Abstract
Background:
Effective and easy to implement interventions to improve adherence to antiretroviral therapy are needed.
Objective:
To compare a site-nurse initiated adherence and symptom support telephone calls for HIV-positive individuals starting antiretroviral therapy compare to the study site’s standard of care.
Methods:
A randomized controlled trial of site-nurse initiated adherence and symptom support telephone calls for HIV-positive individuals starting antiretrovirals. Subjects were randomized to receive site-nurse initiated telephone calls (intervention) or no additional calls above the site’s standard of care (control). Subjects received calls 1-3 days after initiating antiretrovirals, weeks 1, 2, 3, 6, 10, 14, 18, 22, 26, and every 8 weeks thereafter. Self-reported adherence was captured during study visits.
Results:
A total of 333 subjects starting antiretrovirals as part of ACTG 384 were co-enrolled into ACTG 5031. Subjects were followed for up to 160 weeks and were contacted for 74% of scheduled calls. There was no significant difference in proportion of patients with >95% mean Total Adherence, 87.9% and 91.2% (p=0.34) and mean self-reported Total Adherence, 97.9% and 98.4% in the intervention and control, respectively, or in symptom distress and clinical endpoints.
Conclusions:
In the context of a clinical trial, where self-reported adherence was exceptionally high, the site-nurse initiated telephone calls did not further improve self-reported adherence, symptom distress or clinical outcomes.
Keywords: Adherence intervention, Nursing telephone support, Randomized Controlled Trial, Antiretroviral Therapy
Human immunodeficiency virus (HIV) Combination antiretroviral therapy dramatically decreases HIV mortality and improves quality of life for individuals living with HIV.1 Adherence to HIV medications is one of the most critical determinants for sustained virologic suppression.2,3 Near perfect adherence (taking 90 to 95 percent of the prescribed doses of antiretrovirals) increases the probability to maintain viral suppression.4-6 Lower levels of adherence can lead to regimen failure, acquisition of HIV mutations conferring drug resistance to other antiretrovirals, and increased mortality.7-10 Adherence interventions offer the potential to decrease drug resistance and mortality.11-13
Various adherence interventions have been evaluated including memory aids, timers, beepers, pill boxes, medication counselors, buddy systems, and behavioral interventions.14 A meta-analysis of 1839 subjects in 19 antiretroviral (ART) adherence studies reported that individuals randomized to the intervention arms were 1.5 times more likely to report ≥95% adherence and 1.25 times more likely to achieve an undetectable HIV viral load then controls.15,16 Several studies have used nursing telephone calls to improve HIV adherence. One Adult AIDS Clinical Trial Group (ACTG) study, protocol 388 (ACTG 388), examined the utility of additional phone calls by site personnel.17 Despite a high call completion rate (75%), there was no significant difference in virologic failure. Self-reported adherence, a secondary endpoint, was slightly better, but this difference did not reach statistical significance.
This study was designed to determine if a series of structured telephone calls initiated by nurses from the subjects’ study sites that provided both adherence and symptom support would decrease symptom distress and improve adherence to antiretrovirals. This study was similar to another concurrent ACTG 384 adherence substudy, ACTG 731, where telephone support calls were made from a central call center that was disassociated from subjects’ study sites.
METHODS
Study Design
ACTG 5031 was a randomized controlled trial of site-nurse initiated adherence and symptom support telephone calls (intervention) versus no additional calls above the site’s standard of care (control); all patients received the AIDS Clinical Trial Site’s standard adherence counseling and care. This was a substudy of ACTG 384, a multi-center trial that compared different antiretroviral treatment strategies in treatment naïve individuals.18,19 Primary inclusion and exclusion criteria included: HIV+, HIV RNA ≥ 500 copies/mL, <7 days of prior antiretroviral therapy, no serious acute illnesses or laboratory abnormality within 14 days of entry. A total of 980 subjects from 58 sites in the United States and 23 sites in Italy were randomized to either a 4-drug or sequential 3-drug regimen. Subjects experiencing a virologic or regimen failure were switched to another study-specified regimen designed to avoid cross-resistance. The ACTG 5031 adherence substudy was open to US sites, except those that participated in another adherence substudy of ACTG 384, ACTG 731 (Ohio State, North Carolina, Pennsylvania, and Cincinnati), which involved centralized nurse-initiated structured telephone calls for adherence support.20 Unlike ACTG 5031, these telephone calls originated from one centralized location not affiliated with the study-sites. Structured interventions were similar, although ACTG 731 calls were made only during the start of the study, weekly through week 12 and then on weeks 14 and 16. In contrast ACTG 5031 telephone calls continued for the entire study, up to 160 weeks, and emphasized symptom support in addition to adherence counseling. For this substudy, subjects also needed to be able to receive phone calls, either a landline or cell phone. All patients signed an informed consent approved by their institutions’ review boards.
ACTG 384 and 5031 randomization was done electronically by the data management center at Frontier Science. Subjects co-enrolling into the ACTG 5031 substudy were randomized in a 1:1 fashion to the adherence intervention or control arm using a dynamic randomization scheme to ensure the difference in group assignment at a particular site was never greater than 2. For subjects randomized to the intervention arm, the initial telephone call was made 1 to 3 days after starting antiretrovirals, and then on weeks 1, 2, 3, 6, 10, 14, 18, 22, 26, and every 8 weeks thereafter. Calls were scheduled to fall between study visits. Sites were allowed to make one additional call to subjects beginning a new regimen (ACTG 384, step 2 or 3)18,19, otherwise calls continued irrespective of changing regimens. Study personnel were asked not to use the telephone script for subjects randomized to the control arm but were allowed to call subjects if this was part of that site’s standard of care.
Phone Adherence Intervention
The intervention telephone calls designed to improve adherence and help patients self-manage medication side effects were performed by site personnel, typically study nurses, using a structured script (Appendix A). The intervention telephone script was the same for all calls; nurses adapted the calls depending on the patients’ adherence issues and symptoms. At the start of each call, subjects were told that missing pills would not affect their participation in the main study and that “sometimes people come across problems when taking their medications.” Adherence to study antiretrovirals over the last 2 days was assessed and individualized recommendations on how to improve adherence were provided when subjects reported less than 100% adherence. Subjects were asked whether there were specific times or doses that were harder to remember, and then a series of suggested strategies were discussed, e.g. using reminders and prompts for when to take medications, keeping extra medications on hand, and considering alarm watches or other adherence aids. In addition to adherence, subjects were asked about common side effects, diarrhea, fatigue, “feeling different,” headache, nausea, numbness/tingling, and rash, and were offered simple non-pharmacologic self-management strategies to minimize these symptoms. The script for the phone intervention was based on an earlier adherence substudy of ACTG 388, Life-Steps, and work by the ACTG Recruitment, Adherence, and Retention Subcommittee.17,21,22
Site personnel were asked to make a “reasonable” number of attempts to reach the subject for each call, and were in general not to exceed 5 attempts. The status of each call was recorded, including whether the subject was contacted, the call was completed, a message was left, the site personnel was unable to leave a message, or if the call was not attempted.
Study Endpoints
The primary study endpoint was the proportion of subjects reporting near perfect, defined as >95% Total Adherence (over the entire study). Planned secondary endpoints included mean Total Adherence (over the entire study), Early Adherence (over the first 32 weeks), quality of life, self-reported symptom distress, and ACTG 384 study endpoints: primary endpoint (first four-drug or second three-drug regimen failure), first regimen failure, and first virologic failure.18,19
Self-reported Adherence, Quality of Life and Symptoms Distress
Adherence on all subjects was collected as part of ACTG 384 using the AIDS Clinical Trials Group (ACTG) Adherence Questionnaire II (QL0702)23,24 This instrument captures subject self-report of the number of missed doses for each study medication over the previous four days, several general questions about adherence, and reasons why they may have missed taking their medications. Subjects were asked to complete this questionnaire during their visits at weeks 4, 16, 32, 48, and then every 16 weeks thereafter. This schedule was restarted if subjects were switched to a new ART regimen. At these visits, quality of life and symptoms distress were also captured using the ACTG Multidimensional Health Status (QL0601-0602) and the Quality of Life and Symptoms Distress (QL0730) questionnaires. Depression was assessed using a “CES-D score.”
Data obtained from the 4-day adherence recall (item 1 of the QL0702) were used to derive summary measures of adherence rate, the percent of the prescribed regimen taken [(1-proportion pills missed)*100] over the preceding four days, where percentages closer to 100% indicate better adherence. “Total Adherence” was defined as the average 4-day adherence recall for the entire study and “Early Adherence” as the 4-day average adherence recall at weeks 4, 16, and 32. Subjects on protocol mandated treatment holds were considered fully adherent.
Site “Standard of Care”
Approximately halfway through the study, sites were polled to determine their standard of care for treatment of naïve ACTG trial participants starting antiretroviral therapy (Appendix B). A “baseline adherence score” (1-8) was calculated mid-study based on whether sites routinely gave patients initiating antiretrovirals written materials on adherence and the amount of time spent specifically discussing adherence. A “follow-up adherence support score” was calculated from the total number of adherence-related phone calls a site typically made to their subject after starting a new regimen. For subjects in the phone intervention group, the “follow-up adherence support score” was set to zero since we assumed that they would not receive the standard follow-up care.
Study Analysis
Differences in baseline health dimensions between the follow-up groups were compared using ANOVA and non-parametric Mann-Whitney tests, where appropriate. Pearson chi-square tests were performed to compare differences in total adherence dichotomized to greater than 95% between the two study arms. Mann-Whitney test were used to compare Total Adherence and Early Adherence. Pearson’s chi-square statistic using continuity correction was calculated as a post-hoc analysis dichotomizing the adherence scores using the mean overall adherence for the ACTG 384 study (98.9%).
Time to ACTG 384 clinical endpoints (first-four drug or second-three drug regiment failure, first regimen failure, or first virologic failure) were compared using Mantel-Haenszel log-rank tests; estimates of hazard ratios and associated 95% confidence intervals were obtained with Cox proportional-hazard models adjusting for HIV RNA strata (>100,000 c/mL), baseline CD4 (continuous), gender, age (continuous), ethnic group, and randomized ACTG 384 treatment arm.
Adherence rates were analyzed with a linear model for repeated measures with mixed effects for treatment, time, and treatment-by-time interaction. The correlation structure of within-subject repeated measures, determined by comparing Akaike’s information criterion (AIC) from competing covariance structures, was used to achieve valid inferences on the fixed effects. The longitudinal model was fit using maximum likelihood estimation, which takes into account the correlation among the repeated measures. Subjects missing 1 or more adherence scores were included in the analysis with no imputation for missing scores. This estimation technique provides valid estimates of the model parameters even if missing data was dependent on the observed data rather than completely random.
A post-hoc analysis was performed to determine whether or not differences in the Standard of Care at the sites could have explained the higher adherence rates seen in the control group. In order to assess the effect of the Standard of Care on the efficacy of the adherence intervention, a linear regression model for the “Early Adherence” endpoint as a function of the “baseline adherence score,” “follow-up adherence support score” and treatment group was fit.
All analyses were intent-to-treat (ITT) analyses using SAS (9.3, Cary, NC).
Study Power
The study was designed to have 84 percent (two-sided) or 90 percent (one-sided) power assuming 175 subjects to detect an improvement in the percentage of subjects reporting near perfect adherence, no missed doses over the last four days, from 85 to 95 percent of subjects, or conversely, a decrease in the percentage of subjects reporting 1 or more missed antiretroviral doses over the 4-day recall from 15% to 5%. Assuming a higher enrollment of 225 subjects, the estimated power to detect 10% improvement in subjects reporting no missed doses was 95 percent.
RESULTS
Study Follow-up
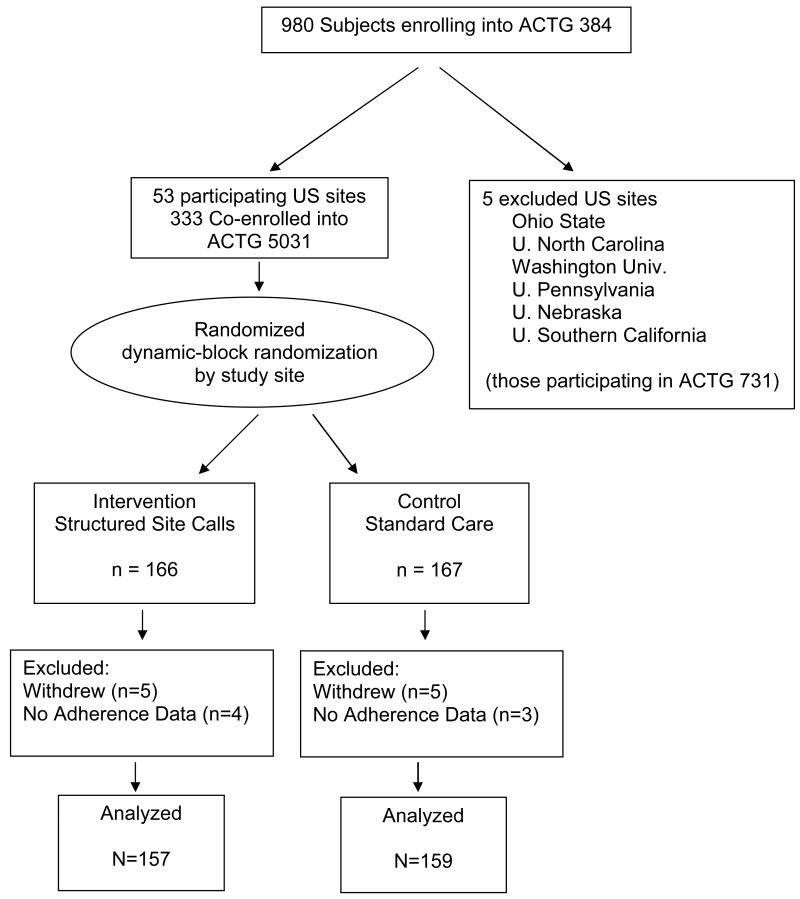
From October 1999 to November 2000, 333 subjects were co-enrolled into the ACTG 5031 adherence substudy (166 intervention, 167 control; Figure 1). Demographics of subjects in both groups were similar (Table 1). Subjects randomized to the intervention arm were more depressed at entry (p=0.003) but there were no other significant differences in additional baseline health dimensions. Two hundred eighty-one subjects completed the adherence questionnaire at week 4, 87.3% of the subjects of ACTG 5031 who were still on the main study (ACTG 384). By week 64 this number had decreased to 172, 73.8% of those remaining on study (Table 2). There was no significant difference in completion of the adherence questionnaires by study arm.
Figure 1.
Consort Flow Diagram
Table 1.
Baseline characteristics
| Intervention Group | Standard Care Group | |
|---|---|---|
| n = 166 | n = 167 | |
| Median Age (yrs) (IQR) |
36 (30 – 44.25) |
36 (31 – 42.75) |
| Male (%) | 78 | 81 |
| Race/Ethnicity (%) | ||
| White | 36 | 43 |
| Black | 40 | 32 |
| Hispanic | 21 | 23 |
| Others | 3 | 2 |
| Median RNA (c/mL) (IQR) |
5.1 log10
(4.3 – 5.7) |
5.1 log10
(4.3 – 5.5) |
| Median CD4 cell count (cells/mm3) (IQR) |
265.8 (83.4 – 437.1) |
268.8 (79.3 – 423.8) |
Table 2.
Study follow-up
| Week | Completed Questionnaires* |
Available Subjects# |
Percent completing the questionnaire (%) |
|---|---|---|---|
| 4 | 281 | 322 | 87 |
| 16 | 250 | 304 | 82 |
| 32 | 215 | 278 | 77 |
| 48 | 194 | 254 | 76 |
| 64 | 172 | 233 | 74 |
| 80 | 156 | 216 | 72 |
| 96 | 133 | 201 | 66 |
| 112 | 110 | 159 | 69 |
| 128 | 85 | 123 | 69 |
| 144 | 46 | 59 | 78 |
| 160 | 4 | 5 | 80 |
Number of subjects completing adherence questionnaire
Number of subjects still on study and w/o primary endpoint
Telephone Calls
Subjects assigned to the intervention telephone support calls were contacted for 74.4% of scheduled calls, and for 71% of these calls, the script was completed. The most common reasons for incomplete calls were inability to contact or leave a message, or subjects failed to call back.
Self-Reported Adherence
Mean and median self-reported adherence for all subjects were 98.2% and 99.6%, respectively. Many subjects reported perfect (100%) adherence on the ACTG adherence questionnaire (four-days prior to study visits): 60% reported perfect adherence during the first 32 weeks, while 40% reported perfect adherence at each assessment for the entire study. Mean adherence improved slightly from the first 48 weeks, 98.5% to 99.3%, and then fluctuated between 99.2% and 97.3% through week 160. The percent of subjects completing the self-report are shown in Table 2 and the adherence percentiles for Early Adherence (weeks 4, 16, 32) and Total Adherence are in Table 3.
Table 3.
Adherence by percentiles by group for Early and Total Adherence
| Percentiles | |||||
|---|---|---|---|---|---|
| 5 | 10 | 25 | 50 | 75 | |
|
Early Adherence
(week 4-32) |
|||||
| Intervention | 89.3 | 93.7 | 98.0 | 100 | 100 |
| Control | 92.8 | 94.5 | 99.2 | 100 | 100 |
|
Total Adherence
(weeks 4-160) |
|||||
| Intervention | 90.6 | 93.8 | 97.8 | 99.5 | 100 |
| Control | 91.9 | 95.2 | 97.8 | 99.7 | 100 |
Primary and Secondary Analyses
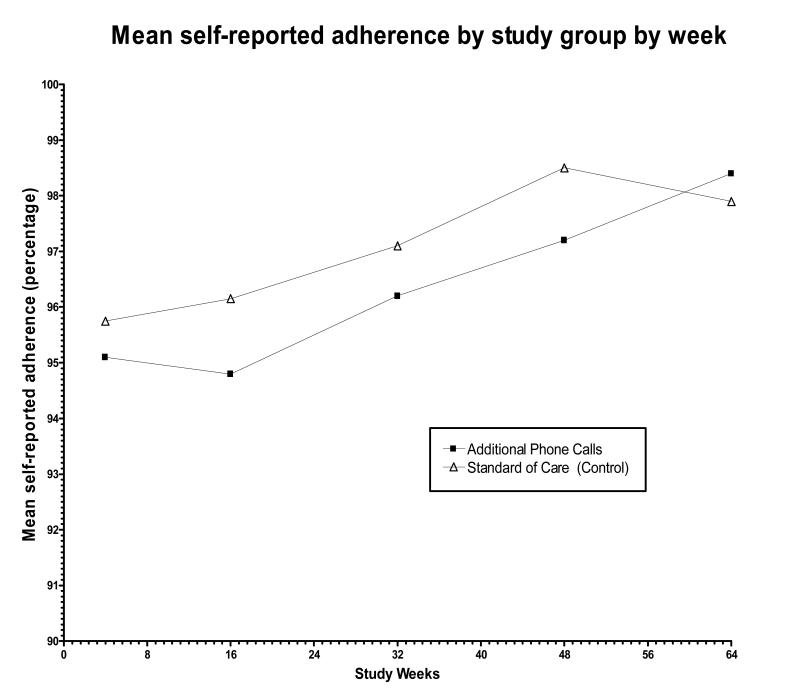
There were no significant difference in the proportion of patients with near perfect self-reported adherence, >95%Total Adherence, in the intervention (87.9%) and control arms (91.2%; p=0.34, Chi-Square). Self-reported mean Total Adherence by study week was similar in the intervention (97.9%, S.D. 3.94) and control (98.4%, S.D. 2.82) (Table 2, Figure 2). Because adherence rates were unevenly distributed, most reporting near perfect adherence, nonparametric testing was performed. Comparing ranked self-reported Total Adherence there was no significant difference (p=0.20, Mann-Whitney) between the two arms. Comparing differences in Early Adherence (weeks 4, 16, and 32), subjects in the intervention arm reported significantly lower adherence 98.0% vs. 98.6% control arm (p=0.01, Mann-Whitney). However, this mean difference was small (0.6%). In a post-hoc analysis, dichotomizing adherence scores using the mean for the overall ACTG 384 study (98.9%), a smaller percentage of the intervention arm had a mean adherence of >98.9% (62.7% versus 72.5%, p=0.07, Pearson’s Chi-square with continuity correction), but this difference was not significant. These results are similar to the primary endpoint (> 95%Total Adherence) where intervention arm had lower adherence than control, although none were statistically significant. Using a random effects model there was no evidence for a time-by-treatment interaction (p=0.88). There were no significant differences in quality of life or symptom distress.
Figure 2.
Mean self-reported adherence by study group by week. Differences between the two adherence arms were not significant. Adherence increased over the first 48 weeks and then remained high during the 160 weeks of follow-up.
Time-to ACTG 384 Study Endpoints
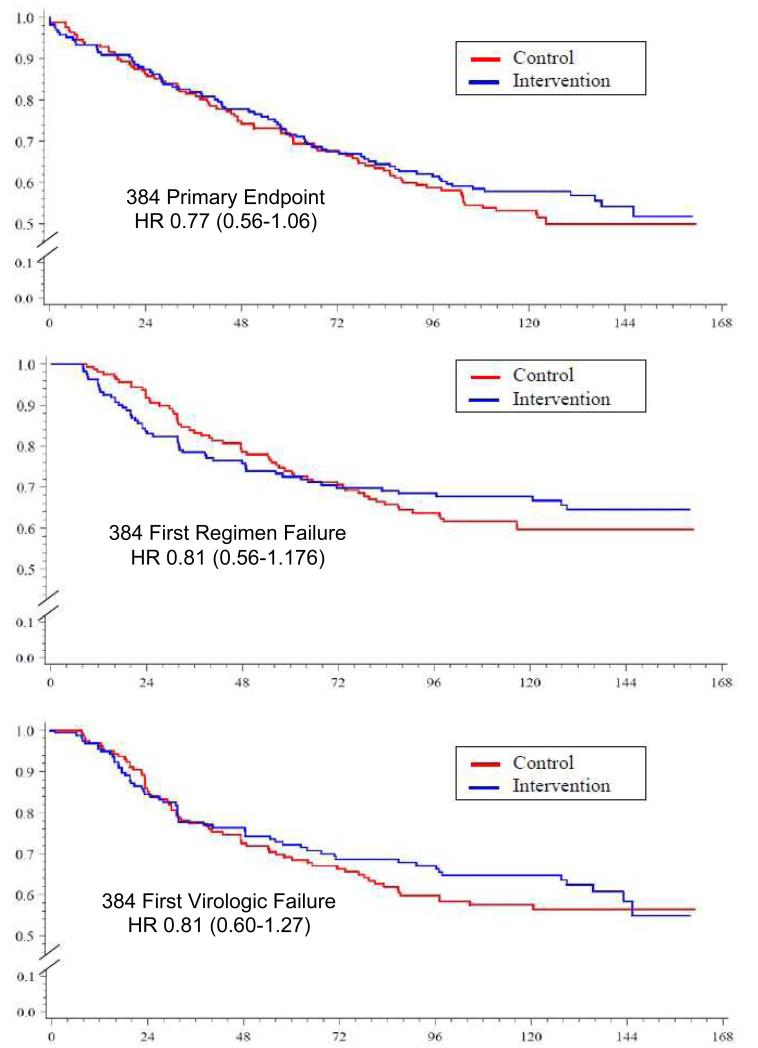
There was no significant difference in time to ACTG 384 endpoints in the intervention arm vs. control: first four-drug or second three-drug regimen failure (HR 0.77 (95% CI: 0.56-1.06), p=0.11, log-rank), first virologic failure (0.77 (95% CI: 0.52-1.13), or first regimen failure (HR 0.73 (95% CI: 0.51-1.06), p=0.10), or p=0.13; Figure 3a, 3b, 3c). There was also no significant difference in time to first HIV RNA <200 copies/mL, or proportion <200 copies/mL, or delta CD4 at week 48.
Figure 3.
Time to primary 384 endpoint, first four-drug or second three-drug regimen failure (3a), Time to 1st virologic failure (3b), and Time to first regimen failure (3c). Subjects participating in the 5031 Adherence substudy are shown: Additional phone support group (red) and standard care group (blue). For the 384 clinical endpoints differences between the two adherence arms were not significant.
Site Standard of Care
A linear regression model was fit for Early Adherence and Total Adherence using the estimates of sites’ Standard of Care. Site “baseline adherence score” varied from a low of 1 to a maximum of 8. There was no significant difference in efficacy of site calls based on differences in Site’s baseline adherence scores. However, sites with higher “baseline adherence score” tended to have subjects reporting higher “Early Adherence” (parameter estimate 0.17, p=0.09). This suggests that better site baseline adherence counseling tended to correlate with better self-reported adherence.
DISCUSSION
In one of the largest adherence studies to date, we found that site-nurse initiated telephone calls were well received and completed for the majority of patients still enrolled in ACTG 384 throughout the almost 3 year study. However, participants in both study arms evidenced exceptionally high adherence; hence, there was a ceiling effect that may have limited the ability for the intervention telephone calls to demonstrate a significant additional benefit in self-reported adherence, quality of life, symptom distress or clinical endpoints. Interestingly, there was an unexplained small negative association on self-reported adherence during the first 32 weeks of the study. This study confirms the feasibility of site-nurse initiated telephone calls, but these calls had little measurable impact in the context of a clinical trial where self-reported adherence was already exceptionally high.
Rates of self-reported adherence for the four days prior to study visits were higher than anticipated. Sixty percent reported perfect adherence over the first 32 weeks, but only 40% were able to maintain perfect adherence for the entire study. A meta-analysis of North American studies estimated that only 55% of patients are able to maintain adequate levels of adherence.25 Gardner et. al. found that 29% of subjects on a large antiretroviral study reported differential adherence over the course of the study.26 Medication fatigue is a well documented phenomena across other disciplines of medicine,27 and our results demonstrate that medication adherence fatigue is common even among those who initially report perfect adherence.
Our study results are in stark contrast to those of ACTG 731, a concurrent ACTG 384 substudy that utilized similar nursing support calls.20 This study enrolled 109 subjects at 5 sites that did not participate in ACTG 5031. Calls made from a central site by trained nurses, not affiliated with the study site, resulted in significantly higher self-reported adherence (p=0.023). Reasons for the disparate results are unclear, but may be multifactorial. One key design difference is that contamination may have occurred in our study and some control patients may have inadvertently received the intervention phone script. In addition, the central study nurses phone intervention utilized in ACTG 731 was more intensive and much more standardized, which may have delivered a more consistent and effective intervention. ACTG 731 subjects were also able to discuss adherence freely, knowing that their discussions were not shared with sites. In contrast, ACTG 5031 subjects discussed adherence with site personnel and it is possible that this either resulted in an unexpected negative impact on adherence or alternatively subjects may have been more honest in reporting non-adherence – so that a small positive effect on adherence could have been missed.
There are several limitations to this study. First, there was a strong ceiling effect that limited the ability to detect a difference. Self-reported adherence was also dramatically higher and better maintained than those generally reported in routine clinical care. Standard adherence counseling at ACTG sites varied and we had only a single mid-study assessment of site’s “standard care.” Overall the adherence counseling was higher than expected, with many sites utilizing written instructions and phone support. Site patients in the intervention arm were more depressed at baseline, although the reasons are unclear. Self-reported adherence is only a surrogate for adherence and more recent studies have suggested 30 day recall may be better than the 4-day ACTG questionnaire.28,29 Finally, phone interventions were not monitored to ensure compliance with the script, and as discussed above, there was the potential for control subjects to receive the intervention telephone calls. Many of these limitations are likely to be similar to those encountered when generalizing behavioral interventions to routine clinical practice. Additionally, the baseline level of standard of care was not the same for all the sites. A meta-analysis of twenty randomized controlled trials found that even though the interventions were helpful in predicting viral load and adherence, as the comparative standard of care increases, the effectiveness of the intervention decreases.30
In summary, in contrast to ACTG 731, a successful study of centralized nursing support phone calls, this randomized controlled trial of site-nurse initiated telephone calls failed to demonstrate improved self-reported adherence, symptom distress or clinical endpoints in a study population with already exceptionally high adherence rates. This study raises concerns about assessment of adherence in the context of site-delivered interventions. These results also demonstrate that even individuals with high adherence are at risk for medication adherence fatigue. Future multi-site adherence intervention trials should standardize and monitor the quality and of phone interventions.
Supplementary Material
Acknowledgements
Support: NIAID, Adult AIDS Clinical Trials Group (AI38858), Harvard University (AI27659), New York University, Stanford University, University California, Los Angeles, University California, San Diego, University California, San Francisco, University of Miami, University of Rochester Medical Center, University of Southern California, University of Washington, University of Minnesota, Tulane University, University of Cincinnati, Case Western Reserve University, Indiana University, Northwestern University, Beth Israel Medical Center, University of Hawaii, Howard University, University of Puerto Rico, University of Alabama, University of Colorado, University of Texas, Galveston, Columbia University.
We wish to thank the study volunteers and participating ACTU personnel. We would also like to acknowledge the contributions of the Lisa Johnson, Sally Snyder, Mark Shiner, and members of the ACTG Outcomes Committee for their efforts in developing structured phone interventions and adherence questionnaires, and the ACTG 384 team: Laura M. Smeaton, M.S., Victor De Gruttola, Sc.D., Sally W. Snyder, Carla Pettinelli, M.D., Ph.D., Ana I. Martinez, R.Ph., Michael P. Dubé, M.D., Margaret A. Fischl, M.D., Richard B. Pollard, M.D., Mostafa A. Nokta, M.D., Linda Gedeon, Tom Nevin, Mark I. Becker, Pharm.D., Mary Swingle, R.N., S. Debra McCarty, M.D. Richard T. D'Aquila, M.D., Stefano Vella, M.D., Thomas C. Merigan, M.D., and Martin S. Hirsch, M.D.
Footnotes
Conflicts/Disclosures: None.
References
- 1.Walensky RP, Paltiel AD, Losina E, et al. The survival benefits of AIDS treatment in the united states. J Infect Dis. 2006;194(1):11–19. doi: 10.1086/505147. [DOI] [PubMed] [Google Scholar]
- 2.Chesney MA, Ickovics J, Hecht FM, Sikipa G, Rabkin J. Adherence: A necessity for successful HIV combination therapy. AIDS. 1999;13(Suppl A):S271–8. [PubMed] [Google Scholar]
- 3.Reynolds NR. Adherence to antiretroviral therapies: State of the science. Curr HIV Res. 2004;2(3):207–214. doi: 10.2174/1570162043351309. [DOI] [PubMed] [Google Scholar]
- 4.Paterson DL, Swindells S, Mohr J, et al. Adherence to protease inhibitor therapy and outcomes in patients with HIV infection. Ann Intern Med. 2000;133(1):21–30. doi: 10.7326/0003-4819-133-1-200007040-00004. [DOI] [PubMed] [Google Scholar]
- 5.Bangsberg DR. Less than 95% adherence to nonnucleoside reverse-transcriptase inhibitor therapy can lead to viral suppression. Clin Infect Dis. 2006;43(7):939–941. doi: 10.1086/507526. [DOI] [PubMed] [Google Scholar]
- 6.Simoni JM, Pearson CR, Pantalone DW, Marks G, Crepaz N. Efficacy of interventions in improving highly active antiretroviral therapy adherence and HIV-1 RNA viral load. A meta-analytic review of randomized controlled trials. J Acquir Immune Defic Syndr. 2006;43(Suppl 1):S23–35. doi: 10.1097/01.qai.0000248342.05438.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bangsberg DR, Perry S, Charlebois ED, et al. Non-adherence to highly active antiretroviral therapy predicts progression to AIDS. AIDS. 2001;15(9):1181–1183. doi: 10.1097/00002030-200106150-00015. [DOI] [PubMed] [Google Scholar]
- 8.Garcia de Olalla, Knobel H, Carmona A, Guelar A, Lopez-Colomes JL, Cayla JA. Impact of adherence and highly active antiretroviral therapy on survival in HIV-infected patients. J Acquir Immune Defic Syndr. 2002;30(1):105–110. doi: 10.1097/00042560-200205010-00014. [DOI] [PubMed] [Google Scholar]
- 9.Hogg RS, Heath K, Bangsberg D, et al. Intermittent use of triple-combination therapy is predictive of mortality at baseline and after 1 year of follow-up. AIDS. 2002;16(7):1051–1058. doi: 10.1097/00002030-200205030-00012. [DOI] [PubMed] [Google Scholar]
- 10.Hogg RS, Bangsberg DR, Lima VD, et al. Emergence of drug resistance is associated with an increased risk of death among patients first starting HAART. PLoS Med. 2006;3(9):e356. doi: 10.1371/journal.pmed.0030356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chesney MA, Morin M, Sherr L. Adherence to HIV combination therapy. Soc Sci Med. 2000;50(11):1599–1605. doi: 10.1016/s0277-9536(99)00468-2. [DOI] [PubMed] [Google Scholar]
- 12.Heckman BD, Catz SL, Heckman TG, Miller JG, Kalichman SC. Adherence to antiretroviral therapy in rural persons living with HIV disease in the united states. AIDS Care. 2004;16(2):219–230. doi: 10.1080/09540120410001641066. [DOI] [PubMed] [Google Scholar]
- 13.Simoni JM, Frick PA, Pantalone DW, Turner BJ. Antiretroviral adherence interventions: A review of current literature and ongoing studies. Top HIV Med. 2003;11(6):185–198. [PubMed] [Google Scholar]
- 14.Simoni JM, Amico KR, Pearson CR, Malow R. Strategies for promoting adherence to antiretroviral therapy: A review of the literature. Curr Infect Dis Rep. 2008;10(6):515–521. doi: 10.1007/s11908-008-0083-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Simoni JM, Kurth AE, Pearson CR, Pantalone DW, Merrill JO, Frick PA. Self-report measures of antiretroviral therapy adherence: A review with recommendations for HIV research and clinical management. AIDS Behav. 2006;10(3):227–245. doi: 10.1007/s10461-006-9078-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Simoni JM, Amico KR, Smith L, Nelson K. Antiretroviral adherence interventions: Translating research findings to the real world clinic. Curr HIV/AIDS Rep. 2010;7(1):44–51. doi: 10.1007/s11904-009-0037-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Collier AC, Ribaudo H, Mukherjee AL, et al. A randomized study of serial telephone call support to increase adherence and thereby improve virologic outcome in persons initiating antiretroviral therapy. J Infect Dis. 2005;192(8):1398–1406. doi: 10.1086/466526. [DOI] [PubMed] [Google Scholar]
- 18.Robbins GK, De Gruttola V, Shafer RW, et al. Comparison of sequential three-drug regimens as initial therapy for HIV-1 infection. N Engl J Med. 2003;349(24):2293–2303. doi: 10.1056/NEJMoa030264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shafer RW, Smeaton LM, Robbins GK, et al. Comparison of four-drug regimens and pairs of sequential three-drug regimens as initial therapy for HIV-1 infection. N Engl J Med. 2003;349(24):2304–2315. doi: 10.1056/NEJMoa030265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reynolds NR, Testa MA, Su M, et al. Telephone support to improve antiretroviral medication adherence: A multisite, randomized controlled trial. J Acquir Immune Defic Syndr. 2008;47(1):62–68. doi: 10.1097/QAI.0b013e3181582d54. [DOI] [PubMed] [Google Scholar]
- 21.Safren SA, Otto MW, Worth JL, et al. Two strategies to increase adherence to HIV antiretroviral medication: Life-steps and medication monitoring. Behav Res Ther. 2001;39(10):1151–1162. doi: 10.1016/s0005-7967(00)00091-7. [DOI] [PubMed] [Google Scholar]
- 22.Safren SA, Otto MW, Worth JL. Life-steps: Applying cognitive behavioral therapy to HIV medication adherence. Cognitive and Behavioral Practice. 1999;6(4):332–341. [Google Scholar]
- 23.Chesney MA, Ickovics JR, Chambers DB, et al. Self-reported adherence to antiretroviral medications among participants in HIV clinical trials: The AACTG adherence instruments. patient care committee & adherence working group of the outcomes committee of the adult AIDS clinical trials group (AACTG) AIDS Care. 2000;12(3):255–266. doi: 10.1080/09540120050042891. [DOI] [PubMed] [Google Scholar]
- 24.Reynolds NR, Sun J, Nagaraja HN, Gifford AL, Wu AW, Chesney MA. Optimizing measurement of self-reported adherence with the ACTG adherence questionnaire: A cross-protocol analysis. J Acquir Immune Defic Syndr. 2007;46(4):402–409. doi: 10.1097/qai.0b013e318158a44f. [DOI] [PubMed] [Google Scholar]
- 25.Mills EJ, Nachega JB, Buchan I, et al. Adherence to antiretroviral therapy in sub-saharan africa and north america: A meta-analysis. JAMA. 2006;296(6):679–690. doi: 10.1001/jama.296.6.679. [DOI] [PubMed] [Google Scholar]
- 26.Gardner EM, Sharma S, Peng G, et al. Differential adherence to combination antiretroviral therapy is associated with virological failure with resistance. AIDS. 2008;22(1):75–82. doi: 10.1097/QAD.0b013e3282f366ff. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Osterberg L, Blaschke T. Adherence to medication. N Engl J Med. 2005;353(5):487–497. doi: 10.1056/NEJMra050100. [DOI] [PubMed] [Google Scholar]
- 28.Amico KR, Zuniga JM, Wilson IB, Gross R, Young B. Provider guidance for linking patients to antiretroviral therapy adherence interventions: Recommendations from an IAPAC advisory committee on adherence monitoring and support. J Int Assoc Provid AIDS Care. 2013;12(2):79–83. doi: 10.1177/1545109712474844. [DOI] [PubMed] [Google Scholar]
- 29.Lu M, Safren SA, Skolnik PR, et al. Optimal recall period and response task for self-reported HIV medication adherence. AIDS Behav. 2008;12(1):86–94. doi: 10.1007/s10461-007-9261-4. [DOI] [PubMed] [Google Scholar]
- 30.de Bruin M, Viechtbauer W, Schaalma HP, Kok G, Abraham C, Hospers HJ. Standard care impact on effects of highly active antiretroviral therapy adherence interventions: A meta-analysis of randomized controlled trials. Arch Intern Med. 2010;170(3):240–250. doi: 10.1001/archinternmed.2009.536. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.