Abstract
Although previous studies have shown that GATA1 is required for mast cell differentiation, the effects of the complete ablation of GATA1 in mast cells have not been examined. Using conditional Gata1 knockout mice (Gata1−/y), we demonstrate here that the complete ablation of GATA1 has a minimal effect on the number and distribution of peripheral tissue mast cells in adult mice. The Gata1−/y bone marrow cells were capable of differentiating into mast cells ex vivo. Microarray analyses showed that the repression of GATA1 in bone marrow mast cells (BMMCs) has a small impact on the mast cell-specific gene expression in most cases. Interestingly, however, the expression levels of mast cell tryptases in the mouse chromosome 17A3.3 were uniformly reduced in the GATA1 knockdown cells, and GATA1 was found to bind to a 500-bp region at the 5′ end of this locus. Revealing a sharp contrast to that observed in the Gata1-null BMMCs, GATA2 deficiency resulted in a significant loss of the c-Kit+ FcεRIα+ mast cell fraction and a reduced expression of several mast cell-specific genes. Collectively, GATA2 plays a more important role than GATA1 in the regulation of most mast cell-specific genes, while GATA1 might play specific roles in mast cell functions.
INTRODUCTION
GATA1 is a key transcription factor involved in the differentiation of several hematopoietic cell lineages, including erythroid cells, megakaryocytes, eosinophils, and dendritic cells (1, 2). Gata1-deficient mice die in utero around embryonic day 11.5 (E11.5) due to defective primitive erythropoiesis in the yolk sac (3). Similarly, Gata1 knockdown mice, referred to as Gata1G1.05/Y mice, generated by targeted inactivation of the hematopoietic-specific exon 1 (IE) promoter, die by E12.5 (4). Cre-loxP-mediated Gata1 gene deletion in adult mice results in erythroid aplasia and defective stress erythropoiesis, indicating that GATA1 is also necessary for postnatal erythropoiesis (5). The requirements of GATA1 for megakaryocyte differentiation and specification of the eosinophil lineage were demonstrated through the use of megakaryocyte- and eosinophil-specific GATA1 knockdown mice, respectively (6, 7).
In addition to these cell lineages, GATA1 is also expressed in mast cells. These cells have a central role in the innate immune system and allergic diseases (8). Several studies shown that GATA1 is not essential for the specification of the mast cell lineage but is critical for the later stage of mast cell development (9–14). In addition, several mast cell-specific genes, such as FcerIa and FcerIb, encoding the α and β subunits of the IgE receptor (FcεRIα, FcεRIβ), and Cpa3, encoding mast cell carboxypeptidase, have been shown to be regulated by GATA1 (13–15). Of note, these studies were conducted using Gata1 knockdown mice or cultured mast cell lines. The consequences of complete ablation of GATA1 on mast cell differentiation have never been examined. We previously noted that GATA2, another GATA family member, is abundantly expressed in mast cells, implying a functional redundancy between GATA1 and GATA2 (16).
GATA2 is essential for mast cell lineage specification in the in vitro differentiation of embryonic stem cells (17). We recently revealed that the GATA2 mRNA level was significantly increased, while GATA1 mRNA expression was maintained at low levels during the differentiation of mast cells derived from mouse bone marrow (BMMCs) (16). Furthermore, in a coculture system with Swiss 3T3 fibroblasts, Takano et al. reported that the expression level of GATA1 further declines to an undetectable level when BMMCs mature into connective tissue-type mast cells (18). Collectively, these data prompted us to reassess whether GATA1 plays an essential role in BMMCs.
In contrast to BMMC differentiation, the GATA2 expression in multilineage progenitors declines upon commitment to the erythroid lineage and is switched for GATA1 expression, which peaks at the late erythroid progenitor and proerythroblast stages. This dynamic transition of GATA factor expression is essential for correct erythroid differentiation and has been referred to as “GATA factor switching” (19, 20), which is mediated by two key cis-regulatory mechanisms through highly conserved GATA binding sites in the Gata1 and Gata2 loci. One is a direct repression of Gata2 gene expression by GATA1 through the conserved GATA boxes within the Gata2 locus (21, 22). The other is a positive autoregulation of Gata1 through several conserved GATA boxes, including the Gata1 gene hematopoietic enhancer (G1HE, also referred to as HS1 or mHS-3.5) located 3.9 kb upstream of IE (23, 24). Importantly, we showed that neither forced expression nor small interfering RNA (siRNA)-mediated knockdown of GATA1 affected the Gata2 gene expression in BMMCs, indicating that the GATA1-mediated Gata2 repression does not take place in mast cells (16). Furthermore, we found that the G1HE region is epigenetically inactivated and is dispensable for Gata1 gene expression in BMMCs and peritoneal mast cells by performing transgenic reporter mouse assays (16). Taking these findings into account, we surmised that, unlike erythroid differentiation, GATA2 might play a predominant role over GATA1 in mast cell differentiation.
In the present study, we wanted to define the specific roles of GATA1 in mast cell development. To this end, we examined the effects of complete ablation of GATA1 in mast cell differentiation using tamoxifen-inducible Gata1 knockout mice (Gata1−/y). We demonstrate here that the role of GATA1 in mast cell differentiation is more limited than previously anticipated and that the null mutation of Gata1 is likely compensated for by GATA2.
MATERIALS AND METHODS
Mice.
Conditional Gata1 knockout mice (Gata1flox/y) were generated as described previously (5). The knockin mice expressing a 4-hydroxy tamoxifen (4-OHT)-inducible Cre recombinase gene under the control of the Rosa26 promoter (Rosa26CreERT2) were kindly provided by Anton Berns, Netherlands Cancer Institute. Since the Gata1 gene is X linked, the Gata1 knockout phenotype was examined in hemizygous male mice (Gata1flox/y) expressing CreERT2. These mice had been bred on a BDF1 background and were subsequently backcrossed to a C57BL/6 background. Cre-mediated recombination of the Gata1 gene was determined by genomic PCR, as described previously (16). Gata2flox mice (25) were kindly provided by S. A. Camper, University of Michigan. C57BL/6-KitW-sh/W-sh mice were purchased from RIKEN BRC. Mice were maintained in the animal facility of Takasaki University of Health and Welfare in accordance with institutional guidelines.
Induction of the Cre transgenes in vivo.
To induce Cre recombinase, mice (8 to 10 weeks of age) were injected subcutaneously with tamoxifen (0.1 mg/g [body weight]; Sigma) dissolved in sunflower oil on experimental days 1 to 5 and 8 to 12. The body weight and hematocrit level were monitored weekly. The mice were euthanized and used for the analysis on experimental days 28 to 35.
Hematological analyses.
Blood samples were taken from the tail vein using heparin-coated microtubes. The hematocrit values were measured using a micro-hematocrit centrifuge (MC-150; Tomy Seiko).
qRT-PCR.
Total RNA was extracted from cells using NucleoSpin RNA II (TaKaRa). Reverse transcription (RT) reactions were performed using a ReverTra Ace qPCR RT kit (Toyobo) according to the manufacturer's instructions. Quantitative RT-PCR (qRT-PCR) was performed using the Go Taq qPCR master mix (Promega) and an Mx3000P real-time PCR system (Stratagene), as described previously (26). The data were normalized to the 18S rRNA or GAPDH (glyceraldehyde-3-phosphate dehydrogenase) levels and are shown as the averages ± the standard deviations (SD). The primer sequences used for PCR are shown in Table 1, unless previously described (16).
TABLE 1.
Primer sequences for qRT-PCR and qChIP analyses
| Analysis and gene | Locus | Orientation | Sequence |
|---|---|---|---|
| qRT-PCR | |||
| Il1a | 5′ | TCGGGAGGAGACGACTCTAA | |
| 3′ | GTGCACCCGACTTTGTTCTT | ||
| Epor | 5′ | GACCCCAAGTTTGAGAGCAA | |
| 3′ | TGCAGGCTACATGACTTTCG | ||
| Ahr | 5′ | ATGGCTTTGTGCTGGGTTGTCACAG | |
| 3′ | ACTCCTTGTGCAGAGTCTGGGTTT | ||
| Il18 | 5′ | ACGTGTTCCAGGACACAACA | |
| 3′ | ACAAACCCTCCCCACCTAAC | ||
| Tnfrsf11b | 5′ | ATGAACAAGTGGCTGTGCTG | |
| 3′ | CCTCACTGTGCAGTGCTGTT | ||
| Creg2 | 5′ | GTGTCCACCCACGAAAAGAT | |
| 3′ | GTCTTCTGGGTCGACGATGT | ||
| Fceria | 5′ | AGAGCAAACCTGTGTACTTG | |
| 3′ | GTGGACTTTCCATTTCTTCC | ||
| Fcerib | 5′ | AGGCTACCCATTCTGGGGTG | |
| 3′ | GGCTGCCTCTCACCAGATAC | ||
| Fcerig | 5′ | CTCCTTTTGGTGGAAGAAGC | |
| 3′ | TGAGTCGACAGTAGAGCAGG | ||
| Mcpt1 | 5′ | TTCCCTTGCCTGGTCCCT | |
| 3′ | GTTTTCCCCCAGCCAGCT | ||
| Mcpt4 | 5′ | GAAGTGAAAAGCCTGACCTGC | |
| 3′ | CATGCTTTGTTGAACCCAAGG | ||
| Mcp9 | 5′ | GGGTGGCCCATGGTATTGTA | |
| 3′ | CGGGTGAAGATTGCAGGG | ||
| Tpsg1 | 5′ | GGTCACACTGTCTCCCCACT | |
| 3′ | GCATCCCAGGGTAGAAGTCA | ||
| Tpsb2 | 5′ | CGACATTGATAATGACGAGCCTC | |
| 3′ | ACAGGCTGTTTTCCACAATGG | ||
| Tpsab1 | 5′ | ATGACCACCTGATGACTGTGAGCCAG | |
| 3′ | AGGAACGGAGGTCATCCTGGATGTG | ||
| qChIP | |||
| Chr17A3.3a | Region A a | 5′ | AACCTTCGACGTGACCTTTG |
| 3′ | GGCACAGGATTTGTGAGACC | ||
| Region A b | 5′ | TGGCTACATGTTCCCTACCC | |
| 3′ | CAAAGGTCACGTCGAAGGTT | ||
| Kit | −114Ka | 5′ | AGCAATGGCCTCACGAGTTCTA |
| 3′ | CCAGGAAAAGTTTGGCAGGAT | ||
| −114Kb | 5′ | CGTGCACACAGGTTTGTTTC | |
| 3′ | TGCTGAGATGTGGCAATAGG | ||
| Promoter | 5′ | CACCTCCACCATAAGCCGAAT | |
| 3′ | CTCCTAGACAATAAAGGACAACCA | ||
| Fcerib | Promoter a | 5′ | ACAGCAAGAGAAAGGAGTCACTGAT |
| 3′ | CATGCGGAACCTACTTGTCAGA | ||
| Promoter b | 5′ | ATGAAGCTGCCAAAGAGCAC | |
| 3′ | CTGCCAGAGATGTGGGATTT | ||
| −2.8K | 5′ | GACAAGTAGGTTCCGCATGAA | |
| 3′ | TGGGCTATCCAGGAATGAAA |
That is, mouse chromosome 17A3.3.
Western blotting.
Cells were lysed by boiling in Laemmli buffer. The lysates were resolved by sodium dodecyl sulfate–10% polyacrylamide gel electrophoresis, and the Western blot analyses were performed as described previously (26) using anti-GATA1 (N6; Santa Cruz), anti-GATA2 (H-116; Santa Cruz), anti-β-tubulin (Sigma), and anti-lamin B (M-20; Santa Cruz) antibodies.
Histological and cytological analyses.
Tissue specimens were fixed in 4% paraformaldehyde, followed by embedding in Tissue-Tek OCT compound (Sakura Finetechnical) and freezing in liquid nitrogen. Cryostat sections were prepared by using a Leica CM1515 cryostat (Leica). Cytospin preparations of the cells (5 × 104 cells) were made using a Shandon Cytospin4 centrifuge (Thermo Electron Corp.). Tissue sections and cytospin preparations were stained with either Wright-Giemsa stain, toluidine blue, or Alcian blue-safranin O.
Cell culture.
Bone marrow mononuclear cells isolated from the femurs and tibiae were cultured for 2 weeks in Roswell Park Memorial Institute (RPMI) 1640 medium supplemented with 10% fetal bovine serum and penicillin-streptomycin in the presence of 10 ng of recombinant murine interleukin-3 (IL-3; Peprotech)/ml and subsequently cultured for 2 weeks with IL-3 and 10 ng of recombinant murine stem cell factor (SCF; Peprotech)/ml. The cultures were replenished with fresh medium every 4 days and maintained at a cell density of 5 × 105/ml.
FACS analysis.
For fluorescence-activated cell sorting (FACS) analysis, cells were stained with fluorescence-conjugated antibodies for 30 min and analyzed using a FACSCantoII flow cytometer (BD Biosciences). The following antibodies were used for the analyses: allophycocyanin-conjugated rat anti-mouse CD117 (c-Kit), phycoerythrin (PE)-conjugated mouse anti-mouse FcεRIα (eBioscience), fluorescein isothiocyanate-conjugated rat anti-mouse CD11b (MacI), and PE-conjugated mouse anti-mouse Ly6C/G (Gr1). The anti-mouse FcεRIα antibody was purchased from eBioscience, and the other antibodies were purchased from BD Pharmingen.
Degranulation assay.
The degranulation of mast cells was measured by the release of β-hexosaminidase, as described previously (26). Briefly, BMMCs were sensitized with 0.5 μg of monoclonal anti-dinitrophenyl (DNP) murine IgE antibody (clone SPE-7; Sigma)/ml overnight and stimulated with 200 ng of DNP-BSA (Calbiochem)/ml at 37°C for 30 min. After stimulation, supernatants were collected, and the cells were lysed with 0.5% Triton X-100 in Tyrode buffer. The supernatants and cell lysates were incubated with 2 mM N-acetyl-β-d-glucosamide (Sigma) in 0.1 M citrate buffer, and the absorbance at 405 nm was measured using a Sunrise Rainbow RC microplate reader (Tekan).
Reconstitution of mast cells in mast cell-deficient mice.
BMMCs (2.0 × 106 cells) in 200 ml of phosphate-buffered saline (PBS) were injected intraperitoneally into 6-week-old male KitW-sh/W-sh mice, and the mice were used for the experiments 6 to 8 weeks after the injection.
Transfection of siRNA.
The siRNA duplexes for mouse Gata1 and Gata2 were purchased from Invitrogen. The control siRNA was purchased from Sigma. BMMCs (2.0 × 106 cells) were transfected with 200 pmol of siRNA by electroporation using an Amaxa Nucleofector (Lonza). The cells were harvested 24 h after transfection and used for the analyses.
Methylcellulose colony formation assay.
Methylcellulose colony formation assays were performed with MethoCult M3434 (Stem Cell Corporations) according to the manufacturer's instructions. Bone marrow cells were suspended in culture medium containing methylcellulose and plated onto 35-mm culture dishes at a density of 2 × 104 cells/dish. Colonies were counted after 8 days of incubation at 37°C.
Microarray analysis.
Wild-type (WT) BMMCs were transfected with control or Gata1 siRNA (200 pmol), and total RNA was isolated 24 h after transfection, as described above. The quality of the RNA samples was checked using an Agilent 2100 bioanalyzer platform (Agilent Technologies). For each siRNA treatment, two replicate samples from different animals were pooled and used for the analysis. Cy3 labeling of cRNA, hybridization, scanning, and data analysis were done by Miltenyi Biotec using Agilent whole-mouse-genome Oligo Microarrays 8x60K. The expression data were analyzed using the Gene Spring software program (Agilent Technologies). Heat maps were generated using the Cluster 3.0 (http://bonsai.hgc.jp/∼mdehoon/software/cluster/) and JAVA TreeView (http://jtreeview.sourceforge.net/) software programs. The classification of the selected genes according to their functions was done using the Ingenuity Pathway Analysis (IPA) software program (Ingenuity System). The P values, represented as the negative log ratio of the IPA results, were the probability based on the Fisher exact test.
ChIP.
A chromatin immunoprecipitation (ChIP) assay was performed using anti-GATA-1 (N6; Santa Cruz) and anti-GATA2 (H-116; Santa Cruz) antibodies as in a prior report (26). As described previously, the GATA1 ChIP assay was performed using an anti-rat IgG rabbit antibody (Jackson ImmunoResearch) as a secondary antibody to precipitate the immune complex (26). The GATA2 ChIP assay was performed without the use of a secondary antibody. The quantitative analyses of DNA purified from the ChIP samples were conducted as previously described (16). The primer sequences used for PCR are shown in Table 1, unless previously described (16).
Statistical analysis.
Comparisons between two groups were made using Student t test. The data are presented as the means ± the SD. For all of the analyses, statistical significance was defined as a P < 0.05.
Accession numbers for the microarray data.
The microarray data were deposited in the Gene Expression Omnibus under GEO accession number GSE52254.
RESULTS
The systemic administration of tamoxifen resulted in the complete ablation of GATA1 expression in the hematopoietic tissues of adult Gata1−/y mice.
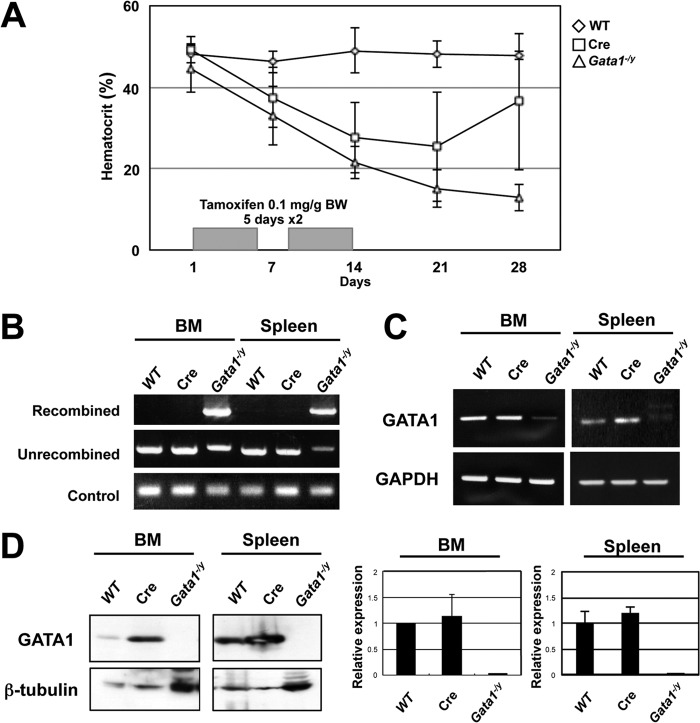
To examine the roles of GATA1 in mast cell differentiation in adult mice, we used conditional Gata1 knockout mice expressing tamoxifen-inducible Cre recombinase (Gata1fl/y::ROSA26CreERT2). We previously examined the roles of GATA1 in adult erythropoiesis by using a similar compound mutant mouse model (Gata1fl/y::ROSA26CreER [5]). Consistent with our previous data (5), systemic administration of tamoxifen led to progressive anemia by 28 days after the first tamoxifen treatment (Fig. 1A). The PCR analyses using genomic DNA revealed that recombination of the Gata1 gene in Gata1fl/y::ROSA26CreERT2 mice occurred efficiently in the bone marrow (BM) and spleen by day 28 (Fig. 1B). Thus, we here refer to the Gata1fl/y::ROSA26CreERT2 mice treated with tamoxifen as Gata1−/y mice. Mice expressing Cre recombinase with the normal Gata1 allele (Cre) also showed anemia to various extents, probably due to “Cre toxicity,” as previously reported (27, 28) (Fig. 1A). However, unlike the Gata1−/y mice, all Cre mice showed a recovery from anemia by day 28 and had normal hematocrit levels thereafter. The Gata1 mRNA level was significantly decreased in the BM and spleen of Gata1−/y mice, as shown by both the conventional and qRT-PCR analyses (Fig. 1C). Consistently, the GATA1 protein was undetectable in the BM and spleen of Gata1−/y mice by a Western blot analysis (Fig. 1D). These results demonstrate that, upon tamoxifen treatment, GATA1 was almost completely ablated in the hematopoietic tissues of Gata1−/y mice.
FIG 1.
The GATA1 expression in hematopoietic tissues was completely ablated by the administration of tamoxifen to Gata1−/y mice. (A) Average change in the hematocrit levels of WT, Cre, and Gata1−/y mice treated with tamoxifen (n = 4 for each group). (B) Results of the PCR analysis of recombination in the genomic DNA isolated from the BM and spleens of WT, Cre, and Gata1−/y mice. Genomic DNA was isolated 28 days after the start of the tamoxifen treatment and was used for the PCR analysis. PCR amplicons of the G1HE region are shown as controls. (C) mRNA levels of GATA1 in the BM and spleens of WT, Cre, and Gata1−/y mice measured by conventional (upper panels) and quantitative (lower panels) PCR analyses. PCR amplicons of GAPDH are shown as controls. For qRT-PCR, the value from WT mice was set to 1. The data were obtained from three independent experiments. (D) Protein levels of GATA1 and β-tubulin (control) in the BM and spleens of WT, Cre, and Gata1−/y mice measured by a Western blot analysis.
The mast cell number and distribution in peripheral tissues were not affected by GATA1 ablation.
We next examined the number and distribution of mast cells in the peripheral tissues of Gata1−/y mice (Fig. 2). Wild-type (WT) and Cre mice treated with tamoxifen were used as controls. The histological analyses of the ear pinna using both toluidine blue and Alcian blue-safranin O staining showed that the number and distribution of mast cells were comparable between all genotypes of mice (Fig. 2A and B). The FACS analysis demonstrated that c-Kit+ FcεRIα+ mast cells were detectable in the peritoneal fluid of Gata1−/y mice. The average frequency of c-Kit+ FcεRIα+ cells was lower in Gata1−/y mice (1.60% ± 0.70%) than in WT and Cre mice (2.39% ± 1.06% and 2.98% ± 2.76%, respectively), although the difference was not statistically significant (P = 0.113) (Fig. 2C, left panels, and D). Alcian blue-safranin O staining of the cytospin preparations of peritoneal cells showed differentiated mast cells containing safranin O-positive granules in all three genotypes of mice (Fig. 2C, right panels). The number and distribution of mast cells in the forestomach and glandular stomach were also similar in all three genotypes, as assessed by toluidine blue staining (Fig. 2E and F). Collectively, these results suggest that the ablation of GATA1 in adult mice does not significantly affect the number or distribution of peripheral tissue mast cells.
FIG 2.
The number and distribution of mast cells in peripheral tissues were not affected by Gata1 disruption. (A) Ear skin sections prepared from WT, Cre, and Gata1−/y mice were stained with toluidine blue (left) and Alcian blue-safranin O (right). Mast cells are indicated by arrows. The scale bar represents 100 μm. (B) Mast cell numbers in ear skin sections. The values represent the average cell numbers ± the SD per field in three separate fields per section. The data were obtained from three mice per group. (C) In the left panels, representative flow cytometry plots show the expression of c-Kit and FcεRIα on peritoneal mast cells isolated from WT, Cre, and Gata1−/y mice. In the right panels, cytospin preparations of peritoneal mast cells were stained with Alcian blue-safranin O. Original magnification, ×100. (D) Percentages of c-Kit/FcεRIα double-positive cells in peritoneal fluid isolated from WT, Cre, and Gata1−/y mice. The data are average ± the SD obtained from seven mice per group. (E) Forestomach and glandular-stomach sections prepared from WT, Cre, and Gata1−/y mice were stained with toluidine blue. Mast cells are indicated by arrows. (F) Mast cell numbers in stomach sections. The values represent the average cell numbers ± the SD per square millimeter of sections stained with toluidine blue. The data were obtained from four mice per group.
Gata1-null bone marrow cells are able to differentiate into mast cells in vitro.
To test the ability of GATA1-null BM cells to differentiate into mast cells in vitro, total BM cells were cultured in the presence of IL-3 and SCF, and then the frequency of c-Kit+ FcεRIα+ cells was examined by FACS on days 14 and 28 of culture (Fig. 3A). On day 14, the c-Kit+ FcεRIα+ cells appeared at a frequency of 20 to 30% in all three genotypes (Fig. 3A, upper panels). By day 28, the frequency of c-Kit+ FcεRIα+ cells was increased in all of the genotypes, but the average frequency of these cells was lower in the Gata1−/y mice compared to the WT mice (86.7% ± 1.68% and 95.7% ± 1.85%, respectively; P = 0.009) (Fig. 3B). Wright-Giemsa staining of the cytospin preparations showed that the BMMCs were morphologically indistinguishable among these three genotypes (Fig. 3C).
FIG 3.
Gata1-null bone marrow cells differentiate into mast cells in vitro in the presence of IL-3 and SCF. (A) Representative flow cytometry plots showing the expression of c-Kit and FcεRIα on BM cells isolated from WT, Cre, and Gata1−/y mice on culture day 14 and day 28. (B) Percentage of c-Kit/FcεRIα double-positive cells in BM cells prepared from WT, Cre, and Gata1−/y mice on culture days 14 and 28. The values represent average cell numbers ± the SD. The data were obtained from four mice per group. **, P < 0.01 (compared to the data for WT day 28). (C) Cytospin preparations of BM cells stained with Wright-Giemsa stain. The scale bar represents 50 mm. (D) FcεRI-dependent β-hexosaminidase release from WT and Gata1−/y BMMCs on culture days 14 and 28.
We next measured the release of β-hexosaminidase in WT and Gata1−/y BMMCs to determine whether the antigen-induced mast cell degranulation was affected by the GATA1 ablation (Fig. 3D). The average percentage of β-hexosaminidase release was similar in WT and Gata1−/y BMMCs (Fig. 3D). Thus, although GATA1 might play a facilitative role, it is not necessary for the formation and maturation of BMMCs.
GATA1 is dispensable for maintaining the mast cell characteristics in BMMCs.
To test whether GATA1 is required for maintaining the mast cell phenotype in BMMCs, BMMCs were generated from Gata1flox/y mice without tamoxifen treatment, and then 4-hydroxy tamoxifen (4-OHT) was added to the culture medium on day 28 of culture. Twelve days after the first 4-OHT treatment, the Gata1 mRNA level was reduced to 26% of that observed in WT cultures (P = 0.004) (Fig. 4A). Consistent with this finding, the GATA1 protein was undetectable by a Western blot analysis (Fig. 4B). Despite the almost complete GATA1 ablation, most of the cells sustained both c-Kit and FcεRIα expression, as assessed by a FACS analysis (Fig. 4C). Moreover, toluidine blue staining of the cytospin preparations showed that the Gata1−/y cells contained metachromatic granules in the cytoplasm similar to those observed in the WT and Cre BMMCs (Fig. 4D).
FIG 4.
GATA1 is dispensable for maintaining mast cell characteristics in BMMCs. (A) GATA1 mRNA levels in BMMCs isolated from WT, Cre, and Gata1−/y mice measured by qPCR, with 18S rRNA used as an internal control. Total RNA was isolated on day 12 after the first 4-OHT treatment. *, P < 0.05 (compared to the data for WT). (B) The protein levels of GATA1 and lamin B (control) in nuclear extracts prepared from WT, Cre, and Gata1−/y BMMCs were examined by Western blot analysis. The preparation of nuclear protein was done 12 days after the first 4-OHT treatment. (C) Representative flow cytometry plots show the expression of c-Kit and FcεRIα on BMMCs isolated from WT, Cre, and Gata1−/y mice on days 0 and 12 of 4-OHT treatment. (D) Cytospin preparations of BMMCs stained with Wright-Giemsa stain on days 0 and 12 of 4-OHT treatment. (E) mRNA levels of Gata2, Tal1, Kit, and Cpa3 in Cre and Gata1−/y BMMCs examined by qRT-PCR. The values from WT BMMCs are set to 1.0, and the relative values are shown. (F) BMMCs (2.0 × 106 cells) isolated from WT or Gata1−/y mice were injected intraperitoneally into KitW-sh/W-sh mice, and the peritoneal fluid was collected 6 to 8 weeks after the first injection. Upper panels show the results of the flow cytometric analysis of the peritoneal cells isolated from KitW-sh/W-sh mice injected with PBS, WT BMMCs, or Gata1−/y BMMCs. Lower panels show cytospin preparations of peritoneal cells stained with Alcian blue-safranin O. Original magnification, ×100.
We then examined the expression levels of the GATA2 and Tal1/Scl transcription factors, both of which are critical for mast cell development (17, 29). As shown in Fig. 4E, the qRT-PCR analysis revealed that the expression of these genes was not affected by the GATA1 ablation. A previous study showed that GATA1 binds to the promoter region of the Kit and Cpa3 genes in BMMCs (30). Despite the fact that the GATA1 protein expression was decreased to an undetectable level, the expression levels of Kit and Cpa3 were unaffected in BMMCs (Fig. 4E). Collectively, these results indicated that GATA1 is not necessary for maintaining the general characteristics of mast cell in BMMCs.
We further examined whether Gata1−/y BMMCs are able to terminally differentiate into mast cells in vivo. To this end, WT and Gata1−/y BMMCs on day 12 of 4-OHT treatment were transferred into the peritoneal cavity of mast cell-deficient KitW-sh/W-sh mice. At 6 to 8 weeks after transplantation, c-Kit+ FcεRIα+ mast cells were detectable in the peritoneal fluid of the BMMC-transplanted KitW-sh/W-sh mice but not of the PBS-injected control mice. Furthermore, the peritoneal fluid of KitW-sh/W-sh mice transplanted with either WT or Gata1−/y BMMC cells contained safranin O-positive differentiated mast cells (Fig. 4F). Therefore, Gata1−/y BMMCs appear to be capable of further differentiating into peritoneal mast cells in vivo.
Mast cell colonies developed at a high frequency from Gata1−/y BM cells in methylcellulose media.
A previous study showed that putative mast cell colonies were frequently formed in agar cultures of BM cells from heterozygous Gata1Plt13/+ mice, which carry an N-ethyl-N-nitrosurea-induced mutation at the translation initiation codon of Gata1 (31). Because the mouse Gata1 gene is located on the X chromosome, cells having an activated Gata1Plt13 allele do not produce detectable amounts of GATA1 protein. The mast cell colony-forming cells from the Gata1Plt13/+ mice exclusively carried the active Gata1Plt13 mutant allele in spite of the random X inactivation (31). Interestingly, despite the high frequency of mast cell colony-forming cells in the Gata1Plt13/+ BM, the mast cell population in the peripheral tissues was normal in the Gata1Plt13/+ mice. Based on this observation, we assumed that the number of mast cell colony-forming cells might be increased in the Gata1−/y BM, even though the number of peripheral tissue mast cells was not significantly affected by the GATA1 ablation. Of note, the methylcellulose colony assays showed a significantly increased frequency of colony-forming cells with aberrant morphology in the Gata1−/y BM, while the total colony numbers were comparable among all of the genotypes of BM cells (Fig. 5A and B).
FIG 5.
Mast cell colonies appeared at a high frequency from Gata1−/y BM cells in methylcellulose culture. (A) Total numbers of colonies grown from WT, Cre, and Gata1−/y BM cells in methylcellulose culture. Colony counts were performed on the eighth day of culture. (B) Frequencies of colony-forming cells in the WT, Cre, and Gata1−/y BM. Colony counts were performed on the eighth day of methylcellulose culture. (C) Phase-contrast photomicrographs (left) and cytospin preparations (right) of CFU-Mast and CFU-GM colonies. For cytospin preparations, cells were stained with Wright-Giemsa stain. (D) Results of flow cytometric analysis of colony-forming cells stained for c-Kit/FcεRIα (left panels) or Mac1/Gr1 (right panels). Cells were picked up from CFU-Mast or CFU-GM colonies on the eighth day of methylcellulose culture.
The aberrant colonies developed from Gata1−/y BM cells showed a large and loosely dispersed appearance, which is compatible with the characteristics of GATA1-deficient mast cell colonies described in a previous study (31) (Fig. 5C). Thus, we designated these colonies CFU-Mast. To further characterize the cellular morphology of the CFU-Mast, the colony-forming cells were picked off and stained with Wright-Giemsa stain on cytospin preparations. The cells from typical CFU-GM colonies were stained with Wright-Giemsa stain as controls (Fig. 5C, right panels). Most of the cells from CFU-GM showed characteristics of macrophages, with condensed nuclei in the peripheral region. Granulocyte-like cells with segmented nuclei were also observed, albeit at a lower frequency. In contrast, the cells from CFU-Mast were uniformly shaped, with rounded nuclei in the central region of the cells. Secretory granules were rarely developed in these cells.
Next, the cells picked up from the colonies were examined by a FACS analysis (Fig. 5D). The frequency of c-Kit+ FcεRIα+ cells was higher in the cells from CFU-Mast compared to those from CFU-GM (Fig. 5D, left panels). In contrast, the frequency of cells expressing myeloid cell markers, Mac1 and Gr1, was higher in the cells from CFU-GM compared to those from CFU-Mast (Fig. 5D, right panels). Taken together, these results demonstrated that GATA1 ablation led to an increase in CFU-Mast formation in methylcellulose cultures. Consistent with the known roles of GATA1 in erythropoiesis, no erythroid colony was formed from the Gata1−/y BM cells (Fig. 5B).
GATA1 might play a specific role in the regulation of tryptase genes on Chr17A3.3.
Although the general characteristics of the mast cells were mostly maintained in the GATA1-deficient BMMCs, we assumed that GATA1 deficiency might affect more specific mast cell functions. To explore this issue, we comprehensively examined the gene expression profiles in GATA1-deficient BMMCs by means of microarray analyses using BMMCs transduced with either control or Gata1 siRNA. Because BMMCs are primary cultured cells, we were concerned about nonspecific, individual variability in the transcript abundance. Thus, instead of comparing BMMCs prepared from Gata1−/y and WT mice, BMMCs prepared from the same individuals were transfected with either control or Gata1 siRNA and used for the microarray analysis. The transduction of Gata1 siRNA resulted in a 4.9-fold decrease in the Gata1 mRNA level compared to control cells. This analysis identified 1,670 differentially expressed genes (768 downregulated and 902 upregulated genes) in the Gata1 knockdown cells.
To gain further insight into the roles of GATA1 in mast cell gene expression, we functionally categorized these genes using the IPA software program. We found that the differentially expressed genes were strongly associated with hematological, inflammatory, and immunological diseases (Fig. 6A). However, only a few mast cell-related genes were listed among the differentially expressed genes in these categories (Fig. 6B). Consistent with the microarray data, the downregulation of Il1a, Epor, and Ahr and the upregulation of Il18, Tnfrsf11b, and Creg2 were confirmed by using qRT-PCR analyses (Fig. 6C).
FIG 6.
Large-scale gene expression profiles in BMMCs transfected with Gata1 siRNA. WT BMMCs were transfected with control or Gata1 siRNA (200 pmol), and total RNA was isolated 24 h after transfection. (A) Pathway analysis was performed using the IPA software program. A comparison of disease-related and biological functions among genes affected in the GATA1 knockdown BMMCs is shown. The red line indicates the threshold for a significant difference (P < 0.05) compared to the BMMCs transfected with control siRNA. (B) The expression levels of the genes listed in the categories of hematological and inflammatory diseases were compared in control and Gata1 siRNA-transfected BMMCs. Heat map comparisons of genes significantly affected (P < 0.05, 2-fold or larger change) in GATA1 siRNA-transfected BMMCs compared to controls are shown. Red indicates relatively high expression, green indicates low expression, and black indicates intermediate expression in GATA1 siRNA-transfected BMMCs compared to controls, as indicated by the color bar. (C) Results of qRT-PCR analysis of three downregulated (Il1a, Epor, and Ahr) and three upregulated (Il18, Tnfrsf11b, and Creg2) genes. The values of the wild-type cells were set to 1.0, and the relative values are shown. (D) Results of qRT-PCR analysis of mast cell-related genes. The values of the wild-type cells were set to 1.0, and the relative values are shown. (E) Configurations of group A tryptase gene loci on mouse chromosome 17A3.3. “Region A” located at the 5′ end of the gene cluster is indicated. (F) Nucleotide sequence of region A. Seven WGATAR sequence motifs are boxed and numbered. All GATA motifs except motif 1 were found to be evolutionally conserved between mouse and rat. The GATA motifs 2, 4, and 6 (in the red box) were identified in recent ChIP-seq studies. (G) The DNA-binding activity of GATA1 to region A, the double GATA region (dblGATA, positive control), and the sixth exon (Gata1 ex6, negative control) of the Gata1 gene was examined using qChIP analysis. The control experiments were performed using rat IgG in place of anti-GATA1 antibodies (N6). *, P < 0.05; **, P < 0.01 (compared to the data for IgG).
We then investigated the expression of 37 mast cell-related genes whose expression and/or function in mast cells have been previously reported (32–50) (Table 2). Again, we found that the fold change values were <2.0 for most of the mast cell-related genes on the list (Table 2). Nevertheless, we noted that the expression levels of mast cell proteases were uniformly decreased, except for that of carboxypeptidase E (Table 2). Moreover, the qRT-PCR analyses revealed that the expression levels of three mast cell tryptases—Tpsg1, Tpsb2 (Mcpt6), and Tpsab1 (Mcpt7)—in the Gata1 knockdown cells were reduced to less than half of those of the control siRNA-treated cells (0.46 ± 0.10, 0.47 ± 0.02, and 0.43 ± 0.10, respectively [n = 4]) (Fig. 6D). In contrast, the expression levels of three mast cell chymases Mcpt1, Mcpt4, and Cma1 were less affected by the Gata1 siRNA treatment (0.81 ± 0.19, 0.64 ± 0.09, and 1.14 ± 0.26, respectively [n = 4]) (Fig. 6D). Similarly, the expression levels of Fceria, Fcerig, and Cpa3 were not reduced in the Gata1 knockdown cells (Fig. 6D). It has been reported that the Tpsg1, Tpsb2, and Tpsab1 genes can be categorized as group 2 tryptase genes residing on mouse chromosome 17A3.3 (51, 52) (Fig. 6E). In order to examine whether GATA1 directly regulates the expression of these genes, we searched for consensus GATA-binding motifs that are evolutionally conserved among mammalian species on this region. We identified a 500-bp region that contains seven repetitive WGATAR sequences, six of which are conserved between mouse and rat, at the 5′ end of the group A tryptase gene cluster (here referred to as region A) (Fig. 6E and F). Furthermore, quantitative ChIP (qChIP) assays showed that the binding activity of GATA1 to this region was comparable to that of the double GATA site of the Gata1 gene, a previously characterized GATA1-binding region in BMMCs (16) (Fig. 6G). Interestingly, this region contains two “chromatin occupancy” consensus sequences for GATA1, (C/G)(A/T)GATAA(G/A/C)(G/A/C), and a composite half E-box-GATA motif (CTGN8WGATAA), both of which are characterized as functional GATA-binding motifs according to genome-wide ChIP-seq studies (53–55). Therefore, while siRNA-mediated GATA1 knockdown has only a small impact on most mast cell-affiliated genes in BMMCs, GATA1 might play a role in more specific functions of mast cells by regulating group A tryptase genes.
TABLE 2.
Expression of mast cell-enriched genes in the control or GATA1 siRNA-treated BMMCs
| Gene | Entrez full name | Gene ID | Fold change | P | Reference |
|---|---|---|---|---|---|
| Gata1 | GATA binding protein 1 | 14460 | –4.88 | 6.8E–15 | |
| Mast cell protease genes | |||||
| Mcpt2 | Mast cell protease 2 | 17225 | –1.61 | 1.4E–03 | |
| Mcpt4 | Mast cell protease 4 | 17227 | –1.91 | 2.0E–05 | |
| Cma2 | Chymase 2, mast cell | 545055 | –1.57 | 2.3E–03 | |
| Tpsab1 | Tryptase alpha/beta 1 (Tpsab1) | 100503895 | –2.22 | 5.2E–07 | |
| Tpsb2 | Tryptase beta 2 | 17229 | –1.53 | 3.6E–03 | |
| Cpe | Carboxypeptidase E (Cpe) | 12876 | 1.91 | 4.0E–05 | |
| Mast cell proteoglycan genes | |||||
| Prg2 | Proteoglycan 2 | 19074 | –1.46 | 8.9E–03 | 32 |
| Agrn | Agrin | 11603 | –2.19 | 3.0E–02 | 32 |
| Gpc2 | Glypican 2 (cerebroglycan) | 71951 | 4.00 | 3.3E–02 | 32 |
| Chad | Chondroadherin | 12643 | 1.39 | 3.5E–02 | 32 |
| Gpc1 | Glypican 1 | 14733 | 1.35 | 3.8E–02 | 32 |
| Other mast cell-related genes | |||||
| CtsD | Cathepsin D | 13033 | 1.40 | 2.1E–02 | 33 |
| Ctsg | Cathepsin G | 13035 | –1.35 | 3.7E–02 | 34 |
| Timp3 | Tissue inhibitor of metalloproteinase 3 | 21859 | 1.29 | 1.9E–02 | 34 |
| pld2 | Phospholipase D family, member 2 | 18806 | –1.45 | 1.0E–02 | 35 |
| Dusp14 | Dual specificity phosphatase 14 | 56405 | 1.71 | 4.5E–04 | 34 |
| Maob | Monoamine oxidase B | 109731 | –1.98 | 1.0E–05 | 34 |
| Trib2 | Tribbles homolog 2 (Drosophila) | 217410 | –1.43 | 4.8E–02 | 34 |
| Trib3 | Tribbles homolog 3 | 228775 | –1.41 | 1.8E–02 | 36 |
| C3 | Complement component 3 (C3) | 12266 | 1.48 | 2.2E–16 | 37 |
| C5ar1 | Complement component 5a receptor 1 | 12273 | 1.47 | 1.2E–02 | 38 |
| Grk5 | G protein-coupled receptor kinase 5 | 14773 | –1.64 | 8.6E–04 | 39 |
| Tlr1 | Toll-like receptor 1 | 21897 | 1.37 | 3.2E–02 | 40 |
| Tlr2 | Toll-like receptor 2 | 24088 | 1.24 | 2.1E–06 | 40 |
| myd88 | Myeloid differentiation primary response gene 88 | 17874 | 1.10 | 3.3E–02 | 40 |
| Il13 | Interleukin-13 | 16163 | –1.42 | 1.6E–02 | 41 |
| fgf2 | Fibroblast growth factor 2 | 14173 | –2.05 | 2.8E–45 | 42 |
| Ccr2 | Chemokine (C-C motif) receptor 2 | 12772 | 1.61 | 1.3E–03 | 43 |
| Cx3cr1 | Chemokine (C-X3-C) receptor 1 | 13051 | –1.87 | 7.0E–05 | 44 |
| Fyb | FYN binding protein | 23880 | –1.54 | 3.3E–03 | 45 |
| Orai1 | ORAI calcium release-activated calcium modulator 1 | 109305 | 1.44 | 1.3E–02 | 46 |
| Mt1 | Metallothionein 1 | 17748 | 1.03 | 1.0E–02 | 34 |
| Fst | Follistatin | 14313 | –1.34 | 4.8E–02 | 34 |
| Nlrp3 | NLR family, pyrin domain containing 3 | 216799 | –1.45 | 2.3E–02 | 47 |
| Socs3 | Suppressor of cytokine signaling 3 | 12702 | 1.38 | 2.6E–02 | 48 |
| CD52 | CD52 antigen | 23833 | 1.75 | 1.9E–04 | 49 |
| CD69 | CD69 antigen | 12515 | 1.44 | 1.2E–02 | 50 |
GATA2 plays a more important role than GATA1 in the regulation of most mast cell-specific genes.
We previously reported that the Gata2 mRNA levels are significantly increased during the maturation of BMMCs, whereas the Gata1 gene expression remains largely unaffected throughout BMMC differentiation (16). Given the robust GATA2 induction observed in the BMMCs, we hypothesized that GATA2 might compensate for GATA1 loss in the GATA1 knockdown BMMCs. To test this hypothesis, we examined the expression profiles of five mast cell-related genes, i.e., Kit, Ms4a2 (Fcer1b), Mcp9, Mitf, and Cpa3, in the BMMCs, in which either or both the levels of Gata1 and Gata2 were reduced using siRNAs. The downregulation of the Gata1 and Gata2 expression was confirmed by qRT-PCR (Fig. 7A), as well as Western blot analysis (Fig. 7B). The qRT-PCR analyses showed that expression levels of all mast cell-specific genes were most significantly decreased when both GATA factors were knocked down in comparison with that observed upon the knockdown of either GATA factor individually (Fig. 7C). Furthermore, the qChIP assays showed that both GATA1 and GATA2 bind to the Kit −114Kb enhancer and the Ms4a2 promoter regions, both of which harbor functional GATA-binding sites (30). These results clearly indicate the contribution of GATA1 to the regulation of these genes. Meanwhile, there remains a question as to whether GATA1 and GATA2 equally contribute to the expression of these mast cell-specific genes, or whether GATA2 plays a more dominant role than GATA1. In order to address this question, we used the Gata2flox allele to conditionally disrupt the GATA2 activity (25). This mutant allele was designed to conditionally delete the Gata2 gene fifth exon encoding the DNA-binding C-finger domain. As described earlier, Gata2flox mice were crossed with ROSA26CreERT2 mice, and BMMCs were subsequently generated from the Gata2flox/flox::ROSA26CreERT2 mice. At 4 days after the 4-OHT treatment, we confirmed that the transcripts derived from the Gata2 fifth exon sequences were undetectable in the qRT-PCR analysis (Fig. 7F, GATA2ΔCF). Surprisingly, the FACS analysis performed on day 4 of the 4-OHT treatment showed that the frequency of c-Kit+ FcεRIα+ cells was significantly reduced in the GATA2ΔCF samples compared to that observed in the control BMMCs obtained from the ROSA26CreERT2 mice (58.7% ± 6.9% and 92.7% ± 5.9%, respectively, P = 0.003 [n = 3]) (Fig. 7E). This observation reveals a sharp contrast with the subtle effects of Gata1-null deficiency noted in the BMMCs. Furthermore, the mRNA levels of Kit, Ms4a2, Mcp9, and Mitf were significantly reduced in the GATA2ΔCF cells, while the Gata1 expression was largely maintained at the wild-type level (Fig. 7F). Collectively, our data suggest that GATA2 plays a more important role than GATA1 in the regulation of most mast cell-specific genes.
FIG 7.
GATA2 plays a more important role than GATA1 in the regulation of most mast cell-specific genes. (A) mRNA levels of Gata1 and Gata2 in BMMCs transfected with the indicated siRNAs. The values from control siRNA-transfected cells were set to 1.0, and the relative values are shown. *, P < 0.05; **, P < 0.01 (versus control; n = 3 for each group). (B) Protein levels of GATA1, GATA2, and lamin B (control) in BMMCs transfected with the indicated siRNAs. (C) mRNA expression levels of Kit, Ms4a2 (Fcer1b), Mcp9, MITF, and Cpa3 genes in cells transfected with the indicated siRNAs. The values from control siRNA-transfected cells were set to 1.0, and the relative values are shown. *, P < 0.05 for the comparisons indicated (n = 3 for each group). (D) The DNA-binding activity of GATA1 and GATA2 to the Kit and Ms4a2 (Fcer1b) loci. Two primer sets were used to verify the Kit −114Kb region and the Ms4a2 promoter. The Kit promoter and the Ms4a2 −2.8Kb region were examined as negative regions. The gray bars indicate the results using rat (GATA1) or rabbit (GATA2) IgG in place of specific antibodies. *, P < 0.05; **, P < 0.01 (compared to the data for IgG). (E) Representative flow cytometry plots showing the expression of c-Kit and FcεRIα in the BMMCs isolated from Cre and GATA2ΔCF mice on days 0 and 4 of 4-OHT treatment. The percentage of c-Kit/FcεRIα double-positive cells among the Gata2ΔCF cells on days 0 and 4 is indicated (n = 3). (F) The mRNA levels of Gata1, the fifth exon of the Gata2 gene (Gata2 ex5), c-Kit, Ms4a2, Mcp9, and MITF in the Gata2ΔCF cells treated with 4-OHT. The ratios of the values obtained on day 4 versus day 0 are shown.
DISCUSSION
The present study demonstrates that the role of GATA1 in mast cell differentiation is more limited than previously anticipated based on in vivo (9–11) and in vitro (12, 56) studies. There are two plausible explanations for this discrepancy. First, while we examined mice lacking an entire Gata1 coding region, the preceding loss-of-function studies of GATA1 were performed using Gata1 knockdown mouse models with intact coding sequences. It was reported that there was defective mast cell differentiation in Gata1low mutant mice (9), which were generated by a targeted deletion of Gata1 gene enhancer region designated as Gata1 hematopoietic enhancer (G1HE, also referred to as HS1) (6). We and another group (11, 12) previously showed that GATA1 plays an important role in mast cell differentiation using heterozygous Gata1 knockdown mice generated by the targeted inactivation of the hematopoietic specific IE promoter (4). In both mutant mouse models, the Gata1 mRNA expression was severely reduced because of the lack of a promoter or critical regulatory sequences.
The important point here is that the regulation of Gata1 gene expression is distinct between erythroid and mast cell lineages. We recently showed that the proximal double GATA region, but not G1HE, is essential for the Gata1 gene expression in BMMCs and peritoneal mast cells in vivo using transgenic mouse reporter assays (16). Interestingly, the targeted disruption of this region in mice (ΔdblGATA) resulted in an eosinophil-deficient phenotype in vivo, although the BM cells of these mutant mice are able to differentiate into eosinophils ex vivo (7, 57). The BM cells of ΔdblGATA mice are also capable of differentiating into mast cells ex vivo (7), while the formation of peripheral tissue mast cells in vivo has not been examined. Therefore, the precise roles of these regulatory regions in the Gata1 gene expression in mast cells can be determined by comparing mast cell formation between the two mutant mouse models, Gata1low and ΔdblGATA, in vivo.
With regard to the IE promoter, a previous study demonstrated that the promoter usage of Gata1 gene in mast cells is distinct from that in the erythroid lineage cells, and a considerable amount of Gata1 mRNA is transcribed from an alternatively spliced first exon Ib that is regulated by PU.1 (58). Therefore, the mast cell defects reported in these previous studies using mice with a targeted Gata1 gene regulatory sequence or promoter might have resulted from the aberrant spatial and temporal regulation of Gata1 gene expression rather than reflecting the simple absence of GATA1 expression in differentiated mast cells.
Second, whereas we examined the effects of postnatal Gata1 inactivation in mast cells using inducible gene targeting in the present study, the Gata1 expression was attenuated from the embryonic stage onward in the previous mouse models. Thus, the differences in the results might have been due to the differential roles of GATA1 in mast cell differentiation during embryonic and postnatal hematopoiesis. Importantly, our data suggest a novel role of GATA1 in the regulation of group A tryptase genes located on chromosome 17A3.3. Recent gene targeting studies have shown that mMCP-6, a group A tryptase, plays a key role in the innate immune response against bacterial and parasitic infection (59, 60), as well as the formation of abdominal aortic aneurysms (61). Taken together, with our microarray data revealing that the expression of genes related to hematological, inflammatory, and immunological diseases was affected in the Gata1 knockdown BMMCs, GATA1 might play a central role in mast cell function under certain pathological conditions in adult mice. Because of their progressive anemia, we had limitations when trying to examine Gata1−/y (Gata1fl/y::ROSA26CreERT2) mice under pathological conditions. Recently, several groups have succeeded in performing Cre-loxP-mediated gene targeting, specifically in mast cells, using the regulatory regions of the cpa3, mcpt5, and il4 genes (see references 62, 63, and 64, respectively). These strategies could be applied for generating mast cell-specific Gata1 knockout mice to examine their innate immune responses against bacterial and/or parasitic infection and a variety of mast cell functions observed under pathological conditions.
Our data from the methylcellulose colony assays using bone marrow cells from Gata1−/y mice are consistent with the previous report of mast cell colony forming cells from chimeric Gata1Plt13/+ bone marrow cells in agar cultures (31). Interestingly, the methylcellulose colony assays of bone marrow and spleen cells from Gata1low mice resulted in an abnormal colony formation, with trilineage colonies containing erythroblasts, megakaryocytes, and mast cells (9, 19). Taken together with our present results, these findings suggest that residual low-level GATA1 expression level is required for the formation of this trilineage progenitor. An independent line of evidence also suggests that enhanced proliferation of progenitor cells in vitro might be a principle feature of GATA1 loss, because Gata1-null proerythroblasts cultured on OP9-stroma cells displayed high proliferative activity (65). Notably, these cells differentiate into mast cells in the presence of IL-3 without a restoration of GATA1 (65), supporting our contention that GATA1 is not essential for mast cell differentiation. It remains to be elucidated why Gata1−/y mice showed normal mast cell numbers in vivo.
We have presented several lines of evidence suggesting that GATA1 contributes to the expression of mast cell-specific genes, although the vast majority of mast cell-specific genes evaluated here were not affected by GATA1 loss. Our data for the GATA2ΔCF BMMCs indicate that the null mutation of Gata1 is likely compensated for by GATA2, with the exception of the regulation of a limited group of mast cell-specific genes, such as mast cell tryptases. The sequence analysis of region A on chromosome 17A3.3 raised an interesting possibility regarding the DNA-binding specificity of GATA1 and GATA2 in the hematopoietic cell lineages. The preceding genome-wide studies of GATA1 ChIP-seq were conducted using either erythroid cell lines (53–55) or a multipotent progenitor cell line harboring the potential to differentiate into erythroid cells (66). Notably, region A contains two “chromatin occupancy” consensus sequences for GATA1, (C/G)(A/T)GATAA(G/A/C)(G/A/C), and a composite half E-box-GATA motif (CTGN8WGATAA), both of which were characterized as functional GATA-binding motifs in these studies. The former motif has been identified to be the most frequent sequence among the WGATAR-containing peaks bound by GATA1 (53), while the latter has been found to be enriched in de novo GATA1 peaks without prior binding of GATA2 in differentiating erythroid progenitor cells (66). Interestingly, the latter sequence motif is also found to be frequently cooccupied by GATA1 and Scl/Tal1 (55). Therefore, it is conceivable that the properties of GATA factor binding specificity are conserved between erythroid and mast cell lineages and that GATA1, rather than GATA2, preferentially acts on this chromatin region.
In the final experiment, we showed that the ablation of the DNA-binding activity of GATA2 resulted in a significant reduction of the c-Kit+ FcεRIα+ mast cell fraction and a reduced expression of several mast cell-specific genes, suggesting that GATA2 plays a more important role than GATA1 in the regulation of most mast cell-specific genes. These data suggest that GATA2 is required for the maintenance of the mast cell identity in the differentiated state, in addition to cell lineage specification, as previously reported (17). Previous studies have demonstrated that the downregulation of a cofactor FOG-1 or the CEBP/α (CCAAT enhancer-binding protein α) is required for mast cell lineage specification (67, 68). Therefore, it is likely that GATA2 plays a role in maintaining the repressive state of these genes. Future analyses of GATA2ΔCF cells are expected to reveal mechanistic insight into the cell type-specific gene regulation of these critical innate immune cells.
ACKNOWLEDGMENTS
We thank Sally A. Camper (University of Michigan) for providing the Gata2flox mice. We also thank Takuya Matsumoto, Kae Nakajima, Chika Tonokura, Aya Makishima, Aoi Tamba, and Shogo Ikeda for technical assistance.
This study was supported by a Grant-in-Aid for Scientific Research on Innovative Areas from the Ministry of Education, Culture, Sports, Science, and Technology (K.O.), a Grant-in-Aid for Scientific Research (C) (K.O.), and a Grant-in-Aid for Young Scientists (B) (S.O.) from the Japan Society for the Promotion of Science.
Footnotes
Published ahead of print 10 March 2014
REFERENCES
- 1.Ferreira R, Ohneda K, Yamamoto M, Philipsen S. 2005. GATA1 function, a paradigm for transcription factors in hematopoiesis. Mol. Cell. Biol. 25:1215–1227. 10.1128/MCB.25.4.1215-1227.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gutiérrez L, Nikolic T, van Dijk TB, Hammad H, Vos N, Willart M, Grosveld F, Philipsen S, Lambrecht BN. 2007. Gata1 regulates dendritic cell development and survival. Blood 110:1933–1941. 10.1182/blood-2006-09-048322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fujiwara Y, Browne CP, Cunniff K, Goff SC, Orkin SH. 1996. Arrested development of embryonic red cell precursors in mouse embryos lacking transcription factor GATA-1. Proc. Natl. Acad. Sci. U. S. A. 93:12355–12358. 10.1073/pnas.93.22.12355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Takahashi S, Onodera K, Motohashi H, Suwabe N, Hayashi N, Yanai N, Nabesima Y, Yamamoto M. 1997. Arrest in primitive erythroid cell development caused by promoter-specific disruption of the GATA-1 gene. J. Biol. Chem. 272:12611–12615. 10.1074/jbc.272.19.12611 [DOI] [PubMed] [Google Scholar]
- 5.Gutiérrez L, Tsukamoto S, Suzuki M, Yamamoto-Mukai H, Yamamoto M, Philipsen S, Ohneda K. 2008. Ablation of Gata1 in adult mice results in aplastic crisis, revealing its essential role in steady-state and stress erythropoiesis. Blood 111:4375–4385. 10.1182/blood-2007-09-115121 [DOI] [PubMed] [Google Scholar]
- 6.Shivdasani RA, Fujiwara Y, McDevitt MA, Orkin SH. 1997. A lineage-selective knockout establishes the critical role of transcription factor GATA-1 in megakaryocyte growth and platelet development. EMBO J. 16:3965–3973. 10.1093/emboj/16.13.3965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yu C, Cantor AB, Yang H, Browne C, Wells RA, Fujiwara Y, Orkin SH. 2002. Targeted deletion of a high-affinity GATA-binding site in the GATA-1 promoter leads to selective loss of the eosinophil lineage in vivo. J. Exp. Med. 195:1387–1395. 10.1084/jem.20020656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Galli SJ, Tsai M. 2012. IgE and mast cells in allergic disease. Nat. Med. 18:693–704. 10.1038/nm.2755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Migliaccio AR, Rana RA, Sanchez M, Lorenzini R, Centurione L, Bianchi L, Vannucchi AM, Migliaccio G, Orkin SH. 2003. GATA-1 as a regulator of mast cell differentiation revealed by the phenotype of the GATA-1low mouse mutant. J. Exp. Med. 197:281–296. 10.1084/jem.20021149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ghinassi B, Sanchez M, Martelli F, Amabile G, Vannucchi AM, Migliaccio G, Orkin SH, Migliaccio AR. 2007. The hypomorphic Gata1low mutation alters the proliferation/differentiation potential of the common megakaryocytic-erythroid progenitor. Blood 109:1460–1471. 10.1182/blood-2006-07-030726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harigae H, Takahashi S, Suwabe N, Ohtsu H, Gu L, Yang Z, Tsai FY, Kitamura Y, Engel JD, Yamamoto M. 1998. Differential roles of GATA-1 and GATA-2 in growth and differentiation of mast cells. Genes Cells 3:39–50. 10.1046/j.1365-2443.1998.00166.x [DOI] [PubMed] [Google Scholar]
- 12.Nishiyama C, Ito T, Nishiyama M, Masaki S, Maeda K, Nakano N, Ng W, Fukuyama K, Yamamoto M, Okumura K, Ogawa H. 2005. GATA-1 is required for expression of FcεRI on mast cells: analysis of mast cells derived from GATA-1 knockdown mouse bone marrow. Int. Immunol. 17:847–856. 10.1093/intimm/dxh278 [DOI] [PubMed] [Google Scholar]
- 13.Nishiyama C, Yokota T, Okumura K, Ra C. 1999. The transcription factors Elf-1 and GATA-1 bind to cell-specific enhancer elements of human high-affinity IgE receptor alpha-chain gene. J. Immunol. 163:623–630 [PubMed] [Google Scholar]
- 14.Maeda K, Nishiyama C, Tokura T, Akizawa Y, Nishiyama M, Ogawa H, Okumura K, Ra C. 2003. Regulation of cell type-specific mouse Fc epsilon RI beta-chain gene expression by GATA-1 via four GATA motifs in the promoter. J. Immunol. 170:334–340 [DOI] [PubMed] [Google Scholar]
- 15.Zon LI, Gurish MF, Stevens RL, Mather C, Reynolds DS, Austen KF, Orkin SH. 1991. GATA-binding transcription factors in mast cells regulate the promoter of the mast cell carboxypeptidase A gene. J. Biol. Chem. 266:22948–22953 [PubMed] [Google Scholar]
- 16.Ohmori S, Takai J, Ishijima Y, Suzuki M, Moriguchi T, Philipsen S, Yamamoto M, Ohneda K. 2012. Regulation of GATA factor expression is distinct between erythroid and mast cell lineages. Mol. Cell. Biol. 32:4742–4755. 10.1128/MCB.00718-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tsai FY, Orkin SH. 1997. Transcription factor GATA-2 is required for proliferation/survival of early hematopoietic cells and mast cell formation, but not for erythroid and myeloid terminal differentiation. Blood 89:3636–3643 [PubMed] [Google Scholar]
- 18.Takano H, Nakazawa S, Okuno Y, Shirata N, Tsuchiya S, Kainoh T, Takamatsu S, Furuta K, Taketomi Y, Naito Y, Takematsu H, Kozutsumi Y, Tsujimoto G, Murakami M, Kudo I, Ichikawa A, Nakayama K, Sugimoto Y, Tanaka S. 2008. Establishment of the culture model system that reflects the process of terminal differentiation of connective tissue-type mast cells. FEBS Lett. 582:1444–1450. 10.1016/j.febslet.2008.03.033 [DOI] [PubMed] [Google Scholar]
- 19.Bresnick EH, Lee HY, Fujiwara T, Johnson KD, Keles S. 2010. GATA switches as developmental drivers. J. Biol. Chem. 285:31087–31093. 10.1074/jbc.R110.159079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Suzuki M, Kobayashi-Osaki M, Tsutsumi S, Pan X, Ohmori S, Takai J, Moriguchi T, Ohneda O, Ohneda K, Shimizu R, Kanki Y, Kodama T, Aburatani H, Yamamoto M. 2013. GATA factor switching from GATA2 to GATA1 contributes to erythroid differentiation. Genes Cells 10.1111/gtc.12086 [DOI] [PubMed] [Google Scholar]
- 21.Grass JA, Boyer ME, Pal S, Wu J, Weiss MJ, Bresnick EH. 2003. GATA-1-dependent transcriptional repression of GATA-2 via disruption of positive autoregulation and domain-wide chromatin remodeling. Proc. Natl. Acad. Sci. U. S. A. 100:8811–8816. 10.1073/pnas.1432147100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Martowicz ML, Grass JA, Boyer ME, Guend H, Bresnick EH. 2005. Dynamic GATA factor interplay at a multicomponent regulatory region of the GATA-2 locus. J. Biol. Chem. 280:1724–1732. 10.1074/jbc.M406038200 [DOI] [PubMed] [Google Scholar]
- 23.Vyas P, McDevitt MA, Cantor AB, Katz SG, Fujiwara Y, Orkin SH. 1999. Different sequence requirements for expression in erythroid and megakaryocytic cells within a regulatory element upstream of the GATA-1 gene. Development 126:2799–2811 [DOI] [PubMed] [Google Scholar]
- 24.Nishimura S, Takahashi S, Kuroha T, Suwabe N, Nagasawa T, Trainor C, Yamamoto M. 2000. A GATA box in the GATA-1 gene hematopoietic enhancer is a critical element in the network of GATA factors and sites that regulate this gene. Mol. Cell. Biol. 20:713–723. 10.1128/MCB.20.2.713-723.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Charles MA, Saunders TL, Wood WM, Owens K, Parlow AF, Camper SA, Ridgway EC, Gordon DF. 2006. Pituitary-specific Gata2 knockout: effects on gonadotrope and thyrotrope function. Mol. Endocrinol. 20:1366–1377. 10.1210/me.2005-0378 [DOI] [PubMed] [Google Scholar]
- 26.Ishijima Y, Ohmori S, Uenishi A, Ohneda K. 2012. GATA transcription factors are involved in IgE-dependent mast cell degranulation by enhancing the expression of phospholipase C-γ1. Genes Cells 17:285–301. 10.1111/j.1365-2443.2012.01588.x [DOI] [PubMed] [Google Scholar]
- 27.Higashi AY, Ikawa T, Muramatsu M, Economides AN, Niwa A, Okuda T, Murphy AJ, Rojas J, Heike T, Nakahata T, Kawamoto H, Kita T, Yanagita M. 2009. Direct hematological toxicity and illegitimate chromosomal recombination caused by the systemic activation of CreERT2. J. Immunol. 182:5633–5640. 10.4049/jimmunol.0802413 [DOI] [PubMed] [Google Scholar]
- 28.Naiche LA, Papaioannou VE. 2007. Cre activity causes widespread apoptosis and lethal anemia during embryonic development. Genesis 45:768–775. 10.1002/dvg.20353 [DOI] [PubMed] [Google Scholar]
- 29.Salmon JM, Slater NJ, Hall MA, McCormack MP, Nutt SL, Jane SM, Curtis DJ. 2007. Aberrant mast-cell differentiation in mice lacking the stem-cell leukemia gene. Blood 110:3573–3581. 10.1182/blood-2006-10-053124 [DOI] [PubMed] [Google Scholar]
- 30.Tripic T, Deng W, Cheng Y, Zhang Y, Vakoc CR, Gregory GD, Hardison RC, Blobel GA. 2009. SCL and associated proteins distinguish active from repressive GATA transcription factor complexes. Blood 113:2191–2201. 10.1182/blood-2008-07-169417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Metcalf D, Majewski I, Mifsud S, Di Rago L, Alexander WS. 2007. Clonogenic mast cell progenitors and their excess numbers in chimeric BALB/c mice with inactivated GATA-1. Proc. Natl. Acad. Sci. U. S. A. 104:18642–18647. 10.1073/pnas.0709625104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ronnberg E, Melo FR, Pejler G. 2012. Mast cell proteoglycans. J. Histochem. Cytochem. 60:950–962. 10.1369/0022155412458927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mirghomizadeh F, Winoto-Morbach S, Orinska Z, Lee KH, Schutze S, Bulfone-Paus S. 2009. Intracellular IL-15 controls mast cell survival. Exp. Cell Res. 315:3064–3075. 10.1016/j.yexcr.2009.07.019 [DOI] [PubMed] [Google Scholar]
- 34.Nakajima T, Matsumoto K, Suto H, Tanaka K, Ebisawa M, Tomita H, Yuki K, Katsunuma T, Akasawa A, Hashida R, Sugita Y, Ogawa H, Ra C, Saito H. 2001. Gene expression screening of human mast cells and eosinophils using high-density oligonucleotide probe arrays: abundant expression of major basic protein in mast cells. Blood 98:1127–1134. 10.1182/blood.V98.4.1127 [DOI] [PubMed] [Google Scholar]
- 35.Lee JH, Kim YM, Kim NW, Kim JW, Her E, Kim BK, Kim JH, Ryu SH, Park JW, Seo DW, Han JW, Beaven MA, Choi WS. 2006. Phospholipase D2 acts as an essential adaptor protein in the activation of Syk in antigen-stimulated mast cells. Blood 108:956–964. 10.1182/blood-2005-10-009159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ord T, Ord D, Kuuse S, Plaas M, Ord T. 2012. Trib3 is regulated by IL-3 and affects bone marrow-derived mast cell survival and function. Cell. Immunol. 280:68–75. 10.1016/j.cellimm.2012.11.011 [DOI] [PubMed] [Google Scholar]
- 37.Prodeus AP, Zhou X, Maurer M, Galli SJ, Carroll MC. 1997. Impaired mast cell-dependent natural immunity in complement C3-deficient mice. Nature 390:172–175. 10.1038/36586 [DOI] [PubMed] [Google Scholar]
- 38.Fureder W, Agis H, Willheim M, Bankl HC, Maier U, Kishi K, Muller MR, Czerwenka K, Radaszkiewicz T, Butterfield JH, Klappacher GW, Sperr WR, Oppermann M, Lechner K, Valent P. 1995. Differential expression of complement receptors on human basophils and mast cells. Evidence for mast cell heterogeneity and CD88/C5aR expression on skin mast cells. J. Immunol. 155:3152–3160 [PubMed] [Google Scholar]
- 39.Guo Q, Subramanian H, Gupta K, Ali H. 2011. Regulation of C3a receptor signaling in human mast cells by G protein coupled receptor kinases. PLoS One 6:e22559. 10.1371/journal.pone.0022559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Saluja R, Delin I, Nilsson GP, Adner M. 2012. FεRI-mediated mast cell reactivity is amplified through prolonged Toll-like receptor-ligand treatment. PLoS One 7:e43547. 10.1371/journal.pone.0043547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Burd PR, Thompson WC, Max EE, Mills FC. 1995. Activated mast cells produce interleukin 13. J. Exp. Med. 181:1373–1380. 10.1084/jem.181.4.1373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Reed JA, Albino AP, McNutt NS. 1995. Human cutaneous mast cells express basic fibroblast growth factor. Lab. Invest. 72:215–222 [PubMed] [Google Scholar]
- 43.Collington SJ, Hallgren J, Pease JE, Jones TG, Rollins BJ, Westwick J, Austen KF, Williams TJ, Gurish MF, Weller CL. 2010. The role of the CCL2/CCR2 axis in mouse mast cell migration in vitro and in vivo. J. Immunol. 184:6114–6123. 10.4049/jimmunol.0904177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Juremalm M, Nilsson G. 2005. Chemokine receptor expression by mast cells. Chem. Immunol. Allergy 87:130–144. 10.1159/000087640 [DOI] [PubMed] [Google Scholar]
- 45.Geng L, Pfister S, Kraeft SK, Rudd CE. 2001. Adaptor FYB (Fyn-binding protein) regulates integrin-mediated adhesion and mediator release: differential involvement of the FYB SH3 domain. Proc. Natl. Acad. Sci. U. S. A. 98:11527–11532. 10.1073/pnas.191378198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vig M, DeHaven WI, Bird GS, Billingsley JM, Wang H, Rao PE, Hutchings AB, Jouvin MH, Putney JW, Kinet JP. 2008. Defective mast cell effector functions in mice lacking the CRACM1 pore subunit of store-operated calcium release-activated calcium channels. Nat. Immunol. 9:89–96. 10.1038/ni1550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nakamura Y, Franchi L, Kambe N, Meng G, Strober W, Nunez G. 2012. Critical role for mast cells in interleukin-1β-driven skin inflammation associated with an activating mutation in the nlrp3 protein. Immunity. 37:85–95. 10.1016/j.immuni.2012.04.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kim D, Kim SH, Cho SH, Shin K, Kim S. 2011. SOCS3 suppresses the expression of IL-4 cytokine by inhibiting the phosphorylation of c-Jun through the ERK signaling pathway in rat mast cell line RBL-2H3. Mol. Immunol. 48:776–781. 10.1016/j.molimm.2010.11.005 [DOI] [PubMed] [Google Scholar]
- 49.Santos DD, Hatjiharissi E, Tournilhac O, Chemaly MZ, Leleu X, Xu L, Patterson C, Branagan AR, Manning RJ, Ho AW, Hunter ZR, Dimmock EA, Kutok JL, Churchill WH, Castells MC, Tai YT, Anderson KC, Treon SP. 2006. CD52 is expressed on human mast cells and is a potential therapeutic target in Waldenstrom's macroglobulinemia and mast cell disorders. Clin. Lymphoma Myeloma 6:478–483. 10.3816/CLM.2006.n.029 [DOI] [PubMed] [Google Scholar]
- 50.Diaz-Agustin B, Escribano L, Bravo P, Herrero S, Nunez R, Navalon R, Navarro L, Torrelo A, Cantalapiedra A, Del Castillo L, Villarrubia J, Navarro JL, San Miguel JF, Orfao A. 1999. The CD69 early activation molecule is overexpressed in human bone marrow mast cells from adults with indolent systemic mast cell disease. Br. J. Haematol. 106:400–405. 10.1046/j.1365-2141.1999.01572.x [DOI] [PubMed] [Google Scholar]
- 51.Wong GW, Yasuda S, Morokawa N, Li L, Stevens RL. 2004. Mouse chromosome 17A3.3 contains 13 genes that encode functional tryptic-like serine proteases with distinct tissue and cell expression patterns. J. Biol. Chem. 279:2438–2452. 10.1074/jbc.M308209200 [DOI] [PubMed] [Google Scholar]
- 52.Reimer JM, Samollow PB, Hellman L. 2010. High degree of conservation of the multigene tryptase locus over the past 150 to 200 million years of mammalian evolution. Immunogenetics 62:369–382. 10.1007/s00251-010-0443-2 [DOI] [PubMed] [Google Scholar]
- 53.Fujiwara T, O'Geen H, Keles S, Blahnik K, Linnemann AK, Kang YA, Choi K, Farnham PJ, Bresnick EH. 2009. Discovering hematopoietic mechanisms through genome-wide analysis of GATA factor chromatin occupancy. Mol. Cell 36:667–681. 10.1016/j.molcel.2009.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yu M, Riva L, Xie H, Schindler Y, Moran TB, Cheng Y, Yu D, Hardison R, Weiss MJ, Orkin SH, Bernstein BE, Fraenkel E, Cantor AB. 2009. Insights into GATA-1-mediated gene activation versus repression via genome-wide chromatin occupancy analysis. Mol. Cell 36:682–695. 10.1016/j.molcel.2009.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Soler E, Andrieu-Soler C, de Boer E, Bryne JC, Thongjuea S, Stadhouders R, Palstra RJ, Stevens M, Kockx C, van Ijcken W, Hou J, Steinhoff C, Rijkers E, Lenhard B, Grosveld F. 2010. The genome-wide dynamics of the binding of Ldb1 complexes during erythroid differentiation. Genes Dev. 24:277–289. 10.1101/gad.551810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Masuda A, Hashimoto K, Yokoi T, Doi T, Kodama T, Kume H, Ohno K, Matsuguchi T. 2007. Essential role of GATA transcriptional factors in the activation of mast cells. J. Immunol. 178:360–368 [DOI] [PubMed] [Google Scholar]
- 57.Dyer KD, Czapiga M, Foster B, Foster PS, Kang EM, Lappas CM, Moser JM, Naumann N, Percopo CM, Siegel SJ, Swartz JM, Ting-De Ravin S, Rosenberg HF. 2007. Eosinophils from lineage-ablated Delta dblGATA bone marrow progenitors: the dblGATA enhancer in the promoter of GATA-1 is not essential for differentiation ex vivo. J. Immunol. 179:1693–1699 [DOI] [PubMed] [Google Scholar]
- 58.Takemoto CM, Brandal S, Jegga AG, Lee YN, Shahlaee A, Ying Y, Dekoter R, McDevitt MA. 2010. PU.1 positively regulates GATA-1 expression in mast cells. J. Immunol. 184:4349–4361. 10.4049/jimmunol.0900927 [DOI] [PubMed] [Google Scholar]
- 59.Thakurdas SM, Melicoff E, Sansores-Garcia L, Moreira DC, Petrova Y, Stevens RL, Adachi R. 2007. The mast cell-restricted tryptase mMCP-6 has a critical immunoprotective role in bacterial infections. J. Biol. Chem. 282:20809–20815. 10.1074/jbc.M611842200 [DOI] [PubMed] [Google Scholar]
- 60.Shin K, Watts GF, Oettgen HC, Friend DS, Pemberton AD, Gurish MF, Lee DM. 2008. Mouse mast cell tryptase mMCP-6 is a critical link between adaptive and innate immunity in the chronic phase of Trichinella spiralis infection. J. Immunol. 180:4885–4891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhang J, Sun J, Lindholt JS, Sukhova GK, Sinnamon M, Stevens RL, Adachi R, Libby P, Thompson RW, Shi GP. 2011. Mast cell tryptase deficiency attenuates mouse abdominal aortic aneurysm formation. Circ. Res. 108:1316–1327. 10.1161/CIRCRESAHA.111.243758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lilla JN, Chen CC, Mukai K, BenBarak MJ, Franco CB, Kalesnikoff J, Yu M, Tsai M, Piliponsky AM, Galli SJ. 2011. Reduced mast cell and basophil numbers and function in Cpa3-Cre; Mcl-1fl/fl mice. Blood 118:6930–6938. 10.1182/blood-2011-03-343962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Scholten J, Hartmann K, Gerbaulet A, Krieg T, Muller W, Testa G, Roers A. 2008. Mast cell-specific Cre/loxP-mediated recombination in vivo. Transgenic Res. 17:307–315. 10.1007/s11248-007-9153-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Otsuka A, Kubo M, Honda T, Egawa G, Nakajima S, Tanizaki H, Kim B, Matsuoka S, Watanabe T, Nakae S, Miyachi Y, Kabashima K. 2011. Requirement of interaction between mast cells and skin dendritic cells to establish contact hypersensitivity. PLoS One 6:e25538. 10.1371/journal.pone.0025538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kitajima K, Zheng J, Yen H, Sugiyama D, Nakano T. 2006. Multipotential differentiation ability of GATA-1-null erythroid-committed cells. Genes Dev. 20:654–659. 10.1101/gad.1378206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.May G, Soneji S, Tipping AJ, Teles J, McGowan SJ, Wu M, Guo Y, Fugazza C, Brown J, Karlsson G, Pina C, Olariu V, Taylor S, Tenen DG, Peterson C, Enver T. 2013. Dynamic analysis of gene expression and genome-wide transcription factor binding during lineage specification of multipotent progenitors. Cell. Stem Cell 13:754–768. 10.1016/j.stem.2013.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cantor AB, Iwasaki H, Arinobu Y, Moran TB, Shigematsu H, Sullivan MR, Akashi K, Orkin SH. 2008. Antagonism of FOG-1 and GATA factors in fate choice for the mast cell lineage. J. Exp. Med. 205:611–624. 10.1084/jem.20070544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Arinobu Y, Iwasaki H, Gurish MF, Mizuno S, Shigematsu H, Ozawa H, Tenen DG, Austen KF, Akashi K. 2005. Developmental checkpoints of the basophil/mast cell lineages in adult murine hematopoiesis. Proc. Natl. Acad. Sci. U. S. A. 102:18105–18110. 10.1073/pnas.0509148102 [DOI] [PMC free article] [PubMed] [Google Scholar]