitrogen). The plates were supplemented to a final concentration of 10% FBS 6 h after transfection, and the medium was replaced with normal medium 24 h after transfection.
In order to functionally characterize 5-HT1αPan and 5-HT1αPro, we first generated full-length constructs using standard recombinant techniques, as described above. The 5-HT1α receptors were first cloned into pIRESneo and stably transfected into several cell lines using lipofectamine as detailed in the Results. Immunoblotting experiments coupled with the lack of growth after several weeks of selection suggested that none of these cell lines could stably express either the 5-HT1αPan or the 5-HT1αPro receptor. This did not appear to be a general phenomenon associated with crustacean receptors, as we successfully expressed 5HT2β receptors and crustacean dopamine receptors in these cell lines. The most reasonable explanation for our finding was that the cell lines could not tolerate high levels of the 5-HT1α receptor, and for reasons unknown, cells expressing 5-HT1α receptors were selected against. Inducible expression systems have been developed to deal with this type of problem.
We next expressed the 5-HT1α constructs in an inducible expression system. In this system the 5-HT1α cDNA is stably integrated into the genome of the parental HEK cells, but it is not transcribed unless tetracycline is present in the medium. Using this system we could express 5-HT1α receptors for a defined time interval, and then perform the assays before the cells were selected against. 5-HT1α cDNA was cloned into the inducible expression plasmid, pDNA4/TO, behind the tetracycline operator. The new constructs were then transfected into HEK293-TR cells stably expressing the tetracycline repressor protein. Stably transfected cells (i.e. those in which the plasmid carrying the receptor was incorporated into the HEK cell genome) were selected for >4 weeks in DMEM supplemented with 10% dialyzed fetal bovine serum, 5 μg ml−1 blasticidin and 300 μg ml−1 zeocin (complete medium; TRex regulated expression system, Invitrogen). Confluent plates were easily obtained after 4 weeks, suggesting that the 5-HT1α cDNA could be maintained in tissue culture cells as long as it was not transcribed and translated. Next, western blots were used to confirm tet-repressor-regulated 5-HT1αPan expression as follows. Cells were plated in 60 mm dishes and induced with 1 μmol l−1 tetracycline 0, 6, 8, 12 and 24 h before collection and isolation of protein by previously described methods (Clark et al., 2004; Sosa et al., 2004). Protein preparations were run on a 10% SDS-PAGE gel, transferred to PVDF membranes and probed with a custom-made rabbit anti-5-HT1αCrust antibody (Sosa et al., 2004). Bands were visualized using chemiluminescence (Immun-Star, BioRad, Hercules, CA, USA). 5-HT1αPan production commences within 6 h after induction and lasts through 24 h of induction (Fig. 1). Crude protein prepared from lobster nervous system was run as a positive control. Similar results were found for induction of 5-HT1αPro in stably transfected 293-TR-5-HT1αPro lines. Based on these findings we induced cells for functional assays for 18–20 h (below).
Fig. 1.

5-HT1αPan can be stably expressed in an inducible tissue culture system. Western blot with anti-5-HT1αCrust showing expression of 5-HT1αPan in the lobster nervous system (Lns) and in stably transfected 293-TR-5-HT1αPan cells after induction with tetracycline for the indicated times (24, 12, 8, 6 h) and in non-induced cells (NI).
Assay of IP release in cells expressing 5-HT2βPan and 5-HT2βPro
Inositol phosphate (IP) release was assayed as previously described (Clark et al., 2004). Briefly, transiently transfected cells were divided among wells on a 24-well plate with 1 μCi ml−1 of [3H]myoinositol (Amersham, Piscataway, NJ, USA) and allowed to grow to 95–100% confluency over 48 h. The cells were washed with fresh EMEM and then exposed to 10 mmol l−1 LiCl in EMEM for 20 min at 37°C. As applicable, antagonists were added to individual wells and allowed to incubate for an additional 10 min. 5-HT or agonist drugs were added to test well contents and cells were returned to 37°C for 60 min. The medium was removed and replaced with ice-cold 20 mmol l−1 formic acid. Plates were then placed on ice for 30 min. The cell lysate was collected and applied to AG1-X8 columns (BioRad, Hercules, CA, USA) equilibrated with 20 mmol l−1 formic acid. The columns were washed with 50 mmol l−1 ammonium hydroxide followed by elution of inositol phosphates (IP) with 10 ml of 1 mol l−1 ammonium formate–0.1 mol l−1 formic acid. The IP fraction was scintillation counted. Membranes attached to the wells were dissolved in 1 mol l−1 NaOH and scintillation counted as total phosphatidyl inositols (PI). Activation results are expressed as the fraction of radioactivity incorporated in IP over that in IP+PI and normalized to activity observed in negative control wells (no drug) for every experiment.
[cAMP] determinations in cells expressing 5-HT1αPan or 5-HT1αPro
Cyclic AMP levels in HEK293-TR cells stably expressing 5-HT1αPan or 5-HT1αPro were determined using a Direct cAMP kit (Assay Designs, Ann Arbor, MI, USA) as previously described (Clark et al., 2004). For receptor activation assays, stably transfected cells were plated in 24-well plates and allowed to grow to 100% confluency. The medium was replaced with 1 ml of complete medium containing 1 μg ml−1 tetracycline to induce expression of receptor protein. After 18–20 h the medium was replaced with 1 ml of fresh DMEM containing 2.5 mmol l−1 3-isobutyl-1-methylxanthine to block phosphodiesterase activity and plates were incubated for 10 min. Antagonists were added to individual wells (if applicable) and allowed to incubate for an additional 10 min. 5-HT or agonists and forskolin (250 nmol l−1), a nonspecific activator of adenylyl cyclase, were then added to individual wells and left at 37°C for 30 min. The medium was removed and replaced with 0.5 ml of 0.1 mol l−1 HCl containing 0.8% Triton X-100. Plates were shaken for 30 min at room temperature, the lysate collected, centrifuged for 5 min at 600 g and the supernatant assayed for [cAMP] using the Direct cAMP kit and [protein] using a BCA Protein Assay Kit (Pierce, Rockford, IL, USA). Data are presented as pmoles of cAMP per milligram of protein.
Heterologous expression system data analysis
Data for all pharmacology assays involving the heterologous expression systems were plotted and analyzed in GraphPad Prism v.4. Statistics for bar graphs were calculated using a two-way ANOVA with a Bonferroni post-test. Dose–response curves were fitted with a standard slope top–bottom or bottom–top dose–response curve to calculate EC/IC50 and efficacy values.
RESULTS
Molecular structure of crustacean 5-HT receptors
The crustacean 5-HT2β receptor was recently cloned from Panulirus interruptus and characterized with respect to its signaling properties (Clark et al., 2004) and pharmacological profile (N.S. and D.J.B., unpublished). We have used degenerate RT-PCR and RACE to clone the Procambarus clarkii ortholog, 5-HT2βPro, from crayfish nervous system cDNA. The full-length 5-HT1α cDNA was previously cloned from Panulirus and the partial sequence was reported for Macrobrachium rosenbergii and Procambarus clarkii (Sosa et al., 2004). In this study we completed the cloning and sequencing of the Procambarus 5-HT1α ortholog using RACE. The predicted amino acid sequences of 5-HT2β and 5-HT1α orthologs were very well conserved between Panulirus and Procambarus; 72 and 90%, respectively, over the entire protein (Figs 2 and 3, Table 1).
Fig. 2.
5-HT2β receptors contain key structural elements typical of the 5-HT receptor superfamily and are conserved among arthropods. Predicted protein sequences of 5-HT2β from Panulirus and Procambarus are aligned with their ortholog from Drosophila and the human 5-HT2C receptor. Residues identical to the 5-HT2βPro sequence are indicated with thin lined boxes. Transmembrane domains are indicated with black bars above the sequence and the reference residue for numbering in each is shaded (see Results). Black ovals surround amino acids important for 5-HT ligand binding. Grey boxes surround areas important for G protein coupling or activation. Asterisks above the sequences indicate consensus sites for N-linked glycosylations in crustacean sequences. A black dot indicates the evolutionary change from DRY to DRF in crustacean sequences (see Results).
Fig. 3.
5-HT1α receptors contain key structural elements typical of the 5-HT receptor superfamily and are conserved among arthropods. Predicted protein sequences of 5-HT1α from Panulirus and Procambarus are aligned with their orthologs from Drosophila, Heliothis and Papilio and the human 5-HT1A receptor. Residues identical to the 5-HT1αPro sequence are boxed with thin lines. Transmembrane domains are indicated with black bars above the sequence and the reference residue for numbering in each is shaded (see Results). Black ovals surround amino acids important for 5-HT ligand binding. Grey boxes surround areas important for G protein coupling or activation. Asterisks above the sequences indicate consensus sites for N-linked glycosylations in crustacean sequences.
Table 1.
Predicted protein sequences of arthropod 5-HT2β and 5-HT1α receptors are well conserved
| 2BHum | 2βDro | 2βPan | 2βPro | 1αHel | 1αDro | 1AHum | 1αPan | 1αPro | |
|---|---|---|---|---|---|---|---|---|---|
| 2BHum | X | X | X | X | X | X | X | X | X |
| 2βDro | 31 (47) | X | X | X | X | X | X | X | X |
| 2βPan | 29 (43) | 46 (68) | X | X | X | X | X | X | X |
| 2βPro | 28 (43) | 45 (68) | 72 (97) | X | X | X | X | X | X |
| 1αHel | 21 (33) | 21 (35) | 16 (34) | 16 (34) | X | X | X | X | X |
| 1αDro | 22 (33) | 25 (37) | 17 (33) | 18 (33) | 60 (81) | X | X | X | X |
| 1AHum | 21 (33) | 21 (36) | 15 (33) | 15 (33) | 33 (50) | 18 (49) | X | X | X |
| 1αPan | 22 (34) | 21 (36) | 14 (31) | 15 (32) | 53 (77) | 29 (76) | 36 (50) | X | X |
| 1αPro | 22 (34) | 22 (36) | 14 (31) | 14 (32) | 53 (77) | 30 (77) | 37 (49) | 90 (98) | X |
| 1αPap | 21 (33) | 21 (34) | 16 (33) | 16 (34) | 92 (98) | 35 (82) | 37 (50) | 56 (76) | 53 (77) |
Percentage identities between 5-HT2 and 5-HT1 receptors from Human (Hum), Drosophila (Dro), Panulirus (Pan) and Procambarus (Pro). 5-HT1α receptors from two other arthropods, the tobacco budworm Heliothis virescens (Hel) and the swallowtail butterfly Papilio xuthus (Pap), are also included. Identity was determined for the entire protein and the core region (parentheses) using the ClustalW algorithm in MegAlign (Lasergene). Comparisons amongst 5-HT2 and 5-HT1 receptors are shaded and crustacean ortholog comparisons are in bold. Accession numbers are human (5-HT2BHum, NM_000868; 5-HT1A, AAH69159), Drosophila (5-HT2βDro, NP_731257 plus NP_649805; 5-HT1αDro P28285), Panulirus (5-HT 2βPan, AY550910; 5-HT1αPan, AY528823), Heliothis (5-HT1Hel, CAA64863), Papilio (5-HT1Pap, BAD72868), Procambarus (5-HT2βPro, EU131666; 5-HT1αPro, EU131667).
When comparing 5-HT receptors from various species, the overall sequence identity can fall very quickly. This is mainly due to the N- and C-terminal domains and the majority of the third intracellular loop, all of which are highly variable in 5-HT receptors. These regions are not thought to be critical to signaling or pharmacology (Kroeze et al., 2002; Saudou et al., 1992; Witz et al., 1990). When these variable regions were excluded from the alignment, a core region representing 34–59% of the protein remained. This core consisted of transmembrane domains and short linker regions important for maintenance of protein structure, ligand binding and signaling. The identity between Panulirus and Procambarus orthologs in the core protein was very high at 97% for 5-HT2β and 98% for 5-HT1α (Table 1). The complete 5-HT2β sequence from each crustacean was 45% identical to the predicted protein sequence of their ortholog from the fruit fly, with an increase to 68% for the core protein. Similarly, crustacean 5-HT1α receptors showed 29–53% identity to orthologs from fly, budworm and butterfly with the core protein sharing at least 76% identity between any of these five arthropod species. In addition, the cores of both crustacean receptors had greater than 40% identity to their human homologs.
5-HT receptors from all species share key conserved residues in their transmembrane domains that are responsible for forming the ligand binding pocket. Because the N-terminal and extra-membrane loops of diverse GPCRs are of variable lengths, referring to residues by their absolute position within these proteins does not allow for comparisons of structural domains between proteins. The Ballesteros-Weinstein numbering scheme used here identified a crucial and conserved characteristic residue common to all G protein coupled receptors (GPCRs) within each transmembrane domain (TM) that was arbitrarily assigned the number 50 (grey, Figs 2 and 3). Other residues within that TM domain were then numbered with the TM number followed by the residue number in relation to this identified amino acid [i.e. Phe (6.51) immediately follows the reference residue in TM6, Pro (6.50)] (Ballesteros and Weinstein, 1995). In biogenic amine receptors the charged residue Asp (3.32) is thought to act as a counterion for the protonated amine moiety of amine ligands, agonists and antagonists and is required for ligand binding but not receptor activation (Kristiansen et al., 2000). The presence of Asp (3.32) in combination with Trp (7.40) is considered a unique fingerprint for biogenic and trace amine GPCRs. These amino acids are involved in ligand binding and receptor activation. In addition to these residues, 5-HT receptors typically have a conserved group of hydrophobic amino acids [Trp (3.28), Phe (5.47), Phe (5.48), Trp (6.48), Phe (6.51), Phe (6.52), Trp (7.40), Tyr (7.43)] that form the hydrophobic ligand-binding pocket within the tertiary structure of the receptor [Kristiansen (Kristiansen, 2004) and references therein; (Roth et al., 1997)]. The hydroxyl group of 5-HT is thought to be stabilized by the Ser (5.43) residue in transmembrane helix 5. Finally, a disulfide bridge formed between Cys (3.25) and a Cys in extracellular loop 2 (EL 2) is important in maintaining tertiary structure and stabilizing the ligand binding pocket. All of these key amino acids (first defined in mammalian receptors) were conserved in 5-HT2β and 5-HT1α from crayfish and spiny lobster (Figs 2 and 3).
Crucial residues for Gα subunit binding specificity are located in cytoplasmic amphipathic α-helical extensions of TM5 and 6 in intracellular loop 3 (IL3) (reviewed by Blenau and Baumann, 2001; Gether, 2000; Kristiansen, 2004; Strader et al., 1995). The C-terminal region of IL3 near the membrane interface with TM6 interacts with the C-terminal end of the Gα protein, helping to confer the receptor’s specificity for a specific Gα subtype. Again, these regions were very well conserved when comparing the crustacean receptor orthologs to each other, to orthologs from other arthropods and to a human homolog (Figs 2 and 3). Although IL2 does not appear to be involved in determining Gα specificity, this loop contains an α-helix that is thought to act in conjunction with the adjacent DRY motif as a switch between active and inactive states making this loop important for efficient G protein activation. Ligand binding results in protonation of the Asp (3.49) in the DRY motif and in significant rotation of TM3 and TM6 and transition to the active state of the receptor. In addition, the DRY motif and the residues surrounding it are important for constitutive activation in 5-HT receptors (Gether, 2000; Shapiro et al., 2002). Interestingly, the 5-HT2β receptor cloned from Panulirus has evolved a DRF sequence in place of the DRY that confers increased basal activity of the receptor when stably expressed in cell culture (Clark et al., 2004); this sequence alteration was conserved in 5-HT2βPro from crayfish (Fig. 2).
Serotonin receptors are extensively post-translationally modified by several mechanisms. Most known GPCRs, including 5-HT2βPro and 5-HT1βPro have several consensus sites for N-linked glycosylation (Asn–X–Ser/Thr) in the N-terminal tail (Figs 2 and 3) and sometimes in other extracellular regions such as EL2. Proper glycosylation of at least some of these sites is required to obtain appropriate levels of receptor expression on the cell surface (Lanctot et al., 2006). Efficiency of ligand binding and functional activity of receptors are not known to be affected by the glycosylation state in receptors that are expressed in the membrane. The putative glycosylation sites of 5-HT2β and 5-HT1α were conserved between orthologs from Panulirus and Procambarus (Figs 2 and 3). Many GPCRs also have a Cys residue in the proximal region of the C-terminal tail that is a putative palmitoylation site; this creates a membrane anchor, generating an additional cytoplasmic loop. This site was present in crustacean 5-HT2β receptors but not in 5-HT1α.
The high degree of conservation of the key structures within the crustacean receptors led us to predict that their signaling pathways will also be the same as those of their vertebrate and invertebrate homologs. Because of the high level of overall conservation, we also might expect the spiny lobster and crayfish orthologs of 5-HT2β and 5-HT1α to exhibit similar pharmacological profiles. In order to compare the functional properties of Panulirus and Procambarus 5-HT receptors, we heterologously expressed the proteins in cell culture and used second messenger assays to determine their ligand specificity, signaling and pharmacological properties.
Amine specificity of 5-HT2βPan and 5-HT2βPro
The arthropod 5-HT2β receptor was initially cloned from Panulirus and shown to be specific for 5-HT over other biogenic amines (Clark et al., 2004). When stably expressed in HEK cells, activation of this receptor resulted in increased intracellular levels of inositol phosphates (IP), activation of protein kinase C (PKC) and no change in cAMP levels. In addition, stably expressed 5-HT2βPan demonstrated constitutive activity conferred by the DRY motif (Clark et al., 2004). In this study we transiently expressed 5-HT2βPan in HEK cells and measured IP release in response to amines and putative pharmacological agents. Interestingly, unlike stably transfected cultures, we found no constitutive activity of 5-HT2βPan when the receptor was transiently expressed (see Discussion).
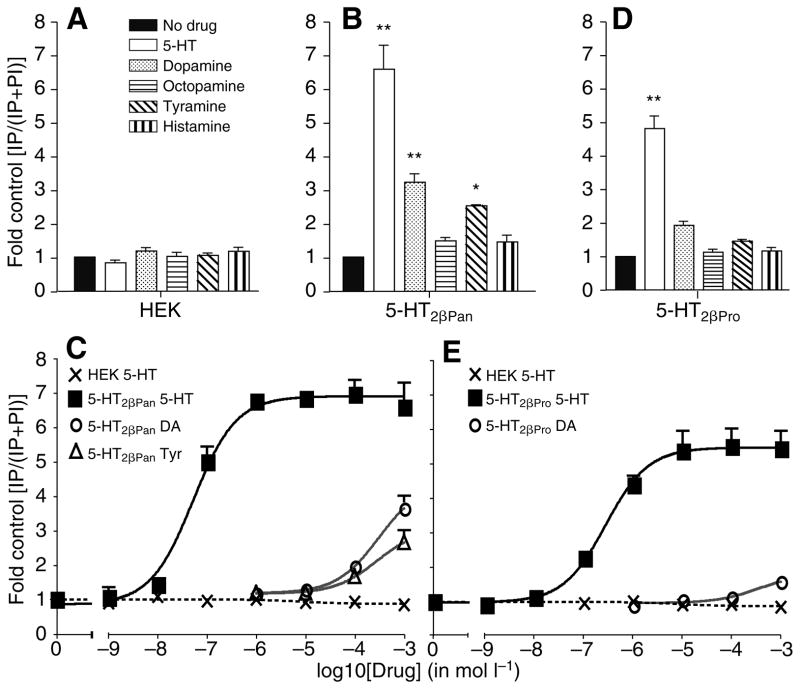
Non-transfected parental HEK cells did not respond to 1 mmol l−1 concentrations of any of the monoamines in the IP assay (Fig. 4A). 5-HT2βPan responded to 5-HT, dopamine and tyramine with IP release (Fig. 4B). The EC50 for 5-HT was 52 nmol l−1 whereas greater than 50 μmol l−1 dopamine (DA) and tyramine (Tyr) were required to activate 5-HT2βPan (Fig. 4C). At 1 mmol l−1 these amines also had an efficacy less than 55% that of 5-HT (Table 2), indicating that 5-HT is the preferred functional ligand for the 5-HT2βPan receptor. As observed for transiently expressed 5-HT2βPan, we found no constitutive activity of transiently expressed 5-HT2βPro. 5-HT2βPro responded strongly to 5-HT with a smaller response to DA (Fig. 4D). The EC50 for 5-HT2βPro was 270 nmol l−1 whereas 1 mmol l−1 DA elicited only a minimal response (Fig. 4E, Table 2).
Fig. 4.
5-HT is the only biogenic amine that acts as a potent agonist at 5-HT2βPan and 5-HT2βPro. (A) Non-transfected parental HEK cells do not show significant IP responses to any of the amines tested. (B) IP release in response to biogenic amines (10−3 mol l−1) in cells transiently expressing 5-HT2βPan. Cells expressing 5-HT2βPan demonstrate a greater than sixfold increase in IPrelease in response to 5-HT and a smaller but significant increase in response to dopamine and tyramine. Values are means ± s.e.m., N=3, **P<0.001 and *P<0.05 versus no drug control. (C) Dose–response curves of 5-HT2βPan to biogenic amines in IP assay. 5-HT2βPan responds to 5-HT (squares) with an EC50 of 52 nmol l−1. Dopamine (DA; circles) and tyramine (Tyr; triangles) activate the receptors only at very high concentrations. Non-transfected HEK cells do not respond to 5-HT (crosses). Values are means ± s.e.m., N=3. (D) IP release in response to biogenic amines (10−3 mol l−1) in cells transiently expressing 5-HT2βPro. IP release is increased more than fourfold in response to 1 mmol l−1 5-HT in cells expressing 5-HT2βPro. A smaller but significant increase in response to dopamine is observed. Values are means ± s.e.m., N=3, **P<0.001 vs no drug control. (E) Dose–response curves of 5-HT2βPro to biogenic amines in IP assay. 5-HT2βPro responds to 5-HT (squares) with an EC50 of 270 nmol l−1. Dopamine (circles) activates the receptor only at very high concentrations. Non-transfected HEK cells do not respond to 5-HT (crosses). Values are means ± s.e.m., N=3.
Table 2.
Agonist profiles of 5-HT2β and 5-HT1α from Panulirus and Procambarus are very similar
| Drug | Potency (EC50, μmol l−1)
|
Efficacy (%5-HT effect)
|
||||||
|---|---|---|---|---|---|---|---|---|
| 5-HT2βPan | 5-HT2βPro | 5-HT1αPan | 5-HT1αPro | 5-HT2βPan | 5-HT2βPro | 5-HT1αPan | 5-HT1αPro | |
| 5-HT | 0.052 | 0.27 | 0.0084 | 0.031 | 100 | 100 | 100 | 100 |
| Dopamine | 310 | 362 | IA | IA | 54 | 19 | IA | IA |
| Octopamine | IA | IA | IA | IA | IA | IA | IA | IA |
| Tyramine | 283 | IA | IA | 30 | 32 | IA | IA | 67 |
| Histamine | IA | IA | IA | IA | IA | IA | IA | IA |
| DOI | 4.5 | IA | Bkd | NS | 32 | IA | Bkd | NS |
| 5-CT | 6.1 | 4.6 | 2.2 | NS | 79 | 50 | 100 | NS |
| 2-Me-5-HT | 0.78 | 5.2 | Bkd | 0.043 | 96 | 104 | Bkd | 73 |
| MeOTryp | 1.0 | 1.5 | 4.2 | NS | 80 | 29 | 94 | NS |
| N-acetyl-5-HT | IA | IA | IA | NS | IA | IA | IA | NS |
| Quipazine | IA | IA | Bkd | 111 | IA | IA | Bkd | 92 |
| α-Me-HT | 1.5 | 7.3 | 1.1 | 0.22 | 79 | 76 | 120 | 67 |
| 8-OH-DPAT | 0.27 | 1.1 | 7.6 | 65 | 77 | 64 | 105 | 68 |
| mCPP | IA | IA | 139 | 109 | IA | IA | 97 | 70 |
| Methysergide | 0.11 | 0.11 | 0.089 | 0.42 | 48 | 19 | 81 | 109 |
EC50 values (potency) and relative efficacy were calculated from dose–response curves for each drug. Efficacy is presented as a given drug’s ability to activate the receptor compared to the maximum activation obtained from 5-HT (100%). Drugs that activate one and not the other of 5-HT2β and 5-HT1α for each species are indicated in bold.
IA, inactive; Bkd, drug has background activity on non-induced cells and was not tested; NS, curve could not be fit because of complex effects of the drug. N≥3 separate experiments for each drug.
DOI, 2,5-dimethoxy-4-iodoamphetamine; 5-CT, 5-carboxamidotryptamine; 2-Me-5-HT, 2-methyl-serotonin; MeOTryp, 5-methoxytryptamine; α-Me-5-HT, α-methyl-serotonin; 8-OH-DPAT, (±)-8-hydroxy-2-(di-n-dipropylamino) tetralin; mCPP, 1-(m-chlorophenyl)-piperazine.
Amine specificity of 5-HT1αPan and 5-HT1αPro
We were not able to express 5-HT1αPan or 5-HT1αPro in traditional systems including HEK293, NIH3T3, MDCK or COS-7 cells; all cells that produced the receptor protein, as determined by western blot analysis, were unhealthy and did not grow beyond 3 weeks. In the few cases where a stable cell line was generated, 5-HT1α protein could not be detected by western blot analysis, suggesting rearrangements in the DNA construct. We do not understand why mammalian cell lines were unable to stably express 5-HT1α using traditional methods. 5-HT1αPan and 5-HT1αPro appeared to be constitutively active (below) so high levels of expression of the receptors in standard expression systems may have resulted in toxicity. Alternatively, the protein synthesis, export or turnover machineries of the cells may have been overly taxed by high levels of 5-HT1α expression such that they were not able to maintain normal functions.
In order to functionally characterize 5-HT1αPan and 5-HT1αPro, we therefore employed an inducible expression system. 5-HT1αPan or 5-HT1αPro constructs were stably transfected into 293-TR cells expressing the tetracycline repressor protein. In this system the 5-HT1α construct is under control of the Tet operator sequence, which binds the repressor protein in the absence of tetracycline, thereby preventing expression of 5-HT1α. Upon addition of tetracycline to the media, the repressor protein dissociates and 5-HT1α is transcribed and translated into protein. The western blot in Fig. 1 indicates that non-induced cells (tetracycline absent) did not express detectable levels of 5-HT1αPan. After induction (tetracycline present) we were able to obtain high levels of 5-HT1αPan expression within 6 h that lasted for at least 24 h (Fig. 1). Similar results were obtained for cells induced to express 5-HT1αPro (not shown).
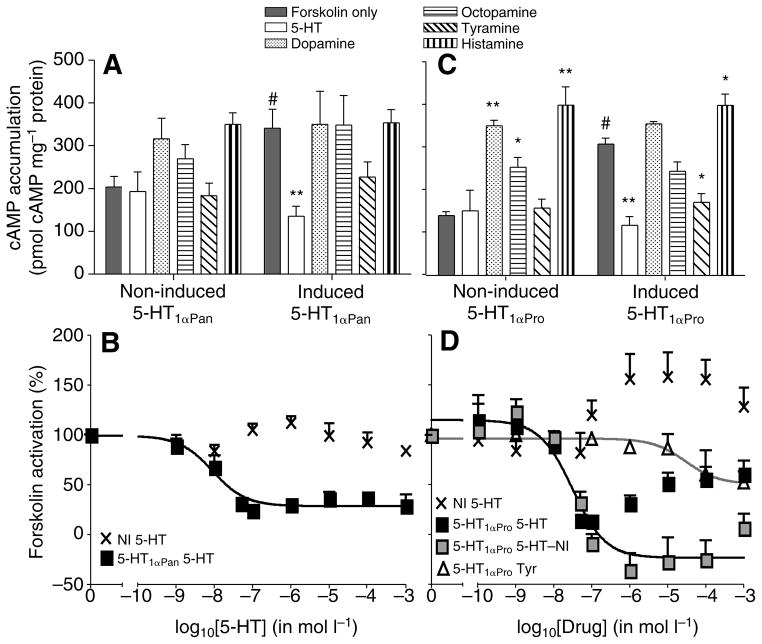
When compared with non-induced cells, induced cells expressing either 5-HT1αPan or 5-HT1αPro showed an increased sensitivity to forskolin, a non-specific activator of adenylyl cyclase (Fig. 5A,C). This supersensitization of adenylyl cyclase is typical of cells expressing a constitutively active Gi/o-coupled receptor (Johnston and Watts, 2003). Constitutive activity has been observed in mammalian 5-HT1 receptors as well as other G protein-coupled receptors (Berg et al., 2005; Cosi and Koek, 2000; Johnston and Watts, 2003; Liu et al., 1999).
Fig. 5.
5-HT is the only biogenic amine that acts as a potent agonist at 5-HT1αPan and 5-HT1αPro. (A) Inhibition of forskolin-stimulated cAMP accumulation in response to biogenic amines (10−3 mol l−1) in cells induced to express 5-HT1αPan. Non-induced (NI) cells do not significantly respond to biogenic amines (left). Induced cells show supersensitivity and accumulate significantly higher levels of cAMP in response to forskolin only (grey bars). In induced cells adenylyl cyclase is significantly inhibited in response to 1 mmol l−1 5-HT but no other biogenic amines (right). Values are means ± s.e.m., N=3, #P<0.05 vs non-induced, **P<0.001 vs forskolin only. (B) Dose–response curve of 5-HT1αPan (squares) to 5-HT. The EC50 of 5-HT at 5-HT1αPan is 8.4 nmol l−1. Non-induced cells do not show significant changes in forskolin-stimulated cAMP accumulation in response to 5-HT (crosses). Values are means ± s.e.m., N=3. (C) Inhibition of forskolin-stimulated cAMP accumulation in response to amines (10−3 mol l−1) in cells induced to express 5-HT1αPro. Adenylyl cyclase in non-induced cells is activated by dopamine, octopamine and histamine (left). Induced cells accumulate significantly higher levels of cAMP in response to forskolin only (grey bars). In induced cells adenylyl cyclase is significantly inhibited in response to 1 mmol l−1 5-HT and tyramine but no other biogenic amines (right). As observed in non-induced cells, histamine elicits an increase in cAMP levels in cells expressing 5-HT1αPro. Values are means ± s.e.m. N=3, #P<0.001 vs non-induced, **P<0.001 and *P<0.05 vs forskolin only. (D) Non-induced (NI) cells show a positive response in cAMP levels at high concentrations of 5-HT (crosses). Owing to this background activity, the dose–response curve of 5-HT1αPro (black squares) to 5-HT is biphasic. When the activity of non-induced cells in response to 5-HT is subtracted from the response of induced cells (5-HT–NI; grey squares), a sigmoidal dose–response curve is obtained. This curve has an EC50 for 5-HT at 5-HT1αPro of 31 nmol l−1. Even at high concentrations tyramine has a minimal effect on 5-HT1αPro (triangles). Values are means ± s.e.m., N=3.
All known vertebrate and invertebrate 5-HT1 receptors inhibit adenylyl cyclase, resulting in decreased cAMP levels after stimulation with forskolin (Hoyer et al., 2002; Tierney, 2001). As expected for a 5-HT1 receptor expressed in a HEK293 cell line, 5-HT1αPan activation with 5-HT inhibited forskolin-stimulated cAMP accumulation, presumably via Gi/o inhibition of adenylyl cyclase. At 1 mmol l−1 concentrations, 5-HT was the only monoamine to significantly activate 5-HT1αPan (Fig. 5A). 5-HT is a highly effective ligand at 5-HT1αPan with an EC50 of 8.4 nmol l−1 (Fig. 5B, Table 2). No significant change in cAMP levels was observed with any biogenic amine in non-induced 293-TR-5-HT1αPan cells (Fig. 5A).
Similarly, 5-HT1αPro activation with 1 mmol l−1 5-HT also blocks forskolin-stimulated cAMP accumulation. Tyr was the only other biogenic amine that resulted in a significant decrease of cAMP in cells expressing 5-HT1αPro (Fig. 5C). Serotonin and tyramine are therefore the only amines that significantly inhibit adenylyl cyclase via activation of the 5-HT1αPro receptor. Histamine, dopamine and octopamine all produced an increase in cAMP levels in non-induced cells. Similar levels of cAMP were observed in the induced cells, suggesting that these amines do not act at the 5-HT1αPro receptor. Non-induced 293-TR-5-HT1αPro cells gave a positive cAMP response at high 5-HT concentrations (Fig. 5D, crosses). Unlike the cell line expressing Panulirus 5-HT1α, during the selection period, the 5-HT1αPro cell line appears to have initiated expression of an endogenous 5-HT receptor that is positively coupled to adenylyl cyclase. It is not unusual for cell cultures to change their karyotypes or expression profiles over time due to the lack of selection pressure to maintain a constant genome. Such changes can be significant and can lead to different net signaling effects for the same protein (Clark and Baro, 2007; Friedman et al., 2002). Cells that were induced to produce 5-HT1αPro responded to low 5-HT concentrations with a decrease in forskolin-induced cAMP mediated by 5-HT1αPro. This effect was dampened and partially reversed at higher 5-HT concentrations through activation of the endogenous receptor (Fig. 5D, black squares). Subtraction of the 5-HT curves of non-induced cells (Fig. 5D, crosses) from induced cells (Fig. 5D, black squares) resulted in a sigmoidal dose–response for 5-HT at 5-HT1αPro with an EC50 of 31 nmol l−1 (Fig. 5D, grey squares). Tyramine was an inefficient agonist and slightly reduced cAMP accumulation only at high concentrations (Fig. 5D, triangles) indicating that 5-HT is the preferred functional ligand for 5-HT1αPro.
Agonist drugs of crustacean 5-HT receptors
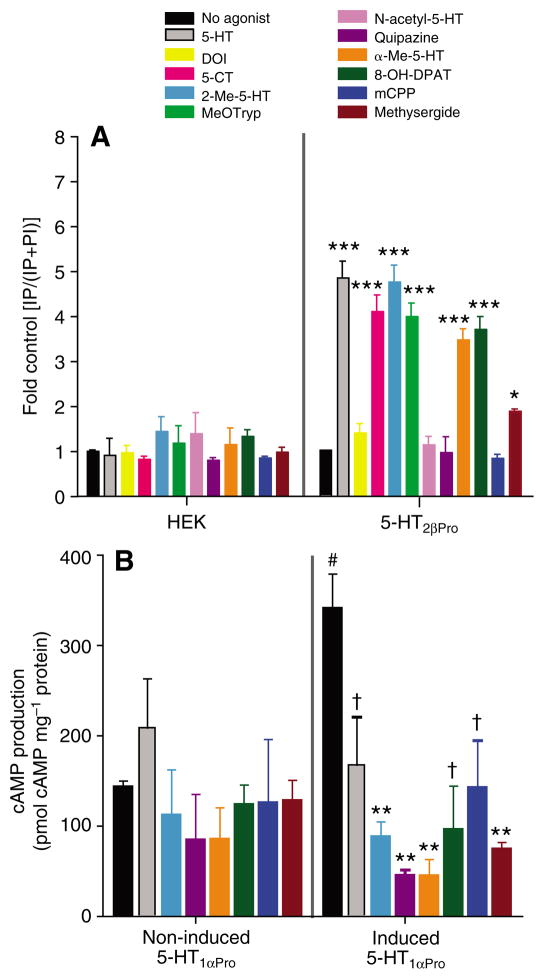
In order to determine if the conservation in sequence and signaling extends to the receptors’ responses to pharmacological agents, we tested crayfish 5-HT2βPro and 5-HT1αPro with a suite of agonist drugs. All drugs that showed significant activity at 10−3 mol l−1 in an initial overview (Fig. 6) were tested in dose–response curves (see Fig. 7 for examples), which were then used to determine the potency and efficacy (% of maximum 5-HT response at highest concentration) of the drugs. The EC50 is a measure of the potency of a drug and reflects its binding affinity at the receptor. Because the maximum effect, or efficacy, achieved by any drug is dependent on the number of receptors expressed we ran a parallel dose–response curve for 5-HT in every experiment and normalized all agonist maximum effects to the maximum 5-HT response, set at 100%. These data have been previously reported for the Panulirus receptor (N.S. and D.J.B., unpublished) and are summarized with the Procambarus receptor data in Table 2 for comparison.
Fig. 6.
Specific drugs have differential agonist activity at 5-HT2β and 5-HT1α. (A) Release of IP in cells expressing 5-HT2βPro (right) in response to various putative agonist drugs (10−3 mol l−1, except methysergide, 10−5 mol l−1). Non-transfected HEK cells (left) show no IP release in response to the drugs. Values are means ± s.e.m., N=3, ***P<0.001 and *P<0.05 vs no drug. (B) Inhibition of forskolin-stimulated cAMP production in response to the same drugs in cells induced to express 5-HT1αPro. Values are means ± s.e.m., N=3, #P<0.05 vs non-induced, **P<0.001 and †P<0.05 vs forskolin only.
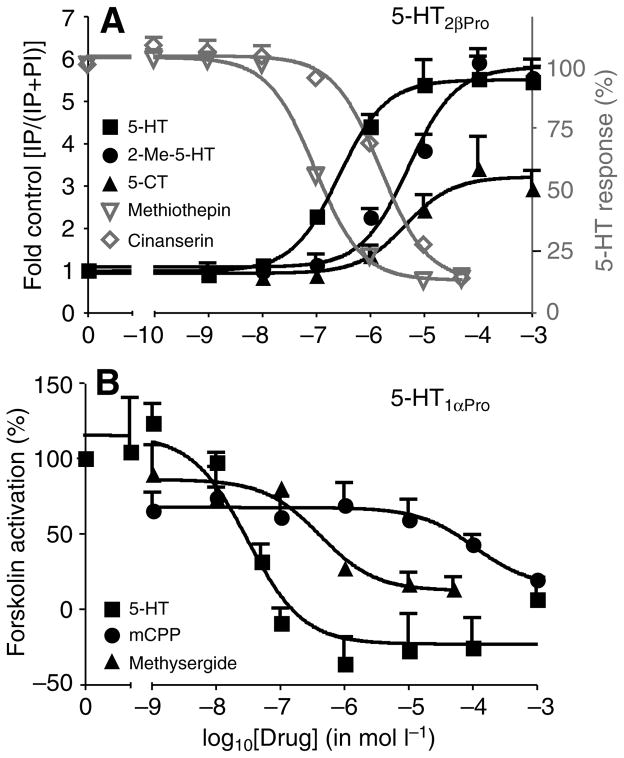
Fig. 7.
Example dose–response curves for agonists and antagonists at 5-HT2βPro and 5-HT1αPro. (A) Cells expressing 5-HT2βPro respond with a dose-dependent increase in IP levels in response to 5-HT (10−3 mol l−1; black squares) and two agonists with different potencies and efficacies, 2-methyl-5-HT (black circles) and 5-XT (black triangles). The 5-HT-indexed IP response is blocked by increasing concentrations of two antagonists, methiothepin (grey inverted triangles) and cinanserin (grey diamonds). Values are means ± s.e.m., N=3. (B) Dose-dependent responses to 5-HT (squares) and two agonists, mCPP (circles) and methysergide (triangles) with different potencies and efficacies in cells induced to express 5-HT1aPro. Values are means ± s.e.m., N=3.
5-HT2β agonists
Most of the drugs that activated 5-HT2βPro had an efficacy close to that of 5-HT (Fig. 6A, Table 2). Methysergide (EC50=110 nmol l−1) was more potent than 5-HT (EC50=270 nmol l−1), however, it elicited only 19% of the total response obtained with 5-HT. Thus, when both potency and efficacy are considered, 5-HT is the strongest ligand at 5-HT2βPro. The rank potency of effective agonists at 5-HT2βPro was methysergide>5-HT>8-OH-DPAT>MeOTryp>5-CT>2-Me-5-HT>α-Me-5-HT. This was very similar to the potency ranking of these drugs at 5-HT2βPan. However, as can be seen in Table 2, the potency of drugs at the crayfish receptor was generally lower than in lobster, with 5-HT being five times less potent. The crayfish ortholog did not respond to 2,5-dimethoxy-4-iodoamphetamine (DOI), which was a relatively weak agonist of 5-HT2βPan. The potencies of methysergide, 5-carboxamido-tryptamine (5-CT) and 5-methoxytryptamine (MeOTryp) were similar for the two orthologs, but their efficacies were twofold lower at the 5-HT2βPro ortholog (Table 2). However, 2-methyl-serotonin (2-Me-5-HT) was less potent at 5-HT2βPro, whereas ortholog efficacies were equivalent. Together these data indicate that, whereas the pharmacological profiles of 5-HT2βPro and 5-HT2βPan are conserved in terms of which drugs are active, 5-HT2βPro was consistently less sensitive to agonist stimulation. As observed for 5-HT2βPan, no change in IP level was detected in cells expressing 5-HT2γPro after application of 10–3 mol l−1 N-acetyl-5-HT, quipazine, or 1-(m-chlorophenyl)-piperazine (mCPP). None of the drugs had significant effects on non-transfected parental HEK cells (Fig. 6A).
5-HT1α agonists
Most of the putative agonists tested (at 1 mmol l−1) resulted in some activation of the 5-HT1αPro receptor (Fig. 6B, Table 2). The rank potency of effective agonists at 5-HT1αPro was 5-HT > 2-Me-5-HT > α-Me-5-HT > methysergide > tyramine > 8-OH-DPAT > mCPP > quipazine. The agonist 2-Me-5-HT was almost as potent but only 73% as efficacious as 5-HT. This pharmacological profile is very similar to that of its lobster ortholog, 5-HT1αPan. However, as for the 5-HT2βPan receptor, the crayfish ortholog of 5-HT1αPro was generally less sensitive to agonists than the lobster ortholog. ±-Methyl-serotonin (α-Me-5-HT) was more potent, and (±)-8-hydroxy-2-(di-n-dipropylamino) tetralin (8-OH-DPAT) was less potent at 5-HT1αPro relative to 5-HT1αPan, however, both these drugs had lower efficacies at the crayfish ortholog. The potency of mCPP was comparable for Panulirus and Procambarus 5-HT1α, although it was less efficient at the crayfish ortholog. Methysergide was less potent but had a higher efficacy at 5-HT1αPro compared to 5-HT1αPan. Two drugs that could not be tested at 5-HT1αPan because they altered cAMP levels in non-induced 293-TR-5-HT1αPan cells, 2-Me-5-HT and quipazine, are effective agonists of 5-HT1αPro. Several drugs (DOI, 5-CT, MeOTryp, N-acetyl-5-HT) had complex effects on induced 293-TR-5-HT1αPro cells that could not be fitted with standard dose–response curves. These complex effects are probably due to endogenous 5-HT receptors expressed by the non-induced cell line as observed in the 5-HT dose–response curve above (Fig. 5D, crosses). We were therefore not able to determine an EC50 or relative efficacy measurements for these drugs.
In summary, we identified two agonists that would activate 5-HT1α but not 5-HT2β (Table 2). mCPP is an agonist of 5-HT1α but not 5-HT2β in Procambarus and Panulirus whereas quipazine is also inactive at 5-HT2β from either species and activates 5-HT1αPro but could not be tested on 5-HT1αPan.
Antagonist drugs of crustacean 5-HT receptors
Because pharmacological agents can be active at multiple 5-HT receptors, strategic combinations of drugs will be necessary to identify the receptors involved in physiological 5-HT effects. In addition to agonists, we therefore tested a suite of putative antagonists on 5-HT2β and 5-HT1α from Procambarus and compared them to the antagonist profile for Panulirus receptors (N.S. and D.J.B., unpublished).
Antagonists were applied to cells 10 min before 5-HT application and second messenger assays were used to test receptor activation. Antagonists were first screened at 10−5 mol l−1 (Fig. 8) and then dose–response curves were generated for any drugs that significantly blocked 5-HT activation of second messengers at that concentration (see Fig. 7 for examples). The IC50 was calculated and is reported as a measure of potency for the drug. The efficacy, or maximum effect, for each drug again depends on receptor expression levels and is therefore reported as a percentage reduction from the level of receptor activation achieved by 5-HT alone in the same experiment (Table 3).
Fig. 8.
Identification of antagonists of 5-HT2β and 5-HT1α. (A) Putative antagonists have no effect on the parental HEK cell line (left). Several of the antagonists (10−5 mol l−1) block 5-HT (1 mmol l−1)-stimulated increases in IP levels in cells expressing 5-HT2βPro (right). Values are means ± s.e.m., N=3, *P<0.05 vs 5-HT only. (B) Antagonist drugs have no significant effect on non-induced 5-HT1αPro cells (left). None of these drugs (10 mmol l−1) blocks inhibition of adenylyl cyclase by 10−5 mol l−1 5-HT in cells induced to express 5-HT1αPro (right). Some putative antagonists actually increase the efficacy of the 5-HT effect. Values are means ± s.e.m., N=3, *P<0.05 vs 5-HT only.
Table 3.
Antagonist profiles of 5-HT2β and 5-HT1α from Panulirus and Procambarus are well conserved
| Drug | Potency (IC50, μmol l−1)
|
Efficacy (% reduction)
|
||||
|---|---|---|---|---|---|---|
| 5-HT2βPan | 5-HT2βPro | 5-HT1αPan | 5-HT1αPro | 5-HT2βPan | 5-HT2βPro | |
| Clozapine | 6.8 | 2.5 | Bkd | Bkd | 79 | 86 |
| Ritanserin | 0.57 | 0.18 | IA | Bkd | 58 | 86 |
| Methiothepin | 0.66 | 0.097 | Bkd | IA | 84 | 88 |
| (+)Butaclamol | 0.14 | 0.017 | IA | Bkd | 48 | 82 |
| Cinanserin | 1.2 | 1.6 | IA | IA | 73 | 88 |
| Gramine | IA | IA | IA | Bkd | IA | IA |
| (−)Butaclamol | IA | IA | IA | IA | IA | IA |
| Ketanserin | IA | IA | IA | IA | IA | IA |
| Spiperone | IA | IA | IA | IA | IA | ND |
| Prazosin | IA | IA | IA | Bkd | IA | IA |
| Atropine | IA | IA | IA | Bkd | IA | IA |
| Chlorpromazine | ND | ND | IA | IA | ND | ND |
| Flupenthixol | ND | ND | IA | Bkd | ND | ND |
| Domperidone | ND | ND | IA | Bkd | ND | ND |
| Fluphenazine | ND | ND | IA | Bkd | ND | ND |
| Haloperidol | ND | ND | IA | IA | ND | ND |
| Metoclopride | ND | ND | IA | IA | ND | ND |
| (−)Sulpiride | ND | ND | IA | Bkd | ND | ND |
| WAY100635 | ND | ND | IA | IA | ND | ND |
| Yohimbine | ND | ND | IA | IA | ND | ND |
| S(−)Propanolol | ND | ND | Bkd | IA | ND | ND |
| SB269970 | ND | ND | IA | IA | ND | ND |
| Metergoline | ND | ND | Bkd | Bkd | ND | ND |
| Cyproheptadine | ND | ND | Bkd | Bkd | ND | ND |
| SB224289 | ND | ND | IA | Bkd | ND | ND |
| BRL15572 | ND | ND | IA | IA | ND | ND |
| TFMPP | ND | ND | IA | IA | ND | ND |
| SCH23390 | ND | ND | Bkd | Bkd | ND | ND |
| S(−)eticlopride | ND | ND | Bkd | Bkd | ND | ND |
IC50 values (potency) and relative efficacy were calculated from dose–response curves for each drug. Efficacy is presented as the percentage reduction of the total effect obtained from 5-HT in the absence of antagonist. Drugs that block one and not the other of 5-HT2β and 5-HT1α for each species are indicated in bold. N≥3 separate experiments for each drug.
IA, inactive; Bkd, drug has background activity on non-induced cells and was not tested on induced cells, ND, not determined.
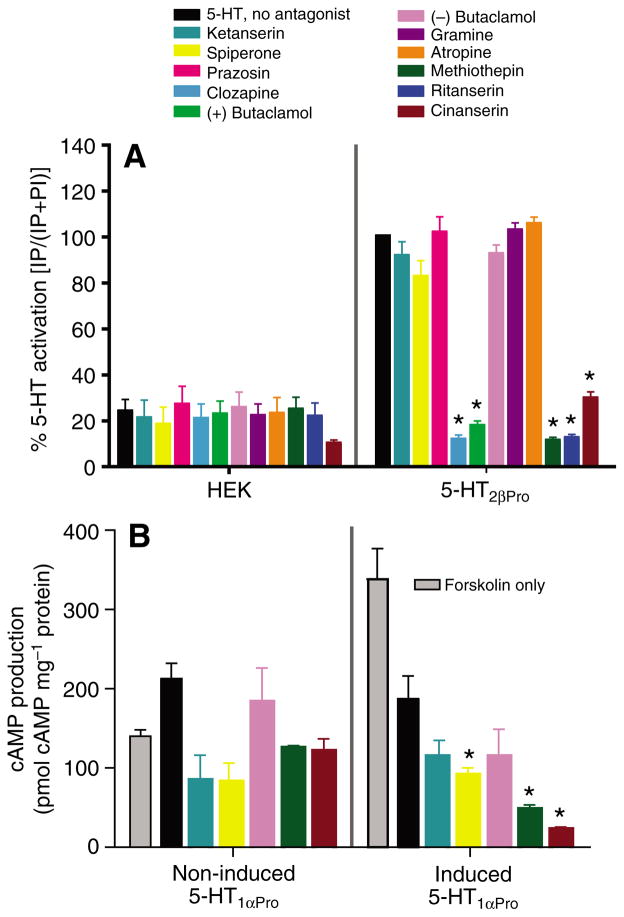
5-HT2β antagonists
Putative antagonists had no effect on parental HEK cells (Fig. 8A). Several antagonists (at 10 μmol l−1) significantly reduced 5-HT-induced (1 mmol l−1) IP release in HEK cells expressing 5-HT2βPro (Fig. 8A, Table 3). The rank potency of effective antagonists at 5-HT2βPro was (+)butaclamol > methiothepin > ritanserin > cinanserin > clozapine (Table 3). Of the antagonists tested, ketanserin, spiperone, prazosin, (−)butaclamol, gramine and atropine had no effect at 10−5 mol l−1. The potency profile of active antagonists was nearly conserved between 5-HT2β from crayfish and lobster, but four of the five drugs had lower IC50s at the crayfish ortholog. In addition, all of the drugs blocked the crayfish receptor by >80%, but the lobster receptor by only 48–84%. 5-HT2β from lobster and crayfish diverge by almost 30% in the variable regions (Table 1) and these differences could contribute to the increase in potency and efficacy of antagonists at 5-HT2βPro.
5-HT1α antagonists
Twenty-nine putative antagonists, including those that were characterized at 5-HT2βPro, were tested on 5-HT1αPro (Fig. 8B, Table 3). None of 14 putative antagonists (at 10 μmol l−1) were able to block 5-HT (at 10 μmol l−1) activation of 5-HT1αPro. The antagonists were ineffective even at 10 nmol l−1 (data not shown). These results agree with what we found for 5-HT1αPan from Panulirus where the same putative antagonists also did not block activation of the receptor (Table 3). Because of background activity, presumably at endogenous GPCRs in the non-induced cell line, we were not able to test the 15 other putative antagonists on cells induced to express 5-HT1αPro (Table 3). It is not surprising that the uninduced Pan and Pro cell lines showed different background activities, as it is well established that cell lines diverge with time in culture (Clark and Baro, 2007). Further, multiple copies of the plasmid DNA can be integrated into the host genome, and this has the potential to alter expression of nearby genes. It is unlikely that the number or location of plasmid insertions would be identical in the two cell lines.
As was seen at 5-HT1αPan, several putative antagonists appeared to increase the efficacy of the 5-HT effect on cAMP accumulation in cells expressing 5-HT1αPro (Fig. 8B). When tested without 5-HT, however, these drugs had no significant effect on forskolin-stimulated cAMP production (not shown), indicating that they are not acting as agonists. Further studies will be necessary to determine the relationship between antagonist drugs and the crustacean 5-HT1α receptors.
Although we could not find effective 5-HT1αPro antagonists, we were able to identify several drugs that would block 5-HT2β while not affecting 5-HT activation of 5-HT1α (Table 3). Methiothepin and cinanserin efficiently blocked 5-HT2βPro but had no activity at 5-HT1αPro. Similarly, cinanserin, (+)butaclamol and ritanserin all blocked 5-HT2βPan but not 5-HT1αPan. All four of these drugs are effective 5-HT2β antagonists but some could not be tested at the 5-HT1α receptor from one or the other species to confirm specificity because of differences in the parental 5-HT1α cell lines.
DISCUSSION
Two 5-HT receptors from two infraorders of decapod crustaceans, Panulirus (Achelata) and Procambarus (Astacidea) are highly conserved in their sequence, signaling and pharmacological properties. A significant portion of the 5-HT1α receptor from the giant freshwater prawn Macrobrachium rosenbergii, has also been cloned and is also highly conserved with its lobster and crayfish orthologs (Sosa et al., 2004). Together, these data suggest that the conservation of pharmacological function observed between the species studied here may extend beyond reptantian crustaceans, in which case the drugs identified here could perhaps be useful for dissecting 5-HT mechanisms in diverse crustacean systems. We have identified several antagonists, (+)butaclamol, cinanserin and ritanserin for Panulirus and methiothepin and cinanserin for Procambarus, that will block 5-HT2β while leaving 5-HT1α unaffected. In addition, mCPP weakly activates 5-HT1α while having no effect on 5-HT2β for both species whereas quipazine activates 5-HT1βPro but not 5-HT2βPro. Combinations of these drugs can be applied in studies of the mechanisms underlying 5-HT modulation in identified circuits and cells of the California spiny lobster, crayfish and related crustacean nervous systems.
Conservation of crustacean 5-HT receptor structure and signaling
Panulirus and Procambarus orthologs of 5-HT2β and 5-HT1α have the 5-HT signature sequences required for ligand binding and G protein coupling. Most of the sequence differences between receptor orthologs were located in the amino termini and the nonconserved center of the third intracellular loop. These regions may contribute to differences in sensitivity to pharmacological agents observed between receptor orthologs; however, no specific function has been ascribed to these regions to date (Kroeze et al., 2002). Indeed, in characterizing the Drosophila 5-HT1 and 5-HT7 receptors, the amino termini were removed to increase expression levels in cell culture, with no apparent effect on receptor function (Saudou et al., 1992; Witz et al., 1990).
5-HT was the only biogenic amine to significantly activate 5-HT2βPro, however, DA and Tyr were also weak agonists of 5-HT2βPan, suggesting that these amines could activate 5-HT2βPan in a physiological context. By contrast, Clark et al. (Clark et al., 2004) found that 5-HT was the only biogenic amine to significantly activate 5-HT2βPan. Thus, it is not clear whether or not DA and Tyr have any effect on the 5-HT2βPan receptor in the native system. If these amines do activate native receptors, then is likely to be a concern only at synaptic sites where concentrations can reach up to 1 mmol l−1 [(Clements, 1996; Frerking and Wilson, 1996) and references therein] because, high levels of DA and Tyr are required to activate 5-HT2βPan. The difference between the two studies may be because we measured IP release [i.e. phospholipase Cβ (PLCβ) activity] whereas the previous study measured PKC activity. Because PKC is downstream of PLCβ and dependent on the consequent release of Ca2+, low levels of PLCβ activation by DA and Tyr may not be sufficient to initiate a cascade culminating in PKC activation. Alternatively, the difference may stem from our use of transiently transfected cells whereas Clark et al. (Clark et al., 2004) studied stable transfectants. In addition, 5-HT2βPan was found to be constitutively active when stably expressed in HEK293 cells (Clark et al., 2004). However, in our transient transfections with 5-HT2βPan and 5-HT2βPro we found no constitutive activity. The reason for the difference is unclear.
Crustacean 5-HT1α receptors are typical type 1 5-HT receptors, preferentially responding to 5-HT over other biogenic amines with EC50 values of 8.4 and 31 nmol l−1, respectively. Other arthropod orthologs of 5-HT1αPan and 5-HT1αPro have been cloned and characterized from Drosophila (5-HT1ADro, originally 5-HTdro2A) and Boophilus microplus and these have comparable EC50 values of 30 and 83 nmol l−1 for 5-HT, respectively (Chen et al., 2004; Saudou et al., 1992). Whereas the lobster ortholog responded only to 5-HT, the crayfish ortholog was also activated by Tyr. Activation of 5-HT1 receptors, including those described here, results in inhibition of forskolin-stimulated cAMP accumulation. In addition, the crustacean 5-HT1α receptor shows agonist-independent activity when expressed in HEK cells.
Conservation of crustacean 5-HT receptor pharmacological profiles
The pharmacological profiles of 5-HT receptors are very similar for Procambarus and Panulirus, but show more variability when compared with homologs from other invertebrates. Agonist activity at crustacean 5-HT2β and 5-HT1α orthologs is quite conserved. Two agonists that would differentiate between 5-HT2β and 5-HT1α were identified. mCPP activates 5-HT1α but not 5-HT2β in both Procambarus and Panulirus. Quipazine is also specific to 5-HT1αPro over 5-HT2βPro and, similarly, is inactive at 5-HT2βPan but could not be tested on 5-HT1αPan. Quipazine binds weakly to a molluskan 5-HT1 receptor (Sugamori et al., 1993) and to 5-HT2αDro (Colas et al., 1995), indicating that its specificity may not be highly conserved among invertebrates. 8-OH-DPAT was an effective agonist of both crustacean 5-HT2β and 5-HT1α, and is also the only agonist reported to bind 5-HT1αDro (Saudou et al., 1992). 8-OH-DPAT was considered a specific mammalian 5-HT1 agonist but was later found to also activate 5-HT7 receptors (Bard et al., 1993; Sprouse et al., 2004) and it activates Drosophila 5-HT7Dro (Witz et al., 1990). Interestingly, methysergide is an agonist at crustacean 5-HT2β and 5-HT1α. This drug is a functional antagonist of 5-HT7Dro (Witz et al., 1990) and of vertebrate 5-HT2 receptors but has agonist activity at some vertebrate 5-HT1 receptors (Silberstein, 1998).
The antagonist profiles of 5-HT2β receptors from Panulirus and Procambarus are also very well conserved. Cinanserin, (+)butaclamol, ritanserin and methiothepin were identified as antagonists that would block crustacean 5-HT2β but not 5-HT1α receptors, however not all of these could be tested at 5-HT1α from both species. The antagonist profiles of the crustacean receptors could not be compared to other arthropod orthologs, as 5-HT2β receptors have only been cloned from crustaceans, though they have been found in the fruit fly, honey bee and mosquito sequence databases. A Drosophila paralog of the 5-HT2β receptor, 5-HT2αDro [originally 5-HT2Dro (see Tierney, 2001; Clark et al., 2004)] binds strongly to α-Me-5-HT, 2-Me-5-HT, ritanserin and methysergide (Colas et al., 1995), all of which we found to be functionally active at 5-HT2βPan and 5-HT2βPro. These drugs may therefore, be 5-HT2-specific antagonists in arthropods. N-acetyl-5-HT and ketanserin were completely inactive at crustacean 5-HT2β but have very high binding constants at 5-HT2αDro. Effective binding of a drug to a receptor may not directly reflect that drug’s functional properties at the receptor. 5-HT2β and 5-HT2α may therefore be more or less similar, functionally, than suggested by these comparisons. Looking beyond arthropods, several 5-HT1 and 5-HT2 type receptors from mollusks and nematodes, also bind methiothepin or ritanserin (Angers et al., 1998; Gerhardt et al., 1996; Hamdan et al., 1999; Olde and McCombie, 1997; Sugamori et al., 1993). Similarly, (+)butaclamol binds a variety of 5-HT receptor subtypes from diverse species (Hamdan et al., 1999; Olde and McCombie, 1997; Saudou et al., 1992). In sum, the antagonist profile of 5-HT receptors appears to be well conserved among crustaceans but this may not extend between phyla.
Crustacean 5-HT1α receptors are highly resistant to antagonists. Even the potent and selective mammalian 5-HT1A blocker WAY100635 (Hoyer et al., 2002) was ineffective at 5-HT1αPan. Prazosin, which functionally blocks 5-HT1Dro (Saudou et al., 1992), also had no effect on crustacean 5-HT1α. Many of these putative antagonists block functional activation of mammalian (Hoyer et al., 2002) and invertebrate (Barbas et al., 2002; Hobson et al., 2003; Li et al., 1995; Saudou et al., 1992; Witz et al., 1990) 5-HT receptors. Although some of these may act as allosteric modulators and therefore be sensitive to the high variability of the N-terminal tail amongst 5-HT receptors (May et al., 2004), several of the drugs do efficiently displace radioligands at arthropod receptors (Tierney, 2001) and therefore presumably bind at or near the ligand binding pocket. For none of these to be capable of blocking 5-HT activation of crustacean 5-HT1α is unexpected. 5-HT1α may have an extraordinarily high affinity and selectivity for 5-HT such that the antagonists are not able to overcome the binding of and/or conformational changes elicited by 5-HT. Radioligand studies done on other arthropod 5-HT1α receptors, however, do not fully support this hypothesis as some putative antagonist drugs bind more strongly than 5-HT (Colas et al., 1995; Saudou et al., 1992). Alternatively, the exogenously expressed crustacean 5-HT1α may be in a hyperactive state that overcomes any antagonist effects. This might explain the high response to almost all the putative agonists we tested.
Putative function of characterized 5-HT receptors in crustacean physiological systems
We previously showed that 5-HT1α was extensively expressed in thoracic ganglia of crayfish and Macrobrachium rosenbergii, the giant freshwater prawn, in similar patterns (Sosa et al., 2004; Spitzer et al., 2005). Crustacean 5-HT receptors may therefore be conserved in their expression patterns as well as in their molecular structure and function. In crayfish, 5-HT1α is observed in somata and the neuropil throughout the central nerve cord. It is also localized to processes surrounding the nerve roots, to superficial flexor muscles of the abdomen and to processes on the vasculature (Spitzer et al., 2005). The 5-HT1α receptor therefore provides numerous targets for 5-HT modulation in the crayfish.
Several of the drugs characterized here have been used to investigate physiological mechanisms of 5-HT neuromodulation in crustaceans. Based on the pharmacological characterization of crustacean 5-HT2β and 5-HT1α we can now hypothesize which receptors might be involved in mediating specific effects and behaviors. In the lateral giant neuron of crayfish, mCPP elicited the inhibitory component of the 5-HT response (Yeh et al., 1997) indicating that 5-HT1αPro may be responsible. The facilitatory component of the 5-HT response was elicited by α-Me-5-HT (Yeh et al., 1997), an effective agonist of both 5-HT2βPro and 5-HT1αPro. One or both of these receptors could therefore contribute to facilitation of the circuit. In freely behaving crayfish, mCPP injection mimicked the effects of 5-HT on posture (Tierney et al., 2004; Tierney and Mangiamele, 2001) indicating that 5-HT1αPro may mediate 5-HT effects on posture. Crayfish abdominal muscles, essential to posture, are modulated by 5-HT at the motor neurons and the neuromuscular junction (Strawn et al., 2000). 5-HT1αPro receptors are localized on abdominal superficial flexor motorneurons (Spitzer et al., 2005) and could mediate this effect. In addition, 5-CT enhances agonistic behavior in Procambarus (Tierney and Mangiamele, 2001), indicating that 5-HT2βPro and/or 5-HT1αPro could mediate 5-HT signals eliciting these behaviors. Because posture is an essential component of agonistic behavior, these receptors may therefore contribute to different aspects of the agonistic behavioral program (Edwards and Kravitz, 1997; Huber et al., 1997; Kravitz, 2000). Although 5-HT1αPro is expressed by neurons throughout the crayfish eyestalk (Spitzer et al., 2005), it may not mediate 5-HT-elicited hyperglycemia as this was not mimicked by mCPP (Lee et al., 2000). 5-HT2βPro may, however, be involved as the response was evoked by both 5-CT and 8-OH-DPAT. In the stomatogastric nervous system of the crab, Cancer borealis, cinanserin blocked 5-HT-mediated acceleration of the pyloric rhythm (Zhang and Harris-Warrick, 1994) suggesting that the crab 5-HT2β ortholog may play a role in modulation of pyloric frequency. Indeed, we have identified roles for 5-HT2βPan, 5-HT1αPan and an unidentified 5-HT receptor in modulation of the pyloric rhythm in Panulirus using the drugs identified here (N.S. and D.J.B., unpublished).
Considering uncharacterized 5-HT receptors
At least three crustacean 5-HT receptors (5-HT1β, 5-HT2α, 5-HT7) remain uncharacterized (Clark et al., 2004; Tierney, 2001) and, as we demonstrate in comparing crustacean 5-HT2β and 5-HT1α receptor pharmacology, many drugs are active at multiple 5-HT receptor subtypes. Uncharacterized 5-HT receptors could therefore be contributing to observed effects. Especially when using a single agonist drug to elicit a ‘5-HT effect’, the possibility of activating multiple 5-HT or other aminergic receptors must be considered. In addition, radioligand displacement assays of Drosophila 5-HT1α and 5-HT1β, indicated that arthropod 5-HT receptor paralogs can have similar pharmacological properties (Saudou et al., 1992); their effects may therefore be difficult to tease apart even using several drugs. Combinations of drugs were, however, used to assign specific modulatory roles to 5-HT2β, 5-HT1α and an uncharacterized receptor in the stomatogastric nervous system of Panulirus (N.S. and D.J.B., unpublished). Profiling of additional receptor subtypes, the use of multiple characterized drugs and expression studies should allow greater confidence in assigning specific receptor types to behavioral effects.
Conclusion
In conclusion, we have shown that crustacean 5-HT1α receptors are functionally similar to homologous receptors across multiple vertebrate and invertebrate species; furthermore, 5-HT receptors are conserved in protein structure and signaling properties. For 15 drugs that we were able to test at both receptors in both species, the effects on Panulirus and Procambarus receptors were very comparable. Furthermore, the effects of 19 additional drugs that could only be compared for one receptor type were also similar between the two species. These comparisons suggest that the pharmacological profiles of 5-HT receptor orthologs between these two infraorders are relatively well conserved. Finally, we have identified drugs specific to 5-HT receptor subtypes and thus developed a pharmacological toolset for use in crustacean physiological preparations.
Acknowledgments
We are grateful to Merry Clark for training and insightful discussions and thank Jin Kim and Ping Jiang for excellent technical support. N.S. was a scholar in the Brains and Behavior Program and the Center for Behavioral Neuroscience at Georgia State University. This work was funded by NIH NS38770 to D.J.B., NIH MH62167 to D.H.E. and NSERC PGS-D2-304455-2004 to N.S. Partial support came from the NSF under agreement IBN-9876754 to the Center for Behavioral Neuroscience.
References
- Angers A, Storozhuk MV, Duchaine T, Castellucci VF, DesGroseillers L. Cloning and functional expression of an Aplysia 5HT receptor negatively coupled to adenylate cyclase. J Neurosci. 1998;18:5586–5593. doi: 10.1523/JNEUROSCI.18-15-05586.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ausubel A, Brent R, Kinston R, Moore D, Seidman J, Smith JA, Struhl K. Current Protocols in Molecular Biology. New York: Greene Publishing Associates and Wiley Interscience; 1990. [Google Scholar]
- Ballesteros JA, Weinstein H. Integrated methods for the construction of three-dimensional models and computational probing of structure-function relations in G protein coupled receptors. Methods Neurosci. 1995;25:366–428. [Google Scholar]
- Barbas D, Zappulla JP, Angers S, Bouvier M, Castellucci VF, DesGroseillers L. Functional characterization of a novel serotonin receptor (5-HTap2) expressed in the CNS of Aplysia californica. J Neurochem. 2002;80:335–345. doi: 10.1046/j.0022-3042.2001.00703.x. [DOI] [PubMed] [Google Scholar]
- Barbas D, DesGroseillers L, Castellucci VF, Carew TJ, Marinesco S. Multiple serotonergic mechanisms contributing to sensitization in Aplysia: evidence of diverse serotonin receptor subtypes. Learn Mem. 2003;10:373–386. doi: 10.1101/lm.66103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bard JA, Zgombick J, Adham N, Vaysse P, Branchek TA, Weinshank RL. Cloning of a novel human serotonin receptor (5-HT7) positively linked to adenylate cyclase. J Biol Chem. 1993;268:23422–23426. [PubMed] [Google Scholar]
- Baro DJ, Cole CL, Zarrin AR, Hughes S, Harris-Warrick RM. Shab gene expression in identified neurons of the pyloric network in the lobster stomatogastric ganglion. Recept Channels. 1994;2:193–205. [PubMed] [Google Scholar]
- Berg KA, Harvey JA, Spampinato U, Clarke WP. Physiological relevance of constitutive activity of 5-HT2A and 5-HT2C receptors. Trends Pharmacol Sci. 2005;26:625–630. doi: 10.1016/j.tips.2005.10.008. [DOI] [PubMed] [Google Scholar]
- Bicker G. Biogenic amines in the brain of the honeybee: cellular distribution, development, and behavioral functions. Microsc Res Tech. 1999;44:166–178. doi: 10.1002/(SICI)1097-0029(19990115/01)44:2/3<166::AID-JEMT8>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- Blenau W, Baumann A. Molecular and pharmacological properties of insect biogenic amine receptors: lessons from Drosophila melanogaster and Apis mellifera. Arch Insect Biochem Physiol. 2001;48:13–38. doi: 10.1002/arch.1055. [DOI] [PubMed] [Google Scholar]
- Chen A, Holmes SP, Pietrantonio PV. Molecular cloning and functional expression of a serotonin receptor from the Southern cattle tick, Boophilus microplus (Acari: Ixodidae) Insect Mol Biol. 2004;13:45–54. doi: 10.1111/j.1365-2583.2004.00457.x. [DOI] [PubMed] [Google Scholar]
- Clark MC, Baro DJ. Arthropod D2 receptors positively couple with cAMP through the Gi/o protein family. Comp Biochem Physiol. 2007;146B:9–19. doi: 10.1016/j.cbpb.2006.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark MC, Dever TE, Dever JJ, Xu P, Rehder V, Sosa MA, Baro DJ. Arthropod 5-HT2 receptors: a neurohormonal receptor in decapod crustaceans that displays agonist independent activity resulting from an evolutionary alteration to the DRY motif. J Neurosci. 2004;24:3421–3435. doi: 10.1523/JNEUROSCI.0062-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clements JD. Transmitter timecourse in the synaptic cleft: its role in central synaptic function. Trends Neurosci. 1996;19:163–171. doi: 10.1016/s0166-2236(96)10024-2. [DOI] [PubMed] [Google Scholar]
- Colas JF, Launay JM, Kellerman O, Rosay P, Maroteaux L. Drosophila 5-HT2 serotonin receptor: Coexpression with fushi-tarazu during segmentation. Proc Natl Acad Sci USA. 1995;92:5441–5445. doi: 10.1073/pnas.92.12.5441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosi C, Koek W. The putative “silent” 5-HT (1A) receptor antagonist, WAY 100635, has inverse agonist properties at cloned human 5-HT (1A) receptors. Eur J Pharmacol. 2000;401:9–15. doi: 10.1016/s0014-2999(00)00410-6. [DOI] [PubMed] [Google Scholar]
- Edwards DH, Kravitz EA. Serotonin, social status and aggression. Curr Opin Neurobiol. 1997;7:812–819. doi: 10.1016/s0959-4388(97)80140-7. [DOI] [PubMed] [Google Scholar]
- Edwards DH, Heitler WJ, Krasne FB. Fifty years of a command neuron: the neurobiology of escape behaviour in the crayfish. Trends Neurosci. 1999;22:153–161. doi: 10.1016/s0166-2236(98)01340-x. [DOI] [PubMed] [Google Scholar]
- Flamm RE, Harris-Warrick RM. Aminergic modulation in lobster stomatogastric ganglion. II Target neurons of dopamine, octopamine, and serotonin within the pyloric circuit. J Neurophysiol. 1986;55:866–881. doi: 10.1152/jn.1986.55.5.866. [DOI] [PubMed] [Google Scholar]
- Frerking M, Wilson M. Saturation of postsynaptic receptors at central synapses? Curr Opin Neurobiol. 1996;6:395–403. doi: 10.1016/s0959-4388(96)80125-5. [DOI] [PubMed] [Google Scholar]
- Friedman J, Babu B, Clark RB. Beta (2)-adrenergic receptor lacking the cyclic AMP-dependent protein kinase consensus sites fully activates extracellular signal-regulated kinase 1/2 in human embryonic kidney 293 cells: lack of evidence for G (s)/G (i) switching. Mol Pharmacol. 2002;62:1094–1102. doi: 10.1124/mol.62.5.1094. [DOI] [PubMed] [Google Scholar]
- Gerhardt CC, van Heerikhuizen H. Functional characteristics of heterologously expressed 5-HT receptors. Eur J Pharmacol. 1997;334:1–23. doi: 10.1016/s0014-2999(97)01115-1. [DOI] [PubMed] [Google Scholar]
- Gerhardt CC, Leysen JE, Planta RJ, Vreugdenhil E, van Heerikhuizen H. Functional characterization of a 5-HT2 receptor cDNA cloned from Lymnaea stagnalis. Eur J Pharmacol. 1996;311:249–258. doi: 10.1016/0014-2999(96)00410-4. [DOI] [PubMed] [Google Scholar]
- Gether U. Uncovering molecular mechanisms involved in activation of G protein-coupled receptors. Endocr Rev. 2000;21:90–113. doi: 10.1210/edrv.21.1.0390. [DOI] [PubMed] [Google Scholar]
- Hamdan FF, Ungrin MD, Abramovitz M, Ribeiro P. Characterization of a novel serotonin receptor from Caenorhabditis elegans: cloning and expression of two splice variants. J Neurochem. 1999;72:1372–1383. doi: 10.1046/j.1471-4159.1999.721372.x. [DOI] [PubMed] [Google Scholar]
- Hobson RJ, Geng J, Gray AD, Komuniecki RW. SER-7b, a constitutively active Galphas coupled 5-HT7-like receptor expressed in the Caenorhabditis elegans M4 pharyngeal motorneuron. J Neurochem. 2003;87:22–29. doi: 10.1046/j.1471-4159.2003.01967.x. [DOI] [PubMed] [Google Scholar]
- Hooper SL, DiCaprio RA. Crustacean motor pattern generator networks. Neurosignals. 2004;13:50–69. doi: 10.1159/000076158. [DOI] [PubMed] [Google Scholar]
- Hoyer D, Hannon JP, Martin GR. Molecular, pharmacological and functional diversity of 5-HT receptors. Pharmacol Biochem Behav. 2002;71:533–554. doi: 10.1016/s0091-3057(01)00746-8. [DOI] [PubMed] [Google Scholar]
- Huber R, Smith K, Delago A, Isaksson K, Kravitz EA. Serotonin and aggressive motivation in crustaceans: altering the decision to retreat. Proc Natl Acad Sci USA. 1997;94:5939–5942. doi: 10.1073/pnas.94.11.5939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston CA, Watts VJ. Sensitization of adenylate cyclase: a general mechanism of neuroadaptation to persistent activation of Galpha (i/o)-coupled receptors? Life Sci. 2003;73:2913–2925. doi: 10.1016/s0024-3205(03)00703-3. [DOI] [PubMed] [Google Scholar]
- Kandel ER, Schwartz JH. Molecular biology of learning: modulation of transmitter release. Science. 1982;218:433–443. doi: 10.1126/science.6289442. [DOI] [PubMed] [Google Scholar]
- Katz PS, Tazaki K. Comparative and evolutionary aspects of the crustacean stomatogastric system. In: Harris-Warrick RM, Marder E, Selverston AI, Moulins M, editors. Dynamic Biological Networks: The Stomatogastric Nervous System. Cambridge, MA: MIT; 1992. pp. 221–261. [Google Scholar]
- Kravitz EA. Serotonin and aggression: insights gained from a lobster model system and speculations on the role of amine neurons in a complex behavior. J Comp Physiol A. 2000;186:221–238. doi: 10.1007/s003590050423. [DOI] [PubMed] [Google Scholar]
- Kristiansen K. Molecular mechanisms of ligand binding, signaling, and regulation within the superfamily of G-protein-coupled receptors: molecular modeling and mutagenesis approaches to receptor structure and function. Pharmacol Ther. 2004;103:21–80. doi: 10.1016/j.pharmthera.2004.05.002. [DOI] [PubMed] [Google Scholar]
- Kristiansen K, Kroeze WK, Willins DL, Gelber EI, Savage JE, Glennon RA, Roth BL. A highly conserved aspartic acid (Asp-155) anchors the terminal amine moiety of tryptamines and is involved in membrane targeting of the 5-HT (2A) serotonin receptor but does not participate in activation via a “salt-bridge disruption” mechanism. J Pharmacol Exp Ther. 2000;293:735–746. [PubMed] [Google Scholar]
- Kroeze WK, Kristiansen K, Roth BL. Molecular biology of serotonin receptors structure and function at the molecular level. Curr Top Med Chem. 2002;2:507–528. doi: 10.2174/1568026023393796. [DOI] [PubMed] [Google Scholar]
- Lanctot PM, Leclerc PC, Escher E, Guillemette G, Leduc R. Role of N-glycan-dependent quality control in the cell-surface expression of the AT1 receptor. Biochem Biophys Res Commun. 2006;340:395–402. doi: 10.1016/j.bbrc.2005.12.016. [DOI] [PubMed] [Google Scholar]
- Lee CY, Yau SM, Liau CS, Huang WJ. Serotonergic regulation of blood glucose levels in the crayfish, Procambarus clarkii: site of action and receptor characterization. J Exp Zool. 2000;286:596–605. doi: 10.1002/(sici)1097-010x(20000501)286:6<596::aid-jez6>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Li XC, Giot JF, Kuhl D, Hen R, Kandel ER. Cloning and characterization of two related serotonergic receptors from the brain and the reproductive system of Aplysia that activate phospholipase C. J Neurosci. 1995;15:7585–7591. doi: 10.1523/JNEUROSCI.15-11-07585.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu YF, Ghahremani MH, Rasenick MM, Jakobs KH, Albert PR. Stimulation of cAMP synthesis by Gi-coupled receptors upon ablation of distinct Galphai protein expression. Gi subtype specificity of the 5-HT1A receptor. J Biol Chem. 1999;274:16444–16450. doi: 10.1074/jbc.274.23.16444. [DOI] [PubMed] [Google Scholar]
- May LT, Avlani VA, Sexton PM, Christopoulos A. Allosteric modulation of G protein-coupled receptors. Curr Pharm Des. 2004;10:2003–2013. doi: 10.2174/1381612043384303. [DOI] [PubMed] [Google Scholar]
- Olde B, McCombie WR. Molecular cloning and functional expression of a serotonin receptor from Caenorhabditis elegans. J Mol Neurosci. 1997;8:53–62. doi: 10.1007/BF02736863. [DOI] [PubMed] [Google Scholar]
- Roth BL, Shoham M, Choudhary MS, Khan N. Identification of conserved aromatic residues essential for agonist binding and second messenger production at 5-hydroxytryptamine2A receptors. Mol Pharmacol. 1997;52:259–266. doi: 10.1124/mol.52.2.259. [DOI] [PubMed] [Google Scholar]
- Saudou F, Boschert U, Amlaiky N, Plassat JL, Hen R. A family of Drosophila serotonin receptors with distinct intracellular signalling properties and expression patterns. EMBO J. 1992;11:7–17. doi: 10.1002/j.1460-2075.1992.tb05021.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro DA, Kristiansen K, Weiner DM, Kroeze WK, Roth BL. Evidence for a model of agonist-induced activation of 5-hydroxytryptamine 2A serotonin receptors that involves the disruption of a strong ionic interaction between helices 3 and 6. J Biol Chem. 2002;277:11441–11449. doi: 10.1074/jbc.M111675200. [DOI] [PubMed] [Google Scholar]
- Silberstein SD. Methysergide. Cephalalgia. 1998;18:421–435. doi: 10.1046/j.1468-2982.1998.1807421.x. [DOI] [PubMed] [Google Scholar]
- Sosa MA, Spitzer N, Edwards DH, Baro DJ. A crustacean serotonin receptor: cloning and distribution in the thoracic ganglia of crayfish and freshwater prawn. J Comp Neurol. 2004;473:526–537. doi: 10.1002/cne.20092. [DOI] [PubMed] [Google Scholar]
- Spitzer N, Antonsen BL, Edwards DH. Immunocytochemical mapping and quantification of expression of a putative type 1 serotonin receptor in the crayfish nervous system. J Comp Neurol. 2005;484:261–282. doi: 10.1002/cne.20456. [DOI] [PubMed] [Google Scholar]
- Sprouse J, Reynolds L, Li X, Braselton J, Schmidt A. 8-OH-DPAT as a 5-HT7 agonist: phase shifts of the circadian biological clock through increases in cAMP production. Neuropharmacology. 2004;46:52–62. doi: 10.1016/j.neuropharm.2003.08.007. [DOI] [PubMed] [Google Scholar]
- Strader CD, Fong TM, Graziano MP, Tota MR. The family of G-protein-coupled receptors. FASEB J. 1995;9:745–754. [PubMed] [Google Scholar]
- Strawn JR, Neckameyer WS, Cooper RL. The effects of 5-HT on sensory, central and motor neurons driving the abdominal superficial flexor muscles in the crayfish. Comp Biochem Physiol. 2000;127B:533–550. doi: 10.1016/s0305-0491(00)00287-x. [DOI] [PubMed] [Google Scholar]
- Sugamori KS, Sunahara RK, Guan HC, Bulloch AGM, Tensen CP, Seeman P, Niznik HB, Van Tol HHM. Serotonin receptor cDNA cloned from Lymnaea stagnalis. Proc Natl Acad Sci USA. 1993;90:11–15. doi: 10.1073/pnas.90.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teshiba T, Shamsian A, Yashar B, Yeh SR, Edwards DH, Krasne FB. Dual and opposing modulatory effects of serotonin on crayfish lateral giant escape command neurons. J Neurosci. 2001;21:4523–4529. doi: 10.1523/JNEUROSCI.21-12-04523.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tierney AJ. Structure and function of invertebrate 5-HT receptors: a review. Comp Biochem Physiol. 2001;128A:791–804. doi: 10.1016/s1095-6433(00)00320-2. [DOI] [PubMed] [Google Scholar]
- Tierney AJ, Mangiamele LA. Effects of serotonin and serotonin analogs on posture and agonistic behavior in crayfish. J Comp Physiol A. 2001;187:757–767. doi: 10.1007/s00359-001-0246-x. [DOI] [PubMed] [Google Scholar]
- Tierney AJ, Greenlaw MA, Dams-O’Connor K, Aig SD, Perna AM. Behavioral effects of serotonin and serotonin agonists in two crayfish species, Procambarus clarkii and Orconectes rusticus. Comp Biochem Physiol. 2004;139A:495–502. doi: 10.1016/j.cbpb.2004.10.010. [DOI] [PubMed] [Google Scholar]
- Witz P, Amlaiky N, Plassat JL, Maroteaux L, Borrelli E, Hen R. Cloning and characterization of a Drosophila serotonin receptor that activates adenylate cyclase. Proc Natl Acad Sci USA. 1990;87:8940–8944. doi: 10.1073/pnas.87.22.8940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh SR, Fricke RA, Edwards DH. The effect of social experience on serotonergic modulation of the escape circuit of crayfish. Science. 1996;271:366–369. doi: 10.1126/science.271.5247.366. [DOI] [PubMed] [Google Scholar]
- Yeh SR, Musolf BE, Edwards DH. Neuronal adaptations to changes in the social dominance status of crayfish. J Neurosci. 1997;17:697–708. doi: 10.1523/JNEUROSCI.17-02-00697.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B, Harris-Warrick RM. Multiple receptors mediate the modulatory effects of serotonergic neurons in a small neural network. J Exp Biol. 1994;190:55–77. doi: 10.1242/jeb.190.1.55. [DOI] [PubMed] [Google Scholar]