Abstract
Hypothalamic gonadotropin-releasing hormone (GnRH) plays a critical role in reproductive physiology by regulating follicle-stimulating hormone (FSH) and luteinizing hormone (LH) gene expression in the pituitary. Analysis of gonadotrope deep-sequencing data identified a global regulation of pre-mRNA splicing by GnRH. Homer1, a gene encoding a postsynaptic density scaffolding protein, was selected for further study. Homer1 expresses a short splice form, Homer1a, and more-abundant long transcripts Homer1b/c. GnRH induced a modest increase in Homer1b/c expression and a dramatic increase in the Homer1a splice form. G protein knockdown studies suggested that the Homer1 induction, but not the regulated splicing, was Gαq/11 dependent. Phosphorylation of the splicing regulator SRp20 was found to be induced by GnRH. SRp20 depletion attenuated the GnRH-induced increase in the Homer1a-to-Homer1b/c ratio and modulated the effects of GnRH on FSHβ and LHβ expression. Homer1 gene knockdown resulted in increased GnRH-induced FSHβ and LHβ transcript levels. Furthermore, splice-form-specific reduction of Homer1b/c increased both FSHβ and LHβ mRNA induction, whereas reduction of Homer1a had the opposite effect on FSHβ induction. These results indicate that the regulation of Homer1 splicing by GnRH contributes to gonadotropin gene control.
INTRODUCTION
Gonadotropin-releasing hormone (GnRH) plays a central role in the control of normal reproductive function by regulating the synthesis and release of the gonadotropins luteinizing hormone (LH) and follicle-stimulating hormone (FSH) by pituitary gonadotropes. These two hormones are heterodimeric proteins composed of a common α subunit and a specific β subunit (1). The control of FSHβ and LHβ gene expression by GnRH is critical during the menstrual cycle, including ovulation, and through the various stages of reproductive life (2). Impairment of the gonadotrope response to GnRH results in reproductive disorders such as polycystic ovary syndrome (3) and provides the basis for treatment of gonadal hormone-sensitive cancers (4, 5). Therefore, understanding the molecular mechanisms involved in the response of gonadotropins to GnRH may help discover new therapeutic targets for reproductive disorders and hormone-dependent malignancies.
The development of the immortalized gonadotrope cell lines αT3-1 and LβT2 has facilitated the characterization of the mechanisms regulating gonadotropin subunit gene transcription (6–9). A member of the G-protein-coupled receptor family, the GnRH receptor (GnRHR) is coupled to both Gαq/11 and Gαs, which activate protein kinase C/mitogen-activated protein kinase (PKC/MAPK)- and 3′-5′-cyclic AMP (cAMP)/protein kinase A-dependent pathways, respectively (for a review, see reference 10). GnRH induction of LHβ involves PKC/MAPK signaling and the synergistic interaction of early growth response 1, steroidogenic factor 1, and paired-like homeodomain transcription factor 1 (11). Additionally, cAMP may augment LHβ gene transcription (12, 13). GnRH induction of FSHβ is mediated by the PKC and MAPK signaling pathways and notably activator protein 1 (14, 15). The mechanisms underlying gonadotropin regulation by GnRH are incompletely known (16). We recently reported the role of inhibin α (12) and β-catenin (17) in FSHβ induction by GnRH.
Alternative splicing is a posttranscriptional mechanism that selectively joins exons together to form distinct mature mRNA species, thus enhancing mRNA variety and protein diversity. This process can lead to the expression of functionally distinct proteins from the same gene in a temporal or tissue-specific fashion. About 95% of human genes undergo alternative splicing (18). Mechanistically, splicing regulators are key modulators of splice site choice via interaction with the splicing machinery and cis-regulatory elements. The most common splicing regulators are serine/arginine-rich RNA-binding proteins (SR proteins), which are thought to recognize exonic/intronic splicing enhancer elements (ESEs/ISEs) and to promote sequence inclusion, while heterogeneous nuclear ribonucleoproteins (hnRNPs) bind to exonic/intronic splicing silencer elements (ESSs/ISSs) and act in a fashion opposite to that of SR proteins (19).
Several studies previously demonstrated that hormonal inputs alter the splicing pattern of a variety of genes (20–22). For instance, injection of estrogen to ovariectomized ewes that were administered GnRH following hypothalamic-pituitary disconnection increased the levels of GnRHR transcript variants of 1.5, 2.3, and 3.7 kb, while leaving the 1.2- and 5.6-kb variants unchanged (23). This suggested that estrogen had a direct effect on GnRHR mRNA splicing in the pituitary. We wondered if the GnRH stimulus could induce changes in global splicing pattern in LβT2 gonadotrope cells and whether such changes may influence gonadotropin subunit gene regulation.
In the present study, we utilize deep sequencing in combination with computational analysis to profile GnRH-mediated splicing changes and determine how splice variants affect gonadotropin subunit gene regulation. We demonstrate for the first time that GnRH induces a global change in splicing pattern and report that novel signaling components SRp20, a splicing regulator protein, and Homer1, a scaffolding protein implicated in calcium regulation, are involved in the regulation of gonadotropin subunit gene expression. Furthermore, we identify splicing isoforms Homer1a and Homer1b/c as mediators of the SRp20 effect on GnRH-induced FSHβ and LHβ gene expression.
MATERIALS AND METHODS
Cell culture.
LβT2 cells obtained from Pamela Mellon (University of California, San Diego) were maintained at 37°C in a humidified air atmosphere of 5% CO2 in Dulbecco's modified Eagle's medium (Mediatech, Herndon, VA) supplemented with 10% fetal bovine serum (Gemini, Calabasas, CA) and l-glutamine (Gibco Invitrogen, Carlsbad, CA).
Transfection and siRNA interference.
Cells were transfected in an Amaxa Nucleofector 96-well electroporation shuttle according to the manufacturer's instructions (Lonza Walkersville Inc., Walkersville, MD). Briefly, 1 million LβT2 cells were transfected with 600 ng of small interfering RNA (siRNA) (2 μl of a 20 μM stock in a 20-μl reaction volume) using Nucleofector program DS-137. Immediately after transfection, cells were plated and incubated in fresh medium for 48 h. Cells were then serum starved and ultimately subjected to hormonal treatment. siRNAs against mouse SRp20 (sc-38339) and Homer1 (sc-35582) were purchased from Santa Cruz Biotech. siRNAs against mouse Gαs and Gαq/11 were obtained from Dharmacon On-Target plus siRNA SMARTpool. siRNAs against specific Homer1 isoforms were customized from Dharmacon, and sequences were as follows: Homer1a_1, 5′-GAU UAA GUA AGG UGG AUA AUU-3′; Homer1a_2, 5′-CCA CAU AGG UAC ACA UUC AUU-3′; Homer1a_3, 5′-UGA UUA AGU AAG GUG GAU AUU-3′; Homer1b/c_1, 5′-AAG UAA AGG AAG AGG AAA UUU-3′; Homer1b/c_2, 5′-GAA GAG ACC CUG AAA GUA AUU-3′; and Homer1b/c_3, 5′-GAA AGU AAA GGA AGA GGA AUU-3′. Three siRNAs against each isoform were mixed in equal molarity as a pool to increase knockdown efficiency.
RNA sequencing experiments.
LβT2 cells were seeded for 48 h, serum starved overnight, and stimulated with 5 nM GnRH or vehicle for either 45 min or 2 h. A total of 3 different groups or experimental conditions were used, and each group was comprised of four replicates. Total RNA (2.5 μg) from each replicate was sequenced at the Mount Sinai Genomics Core Facility using an Illumina platform (Illumina, Inc., San Diego, CA) and a HiSeq 2000 sequencing system (100-nucleotide length, single-read type). Reads were processed using the RUM pipeline using mm9 annotations (24). Transcripts with log2 (RPKM) of <2 where excluded from further analysis. Exon inclusion values were calculated by dividing the exon-specific counts by the total transcript counts. The resulting exon inclusion matrix was processed using the limma pipeline (25) for differential expression. Pathway enrichment analysis for differentially spliced genes was evaluated using canonical pathways from the mSigDB (26) database and protein domains from InterPro (27). The RNA sequencing data were previously deposited in Genome Expression Omnibus (GSE42120).
Western blot analysis.
Western blot analysis was performed as previously described (28). Protein concentrations were measured using the Bio-Rad protein assay (Bio-Rad, Hercules, CA). Briefly, 50 μg proteins was loaded onto a 4 to 20% precasted sodium dodecyl sulfate-polyacrylamide gel electrophoresis gel (Bio-Rad). After transfer to a nitrocellulose membrane (Li-Cor Biosciences, Lincoln, NE), blocking for 1 h at room temperature using blocking buffer (Li-Cor Biosciences) was followed by overnight incubation with primary antibody at 4°C. Primary antibodies against phosphoepitope SR proteins (clone 1H4, MABE50; EMD Millipore Corporation, MA) were used at a 1:3,000 dilution. Anti-SRp20 (ab73891; Abcam) was used at a 1:3,000 dilution. Homer1a and Homer1 antibodies were used at 1:500 dilutions (sc-8922 and sc-136358; Santa Cruz Biotechnology). Anti-lysine-specific demethylase 1 (anti-LSD1; number 2139; Cell Signaling Technology, Inc., MA) antibodies were used at a 1:5,000 dilution. Fluorophore-conjugated secondary antibodies (Li-Cor Biosciences) were diluted at 1:5,000. Incubation with the secondary antibody was performed at room temperature for 2 h. Odyssey infrared fluorescent Western blot system (Li-Cor Biosciences) was used to detect and quantify protein bands of interest.
RT-qPCR.
For real-time quantitative PCR (RT-qPCR) experiments, total RNA was isolated using the Stratagene Absolutely 96 RNA Microprep kit (Stratagene, La Jolla, CA). RNA quantity and quality were assessed with NanoDrop (NanoDrop Technologies, Inc., Wilmington, DE). After reverse transcription of 1 μg of RNA, cDNA samples were diluted to 1:50 in distilled water (dH2O). SYBR green quantitative PCR was performed according to the manufacturer's recommendations, using 5 μl of cDNA template and 5 μl of master mix containing specific primers for the targeted gene. The PCR program was 95°C for 2 min, followed by 40 cycles of 95°C for 15 s, 55°C for 15 s, and 72°C for 30 s. Samples were measured in triplicate using the ABI Prism 7900HT detection system and software version 2.2.2 (Applied Biosystems, Foster City, CA). Results were exported as threshold cycle (CT) values for subsequent analysis. Data were normalized to either ribosomal protein S11 (RPS11) or the glyceraldehyde-3-phosphate dehydrogenase (GAPDH) housekeeping gene. Primer sequences were as follows: FSHβ/Sense, 5′-TGG AGA CTC TGG CAT GAT TG-3′; FSHβ/Antisense, 5′-GAG TTG AGC AGC CTA ACC TT-3′; LHβ/Sense, 5′-CTC CCG GTA GGT GCA CAC T-3′; LHβ/Antisense, 5′-CCT TCA CCA CCA GCA TCT GT-3′; rps11/Sense, 5′-CGT GAC GAA GAT GAA GAT GC-3′; rps11/Antisense, 5′-GCA CAT TGA ATC GCA CAG TC-3′; GAPDH/Sense, 5′-TGC GAC TTC AAC AGC AAC TC-3′; GAPDH/Antisense, 5′-CTT GCT CAG TGT CCT TGC TG-3′; Homer1a/Sense, 5′-TGG GAC AGA CGA TGA GAG AAC AC-3′; Homer1a/Antisense, 5′-CAG TCA ACT TGA GCA ACC AAG AAC-3′; Homer1c/Sense, 5′-GAG AAG TCG CAG GAG AAG ATG-3′; Homer1c/Antisense, 5′-TTG CTG AAC TAG CAT GAG AGA G-3′; Homer1b/sense, 5′-CAA CAA CGG AGC AAC CTA TCT-3′; Homer1b/Antisense, 5′-GAG GAG AAT CCC AGT CCA TAA AC-3′; Gαs/Sense, GCT ACC CTC ACT TTA CCT GC; Gαs/Antisense, 5′-CAG CTC GTA TTG GCG GAG AT-3′; Gαq/Sense, 5′-ACG CCC ATG CAG AAG TCA GT-3′; Gαq/Antisense, 5′-GAT GCC ACC TGC CAG TAA AC-3′; Gα11/Sense, 5′-CAC TCA GAC ACA CGC CAA TC-3′; and Gα11/Antisense, 5′-ACA GAT CGG ACC CTC AAC TG-3′.
Statistical analysis.
Statistical analyses for GnRH-induced SRp20 phosphorylation and endogenous FSHβ, LHβ, and Homer1 gene expression were carried out using GraphPad Prism version 5.04. One-way and two-way analyses of variance (ANOVA) were applied for overall effect, and specific comparison was examined with Bonferroni's corrections. Statistical significance was set as indicated in each figure.
RESULTS
GnRH induces changes in global splicing pattern in LβT2 pituitary gonadotropes.
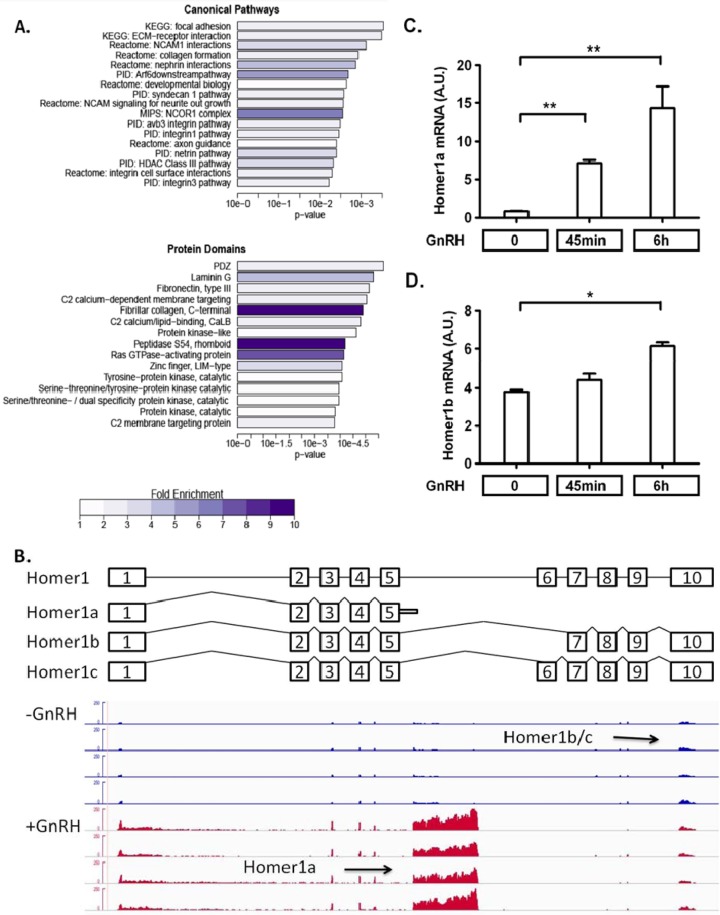
We performed an in-depth RNA sequencing experiment in LβT2 cells, which is an immortalized mature mouse gonadotrope cell line. Two time points (45 min and 2 h) were chosen to examine the changes of immediate early gene responses and secondary genes whose expression is required for gonadotropin gene expression (29). Based on the RNA sequencing data, 26 genes and 1,341 genes showed significant differences in exon inclusion following 45-min and 2-h GnRH stimulations, respectively. To understand which specific functions were affected by GnRH-induced alternative splicing, the genes with altered exon usage patterns were evaluated for annotation enrichment using canonical pathways from the mSigDB database and protein domains from InterPro (Fig. 1A). Interestingly, these genes with GnRH-regulated splicing were enriched for membrane protein pathways.
FIG 1.
GnRH induces global changes in RNA splicing. (A) The 1,341 genes that had altered exon usage patterns following GnRH stimulation were evaluated for annotation enrichment using canonical pathways from the mSigDB database and protein domains from InterPro. For the canonical pathways, all pathways with significant enrichment at a q value of 0.1 are shown, while for domain enrichment the list is truncated to the top 15 terms. Bars represent the significance of enrichment (using Fisher's exact test), while the color scale indicates the raw fold enrichment over genome background. (B) RNA sequencing data analysis showing differential exon usage of the Homer1 gene in either the presence (+GnRH) or absence (−GnRH) of GnRH. A schematic of the structure of the Homer1 gene and the exonic structures of its isoforms is provided above the RNA sequencing data. (C and D) RT-qPCR validation of differential regulation of Homer1 splice variants Homer1a (C) and Homer1b (D) by GnRH. LβT2 cells were seeded in a 96-well plate, cultured for 24 h, and serum starved overnight. Cells were then treated with 5 nM GnRH for the indicated times. The data shown are means ± standard errors of the means (SEM) of triplicate samples from one experiment and are representative of three independent experiments. One-way ANOVA with Bonferroni's posttest corrections; *, P < 0.05; **, P < 0.01. A.U., arbitrary units.
GnRH strongly induces splice variant Homer1a.
One gene showing a high level of regulated alternative splicing was Homer1. Homer1 splicing isoforms have been reported to affect Ca2+ influx by interaction with mGluR (30), and Ca2+ plays an important role in gonadotropin gene regulation (31). Because of its high level of splicing regulation by GnRH and its role in other systems, Homer1 was studied further. GnRH induced a shift in expression from the longer forms, Homer1b/c, to the short form, Homer1a (Fig. 1B). These changes were confirmed by splice-specific RT-qPCRs. The long forms of Homer1, Homer1b and Homer 1c, are considered constitutively expressed (32), and they showed similar levels of expression by RT-qPCR (data not shown). Homer1a expression rose ∼7-fold 45 min after GnRH exposure and ∼14-fold after 6 h (Fig. 1C). Homer1b, in contrast, was only slightly increased following GnRH exposure (Fig. 1D).
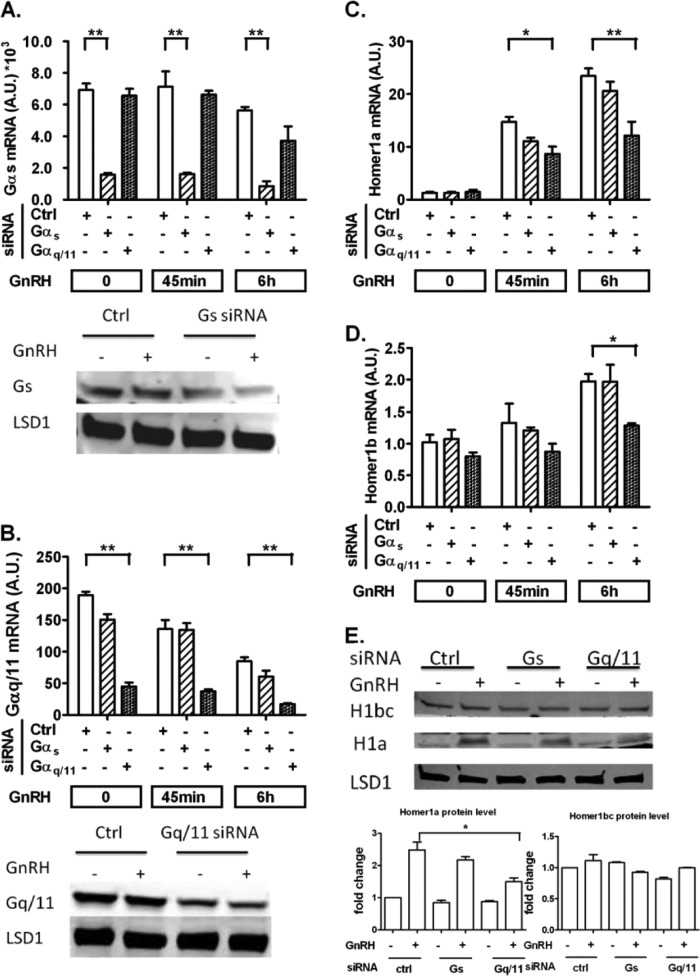
To study the signaling pathways involved in Homer1 regulation, we inactivated G proteins Gαs and Gαq/11 by siRNA knockdown (Fig. 2A and B). Inactivation of Gαq/11 resulted in a similar reduction in Homer1a and Homer1b following GnRH exposure, without suppressing the regulation of splicing by GnRH causing a relative increase in Homer1a (Fig. 2C and D). The changes in the protein level of Homer1a corresponded to the changes in its mRNA levels. Homer1b/c protein was not significantly affected by Gq/11 knockdown (Fig. 2E). These results suggest that the induction of Homer1 depends predominantly on Gαq/11-mediated signaling but the regulation of splicing involves other proximal signaling mechanisms.
FIG 2.
GnRH induces Homer1 induction via the Gαq/11-dependent pathway. On day 1, LβT2 cells were transfected with either scrambled (Ctrl), Gαs, or Gαq/11 siRNA for 48 h. On day 2, cells were serum starved overnight. On day 3, cells were stimulated with 5 nM GnRH for either 45 min or 6 h. (A and B) Knockdown efficiency and specificity of Gαs and Gαq/11 siRNA on both mRNA and protein levels. (C and D) Homer1a (C) and Homer1b (D) mRNA levels were determined by RT-qPCR. (E) Protein levels of Homer1 under G protein knockdown. Cells were treated with 5 nM GnRH for 3 h. For RT-qPCR, the data shown are means ± SEM of triplicate samples from one experiment and are representative of three independent experiments. For Western blots, a representative figure is shown and densitometry is based on three independent experiments. Two-way ANOVA with Bonferroni's posttest corrections; *, P < 0.05; **, P < 0.01.
GnRH activates the phosphorylation of splicing regulator SRp20 in a time-dependent manner.
Because alternative splicing can be influenced by splicing regulators (SR proteins [33]), we studied whether GnRH regulated the phosphorylation of SR proteins. The phosphorylation levels of SR protein family members (SRp75, SRp55, SRp40, SRp30, and SRp20) in response to GnRH stimulation were assayed by Western blotting. Using LSD1 as a control, total SRp20 was not affected by GnRH, while phosphorylated SRp20 increased significantly following a 45-min GnRH treatment (Fig. 3A to C). These results led us to test whether the GnRH-induced changes in the Homer1 splicing pattern were mediated by SRp20.
FIG 3.
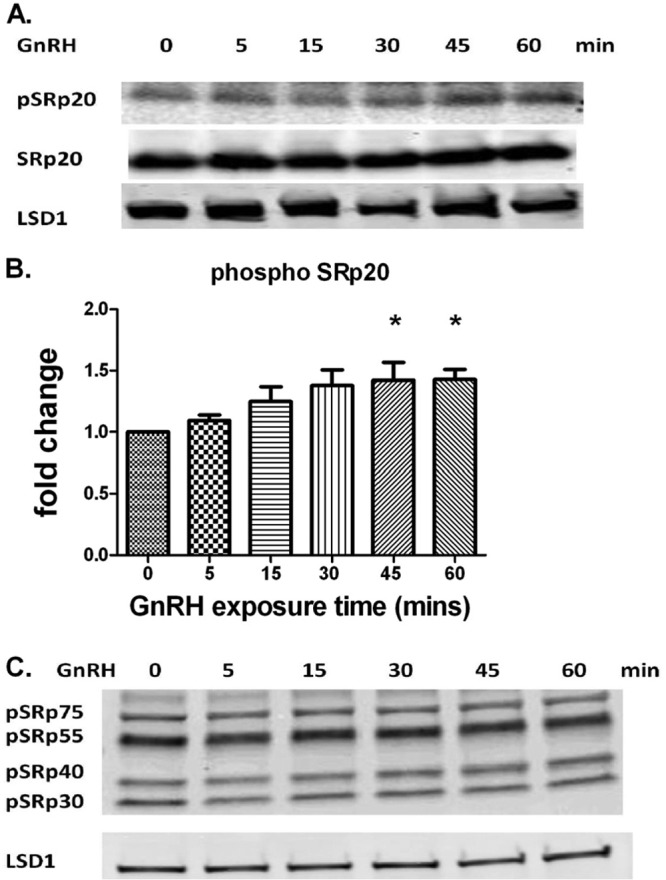
GnRH induces the phosphorylation of SRp20. LβT2 cells were seeded in a 12-well plate, cultured for 24 h, and serum starved overnight. Cells were then treated with 5 nM GnRH for the indicated times. Whole-cell lysates were subjected to a quantitative Western blot analysis. Phosphorylated and total SRp20 are shown in the upper panels. LSD1 in the lower panel was used as a loading control. (A and B) GnRH induces SRp20 phosphorylation in a time-dependent manner. Note that the images shown in panel A are from the same gel but are shown separately due to the large differences in exposure required for visualizing the different signals. The data shown are means ± SEM obtained from four independent experiments. One-way ANOVA with Bonferroni's posttest corrections; *, P < 0.05. (C) Phosphorylation of other SR proteins is not affected by GnRH. The two panels shown are from the same gel at different exposures.
SRp20 inactivation attenuates GnRH-mediated increase in Homer1 splice variant ratio.
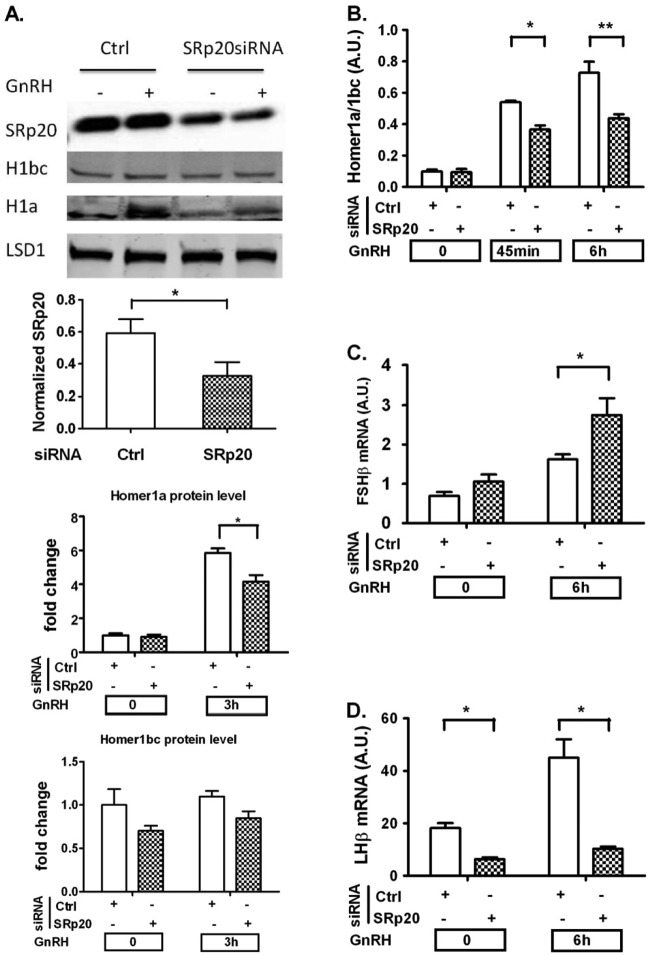
We determined whether depletion of SRp20 would affect the splicing pattern of Homer1. SRp20 siRNA knockdown resulted in an approximately 60% decrease in SRp20 protein levels (Fig. 4A). SRp20 depletion caused a significant reduction in the Homer1a-to-Homer1b/c splicing isoform ratio after 45-min and 6-h exposures to GnRH (Fig. 4B). Western blots from whole-cell lysates after 3-h GnRH treatment showed that knockdown of SRp20 attenuated the increase in Homer1a protein levels and had no significant effects on Homer1bc protein levels (Fig. 4A), which is consistent with the mRNA measurements. Thus, these results provide evidence for the involvement of SRp20 in GnRH-induced alternative splicing of Homer1.
FIG 4.
SRp20 knockdown affects GnRH-induced Homer1 splicing pattern and gonadotropin β-subunit gene expression. On day 1, cells were transfected with either scrambled (Ctrl) or SRp20 siRNA for 48 h. On day 2, cells were serum starved overnight. On day 3, cells were stimulated with 5 nM GnRH for the indicated times. (A) Knockdown efficiency and effect of SRp20 knockdown on Homer1 isoforms determined by quantitative Western blot analysis. LSD1 was used as a loading control. (B) Effects of SRp20 knockdown on GnRH-induced Homer1 splicing pattern after 45-min and 6-h GnRH exposures. (C) SRp20 knockdown upregulates GnRH-mediated FSHβ gene expression. (D) SRp20 knockdown reduces GnRH-induced LHβ gene expression. For RT-qPCR, the data shown are means ± SEM of triplicate samples from one experiment and are representative of three independent experiments. For Western blots, a representative figure is shown and densitometry is based on three independent experiments. Two-way ANOVA with Bonferroni's posttest corrections; *, P < 0.05; **, P < 0.01.
SRp20 protein and Homer1 are involved in the regulation of gonadotropin genes by GnRH.
We investigated whether SRp20 was involved in the transcriptional response of gonadotropin subunit genes to GnRH. LβT2 cells were transfected with siRNA targeting SRp20 and stimulated with GnRH. SRp20 depletion had different effects on FSHβ and LHβ expression. SRp20 knockdown resulted in an approximately 70% increase in GnRH-induced FSHβ transcript levels (Fig. 4C). Both basal and GnRH-stimulated LHβ expression levels were significantly decreased by SRp20 knockdown (Fig. 4D).
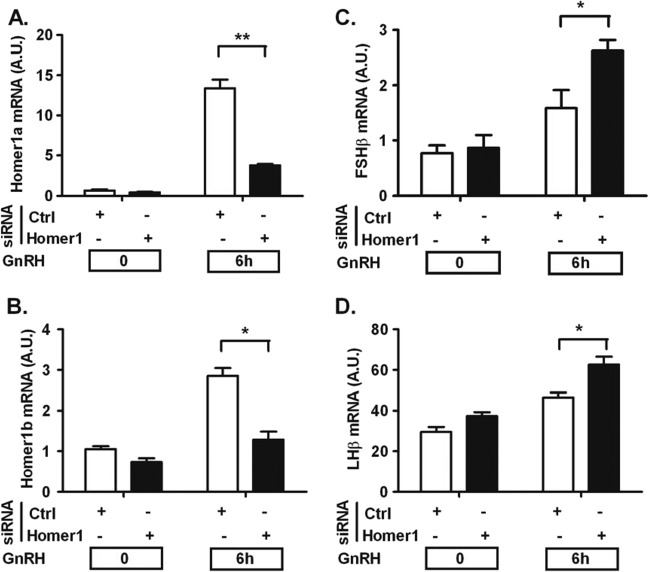
We also studied whether Homer1 was involved in the transcriptional response of gonadotropin subunit genes to GnRH. Cells were transfected with siRNA against the Homer1 gene and stimulated with GnRH. As shown in Fig. 5A and B, knockdown efficiency was high, with Homer1a and Homer1b/c splicing isoforms being reduced by ∼80% and 60%, respectively. Homer1 knockdown resulted in a significant increase in GnRH-induced FSHβ (Fig. 5C) and LHβ mRNA expression (Fig. 5D). These results (i) suggest that other factors besides Homer1 contributing to FSHβ expression are regulated by SRp20 and (ii) implicate Homer1 in the regulation of both gonadotropin genes (see Discussion).
FIG 5.
Homer1 knockdown upregulates gonadotropin β-subunit gene expression. On day 1, cells were transfected with either scrambled (Ctrl) or Homer1 siRNA for 48 h. On day 2, cells were serum starved overnight. On day 3, cells were stimulated with 5 nM GnRH for the indicated times. (A and B) Knockdown efficiency of Homer1 siRNA on the splicing isoforms. (C and D) Homer1 knockdown upregulates GnRH-mediated FSHβ and LHβ gene expression. The data shown are means ± SEM of triplicate samples from one experiment and are representative of three independent experiments. Two-way ANOVA with Bonferroni's posttest corrections; * P < 0.05; **, P < 0.01.
Inactivation of splicing isoform Homer1b/c results in a significant increase in GnRH-induced FSHβ and LHβ mRNAs.
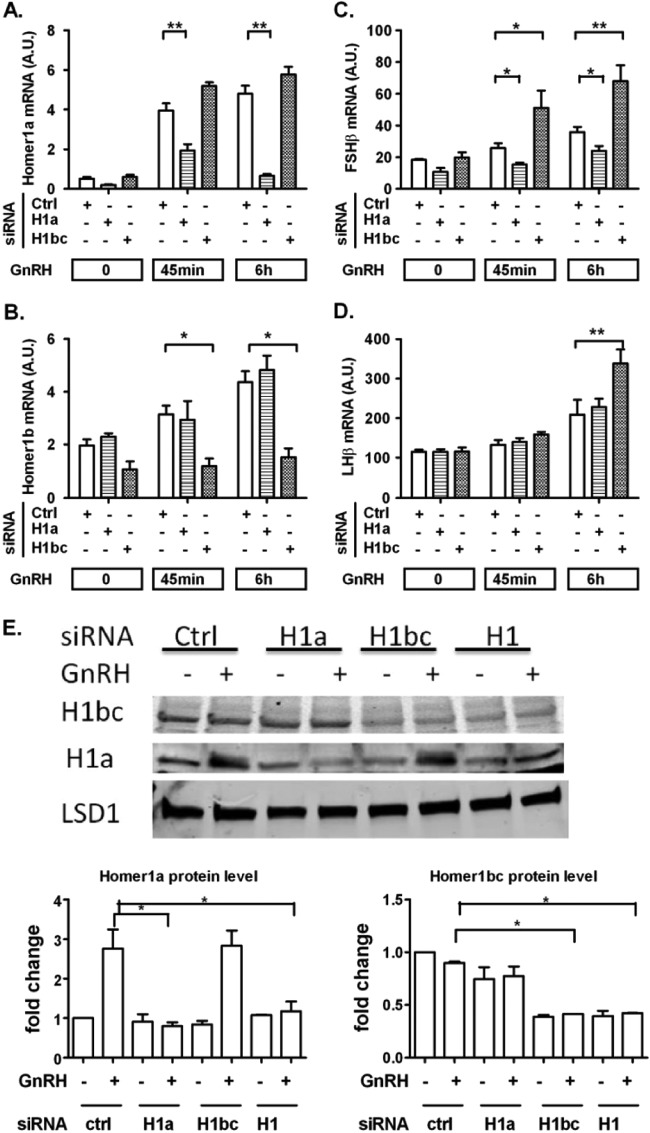
To determine the roles of the different Homer1 splice variants in GnRH-induced FSHβ and LHβ expression, we designed siRNA duplex pools that specifically targeted either Homer1a or Homer1b/c. As shown in Fig. 6A, B, and E, the siRNAs were highly specific for their respective targets. Knockdown efficiency was 80% for Homer1a and 50% for Homer1b/c. Homer1a knockdown significantly attenuated GnRH-stimulated FSHβ expression. Conversely, Homer1b/c knockdown resulted in a marked increase in FSHβ expression in GnRH-stimulated cells (Fig. 6C). Knockdown of Homer1b/c also caused an increase in LHβ gene expression in GnRH-stimulated cells, while an effect of Homer1a knockdown was not detectable (Fig. 6D). Overall, the Homer1b/c data are similar to the results obtained with Homer1 gene knockdown. Moreover, they suggest that Homer1b/c may function as a suppressor of FSHβ and LHβ whereas Homer1a has the opposite effect on FSHβ (see Discussion).
FIG 6.
Opposite effects of Homer1a- and Homer1b/c-specific knockdown on GnRH-induced gonadotropin β-subunit gene expression. On day 1, cells were transfected with either scrambled (Ctrl), Homer1a (H1a), or Homer1b/c (H1bc) siRNA for 48 h. On day 2, cells were serum starved overnight. On day 3, cells were stimulated with 5 nM GnRH for the indicated times. (A, B, and E) Knockdown efficiency and siRNA specificity of Homer1a and Homer1b/c siRNAs on mRNA level (A and B) and on protein level (E). (C and D) Knockdown of Homer1b/c upregulates GnRH-mediated FSHβ and LHβ gene expression, while knockdown of Homer1a reduces FSHβ gene expression but does not alter LHβ. For RT-qPCR, the data shown are means ± SEM of triplicate samples from one experiment and are representative of three independent experiments. For Western blots, a representative figure is shown and densitometry is based on three independent experiments. Two-way ANOVA with Bonferroni posttest corrections; *, P < 0.05; **, P < 0.01.
DISCUSSION
Alternative splicing of primary transcripts has been increasingly recognized as a critical constituent of gene regulation, and a number of signaling molecules have been shown to affect this cotranscriptional process (for a review, see reference 34). We find that GnRH increases phosphorylation of the splicing regulator SRp20 and that SRp20 contributes to the regulated splicing of Homer1. Our data provide the first evidence that GnRH induces global changes in alternative splicing and that the regulation of Homer1 splice variants is involved in FSHβ and LHβ gene control.
Homer1 is known as a postsynaptic scaffold protein. It has two isoforms, short variant Homer1a and long variant Homer1b/c, which are expressed mainly in the nervous system and play major roles in synaptic plasticity and transduction of intracellular signaling (for a review, see reference 35). While both isoforms have a highly conserved N-terminal region comprised of an EVH1 domain and a proline-containing motif, Homer1b/c has a specific coiled-coil C-terminal domain, which allows multimerization with itself or other long Homer proteins. In contrast, the EVH1 domain is a ligand-binding domain that interacts with target proteins such as the metabotropic glutamate receptor and inositol 1,4,5-triphosphate receptor. As Homer1a cannot form dimers, it functions as a dominant negative protein by competitively binding the target proteins, thereby disrupting the synaptic molecular complexes formed by Homer1b/c.
The overall effect of Homer1 expression and of the Homer1b/c isoform is to suppress FSHβ and to a lesser extent LHβ expression. SRp20 phosphorylation by GnRH signaling increases the Homer1a-to-Homer1b/c ratio, which favors the induction of FSHβ and to a lesser degree that of LHβ (Fig. 7). SRp20 knockdown decreases LHβ expression. However, SRp20 knockdown increases FSHβ expression, indicating that SRp20 is likely to affect other genes that suppress FSHβ. The global nature of the splicing altered by GnRH (Fig. 1) is consistent with the possibility that other spliced genes besides Homer1 contribute to the regulation of FSHβ.
FIG 7.
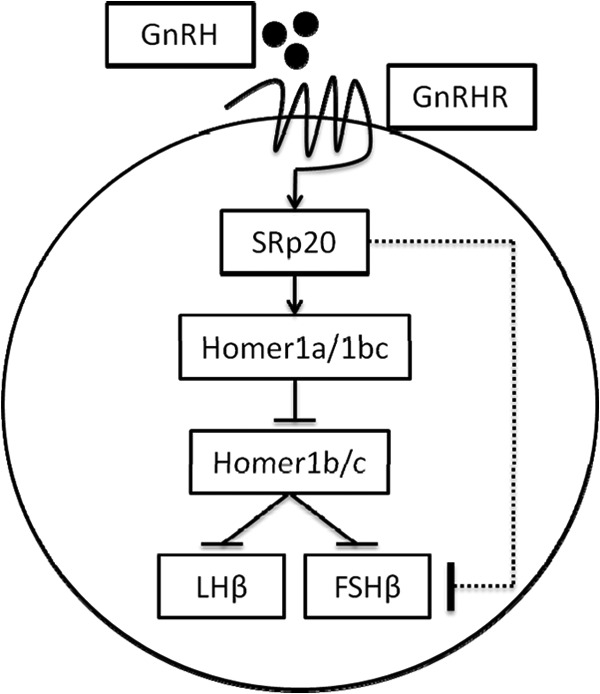
SRp20 and Homer1 regulate gonadotropin β-subunit gene expression (schematic). Upon activation by its ligand, the GnRH receptor (GnRHR) activates the phosphorylation of SRp20, which in turn increases the Homer1a-to-Homer1b/c splice variant ratio, resulting in the repression of Homer1b/c. Homer1b/c has an inhibitory effect on FSHβ and LHβ gene transcription. The finding that both SRp20 knockdown and Homer1b/c knockdown increase FSHβ suggests the presence of an inhibitory pathway downstream of SRp20, as indicated by the dotted line.
Splicing regulator SRp20 could have a direct effect on Homer1 pre-mRNA, as sequence analysis of the precursor RNA has identified multiple consensus high-affinity SRp20 binding sites (CUCKUCY [36]). Additionally, SRp20 regulates polyadenylation, which occurs during posttranscriptional pre-mRNA processing (37), and is involved in the process of transcription termination, possibly by binding to the RNA downstream of the 3′-end cleavage site (38).
Isoform-specific knockdown experiments show different effects of reducing the expression of long and short Homer1 isoforms. While Homer1b/c knockdown increases both FSHβ and LHβ expression, knockdown of Homer1a decreases FSHβ without changing LHβ. The differing effects of the isoforms on FSHβ are consistent with previous work showing that Homer1a can function as a negative regulator of Homer1b/c by disrupting its tetrameric structure due to its lack of the coiled-coil region necessary for tetramerization (39). The effects of knockdown experiments are sensitive to the stoichiometry and concentration response characteristics of the mRNA targets. Thus, it is possible that Homer1a also opposes the effect of Homer1b on LHβ, but the effect cannot be detected at the knockdown efficiencies achieved in these experiments.
Previous studies suggest that Homer1a stimulates and Homer1b/c inhibits calcium (Ca2+) responses in cerebellar neurons (30). GnRH-induced relief of histone deacetylase-dependent repression of FSHβ is mediated by Ca2+/Ca2+/calmodulin-dependent protein kinase signaling (31). Thus, it is conceivable that GnRH-mediated increase in the Homer1 splice variant ratio may lead to activation of Ca2+/Ca2+/calmodulin-dependent protein kinase signaling and subsequent derepression of the FSHβ gene. Ca2+ is also reported to be involved in LHβ regulation, as a Ca2+ chelator greatly inhibited GnRH-induced LHβ expression (40). These data suggest a role of Homer1-Ca2+ signaling in GnRH-mediated gonadotropin gene regulation and are consistent with our findings in Homer1 knockdown experiments. The role of Homer1 in reproductive function is supported by the finding that homozygous Homer1 knockout mice are unable to breed, despite normal ovarian and testes histology (41).
Interestingly, several signaling components of the GnRH network were previously reported to have splice variants that may impact gonadotrope cell function. GnRH itself may undergo alternative splicing. Intron A retention is a key mechanism that keeps GnRH levels low in newborns. With the increased expression of splicing regulators, the rate of intron A excision increases to allow accumulation of mature GnRH mRNA during postnatal development (42). The Pvr1 gene encodes four major splice variant forms of the pituitary adenylate cyclase-activating polypeptide receptor; these variants differ in the sequence of the third intracellular loop, which is critical for G protein binding. As these variants display differences of affinity for G proteins and in their downstream signaling, this results in heterogeneous ligand responses in individual rat gonadotropes (43). In pituitary gonadotropes, a novel splice variant, Pitx2b2, employs the same 5′ donor site as Pitx2b at intron 2 but an alternative 3′ acceptor site, resulting in a 39-bp in-frame deletion. Nevertheless, the truncated protein has DNA binding and transactivation properties similar to those of other isoforms of paired-like homeodomain transcription factor 2 in vitro (44). The present study shows the breadth of GnRH-regulated splicing in the gonadotrope and increases the importance of splicing in contributing to gonadotropin gene regulation.
ACKNOWLEDGMENT
This work was supported by National Institutes of Health grant DK46943.
Footnotes
Published ahead of print 3 March 2014
REFERENCES
- 1.Pierce JG, Parsons TF. 1981. Glycoprotein hormones: structure and function. Annu. Rev. Biochem. 50:465–495. 10.1146/annurev.bi.50.070181.002341 [DOI] [PubMed] [Google Scholar]
- 2.Burger LL, Haisenleder DJ, Dalkin AC, Marshall JC. 2004. Regulation of gonadotropin subunit gene transcription. J. Mol. Endocrinol. 33:559–584. 10.1677/jme.1.01600 [DOI] [PubMed] [Google Scholar]
- 3.Buckler HM, Phillips SE, Kovacs GT, Burger HG, Healy DL. 1989. GnRH agonist administration in polycystic ovary syndrome. Clin. Endocrinol. (Oxf.) 31:151–165. 10.1111/j.1365-2265.1989.tb01238.x [DOI] [PubMed] [Google Scholar]
- 4.Del Mastro L, Catzeddu T, Venturini M. 2006. Infertility and pregnancy after breast cancer: current knowledge and future perspectives. Cancer Treat. Rev. 32:417–422. 10.1016/j.ctrv.2006.05.004 [DOI] [PubMed] [Google Scholar]
- 5.Kim DK, Yang JS, Maiti K, Hwang JI, Kim K, Seen D, Ahn Y, Lee C, Kang BC, Kwon HB, Cheon J, Seong JY. 2009. A gonadotropin-releasing hormone-II antagonist induces autophagy of prostate cancer cells. Cancer Res. 69:923–931. 10.1158/0008-5472.CAN-08-2115 [DOI] [PubMed] [Google Scholar]
- 6.Alarid ET, Windle JJ, Whyte DB, Mellon PL. 1996. Immortalization of pituitary cells at discrete stages of development by directed oncogenesis in transgenic mice. Development 122:3319–3329 [DOI] [PubMed] [Google Scholar]
- 7.Thomas P, Mellon PL, Turgeon J, Waring DW. 1996. The L beta T2 clonal gonadotrope: a model for single cell studies of endocrine cell secretion. Endocrinology 137:2979–2989. 10.1210/en.137.7.2979 [DOI] [PubMed] [Google Scholar]
- 8.Turgeon JL, Kimura Y, Waring DW, Mellon PL. 1996. Steroid and pulsatile gonadotropin-releasing hormone (GnRH) regulation of luteinizing hormone and GnRH receptor in a novel gonadotrope cell line. Mol. Endocrinol. 10:439–450 [DOI] [PubMed] [Google Scholar]
- 9.Windle JJ, Weiner RI, Mellon PL. 1990. Cell lines of the pituitary gonadotrope lineage derived by targeted oncogenesis in transgenic mice. Mol. Endocrinol. 4:597–603. 10.1210/mend-4-4-597 [DOI] [PubMed] [Google Scholar]
- 10.Naor Z. 2009. Signaling by G-protein-coupled receptor (GPCR): studies on the GnRH receptor. Front. Neuroendocrinol. 30:10–29. 10.1016/j.yfrne.2008.07.001 [DOI] [PubMed] [Google Scholar]
- 11.Thackray VG, Mellon PL, Coss D. 2010. Hormones in synergy: regulation of the pituitary gonadotropin genes. Mol. Cell. Endocrinol. 314:192–203. 10.1016/j.mce.2009.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Choi SG, Jia J, Pfeffer RL, Sealfon SC. 2012. G proteins and autocrine signaling differentially regulate gonadotropin subunit expression in the pituitary gonadotrope. J. Biol. Chem. 287:21550–21560. 10.1074/jbc.M112.348607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ferris HA, Walsh HE, Stevens J, Fallest PC, Shupnik MA. 2007. Luteinizing hormone beta promoter stimulation by adenylyl cyclase and cooperation with gonadotropin-releasing hormone 1 in transgenic mice and LBetaT2 cells. Biol. Reprod. 77:1073–1080. 10.1095/biolreprod.107.064139 [DOI] [PubMed] [Google Scholar]
- 14.Coss D, Jacobs SB, Bender CE, Mellon PL. 2004. A novel AP-1 site is critical for maximal induction of the follicle-stimulating hormone beta gene by gonadotropin-releasing hormone. J. Biol. Chem. 279:152–162. 10.1074/jbc.M304697200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mistry DS, Tsutsumi R, Fernandez M, Sharma S, Cardenas SA, Lawson MA, Webster NJ. 2011. Gonadotropin-releasing hormone pulse sensitivity of follicle-stimulating hormone-{beta} gene is mediated by differential expression of positive regulatory activator protein 1 factors and corepressors SKIL and TGIF1. Mol. Endocrinol. 25:1387–1403. 10.1210/me.2011-0032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bernard DJ, Fortin J, Wang Y, Lamba P. 2010. Mechanisms of FSH synthesis: what we know, what we don't, and why you should care. Fertil. Steril. 93:2465–2485. 10.1016/j.fertnstert.2010.03.034 [DOI] [PubMed] [Google Scholar]
- 17.Wang Q, Chikina M, Zaslavsky E, Pincas H, Sealfon SC. 2013. beta-catenin regulates GnRH-induced FSHbeta gene expression. Mol. Endocrinol. 27:224–237. 10.1210/me.2012-1310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ward AJ, Cooper TA. 2010. The pathobiology of splicing. J. Pathol. 220:152–163. 10.1002/path.2649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Busch A, Hertel KJ. 2012. Evolution of SR protein and hnRNP splicing regulatory factors. Wiley Interdiscip. Rev. RNA 3:1–12. 10.1002/wrna.100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guivarc'h D, Vincent JD, Vernier P. 1998. Alternative splicing of the D2 dopamine receptor messenger ribonucleic acid is modulated by activated sex steroid receptors in the MMQ prolactin cell line. Endocrinology 139:4213–4221. 10.1210/en.139.10.4213, [DOI] [PubMed] [Google Scholar]
- 21.Kawamoto S. 1996. Neuron-specific alternative splicing of nonmuscle myosin II heavy chain-B pre-mRNA requires a cis-acting intron sequence. J. Biol. Chem. 271:17613–17616 [PubMed] [Google Scholar]
- 22.Xie J, McCobb DP. 1998. Control of alternative splicing of potassium channels by stress hormones. Science 280:443–446. 10.1126/science.280.5362.443 [DOI] [PubMed] [Google Scholar]
- 23.Cowley MA, Rao A, Wright PJ, Illing N, Millar RP, Clarke IJ. 1998. Evidence for differential regulation of multiple transcripts of the gonadotropin releasing hormone receptor in the ovine pituitary gland; effect of estrogen. Mol. Cell. Endocrinol. 146:141–149. 10.1016/S0303-7207(98)00162-2 [DOI] [PubMed] [Google Scholar]
- 24.Grant GR, Farkas MH, Pizarro AD, Lahens NF, Schug J, Brunk BP, Stoeckert CJ, Hogenesch JB, Pierce EA. 2011. Comparative analysis of RNA-Seq alignment algorithms and the RNA-Seq unified mapper (RUM). Bioinformatics 27:2518–2528. 10.1093/bioinformatics/btr427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Smyth GK, Michaud J, Scott HS. 2005. Use of within-array replicate spots for assessing differential expression in microarray experiments. Bioinformatics 21:2067–2075. 10.1093/bioinformatics/bti270 [DOI] [PubMed] [Google Scholar]
- 26.Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette MA, Paulovich A, Pomeroy SL, Golub TR, Lander ES, Mesirov JP. 2005. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl. Acad. Sci. U. S. A. 102:15545–15550. 10.1073/pnas.0506580102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hunter S, Jones P, Mitchell A, Apweiler R, Attwood TK, Bateman A, Bernard T, Binns D, Bork P, Burge S, de Castro E, Coggill P, Corbett M, Das U, Daugherty L, Duquenne L, Finn RD, Fraser M, Gough J, Haft D, Hulo N, Kahn D, Kelly E, Letunic I, Lonsdale D, Lopez R, Madera M, Maslen J, McAnulla C, McDowall J, McMenamin C, Mi H, Mutowo-Muellenet P, Mulder N, Natale D, Orengo C, Pesseat S, Punta M, Quinn AF, Rivoire C, Sangrador-Vegas A, Selengut JD, Sigrist CJ, Scheremetjew M, Tate J, Thimmajanarthanan M, Thomas PD, Wu CH, Yeats C, Yong SY. 2012. InterPro in 2011: new developments in the family and domain prediction database. Nucleic Acids Res. 40:D306–D312. 10.1093/nar/gkr948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Choi SG, Ruf-Zamojski F, Pincas H, Roysam B, Sealfon SC. 2011. Characterization of a MAPK scaffolding protein logic gate in gonadotropes. Mol. Endocrinol. 25:1027–1039. 10.1210/me.2010-0387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ruf F, Fink MY, Sealfon SC. 2003. Structure of the GnRH receptor-stimulated signaling network: insights from genomics. Front. Neuroendocrinol. 24:181–199. 10.1016/S0091-3022(03)00027-X [DOI] [PubMed] [Google Scholar]
- 30.Minami I, Kengaku M, Smitt PS, Shigemoto R, Hirano T. 2003. Long-term potentiation of mGluR1 activity by depolarization-induced Homer1a in mouse cerebellar Purkinje neurons. Eur. J. Neurosci. 17:1023–1032. 10.1046/j.1460-9568.2003.02499.x [DOI] [PubMed] [Google Scholar]
- 31.Lim S, Luo M, Koh M, Yang M, bin Abdul Kadir MN, Tan JH, Ye Z, Wang W, Melamed P. 2007. Distinct mechanisms involving diverse histone deacetylases repress expression of the two gonadotropin beta-subunit genes in immature gonadotropes, and their actions are overcome by gonadotropin-releasing hormone. Mol. Cell. Biol. 27:4105–4120. 10.1128/MCB.00248-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ghasemzadeh MB, Windham LK, Lake RW, Acker CJ, Kalivas PW. 2009. Cocaine activates Homer1 immediate early gene transcription in the mesocorticolimbic circuit: differential regulation by dopamine and glutamate signaling. Synapse 63:42–53. 10.1002/syn.20577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Matlin AJ, Clark F, Smith CW. 2005. Understanding alternative splicing: towards a cellular code. Nat. Rev. Mol. Cell Biol. 6:386–398. 10.1038/nrm1645 [DOI] [PubMed] [Google Scholar]
- 34.Kornblihtt AR, Schor IE, Allo M, Dujardin G, Petrillo E, Munoz MJ. 2013. Alternative splicing: a pivotal step between eukaryotic transcription and translation. Nat. Rev. Mol. Cell Biol. 14:153–165. 10.1038/nrm3525 [DOI] [PubMed] [Google Scholar]
- 35.Luo P, Li X, Fei Z, Poon W. 2012. Scaffold protein Homer 1: implications for neurological diseases. Neurochem. Int. 61:731–738. 10.1016/j.neuint.2012.06.014 [DOI] [PubMed] [Google Scholar]
- 36.Schaal TD, Maniatis T. 1999. Selection and characterization of pre-mRNA splicing enhancers: identification of novel SR protein-specific enhancer sequences. Mol. Cell. Biol. 19:1705–1719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lou H, Neugebauer KM, Gagel RF, Berget SM. 1998. Regulation of alternative polyadenylation by U1 snRNPs and SRp20. Mol. Cell. Biol. 18:4977–4985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cui M, Allen MA, Larsen A, Macmorris M, Han M, Blumenthal T. 2008. Genes involved in pre-mRNA 3′-end formation and transcription termination revealed by a lin-15 operon Muv suppressor screen. Proc. Natl. Acad. Sci. U. S. A. 105:16665–16670. 10.1073/pnas.0807104105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hayashi MK, Tang C, Verpelli C, Narayanan R, Stearns MH, Xu RM, Li H, Sala C, Hayashi Y. 2009. The postsynaptic density proteins Homer and Shank form a polymeric network structure. Cell 137:159–171. 10.1016/j.cell.2009.01.050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kawaminami M, Uematsu N, Funahashi K, Kokubun R, Kurusu S. 2008. Gonadotropin releasing hormone (GnRH) enhances annexin A5 mRNA expression through mitogen activated protein kinase (MAPK) in LbetaT2 pituitary gonadotrope cells. Endocr. J. 55:1005–1014. 10.1507/endocrj.K08E-131 [DOI] [PubMed] [Google Scholar]
- 41.Grinevich V, Jezova D, Gambaryan S, Illarionova A, Kolleker A, Seeburg PH, Schwarz MK. Hypertrophy and altered activity of the adrenal cortex in Homer 1 knockout mice. Horm. Metab. Res. 43:551–556. 10.1055/s-0031-1280828 [DOI] [PubMed] [Google Scholar]
- 42.Han J, Son GH, Seong JY, Kim K. 2002. GnRH pre-mRNA splicing: role of exonic splicing enhancer. Prog. Brain Res. 141:209–219. 10.1016/S0079-6123(02)41095-3 [DOI] [PubMed] [Google Scholar]
- 43.Bresson-Bepoldin L, Jacquot MC, Schlegel W, Rawlings SR. 1998. Multiple splice variants of the pituitary adenylate cyclase-activating polypeptide type 1 receptor detected by RT-PCR in single rat pituitary cells. J. Mol. Endocrinol. 21:109–120. 10.1677/jme.0.0210109 [DOI] [PubMed] [Google Scholar]
- 44.Lamba P, Hjalt TA, Bernard DJ. 2008. Novel forms of Paired-like homeodomain transcription factor 2 (PITX2): generation by alternative translation initiation and mRNA splicing. BMC Mol. Biol. 9:31. 10.1186/1471-2199-9-31 [DOI] [PMC free article] [PubMed] [Google Scholar]