Abstract
Context
Autism is a heterogeneous disorder with genetic and environmental factors likely contributing to its origins. Examination of hazardous pollutants has suggested the importance of air toxics in autism etiology, yet little research has examined local level air pollution associations using residence-specific exposure assignments.
Objective
To examine the relationship between traffic-related air pollution (TRP), air quality, and autism.
Design, Setting and Population
This study includes data on 279 autism cases and 245 typically developing controls enrolled in the Childhood Autism Risks from Genetics and the Environment (CHARGE) Study in California. The mother’s address from the birth certificate and addresses reported from a residential history questionnaire were used to estimate exposure for each trimester of pregnancy and first year of life. TRP was assigned to each location using a line-source air-quality dispersion model. Regional air pollutant measures were based on the Environmental Protection Agency’s Air Quality System data. Logistic regression models compared estimated and measured pollutant levels for autism cases and typically developing controls.
Main Outcome Measures
Crude and multivariable-adjusted odds ratios (OR) for autism.
Results
Cases were more likely to live at residences in the highest quartile TRP exposure during pregnancy (OR=1.98, 95%CI 1.20–3.31) and the first year of life (OR=3.10, 1.76–5.57) compared to controls. Regional exposure measures of nitrogen dioxide (NO2) and particulate matter less than 2.5 and 10 microns in diameter (PM2.5 and PM10) were also associated with autism during gestation (NO2 OR=1.81/2SD, 95%CI 1.37–3.09; PM2.5 OR=2.08/2SD, 95%CI 1.93–2.25; PM10 OR=2.17/2SD, 95%CI 1.49–3.16) and the first year of life (NO2 OR=2.06, 95%CI 1.37–3.09; PM2.5 OR=2.12, 95%CI 1.45–3.10; PM10 OR=2.14, 95%CI 1.46–3.12).
Conclusions
Exposure to TRP, NO2, PM2.5, and PM10 during pregnancy and the first year of life was associated with autism. Further epidemiological and toxicological examination of likely biological pathways will help determine whether these associations are causal.
Introduction
Autism spectrum disorders (ASDs) are a group of developmental disorders commonly characterized by problems in communication, social interaction, and repetitive behaviors or restricted interests.1 While the severity of impairment for the ASDs varies across the spectrum (full syndrome autism being the most severe), the incidence rate of all ASDs is now reported to be as high as 1 in 110 children.2 Emerging evidence suggests environment plays a role in autism, yet at this stage, only limited information is available as to what exposures are relevant, their mechanisms of action, stages of development in which they act, and then how to develop effective preventive measures.
Recently, air pollution has been examined as a potential risk factor for autism. Using the Environmental Protection Agency’s (EPA) dispersion model-estimates of ambient concentrations of Hazardous Air Pollutants (HAPs), Windham and colleagues identified an increased autism risk based on exposure to diesel exhaust particles, metals (mercury, cadmium and nickel) and chlorinated solvents in Northern California census tracts.3 Additional research using dispersion model-estimates of HAPs also reported associations between autism and air toxics at the birth residence of children from North Carolina and West Virginia.4 These epidemiologic findings on autism are supported by additional research describing other physical and developmental effects of air pollution due to prenatal and early life exposure. For example, high levels of air pollutants have been associated with poor birth outcomes, immunologic changes, and decreased cognitive abilities.5,6
Recently, we reported an association between autism risk and early life residence within 309 meters of a freeway in the Childhood Autism Risks from Genetics and the Environment (CHARGE) study.7 The near source traffic-related air pollutant (TRP) mixture has large spatial variation, returning to near background daytime levels beyond this distance.8,9 Here we report associations of autism with estimates of exposure to the mixture of TRP and with regional measures of nitrogen dioxide (NO2), particulate matter < 2.5 μm aerodynamic diameter (PM2.5), and particulate matter < 10 μm aerodynamic diameter (PM10) in the CHARGE sample.
Methods
The CHARGE study is a population-based case-control study of preschool children. The study design is described in detail elsewhere.10 Briefly, CHARGE subjects were between the ages of 24 and 60 months at the time of recruitment, lived with at least one English- or Spanish-speaking biologic parent, were born in California, and lived in one of the study catchment areas. Recruitment was facilitated by the California Department of Developmental Services (DDS), the Regional Centers with which they contract to coordinate services for persons with developmental disabilities, and referrals from the M.I.N.D. Institute clinic at the University of California, Davis (UCD) and from other research studies. Population-based controls were recruited from the sampling frame of birth files from the state of California, and were frequency matched by gender, age, and broad geographic area to the autism cases.
Each participating family was evaluated in person. Children with a previous diagnosis of autism were evaluated using the Autism Diagnostic Observation Schedules (ADOS) and parents were administered the Autism Diagnostic Interview-Revised (ADI-R).11,12 Children with diagnosed developmental delay and general population controls were given the Social Communication Questionnaire (SCQ) to screen for the presence of autistic features.13 If the SCQ score was 15 or greater, the child was then given the ADOS and the parent the ADI-R. In our study, autism cases were children with a diagnosis of full syndrome autism from both the ADOS and the ADI-R. All children were also assessed using the Mullen Scales of Early Learning (MSEL) and the Vineland Adaptive Behavior Scales (VABS) to collect information on motor skills, language, socialization, and daily living skills.14,15 Controls were children sampled from the general population set who received a score less than 15 on the SCQ and who also showed no evidence of other types of delay (cognitive or adaptive).
Parents were interviewed to obtain demographic and medical information, and, among other factors, residential histories. Race/ethnicity data were collected by self-report in categories defined by the US Census (Table 1). The residential data captured addresses and corresponding dates the mother and child lived at each location beginning 3 months before conception and extending to the most recent place of residence. Further details about the collection of clinical and exposure data have been previously reported.10
Table 1.
Spearman correlations (r) of traffic related pollution (TRP) and regional pollutants (N=524)*. Light grey shading reflects correlations across pollutants within pregnancy. Dark grey shading reflects correlations across pollutants within the first year of life. Values reported in the white boxes report correlations of the same pollutant across time periods.
| All Pregnancy | |||||
|---|---|---|---|---|---|
|
| |||||
| TRP | PM2.5 | PM10 | Ozone | Nitrogen Dioxide | |
| TRP | 0.92 | 0.36 | 0.33 | −0.36 | 0.60 |
| PM2.5 | 0.25 | 0.67 | 0.77 | −0.11 | 0.63 |
| PM10 | 0.27 | 0.84 | 0.82 | 0.13 | 0.66 |
| Ozone | −0.31 | 0.26 | 0.27 | 0.74 | −0.29 |
| Nitrogen Dioxide | 0.58 | 0.60 | 0.64 | −0.19 | 0.89 |
|
| |||||
| Year 1 | |||||
All correlation measures were statistically significant (p<0.05).
PM2.5 = particulate matter < 2.5μm aerodynamic diameter, PM10 = particulate matter < 10μm aerodynamic diameter.
To obtain model-based estimates of TRP exposure, we applied the CALINE4 line-source air-quality dispersion model.16 The dispersion model was used to estimate average concentrations for the specific locations and time periods (trimesters of gestation and first year of life) for each subject. The principal model inputs are roadway geometry, link-based traffic volumes, period-specific meteorological conditions (wind speed and direction, atmospheric stability, and mixing heights), and vehicle emission rates. Detailed roadway geometry data and annual average daily traffic counts were obtained from Tele Atlas/Geographic Data Technology (GDT) in 2005. These data represent an integration of state-, county-, and city-level traffic counts collected between 1995 and 2000. Because our period of interest was 1997 to 2008, the counts were scaled to represent individual years based on estimated growth in county average vehicle-miles-traveled (VMT) data.17 Traffic counts were assigned to roadways based on location and street names. Traffic volumes on roadways without count data (mostly small roads) were estimated based on median volumes for similar class roads in small geographic regions. Meteorological data from 56 local monitoring stations were matched to the dates and locations of interest. Vehicle fleet average emission factors were based on the California Air Resource Board’s EMFAC2007 (version 2.3) model. Annual average emission factors were calculated by year (1997–2008) for travel on freeways (65 mph), state highways (50 mph), arterials (35 mph), and collectors (30 mph). We used the CALINE4 model to estimate locally varying ambient concentrations of nitrogen oxides (NOx) contributed by freeways, non-freeways, and all roads located within 5 km of each child’s home. Previously, we have used the CALINE4 model to estimate concentrations of other traffic-related pollutants, including elemental carbon and carbon monoxide; and found that they were almost perfectly correlated (around 0.99) with estimates for nitrogen oxides. Thus, our model-based concentrations should be viewed as an indicator of the TRP mixture rather than any pollutant specifically.
A second approach was to use the regional air quality data for the exposure assignments for PM2.5, PM10, ozone (O3) and NO2. These were derived from the US EPA’s Air Quality System (AQS) data (www.epa.gov/ttn/airs/airsaqs) supplemented by USC’s Children’s Health Study (CHS) data for 1997–2009.18 CHS continuous PM data were used for a given monitoring station when no Federal Reference/Equivalent Method data for PM were available from AQS. The monthly air quality data from monitoring stations located within 50 km of each residence were made available for spatial interpolation of ambient concentrations. The spatial interpolations were based on inverse distance-squared weighting (IDW2) of data from up to four closest stations located within 50 km of each participant residence; however, if one or more stations were located within 5 km of a residence then only data from the stations within 5 km were used for the interpolation. Because special studies have shown large offshore to onshore pollutant gradients along the southern California coast, the interpolations were carried out with pseudo-stations, or theoretical locations used for estimating pollution gradients from extant data when geography did not permit observed data, located ~ 20–40 km offshore that had background concentrations based on long-term measurements (1994–2003) at clean coastal locations (i.e., Lompoc, CA).
Periods and locations relevant to the modeled traffic exposure were identified based on dates and addresses recorded on the birth certificate and from the residential history questionnaire. The birth certificate addresses corresponded to the mother’s residence at the time of child’s birth while the residential history captures both mother’s residences during pregnancy (required for estimation of prenatal exposure) and child’s residences after birth through the time of study enrollment. We determined the conception date for each child using gestational age from ultrasound measurements or the date of last menstrual period, as determined from prenatal records. We used these locations and dates to estimate exposure for the first year of life, the entire pregnancy period, and each trimester of pregnancy. When more than 1 address fell into a time interval, we created a weighted average to reflect the exposure level of the participant across the time of interest taking into account changes in residence. TRP was determined based on the required inputs reflecting change in each address over the study period. For the regional pollutant measures, we assigned PM2.5, PM10, and NO2 measurements based on average concentrations for the time period of interest. For O3, we calculated the averages for the period of interest based on the 1000–1800 hours (reflecting the high 8 hour daytime) average. Based on these methods, we were able to assign TRP estimates and regional pollutant measures for 524 mother-child pairs.
Spearman correlations were calculated pairwise between TRP estimates and regional pollution measures for pregnancy and the first year of life to assess independence of these exposure metrics. We used logistic regression to examine the association between exposure to traffic-related air pollution and autism risk. Models of autism risk as a function of TRP exposure levels from all road types were fitted separately for each time period. Categories of exposure were formed based on quartiles of the TRP distribution for all pregnancy as this provided the most comprehensive data for each child. Levels of regional pollutants were examined as continuous variables and effect estimates scaled to twice the standard deviation of the distribution for the all pregnancy estimates. When levels of correlation permitted, we examined both TRP and regional pollutants in a single model. Pertinent covariates were included in each model to adjust for potential confounding due to socio-demographic and lifestyle characteristics. We included children’s gender and ethnicity, maximum education level of the parents, maternal age, and maternal smoking during pregnancy as described previously.7 To examine if our findings were affected by living in an urban or rural area, we included population density obtained from Environmental Systems Research Institute Inc.’s 2008 estimates of people per square meter (p/m2) using ArcGIS software (version 9.2). We used the United States Census Bureau cut off of 2,500 p/m2 to categorize population density into urban vs. rural areas and included this variable as a covariate in analysis of air pollution effects from the first year of life since these residences were the most recent recorded.
We also fitted logistic additive models to evaluate the relationship between autism and TRP. These models used the smoothing spline with three degrees of freedom for continuous TRP and used the same adjustment variables as in the linear logistic models described above. Statistical tests were conducted using an alpha level of 0.05 and 95% confidence limits were used to measure precision. All analyses were conducted using the R package version 2.9.2 (www.r-project.org). Institutional review boards of the University of Southern California and UCD approved the research.
Results
Children in this study were predominantly male (84%) and most were non-Hispanic Caucasian (50%) or Hispanic (30%). No differences were found between cases and controls for any demographic, socioeconomic, or lifestyle variables we examined (eTable 1). Details regarding the exposure distributions are presented in eFigures 1a and 1b. Spearman correlations calculated for the first year of life and pregnancy time periods are presented in Table 1. During pregnancy and the first year TRP was moderately correlated with PM2.5 and PM10, highly correlated with NO2, but inversely correlated with O3. Among the regional pollutant measures, PM2.5 and PM10 were nearly perfectly correlated and both were highly correlated with NO2. Correlations with O3 were low and often negative, demonstrating an inverse relationship. We also examined correlations of each pollutant across time periods and high correlations were identified.
Traffic Related Air Pollution Exposure
Increased autism risk was associated with exposure to traffic related air pollution during the first year of life. Children residing in homes with the highest levels of modeled TRP were three times as likely to have autism compared to children with the lowest exposure (Table 2). Exposure in the middle quartile groups (2nd and 3rd) was not associated with an increased risk of autism. In our analysis including population density, this association with the highest quartile of exposure was still evident (OR=3.48, 95%CI 1.81–6.83) and living in an urban area, compared to rural, was not associated with autism (OR=0.86, 95%CI 0.56–1.31). When we examined TRP exposures during pregnancy, the highest quartile was also associated with autism risk (OR=1.98, 95%CI 1.20–3.31) compared to the lowest quartile. We further divided the pregnancy into three trimesters and modeled TRP based on these intervals. During all three trimesters of pregnancy, we found associations with the highest quartile of exposure (≥31.8 ppb), compared to the lowest quartile (≤9.7 ppb), and autism (Table 2). Inclusion of demographic and socioeconomic variables in the models did not greatly alter these associations (Table 2).
Table 2.
Odds ratios (OR) and 95% confidence intervals for autism, by quartile** of modeled traffic related air pollution (TRP) exposure from all road types (N=524).
| 4th quartile | 3rd quartile | 2nd quartile | ||
|---|---|---|---|---|
| First Year of Life | Crude | 2.97 (1.71–5.27) | 1.00 (0.63–1.60) | 0.88 (0.55–1.42) |
| Adjusted* | 3.10 (1.76–5.57) | 1.00 (0.62–1.62) | 0.91 (0.56–1.47) | |
|
| ||||
| All Pregnancy | Crude | 1.99 (1.22–3.28) | 1.10 (0.67–1.78) | 1.20 (0.74–1.95) |
| Adjusted* | 1.98 (1.20–3.31) | 1.09 (0.67–1.79) | 1.26 (0.77–2.06) | |
|
| ||||
| First Trimester | Crude | 1.91 (1.67–3.14) | 1.28 (0.80–2.06) | 1.28 (0.77–2.14) |
| Adjusted* | 1.85 (1.11–3.08) | 1.28 (0.79–2.08) | 1.28 (0.77–2.15) | |
|
| ||||
| Second Trimester | Crude | 1.69 (1.04–2.78) | 1.15 (0.71–1.87) | 0.89 (0.54–1.47) |
| Adjusted* | 1.65 (1.00–2.74) | 1.13 (0.69–1.84) | 0.90 (0.54–1.49) | |
|
| ||||
| Third Trimester | Crude | 2.04 (1.25–3.38) | 0.92 (0.57–1.48) | 1.12 (0.68–1.84) |
| Adjusted* | 2.10 (1.27–3.51) | 0.91 (0.56–1.46) | 1.17 (0.71–1.93) | |
Model adjusted for child male gender, child ethnicity (Hispanic vs. White, Black/Asian/Other vs. White), maximum education of parents (parent with highest of four levels: college degree or higher vs. some high school, high school degree, or some college education), maternal age ( >35 years vs. ≤ 35years), and prenatal smoking (self report of ever vs. never smoked while pregnant).
Quartile cut points correspond to TRP levels of 31.8 ppb or greater (4th quartile), 16.9–31.8 ppb (3rd quartile), and 9.7–16.9 ppb (2nd quartile), compared to 9.7 ppb or less (1st quartile, reference group).
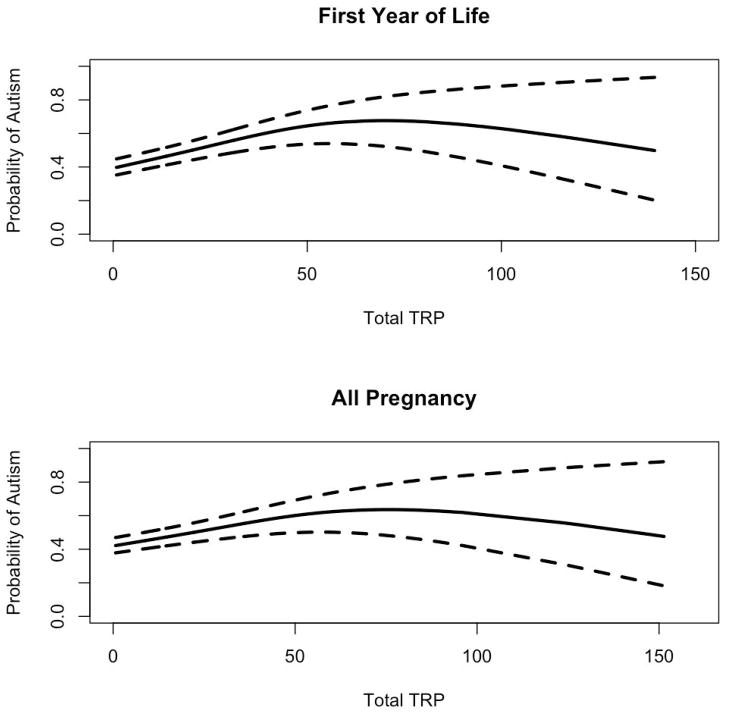
Since our quartile-based categories indicated that there is a threshold upon which TRP exposure is detrimental, we also examined the relationship of TRP exposure and autism using smoothed models for the first year of life and all of pregnancy. An increasing probability of autism was seen with increasing TRP estimates, with the odds reaching a plateau when TRP estimates were above 25–30 ppb (Figure 1).
Figure 1.
Probability of autism by increasing level of traffic related air pollution (TRP) exposure for the first year of life and pregnancy. Dotted lines indicate 95% confidence interval.
Regional Air Pollutant Exposure
Higher levels of exposure to PM2.5, PM10, and NO2 based on the EPA’s regional air quality monitoring program were associated with increased risk of autism (Table 3). Specifically for an 8.7 unit increase (μg/m3) in PM2.5 (corresponding to twice the standard deviation of the PM2.5 distribution) exposure during the first year of life, children were 2.12 times more likely to have autism. Increases were also present for pregnancy and trimester-specific estimates of PM2.5 with the smallest effects present in the first trimester. For PM10, a 14.6 unit increase (μg/m3) during the first year was associated with twice the risk of autism (Table 3). Associations were present for pregnancy and each trimester with the first trimester having the smallest magnitude. We did not find associations between levels of regional O3 and autism. Regional NO2 exposure during the first year was associated with a two-fold autism risk. Similar effects were identified for NO2 exposure during pregnancy. While exposure during each of the three trimesters was associated with autism, effects of the first trimester were the smallest. For all regional pollutant measures, adjustment for demographic and socioeconomic variables did not alter the associations. As with TRP, when we included population density in the models including exposure during the first year of life associations with PM2.5, PM10, and NO2 did not change, nor did they change when living in an urban area vs. a rural area was included (data not shown).
Table 3.
Odds ratios (OR) and 95% confidence intervals for autism based on continuous regional pollutant exposure (N=524)**.
| Regional Pollutant | |||||
|---|---|---|---|---|---|
|
| |||||
| PM2.5 | PM10 | O3 | Nitrogen Dioxide | ||
| First Year | Crude | 2.14 (1.48–3.09) | 2.14 (1.47–3.10) | 1.15 (0.72–1.84) | 2.06 (1.39–3.06) |
| Adjusted* | 2.12 (1.45–3.10) | 2.14 (1.46–3.12) | 1.15 (0.72–1.86) | 2.06 (1.37–3.09) | |
|
| |||||
| All Pregnancy | Crude | 2.11 (1.46–3.03) | 2.17 (1.50–3.13) | 1.08 (0.76–1.52) | 1.82 (1.26–2.64) |
| Adjusted* | 2.08 (1.93–2.25) | 2.17 (1.49–3.16) | 1.09 (0.76–1.55) | 1.81 (1.23–2.65) | |
|
| |||||
| First Trimester | Crude | 1.24 (0.99–1.56) | 1.47 (1.10–1.98) | 1.07 (0.86–1.33) | 1.47 (1.07–2.01) |
| Adjusted* | 1.22 (0.96–1.53) | 1.44 (1.07–1.96) | 1.08 (0.86–1.35) | 1.44 (1.05–1.20) | |
|
| |||||
| Second Trimester | Crude | 1.50 (1.16–1.93) | 1.82 (1.35–2.45) | 1.03 (0.84–1.27) | 1.62 (1.17–2.25) |
| Adjusted* | 1.48 (1.40–1.57) | 1.83 (1.35–2.47) | 1.04 (0.84–1.29) | 1.61 (1.15–2.25) | |
|
| |||||
| Third Trimester | Crude | 1.39 (1.11–1.75) | 1.61 (1.21–2.13) | 1.03 (0.84–1.27) | 1.65 (1.19–2.27) |
| Adjusted* | 1.40 (1.11–1.77) | 1.61 (1.20–2.14) | 1.03 (0.83–1.26) | 1.64 (1.18–2.29) | |
PM2.5 = particulate matter < 2.5μm aerodynamic diameter, PM10 = particulate matter < 10μm aerodynamic diameter. Models adjusted for child male gender, child ethnicity (Hispanic vs. White, Black/Asian/Other vs. White), maximum education of parents (parent with highest of four levels: college degree or higher vs. some high school, high school degree, or some college education), maternal age ( >35 years vs. ≤ 35years), and prenatal smoking (self report of ever vs. never smoked while pregnant).
Regional pollution effects reflect risk of autism based on 2 standard deviations from the mean value, specifically per increase of 8.7 μg/m3 PM2.5, 14.6 μg/m3 PM10, 14.1 ppb Nitrogen Dioxide, and 16.1 ppb Ozone.
TRP, PM2.5, and PM10
Because pairwise correlations between TRP and PM2.5 and TRP and PM10 were moderate, we included both in models to examine if local pollution estimates (TRP) and regional pollution measures (PM2.5 and PM10) were independently associated with autism. In these analyses, we included the same set of covariates described above in the single pollutant analysis. When examined in the same model, both the top quartile of TRP (OR=2.37, 95%CI 1.28–4.45) and PM2.5 (OR=1.58/2SD, 95%CI 1.03–2.42) exposure during the first year of life remained associated with autism. Examining both TRP and PM10, we found that the top quartile of TRP (OR=2.36, 95%CI 1.28–4.43) and PM10 (OR=1.61, 95%CI 1.06–2.47) remained associated with autism. For all pregnancy, we found that both the top quartile of TRP (OR=2.42, 95%CI 1.32–4.50) and PM2.5 (OR=1.60, 95%CI 1.07–2.40) were associated with autism when examined in the same model. Similarly, both the top quartile of TRP (OR=2.33, 95%CI 1.27–4.36) and PM10 (OR=1.68, 95%CI 1.11–2.53) remained associated with autism when examined jointly.
Discussion
This study found that local estimates of TRP and regional measures of PM2.5, PM10, and NO2 at residences were higher in children with autism. The magnitude of these associations appear to be most pronounced during late gestation and early life, though it was not possible to adequately distinguish a period critical to exposure. Children with autism were three times as likely to have been exposed during the first year of life to higher modeled traffic-related air pollution as compared with typically developing controls. Similarly, exposure to TRP during pregnancy was also associated with autism. Examination of TRP using an additive logistic model demonstrated a potential threshold near 25–30ppb beyond which the probability of autism did not increase. Exposure to high levels of regional PM2.5, PM10, and NO2 were also associated with autism. When we examined PM2.5 or PM10 exposure jointly with TRP, both regional and local pollutants remained associated with autism though the magnitude of effects decreased.
We previously reported an association between living near a freeway, based on the location of the birth and third trimester address, and autism.7 That result relied on simple distance metrics as a proxy for exposure to traffic related air pollution. The present study builds on that result, demonstrating associations with both regional particulate and NO2 exposure and to dispersion-modeled exposure to the near-roadway traffic mixture accounting for traffic volume, fleet emission factors and wind speed and direction, in addition to traffic proximity. The results provide more convincing evidence that exposure to local air pollution from traffic may increase risk of autism. Demographic or socio-economic factors did not explain these associations.
Toxicological and genetic research suggests possible biologically plausible pathways to explain these results. Concentrations of many air pollutants, including diesel exhaust particles (DEP) and other PM constituents, are increased near freeways and other major roads, and DEP and the polycyclic aromatic hydrocarbons (PAHs) commonly present in DEP affect brain function and activity in toxicological studies. 19–23 PAHs have been shown to reduce expression of the MET receptor tyrosine kinase gene, which is important in early life neurodevelopment and is markedly reduced in autistic brains.24,25 Other research indicates that TRPs induce inflammation and oxidative stress after both short term and long term exposures, processes which mediate effects of air pollution on respiratory and cardiovascular disease and other neurological outcomes.26–29 Data examining biomarkers suggests that oxidative stress and inflammation may also be involved in the pathogenesis of autism.30–33
Emerging evidence suggests that systemic inflammation may also result in damage to endothelial cells in the brain and compromise the blood-brain barrier.29 Systemic inflammatory mediators may cross the blood-brain barrier, activating brain microglia, and peripheral monocytes may migrate into the pool of microglia.34–36 In addition, ultrafine particles (PM0.1) may penetrate cellular membranes.37,38 These particles translocate indirectly through the lungs and from the systemic circulation or directly via the nasal mucosa and the olfactory bulb into the brain.39,40 Toxicity may be mediated by physical properties of PM, or by the diverse mixture of organic compounds, including PAHs, and oxidant metals adsorbed to the surface.29 Neurodevelopmental effects of PAHs may be mediated by aryl hydrocarbon hydroxylase induction in placenta, decreased exchange of oxygen secondary to disruption of placental growth factor receptors, endocrine disruption, activation of apoptotic pathways, inhibition of the brain antioxidant-scavenging system resulting in oxidative stress, or epigenetic effects.21
This study draws on a rich record of residential locations of typically developing children and children with autism across California, allowing us to assign modeled pollutant exposures for developmentally relevant time points. However, our results could also be affected by unmeasured confounding factors associated with both autism and traffic related air pollution exposure. While we did not find that including demographic or socio-economic variables altered our estimates of effect, confounding by other factors could still occur. These might include lifestyle, nutritional, or other residential exposures, if they were associated with TRP or PM. We have also not explored indoor sources of pollution, such as indoor NO or second-hand tobacco smoke, though prenatal smoking was examined and did not influence the associations of ambient pollution with autism. Additionally, confounding could have occurred if proximity to diagnosing physicians or treatment centers were also associated with exposure. We included population density as an adjustment in an analysis using estimates from the first year of life to examine the sensitivity of our results to urban or rural locations, for which population density is a surrogate. We did not find that living in a more densely populated area altered the association between autism risk and TRP or regional pollutants. Despite our attempts to use a residential history to examine specific time windows of vulnerability, incorporation of meteorology into our TRP models, and inclusion of pollutants with seasonal variation, we are currently unable to disentangle effects trimester-specific effects or during the first year of life because of the high correlation across these time periods.
Exposure to TRP, PM, and NO2 were associated with increased autism risk. These effects were observed from measures of air pollution with variation on both local and regional levels suggesting the need for further study to understand both individual pollutant contributions and the effects of pollutant mixtures on disease. Research on pollutant exposure effects and their interaction with susceptibility factors may lead to identification of biologic pathways activated in autism and improved prevention and therapeutic strategies. While additional research and replication of these findings is needed, the public health implications of these findings are large because air pollution exposure is common and may have lasting neurological effects.
Supplementary Material
Acknowledgments
This work was supported by NIEHS grants ES019002, ES013578, ES007048, ES11269, ES015359, ES016535, ES011627, EPA Star-R-823392, EPA Star-R-833292, and the MIND Institute matching funds and pilot grant program. These funders did not in any way influence the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
Fred Lurmann and Bryan Penfold are employed by Sonoma Technology Inc., Petaluma, CA. Rob McConnell has received support from an air quality violations settlement agreement between the South Coast Air Quality Management District, a California state regulatory agency, and BP.
Footnotes
A portion of this research was presented at the International Meeting for Autism Research on May 14, 2011 in San Diego, California and at the meeting of the International Society for Environmental Epidemiology on September 16, 2011 in Barcelona, Spain.
The other authors declare no competing financial interests.
Author Contributions: Dr. Volk had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Volk, McConnell
Acquisition of data: Lurmann, Penfold, Hertz-Picciotto
Analysis and interpretation of data: Volk, McConnell, Hertz-Picciotto, Lurmann
Drafting of the manuscript: Volk
Critical revision of the manuscript for important intellectual content: Volk, Hertz-Picciotto, McConnell, Lurmann, Penfold
Statistical analysis: Volk
Obtained funding: Hertz-Picciotto, McConnell, Volk
Study Supervision: Volk
Financial Disclosures: Fred Lurmann and Bryan Penfold are employed by Sonoma Technology Inc., Petaluma, CA. Rob McConnell has received support from an air quality violations settlement agreement between the South Coast Air Quality Management District, a California state regulatory agency, and BP. The other authors declare no competing financial interests.
References
- 1.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4. Washington, DC: American Psychiatric Association; 2000. Text Revision. [Google Scholar]
- 2.Centers for Disease Control and Prevention. Prevalence of autism spectrum disorders - Autism and Developmental Disabilities Monitoring Network, United States, 2006. MMWR Surveill Summ. 2009;58(10):1–20. [PubMed] [Google Scholar]
- 3.Windham GC, Zhang L, Gunier R, Croen LA, Grether JK. Autism spectrum disorders in relation to distribution of hazardous air pollutants in the San Francisco bay area. Environ Health Perspect. 2006;114(9):1438–44. doi: 10.1289/ehp.9120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kalkbrenner AE, Daniels JL, Chen JC, Poole C, Emch M, Morrissey J. Perinatal Exposure to Hazardous Air Pollutants and Autism Spectrum Disorders at Age 8. Epidemiology. 2010;20(7):615–24. doi: 10.1097/EDE.0b013e3181e65d76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Currie J, Neidell M, Schmieder JF. Air pollution and infant health: Lessons from New Jersey. J Health Econ. 2009;28(3):688–703. doi: 10.1016/j.jhealeco.2009.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hansen CA, Barnett AG, Pritchard G. The effect of ambient air pollution during early pregnancy on fetal ultrasonic measurements during mid-pregnancy. Environ Health Perspect. 2008;116(3):362–9. doi: 10.1289/ehp.10720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Volk HE, Hertz-Picciotto I, Delwiche L, Lurmann F, McConnell R. Residential Proximity to Freeways and Autism in the CHARGE study. Environ Health Perspect. 2011;119(6):873–7. doi: 10.1289/ehp.1002835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhu YF, Hinds WC, Kim S, Sioutas C. Concentration and size distribution of ultrafine particles near a major highway. Journal of the Air & Waste Management Association. 2002;52:1032–1042. doi: 10.1080/10473289.2002.10470842. [DOI] [PubMed] [Google Scholar]
- 9.Zhu Y, Hinds WC, Kim S, Shen S, Sioutas C. Study of ultrafine particles near a major highway with heavy-duty diesel traffic. Atmospheric Environment. 2002;36(27):4323–4335. [Google Scholar]
- 10.Hertz-Picciotto I, Croen LA, Hansen R, Jones CR, van de Water J, Pessah IN. The CHARGE study: an epidemiologic investigation of genetic and environmental factors contributing to autism. Environ Health Perspect. 2006;114(7):1119–25. doi: 10.1289/ehp.8483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Le Couteur A, Lord C, Rutter M. Autism Diagnostic Interview-Revised (ADI-R) Los Angeles: Western Psychological Services; 2003. [Google Scholar]
- 12.Lord C, Rutter M, DiLavore P, Risi S. Autism Diagnostic Observation Schedule Manual. Los Angeles: Western Psychological Services; 2003. [Google Scholar]
- 13.Rutter M, Bailey A, Lord C. A Social Communication Questionnaire (SCQ) Los Angeles: Western Psychological Services; 2003. [Google Scholar]
- 14.Sparrow S, Cicchettim D, Balla D. Vineland Adaptive Behavior Scales Interview Edition Expanded Form Manual. Circle Pines, MN: American Guidance Services Inc; 1984. [Google Scholar]
- 15.Mullen E. Mullen Scales of Early Learning. Circle Pines, MN: American Guidance Services Inc; 1995. [Google Scholar]
- 16.Bensen PE. A review of the development and application of the CA-LINE3 and 4 models. Atmos Environ. 1992;26B:379–390. [Google Scholar]
- 17.California Department of Transportation. California motor vehicle stock, travel and fuel forecast. 2004. [Google Scholar]
- 18.Alcorn SH, Lurmann FW. Southern California Children’s Health Study exposure database. 2003 [Google Scholar]
- 19.Ntziachristos L, Geller MD, Sioutas C. Particle concentration and characteristics near a major freeway with heavy-duty diesel traffic. Environ Sci Technol. 2007;41(7):2223–2230. doi: 10.1021/es062590s. [DOI] [PubMed] [Google Scholar]
- 20.Perera FP, Rauh V, Whyatt RM, Tsai WY, Tang D, Diaz D, Hoepner L, Barr D, Tu YH, Camann D, Kinney P. Effect of prenatal exposure to airborne polycyclic aromatic hydrocarbons on neurodevelopment in the first 3 years of life among inner-city children. Environ Health Perspect. 2006;114(8):1287–92. doi: 10.1289/ehp.9084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Perera FP, Li Z, Whyatt R, Hoepner L, Wang S, Camann D, Rauh V. Prenatal airborne polycyclic aromatic hydrocarbon exposure and child IQ at age 5 years. Pediatrics. 2009;124(2):e195–202. doi: 10.1542/peds.2008-3506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hougaard KS, Jensen KA, Nordly P, Taxvig C, Vogel U, Saber AT, Wallin H. Effects of prenatal exposure to diesel exhaust particles on postnatal development, behavior, genotoxicity and inflammation in mice. Part Fibre Toxicol. 2008;5:3. doi: 10.1186/1743-8977-5-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brown LA, Khousbouei H, Goodwin JS, Irvin-Wilson CV, Ramesh A, Sheng L, McCallister MM, Jiang GC, Aschner M, Hood DB. Down-regulation of early ionotrophic glutamate receptor subunit developmental expression as a mechanism for observed plasticity deficits following gestational exposure to benzo(a)pyrene. Neurotoxicology. 2007;28(5):965–78. doi: 10.1016/j.neuro.2007.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Levitt P, Campbell DB. The genetic and neurobiologic compass points toward common signaling dysfunctions in autism spectrum disorders. J Clin Invest. 2009;119(4):747–54. doi: 10.1172/JCI37934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Campbell DB, D’Oronzio R, Garbett K, Ebert PJ, Mirnics K, Levitt P, Persico AM. Disruption of cerebral cortex MET signaling in autism spectrum disorder. Ann Neurol. 2007;62(3):243–50. doi: 10.1002/ana.21180. [DOI] [PubMed] [Google Scholar]
- 26.Castro-Giner F, Kunzli N, Jacquemin B, Forsberg B, de Cid R, Sunyer J, Jarvis D, Briggs D, Vienneau D, Norback D, Gonzalez JR, Guerra S, Janson C, Anto JM, Wjst M, Heinrich J, Estivill X, Kogevinas M. Traffic-related air pollution, oxidative stress genes, and asthma (ECHRS) Environ Health Perspect. 2009;117(12):1919–24. doi: 10.1289/ehp.0900589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gilliland FD, Li YF, Saxon A, Diaz-Sanchez D. Effect of glutathione-S-transferase M1 and P1 genotypes on xenobiotic enhancement of allergic responses: randomised, placebo-controlled crossover study. Lancet. 2004;363(9403):119–25. doi: 10.1016/S0140-6736(03)15262-2. [DOI] [PubMed] [Google Scholar]
- 28.Kunzli N, Jerrett M, Garcia-Esteban R, Basagana X, Beckermann B, Gilliland F, Medina M, Peters J, Hodis HN, Mack WJ. Ambient air pollution and the progression of atherosclerosis in adults. PLoS One. 5(2):e9096. doi: 10.1371/journal.pone.0009096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Block ML, Calderon-Garciduenas L. Air pollution: mechanisms of neuroinflammation and CNS disease. Trends Neurosci. 2009;32(9):506–16. doi: 10.1016/j.tins.2009.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Enstrom A, Krakowiak P, Onore C, Pessah IN, Hertz-Picciotto I, Hansen RL, Van de Water JA, Ashwood P. Increased IgG4 levels in children with autism disorder. Brain Behav Immun. 2009;23(3):389–95. doi: 10.1016/j.bbi.2008.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.James SJ, Rose S, Melnyk S, Jernigan S, Blossom S, Pavliv O, Gaylor DW. Cellular and mitochondrial glutathione redox imbalance in lymphoblastoid cells derived from children with autism. FASEB J. 2009;23(8):2374–83. doi: 10.1096/fj.08-128926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li X, Chauhan A, Sheikh AM, Patil S, Chauhan V, Li XM, Ji L, Brown T, Malik M. Elevated immune response in the brain of autistic patients. J Neuroimmunol. 2009;207(1–2):111–6. doi: 10.1016/j.jneuroim.2008.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ashwood P, Enstrom A, Krakowiak P, Hertz-Picciotto I, Hansen RL, Croen LA, Ozonoff S, Pessah IN, Van de Water J. Decreased transforming growth factor beta1 in autism: a potential link between immune dysregulation and impairment in clinical behavioral outcomes. J Neuroimmunol. 2008;204(1–2):149–53. doi: 10.1016/j.jneuroim.2008.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nguyen MD, Julien JP, Rivest S. Innate immunity: the missing link in neuroprotection and neurodegeneration? Nat Rev Neurosci. 2002;3(3):216–27. doi: 10.1038/nrn752. [DOI] [PubMed] [Google Scholar]
- 35.Banks WA, Farr SA, Morley JE. Entry of blood-borne cytokines into the central nervous system: effects on cognitive processes. Neuroimmunomodulation. 2002;10(6):319–27. doi: 10.1159/000071472. [DOI] [PubMed] [Google Scholar]
- 36.D’Mello C, Le T, Swain MG. Cerebral microglia recruit monocytes into the brain in response to tumor necrosis factoralpha signaling during peripheral organ inflammation. J Neurosci. 2009;29(7):2089–102. doi: 10.1523/JNEUROSCI.3567-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Geiser M, Rothen-Rutishauser B, Kapp N, Schurch S, Kreyling W, Schulz H, Semmler M, Im Hof V, Heyder J, Gehr P. Ultrafine particles cross cellular membranes by nonphagocytic mechanisms in lungs and in cultured cells. Environ Health Perspect. 2005;113(11):1555–60. doi: 10.1289/ehp.8006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rothen-Rutishauser B, Mueller L, Blank F, Brandenberger C, Muehlfeld C, Gehr P. A newly developed in vitro model of the human epithelial airway barrier to study the toxic potential of nanoparticles. ALTEX. 2008;25(3):191–6. doi: 10.14573/altex.2008.3.191. [DOI] [PubMed] [Google Scholar]
- 39.Campbell A, Araujo JA, Li H, Sioutas C, Kleinman M. Particulate matter induced enhancement of inflammatory markers in the brains of apolipoprotein E knockout mice. J Nanosci Nanotechnol. 2009;9(8):5099–104. doi: 10.1166/jnn.2009.gr07. [DOI] [PubMed] [Google Scholar]
- 40.Oberdorster G, Elder A, Rinderknecht A. Nanoparticles and the brain: cause for concern? J Nanosci Nanotechnol. 2009;9(8):4996–5007. doi: 10.1166/jnn.2009.gr02. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.