Abstract
Following the discovery of leptin in 1994, major research efforts have brought us much closer to a fuller understanding of the cellular and molecular mechanisms underlying the biological effects of the hormone. Interestingly, leptin exerts potent anti-diabetic actions that are independent of its effects on body weight and food intake. In particular, leptin can correct diabetes in animal models of either diabetes mellitus type 1 (T1DM) or type 2 (T2DM). In addition, long-term leptin-replacement therapy is well tolerated and dramatically improves glycemic control, insulin sensitivity, and plasma triglycerides in patients with severe insulin resistance due to lipodystrophy. Together, these results have spurred enthusiasm for the use of leptin therapy to treat humans suffering from diabetes mellitus. Here, we review current understandings of these glucoregulatory functions of leptin, with particular emphasis on its central mechanisms of action, lessons from clinical studies and discuss possible therapeutic applications of leptin in the treatment of T1DM and T2DM.
Even with the improved anti-diabetic drugs, enhanced glycemia monitor systems, easier patient-to-physician accessibility, people who have type 2 diabetes mellitus (T2DM; an illness characterized by insulin resistance, elevated blood levels of glucose, insulin and lipids1 and estimated to affect more than 300 million people worldwide2) (Text box 1) are still at a significantly higher risk of developing cardiovascular disease and cancer compared to non-diabetic subjects3,4. The incidence of coronary artery disease in subjects suffering from type 1 diabetes mellitus (T1DM; an illness characterized by pancreatic β-cell loss, lack of insulin, hyperglycemia, cachexia, and ketoacidosis5,6 and estimated to affect millions worldwide7) (Text box 2) is also remarkably high: >90% after the age of 55 years8,9. In the US, 40% of patients diagnosed with diabetes do not achieve accepted glycemic targets and 80% fail to achieve blood pressure and lipid goals10. Therefore, despite the fact that i) insulin therapy transformed the previously lethal disease of T1DM into a liveable condition and ii) current anti-T2DM drugs achieve improved glycemic control, these interventions do not restore metabolic homeostasis and, in the long run, may cause serious co-morbidities of diabetes. Hence, better anti-diabetic approaches are urgently needed.
Text box 1. Type 2 diabetes mellitus (T2DM): Current treatments, complications and limitations.
T2DM is characterized by insulin resistance, hyperglycemia, and elevated circulating lipid levels; in later stages pancreatic β-cell loss can also occur1. There are several treatments available to patients with T2DM176. The most widely prescribed drug for the management of this metabolic defect is metformin, a biguanide that enhances hepatic insulin sensitivity and hence curtails the elevated hepatic glucose production typical of T2DM subjects177,178. Metformin inhibits complex I of the mitochondrial oxidative phosphorylation machinery and causes increased AMP/ATP ratio179; an effect that brings about activation of the AMP-activated protein kinase (AMPK). The current model predicts that the anti-diabetic action of metformin stems from the activation of hepatic LKB1/AMPK pathways → inhibition of HDACs (histone deacetylases) → suppression of FoxO1 action → reduced gluconeogenic gene expression → diminished glucose output178,180–182. Metformin causes minor side effects (e.g.: gastrointestinal upset) if administered to patients who are not prone to developing lactic acidosis 183,184. Thiazolidinediones (TZDs; rosiglitazone, pioglitazone, troglitazone) are synthetic ligands to the nuclear receptor/transcription factor peroxisome proliferator-activated receptor gamma (PPAR-γ)185 that increase insulin sensitivity186–188. Recent studies unveiled some of the mechanisms underlying the unwanted (e.g.: body adiposity augmenting) and wanted (diabetes improving) actions of TZDs. For example, increase appetite and body fat mass are frequent undesired effects of TZDs189–194. These untoward outcomes are likely due to TZDs action on brain PPAR-γ195,196. TZDs influence PPAR-γ activity and consequentially glucose metabolism by blocking the phosphorylation of PPAR-γ by the cyclin-dependent kinase 5 (Cdk5; an enzyme whose activity increases in adipose tissue of obese and diabetic subjects)188. New compounds tailored to target only the Cdk5-PPAR-γ interaction site have already been developed and successfully tested in pre-clinical studies197. These findings may pave the way to development of better anti-diabetic compounds through PPAR-γ. Indeed, due to known detrimental effects on the heart the use of rosiglitazone-containing medicines was recently restricted in the United States of America and suspended in the European Union198. Other classes of approved anti-T2DM drugs include: i) agonists at the glucagon-like peptide 1 (GLP-1) receptor (e.g.: exenatide and liraglutide) that have moderate efficacy. Exenatide is now approved in both Europe and US at once weekly injection, but can cause nausea and vomiting176, ii) inhibitors of the endogenous GLP1-inactivating protein dipetidyl-peptidase-4 (DDP4) that also have moderate efficacy and can cause serious side effects as for example increased risk of developing pancreatitis176, iii) insulin secretagogues, as for example the sulphonylureas that, by reducing the open probability of ATP-sensitive K+-channels, diminish the threshold for glucose-induced insulin secretion; these compounds have good efficacy but significantly increase the risks of hypoglycemia (an event that can be life-threatening), iv) insulin, that also has moderate efficacy and needs to be injected multiple times at day and can cause increased ectopic lipid deposition and hypoglycemia. Other classes of drugs are currently tested at different clinical trials stages (see these manuscripts that elegantly discuss these other drugs176,199,200). In addition to the drawbacks mentioned above, the requirement of currently available anti-T2DM drugs (either alone or in combination) usually augments with the progression of the disease hence increasing the chances of unwanted effects 200.
Text box 2. Type 1 diabetes mellitus (T1DM): Current treatments, complications and limitations.
T1DM is caused by pancreatic β-cell loss a defect that results in the lack of the hormone insulin and a lethal catabolic outcome if untreated. As a classical endocrinological approach to treating an illness resulting from lack of a given hormone is to replace the hormone therapeutically, T1DM clinical practice strictly abides by this paradigm. Thus, insulin administration is part of the daily activities of virtually all patients with T1DM6,201. Despite its undisputable life-saving action, insulin therapy does not restore metabolic homeostasis in patients with T1DM as these subjects are at a much higher risk of developing challenging morbidities as for example heart disease, blindness, kidney failure, neuropathy, and hypertension compared to normal subjects202–204. Whether these complications are due to the volatility of euglcyemia (wide fluctuations in blood glucose levels are indeed commonly seen in T1DM patients), or are direct consequences of therapy, or a combination of both is a dilemma yet to be resolved. In addition to the euglycemic volatility, drawbacks of insulin therapy stems from its lipogenic actions as insulin stimulates transcription of genes whose products are enzymes involved in the bio-synthesis of lipids205. Thus, long-term insulin treatment may underlie the excessive ectopic lipid deposition (i.e.: in non-adipose tissues)206 and the high incidence of coronary artery disease observed in T1DM patients8,9. Of note, these lipogenic actions of insulin likely promote a vicious cycle of fatty-acid-induced insulin resistance in insulin-target cells (e.g.: hepatocytes, myocytes) and hence lead to increased insulin requirements in the enduring management of diabetes207–209. In part due to the potent, fast-acting, glycemia-lowering effect of the hormone, intensive insulin therapy also significantly increases the risk of hypoglycemia, an event that can be life-threatening6,210–213.
Leptin is a hormone produced by adipose tissue that regulates a number of physiological processes and behaviours including appetite, body weight, neuroendocrine functions and glycemia. These effects are mediated via actions on leptin receptors (LepRs) expressed by neurons in the central nervous system (CNS)11,12. Among several splice variants of the leptin receptor gene13, the long LepRb isoform is thought to mediate all actions of leptin via activation of multiple intracellular signaling pathways (Figure 1). Studies in rodents have identified some of the specific neuronal targets mediating the hormonal effects on the aforementioned parameters. For example, the actions of leptin on body weight are mediated mainly by GABAergic neurons14; the anatomical location(s) of which remain unclear, and its action on puberty by neurons within the ventral premammillary nucleus of the hypothalamus (PMV)15. The potent effects of leptin on glucose homeostasis in the context of obesity and insulin resistance are mediated in large part by pro-opiomelanocortin (POMC)-expressing neurons within the hypothalamic arcuate nucleus (ARH)16,17.
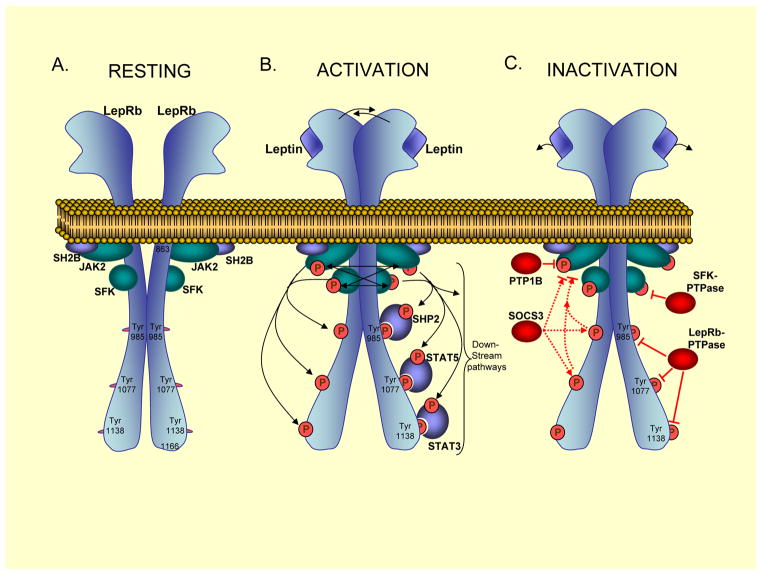
Figure 1. Neuronal Leptin Receptor Activation and Inactivation.
A. In the resting state, the long form leptin receptor (LepRb) exists as a homodimer at the plasma membrane153. The tyrosine kinases Janus Kinase (JAK2) and Src-Family-Kinases (SFKs), and the JAK2-binding protein SH2B, are constitutively associated with membrane-proximal regions of LepRb154,155. B. Upon ligand (leptin) binding to LepRb (1:1 stoichiometry156), the two receptor subunits undergo a conformational change resulting in transphosphorylation and transactivation of JAK2 and SFKs proteins157,158. SH2B enhances JAK2 enzymatic activity159. Activated JAK2 and possibly SFK enzymes, then phosphorylate LepRb tyrosine (Tyr) residues58. Three cytoplasmic Tyr residues of the murine LepRb acts as binding sites for SH2-domain containing proteins. Specifically, SHP2 principally binds to pY985, STAT5 to pY1077 and STAT3 to pY1338160,161. LepRb-bound SHP2, STAT3 and STAT5 proteins then become phosphorylated by JAK2 and SFKs. Additional downstream signalling proteins and pathways include PI3K-Akt; GRB2-Erk1/258,160,162; p90RSK58; p70RSK and ribosomal S6 proteins; and the forkhead box-containing protein O1 (FoxO1) 163,164. These enzymes and pathways ultimately exert regulation of cellular processes, including transcriptional control (socs3165,166, pomc167,168, cFos58, carboxypeptidase E (cpe)169, as well as agrp and npy 168), translational control170–172, and neuronal activity and firing109,173. C. LepRb is inactivated at proximal steps by PTP1B/PTPN1, a tyrosine phosphatase that directly dephosphorylates JAK276. Furthermore, SOCS3 acts to inhibit JAK2 activity by binding directly to JAK2 or indirectly by first binding to Tyr985 or Tyr 1077 of LepRb68,80,161. Finally, yet unidentified protein tyrosine phosphatases are predicted to directly dephosphorylate LepRb and SFKs.
Due to the potent beneficial effects on glucose metabolism, as demonstrated by administration of leptin to diabetic rodents and to humans with insulin-resistant diabetes caused by lipodystrophy18–20 (Text box 3), the 16 kDa polypeptide has the makings to become a novel and an effective anti-diabetic agent. This review presents available results from leptin-based clinical trials. It also examines in detail data from animal studies that led to the current understandings of the underlying mechanisms of leptin-mediated control of glucose balance. In addition, because T2DM patients are commonly obese and display hyperleptinemia and thus resistance to the metabolic actions of leptin, we deliberate on possible mechanisms responsible for leptin resistance. A detailed understanding of this issue is likely critical for successful development and therapeutic use of leptin or compounds targeting down-stream pathways engaged by the hormone as anti-diabetic drugs. Finally, we touch upon the potentials and limitations of leptin to become an addition to the anti-diabetic pharmacopeia.
Text box 3. Lipodystrophy: Current treatments, complications and limitations.
Lipodystrophy refers to a heterogeneous group of disorders characterized by abnormal adipose tissue homeostasis. Lipodystrophy can either be partial (displaying adipose tissue abnormalities in one or more sites in the body) or generalized (displaying a near-total lack of body adipose tissue). Either partial or generalized forms can either be congenital or acquired141. Congenital lipodystrophy is diverse with respect to its underlying molecular origin as mutations in several different genes have been found in people affected by this malady. Nevertheless, a common thread is the altered function of genes known to control adipogenesis and/or lipid storage. Mutations in the gene encoding for PPAR-γ (a key transcription factor for normal lipid uptake and storage and adipocyte differentiation) or Perilipin 1 (a crucial protein for normal lipid droplet formation and lipid storage) represent just few examples of the genetic defects found in the congenital lipodystrophic population214,215. Acquired lipodystrophy is thought to be caused by autoimmune-mediated destruction or iatrogenic-induced dysfunction of adipose tissue. For example, high active antiretroviral therapy used in patients affected by human immunodeficiency virus infection/acquired immunodeficiency syndrome (HIV/AIDS) is thought to underlie the lipodystrophy syndrome seen in these patients33. Possibly, the adverse effects on adipose tissue homeostasis are due to administration of nucleoside reverse transcriptase inhibitors and/or protease inhibitors216,217. Of note, acquired lipodystrophy in HIV-AIDS patients (a peculiar form of lipodystrophy that can lead to subcutaneous fat loss and increased abdominal fat) has become the most prevalent type of lipodystrophy141. Depending on the amount of adipose tissue loss and the body area in which this deficiency occurs lipodystrophy may lead to various degrees of hypoleptinemia and insulin resistance141. In some patients, lipodystrophy can even cause high levels of circulating insulin, lipids and glucose20. Treatment of lipodystrophy can be very challenging and it varies depending on the type of lipodystrophy. It may include life-style changes (dieting and increased physical activity) and/or drug(s) administration (e.g.: statins, and/or metformin, and/or insulin). The administration of leptin significantly improves hyperglycemia and hyperlipidemia in lipodystrophic patients displaying very low levels of circulating leptin20. Nevertheless, the vast majority of lipodystrophic patients is not severely hypoleptinemic and the current treatments fall short of restoring metabolic homeostasis in these subjects. Hence, lipodystrophic patients have higher risk of developing cirrhosis, renal disease, retinopathy, heart/circulatory defects compared to normal subjects141.
Leptin in clinical settings
Results from leptin-based clinical trials available at www.clinicaltrials.gov and http://www.ncbi.nlm.nih.gov/pubmed/ are discussed below (Table 1).
Table 1.
Leptin in clinical settings
| Condition | Basal Serum Leptin Levels | Intervention | Outcome | Reference(s) |
|---|---|---|---|---|
| Obesity (leptin deficiency) | Absent (<0.04 ng/ml) | Leptin | Improves obesity and endocrinological imbalances | 21–23 |
| Obesity | High (>15 ng/ml) | Leptin | Does not improve obesity | 25, 26 |
| Obesity | High (>15 ng/ml) | Leptin and Pramlintide | Modestly improves obesity | 27 |
| Lipodystrophy-induced T2DM | Very Low (<5 ng/ml) | Leptin | Improves lipid and glucose imbalances | 19, 20 |
| HIV and Lipodystrophy-induced T2DM | Very Low (<5 ng/ml) | Leptin | Improves glucose but not lipid imbalances | 34 |
| Lipodystrophy-induced T2DM | Low (~5 ng/ml) | Leptin | Improves lipid but not glucose imbalances | 32 |
| Hypothalamic Amenorrhea | Very Low (<5 ng/ml) | Leptin | Recuperates mestruation and corrects gonadal abnormalities | 37–39 |
| T2DM and Obesity | High (>15 ng/ml) | Leptin | Poorly improves glucose imbalances | 46, 47 |
| T1DM and Lipodystrophy | Very Low (<5 ng/ml) | Leptin | Improves lipid and glucose imbalances | 42 |
Effects on obesity
Due to the reproducible preclinical results showing that leptin exerts potent anti-obesity action, the hormone was initially heralded as the panacea for the obesity pandemic. When tested in humans, leptin therapy indeed rescued the obesity and several other endocrine defects (e.g. pubertal delay, infertility) of the very few obese people affected by congenital leptin deficiency21–23. The vast majority of subjects affected by obesity however displays elevated level of circulating leptin and likely is leptin resistant24. Unfortunately, hyperleptinemic obese patients were found to respond poorly to the anorectic and body-weight-suppressing action of exogenous leptin administration25,26; these results diminished expectations from leptin-based anti-obesity approaches. Nevertheless, more recent clinical data indicated that combination treatment with leptin and pramlintide (an amylin analogue) significantly reduces body weight (up to 12% of pre-treatment body weight) in leptin-resistant obese individuals27,28. Because the duration of therapy was limited to 20 weeks, it is however unclear if following a longer period of combined treatment the body weight loss is maintained or changed and/or side effects caused. Interestingly, attempts to address these issues were made but results from these trials (NCT00819234 and NCT00673387) are yet to be available. Also, the efficacy of leptin mono-therapy or leptin and pramlintide bi-therapy in obese subjects who have almost normal levels of circulating leptin (~10% of the obese population11,24) is unknown. In summary, although there are still chances for possible improvements it appears as if treatment with leptin alone is not an effective approach for the treatment of obesity in the vast majority of obese humans who also display hyperleptinemia (fasting serum leptin > 15 ng/ml).
Effects on lipodystrophy
Following the remarkable preclinical results obtained by Shimomura and colleagues who described that leptin administration corrects the severe insulin resistance and hyperglycemia displayed by rodents with congenital generalized lipodystrophy (Text box 3)18, the effects of the hormone were soon after analyzed in the clinical setting. In this case, the clinical outcomes somewhat satisfied the expectations engendered by the preclinical results. Indeed, the insulin resistance, hyperinsulinemia, hyperglycemia, hypertriglyceridemia displayed by severely hypoleptinemic (fasting serum leptin < 4 ng/ml) lipodystrophic patients were all shown to be improved (without adverse effects) following daily subcutaneous administration of recombinant methionyl human leptin19,20. Of note, the improved diabetes observed in these few leptin-treated lipodystrophic patients was achieved even after discontinuation of previously administered anti-diabetic therapy hence ruling out the possibility of synergistic or additive positive action of leptin and anti-diabetic drugs on glucose and lipid imbalances20. Results from additional studies have established the beneficial effect of leptin therapy on glucose and lipid metabolism in hypoleptinemic lipodystrophic patients29,30. Nevertheless, lipodystrophy refers to a very heterogeneous group of disorders that may be accompanied by diverse changes in the amount of circulating leptin (Text box 3)31. Indeed, lipodystrophic patients do not always display reduced leptinemia and some may have only moderately low levels of leptin in the blood32. Thus, concerns pertinent to the efficacy of leptin therapy in all types of lipodystrophies have been raised; more specifically, the anti-diabetic potential of leptin administration in lipodystrophic patients who are not severely hypoleptinemic has been questioned. Supporting these concerns, recent findings indicate that even though leptin therapy is very effective in ameliorating lipid profiles it does not improve hyperglycemia in lipodystrophic subjects with moderately low levels of circulating leptin (fasting serum leptin ~5 ng/ml)32. Thus, people who develop lipodystrophy without severe hypoleptinemia are expected to poorly respond to the hyperglycemia-lowering action of the hormone while still benefit from its hypertriglyceridemia-lowering effect. This potential limitation of leptin therapy in the context of lipodystrophy underscores the necessity for developing better approaches to treat metabolic imbalance in the thousands affected by this malady.
To this end, combination therapy may be a better option. For example, the most prevalent type of lipodystrophy is the acquired form displayed by high-active-antiretroviral-treated patients affected by human immunodeficiency virus infection/acquired immunodeficiency syndrome (HIV/AIDS)33. These patients usually display subcutaneous fat loss and increased abdominal fat. Results from a small clinical trial indicate that leptin administration improves glucose metabolism but not lipid parameters in lipodystrophic HIV/AIDS patients34. On the other hand, administration of tesamorelin (a growth hormone-releasing factor analogue approved by the US Food and Drug Administration for the treatment of lipodystrophy in HIV/AIDS patients) has been shown to improve visceral fat, triglyceride and cholesterol levels but not glucose parameters in these subjects35. Thus, combination treatment with leptin and tesamorelin may ameliorate both lipid and glucose parameters in lipodystrophic HIV/AIDS patients. Future clinical trials aimed at addressing this possibility are warranted.
Effects on nonalcoholic steatohepatitis (NASH)
The beneficial effects of leptin replacement therapy on fat deposition in the liver of lipodystrophic patients36 spurred enthusiasm on the possibility that leptin administration improves the increased fat content in the liver of non-lipodystrophic NASH patients. To test this possibility a clinical trial enrolled non-lipodystrophic NASH patients with relatively low circulating leptin levels; this trial has recently been completed (NCT00596934), however its results are yet to be published.
Effects on hypothalamic amenorrhea
Hypothalamic amenorrhea is a condition characterized by amenorrhea with anovulatory infertility, moderate to severe hypoleptinemia, and decreased bone mineral density. Leptin administration to strenuously exercising lean women affected by this condition leads to recuperation of menstruation and correction of gonadal abnormalities37. Also, this treatment has a clear positive effect on bone mineral density and content38,39. The latter results on bone homeostasis are somewhat unexpected considering that i) leptin deficiency in mice correlates with increased bone mineral density and mass hence suggesting that leptin may have a suppressive role on those two parameters and ii) humans lacking the hormone usually do not display defects in bone mineral density and mass23. Also, because the two studies mentioned above enrolled less than 20 women combined, it is unclear if the results on bone can be reproduced when the effects of leptin are tested in large cohorts.
Effects on type 1 diabetes mellitus
In mouse models of T1DM leptin mono-therapy (i.e. without the use of exogenously administered insulin and/or other compounds) corrects the diabetes and lethal catabolic consequences of insulin deficiency40,41. One year of treatment with leptin also remarkably improves glucose and lipid profiles in 2 patients affected by both T1DM and acquired generalized lipodystrophy42. Although insulin therapy was not entirely discontinued, leptin treatment improved insulin sensitivity to an extent that insulin doses were significantly reduced (30–50% compared to pre-leptin administration doses) in those subjects42. These pre-clinical and clinical findings spurred enthusiasm on the anti-T1DM therapeutic potentials of leptin. As a result, a clinical trial aimed at determining the safety of the hormone and its efficacy to diminishing the insulin requirements, hyperglycemia, glycemic fluctuations, and circulating lipid levels in T1DM humans (NCT01268644) is ongoing.
Effects on type 2 diabetes mellitus
Results from several pre-clinical studies indicate that leptin improves insulin resistance, and glucose and lipid imbalances in mouse models of T2DM16,43–45. However, the results of two recent clinical trials would indicate that leptin therapy is ineffective (or only marginally effective) in improving diabetes and insulin resistance in obese people affected by T2DM46,47. The anti-T2DM action of leptin may be however unmasked in T2DM patients who are not obese and have normo- or hypo-leptinemia such as for example Asian T2DM populations which in general have low adiposity. Future clinical trials aimed at addressing this possibility are therefore warranted.
In summary, regardless to the disease context in which leptin therapy has been tested a common thread seems to emerge: the failure of leptin administration to achieve improved metabolic imbalance in subjects who are not severely hypoleptinemic. For example, in the obesity context, leptin therapy improves metabolic imbalance in leptin deficient subjects but fails to do so in hyperleptinemic patients. In the lipodystrophy context, leptin therapy improves glucose and lipid imbalances in severely hypoleptinemic patients but fails to do so in the ones with moderately low levels of circulating leptin. Admittedly, the small number of patients enrolled in the clinical trials in which the effects of leptin have been tested and whose results have been made available should guide caution when drawing conclusions. Nevertheless, leptin therapy seems not to be effective in people who are leptin resistant. Moving forward, this information would indicate that i) a better understanding of the mechanisms underlying leptin resistance is needed, ii) ways to improve leptin resistance should be looked for, and iii) research aimed at identifying the molecular mechanisms underpinning the beneficial effects of leptin therapy must be encouraged as results from these endeavours are expected to provide the molecular targets for novel medicines able to circumvent the “leptin resistant” obstacle. Below, we will discuss the current information that could provide aid to addressing these deficiencies.
Leptin as an anti-obesity agent
The anti-obesity actions of leptin
Leptin is secreted by adipocytes proportionally to the amount of body fat and represents one of the key peripheral cues signaling the status of body energy reserves to the brain11,12. For example, in fasted rodents circulating leptin levels falls largely due to diminished leptin release from the decreasing fat depots. This fall in leptin is interpreted by the brain as a signal to increase appetite and food-seeking behavior to ultimately restore body fat depots48. Consistent with this role of leptin, genetic deficiency of this hormone or of its receptors leads to massive hyperphagia and obesity in both rodents and humans21,49,50. Remarkably, daily injection of recombinant leptin into normal mice reduces caloric intake and increases energy expenditure, resulting in near complete elimination of fat tissue within a few days with no apparent signs of toxicity51. Due to this potent anti-obesity action, leptin was initially heralded as the panacea for the obesity pandemic. However, it was soon realized that the vast majority of people affected by obesity has elevated circulating level of leptin24 and responds poorly to the anorectic and body-weight-suppressing actions of exogenous leptin administration25,26. The disappointing results of these initial clinical trials halted progress to use leptin in treatment of common forms of obesity. Nevertheless, if mechanisms of leptin resistance are understood and diminished through drug therapy, exogenous leptin may become an important adjunct tool for reducing adiposity and maintaining lower body weight. Currently, leptin therapy is only effective to treat obesity in the very few who have congenital leptin deficiency21,22.
What do we know about mechanisms causing leptin resistance in obesity?
An impaired response of exogenous leptin to reduce body weight and food intake demonstrates resistance to the anti-obesity actions of leptin52. Also, hyperphagia and increased adiposity in the presence of hyperleptinemia indicates resistance to endogenous leptin. In rodents given free access to a high-fat diet (HFD), leptin resistance manifest after only a few weeks53. Similarly, common obesity in humans is characterized by hyperleptinemia and diminished anorectic and body-weight-suppressing response to exogenous leptin25,26. The vast majority of human obesity cannot be attributed to genetic defects in the genes encoding leptin or its receptors. Therefore, because the anti-obesity actions of leptin are mediated by the brain, suggested mechanisms underlying leptin resistance can, in principle, be divided into three categories: i) impaired transport of leptin across the blood-brain-barrier (BBB), ii) impaired neuronal leptin signalling in target neurons, and iii) altered signaling in downstream target cells and neurocircuits (Figure 2A).
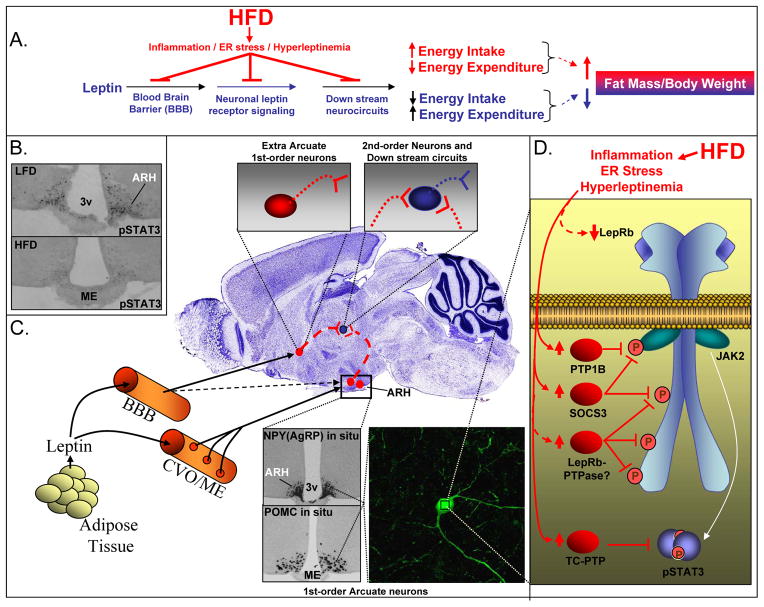
Figure 2. Cellular Mechanisms Causing CNS Leptin Resistance in High-Fat-Diet (HFD)-Induced Obesity in Rodents.
A. Leptin normally inhibits fat accumulation and body weight gain by entering the brain to decrease caloric intake and increase energy expenditure. In rodents, consumption of a HFD causes hyperleptinemia78, hypothalamic endoplasmic reticulum (ER) stress174 and production of pro-inflammatory cytokines175, ultimately leading to neuronal leptin resistance and diminished anti-obesity actions of leptin. HFD-induced leptin resistance causes obesity by a defect in leptin transport across the blood-brain barrier (BBB), leptin receptor signaling in LepRb-expressing neurons and/or in down-stream circuits. B. Leptin activated pSTAT3 immunohistochemistry is reduced in the hypothalamic arcuate nucleus (ARH) of HFD mice for 10 weeks. pSTAT3 is a functional marker for LepRb signalling in LepRb-expressing (1st order) neurons167,60. 3v= 3rd ventricle; ME=median eminence. C. Leptin normally enters most parts of the brain and reaches its target neurons via transport across the blood-brain-barrier (BBB). The ARH however is in close anatomical proximity to the median eminence (ME), a circumventricular organ (CVO) with fenestrated capillaries (red circles), suggesting that leptin may reach its 1st-order LepRb-expressing neurons within the ARH without actively being transported across the BBB66,67. The ARH 1st-order neurons include the POMC and AgRP neurons (lower inserts). D. The HFD-induced hyperleptinemia78, ER stress174 and/or inflammation175 causes leptin resistance within POMC and AgRP neurons61,62. Because activation of pSTAT3 is diminished in these neurons61,62, by exclusion, the signalling defect must be located at a step upstream of STAT3 phosphorylation. HFD mice have increased ARH expression of negative regulators of proximal LepRb-signaling in HFD mice, namely PTP1B, SOCS3 and TC-PTP60,72. Additional possibilities that may explain neuronal leptin resistance are HFD-induced inhibition of LepRb surface expression or increased expression of yet-to-be-identified LepRb-PTPases.
Early evidence supported the possibility of impaired leptin transport across the BBB as a primary defect underlying obesity in humans and rodents. For example, obese humans only have slightly increased leptin content in the cerebrospinal fluid despite their leptin blood levels being greatly elevated54. Also, blood-brain transport of leptin is reduced in diet-induced obese (DIO) animals55. However, more recent rodent data suggest that the impaired BBB leptin transport is acquired during development of obesity, and is therefore a secondary defect56. Consistent with this notion, the anorectic effect of leptin remains reduced in DIO rodents following intracerebroventricular (icv) administration of the hormone57. Altogether these data point to impaired intracellular signalling in neurons that express LepRs (termed neuronal or cellular leptin resistance).
The leptin receptor LepRb isoform has a long intracellular domain capable of activating a number of intracellular signaling pathways, including activation of signal transducer and activator of transcription phosphorylation 3 (STAT3) (Figure 1)58. The LepRb-STAT3 pathway is strictly required for the anti-obesity actions of leptin 59. LepRb is expressed in a relative wide number of hypothalamic and extra-hypothalamic brain regions. Importantly, activation of STAT3 phosphorylation by LepRb is impaired in some, but not all, neuronal groups in the brain of DIO rodents. Specifically, neurons within the ARH exhibit reduced STAT3 activation (Figure 2B), while LepRb-expressing neurons elsewhere in the brain appear to have relatively normal leptin sensitivity60, indicating that ARH LepRb neurons play a key role in development of leptin-resistant obesity. Not surprisingly, the leptin-resistant ARH neurons in DIO mice include the AgRP- and POMC-expressing neurons61,62 which mediate at least part of the anti-obesity effects of leptin63,64.
The ARH is in close anatomical vicinity to the median eminence (ME); a circumventricular organ (i.e. it lacks a BBB); thus, ARH neurons may have direct access to blood-borne leptin. While some studies argue against this possibility65, others support it as large proteins that cannot pass the BBB can reach the ARH by passive diffusion66. In addition, ARH neurons appear more sensitive to low doses of leptin compared to LepRb-expressing neurons located in other brain regions protected by the BBB67. Together the data suggest that leptin resistance of ARH neurons of DIO animals is not caused by defective leptin transport into the brain, but rather by a defect in LepRb-mediated signal transduction in 1st-order neurons (Figure 2C). By exclusion, the blockage site should be localized upstream of STAT3-phosphorylation. Theoretic possibilities include reduced LepRb surface expression, down-regulation of positive regulators or up-regulation of negative regulators of the LepRb-pSTAT3 pathway.
The identification of suppressor of cytokine signaling 3 (SOCS3) as a leptin-inducible feedback inhibitor of Janus kinase 2 (JAK2) activation by LepRb68 and reports demonstrating increased Socs3 mRNA in hypothalamus of leptin-resistant animals60,61 have provided an attractive molecular mechanism to explain neuronal leptin resistance (Figure 2D)69–71. Increased levels of protein tyrosine phosphatase 1B (PTP1B/PTPN1; a JAK2 tyrosine phosphatase) and of the related T- cell protein tyrosine phosphatase (TCPTP/PTPN2; a STAT3 tyrosine phosphatase) in DIO rodents may also contribute to neuronal leptin resistance62,72,73. Despite these advancements, the specific mechanism(s) by which HFD feeding increases hypothalamic SOCS3, PTP1B and TCPTP levels is still unclear. Possibilities include activation of inflammatory pathways74–76 and endoplasmic reticulum (ER) stress77. Although paradoxical and not understood, hyperleptinemia itself may also cause leptin resistance78, possibly via increasing SOCS3 and PTP1B expression79,80. In summary, leptin resistance in the neurons that mediate leptin’s anti-diabetic actions may represent a major roadblock for the therapeutic use of leptin in obese patients.
Discovery of glucoregulatory actions by leptin
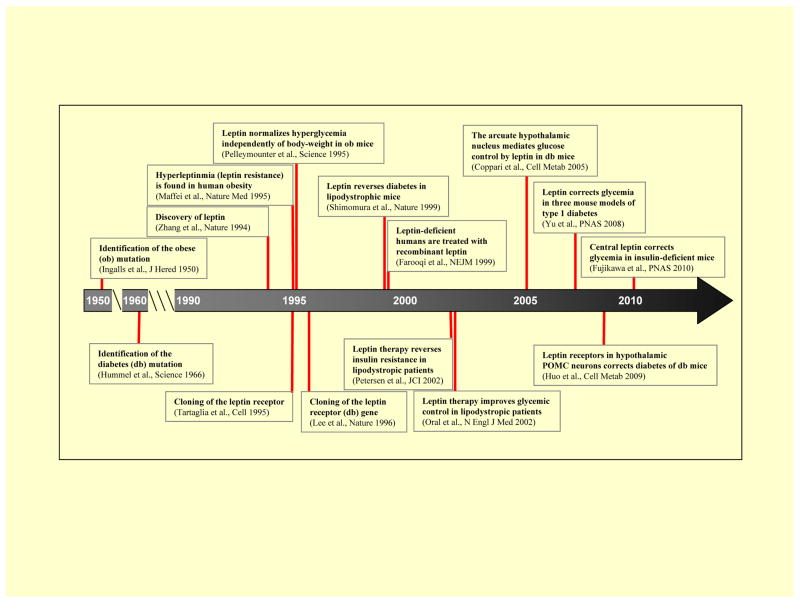
A timeline depicting the landmark discoveries of the beneficial effects of leptin on glucose metabolism is shown in Figure 3. The phenotypes (e.g.: obesity and diabetes) of mice homozygous for the autosomal recessive mutations named “ob” and “db” were first described in 1950 and 1966, respectively81,82. In 1994, Jeffrey Friedman’s group identified the gene mutated in the massively obese (and diabetic) ob/ob mouse49, and named the gene-product leptin. The discovery of leptin receptors was first reported in 199583 and was in 1996 shown to be encoded by the db gene13. After the first batches of recombinant leptin were produced, Pelleymounter and colleagues showed in 1995 that leptin, at a low dose that did not reduce food intake and body weight, almost normalized the severe hyperglycemia displayed by ob/ob mice; this study represents the first demonstration that the effects of leptin on glycemia can be separated from the ones on body weight and food intake84. In 1996, Schwartz and colleagues bolstered this idea by showing that restricting ob/ob mice to consume the amount of food ingested by leptin-treated ob/ob mice was not sufficient to fully recapitulate the glucose-lowering action brought about by leptin therapy85. Additional evidence supporting a direct glucoregulatory action of leptin was shown in 1999 by Shimomura and colleagues who described that leptin administration corrects the severe insulin resistance and hyperglycemia displayed by rodents with lipodystrophy; this effect was also independent of body weight change18. Importantly, beneficial results on insulin sensitivity and glycemic control in humans were obtained in 2002 when leptin was first given to insulin-resistant lipodystrophic patients19,20. However, a significant blow to the prospect of using leptin to treat humans suffering from common T2DM was reported in 2011 when leptin was shown to be ineffective in improving insulin sensitivity and glycemic control in obese and T2DM patients46,47. On a separate front, leptin delivery to hypoinsulinemic T1DM rodent models interestingly showed great efficacy to diminish hyperglycemia86,87; promoting the possibility that leptin might have anti-T1DM actions. This apparent insulin-independent action of leptin in rodents was shown to be mediated by the CNS40.
Figure 3. Key Milestones in the Discovery of Leptin’s Anti-Diabetic Actions.
Following these discoveries, progress has been made in identifying specific neurons capable of mediating glucose regulation by leptin in hyperinsulinemic T2DM rodent models and in understanding CNS effector mechanisms underlying the anti-diabetic effects of leptin in T1DM mouse models that completely lack insulin; these results are discussed below.
CNS and efferent anti-diabetic mechanisms
Central mechanisms
Specific brain sites capable of mediating the anti-diabetic action of leptin in T2DM models were first identified in 2005, when it was reported that restoration of Lepr expression only in ARH neurons completely normalizes hyperglycemia of the obese, hyperinsulinemic and severely diabetic Lepr-null mice in a body-weight- and food-intake-independent fashion45. The search was further narrowed when it was found that selective expression of LepRb only in the POMC-expressing neurons within the ARH of the LepRb-deficient db/db mice was sufficient to mediate this remarkable glucose normalization17. These results identified ARH POMC neurons as principal candidates for mediating the anti-diabetic actions of leptin in the db/db model (Figure 4A).
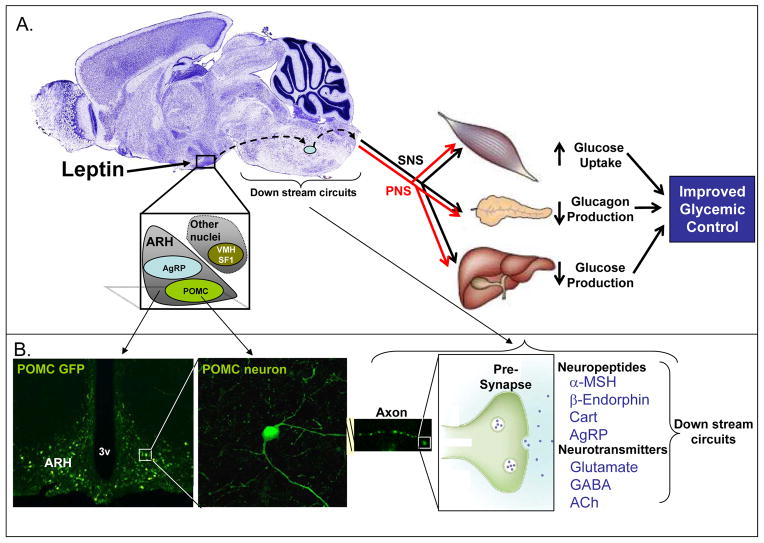
Figure 4. CNS Neuronal Mediators, Efferent Pathways, and Peripheral Mechanisms Underlying the Anti-Diabetic Actions of Leptin.
A. Schematic model of central neuronal pathways and efferent processes whereby leptin exerts its anti-diabetic actions in insulin-resistance and obese mice (e.g. genetically modified db/db animals). ARH POMC-expressing neurons are currently the best candidates responsible for mediating the improved glucose control by leptin in the db/db Type 2 diabetic rodent models, although AgRP-expressing neurons and other hypothalamic and extra-hypothalamic neurons are also likely to play important roles. These neurons act via axonal projections on downstream neurocircuits (e.g. the melanocortin system) to engage efferent pathways. This may include regulation of sympathetic (SNS) and parasympathetic (PNS) branches of the autonomic nervous system, ultimately affecting muscle glucose uptake, pancreatic glucagon production and/or hepatic glucose production. B. ARH leptin-target neurons, including POMC neurons, produce and secrete a number of different molecules from axon terminals, including the POMC-polypeptide-derived neuropeptides; alpha-melanocortin stimulating hormone (α-MSH) and β-Endorphin. Cocaine and amphetamine-regulated transcript (Cart) and Nesfatin-1 is also co-expressed with POMC peptides. Finally, these ARH neurons are heterogeneous with regard to neurotransmitter phenotype; different subpopulations produce glutamate, GABA and acetylcholine (ACh). Combined, these neurotransmitters and neuropeptides are the candidate effector-molecules to mediate glycemic control by leptin in the db/db model.
While the re-expression of LepRb in POMC neurons fully corrects hyperglycemia in very young db/db mice, the effect is only partial with increasing age17. In addition, selective deletion of LepRs from POMC neurons of lean non-db/db mice does not precipitate overt hyperglycemia63. These data suggest redundancy in the system and that other neurons in addition to the POMC- expressing cells also have a role in glycemic control by leptin. The ARH also contains AgRP- expressing neurons which similarly to POMC neurons express LepRb64. AgRP is a key neuropeptide- component of the central melanocortin system, and acts as an antagonist of α-MSH at the melanocortin 4 receptor (MC4R)88. This receptor is widely expressed throughout the CNS in neurons that are targeted by axons from POMC and AgRP neurons89,90, and are as such 2nd-order neurons in respect to 1st-order LepR-expressing neurons. Loss of MC4Rs causes obesity in mice and humans88. Several studies have implicated the central melanocortin system in glycemic control91,92, as further discussed below. In addition, the virally-mediated re-activation of endogenous LepRs in the ARH of Lepr-null mice presumably targeted both POMC and AgRP neurons45. As those mice have complete normalization of blood glucose with increasing age, in contrast to the partial correction of hyperglycemia in same-aged mice with only POMC-selective re-expression of LepRb, these above data combined suggest that AgRP neurons may also have a role in mediating glucose regulation by leptin (Figure 4A). Preliminary experiments (Bjorbaek et al, not shown), by assessing glycemia in db/db mice expressing LepRb only in AgRP neurons, support a significant capacity of LepRb in AgRP neurons in markedly reducing the severe hyperglycemia of db/db mice.
The above studies raise the question of how leptin acts via one group of neurons, (e.g. POMC neurons) to influence both peripheral glucose metabolism and energy balance, independently of each other. Several non-mutually-exclusive possibilities can be proposed: i) POMC neurons are a heterogeneous population of cells, each serving distinct functions; and/or ii) POMC neurons produce and secrete different neurotransmitters and neuropeptides, each serving separate metabolic functions; and/or iii) different intracellular LepRb-signaling pathways regulate the effect of leptin on glucose vs. energy balance. Evidence supports the notion that POMC neurons are highly heterogeneous. For example, leptin activates some (30%–90%), but not all, POMC neurons93. Similarly, insulin has been reported to affect (inhibit) only subsets of POMC neurons93–96. It remains unclear whether the leptin- responsive POMC neurons are insulin sensitive, or not, adding further complexity93,97. In addition, POMC-expressing neurons produce a number of different neuropeptides (i.e. α-MSH, β-MSH, γ-MSH, ACTH, β-Endorphin) derived from the POMC-polypeptide precursor98 along with other neuropeptides including cocaine-amphetamine regulated transcript (Cart)99 and nesfatin-1100. Furthermore, POMC-expressing neurons appear highly heterogeneous with regard to neurotransmitter phenotype with regard to being GABAergic, glutamatergic, or cholinergic14,101,102 (Figure 4B). To understand the specific roles of each of these heterogeneous POMC neuronal populations and POMC- secreted molecules on glucose metabolism vs. energy balance, additional studies are clearly needed.
LepRb regulates several downstream intracellular signaling pathways. In particular, four motifs of the murine leptin receptor [i.e. the proximal JAK2-binding box, and tyrosine(Y)-985, Y1077 and Y1138] regulate largely distinct intracellular signaling pathways (Figure 1). Dissecting the contribution of each motif to specific cellular and whole-body processes is an active area of research. For example, global knock-in of a serine at Y1138, the activation site for STAT3, leads to obesity and hyperphagia that are nearly as severe as that of db/db mice (that entirely lack LepRb signaling)59, suggesting a major role of STAT3 and its nuclear gene-targets in regulation of appetite and whole-body energy balance. Linear growth and fertility however remained intact, indicating that other LepRb-motifs and non-STAT3 pathways regulate those actions of leptin103. Global mutation of all three LepRb Y residues leads to severe hyperphagia and obesity that is similar to that found in mice with mutation of Y1138 alone59. Interestingly, these triple mutant mice reportedly are nearly normoglycemic, indicating that non-tyrosine-mediated signaling events might play a role in mediating the effects of leptin on glucose. Consistent with this notion, C-terminal truncation of LepRb to leave only the proximal JAK2 binding-motif intact delays the onset of diabetes while causes increased adiposity similar to that seen in db/db mice104. Some caution should however be taken with regard to the interpretations of the above data because the diabetic phenotype of db/db (and ob/ob) mice depends greatly on the genetic background105, which may not be the same between studies and even within studies in some cases. Secondly, the identity of the important LepRb-expressing neurons was not identified. Despite these limitations the LepRb mutational studies suggest: i) that different LepRb signaling pathways may regulate largely distinct whole-body metabolic functions; ii) that proximal LepRb signaling-pathways may be of particular importance for glycemic control mediated by leptin.
Neuron-specific genetic manipulations of intracellular proteins in POMC-expressing neurons support an important role of those particular cells in control of glucose homeostasis. For example, SOCS-3 deletion from POMC neurons in mice lowers blood glucose concentrations without influencing body weight and adiposity71. In addition, mice lacking PTP1B in POMC neurons have normal body weight and fat mass, but exhibit increased whole-body insulin-sensitivity106. Although a number of CNS regions and specific neurons are known to act as glucose-sensors, it is interesting that POMC neurons possess glucose-sensing capabilities107. Abolishment of glucose-sensing only in POMC neurons impairs glucose tolerance108. Thus, the anatomical location of POMC neurons near the ME, possibly allowing direct sensing of hormones (e.g. leptin) and metabolites (e.g. glucose) in the circulation, combined with the ability of this group of cells to influence glucose balance, uniquely places them as a key component of both an acute and long-term homeostatic rheostat. A number of studies have investigated the cellular function and whole-body metabolic roles of PI3K in POMC neurons. For example, POMC-neuron-specific deletion of PI3K-subunits (p85) indicates that the PI3K pathway is required for the acute electrical effect of leptin (and glucose) on POMC neurons (i.e. axonal firing)109. Genetic studies of PI3K are however complicated by the existence of different isoforms. In addition, alteration of PI3K activity will not only affect LepRb signaling, but also impact other cellular functions and pathways, including that of insulin. With these limitations in mind, it has been reported that genetically-mediated alteration of PI3K activity in POMC neurons affects circulating insulin levels and hepatic insulin sensitivity, without changes in whole-body energy balance110. This result, combined with the metabolic analyses of mice with various LepRb tyrosine mutations described above, supports the possibility that the PI3K pathway may play a role in glycemic control by POMC neurons. Yet further investigations are required to identify the key downstream PI3K signaling pathways and cellular processes.
Roles of the central melanocortin system, the autonomic nervous system and peripheral target tissues
A substantial body of evidence shows that the autonomic nervous system can exert control on glucose homeostasis via actions on the endocrine pancreas111, skeletal muscle112, liver113, and fat114. It is therefore tempting to speculate that the anti-diabetic actions of CNS leptin are mediated via the sympathetic and/or parasympathetic nervous systems. Indeed, ARH neurons (e.g. POMC and AgRP neurons) send projections to the paraventricular hypothalamic nucleus (PVN) where a group of pre-autonomic (sympathetic and parasympathetic) neurons reside115,116. In addition, a subset of POMC neurons projects directly to the intermediolateral nucleus of the spinal cord (IML) where sympathetic preganglionic cholinergic neurons reside117. Importantly, the PVN and IML neurons express MC4Rs90. Alternatively or in addition, leptin may regulate peripheral glucose metabolism via ARH-mediated synaptic actions on parasympathetic preganglionic neurons located in the dorsal motor nucleus of the vagus nerve (these neurons also express MC4Rs90). Pharmacological and genetic studies shed light on the possible role of the melanocortin system in glycemic control by leptin118,119,120. However, pharmacologically-induced activation of the central melanocortin system is not sufficient to improve STZ-diabetes suggesting that other circuits are important121. Indeed, mice and humans lacking functional MC4Rs are not diabetic122; demonstrating that if defects in the melanocortin system play a role in blood glucose imbalance, other processes must also be defective.
Increased hepatic glucose production (HGP) is the major contributor to fasting hyperglycemia in individuals with diabetes123. Hyperinsulinemic/euglycemic clamp studies in normal rats and in ob/ob mice show that icv leptin primarily inhibits HGP without significant effects on glucose disposal113,124. In addition, virally-mediated re-expression of LepRb in unspecified ARH neurons of LepRb-deficient Koletsky rats inhibits HGP without influencing glucose disposal125. Interestingly, this effect is lost after hepatic vagotomy, indicating a possible requirement of parasympathetic nerve activity; suggesting the existence of a Leptin-ARH-Parasympathetic-Liver axis113,125. However, because nerve branches to other organs may also have been severed in these studies, the possibility of indirect mechanism cannot be excluded. The specific neurons involved have also yet to be identified, although the POMC neurons (and AgRP neurons), are attractive candidates. Contrasting this proposed role of liver parasympathetic nerve activity, other studies point to regulation of hepatic sympathetic activity91. Although ARH LepRb is required for leptin to stimulate sympathetic nerve activity to renal and brown-adipose tissues126, direct measurements of leptin-dependent regulation of hepatic sympathetic and parasympathetic have heretofore not been reported, and will further need to be measured in conscious animals. While the above studies support a role of autonomic innervation of the liver in whole body glucose balance, it is important to consider that denervation of the liver does not alter glycemia in rodents (e.g. following vagotomy) or humans (e.g. following liver transplants). In addition, hepatic deletion of muscarinic acetylcholine receptors (the receptors for the primary neurotransmitter secreted from postganglionic parasympathetic neurons) does not affect glucose homeostasis127. Studies are needed to determine if other neurotransmitters are important for mediating parasympathetic activity and if autonomic control of hepatic glucose metabolism, versus other organs (i.e. pancreas or muscle), is of particular relevance only in pathophysiological states as for example in diabetic or insulin resistant states.
In parallel to the possible direct neural (autonomic) connections listed above, it is well known that the hypothalamus can also affect glycemia through neuroendocrine mechanisms such as the hypothalamus-pituitary-adrenal and the hypothalamus-pituitary-thyroid axes, as well as symapthoadrenal efferents, hence influencing circulating levels of glucocorticoids, thyroid hormones and catecholamines. Despite considerable progress over the past decade, the relative contributions of neuroendocrine versus autonomic pathways in mediating the glucoregulatory actions of leptin are unknown and warrant further research. Insulin-like growth factor binding protein-2 (IGFBP-2) has been reported to mediate anti-diabetic actions of leptin128. Studies aimed at determining a potential involvement of the autonomic system and if IGFBP-2 plays a role downstream of LepRb in POMC neurons, deserve further investigation128,129. Finally, additional studies are required to determine under which specific physiological and pathophysiological circumstances the leptin-hypothalamus-peripheral-tissues axis influences the liver, and/or the endocrine pancreas, and/or skeletal muscle to ultimately govern glucose balance.
Glucoregulatory actions in the absence of insulin
Until recently the general consensus was that circulating insulin is required for the anti-diabetic actions of leptin (e.g. ob/ob and db/db T2DM models and in lipodystrophic patients). Even in STZ-treated rodents, insulin-producing β-cells were not entirely ablated in all studies leaving a residual level of circulating insulin87,130,131. The view that insulin is required for the anti-diabetic effects of leptin is to be amended because leptin administration improves diabetes and survival of animals that completely lack insulin40,41,132,133. Many of metabolic imbalances studied in these papers and the lethal effects of true insulin deficiency were reversed by leptin monotherapy; without the need of insulin co-administration. Furthermore, adenovirally- or pharmacologically-induced hyperleptinemia rescues hyperglycemia in diverse animal models of T1DM, including the NOD mice, and STZ- and Alloxan-induced diabetic rats41,132. Importantly, these effects were not secondary to the anorectic effects of leptin40,41,132. Similar results have also been described when leptin was given subcutaneously in a mouse model of whole-body insulin receptor deficiency; this intervention also led to remarkable beneficial effects on diabetes symptoms133. In addition to the glycemia-lowering action, leptin monotherapy ameliorated several other metabolic defects brought on by insulin deficiency, such as hyperglucagonemia, polyuria, hyperketonemia, and normalized reduced hepatic glycogen content41,132. It must be noted that some of these changes may be the result of the improved hyperglycemia.
Despite the importance of the abovementioned observations, the mechanism(s) underlying the anti-T1DM action of leptin still needs to be determined. For example, is this effect due to the direct action of leptin on glucagon-producing pancreatic α-cells? This possibility seems unlikely because leptin administration does not suppress glucagon secretion from cultured α-cells134. Alternatively, does LepRb signaling in hepatocytes mediate the anti-T1DM action of leptin? This idea seems also unlikely because leptin therapy was recently shown to be able to improve hyperglucagonemia and diabetes in T1DM mice lacking leptin receptors selectively in hepatocytes135. Is LepRb signaling in the brain the key mechanism? This possibility is strongly supported by a study in which CNS-restricted leptin administration normalized hyperglycemia and hyperglucagonemia (as well as other metabolic defects) in T1DM mice; an effect similar to the one observed after systemic leptin administration40.
Because of the aforementioned findings and the importance of hypothalamic LepRb in regulating glucose homeostasis in the T2DM models such as db/db mice 17,44,45,125 it is tempting to speculate that LepRb-expressing neurons within the hypothalamus may also mediate the beneficial effects of leptin in the context of T1DM. Unfortunately, evidence supporting this contention is still lacking. Future studies aimed at testing the anti-T1DM action of leptin in genetically-engineered mice either lacking or expressing LepRb only in discrete hypothalamic neurons will be required to directly test this hypothesis. There is also a need to examine the anti-diabetic potency of leptin in additional animal models of T1DM and T2DM, because the STZ-treated and dbdb mice are not generally representative models of the clinical aetiologies of T1DM and T2DM respectively.
Deciphering the cellular and molecular mechanism(s) by which leptin therapy suppresses hyperglycemia and permits survival of insulin-deficient rodents is likely to be instrumental for developing better anti-diabetic approaches. As it will be discussed more in details below, there are several potential drawbacks of leptin use in humans with diabetes; for example, diabetics are often also obese and leptin resistant and thus poorly responsive to exogenous leptin46. Thus, a detailed understanding of the mechanisms by which leptin exerts its glucose-lowering effect in T1DM and T2DM will be crucial for eventually circumvent these likely shortcomings of leptin therapy in humans. For example, an important role for hyperglucagonemia in the pathophysiology of diabetes has been suggested136, an idea bolstered by the fact that glucagon receptor null mice are protected from developing diabetes137. Therefore, if LepRb on POMC neurons were to mediate the anti-diabetic action of leptin by suppressing glucagon secretion, once identified, this POMC-neuron-dependent pathway can be exploited for development of more effective anti-diabetic agent(s). This idea is not too farfetched as emerging evidence support a role for LepRs in POMC neurons in regulating glucagon secretion138.
Regardless to these issues, the aforementioned findings in T1DM rodents spurred enthusiasm on the therapeutic anti-diabetic potentials of leptin; an interest that resulted in an ongoing clinical trial aimed at determining the safety of the hormone and its efficacy to diminishing the insulin requirements, hyperglycemia, glycemic fluctuations, and circulating lipid levels in T1DM humans (study NCT01268644)139.
Leptin as an anti-diabetic agent: potentials and drawbacks
Leptin is remarkably effective to improve hyperglycemia in some animal models of both T1DM40,41,132 and T2DM43,45,140, and to greatly increase insulin sensitivity in lipodystrophic humans18,20. Whether leptin will be able to exert similar beneficial effects in models that better represent the human aetiology and more importantly, in non-lipodystrophic diabetic humans (who represent the majority of the diabetic population) is the subject of passionate scientific discussions with both optimistic and cautious points of view. Based on the conspicuous data from pre-clinical investigations (mainly rodent studies) and results from human studies (mainly gathered from lipodystrophic subjects), the expected benefits of leptin therapy in T1DM and T2DM can be recapitulated in the following: i) leptin has not been reported to induce hypoglycemia in diabetic rodents40 and human patients141 and as such its slow-acting, glycemia-lowering action would be preferred over the fast-acting, glycemia-lowering action of insulin that is known to trigger hypoglycemic events; ii) because leptin administration decreases lipid contents in adipose and extra-adipose tissues40 its effects on lipid metabolism would also be preferred over the ones exerted by insulin that is known to promote lipid accumulation in adipose and extra-adipose tissues; iii) leptin suppresses appetite40 and this effect is desirable in diabetic people. Therefore, if leptin were to overcome the potential problems discussed below it may become an effective adjuvant or alternative treatment against diabetes without the risk of hypoglycemia and/or lipid-induced cardiovascular defects.
There are however several potential pitfalls that leptin therapy will need to overcome if it is to become an attractive addition to the anti-diabetic pharmacopeia. For example, in rodents, some pharmacological studies show that leptin increases arterial pressure142 although this does not appear to be the case when leptin is co-administered with amylin143. Also, humans lacking leptin have hypotension despite their massive obesity142. Hence, it cannot entirely be ruled out that prolonged leptin administration may cause increased blood pressure in humans; an undesirable effect in the already hypertensive-prone diabetic subjects. Also, leptin has been reported to accelerate autoimmune diabetes in the NOD model of T1DM, possibly by favouring proinflammatory cell responses144. Another obstacle is likely leptin resistance. Obesity and T2DM go hand-in-hand with many obese people being also affected by glucose/insulin imbalance. Because the vast majority of T2DM patients is overweight or obese and is characterized by hyperleptinemia, leptin therapy might be ineffective altogether as the results of recent clinical trials would indicate46. These potential problems may also apply to T1DM people. It is also possible that leptin therapy may cause leptin-induced leptin resistance in humans. If leptin resistance turns out to present a major obstacle in developing leptin-treatment strategies for diabetes as recent clinical results would suggest46, then ways to first dampen leptin resistance and hyperleptinemia may be needed. Finally, not all T2DM diabetics are obese and hyperleptinemic24. This sub-group of patients is therefore presumably relatively normal in this aspect to leptin action and might benefit from leptin therapy.
Leptin resistance may however not prevent the use of leptin as an anti-T2DM drug. For example, data suggest that insulin resistance negatively affects some insulin receptor signaling cascade pathways (e.g.: AKT-FoxO1) while others appear unaffected (e.g.: SREBP1c)1. Although yet speculative, this bifurcation may also exist downstream of LepRb signaling. For example, leptin resistance may selectively affect LepRb signaling cascade pathway(s) that mediate the body-weigh-reducing effect while leaving the pathway(s) important for mediating glucose regulation relatively undamaged. The idea that different intracellular components mediate distinct actions of leptin on body weight vs. glucose homeostasis is supported by experimental data as discussed above. For example, while LepRb-mediated phosphorylation of STAT3 is crucial for leptin to suppress food intake and body weight59, this pathway seems less important for the ability of leptin to improve glucose homeostasis59. Results showing that pharmacological blockade of hypothalamic PI3K signaling impairs the insulin-sensitizing effect of leptin further bolster this concept44. Rodent data also show that low doses of leptin can correct hyperglycemia without affecting body weight or food intake. This result indicates that the pathways underlying the glucose-lowering actions of leptin are more sensitive than the ones underpinning its anti-obesity effects. Therefore, it is tantalizing to insinuate that leptin resistance may not represent an insurmountable obstacle for the anti-diabetic action of leptin. Nevertheless, results from a recently completed clinical trial seem to refute this hypothesis; however, patients enrolled in this trial were massively obese (and likely exaggeratedly leptin resistant) and leptin treatment was very short (i.e.: 2 weeks)46. As such, it will be important to test the effectiveness of leptin therapy in less obese patients with diabetes and also extend the treatment for longer periods (i.e.: several months).
In addition to the aforementioned potential problems of leptin therapy in diabetes, the known stimulatory effects of the hormone on PI3K signaling should not be overlooked. In fact, the PI3K pathway is an important node for normal cellular proliferation145 and unfortunately crucial for the growth of certain types of cancers145,146. Therefore, leptin (as well as insulin) therapy has the potential to facilitate tumor growth by impinging upon this signaling cascade147. If leptin were to be widely prescribed for the management of diabetes, either as an alternative or adjunct approach, a careful analysis of tumor progression in patients who also are affected by cancer will be important. Other potential shortcomings of leptin therapy include i) the generation of neutralizing auto-antibodies against the exogenous hormone29,148,149, ii) increased immune system function and inflammation150, and iii) bone mass loss151,152.
Concluding remarks
Once heralded as the anti-obesity panacea, leptin could become an important asset in the armamentarium against diabetes; for patients with either T1DM, or T2DM, or lipodystrophy-induced diabetes. As discussed above, the failure of leptin as a general anti-obesity medicine probably rests on the fact that the vast majority of people with increased adipose mass are resistant to the food-intake- and body-weight-suppressing action of the hormone. However, not all diabetic patients are obese and hence some may normally respond to the metabolic actions of leptin. The fact that leptin therapy has been shown to have remarkable efficacy in ameliorating or entirely correcting diabetes in some T1DM and T2DM animal models, and that long-term leptin-replacement therapy is well tolerated and markedly improves glycemic control, insulin sensitivity, and plasma triglycerides in patients with severe insulin resistance due to generalized lipodystrophy, development of leptin-based approaches to treat diabetes has generated considerable enthusiasm. Nevertheless, results from recent clinical trials seem to reject the hypothesis that leptin can effectively improve insulin sensitivity in T2DM people who also are overly obese. However because a proportion of the T2DM population is not massively obese, an outstanding question in the field is: does leptin therapy improve insulin sensitivity in non- obese, leptin-sensitive, T2DM subjects? Another important question is: does leptin therapy improve diabetes in T1DM subjects?
In the event that leptin fails to improve diabetes in the vast majority of humans affected by either diabetes, research aimed at uncovering the central circuits, efferent pathways and peripheral processes responsible for mediating the anti-diabetic action of the hormone should continue. If leptin resistance proves an obstacle for the efficacy of leptin as anti-diabetic therapy, further research aimed to unravel the underlying molecular mechanisms is clearly warranted, as this may provide opportunities for identification of new drugs targets and therapeutics that can circumvent the blockade in leptin action and ultimately help improve the quality and quantity of life of people affected by diabetes.
Acknowledgments
This work was supported by the American Heart Association (0930366N to R.C.), the American Diabetes Association (7-09-BS-17 and 7-12-BS-010 to C.B.), the Richard and Susan Smith Family Foundation Pinnacle Program Project (7-05-PPG-02 to C.B.), the Boston Obesity Nutrition Research Center (DK46200 to C.B.) and by grants from the National Institutes of Health (DK080836 to R.C. and DK60673, DK65743 and DK94040 to C.B.).
Footnotes
Competing interests statement
The authors have nothing to declare.
References
- 1.Brown MS, Goldstein JL. Selective versus total insulin resistance: a pathogenic paradox. Cell metabolism. 2008;7:95–96. doi: 10.1016/j.cmet.2007.12.009. [DOI] [PubMed] [Google Scholar]
- 2.Danaei G, et al. National, regional, and global trends in fasting plasma glucose and diabetes prevalence since 1980: systematic analysis of health examination surveys and epidemiological studies with 370 country-years and 2.7 million participants. Lancet. 2011;378:31–40. doi: 10.1016/S0140-6736(11)60679-X. [DOI] [PubMed] [Google Scholar]
- 3.Czyzyk A, Szczepanik Z. Diabetes mellitus and cancer. Eur J Intern Med. 2000;11:245–252. doi: 10.1016/s0953-6205(00)00106-0. [DOI] [PubMed] [Google Scholar]
- 4.Mazzone T, Chait A, Plutzky J. Cardiovascular disease risk in type 2 diabetes mellitus: insights from mechanistic studies. Lancet. 2008;371:1800–1809. doi: 10.1016/S0140-6736(08)60768-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Daneman D. Type 1 diabetes. Lancet. 2006;367:847–858. doi: 10.1016/S0140-6736(06)68341-4. [DOI] [PubMed] [Google Scholar]
- 6.Cryer PE. Diverse causes of hypoglycemia-associated autonomic failure in diabetes. The New England journal of medicine. 2004;350:2272–2279. doi: 10.1056/NEJMra031354. [DOI] [PubMed] [Google Scholar]
- 7.Borchers AT, Uibo R, Gershwin ME. The geoepidemiology of type 1 diabetes. Autoimmunity reviews. 2010;9:A355–365. doi: 10.1016/j.autrev.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 8.Larsen J, et al. Silent coronary atheromatosis in type 1 diabetic patients and its relation to long-term glycemic control. Diabetes. 2002;51:2637–2641. doi: 10.2337/diabetes.51.8.2637. [DOI] [PubMed] [Google Scholar]
- 9.Orchard TJ, et al. Insulin resistance-related factors, but not glycemia, predict coronary artery disease in type 1 diabetes: 10-year follow-up data from the Pittsburgh Epidemiology of Diabetes Complications Study. Diabetes care. 2003;26:1374–1379. doi: 10.2337/diacare.26.5.1374. [DOI] [PubMed] [Google Scholar]
- 10.Hoerger TJ, Segel JE, Gregg EW, Saaddine JB. Is glycemic control improving in U.S. adults? Diabetes care. 2008;31:81–86. doi: 10.2337/dc07-1572. [DOI] [PubMed] [Google Scholar]
- 11.Friedman JM. Leptin at 14 y of age: an ongoing story. Am J Clin Nutr. 2009;89:973S–979S. doi: 10.3945/ajcn.2008.26788B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Flier JS, Maratos-Flier E. Lasker lauds leptin. Cell. 2010;143:9–12. doi: 10.1016/j.cell.2010.09.021. [DOI] [PubMed] [Google Scholar]
- 13.Lee GH, et al. Abnormal splicing of the leptin receptor in diabetic mice. Nature. 1996;379:632–635. doi: 10.1038/379632a0. [DOI] [PubMed] [Google Scholar]
- 14.Vong L, et al. Leptin action on GABAergic neurons prevents obesity and reduces inhibitory tone to POMC neurons. Neuron. 2011;71:142–154. doi: 10.1016/j.neuron.2011.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Donato J, Jr, et al. Leptin’s effect on puberty in mice is relayed by the ventral premammillary nucleus and does not require signaling in Kiss1 neurons. The Journal of clinical investigation. 2011;121:355–368. doi: 10.1172/JCI45106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Berglund ED, et al. Direct leptin action on POMC neurons regulates glucose homeostasis and hepatic insulin sensitivity in mice. The Journal of clinical investigation. 2012;122:1000–1009. doi: 10.1172/JCI59816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huo L, et al. Leptin-dependent control of glucose balance and locomotor activity by POMC neurons. Cell metabolism. 2009;9:537–547. doi: 10.1016/j.cmet.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shimomura I, Hammer RE, Ikemoto S, Brown MS, Goldstein JL. Leptin reverses insulin resistance and diabetes mellitus in mice with congenital lipodystrophy. Nature. 1999;401:73–76. doi: 10.1038/43448. [DOI] [PubMed] [Google Scholar]
- 19.Petersen KF, et al. Leptin reverses insulin resistance and hepatic steatosis in patients with severe lipodystrophy. J Clin Invest. 2002;109:1345–1350. doi: 10.1172/JCI15001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Oral EA, et al. Leptin-replacement therapy for lipodystrophy. The New England journal of medicine. 2002;346:570–578. doi: 10.1056/NEJMoa012437. [DOI] [PubMed] [Google Scholar]
- 21.Farooqi IS, et al. Effects of recombinant leptin therapy in a child with congenital leptin deficiency. The New England journal of medicine. 1999;341:879–884. doi: 10.1056/NEJM199909163411204. [DOI] [PubMed] [Google Scholar]
- 22.Farooqi IS, O’Rahilly S. Leptin: a pivotal regulator of human energy homeostasis. Am J Clin Nutr. 2009;89:980S–984S. doi: 10.3945/ajcn.2008.26788C. [DOI] [PubMed] [Google Scholar]
- 23.Paz-Filho G, Wong ML, Licinio J. Ten years of leptin replacement therapy. Obesity reviews : an official journal of the International Association for the Study of Obesity. 2011;12:e315–323. doi: 10.1111/j.1467-789X.2010.00840.x. [DOI] [PubMed] [Google Scholar]
- 24.Maffei M, et al. Leptin levels in human and rodent: measurement of plasma leptin and ob RNA in obese and weight-reduced subjects. Nature medicine. 1995;1:1155–1161. doi: 10.1038/nm1195-1155. [DOI] [PubMed] [Google Scholar]
- 25.Heymsfield SB, et al. Recombinant leptin for weight loss in obese and lean adults: a randomized, controlled, dose-escalation trial. JAMA : the journal of the American Medical Association. 1999;282:1568–1575. doi: 10.1001/jama.282.16.1568. [DOI] [PubMed] [Google Scholar]
- 26.Hukshorn CJ, et al. Weekly subcutaneous pegylated recombinant native human leptin (PEG-OB) administration in obese men. J Clin Endocrinol Metab. 2000;85:4003–4009. doi: 10.1210/jcem.85.11.6955. [DOI] [PubMed] [Google Scholar]
- 27.Roth JD, et al. Leptin responsiveness restored by amylin agonism in diet-induced obesity: evidence from nonclinical and clinical studies. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:7257–7262. doi: 10.1073/pnas.0706473105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ravussin E, et al. Enhanced weight loss with pramlintide/metreleptin: an integrated neurohormonal approach to obesity pharmacotherapy. Obesity. 2009;17:1736–1743. doi: 10.1038/oby.2009.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ebihara K, et al. Efficacy and safety of leptin-replacement therapy and possible mechanisms of leptin actions in patients with generalized lipodystrophy. The Journal of clinical endocrinology and metabolism. 2007;92:532–541. doi: 10.1210/jc.2006-1546. [DOI] [PubMed] [Google Scholar]
- 30.Chong AY, Lupsa BC, Cochran EK, Gorden P. Efficacy of leptin therapy in the different forms of human lipodystrophy. Diabetologia. 2010;53:27–35. doi: 10.1007/s00125-009-1502-9. [DOI] [PubMed] [Google Scholar]
- 31.Haque WA, Shimomura I, Matsuzawa Y, Garg A. Serum adiponectin and leptin levels in patients with lipodystrophies. The Journal of clinical endocrinology and metabolism. 2002;87:2395. doi: 10.1210/jcem.87.5.8624. [DOI] [PubMed] [Google Scholar]
- 32.Simha V, et al. Comparison of efficacy and safety of leptin replacement therapy in moderately and severely hypoleptinemic patients with familial partial lipodystrophy of the Dunnigan variety. The Journal of clinical endocrinology and metabolism. 2012;97:785–792. doi: 10.1210/jc.2011-2229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Grinspoon S, Carr A. Cardiovascular risk and body-fat abnormalities in HIV-infected adults. The New England journal of medicine. 2005;352:48–62. doi: 10.1056/NEJMra041811. [DOI] [PubMed] [Google Scholar]
- 34.Sekhar RV, et al. Leptin replacement therapy does not improve the abnormal lipid kinetics of hypoleptinemic patients with HIV-associated lipodystrophy syndrome. Metabolism: clinical and experimental. 2012 doi: 10.1016/j.metabol.2012.03.013. [DOI] [PubMed] [Google Scholar]
- 35.Falutz J, et al. Effects of tesamorelin (TH9507), a growth hormone-releasing factor analog, in human immunodeficiency virus-infected patients with excess abdominal fat: a pooled analysis of two multicenter, double-blind placebo-controlled phase 3 trials with safety extension data. The Journal of clinical endocrinology and metabolism. 2010;95:4291–4304. doi: 10.1210/jc.2010-0490. [DOI] [PubMed] [Google Scholar]
- 36.Javor ED, et al. Leptin reverses nonalcoholic steatohepatitis in patients with severe lipodystrophy. Hepatology. 2005;41:753–760. doi: 10.1002/hep.20672. [DOI] [PubMed] [Google Scholar]
- 37.Chou SH, et al. Leptin is an effective treatment for hypothalamic amenorrhea. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:6585–6590. doi: 10.1073/pnas.1015674108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sienkiewicz E, et al. Long-term metreleptin treatment increases bone mineral density and content at the lumbar spine of lean hypoleptinemic women. Metabolism: clinical and experimental. 2011;60:1211–1221. doi: 10.1016/j.metabol.2011.05.016. [DOI] [PubMed] [Google Scholar]
- 39.Welt CK, et al. Recombinant human leptin in women with hypothalamic amenorrhea. The New England journal of medicine. 2004;351:987–997. doi: 10.1056/NEJMoa040388. [DOI] [PubMed] [Google Scholar]
- 40.Fujikawa T, Chuang JC, Sakata I, Ramadori G, Coppari R. Leptin therapy improves insulin-deficient type 1 diabetes by CNS-dependent mechanisms in mice. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:17391–17396. doi: 10.1073/pnas.1008025107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yu X, Park BH, Wang MY, Wang ZV, Unger RH. Making insulin-deficient type 1 diabetic rodents thrive without insulin. Proc Natl Acad Sci U S A. 2008;105:14070–14075. doi: 10.1073/pnas.0806993105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Park JY, et al. Type 1 diabetes associated with acquired generalized lipodystrophy and insulin resistance: the effect of long-term leptin therapy. The Journal of clinical endocrinology and metabolism. 2008;93:26–31. doi: 10.1210/jc.2007-1856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cummings BP, et al. Subcutaneous administration of leptin normalizes fasting plasma glucose in obese type 2 diabetic UCD-T2DM rats. Proc Natl Acad Sci U S A. 2011 doi: 10.1073/pnas.1107163108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Morton GJ, et al. Leptin regulates insulin sensitivity via phosphatidylinositol-3-OH kinase signaling in mediobasal hypothalamic neurons. Cell metabolism. 2005;2:411–420. doi: 10.1016/j.cmet.2005.10.009. [DOI] [PubMed] [Google Scholar]
- 45.Coppari R, et al. The hypothalamic arcuate nucleus: a key site for mediating leptin’s effects on glucose homeostasis and locomotor activity. Cell metabolism. 2005;1:63–72. doi: 10.1016/j.cmet.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 46.Mittendorfer B, et al. Recombinant human leptin treatment does not improve insulin action in obese subjects with type 2 diabetes. Diabetes. 2011;60:1474–1477. doi: 10.2337/db10-1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Moon HS, et al. Efficacy of metreleptin in obese patients with type 2 diabetes: cellular and molecular pathways underlying leptin tolerance. Diabetes. 2011;60:1647–1656. doi: 10.2337/db10-1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ahima RS, et al. Role of leptin in the neuroendocrine response to fasting. Nature. 1996;382:250–252. doi: 10.1038/382250a0. [DOI] [PubMed] [Google Scholar]
- 49.Zhang Y, et al. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372:425–432. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]
- 50.Montague CT, et al. Congenital leptin deficiency is associated with severe early-onset obesity in humans. Nature. 1997;387:903–908. doi: 10.1038/43185. [DOI] [PubMed] [Google Scholar]
- 51.Halaas JL, et al. Weight-reducing effects of the plasma protein encoded by the obese gene. Science. 1995;269:543–546. doi: 10.1126/science.7624777. [DOI] [PubMed] [Google Scholar]
- 52.Bjorbaek C. Central leptin receptor action and resistance in obesity. J Investig Med. 2009;57:789–794. doi: 10.231/JIM.0b013e3181bb0d49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Frederich RC, et al. Leptin levels reflect body lipid content in mice: evidence for diet-induced resistance to leptin action. Nature medicine. 1995;1:1311–1314. doi: 10.1038/nm1295-1311. [DOI] [PubMed] [Google Scholar]
- 54.Caro JF, et al. Decreased cerebrospinal-fluid/serum leptin ratio in obesity: a possible mechanism for leptin resistance. Lancet. 1996;348:159–161. doi: 10.1016/s0140-6736(96)03173-x. [DOI] [PubMed] [Google Scholar]
- 55.Banks WA, DiPalma CR, Farrell CL. Impaired transport of leptin across the blood-brain barrier in obesity. Peptides. 1999;20:1341–1345. doi: 10.1016/s0196-9781(99)00139-4. [DOI] [PubMed] [Google Scholar]
- 56.Banks WA, Farrell CL. Impaired transport of leptin across the blood-brain barrier in obesity is acquired and reversible. American journal of physiology. 2003;285:E10–15. doi: 10.1152/ajpendo.00468.2002. [DOI] [PubMed] [Google Scholar]
- 57.Wilsey J, Zolotukhin S, Prima V, Scarpace PJ. Central leptin gene therapy fails to overcome leptin resistance associated with diet-induced obesity. American journal of physiology. 2003;285:R1011–1020. doi: 10.1152/ajpregu.00193.2003. [DOI] [PubMed] [Google Scholar]
- 58.Bjorbaek C, Uotani S, da Silva B, Flier JS. Divergent signaling capacities of the long and short isoforms of the leptin receptor. J Biol Chem. 1997;272:32686–32695. doi: 10.1074/jbc.272.51.32686. [DOI] [PubMed] [Google Scholar]
- 59.Bates SH, et al. STAT3 signalling is required for leptin regulation of energy balance but not reproduction. Nature. 2003;421:856–859. doi: 10.1038/nature01388. [DOI] [PubMed] [Google Scholar]
- 60.Munzberg H, Flier JS, Bjorbaek C. Region-specific leptin resistance within the hypothalamus of diet-induced obese mice. Endocrinology. 2004;145:4880–4889. doi: 10.1210/en.2004-0726. [DOI] [PubMed] [Google Scholar]
- 61.Enriori PJ, et al. Diet-induced obesity causes severe but reversible leptin resistance in arcuate melanocortin neurons. Cell metabolism. 2007;5:181–194. doi: 10.1016/j.cmet.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 62.Gamber KM, et al. Over-Expression of Leptin Receptors in Hypothalamic POMC Neurons Increases Susceptibility to Diet-Induced Obesity. PLoS One. 2012;7:e30485. doi: 10.1371/journal.pone.0030485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Balthasar N, et al. Leptin receptor signaling in POMC neurons is required for normal body weight homeostasis. Neuron. 2004;42:983–991. doi: 10.1016/j.neuron.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 64.van de Wall E, et al. Collective and individual functions of leptin receptor modulated neurons controlling metabolism and ingestion. Endocrinology. 2008;149:1773–1785. doi: 10.1210/en.2007-1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Banks WA. The blood-brain barrier as a cause of obesity. Current pharmaceutical design. 2008;14:1606–1614. doi: 10.2174/138161208784705496. [DOI] [PubMed] [Google Scholar]
- 66.Herde MK, Geist K, Campbell RE, Herbison AE. Gonadotropin-releasing hormone neurons extend complex highly branched dendritic trees outside the blood-brain barrier. Endocrinology. 2011;152:3832–3841. doi: 10.1210/en.2011-1228. [DOI] [PubMed] [Google Scholar]
- 67.Faouzi M, et al. Differential accessibility of circulating leptin to individual hypothalamic sites. Endocrinology. 2007;148:5414–5423. doi: 10.1210/en.2007-0655. [DOI] [PubMed] [Google Scholar]
- 68.Bjorbaek C, Elmquist JK, Frantz JD, Shoelson SE, Flier JS. Identification of SOCS-3 as a potential mediator of central leptin resistance. Mol Cell. 1998;1:619–625. doi: 10.1016/s1097-2765(00)80062-3. [DOI] [PubMed] [Google Scholar]
- 69.Howard JK, et al. Enhanced leptin sensitivity and attenuation of diet-induced obesity in mice with haploinsufficiency of Socs3. Nature medicine. 2004;10:734–738. doi: 10.1038/nm1072. [DOI] [PubMed] [Google Scholar]
- 70.Mori H, et al. Socs3 deficiency in the brain elevates leptin sensitivity and confers resistance to diet-induced obesity. Nature medicine. 2004;10:739–743. doi: 10.1038/nm1071. [DOI] [PubMed] [Google Scholar]
- 71.Kievit P, et al. Enhanced leptin sensitivity and improved glucose homeostasis in mice lacking suppressor of cytokine signaling-3 in POMC-expressing cells. Cell metabolism. 2006;4:123–132. doi: 10.1016/j.cmet.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 72.Loh K, et al. Elevated Hypothalamic TCPTP in Obesity Contributes to Cellular Leptin Resistance. Cell metabolism. 2011 doi: 10.1016/j.cmet.2011.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bence KK, et al. Neuronal PTP1B regulates body weight, adiposity and leptin action. Nature medicine. 2006;12:917–924. doi: 10.1038/nm1435. [DOI] [PubMed] [Google Scholar]
- 74.Hotamisligil GS, Arner P, Caro JF, Atkinson RL, Spiegelman BM. Increased adipose tissue expression of tumor necrosis factor-alpha in human obesity and insulin resistance. The Journal of clinical investigation. 1995;95:2409–2415. doi: 10.1172/JCI117936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gregor MF, Hotamisligil GS. Inflammatory mechanisms in obesity. Annual review of immunology. 2011;29:415–445. doi: 10.1146/annurev-immunol-031210-101322. [DOI] [PubMed] [Google Scholar]
- 76.Zabolotny JM, et al. Protein-tyrosine phosphatase 1B expression is induced by inflammation in vivo. The Journal of biological chemistry. 2008;283:14230–14241. doi: 10.1074/jbc.M800061200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zhang X, et al. Hypothalamic IKKbeta/NF-kappaB and ER stress link overnutrition to energy imbalance and obesity. Cell. 2008;135:61–73. doi: 10.1016/j.cell.2008.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Knight ZA, Hannan KS, Greenberg ML, Friedman JM. Hyperleptinemia is required for the development of leptin resistance. PLoS One. 2010;5:e11376. doi: 10.1371/journal.pone.0011376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Benomar Y, et al. Leptin but not ciliary neurotrophic factor (CNTF) induces phosphotyrosine phosphatase-1B expression in human neuronal cells (SH-SY5Y): putative explanation of CNTF efficacy in leptin-resistant state. Endocrinology. 2009;150:1182–1191. doi: 10.1210/en.2008-1097. [DOI] [PubMed] [Google Scholar]
- 80.Bjorbaek C, El-Haschimi K, Frantz JD, Flier JS. The role of SOCS-3 in leptin signaling and leptin resistance. J Biol Chem. 1999;274:30059–30065. doi: 10.1074/jbc.274.42.30059. [DOI] [PubMed] [Google Scholar]
- 81.Ingalls AM, Dickie MM, Snell GD. Obese, a new mutation in the house mouse. The Journal of heredity. 1950;41:317–318. doi: 10.1093/oxfordjournals.jhered.a106073. [DOI] [PubMed] [Google Scholar]
- 82.Hummel KP, Dickie MM, Coleman DL. Diabetes, a new mutation in the mouse. Science. 1966;153:1127–1128. doi: 10.1126/science.153.3740.1127. [DOI] [PubMed] [Google Scholar]
- 83.Tartaglia LA, et al. Identification and expression cloning of a leptin receptor, OB-R. Cell. 1995;83:1263–1271. doi: 10.1016/0092-8674(95)90151-5. [DOI] [PubMed] [Google Scholar]
- 84.Pelleymounter MA, et al. Effects of the obese gene product on body weight regulation in ob/ob mice. Science. 1995;269:540–543. doi: 10.1126/science.7624776. [DOI] [PubMed] [Google Scholar]
- 85.Schwartz MW, et al. Specificity of leptin action on elevated blood glucose levels and hypothalamic neuropeptide Y gene expression in ob/ob mice. Diabetes. 1996;45:531–535. doi: 10.2337/diab.45.4.531. [DOI] [PubMed] [Google Scholar]
- 86.Lin CY, Higginbotham DA, Judd RL, White BD. Central leptin increases insulin sensitivity in streptozotocin-induced diabetic rats. American journal of physiology. 2002;282:E1084–1091. doi: 10.1152/ajpendo.00489.2001. [DOI] [PubMed] [Google Scholar]
- 87.Chinookoswong N, Wang JL, Shi ZQ. Leptin restores euglycemia and normalizes glucose turnover in insulin-deficient diabetes in the rat. Diabetes. 1999;48:1487–1492. doi: 10.2337/diabetes.48.7.1487. [DOI] [PubMed] [Google Scholar]
- 88.Cone RD. Anatomy and regulation of the central melanocortin system. Nature neuroscience. 2005;8:571–578. doi: 10.1038/nn1455. [DOI] [PubMed] [Google Scholar]
- 89.Bagnol D, et al. Anatomy of an endogenous antagonist: relationship between Agouti-related protein and proopiomelanocortin in brain. J Neurosci. 1999;19:RC26. doi: 10.1523/JNEUROSCI.19-18-j0004.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kishi T, et al. Expression of melanocortin 4 receptor mRNA in the central nervous system of the rat. J Comp Neurol. 2003;457:213–235. doi: 10.1002/cne.10454. [DOI] [PubMed] [Google Scholar]
- 91.Rossi J, et al. Melanocortin-4 receptors expressed by cholinergic neurons regulate energy balance and glucose homeostasis. Cell metabolism. 2011;13:195–204. doi: 10.1016/j.cmet.2011.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Xu Y, Elmquist JK, Fukuda M. Central nervous control of energy and glucose balance: focus on the central melanocortin system. Annals of the New York Academy of Sciences. 2011;1243:1–14. doi: 10.1111/j.1749-6632.2011.06248.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Williams KW, et al. Segregation of acute leptin and insulin effects in distinct populations of arcuate proopiomelanocortin neurons. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2010;30:2472–2479. doi: 10.1523/JNEUROSCI.3118-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Choudhury AI, et al. The role of insulin receptor substrate 2 in hypothalamic and beta cell function. The Journal of clinical investigation. 2005;115:940–950. doi: 10.1172/JCI24445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Claret M, et al. AMPK is essential for energy homeostasis regulation and glucose sensing by POMC and AgRP neurons. The Journal of clinical investigation. 2007;117:2325–2336. doi: 10.1172/JCI31516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Williams KW, Coppari R, Elmquist JK. AMPing up” our understanding of the hypothalamic control of energy balance. The Journal of clinical investigation. 2007;117:2089–2092. doi: 10.1172/JCI32975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Al-Qassab H, et al. Dominant role of the p110beta isoform of PI3K over p110alpha in energy homeostasis regulation by POMC and AgRP neurons. Cell metabolism. 2009;10:343–354. doi: 10.1016/j.cmet.2009.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Low MJ. Role of proopiomelanocortin neurons and peptides in the regulation of energy homeostasis. Journal of endocrinological investigation. 2004;27:95–100. [PubMed] [Google Scholar]
- 99.Kristensen P, et al. Hypothalamic CART is a new anorectic peptide regulated by leptin. Nature. 1998;393:72–76. doi: 10.1038/29993. [DOI] [PubMed] [Google Scholar]
- 100.Foo KS, Brismar H, Broberger C. Distribution and neuropeptide coexistence of nucleobindin-2 mRNA/nesfatin-like immunoreactivity in the rat CNS. Neuroscience. 2008;156:563–579. doi: 10.1016/j.neuroscience.2008.07.054. [DOI] [PubMed] [Google Scholar]
- 101.Meister B, et al. Hypothalamic proopiomelanocortin (POMC) neurons have a cholinergic phenotype. The European journal of neuroscience. 2006;24:2731–2740. doi: 10.1111/j.1460-9568.2006.05157.x. [DOI] [PubMed] [Google Scholar]
- 102.Hentges ST, Otero-Corchon V, Pennock RL, King CM, Low MJ. Proopiomelanocortin expression in both GABA and glutamate neurons. J Neurosci. 2009;29:13684–13690. doi: 10.1523/JNEUROSCI.3770-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Piper ML, Unger EK, Myers MG, Jr, Xu AW. Specific physiological roles for signal transducer and activator of transcription 3 in leptin receptor-expressing neurons. Molecular endocrinology (Baltimore, Md. 2008;22:751–759. doi: 10.1210/me.2007-0389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Robertson S, et al. Insufficiency of Janus kinase 2-autonomous leptin receptor signals for most physiologic leptin actions. Diabetes. 2010;59:782–790. doi: 10.2337/db09-1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Coleman DL, Hummel KP. The influence of genetic background on the expression of the obese (Ob) gene in the mouse. Diabetologia. 1973;9:287–293. doi: 10.1007/BF01221856. [DOI] [PubMed] [Google Scholar]
- 106.Banno R, et al. PTP1B and SHP2 in POMC neurons reciprocally regulate energy balance in mice. The Journal of clinical investigation. 2010;120:720–734. doi: 10.1172/JCI39620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ibrahim N, et al. Hypothalamic proopiomelanocortin neurons are glucose responsive and express K(ATP) channels. Endocrinology. 2003;144:1331–1340. doi: 10.1210/en.2002-221033. [DOI] [PubMed] [Google Scholar]
- 108.Parton LE, et al. Glucose sensing by POMC neurons regulates glucose homeostasis and is impaired in obesity. Nature. 2007;449:228–232. doi: 10.1038/nature06098. [DOI] [PubMed] [Google Scholar]
- 109.Hill JW, et al. Acute effects of leptin require PI3K signaling in hypothalamic proopiomelanocortin neurons in mice. The Journal of clinical investigation. 2008;118:1796–1805. doi: 10.1172/JCI32964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Hill JW, et al. Phosphatidyl inositol 3-kinase signaling in hypothalamic proopiomelanocortin neurons contributes to the regulation of glucose homeostasis. Endocrinology. 2009;150:4874–4882. doi: 10.1210/en.2009-0454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Malaisse W, Malaisse-Lagae F, Wright PH, Ashmore J. Effects of adrenergic and cholinergic agents upon insulin secretion in vitro. Endocrinology. 1967;80:975–978. doi: 10.1210/endo-80-5-975. [DOI] [PubMed] [Google Scholar]
- 112.Ramadori G, et al. SIRT1 Deacetylase in SF1 Neurons Protects against Metabolic Imbalance. Cell Metab. 2011;14:301–312. doi: 10.1016/j.cmet.2011.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Pocai A, Obici S, Schwartz GJ, Rossetti L. A brain-liver circuit regulates glucose homeostasis. Cell metabolism. 2005;1:53–61. doi: 10.1016/j.cmet.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 114.Buettner C, et al. Leptin controls adipose tissue lipogenesis via central, STAT3-independent mechanisms. Nat Med. 2008;14:667–675. doi: 10.1038/nm1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Uyama N, Geerts A, Reynaert H. Neural connections between the hypothalamus and the liver. The anatomical record. 2004;280:808–820. doi: 10.1002/ar.a.20086. [DOI] [PubMed] [Google Scholar]
- 116.Kalsbeek A, Fliers E, Hofman MA, Swaab DF, Buijs RM. Vasopressin and the output of the hypothalamic biological clock. Journal of neuroendocrinology. 2010;22:362–372. doi: 10.1111/j.1365-2826.2010.01956.x. [DOI] [PubMed] [Google Scholar]
- 117.Elias CF, et al. Leptin activates hypothalamic CART neurons projecting to the spinal cord. Neuron. 1998;21:1375–1385. doi: 10.1016/s0896-6273(00)80656-x. [DOI] [PubMed] [Google Scholar]
- 118.Banno R, et al. Central administration of melanocortin agonist increased insulin sensitivity in diet-induced obese rats. FEBS Lett. 2007;581:1131–1136. doi: 10.1016/j.febslet.2007.02.019. [DOI] [PubMed] [Google Scholar]
- 119.Heijboer AC, et al. Intracerebroventricular administration of melanotan II increases insulin sensitivity of glucose disposal in mice. Diabetologia. 2005;48:1621–1626. doi: 10.1007/s00125-005-1838-8. [DOI] [PubMed] [Google Scholar]
- 120.da Silva AA, do Carmo JM, Freeman JN, Tallam LS, Hall JE. A functional melanocortin system may be required for chronic CNS-mediated antidiabetic and cardiovascular actions of leptin. Diabetes. 2009;58:1749–1756. doi: 10.2337/db08-1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Leckstrom A, Lew PS, Poritsanos NJ, Mizuno TM. Treatment with a melanocortin agonist improves abnormal lipid metabolism in streptozotocin-induced diabetic mice. Neuropeptides. 2011;45:123–129. doi: 10.1016/j.npep.2010.12.005. [DOI] [PubMed] [Google Scholar]
- 122.Cone RD. Studies on the physiological functions of the melanocortin system. Endocr Rev. 2006;27:736–749. doi: 10.1210/er.2006-0034. [DOI] [PubMed] [Google Scholar]
- 123.Taylor GW. Periodontal treatment and its effects on glycemic control: a review of the evidence. Oral surgery, oral medicine, oral pathology, oral radiology, and endodontics. 1999;87:311–316. doi: 10.1016/s1079-2104(99)70214-3. [DOI] [PubMed] [Google Scholar]
- 124.van den Hoek AM, et al. Leptin deficiency per se dictates body composition and insulin action in ob/ob mice. Journal of neuroendocrinology. 2008;20:120–127. doi: 10.1111/j.1365-2826.2007.01626.x. [DOI] [PubMed] [Google Scholar]
- 125.German J, et al. Hypothalamic leptin signaling regulates hepatic insulin sensitivity via a neurocircuit involving the vagus nerve. Endocrinology. 2009;150:4502–4511. doi: 10.1210/en.2009-0445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Harlan SM, et al. Ablation of the leptin receptor in the hypothalamic arcuate nucleus abrogates leptin-induced sympathetic activation. Circ Res. 2011;108:808–812. doi: 10.1161/CIRCRESAHA.111.240226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Li JH, et al. Hepatic muscarinic acetylcholine receptors are not critically involved in maintaining glucose homeostasis in mice. Diabetes. 2009;58:2776–2787. doi: 10.2337/db09-0522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Hedbacker K, et al. Antidiabetic effects of IGFBP2, a leptin-regulated gene. Cell metabolism. 2010;11:11–22. doi: 10.1016/j.cmet.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 129.Levi J, et al. Acute disruption of leptin signaling in vivo leads to increased insulin levels and insulin resistance. Endocrinology. 2011;152:3385–3395. doi: 10.1210/en.2011-0185. [DOI] [PubMed] [Google Scholar]
- 130.Kojima S, et al. Central leptin gene therapy, a substitute for insulin therapy to ameliorate hyperglycemia and hyperphagia, and promote survival in insulin-deficient diabetic mice. Peptides. 2009;30:962–966. doi: 10.1016/j.peptides.2009.01.007. [DOI] [PubMed] [Google Scholar]
- 131.Hidaka S, et al. Chronic central leptin infusion restores hyperglycemia independent of food intake and insulin level in streptozotocin-induced diabetic rats. Faseb J. 2002;16:509–518. doi: 10.1096/fj.01-0164com. [DOI] [PubMed] [Google Scholar]
- 132.Wang MY, et al. Feature Article: Leptin therapy in insulin-deficient type I diabetes. Proceedings of the National Academy of Sciences of the United States of America; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Koch L, et al. Central insulin action regulates peripheral glucose and fat metabolism in mice. The Journal of clinical investigation. 2008;118:2132–2147. doi: 10.1172/JCI31073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Chen L, Philippe J, Unger RH. Glucagon responses of isolated alpha cells to glucose, insulin, somatostatin, and leptin. Endocrine practice : official journal of the American College of Endocrinology and the American Association of Clinical Endocrinologists. 2011;17:819–825. doi: 10.4158/EP11101.OR. [DOI] [PubMed] [Google Scholar]
- 135.Denroche HC, et al. Leptin Therapy Reverses Hyperglycemia in Mice With Streptozotocin-Induced Diabetes, Independent of Hepatic Leptin Signaling. Diabetes. 2011 doi: 10.2337/db10-0958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Unger RH, Cherrington AD. Glucagonocentric restructuring of diabetes: a pathophysiologic and therapeutic makeover. The Journal of clinical investigation. 2012;122:4–12. doi: 10.1172/JCI60016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Lee Y, Wang MY, Du XQ, Charron MJ, Unger RH. Glucagon receptor knockout prevents insulin-deficient type 1 diabetes in mice. Diabetes. 2011;60:391–397. doi: 10.2337/db10-0426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Berglund E, Vianna CR, Coppari R, Elmquist J. Direct leptin action on POMC neurons regulates hepatic insulin sensitivity in mice. Journal of Clinical Investigation. doi: 10.1172/JCI59816. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.http://www.jdrf.org/index.cfm?page_id=114682.
- 140.German JP, et al. Leptin deficiency causes insulin resistance induced by uncontrolled diabetes. Diabetes. 2010;59:1626–1634. doi: 10.2337/db09-1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Garg A. Lipodystrophies: Genetic and Acquired Body Fat Disorders. J Clin Endocrinol Metab. 2011 doi: 10.1210/jc.2011-1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Rahmouni K, Haynes WG. Leptin and the cardiovascular system. Recent Prog Horm Res. 2004;59:225–244. doi: 10.1210/rp.59.1.225. [DOI] [PubMed] [Google Scholar]
- 143.Seth R, Knight WD, Overton JM. Combined amylin-leptin treatment lowers blood pressure and adiposity in lean and obese rats. International journal of obesity. 2011;35:1183–1192. doi: 10.1038/ijo.2010.262. [DOI] [PubMed] [Google Scholar]
- 144.Matarese G, et al. Leptin accelerates autoimmune diabetes in female NOD mice. Diabetes. 2002;51:1356–1361. doi: 10.2337/diabetes.51.5.1356. [DOI] [PubMed] [Google Scholar]
- 145.Engelman JA, Luo J, Cantley LC. The evolution of phosphatidylinositol 3-kinases as regulators of growth and metabolism. Nature reviews. 2006;7:606–619. doi: 10.1038/nrg1879. [DOI] [PubMed] [Google Scholar]
- 146.Wong KK, Engelman JA, Cantley LC. Targeting the PI3K signaling pathway in cancer. Current opinion in genetics & development. 2010;20:87–90. doi: 10.1016/j.gde.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Bowker SL, Majumdar SR, Veugelers P, Johnson JA. Increased cancer-related mortality for patients with type 2 diabetes who use sulfonylureas or insulin. Diabetes care. 2006;29:254–258. doi: 10.2337/diacare.29.02.06.dc05-1558. [DOI] [PubMed] [Google Scholar]
- 148.Farooqi IS, et al. Beneficial effects of leptin on obesity, T cell hyporesponsiveness, and neuroendocrine/metabolic dysfunction of human congenital leptin deficiency. The Journal of clinical investigation. 2002;110:1093–1103. doi: 10.1172/JCI15693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Shetty GK, et al. Leptin administration to overweight and obese subjects for 6 months increases free leptin concentrations but does not alter circulating hormones of the thyroid and IGF axes during weight loss induced by a mild hypocaloric diet. European journal of endocrinology / European Federation of Endocrine Societies. 2011;165:249–254. doi: 10.1530/EJE-11-0252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Vadacca M, Margiotta DP, Navarini L, Afeltra A. Leptin in immuno-rheumatological diseases. Cellular & molecular immunology. 2011;8:203–212. doi: 10.1038/cmi.2010.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Takeda S, et al. Leptin regulates bone formation via the sympathetic nervous system. Cell. 2002;111:305–317. doi: 10.1016/s0092-8674(02)01049-8. [DOI] [PubMed] [Google Scholar]
- 152.Ducy P, et al. Leptin inhibits bone formation through a hypothalamic relay: a central control of bone mass. Cell. 2000;100:197–207. doi: 10.1016/s0092-8674(00)81558-5. [DOI] [PubMed] [Google Scholar]
- 153.Devos R, et al. Ligand-independent dimerization of the extracellular domain of the leptin receptor and determination of the stoichiometry of leptin binding. J Biol Chem. 1997;272:18304–18310. doi: 10.1074/jbc.272.29.18304. [DOI] [PubMed] [Google Scholar]
- 154.Ghilardi N, Skoda RC. The leptin receptor activates janus kinase 2 and signals for proliferation in a factor-dependent cell line. Mol Endocrinol. 1997;11:393–399. doi: 10.1210/mend.11.4.9907. [DOI] [PubMed] [Google Scholar]
- 155.Kurzer JH, et al. Tyrosine 813 is a site of JAK2 autophosphorylation critical for activation of JAK2 by SH2-B beta. Mol Cell Biol. 2004;24:4557–4570. doi: 10.1128/MCB.24.10.4557-4570.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Mistrik P, Moreau F, Allen JM. BiaCore analysis of leptin-leptin receptor interaction: evidence for 1:1 stoichiometry. Anal Biochem. 2004;327:271–277. doi: 10.1016/j.ab.2004.01.022. [DOI] [PubMed] [Google Scholar]
- 157.Couturier C, Jockers R. Activation of the leptin receptor by a ligand-induced conformational change of constitutive receptor dimers. J Biol Chem. 2003;278:26604–26611. doi: 10.1074/jbc.M302002200. [DOI] [PubMed] [Google Scholar]
- 158.Ingley E, Klinken SP. Cross-regulation of JAK and Src kinases. Growth factors. 2006;24:89–95. doi: 10.1080/08977190500368031. [DOI] [PubMed] [Google Scholar]
- 159.Li M, Li Z, Morris DL, Rui L. Identification of SH2B2beta as an inhibitor for SH2B1- and SH2B2alpha-promoted Janus kinase-2 activation and insulin signaling. Endocrinology. 2007;148:1615–1621. doi: 10.1210/en.2006-1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Bjorbaek C, et al. Divergent roles of SHP-2 in ERK activation by leptin receptors. J Biol Chem. 2001;276:4747–4755. doi: 10.1074/jbc.M007439200. [DOI] [PubMed] [Google Scholar]
- 161.De Souza D, et al. SH2 domains from suppressor of cytokine signaling-3 and protein tyrosine phosphatase SHP-2 have similar binding specificities. Biochemistry. 2002;41:9229–9236. doi: 10.1021/bi0259507. [DOI] [PubMed] [Google Scholar]
- 162.Banks AS, Davis SM, Bates SH, Myers MG., Jr Activation of downstream signals by the long form of the leptin receptor. J Biol Chem. 2000;275:14563–14572. doi: 10.1074/jbc.275.19.14563. [DOI] [PubMed] [Google Scholar]
- 163.Fukuda M, et al. Monitoring FoxO1 localization in chemically identified neurons. J Neurosci. 2008;28:13640–13648. doi: 10.1523/JNEUROSCI.4023-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Cao Y, et al. PDK1-Foxo1 in agouti-related peptide neurons regulates energy homeostasis by modulating food intake and energy expenditure. PLoS One. 2011;6:e18324. doi: 10.1371/journal.pone.0018324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Auernhammer CJ, Bousquet C, Melmed S. Autoregulation of pituitary corticotroph SOCS-3 expression: characterization of the murine SOCS-3 promoter. Proc Natl Acad Sci U S A. 1999;96:6964–6969. doi: 10.1073/pnas.96.12.6964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Guo L, Munzberg H, Stuart RC, Nillni EA, Bjorbaek C. N-acetylation of hypothalamic alpha-melanocyte-stimulating hormone and regulation by leptin. Proc Natl Acad Sci U S A. 2004;101:11797–11802. doi: 10.1073/pnas.0403165101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Munzberg H, Huo L, Nillni EA, Hollenberg AN, Bjorbaek C. Role of signal transducer and activator of transcription 3 in regulation of hypothalamic proopiomelanocortin gene expression by leptin. Endocrinology. 2003;144:2121–2131. doi: 10.1210/en.2002-221037. [DOI] [PubMed] [Google Scholar]
- 168.Kitamura T, et al. Forkhead protein FoxO1 mediates Agrp-dependent effects of leptin on food intake. Nat Med. 2006;12:534–540. doi: 10.1038/nm1392. [DOI] [PubMed] [Google Scholar]
- 169.Plum L, et al. The obesity susceptibility gene Cpe links FoxO1 signaling in hypothalamic pro-opiomelanocortin neurons with regulation of food intake. Nature medicine. 2009;15:1195–1201. doi: 10.1038/nm.2026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.van den Brink GR, et al. Leptin signaling in human peripheral blood mononuclear cells, activation of p38 and p42/44 mitogen-activated protein (MAP) kinase and p70 S6 kinase. Molecular cell biology research communications : MCBRC. 2000;4:144–150. doi: 10.1006/mcbr.2001.0270. [DOI] [PubMed] [Google Scholar]
- 171.Roux PP, Ballif BA, Anjum R, Gygi SP, Blenis J. Tumor-promoting phorbol esters and activated Ras inactivate the tuberous sclerosis tumor suppressor complex via p90 ribosomal S6 kinase. Proc Natl Acad Sci U S A. 2004;101:13489–13494. doi: 10.1073/pnas.0405659101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Gong Y, et al. The long form of the leptin receptor regulates STAT5 and ribosomal protein S6 via alternate mechanisms. The Journal of biological chemistry. 2007;282:31019–31027. doi: 10.1074/jbc.M702838200. [DOI] [PubMed] [Google Scholar]
- 173.Cowley MA, et al. Leptin activates anorexigenic POMC neurons through a neural network in the arcuate nucleus. Nature. 2001;411:480–484. doi: 10.1038/35078085. [DOI] [PubMed] [Google Scholar]
- 174.Won JC, et al. Central administration of an endoplasmic reticulum stress inducer inhibits the anorexigenic effects of leptin and insulin. Obesity (Silver Spring) 2009;17:1861–1865. doi: 10.1038/oby.2009.194. [DOI] [PubMed] [Google Scholar]
- 175.De Souza CT, et al. Consumption of a fat-rich diet activates a proinflammatory response and induces insulin resistance in the hypothalamus. Endocrinology. 2005;146:4192–4199. doi: 10.1210/en.2004-1520. [DOI] [PubMed] [Google Scholar]
- 176.Moller DE. Metabolic disease drug discovery- “hitting the target” is easier said than done. Cell metabolism. 2012;15:19–24. doi: 10.1016/j.cmet.2011.10.012. [DOI] [PubMed] [Google Scholar]
- 177.Bailey CJ, Turner RC. Metformin. The New England journal of medicine. 1996;334:574–579. doi: 10.1056/NEJM199602293340906. [DOI] [PubMed] [Google Scholar]
- 178.Shaw RJ, et al. The kinase LKB1 mediates glucose homeostasis in liver and therapeutic effects of metformin. Science. 2005;310:1642–1646. doi: 10.1126/science.1120781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Owen MR, Doran E, Halestrap AP. Evidence that metformin exerts its anti-diabetic effects through inhibition of complex 1 of the mitochondrial respiratory chain. Biochem J. 2000;348(Pt 3):607–614. [PMC free article] [PubMed] [Google Scholar]
- 180.Mihaylova MM, et al. Class IIa Histone Deacetylases Are Hormone-Activated Regulators of FOXO and Mammalian Glucose Homeostasis. Cell. 2011;145:607–621. doi: 10.1016/j.cell.2011.03.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.El-Mir MY, et al. Dimethylbiguanide inhibits cell respiration via an indirect effect targeted on the respiratory chain complex I. The Journal of biological chemistry. 2000;275:223–228. doi: 10.1074/jbc.275.1.223. [DOI] [PubMed] [Google Scholar]
- 182.Zhou G, et al. Role of AMP-activated protein kinase in mechanism of metformin action. The Journal of clinical investigation. 2001;108:1167–1174. doi: 10.1172/JCI13505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Rotella CM, Monami M, Mannucci E. Metformin beyond diabetes: new life for an old drug. Current diabetes reviews. 2006;2:307–315. doi: 10.2174/157339906777950651. [DOI] [PubMed] [Google Scholar]
- 184.Selvin E, et al. Cardiovascular outcomes in trials of oral diabetes medications: a systematic review. Archives of internal medicine. 2008;168:2070–2080. doi: 10.1001/archinte.168.19.2070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Lehmann JM, et al. An antidiabetic thiazolidinedione is a high affinity ligand for peroxisome proliferator-activated receptor gamma (PPAR gamma) The Journal of biological chemistry. 1995;270:12953–12956. doi: 10.1074/jbc.270.22.12953. [DOI] [PubMed] [Google Scholar]
- 186.Semple RK, Chatterjee VK, O’Rahilly S. PPAR gamma and human metabolic disease. The Journal of clinical investigation. 2006;116:581–589. doi: 10.1172/JCI28003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Willson TM, Lambert MH, Kliewer SA. Peroxisome proliferator-activated receptor gamma and metabolic disease. Annu Rev Biochem. 2001;70:341–367. doi: 10.1146/annurev.biochem.70.1.341. [DOI] [PubMed] [Google Scholar]
- 188.Choi JH, et al. Anti-diabetic drugs inhibit obesity-linked phosphorylation of PPARgamma by Cdk5. Nature. 2010;466:451–456. doi: 10.1038/nature09291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Home PD, et al. Rosiglitazone evaluated for cardiovascular outcomes in oral agent combination therapy for type 2 diabetes (RECORD): a multicentre, randomised, open-label trial. Lancet. 2009;373:2125–2135. doi: 10.1016/S0140-6736(09)60953-3. [DOI] [PubMed] [Google Scholar]
- 190.Joosen AM, Bakker AH, Gering MJ, Westerterp KR. The effect of the PPARgamma ligand rosiglitazone on energy balance regulation. Diabetes/metabolism research and reviews. 2006;22:204–210. doi: 10.1002/dmrr.592. [DOI] [PubMed] [Google Scholar]
- 191.Larsen PJ, et al. Differential influences of peroxisome proliferator-activated receptors gamma and -alpha on food intake and energy homeostasis. Diabetes. 2003;52:2249–2259. doi: 10.2337/diabetes.52.9.2249. [DOI] [PubMed] [Google Scholar]
- 192.Kahn SE, et al. Glycemic durability of rosiglitazone, metformin, or glyburide monotherapy. The New England journal of medicine. 2006;355:2427–2443. doi: 10.1056/NEJMoa066224. [DOI] [PubMed] [Google Scholar]
- 193.Festuccia WT, et al. Peroxisome proliferator-activated receptor-gamma-mediated positive energy balance in the rat is associated with reduced sympathetic drive to adipose tissues and thyroid status. Endocrinology. 2008;149:2121–2130. doi: 10.1210/en.2007-1553. [DOI] [PubMed] [Google Scholar]
- 194.Shimizu H, et al. Troglitazone reduces plasma leptin concentration but increases hunger in NIDDM patients. Diabetes care. 1998;21:1470–1474. doi: 10.2337/diacare.21.9.1470. [DOI] [PubMed] [Google Scholar]
- 195.Lu M, et al. Brain PPAR-gamma promotes obesity and is required for the insulin-sensitizing effect of thiazolidinediones. Nat Med. 2011;17:618–622. doi: 10.1038/nm.2332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Ryan KK, et al. A role for central nervous system PPAR-gamma in the regulation of energy balance. Nat Med. 2011;17:623–626. doi: 10.1038/nm.2349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Choi JH, et al. Antidiabetic actions of a non-agonist PPARgamma ligand blocking Cdk5-mediated phosphorylation. Nature. 2011 doi: 10.1038/nature10383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Woodcock J, Sharfstein JM, Hamburg M. Regulatory action on rosiglitazone by the U.S. Food and Drug Administration. The New England journal of medicine. 2010;363:1489–1491. doi: 10.1056/NEJMp1010788. [DOI] [PubMed] [Google Scholar]
- 199.Tahrani AA, Bailey CJ, Del Prato S, Barnett AH. Management of type 2 diabetes: new and future developments in treatment. Lancet. 2011;378:182–197. doi: 10.1016/S0140-6736(11)60207-9. [DOI] [PubMed] [Google Scholar]
- 200.Lebovitz HE. Type 2 diabetes mellitus-current therapies and the emergence of surgical options. Nature reviews. Endocrinology. 2011;7:408–419. doi: 10.1038/nrendo.2011.10. [DOI] [PubMed] [Google Scholar]
- 201.Nathan DM, et al. Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med. 2005;353:2643–2653. doi: 10.1056/NEJMoa052187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Maahs DM, Rewers M. Editorial: Mortality and renal disease in type 1 diabetes mellitus--progress made, more to be done. The Journal of clinical endocrinology and metabolism. 2006;91:3757–3759. doi: 10.1210/jc.2006-1730. [DOI] [PubMed] [Google Scholar]
- 203.Steffes MW, Sibley S, Jackson M, Thomas W. Beta-cell function and the development of diabetes-related complications in the diabetes control and complications trial. Diabetes care. 2003;26:832–836. doi: 10.2337/diacare.26.3.832. [DOI] [PubMed] [Google Scholar]
- 204.Bluestone JA, Herold K, Eisenbarth G. Genetics, pathogenesis and clinical interventions in type 1 diabetes. Nature. 464:1293–1300. doi: 10.1038/nature08933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Horton JD, Goldstein JL, Brown MS. SREBPs: activators of the complete program of cholesterol and fatty acid synthesis in the liver. The Journal of clinical investigation. 2002;109:1125–1131. doi: 10.1172/JCI15593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Liu HY, et al. Insulin is a stronger inducer of insulin resistance than hyperglycemia in mice with type 1 diabetes mellitus (T1DM) The Journal of biological chemistry. 2009;284:27090–27100. doi: 10.1074/jbc.M109.016675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Shulman GI. Cellular mechanisms of insulin resistance. The Journal of clinical investigation. 2000;106:171–176. doi: 10.1172/JCI10583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Randle PJ, Garland PB, Hales CN, Newsholme EA. The glucose fatty-acid cycle. Its role in insulin sensitivity and the metabolic disturbances of diabetes mellitus. Lancet. 1963;1:785–789. doi: 10.1016/s0140-6736(63)91500-9. [DOI] [PubMed] [Google Scholar]
- 209.Boden G, Shulman GI. Free fatty acids in obesity and type 2 diabetes: defining their role in the development of insulin resistance and beta-cell dysfunction. European journal of clinical investigation. 2002;32 (Suppl 3):14–23. doi: 10.1046/j.1365-2362.32.s3.3.x. [DOI] [PubMed] [Google Scholar]
- 210.Cryer PE. Mechanisms of sympathoadrenal failure and hypoglycemia in diabetes. J Clin Invest. 2006;116:1470–1473. doi: 10.1172/JCI28735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Cryer PE. The barrier of hypoglycemia in diabetes. Diabetes. 2008;57:3169–3176. doi: 10.2337/db08-1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Cryer PE. Hypoglycemia: still the limiting factor in the glycemic management of diabetes. Endocr Pract. 2008;14:750–756. doi: 10.4158/EP.14.6.750. [DOI] [PubMed] [Google Scholar]
- 213.Cryer PE. Preventing hypoglycaemia: what is the appropriate glucose alert value? Diabetologia. 2009;52:35–37. doi: 10.1007/s00125-008-1205-7. [DOI] [PubMed] [Google Scholar]
- 214.Agarwal AK, Garg A. A novel heterozygous mutation in peroxisome proliferator-activated receptor-gamma gene in a patient with familial partial lipodystrophy. The Journal of clinical endocrinology and metabolism. 2002;87:408–411. doi: 10.1210/jcem.87.1.8290. [DOI] [PubMed] [Google Scholar]
- 215.Gandotra S, et al. Perilipin deficiency and autosomal dominant partial lipodystrophy. The New England journal of medicine. 2011;364:740–748. doi: 10.1056/NEJMoa1007487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Hudon SE, et al. HIV-protease inhibitors block the enzymatic activity of purified Ste24p. Biochemical and biophysical research communications. 2008;374:365–368. doi: 10.1016/j.bbrc.2008.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Apostolova N, Blas-Garcia A, Esplugues JV. Mitochondrial toxicity in HAART: an overview of in vitro evidence. Current pharmaceutical design. 2011;17:2130–2144. doi: 10.2174/138161211796904731. [DOI] [PubMed] [Google Scholar]