Abstract
Previous work from our lab suggests that a group of interdependent assembly factors (A3 factors) is necessary to create early, stable preribosomes. Many of these proteins bind at or near internal transcribed spacer 2 (ITS2), but in their absence, ITS1 is not removed from rRNA, suggesting long-range communication between these two spacers. By comparing the nonessential assembly factors Nop12 and Pwp1, we show that misfolding of rRNA is sufficient to perturb early steps of biogenesis, but it is the lack of A3 factors that results in turnover of early preribosomes. Deletion of NOP12 significantly inhibits 27SA3 pre-rRNA processing, even though the A3 factors are present in preribosomes. Furthermore, pre-rRNAs are stable, indicating that the block in processing is not sufficient to trigger turnover. This is in contrast to the absence of Pwp1, in which the A3 factors are not present and pre-rRNAs are unstable. In vivo RNA structure probing revealed that the pre-rRNA processing defects are due to misfolding of 5.8S rRNA. In the absence of Nop12 and Pwp1, rRNA helix 5 is not stably formed. Interestingly, the absence of Nop12 results in the formation of an alternative yet unproductive helix 5 when cells are grown at low temperatures.
INTRODUCTION
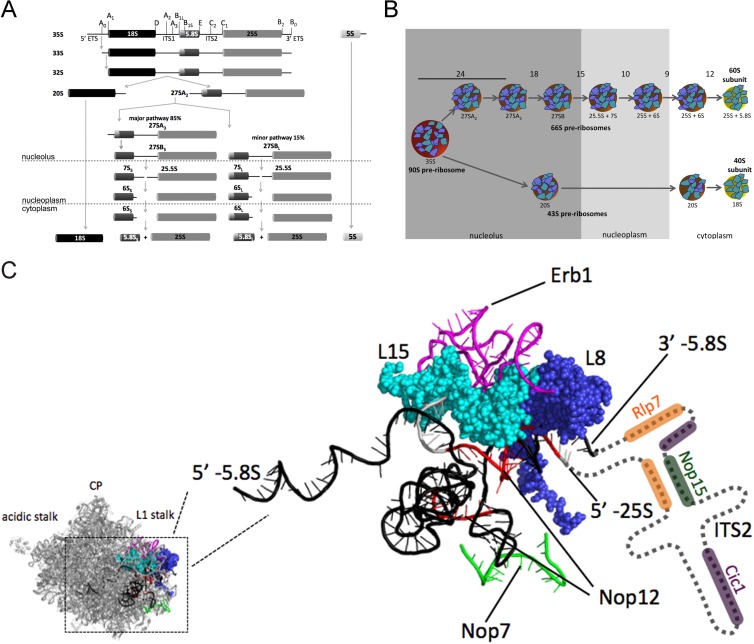
Ribosome assembly is a highly conserved and dynamic process driven by the cooperative transcription, folding, modification, and processing of rRNAs and stable binding of ribosomal proteins (r-proteins) (1, 2). In Saccharomyces cerevisiae, this process begins in the nucleolus with cotranscriptional assembly of early precursor particles. As transcription proceeds, the nascent pre-rRNA is cleaved, separating maturation of early 40S and 60S precursors. A series of endo- and exonucleolytic cleavages remove internal and external transcribed spacer sequences from pre-rRNAs, as the assembling ribosome moves from the nucleolus to the nucleoplasm and is eventually exported to the cytoplasm (Fig. 1A and B).
FIG 1.
Pre-rRNA processing pathway and maturation of preribosomal particles in Saccharomyces cerevisiae. (A) Pre-rRNA processing pathway. 5′ ETS, 5′ external transcribed spacer. (B) Assembly intermediates in the biogenesis of ribosomal subunits. Preribosomal particles that will mature into 60S and 40S subunits are indicated by the pre-rRNAs contained within them. Known numbers of AFs required for each step of 60S subunit assembly are shown. (C) The Pwp1 subcomplex associates with preribosomes near the ITS2-proximal stem. Shown is the crystal structure of the Saccharomyces cerevisiae 60S subunit viewed from the solvent-accessible side (29) (PDB accession no. 3U5H and 3U5I). 5.8S rRNA is shown in black. The ITS2-proximal stem is composed of base pairing between the 5′ end of 25S rRNA and the 3′ end of 5.8S rRNA. r-proteins L8 and L15 are colored dark blue and light blue, respectively. Sequences of rRNA cross-linked to Nop12, Nop7, and Erb1 are shown in red, green, and magenta, respectively (26). ITS2 is represented by the dashed line to indicate the approximate locations of Cic1, Nop15, and Rlp7 (26, 30).
Assembly of yeast ribosomes requires ∼180 trans-acting proteins termed assembly factors (AFs). The majority of these proteins are conserved across eukaryotes, are essential for cell growth, and are thought to function as scaffolding proteins, RNA chaperones, energy-consuming nucleoside triphosphatases (NTPases), nucleases, or posttranslational modifiers (3–5). Purification of ribosome assembly intermediates allowed the identification of most AFs, and initial characterizations have shown in which steps of pre-rRNA processing many of these factors function. However, to begin to understand the function of these proteins on a mechanistic level, one must address the following: the timing of association of these proteins with preribosomes; how they are recruited to preribosomes; their requirement for the stable binding of other proteins; and their role in folding rRNA.
Numerous studies have shown that subsets of AFs can be isolated as subcomplexes (6–23). Proteins within a subcomplex are often required for the same steps of pre-rRNA processing, suggesting that they function together during ribosome biogenesis to achieve a common goal. One subcomplex that functions in assembly of the 60S subunit in Saccharomyces cerevisiae is composed of the AFs Brx1, Ebp2, Nop12, and Pwp1 and r-proteins L8 and L15 (referred to as the Pwp1 subcomplex). High-speed centrifugation, followed by coimmunoprecipitation (co-IP) using epitope-tagged Nop12 or Pwp1, revealed that these proteins interact in a stable complex that can be isolated independently of preribosomes (6, 24). Furthermore, Brx1, Ebp2, Nop12, and L8 have all been shown to be required for processing of 27SA2 and 27SA3 pre-rRNAs (25–28).
In the mature 60S subunit, r-proteins L8 and L15 interact closely with each other as well as with the ITS2-proximal stem (helix 10), a conserved helix formed by base pairing between the 3′ end of 5.8S rRNA and the 5′ end of 25S rRNA (Fig. 1C) (29). These two rRNA ends are generated upon the removal of internal transcribed spacer 2 (ITS2) during processing of 27SB pre-rRNA (Fig. 1A). It was recently reported that Nop12 can be UV cross-linked within the ITS2-proximal stem, as well as a nearby stretch of 5.8S rRNA (helix 8) (Fig. 1C) (26). This result, as well as the known location of L8 and L15 in mature 60S subunits, suggests that the Pwp1 subcomplex binds within or near the ITS2-proximal stem and may play a role in folding rRNA and structuring this neighborhood.
Despite the evidence that the Pwp1 subcomplex binds the ITS2-proximal stem, members of this complex (Ebp2, Brx1, and r-protein L8) are required for removal of ITS1 to generate the 5′ end of 5.8S rRNA (25, 28). In mature ribosomes, the 5′ end of 5.8S rRNA is ∼160 Å from the ITS2-proximal stem. Taken together, these results suggest that removal of ITS1 and ITS2 requires coupling between these sequences that may be located far apart within preribosomes. Consistent with this, depletion of proteins that bind to ITS2 (Cic1, Nop15, and Rlp7) causes ITS2 to misfold but inhibit removal of ITS1 (26, 30–33). By binding to the ITS2-proximal stem, the Pwp1 subcomplex appears to be located in the preribosome at a position to enable long-range communication between these two spacers (Fig. 1C).
Our laboratory has recently established a hierarchy of association between Ebp2, Brx1, r-protein L8, and other factors required for 27SA3 pre-rRNA processing (25, 28, 34). Here we focused on the less characterized Pwp1 subcomplex members, Nop12 and Pwp1, for which an association hierarchy has not been established. Interestingly, both Nop12 and Pwp1 are nonessential proteins containing domains predicted to bind RNA (35–37). Therefore, we rationalized that the changes observed in their absence would be minimal but highly specific. We saw that like other members of the Pwp1 subcomplex, Pwp1 functions in 27SA2 and 27SA3 pre-rRNA processing. Additionally, we have further defined the association hierarchy between the Pwp1 subcomplex and other A3 factors. Most importantly, we have further characterized the relationship between pre-rRNA folding, protein association, and turnover of misassembled ribosomes during this step of ribosome biogenesis. Nop12 and Pwp1 are required for proper folding of 5.8S rRNA and the ITS2-proximal stem. In the absence of either protein, helices in the 3′ half of 5.8S rRNA do not stably form, and in the absence of Nop12, helix 5 is folded into an alternative, unproductive helix. Surprisingly, these misfolded pre-rRNAs are stable in the absence of Nop12 but rapidly turned over when Pwp1 is not present. We have attributed this turnover to a group of proteins that fail to stably associate in the absence of Pwp1 but still readily bind preribosomes in the absence of Nop12.
MATERIALS AND METHODS
Construction of yeast strains.
Yeast strains used in this study were derived from Saccharomyces cerevisiae JWY6147, W303, or S288C and are listed in Table S1 in the supplemental material. nop12Δ and pwp1Δ strains were constructed as described by Longtine et al. (38). PCR products containing the HIS3 gene and sequences complementary to either NOP12 or PWP1 were transformed into diploid W303, and transformants were screened on selective medium lacking histidine. Correct integration of the HIS3 gene at the NOP12 and PWP1 loci was verified by PCR. Diploids were sporulated, and tetrads were dissected. Individual spores were screened for the ability to grow on complete medium lacking histidine (C-his medium), and correct gene disruptions were verified by PCR.
A yeast strain conditional for Pwp1 expression was constructed as described by Longtine et al. (38). Briefly, sequences containing the selectable marker KANMX6, plus the GAL1 promoter sequence followed by an ATG and sequences encoding three hemagglutinins (3HA) were amplified by PCR. PCR products were transformed as described by Rigaut et al. (39) into strain JWY6147, and transformants were screened for their ability to grow on selective medium containing galactose. G418r transformants were screened for correct integration of the GAL1 promoter and 3HA tag by Western blotting with anti-HA sera.
Yeast strains expressing C-terminal tandem affinity purification (TAP)-tagged Rpf2, 13Myc-tagged Nop12, and 3HA-tagged Pwp1 were constructed by PCR of a selectable marker (TAP, URA3; 13Myc and 3HA, KANMX6) and transformation as previously described (38, 39). Transformants that grew on selective medium were screened for correct integration of the PCR product and expression of the tagged protein by SDS-PAGE and Western blotting.
Growth of yeast strains.
Unless otherwise indicated, strains were grown in either YEPGlu (1% yeast extract, 2% peptone, and 2% dextrose) or YEPGal (1% yeast extract, 2% peptone, and 2% galactose) at 30°C. nop12Δ and pwp1Δ strains were grown at the permissive temperature of 30°C and then shifted to either 18°C or 37°C for 4 h. GAL-HA3-PWP1 and GAL-HA3-RLP7 strains were grown in liquid YEPGal, pelleted by centrifugation, suspended in YEPGlu, and grown for 16 h to deplete Pwp1 and Rlp7. Growth of the GAL-HA3-PWP1 strain and depletion of Pwp1 were assessed by growth in liquid YEPGal or YEPGlu. Cells were continually diluted to ensure exponential growth, and growth was monitored with a Genesis 20 spectrophotometer (Thermo Fisher Scientific). Aliquots were taken at the indicated time points and assayed by SDS-PAGE and Western blotting against 3HA-tagged Pwp1 (3HA-Pwp1). An antibody against Sec61 serves as a loading control. Growth of the nop12Δ and pwp1Δ strains was assessed at 18°C, 30°C, and 37°C by spotting serial dilutions (undiluted to 1:100,000) of liquid cultures on solid YEPGlu plates.
RNA analysis.
RNA from whole-cell lysates was extracted and assayed by Northern blotting, primer extension, and pulse-chase as previously described (40). Pulse-chase analysis was performed using [methyl-3H]methionine. For Northern blotting and primer extension, RNA was quantified using a Nano Drop 2000C spectrophotometer (Thermo Fisher Scientific). Five micrograms of RNA was used for reverse transcription reactions. The sequences of the oligonucleotides used for primer extension and Northern blotting are available upon request.
Protein extraction, SDS-PAGE, and Western blot analysis.
Proteins in whole-cell extracts were prepared for gel electrophoresis by dissolving in SDS sample buffer as previously described (41). Proteins were recovered from eluates during affinity purification by precipitation with 10% trichloroacetic acid (TCA) and suspended in SDS sample buffer. The proteins were resolved by electrophoresis on 4% to 20% Tris-glycine Novex gels (Invitrogen). To assay Nog2 by Western blotting, 4% to 12% Bis-Tris gels (Invitrogen) were used, since Nog2 comigrates with IgG on 4% to 20% Tris-glycine gels. Proteins from whole-cell extracts and affinity-purified preribosomes were assayed by Western blotting as previously described (41). To enable detection of multiple proteins from a single blot and to conserve antiserum, after electroblotting, nitrocellulose membranes were cut based on the known mobility of the proteins of interest. Visible cuts in the membrane are indicated with asterisks in the figures. TAP-tagged proteins were detected using alkaline phosphatase conjugated to IgG (Pierce). Mouse monoclonal antibodies 12CA5 (Roche) and 9e10 (Developmental Studies Hybridoma Bank) were used to detect HA-tagged and Myc-tagged proteins, respectively. Otherwise, antibodies specific to r-proteins or AFs were used. Alkaline phosphatase (AP)-conjugated anti-mouse or anti-rabbit secondary antibodies (Promega) were used, and proteins were visualized by colorimetric detection using Nitro Blue Tetrazolium (NBT) and 5-bromo-4-chloro-3-indolylphosphate (BCIP) (Promega).
Affinity purification of preribosomes.
Ribosome assembly intermediates were affinity purified from whole-cell extracts with magnetic Dynabeads (Invitrogen), using TAP-tagged Rpf2, as previously described (34). Rpf2 associates with preribosomes early during biogenesis, with 35S- or 27SA2 pre-rRNA, and is present in each consecutive 66S intermediate. Furthermore, association with preribosomes of Rpf2 is not affected upon depletion of other proteins required for 27SA2 or 27SA3 pre-rRNA processing.
Sucrose gradient assays of ribosomes and polyribosomes.
Preribosomes, ribosomes, and polyribosomes were fractionated from 40 optical density at 254 nm (OD254) units of whole-cell extracts on 7% to 47%(wt/vol) sucrose gradients as described by Deshmukh et al. (42), with the following modifications. Cycloheximide (5 mg) was added to cultures 20 min before harvesting cells. A Teledyne ISCO Foxy R1 density gradient fractionator was used to fractionate and analyze gradients.
In vivo DMS probing.
In vivo structure probing using dimethyl sulfate (DMS) was performed as previously described (43). Briefly, 10 ml of cells was grown in YEPGlu to an OD610 of 0.4 and incubated with 200 μl of a fresh dilution (1:4 [vol/vol] in 95% ethanol) of DMS (Sigma-Aldrich) for 2 min. Reactions were quenched by placing the tubes on ice and adding 5 ml of water-saturated isoamyl alcohol and 5 ml of 0.6 M β-mercaptoethanol. Cells were pelleted and washed again in 0.6 M β-mercaptoethanol, and RNA was immediately phenol extracted.
Nucleotide modifications were assayed by primer extension with Transcriptor reverse transcriptase (Roche) and oligonucleotides complementary to ITS2 as previously described by Liebeg and Waldsich (44) with the following changes. For each reaction, 2.5 μl (3 μg) of whole-cell RNA was incubated with 1 μl (0.2 μM) of 32P-labeled primer and 1 μl of 4.5× hybridization buffer (225 mM HEPES [pH 7.0], 450 mM KCl) at 95°C for 5 min. Reactions were then cooled to the annealing temperature of 54°C for 20 min. Fifteen microliters of prewarmed (54°C) extension mix containing 4 μl of 5× hybridization buffer (Roche Applied Science) (250 mM Tris-HCl, 150 mM KCl, 40 mM MgCl2, pH is approximately 8.5), 2.0 μl of 2.5 mM deoxynucleoside triphosphate (dNTP) mixture, 1.0 μl of 0.1 mM dithiothreitol (DTT), 0.5 μl (20 U) RNasin (Promega), 0.5 μl (10 U) Transcriptor reverse transcriptase (Roche Applied Science), and 7.5 μl of nuclease-free water was added to each reaction mixture. For sequencing reactions, 2 μl of the appropriate dideoxynucleotide (ddNTP) (10 mM) (Roche Applied Science) was also added. Reaction mixtures were incubated at 54°C for 1 h. RNA was degraded by the addition of 3 μl of 1 M NaOH to each reaction mixture and incubated at 55°C for 1 h. The reactions were neutralized by the addition of 3 μl of 1.0 M HCl. cDNAs were precipitated with 1 μl of glycogen (10 mg/ml), 1 μl of 0.5 M EDTA (pH 8.0), 2.8 μl of 3.0 M sodium acetate (NaOAc) (pH 5.0), and 84 μl of ethanol. Following ethanol precipitation, dried cDNA pellets were resuspended in 6 μl of 1× loading dye (45% formamide, 0.01 M EDTA [pH 8.0]), resolved on 6% polyacrylamide–7 M urea gels, and visualized by autoradiography. The intensity of gel bands was quantified using ImageJ (45). Oligonucleotide sequences are available upon request.
Fluorescence microscopy.
Export of preribosomes to the cytoplasm was assayed by monitoring the cellular localization of ribosomal protein (r-protein) L25-enhanced green fluorescent protein (eGFP) as previously described by Babiano et al. (43). Wild-type and pwp1Δ strains were transformed with pRS316 plasmid constructs coexpressing the nucleolar marker monomeric red fluorescent protein (mRFP)-Nop1 and L25-eGFP (46). Cells were imaged using a Carl Zeiss LSM-510 META UV DuoScan inverted spectral confocal microscope and analyzed using ImageJ (45).
RESULTS
Pwp1 is a nonessential protein and functions in 60S subunit biogenesis.
Previously, Nop12 was shown to be a nonessential protein, although deletion causes cold sensitivity and blocks large-subunit pre-rRNA processing (26, 35). Large-scale surveys of the yeast genome reported Pwp1 as an essential protein (47), but closer inspection by Duronio et al. (36) showed that a strain containing an insertion in the PWP1 gene was viable but slow growing. To clarify this, we precisely replaced the PWP1 open reading frame (ORF) with HIS3 in the yeast diploid W303. Upon sporulation and dissection of tetrads, we recovered four viable spores, two of which were extremely slow growing (data not shown). This confirmed previous results that Pwp1 is nonessential. Although pwp1Δ cells were viable, their doubling times were ∼8-fold longer than those of wild-type cells, and they were cold sensitive, i.e., unable to grow at 18°C (see Fig. S1A in the supplemental material).
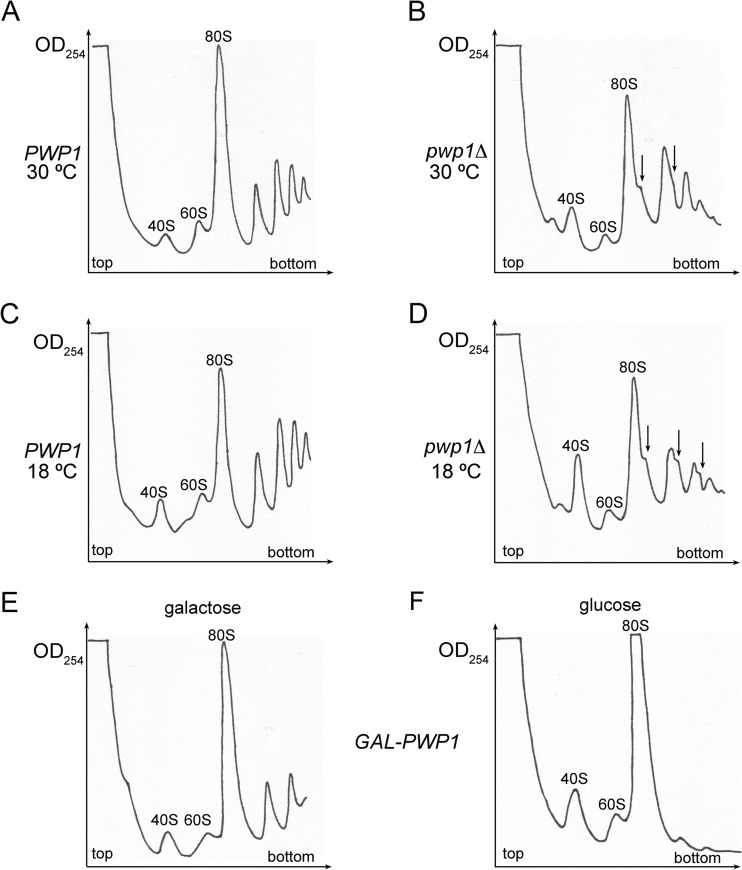
To test the role of Pwp1 in ribosome assembly, we assayed by sucrose gradient centrifugation the amounts of mature ribosomes in wild-type and pwp1Δ strains at 30°C and at the nonpermissive temperature of 18°C. We also assayed the levels of mature ribosomes in a strain conditional for expression of Pwp1, in which the promoter of the PWP1 gene was replaced with the GAL1 promoter to repress Pwp1 expression in glucose-containing medium (see Fig. S1B in the supplemental material). In both cases, in the absence of Pwp1, the amounts of free 60S subunits relative to those of 40S subunits were decreased, and half-mer polyribosomes were present (Fig. 2A to E). The decrease in the ratio of 60S subunits to 40S subunits was exacerbated in the pwp1Δ strain shifted to 18°C, consistent with the cold sensitivity (Fig. 2C and D). Interestingly, an extra peak was present in the pwp1Δ strain, sedimenting near the 43S peak (Fig. 2B and D). This has been previously observed in other ribosome assembly mutants defective in propagating the killer toxin-encoding M1 satellite double-stranded RNA of the L-A virus (48).
FIG 2.
Deletion of Pwp1 affects production of 60S ribosomal subunits. (A to D) Polysome profiles of wild-type and pwp1Δ strains grown at either 30°C (A and B) or shifted to 18°C for 4 h (C and D). (E and F) Polysome profiles of the GAL-HA-PWP1 strain either grown in YEPGal (E) or shifted to YEPGlu (B) for 16 h. Half-mer polyribosomes (polysomes containing a 43S initiation complex bound to an AUG start codon in mRNA without a corresponding 60S subunit) are indicated by small arrows.
Together, these results show that like Nop12, Pwp1 is a nonessential protein that functions in 60S ribosome biogenesis and that deletion of Pwp1 causes cold sensitivity.
Pwp1 is required for efficient processing of 27SA2 and 27SA3 pre-rRNAs.
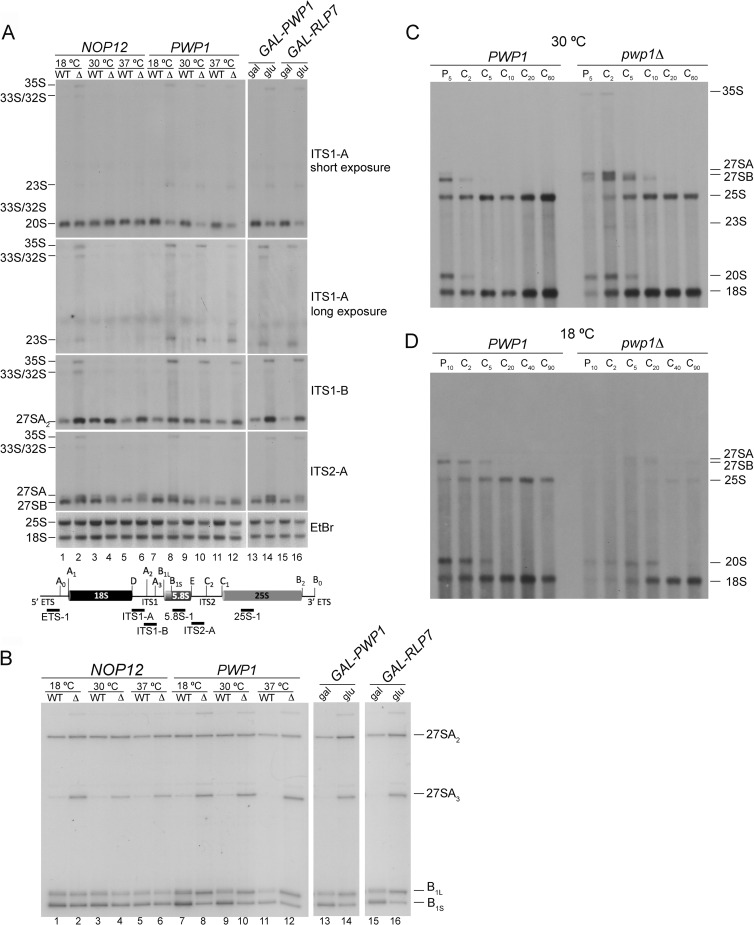
Recently, it was shown that Nop12 is required for processing of 27SA3 pre-rRNA (26). To determine in which step of production of 60S ribosomal subunits Pwp1 functions, we assayed pre-rRNA processing in wild-type cells and pwp1Δ cells grown at either 30°C or shifted to 18°C or 37°C for 4 h. We also examined pre-rRNA processing in the GAL-PWP1 strain either grown at 30°C in YEPGal or shifted to YEPGlu for 16 h to deplete Pwp1. For controls, we compared the defects observed in the absence of Pwp1 to the pre-rRNA processing defects observed in the absence of Nop12 or another A3 factor, Rlp7 (32, 33, 35). Deletion or depletion of Pwp1 resulted in pre-rRNA processing defects similar to those caused by deletion of NOP12 or depletion of Rlp7 (Fig. 3). Consistent with the extremely slow growth phenotype of the pwp1Δ strain, the extent of the pre-rRNA processing defects in the absence of Pwp1 was nearly identical to that in the absence of the essential A3 factor Rlp7 and stronger than that in the absence of Nop12. Furthermore, the observed effects on pre-rRNA processing in the absence of Pwp1 were exacerbated when pwp1Δ cells were grown at 18°C, consistent with the cold-sensitive growth phenotype.
FIG 3.
Pwp1 functions in processing of 27SA2 and 27SA3 pre-rRNAs. (A and B) Steady-state levels of pre-rRNAs and rRNAs extracted from wild-type (WT), pwp1Δ, GAL-HA-PWP1, nop12Δ, and GAL-HA-RLP7 strains assayed by Northern blotting (A) and primer extension (B). The nop12Δ and GAL-HA-RLP7 strains serve as controls of previously characterized mutants defective in processing 27SA3 pre-rRNA. Due to overexposure, 20S pre-rRNA was cropped from the “ITS1-A, long exposure” panel in panel A for clarity. B1S and B1L in panel B refer to the 5′ end of 27SBS plus 7SS and 27SBL plus 7SL, respectively. The positions of oligonucleotide probes used are indicated on the 35S pre-rRNA transcript (A). EtBr, ethidium bromide. (C and D) Kinetics of synthesis and turnover of pre-rRNAs extracted from wild-type and pwp1Δ strains assayed by pulse-chase analysis. Strains were grown at 30°C (C) or shifted to 18°C for 4 h (D). P, pulse time; C, chase time.
The absence of Pwp1 resulted in a marked reduction in the levels of 25S rRNA relative to 18S rRNA (Fig. 3A, lanes 7 to 14). Northern blotting and primer extension revealed an accumulation of 35S, 33S/32S, 27SA2, 27SA3, and 27SBL pre-rRNAs (Fig. 3A and B, lanes 7 to 14). The accumulation of 27SA3 pre-rRNA was accompanied by a corresponding decrease in 27SBs pre-rRNA, suggesting that either 27SA3 pre-rRNA is not being processed or 27SBs pre-rRNA is being turned over. Additionally, we observed a decrease in 7S pre-rRNAs and 5.8S rRNA (data not shown). We also observed an accumulation of 23S pre-rRNA, consistent with a delay in cleavage at sites A0, A1, and A2 (Fig. 3A and B, lanes 7 to 14). A delay in these initial cleavage events was also evident by a decrease in 20S pre-rRNA. Because 18S rRNA levels were relatively unaffected, it is likely that the 23S pre-rRNA generated in the absence of Pwp1 can be processed into 18S rRNA.
To better discern the effects on pre-rRNA processing in the absence of Pwp1, we assayed the kinetics of pre-rRNA synthesis and turnover by pulse-chase analysis. In wild-type cells at both 30°C and 18°C, nascent pre-rRNAs were rapidly converted into 27SA, 27SB, and 20S pre-rRNAs, and these pre-rRNAs were ultimately processed to 25S and 18S rRNAs. In contrast, deletion of PWP1 resulted in a delay in the processing of pre-rRNA intermediates and formation of mature rRNAs (Fig. 3C and D). The levels of 25S rRNA from the pwp1Δ strain grown at 30°C were lower than those in wild-type cells, while 18S rRNA appeared to be relatively unaffected. This was accompanied by an accumulation of 35S, 27SA, and 27SB pre-rRNAs compared to the wild type (Fig. 3C). There was also a slight accumulation of 23S pre-rRNA and a mild delay in processing of 20S pre-rRNA. Notably, at 18°C, deletion of PWP1 caused 27S pre-rRNAs to be turned over, and thus resulted in no 25S rRNA being produced (Fig. 3D). This is not simply a result of a failure to incorporate label into the RNA at 18°C, because we observed the formation of 20S pre-rRNA and 18S rRNA, although at slightly smaller amounts than in wild-type cells. These results are strikingly similar to pulse-chase analyses upon depletion of r-protein L8, a member of the same subcomplex, or of other essential A3 factors or r-proteins that are required for 27SA2 and 27SA3 processing (28, 32, 33, 49).
Taken together, these results demonstrate that Pwp1, like other subcomplex members, participates in early steps of 60S subunit biogenesis, including processing of 27SA2 and 27SA3 pre-rRNAs. Consistent with an early block in pre-rRNA processing, fluorescence microscopy revealed nucleolar accumulation of 66S preribosomes in the absence of Pwp1 (see Fig. S2 in the supplemental material). Similar to the deletion of NOP12, the effects on pre-rRNA processing were most evident when pwp1Δ cells were grown in the cold. Because both Nop12 and Pwp1 have domains proposed to bind RNA, the cold-sensitive phenotype suggests that in the absence of these proteins, pre-rRNAs may be misfolded or unable to undergo a conformational switch (50, 51). In the case of Pwp1, this may lead to turnover of misassembled preribosomes.
Pwp1 associates with 90S preribosomes and is exported to the cytoplasm.
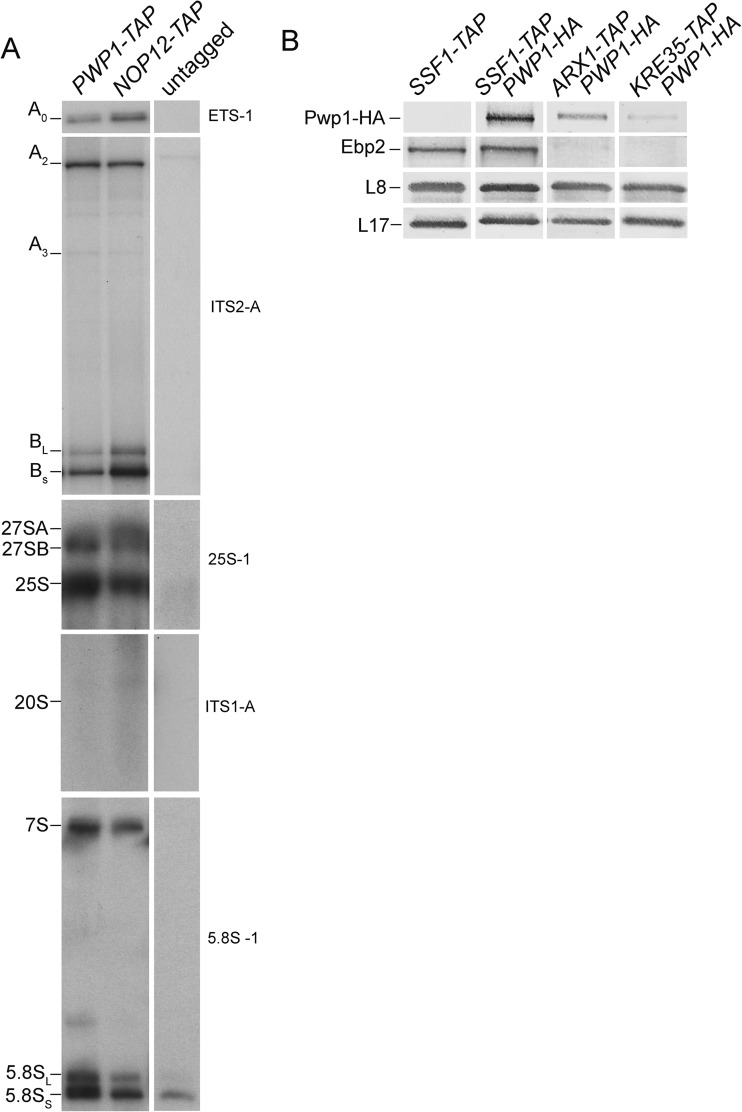
Previous work from our laboratory and others has shown that Ebp2, Brx1, Nop12, and r-protein L8 associate with preribosomes during early steps of 60S subunit assembly and coimmunoprecipitate (co-IP) early 66S pre-rRNA intermediates (25, 26, 28). To better understand when Pwp1 associates with preribosomes, we TAP tagged Pwp1 and purified preribosomes. For a control, we also purified preribosomes using Nop12 tagged with TAP (Nop12-TAP). Like Nop12, Pwp1 associated with preribosomes early in assembly; it co-IPed 27SA, 27SB, and 7S pre-rRNAs above the background amounts that we observed from an untagged strain. Neither Pwp1 nor Nop12 co-IPed 20S pre-rRNA (Fig. 4A) compared to an Enp1-TAP control (data not shown). Furthermore, Pwp1 co-IPed pre-rRNAs with 5′ ends corresponding to 35S pre-rRNA. Last, Pwp1 co-IPed appreciable amounts of mature 5.8S rRNA, suggesting that Pwp1 is associated with 66S preribosomes exported to the cytoplasm. Interestingly, each protein co-IPed slightly different ratios of each pre-rRNA intermediate. This may reflect slight differences in their timing of association or differences in their relative affinity for each of the consecutive preribosomal intermediates.
FIG 4.
Pwp1 associates with preribosomes in the nucleolus and is exported to the cytoplasm. (A) Pre-rRNAs and rRNAs that coimmunoprecipitate with PWP1-TAP and NOP12-TAP (control) were assayed by primer extension and Northern blotting. Detected pre-rRNAs, rRNAs, and pre-rRNA 5′ ends are indicated. B1S and B1L refer to the 5′ end of 27SBS plus 7SS and 27SBL plus 7SL, respectively. Oligonucleotides used are indicated (refer to Fig. 3A). (B) Western blotting for the presence of Pwp1-HA in preribosomes purified using TAP-tagged Ssf1 (nucleolar), Arx1 (nuclear), or Lsg1 (cytoplasmic). L17 serves as a loading control.
As an orthogonal approach to map the timing of association of Pwp1, we purified preribosomes using TAP-tagged Ssf1 (nucleolar), Arx1 (nucleoplasmic), and Lsg1 (cytoplasmic), and assayed by Western blotting for the presence of HA-tagged Pwp1 (52, 53). All three TAP-tagged proteins co-IPed Pwp1-HA (Fig. 4B), corroborating the results that Pwp1-TAP copurifies the earliest pre-rRNA intermediate, as well as mature 5.8S rRNA. Interestingly, we observed a gradual reduction of the amount of Pwp1-HA that copurified with Arx1-TAP and Lsg1-TAP. This suggests that like some other assembly factors, dissociation of Pwp1 from preribosomes might not simply occur at a discrete interval but instead gradually as assembly progresses (54). Alternatively, association of Pwp1 may weaken as assembly proceeds, leading to progressively lower yields when co-IPed with late particles.
Thus, we conclude that like other members of the Pwp1 subcomplex, Pwp1 associates with preribosomes early during biogenesis. Our results indicate that as assembly progresses and particles transition from the nucleus to the cytoplasm, Pwp1 is released from preribosomes (Fig. 1B).
Pwp1 is important for stable association with preribosomes of assembly factors required for 27SA3 pre-rRNA processing.
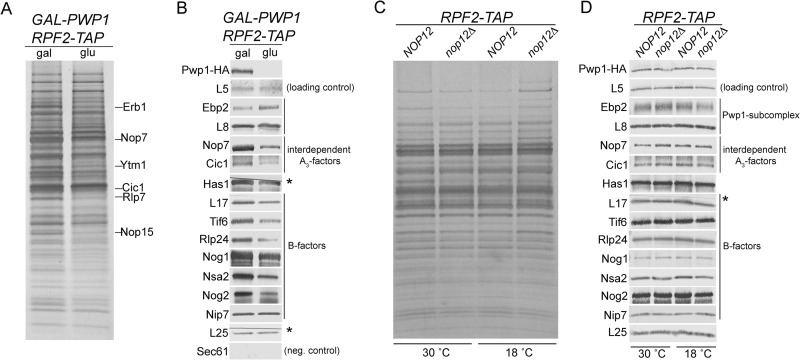
Work from our lab has established a hierarchy in which the Pwp1 subcomplex members Ebp2 and Brx1 are required for stable association of six interdependent A3 factors (Nop7, Erb1, Ytm1, Cic1, Rlp7, and Nop15) with preribosomes, which in turn are necessary for stable association of two DEAD box proteins (DBPs), Has1 and Drs1 (28, 34, 49) (J. Talkish, unpublished data). To better understand the relationship between association of Pwp1 and assembly of other proteins required for 27SA3 processing, we investigated changes in preribosome composition in the absence of Pwp1. Because the extremely slow growth phenotype of the pwp1Δ strain made it difficult to grow large volumes of cells, preribosomes were purified from a GAL-HA-PWP1 strain (as described above). After shifting cells for 16 h to glucose-containing medium, HA-Pwp1 was undetectable by Western blotting (Fig. 5B; see Fig. S1B in the supplemental material).
FIG 5.
Changes in composition of preribosomes in the absence of Pwp1 and Nop12. (A) Preribosome components purified from the GAL-HA-PWP1 RPF2-TAP strain that was grown in YEPGal or shifted to YEPGlu for 16 h were resolved by SDS-PAGE and visualized by silver staining. Stained polypeptide bands containing the interdependent A3 factors are indicated. (B) Preribosome constituents purified in panel A were assayed by Western blotting. Sec61 serves as a negative control for a protein not copurified using Rpf2-TAP. (C) Preribosomes were purified from wild-type and nop12Δ strains using Rpf2-TAP from cells either grown at 30°C or shifted to 18°C for 4 h. Preribosome constituents were resolved by SDS-PAGE and visualized by silver staining. (D) Preribosomes purified in panel C were assayed by Western blotting. Cuts in the nitrocellulose membrane are indicated with an asterisk in panels B and D.
Preribosome constituents were resolved by SDS-PAGE and visualized by silver staining (Fig. 5A). Previous mass spectrometry analysis from our lab has identified many of the polypeptides present in each of the gel bands (7). Depletion of Pwp1 resulted in a characteristic pattern of silver-stained bands similar to depletion of other A3 factors (Fig. 6) (25, 28, 34). Specifically, there was an observable decrease in gel bands corresponding to the interdependent A3 factors Erb1, Nop7, Ytm1, Cic1, Rlp7, and Nop15 (Fig. 5A), suggesting that Pwp1 is important for stable association of these six interdependent proteins with preribosomes.
FIG 6.
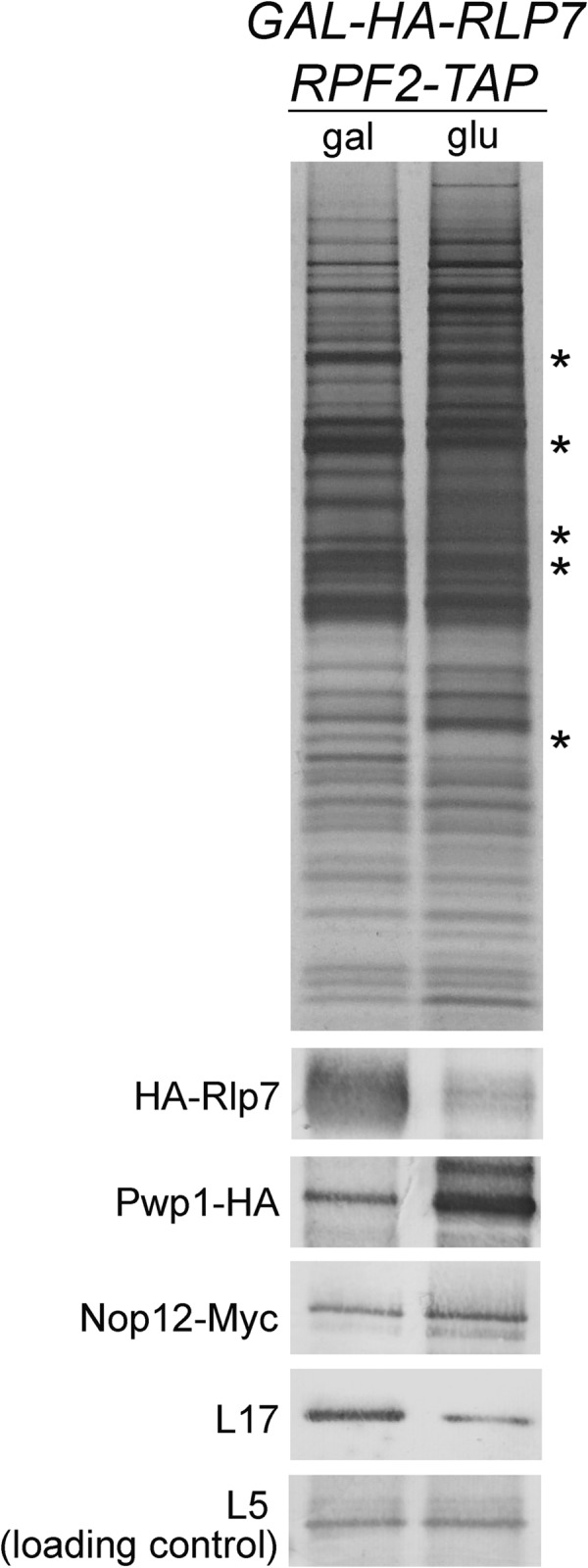
Pwp1 and Nop12 are not dependent on the interdependent A3 factors (asterisks) for association with preribosomes. Preribosome components purified from the GAL-RLP7 RPF2-TAP PWP1-HA strain or GAL-RLP7 RPF2-TAP NOP12-13MYC strain were resolved by SDS-PAGE and visualized by silver staining or subjected to Western analysis after transferring to a nitrocellulose membrane. r-protein L5 serves as a loading control. Pwp1-HA and Nop12-Myc were detected with anti-HA antibody (12CA5) and anti-myc antibody (9e10), respectively.
Western blotting confirmed that in the absence of Pwp1, Nop7 and Cic1 failed to stably associate with preribosomes (Fig. 5B). Consistent with this, we also observed a decrease in the DEAD box protein Has1. Additionally, the levels of several AFs (Tif6, Rlp24, Nog1, Nsa2, and Nog2) and r-protein L17 that function in the next step of ribosome biogenesis were decreased (B factors [Fig. 5B]) (55–59). These proteins have all been shown to be dependent on the other A3 factors to stably bind preribosomes (28, 34, 49) (Talkish, unpublished). In summary, Pwp1 is important for stable association of a group of interdependent A3 factors that function in the same step of pre-rRNA processing, as well as B factors that function in the following step.
To begin to understand the interdependence of the six proteins in the Pwp1 subcomplex, we used available antibodies to assay changes in the levels of Ebp2 and r-protein L8 in preribosomes purified in the presence or absence of Pwp1. Western blotting revealed that upon depletion of Pwp1, both Ebp2 and r-protein L8 associated with preribosomes at wild-type levels (Fig. 5B). We also wanted to test for changes in the levels of Nop12 but were unable to obtain positive transformants after numerous attempts to tag Nop12 with Myc in the GAL-HA-PWP1 strain. However, we consider it likely that Nop12 fails to associate with particles in the absence of Pwp1, because in vivo chemical probing of pre-rRNA structure in the absence of Pwp1 revealed many of the same changes in RNA structure as those in a nop12Δ strain (see Fig. 7). Furthermore, many of the changes in the absence of Pwp1 were at or near the known Nop12 cross-linking site, likely reflecting the failure of Nop12 to associate with preribosomes. These results indicate that Pwp1 is not required to recruit the subcomplex proteins Ebp2 and L8 and suggests a hierarchy in which Pwp1, like Ebp2 and L8, stably associates with preribosomes prior to the interdependent A3 factors (28, 34, 60).
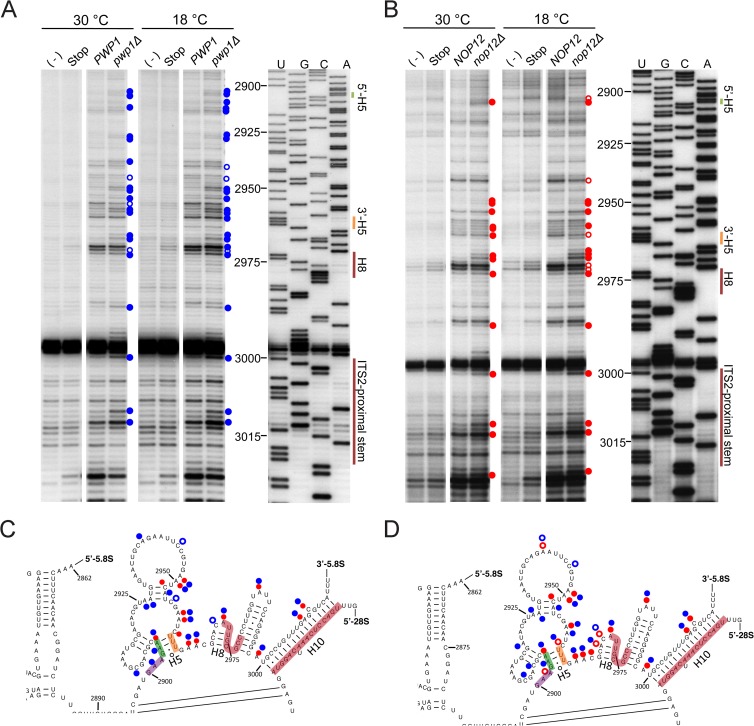
FIG 7.
Pwp1 and Nop12 are important for proper folding of 5.8S rRNA. (A and B) In vivo DMS probing of wild-type, pwp1Δ, and nop12Δ strains visualized by primer extension using an oligonucleotide complementary to the 5′ half of ITS2. The corresponding sequencing lanes (U, G, C, and A) with nucleotide positions are shown. Controls include a no-DMS control [(−) lanes] and a Stop control in which β-mercaptoethanol is added to cells prior to DMS. Nucleotides with enhanced DMS modification in the absence of Nop12 and Pwp1 are indicated with solid red and blue circles, respectively. Nucleotides with decreased DMS modification in the absence of Nop12 and Pwp1 are represented with hollow red and blue circles, respectively. (C and D) Changes in nucleotide reactivity at 30°C (C) and 18°C (D) are indicated on the secondary structure of 5.8S rRNA as described above for panel A (77). The Nop12 cross-linking sites are highlighted in red. The 5′ and 3′ halves of helix 5 (H5) are highlighted in green and orange, respectively. Nucleotides that have the potential to form an alternative helix 5 are shown in purple.
The composition of preribosomes is largely unaffected in the absence of Nop12.
To investigate changes in preribosome composition in the absence of Nop12, we purified preribosomes from wild-type and nop12Δ strains grown at either 30°C or shifted to 18°C for 4 h. SDS-PAGE revealed few changes in the levels of silver-stained gel bands, indicating that preribosomes are largely intact in the absence of Nop12 (Fig. 5C). The pattern of silver-stained bands is clearly different from that observed in the absence of Pwp1 or other essential A3 factors (Fig. 5A and Fig. 6). Western blotting confirmed that, even upon shifting the nop12Δ strain to 18°C, there were no significant changes in the levels of the interdependent A3 factors, the DBP Has1, or the B factors tested (Fig. 5D). This is especially interesting because to our knowledge, this is the first example of a ribosome assembly mutant in which the known A3 factors are associated with preribosomes, but 27SA3 pre-rRNA is not efficiently processed.
To test the requirement of Nop12 for association of other members of the Pwp1 subcomplex, we examined the levels of Ebp2, Pwp1-HA, and r-protein L8 by Western blotting (Fig. 5D). We observed no changes in the levels of these proteins, even from preribosomes purified from nop12Δ cells shifted to the nonpermissive temperature of 18°C (Fig. 5D). These results suggest that Ebp2, Pwp1, and L8 either associate with preribosomes prior to Nop12 or that their association is independent of Nop12.
Taken together, these data suggest that Nop12, although important for processing of 27SA3 pre-rRNA, is not directly involved in recruiting other proteins that function during this step of ribosome biogenesis. In the absence of Nop12 at 18°C, processing of 27SA3 pre-rRNA is blocked at levels comparable to the absence of the essential A3 factors (Fig. 3A and B), yet the protein composition of preribosomes is largely unaffected. Furthermore, this is the first example of a strong block in 27SA3 processing that does not lead to significant turnover of pre-rRNAs (data not shown) (35). This suggests that the observed defects in pre-rRNA processing in the absence of Nop12 are not due to an incomplete complement of proteins, but rather the pre-rRNA may not be in a conformation necessary to facilitate efficient removal of ITS1.
Pwp1 and Nop12 are not dependent on the A3 factors for stable association with preribosomes.
It was previously shown that the Pwp1 subcomplex members Ebp2, Brx1, and L8 were able to stably associate with preribosomes in the absence of the interdependent A3 factors (34). To test the requirement of the A3 factors for stable binding of Pwp1 and Nop12, we HA tagged Pwp1 and Myc tagged Nop12 in an Rpf2-TAP strain conditional for expression of the A3 factor Rlp7. Upon depletion of Rlp7, SDS-PAGE and silver staining of affinity-purified preribosomes revealed the characteristic pattern of gel bands seen upon depletion of any one of the A3 factors (Fig. 6, asterisks). Western blotting demonstrated that in the absence of Rlp7, both Pwp1 and Nop12 remained stably associated with preribosomes (Fig. 6). The observed increase of Pwp1 and Nop12 in the absence of Rlp7 most likely represents the enrichment of early preribosomes, and thus these two proteins.
Together, these and the above data suggest a hierarchy of association in which the Pwp1 subcomplex associates with preribosomes prior to stable binding of the interdependent A3 factors. Nevertheless, association of Nop12 occurs independently of the other A3 factors. Nop12 is able to associate in the absence of these proteins, and conversely, they stably associate when Nop12 is not present. In contrast, Pwp1 is important for stable recruitment into preribosomes of the interdependent A3 factors.
Pwp1 and Nop12 are important for proper folding of 5.8S rRNA.
Pwp1 and Nop12 both contain domains predicted to bind RNA, and Nop12 was shown to cross-link helices 8 and 10 (ITS2-proximal stem) of 5.8S rRNA (26, 35, 36). Furthermore, the absence of either of these proteins results in cold sensitivity, a phenotype often associated with ribonucleoprotein particles (RNPs) in which the RNA is misfolded or unable to undergo a structural transition (50, 61). Thus, we hypothesized that these two proteins might be important for structuring 5.8S rRNA and neighboring domain I of 25S rRNA. To investigate this, we used the small chemical dimethyl sulfate (DMS) to perform in vivo structure probing of pre-rRNAs in the presence or absence of Pwp1 and Nop12 (44, 62). DMS methylates the nitrogenous rings of adenines and cytosines in a manner that impedes reverse transcriptase, which can be visualized as primer extension stops (63). The extent of methylation is dependent upon the environment of each base: nucleotides that are base paired, folded into complex structures, or protected by proteins exhibit low DMS reactivity, whereas unpaired, solvent-accessible bases exhibit high DMS reactivity. To address the roles that Pwp1 and Nop12 play in structuring 5.8S rRNA, we treated wild-type, pwp1Δ, and nop12Δ cells with DMS at 30°C and 18°C. The modified RNA was assayed by primer extension with an oligonucleotide complementary to the 5′ half of ITS2. Using an oligonucleotide in ITS2 ensures that we are assaying the structure of 5.8S rRNA sequences in pre-rRNAs and not mature 5.8S rRNA.
Deletion of Pwp1 at both 30°C and 18°C resulted in differential DMS reactivity of a number of nucleotides in the 3′ half of pre-5.8S rRNA (Fig. 7A, C, and D; see Fig. S3A in the supplemental material). These were highly specific to this RNP neighborhood, as analysis of ITS2 revealed no changes in DMS reactivity (data not shown). The majority of modifications were the same in the absence of Pwp1 at both 30°C and 18°C; however, a greater number of changes were seen at 18°C, consistent with the cold-sensitive phenotype of the pwp1Δ strain. The majority of differentially modified nucleotides exhibited increased modification and lie in single-stranded regions, likely reflecting the footprint of Pwp1, Nop12, and/or the other A3 factors and r-proteins that do not stably associate in the absence of Pwp1. A number of these increased modifications occurred at or near the Nop12 cross-linking site, suggesting that Nop12 also fails to associate with preribosomes in the absence of Pwp1 (Fig. 7C and D). We also observed increased modification of nucleotides within helix 5 (A2905 and A2904), helix 7 (A2953), and the ITS2-proximal stem (A3010), suggesting at least two possibilities. (i) Pwp1 aids in forming these structures during assembly, and in its absence, these helices form less stably. (ii) Deletion of Pwp1 impedes assembly at a step before these helices stably form, and their absence enriches for a population of intermediates prior to the formation of these helices. However, because a number of other assembly factors are diminished from preribosomes in the absence of Pwp1, it is difficult to differentiate direct from indirect effects. This is further complicated by turnover of pre-rRNAs in the absence of Pwp1. We also observed a few nucleotides with decreased modification, suggesting that these nucleotides potentially become base paired in the absence of Pwp1.
Because preribosomes are largely intact in the absence of Nop12, the observed DMS modification patterns in its absence should be highly specific. Similar to the absence of Pwp1, deletion of Nop12 resulted in increased modification of nucleotides at or near the Nop12 cross-linking sites and within helices 5 and 7 and the ITS2-proximal stem (Fig. 7B to D; see Fig. S3B in the supplemental material). Interestingly, we observed nucleotides with decreased reactivity to DMS (A2970 and C2971 in helix 8), but only at 18°C. We also observed decreased DMS modification of nucleotides A2902, A2940, and C2960, indicating that these nucleotides are becoming more protected, possibly through base pairing. In mature 60S subunits, A2902 is single stranded and immediately 5′ of helix 5 of 5.8S rRNA. Helix 5 is three base pairs in length and is formed by pairing of G2903, A2904, and A2905 (Fig. 7C and D, green) with U2963, U2962, and U2961 (Fig. 7C and D, orange), respectively. Interestingly, this sequence (GAA), composing the 5′ half of helix 5, is repeated immediately 5′ of helix 5 (referred to as GAA*). G2900, A2901, and A2902 (Fig. 7C and D, purple) have the potential to base pair with U2963, U2962, and U2961, respectively. This would also allow the formation of three additional interactions between U2899-G2964, G2903-C2960, and A2904-U2959, resulting in a helix extended by 3 bp. The observed pattern of DMS modification at 18°C suggests that in the absence of Nop12, an extended helix 5 is formed by base pairing of GAA* to U2963, U2962, and U2961.
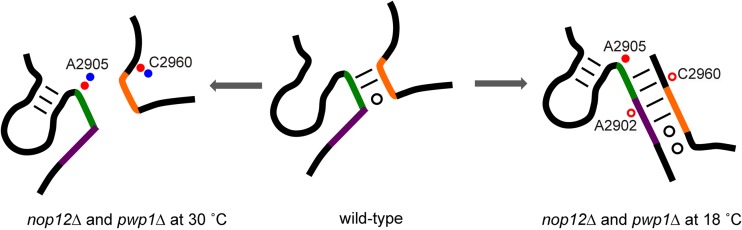
Together, our data suggest the following. (i) In the absence of either protein at 30°C, helix 5 is not stably formed, as evident by increased DMS modification of A2904 and A2905 (Fig. 8, left). (ii) In the absence of Nop12 at 18°C, an alternative yet unproductive helix 5 is formed by pairing of GAA* to the 3′ UUU. This accounts for the decreased DMS modification we observe for A2902 and C2960, and the increased modification of A2905 (Fig. 8, right). Although we observed nucleotides with decreased modification in the absence of Pwp1 at both 30°C and 18°C, they were not entirely consistent with an alternative helix 5. One potential reason is that the formation of this alternative helix requires stable association of the interdependent A3 factors, present only in strains lacking Nop12 but not Pwp1. Furthermore, this alternative helix might not be as evident due to the extensive turnover of pre-rRNAs in the absence of Pwp1 at 18°C.
FIG 8.
Helix 5 of 5.8S rRNA is misfolded in the absence of Pwp1 and Nop12. (A) Model of structural changes that occur in helix 5 in the absence of Nop12 and Pwp1 at 30°C and 18°C. In wild-type cells, helix 5 is formed by base pairing between sequences in green and orange (middle). In the absence of Nop12 and Pwp1, at 30°C, helix 5 is not stably formed, as evident by increased DMS modification of A2905 (left). Decreased modification of A2902 and C2960 indicated that in the absence of Nop12 and Pwp1 at 18°C, an alternative helix 5 is formed between the upstream GAA* (purple) and the 3′ UUU (orange) (right). Changes in DMS reactivity are shown as described in the legend to Fig. 7.
DISCUSSION
Processing of 27SA2 and 27SA3 pre-rRNAs, and subsequent generation of the 5′ end of 5.8S rRNA, requires at least 12 AFs and 10 r-proteins (8, 25, 27, 28, 31–33, 35, 64–68). Numerous physical and genetic interactions exist among these proteins, suggesting that they function in a highly cooperative manner to remove ITS1. At least 12 of these proteins can be isolated in one of three subcomplexes. These subcomplexes include (i) the Pwp1 subcomplex (6, 24); (ii) the Nop7 subcomplex composed of Nop7, Erb1, and Ytm1 (8); and (iii) the ITS2 subcomplex containing Cic1, Nop15, and Rlp7 (30).
Work from our lab has shown that these three subcomplexes assemble into preribosomes in a coordinated and hierarchical manner. We have previously shown the following. (i) Binding of Ebp2 is required for association of Brx1 (25). (ii) Both Ebp2 and Brx1 are required for binding of r-protein L8 (25). (iii) L8 is required for binding of the interdependent A3 factors Nop7, Erb1, Ytm1, Cic1, Nop15, and Rlp7 (28). (iv) All of the aforementioned proteins are required for stable association of the DBPs Drs1 and Has1 (34, 49) (Talkish, unpublished). In the absence of any of these proteins, ITS1 is not removed, and preribosomes are unstable and turned over.
Here we have begun to analyze the function of the previously uncharacterized AF Pwp1 and further investigated the A3 factor Nop12. We show that like Nop12, Pwp1 is a nonessential protein that functions in production of 60S ribosomal subunits. Although cells are viable in the absence of Pwp1, their growth is severely compromised at 30°C, and they are inviable at low temperatures. Like other members of the Pwp1 subcomplex, Pwp1 associates with preribosomes early in biogenesis and is necessary for efficient processing of 27SA2 and 27SA3 pre-rRNAs. We have been able to further refine the association hierarchy of proteins that function in this step of assembly by showing that Pwp1 is upstream of the interdependent A3 factors; Pwp1 is important for stable association of these proteins but not vice versa. Nop12, however, does not play an active role in recruiting other A3 factors. Last, we show that Nop12 and Pwp1 are required for proper folding of 5.8S rRNA and the ITS2-proximal stem. In the absence of these proteins, particularly when cells are grown at low temperature, 5.8S rRNA sequences within pre-rRNAs are misfolded, and 27SA3 pre-rRNA is unable to be efficiently processed.
In most of our assays, deletion of Pwp1 results in the same phenotype as deletion of Nop12, only stronger. The nop12Δ strain behaves like a traditional cold-sensitive mutant, growing at nearly wild-type rates at 30°C but much more slowly when cells are shifted to 18°C. However, deletion of Pwp1, although nonlethal, results in an extremely slow growth phenotype at 30°C and lethality at 18°C. Pwp1 is a WD-40 protein, whereas Nop12 is a RNA recognition motif (RRM)-containing RNA binding protein (35, 36). Pwp1, through its WD-40 motif, may participate in a greater number of interactions with the interdependent A3 factors. Thus, in the absence of Pwp1, one might expect greater perturbations in preribosome structure and thus stronger assembly defects and growth phenotypes. Consistent with this, examination of preribosomes revealed no changes in the interdependent A3 factors in the absence of Nop12 but decreased association of those proteins in the absence of Pwp1. Our recent investigations of A3 factors show that pre-rRNAs are rapidly turned over in their absence; however, it was unclear if this results from a failure to process 27SA3 pre-rRNA or because the A3 factors fail to associate with preribosomes (28, 34, 49). Interestingly, pre-rRNAs are not turned over in the absence of Nop12 (35) (data not shown), suggesting that turnover is a result of an incomplete set of assembly factors in other A3 mutants and not simply due to unprocessed 27SA3 pre-rRNA.
High-resolution crystal structures of the yeast ribosome, as well as newly developed protein-RNA cross-linking approaches, indicate that the Pwp1, Nop7, and ITS2 subcomplexes bind preribosomes adjacent to each other, near domain I of 25S/5.8S rRNA and ITS2 (Fig. 1C). Using UV cross-linking and analysis of cDNA (CRAC), it was shown that Nop12 cross-links to pre-rRNA sequences within the ITS2-proximal stem as well as helix 8 at the 5′ end of 5.8S rRNA (26). This is in close proximity to the binding sites of r-proteins L8 and L15 in mature 60S subunits (29). Two-hybrid interactions between Ebp2 and Brx1 or Nop12 suggest that Brx1 and Ebp2 also occupy this neighborhood (25). The Nop7 subcomplex is thought to lie adjacent to the Pwp1 subcomplex. CRAC analysis revealed that Erb1 and Nop7 cross-link to sequences in domain I and domain III of 25S rRNA, respectively (26). Last, Cic1, Nop15, and Rlp7 cross-link to sequences in ITS2 and the ITS2-proximal stem (26, 30, 69).
Interestingly, these proteins associate with preribosomes near the 3′ end of 5.8S rRNA, yet they are necessary for the removal of ITS1 and generation of the 5′ end of 5.8S rRNA. In mature 60S subunits, the 5′ end of 5.8S rRNA is situated ∼160 Å from the 3′ end. Depletion of the AFs that cluster around the 3′ end of 5.8S rRNA and/or bind to ITS2 causes ITS2 to misfold but results in a failure to remove ITS1 (26, 30–33, 67). These results highlight two key principles that drive processing of 27SA3 pre-rRNA. (i) Proper folding of ITS2 is at least in part a prerequisite for removal of ITS1. (ii) Long-range communication between ITS1 and ITS2 coordinates the removal of these two spacers, and the subsequent generation of the 5′ and 3′ ends of 5.8S rRNA.
Pioneering studies of bacterial ribosome biogenesis from Guthrie and coworkers used genetic screens to identify cold-sensitive mutants defective in ribosome assembly in vivo (51). They rationalized that because in vitro assembly requires heating the reaction at an intermediate step, presumably to cause structural rearrangements of the assembling particle, ribosome assembly in vivo would also be temperature dependent. Thus, defects in ribosome assembly might be exacerbated at low temperatures. The temperature-dependent nature of ribosome assembly reflects the ability of RNA to fold into a myriad of secondary and tertiary structures. As RNA folds, it samples a number of different conformations as it transitions to its native, functional state (70). Many of these conformations are nonfunctional and represent kinetic traps that must be overcome, especially at low temperatures. During ribosome biogenesis, these kinetic traps and energy barriers are thought to be overcome in part by AFs and r-proteins (2, 71).
Although nonessential, deletion of Nop12, especially in the cold, results in the accumulation of unprocessed 27SA3 pre-rRNAs to an extent similar to that of depletion of the essential A3 factors (Fig. 3A and B, compare to GAL-RLP7). Our data suggest that the defects observed in 27SA3 pre-rRNA processing in the absence of Nop12 are not due to an incomplete inventory of AFs and r-proteins but rather are due to misfolded RNA. Analysis of preribosomal particles in the absence of Nop12 revealed few changes in their composition, suggesting that any changes in RNA structure should be highly specific to the absence of Nop12. This is in contrast to depletion of Pwp1 or the essential, interdependent A3 factors that cause a number of changes in preribosome composition, making RNA structure probing studies more difficult to interpret.
In this study, we show that Pwp1 and Nop12 function to assist in folding 5.8S rRNA. In vivo structure probing of pre-rRNAs revealed that in the absence of these proteins, 5.8S sequences in pre-rRNAs are misfolded. The changes we observed in pre-rRNA structure are highly specific to the absence of Pwp1 and/or Nop12 and appear to be localized to the RNP neighborhood in which they bind. Of particular interest is helix 5. In mature 60S subunits, this 3-bp helix is generated by base pairing of a 5′ AAG to a 3′ UUU (Fig. 7C and D, green and orange). Our results suggest that in the absence of Pwp1 or Nop12, at 30°C, helix 5 is not stably formed. This was evident by increased DMS accessibility to A2905 of the 5′ half of helix 5 (Fig. 7B and C). In addition, in the absence of Nop12 at 18°C, our DMS probing data are consistent with the formation of an alternative helix 5 (Fig. 7B and D). This alternative helix 5 is generated by base pairing of an AAG (AAG*), repeated immediately upstream of the AAG that composes helix 5, to the 3′ UUU. Base pairing of the AAG* to the 3′ UUU would allow the original AAG to participate in additional base pairs, resulting in an alternative helix 5 that is extended by 3 bp (Fig. 8). These results are reminiscent of work from Dammel and Noller (50), which showed that a mutation in 16S rRNA that confers cold sensitivity is due to competition between two helices, only one of which is productive. What remains to be determined is whether the alternative helix 5 is a folding intermediate that is on the pathway and that is normally resolved with the assistance of Nop12 or whether this is simply a kinetic trap that occurs at low temperature.
In mature subunits, helix 5 is in close proximity to a number of r-proteins surrounding the polypeptide exit tunnel (r-proteins L17, L26, L35, and L37). While these proteins associate with preribosomes early during assembly, they are not stably incorporated into preribosomes until after removal of ITS1 (68). Rearrangement of pre-rRNA structures during 27SA3 processing might allow these r-proteins to participate in a greater number of protein-RNA interactions and thus integrate more tightly into the ribosome structure. Thus, a more interesting possibility is that the alternative helix 5 is a normally occurring structure that is refolded before or during 27SA3 processing to form the helix 5 observed in the mature 60S subunits and to help tighten the association of some of the surrounding r-proteins. Our working hypothesis is that during and immediately after removal of ITS1, 5.8S rRNA undergoes a series of conformational changes. These conformational changes achieve two goals: (i) efficient removal of ITS1 (and possibly ITS2) and (ii) stable integration of the r-proteins surrounding the exit tunnel.
The complex network of interactions among the A3 factors, as well as their known locations within the preribosome suggest long-range communication between the 5′ and 3′ ends of 5.8S rRNA. Because the removal of spacer sequences is an irreversible process, their excision from pre-rRNAs is thought to act as checkpoints during ribosome biogenesis. By coupling the removal of two spacers (ITS1 and ITS2), the cell may have evolved additional layers of regulation, ensuring that both spacers are competent for removal before the first spacer is excised. Our in vivo RNA structure probing data show that relatively small perturbations in rRNA structure are sufficient to disrupt pre-rRNA processing and spacer removal. This is not surprising, since RNA folding is inherently cooperative, ensuring that RNAs proceed down a smooth folding landscape to form their native structures (2, 72–74). We speculate that the long-range communication between the two ends of 5.8S rRNA is facilitated by an intricate network of protein-protein and protein-RNA interactions between the Pwp1, Nop7, and the ITS2 subcomplexes. The absence of Nop12 and Pwp1 results in a weakening, but not complete loss, of some of these interactions and a breakdown in communication between the 5′ and 3′ ends of 5.8S rRNA. Although the number of changes observed in the secondary structure of pre-rRNAs was small in the absence of Nop12 or Pwp1, we believe that these changes are highly specific and could have profound consequences on the stability of tertiary interactions within 5.8S rRNA. In vitro folding studies of ribozymes have shown that the loss of a single base pair or disruption of a single tertiary interaction can have drastic results and cause global misfolding of RNA (74–76).
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by NIH grant GM28301 to J.L.W. and by funds from the David Scaife Family Charitable Foundation (J.L.W. and J.J.). J.T. was supported by the Richard King Mellon Foundation Presidential Graduate Fellowship in the Life Sciences and the Semon H. Stupakoff Scholarship.
We thank the following people for generously providing antibodies: Jesús de la Cruz and Patrick Linder (Has1), Janine Maddock (Nog1), David Goldfarb (Nip7), Cosmin Saveanu and Micheline Fromont-Racine (Tif6, Rlp24, Nsa2, and Nog2), Michael McAlear (Ebp2), Arlen Johnson (rpL8), Sabine Rospert (rpL17), Katja Siegers (rpL25), and Elizabeth Tosta (Cic1). We also thank Ed Hurt for providing the L25 eGFP Nop1-mRFP plasmid and Haibing Teng of the Molecular Biology Imaging Center at Carnegie Mellon University for training and assistance with microscopy.
Footnotes
Published ahead of print 17 March 2014
Supplemental material for this article may be found at http://dx.doi.org/10.1128/MCB.01322-13.
REFERENCES
- 1.Henras AK, Soudet J, Gerus M, Lebaron S, Caizergues-Ferrer M, Mougin A, Henry Y. 2008. The post-transcriptional steps of eukaryotic ribosome biogenesis. Cell. Mol. Life Sci. 65:2334–2359. 10.1007/s00018-008-8027-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shajani Z, Sykes MT, Williamson JR. 2011. Assembly of bacterial ribosomes. Annu. Rev. Biochem. 80:501–526. 10.1146/annurev-biochem-062608-160432 [DOI] [PubMed] [Google Scholar]
- 3.Karbstein K. 2007. Role of GTPases in ribosome assembly. Biopolymers 87:1–11. 10.1002/bip.20762 [DOI] [PubMed] [Google Scholar]
- 4.Kressler D, Hurt E, Bassler J. 2010. Driving ribosome assembly. Biochim. Biophys. Acta 1803:673–683. 10.1016/j.bbamcr.2009.10.009 [DOI] [PubMed] [Google Scholar]
- 5.Strunk BS, Karbstein K. 2009. Powering through ribosome assembly. RNA 15:2083–2104. 10.1261/rna.1792109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Krogan NJ, Peng WT, Cagney G, Robinson MD, Haw R, Zhong G, Guo X, Zhang X, Canadien V, Richards DP, Beattie BK, Lalev A, Zhang W, Davierwala AP, Mnaimneh S, Starostine A, Tikuisis AP, Grigull J, Datta N, Bray JE, Hughes TR, Emili A, Greenblatt JF. 2004. High-definition macromolecular composition of yeast RNA-processing complexes. Mol. Cell 13:225–239. 10.1016/S1097-2765(04)00003-6 [DOI] [PubMed] [Google Scholar]
- 7.Zhang J, Harnpicharnchai P, Jakovljevic J, Tang L, Guo Y, Oeffinger M, Rout MP, Hiley SL, Hughes T, Woolford JL., Jr 2007. Assembly factors Rpf2 and Rrs1 recruit 5S rRNA and ribosomal proteins rpL5 and rpL11 into nascent ribosomes. Genes Dev. 21:2580–2592. 10.1101/gad.1569307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miles TD, Jakovljevic J, Horsey EW, Harnpicharnchai P, Tang L, Woolford JL., Jr 2005. Ytm1, Nop7, and Erb1 form a complex necessary for maturation of yeast 66S preribosomes. Mol. Cell. Biol. 25:10419–10432. 10.1128/MCB.25.23.10419-10432.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nissan TA, Galani K, Maco B, Tollervey D, Aebi U, Hurt E. 2004. A pre-ribosome with a tadpole-like structure functions in ATP-dependent maturation of 60S subunits. Mol. Cell 15:295–301. 10.1016/j.molcel.2004.06.033 [DOI] [PubMed] [Google Scholar]
- 10.Merl J, Jakob S, Ridinger K, Hierlmeier T, Deutzmann R, Milkereit P, Tschochner H. 2010. Analysis of ribosome biogenesis factor-modules in yeast cells depleted from pre-ribosomes. Nucleic Acids Res. 38:3068–3080. 10.1093/nar/gkp1244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Karbstein K, Doudna JA. 2006. GTP-dependent formation of a ribonucleoprotein subcomplex required for ribosome biogenesis. J. Mol. Biol. 356:432–443. 10.1016/j.jmb.2005.11.052 [DOI] [PubMed] [Google Scholar]
- 12.Granneman S, Gallagher JE, Vogelzangs J, Horstman W, van Venrooij WJ, Baserga SJ, Pruijn GJ. 2003. The human Imp3 and Imp4 proteins form a ternary complex with hMpp10, which only interacts with the U3 snoRNA in 60-80S ribonucleoprotein complexes. Nucleic Acids Res. 31:1877–1887. 10.1093/nar/gkg300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dosil M, Bustelo XR. 2004. Functional characterization of Pwp2, a WD family protein essential for the assembly of the 90 S pre-ribosomal particle. J. Biol. Chem. 279:37385–37397. 10.1074/jbc.M404909200 [DOI] [PubMed] [Google Scholar]
- 14.Rashid R, Liang B, Baker DL, Youssef OA, He Y, Phipps K, Terns RM, Terns MP, Li H. 2006. Crystal structure of a Cbf5-Nop10-Gar1 complex and implications in RNA-guided pseudouridylation and dyskeratosis congenita. Mol. Cell 21:249–260. 10.1016/j.molcel.2005.11.017 [DOI] [PubMed] [Google Scholar]
- 15.Rosado IV, Dez C, Lebaron S, Caizergues-Ferrer M, Henry Y, de la Cruz J. 2007. Characterization of Saccharomyces cerevisiae Npa2p (Urb2p) reveals a low-molecular-mass complex containing Dbp6p, Npa1p (Urb1p), Nop8p, and Rsa3p involved in early steps of 60S ribosomal subunit biogenesis. Mol. Cell. Biol. 27:1207–1221. 10.1128/MCB.01523-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harnpicharnchai P, Jakovljevic J, Horsey E, Miles T, Roman J, Rout M, Meagher D, Imai B, Guo Y, Brame CJ, Shabanowitz J, Hunt DF, Woolford JL., Jr 2001. Composition and functional characterization of yeast 66S ribosome assembly intermediates. Mol. Cell 8:505–515. 10.1016/S1097-2765(01)00344-6 [DOI] [PubMed] [Google Scholar]
- 17.Milkereit P, Gadal O, Podtelejnikov A, Trumtel S, Gas N, Petfalski E, Tollervey D, Mann M, Hurt E, Tschochner H. 2001. Maturation and intranuclear transport of pre-ribosomes requires Noc proteins. Cell 105:499–509. 10.1016/S0092-8674(01)00358-0 [DOI] [PubMed] [Google Scholar]
- 18.Milkereit P, Strauss D, Bassler J, Gadal O, Kuhn H, Schutz S, Gas N, Lechner J, Hurt E, Tschochner H. 2003. A Noc complex specifically involved in the formation and nuclear export of ribosomal 40 S subunits. J. Biol. Chem. 278:4072–4081. 10.1074/jbc.M208898200 [DOI] [PubMed] [Google Scholar]
- 19.Talkish J, Zhang J, Jakovljevic J, Horsey EW, Woolford JL., Jr 2012. Hierarchical recruitment into nascent ribosomes of assembly factors required for 27SB pre-rRNA processing in Saccharomyces cerevisiae. Nucleic Acids Res. 40:8646–8661. 10.1093/nar/gks609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hierlmeier T, Merl J, Sauert M, Perez-Fernandez J, Schultz P, Bruckmann A, Hamperl S, Ohmayer U, Rachel R, Jacob A, Hergert K, Deutzmann R, Griesenbeck J, Hurt E, Milkereit P, Bassler J, Tschochner H. 2013. Rrp5p, Noc1p and Noc2p form a protein module which is part of early large ribosomal subunit precursors in S. cerevisiae. Nucleic Acids Res. 41:1191–1210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lebreton A, Saveanu C, Decourty L, Rain JC, Jacquier A, Fromont-Racine M. 2006. A functional network involved in the recycling of nucleocytoplasmic pre-60S factors. J. Cell Biol. 173:349–360. 10.1083/jcb.200510080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xue Y, Bai X, Lee I, Kallstrom G, Ho J, Brown J, Stevens A, Johnson AW. 2000. Saccharomyces cerevisiae RAI1 (YGL246c) is homologous to human DOM3Z and encodes a protein that binds the nuclear exoribonuclease Rat1p. Mol. Cell. Biol. 20:4006–4015. 10.1128/MCB.20.11.4006-4015.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Perez-Fernandez J, Roman A, De Las Rivas J, Bustelo XR, Dosil M. 2007. The 90S preribosome is a multimodular structure that is assembled through a hierarchical mechanism. Mol. Cell. Biol. 27:5414–5429. 10.1128/MCB.00380-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang W, Morris QD, Chang R, Shai O, Bakowski MA, Mitsakakis N, Mohammad N, Robinson MD, Zirngibl R, Somogyi E, Laurin N, Eftekharpour E, Sat E, Grigull J, Pan Q, Peng WT, Krogan N, Greenblatt J, Fehlings M, van der Kooy D, Aubin J, Bruneau BG, Rossant J, Blencowe BJ, Frey BJ, Hughes TR. 2004. The functional landscape of mouse gene expression. J. Biol. 3:21. 10.1186/jbiol16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shimoji K, Jakovljevic J, Tsuchihashi K, Umeki Y, Wan K, Kawasaki S, Talkish J, Woolford JL, Jr, Mizuta K. 2012. Ebp2 and Brx1 function cooperatively in 60S ribosomal subunit assembly in Saccharomyces cerevisiae. Nucleic Acids Res. 40:4574–4588. 10.1093/nar/gks057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Granneman S, Petfalski E, Tollervey D. 2011. A cluster of ribosome synthesis factors regulate pre-rRNA folding and 5.8S rRNA maturation by the Rat1 exonuclease. EMBO J. 30:4006–4019. 10.1038/emboj.2011.256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Poll G, Braun T, Jakovljevic J, Neueder A, Jakob S, Woolford JL, Jr, Tschochner H, Milkereit P. 2009. rRNA maturation in yeast cells depleted of large ribosomal subunit proteins. PLoS One 4:e8249. 10.1371/journal.pone.0008249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jakovljevic J, Gamalinda M, Talkish J, Alexander L, Linneman J, Milkereit P, Woolford JL., Jr 2012. Ribosomal proteins L7 and L8 function in concert with six A3 assembly factors to propagate assembly of domain I of 25S rRNA in yeast 60S ribosomal subunits. RNA 18:1805–1822. 10.1261/rna.032540.112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ben-Shem A, Garreau de Loubresse N, Melnikov S, Jenner L, Yusupova G, Yusupov M. 2011. The structure of the eukaryotic ribosome at 3.0 A resolution. Science 334:1524–1529. 10.1126/science.1212642 [DOI] [PubMed] [Google Scholar]
- 30.Dembowski JA, Ramesh M, McManus CJ, Woolford JL., Jr 2013. Identification of the binding site of Rlp7 on assembling 60S ribosomal subunits in Saccharomyces cerevisiae. RNA 19:1639–1647. 10.1261/rna.041194.113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fatica A, Oeffinger M, Tollervey D, Bozzoni I. 2003. Cic1p/Nsa3p is required for synthesis and nuclear export of 60S ribosomal subunits. RNA 9:1431–1436. 10.1261/rna.5130503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gadal O, Strauss D, Petfalski E, Gleizes PE, Gas N, Tollervey D, Hurt E. 2002. Rlp7p is associated with 60S preribosomes, restricted to the granular component of the nucleolus, and required for pre-rRNA processing. J. Cell Biol. 157:941–951. 10.1083/jcb.200111039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dunbar DA, Dragon F, Lee SJ, Baserga SJ. 2000. A nucleolar protein related to ribosomal protein L7 is required for an early step in large ribosomal subunit biogenesis. Proc. Natl. Acad. Sci. U. S. A. 97:13027–13032. 10.1073/pnas.97.24.13027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sahasranaman A, Dembowski J, Strahler J, Andrews P, Maddock J, Woolford JL., Jr 2011. Assembly of Saccharomyces cerevisiae 60S ribosomal subunits: role of factors required for 27S pre-rRNA processing. EMBO J. 30:4020–4032. 10.1038/emboj.2011.338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wu K, Wu P, Aris JP. 2001. Nucleolar protein Nop12p participates in synthesis of 25S rRNA in Saccharomyces cerevisiae. Nucleic Acids Res. 29:2938–2949. 10.1093/nar/29.14.2938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Duronio RJ, Gordon JI, Boguski MS. 1992. Comparative analysis of the beta transducin family with identification of several new members including PWP1, a nonessential gene of Saccharomyces cerevisiae that is divergently transcribed from NMT1. Proteins 13:41–56. 10.1002/prot.340130105 [DOI] [PubMed] [Google Scholar]
- 37.Lau CK, Bachorik JL, Dreyfuss G. 2009. Gemin5-snRNA interaction reveals an RNA binding function for WD repeat domains. Nat. Struct. Mol. Biol. 16:486–491. 10.1038/nsmb.1584 [DOI] [PubMed] [Google Scholar]
- 38.Longtine MS, McKenzie A, III, Demarini DJ, Shah NG, Wach A, Brachat A, Philippsen P, Pringle JR. 1998. Additional modules for versatile and economical PCR-based gene deletion and modification in Saccharomyces cerevisiae. Yeast 14:953–961. [DOI] [PubMed] [Google Scholar]
- 39.Rigaut G, Shevchenko A, Rutz B, Wilm M, Mann M, Seraphin B. 1999. A generic protein purification method for protein complex characterization and proteome exploration. Nat. Biotechnol. 17:1030–1032. 10.1038/13732 [DOI] [PubMed] [Google Scholar]
- 40.Horsey EW, Jakovljevic J, Miles TD, Harnpicharnchai P, Woolford JL., Jr 2004. Role of the yeast Rrp1 protein in the dynamics of pre-ribosome maturation. RNA 10:813–827. 10.1261/rna.5255804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ausubel FM, Brent R, Kingston RE, Moore DD, Seidman JG, Smith JA, Struhl K. 1994. Current protocols in molecular biology. Wiley, New York, NY [Google Scholar]
- 42.Deshmukh M, Tsay YF, Paulovich AG, Woolford JL., Jr 1993. Yeast ribosomal protein L1 is required for the stability of newly synthesized 5S rRNA and the assembly of 60S ribosomal subunits. Mol. Cell. Biol. 13:2835–2845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Babiano R, Gamalinda M, Woolford JL, Jr, de la Cruz J. 2012. Saccharomyces cerevisiae ribosomal protein L26 is not essential for ribosome assembly and function. Mol. Cell. Biol. 32:3228–3241. 10.1128/MCB.00539-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liebeg A, Waldsich C. 2009. Probing RNA structure within living cells. Methods Enzymol. 468:219–238. 10.1016/S0076-6879(09)68011-3 [DOI] [PubMed] [Google Scholar]
- 45.Schneider CA, Rasband WS, Eliceiri KW. 2012. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 9:671–675. 10.1038/nmeth.2089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ulbrich C, Diepholz M, Bassler J, Kressler D, Pertschy B, Galani K, Bottcher B, Hurt E. 2009. Mechanochemical removal of ribosome biogenesis factors from nascent 60S ribosomal subunits. Cell 138:911–922. 10.1016/j.cell.2009.06.045 [DOI] [PubMed] [Google Scholar]
- 47.Giaever G, Chu AM, Ni L, Connelly C, Riles L, Veronneau S, Dow S, Lucau-Danila A, Anderson K, Andre B, Arkin AP, Astromoff A, El-Bakkoury M, Bangham R, Benito R, Brachat S, Campanaro S, Curtiss M, Davis K, Deutschbauer A, Entian KD, Flaherty P, Foury F, Garfinkel DJ, Gerstein M, Gotte D, Guldener U, Hegemann JH, Hempel S, Herman Z, Jaramillo DF, Kelly DE, Kelly SL, Kotter P, LaBonte D, Lamb DC, Lan N, Liang H, Liao H, Liu L, Luo C, Lussier M, Mao R, Menard P, Ooi SL, Revuelta JL, Roberts CJ, Rose M, Ross-Macdonald P, Scherens B, Schimmack G, Shafer B, et al. 2002. Functional profiling of the Saccharomyces cerevisiae genome. Nature 418:387–391. 10.1038/nature00935 [DOI] [PubMed] [Google Scholar]
- 48.Ohtake Y, Wickner RB. 1995. Yeast virus propagation depends critically on free 60S ribosomal subunit concentration. Mol. Cell. Biol. 15:2772–2781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dembowski JA, Kuo B, Woolford JL., Jr 2013. Has1 regulates consecutive maturation and processing steps for assembly of 60S ribosomal subunits. Nucleic Acids Res. 41:7889–7904. 10.1093/nar/gkt545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dammel CS, Noller HF. 1993. A cold-sensitive mutation in 16S rRNA provides evidence for helical switching in ribosome assembly. Genes Dev. 7:660–670. 10.1101/gad.7.4.660 [DOI] [PubMed] [Google Scholar]
- 51.Guthrie C, Nashimoto H, Nomura M. 1969. Structure and function of E. coli ribosomes. 8. Cold-sensitive mutants defective in ribosome assembly. Proc. Natl. Acad. Sci. U. S. A. 63:384–391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fatica A, Cronshaw AD, Dlakic M, Tollervey D. 2002. Ssf1p prevents premature processing of an early pre-60S ribosomal particle. Mol. Cell 9:341–351. 10.1016/S1097-2765(02)00458-6 [DOI] [PubMed] [Google Scholar]
- 53.Nissan TA, Bassler J, Petfalski E, Tollervey D, Hurt E. 2002. 60S pre-ribosome formation viewed from assembly in the nucleolus until export to the cytoplasm. EMBO J. 21:5539–5547. 10.1093/emboj/cdf547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bassler J, Kallas M, Pertschy B, Ulbrich C, Thoms M, Hurt E. 2010. The AAA-ATPase Rea1 drives removal of biogenesis factors during multiple stages of 60S ribosome assembly. Mol. Cell 38:712–721. 10.1016/j.molcel.2010.05.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gamalinda M, Jakovljevic J, Babiano R, Talkish J, de la Cruz J, Woolford JL., Jr 2013. Yeast polypeptide exit tunnel ribosomal proteins L17, L35 and L37 are necessary to recruit late-assembling factors required for 27SB pre-rRNA processing. Nucleic Acids Res. 41:1965–1983. 10.1093/nar/gks1272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Basu U, Si K, Warner JR, Maitra U. 2001. The Saccharomyces cerevisiae TIF6 gene encoding translation initiation factor 6 is required for 60S ribosomal subunit biogenesis. Mol. Cell. Biol. 21:1453–1462. 10.1128/MCB.21.5.1453-1462.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Saveanu C, Namane A, Gleizes PE, Lebreton A, Rousselle JC, Noaillac-Depeyre J, Gas N, Jacquier A, Fromont-Racine M. 2003. Sequential protein association with nascent 60S ribosomal particles. Mol. Cell. Biol. 23:4449–4460. 10.1128/MCB.23.13.4449-4460.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lebreton A, Saveanu C, Decourty L, Jacquier A, Fromont-Racine M. 2006. Nsa2 is an unstable, conserved factor required for the maturation of 27 SB pre-rRNAs. J. Biol. Chem. 281:27099–27108. 10.1074/jbc.M602199200 [DOI] [PubMed] [Google Scholar]
- 59.Saveanu C, Bienvenu D, Namane A, Gleizes PE, Gas N, Jacquier A, Fromont-Racine M. 2001. Nog2p, a putative GTPase associated with pre-60S subunits and required for late 60S maturation steps. EMBO J. 20:6475–6484. 10.1093/emboj/20.22.6475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Shoji S, Dambacher CM, Shajani Z, Williamson JR, Schultz PG. 2011. Systematic chromosomal deletion of bacterial ribosomal protein genes. J. Mol. Biol. 413:751–761. 10.1016/j.jmb.2011.09.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Perriman RJ, Ares M., Jr 2007. Rearrangement of competing U2 RNA helices within the spliceosome promotes multiple steps in splicing. Genes Dev. 21:811–820. 10.1101/gad.1524307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wells SE, Hughes JMX, Igel AH, Ares M. 2000. Use of dimethysulfate to probe RNA structure in vivo. Methods Enzymol. 318:479–493. 10.1016/S0076-6879(00)18071-1 [DOI] [PubMed] [Google Scholar]
- 63.Stern S, Moazed D, Noller HF. 1988. Structural analysis of RNA using chemical and enzymatic probing monitored by primer extension. Methods Enzymol. 164:481–489. 10.1016/S0076-6879(88)64064-X [DOI] [PubMed] [Google Scholar]
- 64.Adams CC, Jakovljevic J, Roman J, Harnpicharnchai P, Woolford JL., Jr 2002. Saccharomyces cerevisiae nucleolar protein Nop7p is necessary for biogenesis of 60S ribosomal subunits. RNA 8:150–165. 10.1017/S1355838202010026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Oeffinger M, Leung A, Lamond A, Tollervey D. 2002. Yeast Pescadillo is required for multiple activities during 60S ribosomal subunit synthesis. RNA 8:626–636. 10.1017/S1355838202020022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pestov DG, Stockelman MG, Strezoska Z, Lau LF. 2001. ERB1, the yeast homolog of mammalian Bop1, is an essential gene required for maturation of the 25S and 5.8S ribosomal RNAs. Nucleic Acids Res. 29:3621–3630. 10.1093/nar/29.17.3621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Oeffinger M, Tollervey D. 2003. Yeast Nop15p is an RNA-binding protein required for pre-rRNA processing and cytokinesis. EMBO J. 22:6573–6583. 10.1093/emboj/cdg616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gamalinda M, Ohmayer U, Jakovljevic J, Kumcuoglu B, Woolford J, Mbom B, Lin L, Woolford JL., Jr 2014. A hierarchical model for assembly of eukaryotic 60S ribosomal subunit domains. Genes Dev. 28:198–210. 10.1101/gad.228825.113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Babiano R, Badis G, Saveanu C, Namane A, Doyen A, Diaz-Quintana A, Jacquier A, Fromont-Racine M, de la Cruz J. 2013. Yeast ribosomal protein L7 and its homologue Rlp7 are simultaneously present at distinct sites on pre-60S ribosomal particles. Nucleic Acids Res. 41:9461–9470. 10.1093/nar/gkt726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Woodson SA. 2010. Compact intermediates in RNA folding. Annu. Rev. Biophys. 39:61–77. 10.1146/annurev.biophys.093008.131334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Culver GM. 2003. Assembly of the 30S ribosomal subunit. Biopolymers 68:234–249. 10.1002/bip.10221 [DOI] [PubMed] [Google Scholar]
- 72.Pan J, Thirumalai D, Woodson SA. 1997. Folding of RNA involves parallel pathways. J. Mol. Biol. 273:7–13. 10.1006/jmbi.1997.1311 [DOI] [PubMed] [Google Scholar]
- 73.Dill KA, Chan HS. 1997. From Levinthal to pathways to funnels. Nat. Struct. Biol. 4:10–19. 10.1038/nsb0197-10 [DOI] [PubMed] [Google Scholar]
- 74.Chauhan S, Behrouzi R, Rangan P, Woodson SA. 2009. Structural rearrangements linked to global folding pathways of the Azoarcus group I ribozyme. J. Mol. Biol. 386:1167–1178. 10.1016/j.jmb.2008.12.075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Silverman SK, Zheng M, Wu M, Tinoco I, Jr, Cech TR. 1999. Quantifying the energetic interplay of RNA tertiary and secondary structure interactions. RNA 5:1665–1674. 10.1017/S1355838299991823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Chauhan S, Woodson SA. 2008. Tertiary interactions determine the accuracy of RNA folding. J. Am. Chem. Soc. 130:1296–1303. 10.1021/ja076166i [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cannone JJ, Subramanian S, Schnare MN, Collett JR, D'Souza LM, Du Y, Feng B, Lin N, Madabusi LV, Muller KM, Pande N, Shang Z, Yu N, Gutell RR. 2002. The comparative RNA web (CRW) site: an online database of comparative sequence and structure information for ribosomal, intron, and other RNAs. BMC Bioinformatics 3:2. 10.1186/1471-2105-3-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.