Abstract
Dopamine (DA) differentially modulates identified neurons in the crustacean stomatogastric nervous system (STNS). While the electrophysiological actions of DA have been well characterized, little is known about the dopaminergic transduction cascades operating in this system. As a first step toward illuminating the molecular underpinnings of dopaminergic signal transduction in the crustacean STNS, we have cloned and characterized two type-one DA receptors (DARs) from the spiny lobster (Panulirus interruptus): D1αPan and D1βPan. We found that the structure and function of these arthropod DARs are well conserved across species. Using a heterologous expression system, we determined that DA, but not serotonin, octopamine, tyramine or histamine activates these receptors. When stably expressed in HEK cells, the D1αPan receptor couples with Gs, and DA elicits an increase in [cAMP]. The D1βPan receptor responds to DA with a net increase in [cAMP] that is mediated by Gs and Gz.
Keywords: Central pattern generator, STG, Signal transduction, cAMP, Neuron, Invertebrate, Neuromodulation, G protein coupled receptor
1. Introduction
Monoamines have a variety of physiological and behavioral effects in arthropods (Tierney et al., 2004; Strawn et al., 2000; Cooper and Neckameyer, 1999). The role of neuromodulation in fashioning multiple outputs from a single circuit has long been appreciated. In this regard, important insights have been realized from studies on the STNS in Decapod crustaceans (Hooper and DiCaprio, 2004; Nusbaum and Beenhakker, 2002). Peptidergic and monoaminergic modulation of STNS circuits have been studied extensively at the anatomical and electro-physiological levels (Beltz, 1999; Harris-Warrick et al., 1992; Nusbaum, 2002). DA is known to alter both synaptic and intrinsic properties of stomatogastric neurons in a cell specific manner (Bucher et al., 2003; Cleland and Selverston, 1997; Harris-Warrick et al., 1998; Johnson et al., 2003a; Kloppenburg et al., 1999; Peck et al., 2001); however, little is known about the signal transduction cascades that generate these physiological responses.
Dopaminergic responses are mediated through multiple DARs that comprise an evolutionarily conserved family of G protein coupled receptors (GPCRs). DARs are thought to have evolved initially from gene duplication and drift leading to 2 related paralogous genes defining two different subfamilies: D1 and D2 (Callier et al., 2003; Kapsimali et al., 2003; Le Crom et al., 2003). To date, all DARs can be broadly classified into these two subfamilies on the basis of conserved structure and signaling mechanisms. In general, type 1 DARs preferentially couple to Gs to increase adenylyl cyclase activity while type 2 receptors preferentially couple with Gi/o to decrease adenylyl cyclase activity (Neve et al., 2004). Pharmacology is also used to classify vertebrate DARs. Pharmacological profiles are not conserved across vertebrate/invertebrate lines, however, so vertebrate pharmacology cannot be used when classifying arthropod receptors as D1 vs. D2.
The natural history of D1 receptors has been well studied for vertebrates, but much less is known for vertebrate D2 receptors. In addition, the orthologous relationships for vertebrate and invertebrate DARs are unknown (Kapsimali et al., 2003). Seven DAR subtypes exist in the phylum chordata: four D1 subtypes (D1/D1A, D1B/D5, D1C, D1D) and three D2 subtypes (D2, D3, D4). A given class (e.g., mammal, teleost, reptile, etc.) may possess only a subset of the seven. For example, only five DAR subtypes are represented in mammals: D1/D1A, D1B/D5, D2, D3, D4. There are three well-characterized DARs in the phylum arthropoda (Blenau et al., 1998; Feng et al., 1996; Gotzes et al., 1994; Han et al., 1996; Hearn et al., 2002; Sugamori et al., 1995). Two of these receptors can be classified as type 1, and one of these receptors can be classified as type 2. A fourth arthropod receptor that responds to DA with a slight, but significant increase in cAMP has recently been cloned (Srivastava et al., 2005). This receptor also responds strongly to ecdysteroids, and further characterization is necessary to determine if this receptor should be classified as belonging to the DAR family.
As a first step toward defining the dopaminergic transduction cascades operating in the STNS, we have cloned and characterized the two known arthropod type 1 receptors from the spiny lobster, Panulirus interruptus: D1αPan and D1βPan. In the work presented here, we define the G protein and second messenger couplings for each receptor, and examine which monoamines activate these receptors.
2. Materials and methods
2.1. Cloning and expression in a heterologous system
The three lobster DARs were cloned from nervous tissue of spiny lobster P. interruptus using a degenerate PCR strategy with conventional library screening and RACE technology as previously described (Clark et al., 2004). Full length constructs were created and inserted into a pIRESneo3 vector (B.D. Biosciences Clontech, Palo Alto, CA, USA) using standard recombinant techniques. Constructs were stably expressed in HEK cells using methods previously described (Clark et al., 2004). The data have been submitted to GenBank under accession numbers DQ295790 (D1αPan) and DQ295791 (D1β.1Pan).
2.2. Membrane preparations
Stably transfected cells were harvested with trypsin (ATCC, Manassas, VA, USA) or cell stripper (Media Tech, Herndon, VA, USA). Pellets were homogenized in 20 mM HEPES (pH 7.4) containing 2 mM MgCl2, 1 mM EDTA, 2 mM 1,4-dithiothreitol (DTT), 1 µg/mL leupeptin, 1 µg/mL aprotinin, and 2 mM PMSF. The homogenate was centrifuged at 1000 × g for 5 min. The supernatant was recovered and centrifuged at 20,000 × g for 30 min at 4 °C. Pellets were resuspended in 20 mM HEPES (pH 7.4) containing 0.5% 3-[(3 cholamidopropyl) dimethylammonio]-1-propanesulfonate (CHAPS), and 2 mM EDTA. For some experiments, samples were stored at − 70 °C until assayed. Protein concentrations in each sample were determined using a BCA Protein Assay Kit (Pierce).
2.3. G protein activation assay
Agonist-induced activation of specific G proteins was determined using an assay based on a well-established, previously described protocol (Zhou and Murthy, 2004). In these experiments, individual wells of a 96-well break-apart plate (Fisher Scientific) were UV sterilized in a tissue culture hood for 15 min. At this point, wells were denoted either as blanks or coated. Coated wells received an antibody against one human Gα subunit [EMD/Calbiochem catalog #371778 (G12α), #371723 (Gi1/2α), #371751 (Gqα), #371726 (Gi3/oα), #371732 (Gsα), or #371741 (Gzα)]. Antibodies were diluted to a concentration of 20 µg/mL in sterile phosphate-buffered saline, and 50 µL were aliquoted to separate wells. Plates were incubated on ice. After 2 h, the liquid was removed from the coated wells. Both coated and blank wells were then completely filled with blocking solution (3% BSA, 0.06% sodium azide in phosphate-buffered saline) and incubated on ice for 2 h. During this time, reactions were performed as follows. Membrane preparations from cell lines (1.5 µg/µL of protein) were incubated at 37 °C for 15 min in 10 mM HEPES (pH 7.4) containing 10 mM MgCl2, 100 µMEDTA and 10 nM GTPγ35S (Amersham) with or without DA. Reactions were terminated with ten volumes of termination buffer [10 mM MgCl2, 100 µM GDP, 200 mM NaCl in 100 mM Tris (pH 8.0)]. Fifty microliters of each terminated sample were then aliquoted in triplicate to both coated wells and blank wells (i.e., there are a total of six wells for each sample when measuring the activity of one G protein, nine wells for each sample when measuring the activity of two different G proteins, etc.). Plates were incubated on ice for 2 h. Wells were then rinsed three times with phosphate-buffered saline containing 0.3% Tween-20. Individual wells were placed in scintillation vials containing ScintiSafe Econo 1 (Fisher) and the radioactivity in each well was quantified with a scintillation counter. Resulting cpm from the blank wells were averaged and used as a measure of non-specific binding. The nonspecific binding was subtracted from the average cpm obtained from the coated wells. Data are expressed as cpm/µg of protein.
2.4. cAMP assays
cAMP levels were measured as previously described (Clark et al., 2004). Briefly, 1 × 105 cells were plated in 35 mm dishes and grown to confluence. Cells were washed with 1 mL of medium and preincubated at 37 °C for 10 min in the presence of the phosphodiesterase inhibitor 3-isobutyl-1-methylxanthine (2.5 mM) (Sigma). In some cases, cells were incubated an additional 30 min at 37 °C with or without forskolin (2.5 µM), and varying concentrations of DA. In some experiments, cells were pretreated for 24 h with pertussis toxin (PTX, Calbiochem) or 15 min with 1-O-Octadecyl-2-O-methyl-rac-glycero-3-phosphorylcholine (Et-18-OCH3, Calbiochem). The medium was removed and 0.5 mL of 0.1 M HCl (Sigma) with 0.8% Triton X-100 (Sigma) was added to the plates. After a 30 min incubation at room temperature, the lysate was removed from the plates and centrifuged for 2 min. The supernatant was collected and assayed for cAMP levels using a direct cAMP enzyme immunoassay kit (Assay Designs, Inc.) according to the manufacturer’s instructions. Protein concentrations in each sample were determined using a BCA Protein Assay Kit (Pierce). Data are expressed as picomoles of cAMP/milligram of protein.
2.5. Statistical analyses and curve fitting
Student t-tests were performed with Excel software. Curve fitting and Kruskall–Wallis (ANOVA on ranks) tests were performed with Prism (GraphPad Software, San Diego, CA, USA, www.graphpad.com). In all cases, statistical significance was determined as p<0.05.
3. Results
3.1. The family of dopamine receptors is conserved across different classes of arthropods
To begin to elucidate the dopaminergic systems in the STNS, we cloned the two known arthropod type-one DARs from P. interruptus: D1αPan and D1βPan. The D1αPan receptor is orthologous to the Drosophila receptor DAMB/DopR99B (Feng et al., 1996; Han et al., 1996) and the D1βPan receptor is orthologous to the Drosophila receptor Dmdop1/dDA1 (Blenau et al., 1998; Gotzes et al., 1994; Sugamori et al., 1995). Fig. 1 illustrates that orthologs show high homology. Paired alignments of spiny lobster and Drosophila D1α and D1β orthologs revealed 44% and 37% amino acid identity over the entire protein, respectively. Most differences across species occur in the amino and carboxy termini and intracellular loop 3, which is typical for GPCRs (Clark et al., 2004; Sosa et al., 2004). Indeed, the idea of divergent termini is emphasized by the fact that the gene for the D1β receptor is alternately spliced to produce two proteins with different amino termini, D1β.1Pan and D1β.2Pan (Table 1; Fig. 1). Interestingly, and contrary to the idea that the carboxy termini often diverge, both D1βPan orthologs end in a conserved PDZ domain. However, we have not performed an exhaustive search for alternate splice forms, and it is possible that there are additional alternately spliced exons for both D1αPan and D1βPan receptors.
Fig. 1.
The DAR family is conserved across arthropods. The Panulirus (L) and Drosophila (F) DAR orthologs are aligned. Amino acids that are identical in fly and lobster orthologs are highlighted for each pair of DARs. Black bars approximate the seven transmembrane regions. The point of alternate splicing on lobster D1βPan is indicated by a black arrowhead. The accession numbers are as follows: LD1αPan, DQ295790; FD1αPan, U34383; LD1β.1Pan, DQ295791; FD1βPan, X77234.1.
Table 1.
Alternate splicing of D1βPan
| Location | Isoform | Exon configuration |
Amino acid sequencea |
|---|---|---|---|
| N-terminus | D1β.1Pan | D1β N-terminal exon 1 | MGERPPGDDMSepsdi… |
| D1β.2Pan | D1β N-terminal exon 2 | MKTVIESSAMTNITDDQepsdi… |
Capital letters represent alternately spliced exons, lower case letters represent amino acids present in all isoforms examined.
3.2. D1αPan couples with Gs in HEK cells to produce an increase in [cAMP]i
We next characterized receptor couplings in a heterologous expression system. When bound by ligand, activated DARs function as guanine nucleotide exchange factors (GEFs), causing inactive heterotrimeric G proteins to exchange GDP for GTP. The trimeric G protein then dissociates into Gα and Gβγ subunits, each of which interacts with downstream effectors (Cabrera-Vera et al., 2003). Since vertebrate and insect D1 receptors preferentially couple with Gs to stimulate adenylyl cyclase and increase cAMP levels (Feng et al., 1996; Han et al., 1996; Neve et al., 2004), we predicted that the D1αPan receptor should do likewise. To test this prediction, full-length D1αPan constructs were assembled using standard recombinant techniques. The constructs were then stably expressed in HEK cells, and the resulting cell line, HEK D1αPan, was assayed for changes in G protein activity and cAMP levels.
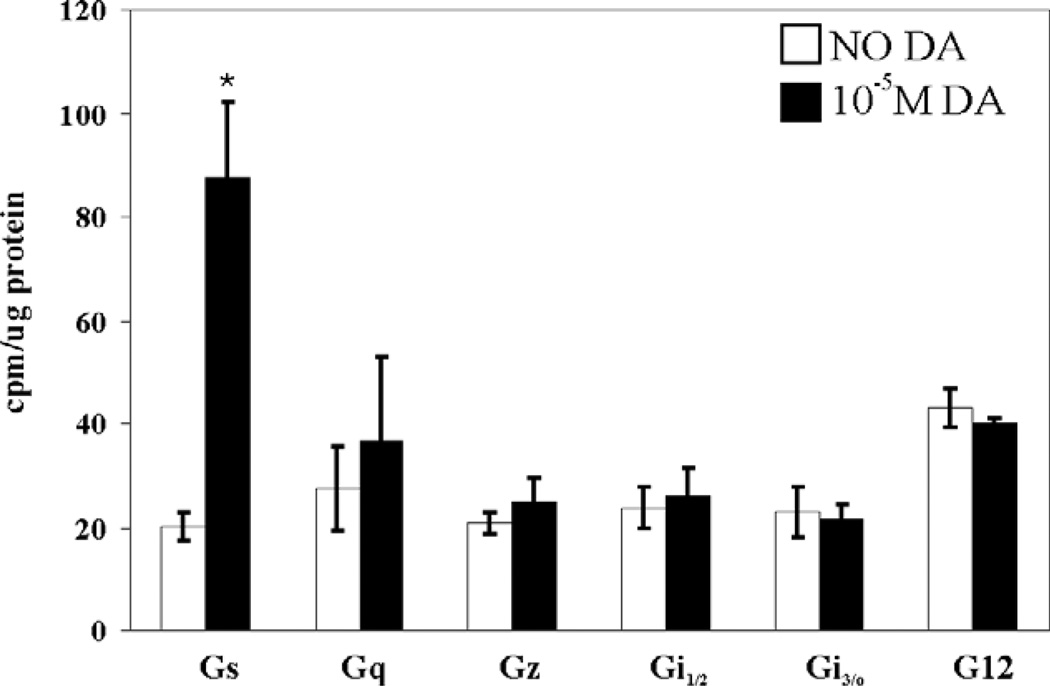
We first developed and performed a “G protein activation assay” based on minor modifications to a previously described protocol (Zhou and Murthy, 2004). In this assay, the wells of a break-apart 96-well plate are pre-coated with commercially available antibodies against the various human G proteins. Membrane fractions of a human cell line stably expressing a lobster receptor are prepared and incubated with or without DA in a solution containing GTPγ35S, a labeled, nonhydrolyzable form of GTP. Activated DARs cause coupled G proteins to exchange GDP for GTPγ35S. The reactions are terminated and dispensed into the pre-coated wells. After two hours the wells are washed to remove material that is not recognized by the anti-G protein antibody, and the bound radioactivity is quantified with a scintillation counter. The cpm associated with each antibody-coated well are a measure of the activation of the corresponding G protein. Fig. 2 illustrates that D1αPan couples exclusively with Gαs. Exposure to 10−5 M DA for 15 min induced a ~4-fold increase in Gαs activity (p<0.3), but did not significantly increase the activity of any of the other G proteins examined (Gαq, Gαi1, Gαi2, Gαi3, Gαo, Gαz, Gα12).
Fig. 2.
The D1αPan receptor couples with Gs. G protein activities in HEK D1αPan membrane preparations were measured in the absence (open bar) vs. the presence (filled bar) of 10−5 M DA for eight G proteins: Gs, Gq, Gz, Gi1, Gi2, Gi3, Go, G12. Data represent the mean±S.E.M., n=3. Statistically significant differences in the activity of a given G protein are indicated with an asterisk (p<0.05).
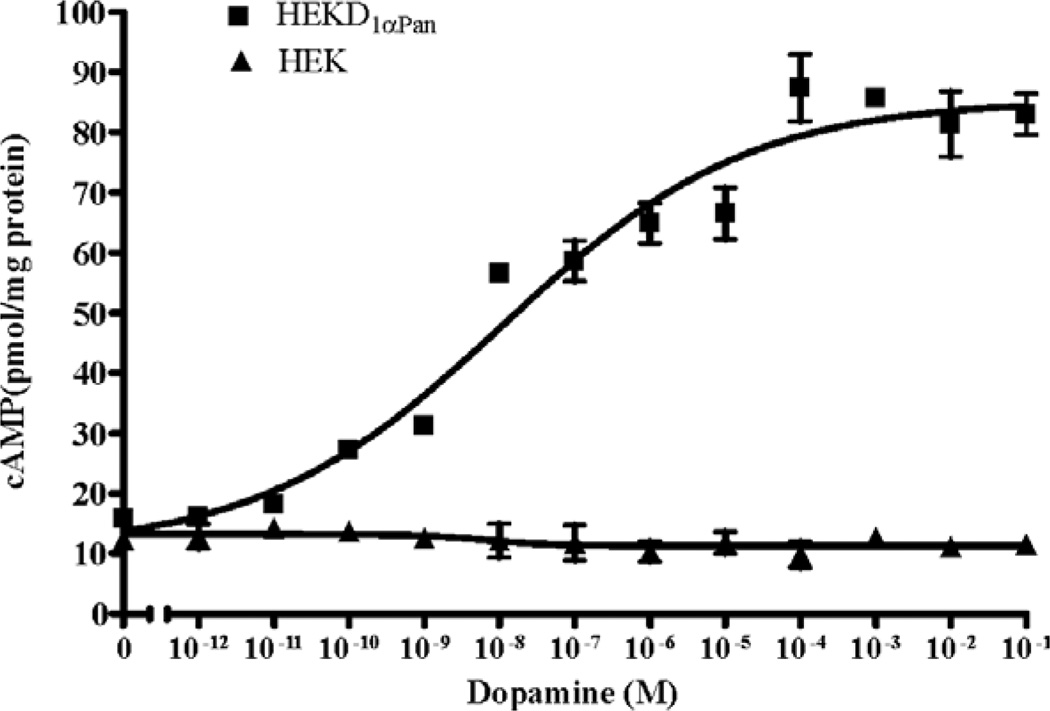
DA induced activation of Gs should produce an increase in cAMP via the positive coupling of Gαs to adenylyl cyclase. We therefore measured cAMP levels in HEKD1αPan and parental HEK cells in response to DA. Fig. 3 illustrates that DA caused a dose dependent increase in HEK D1αPan cAMP levels, with an EC50 of 1.1 × 10−8 M. However, DA had no effect on cAMP levels in the parental HEK cell line. Constitutive activity, or agonist independent activity, is a well documented characteristic of many GPCRs (Seifert and Wenzel-Seifert, 2002). It can be identified as a significant increase in second messenger levels under baseline conditions (no ligand in media) in transfected cell lines expressing a GPCR relative to the parental cell lines that do not express the receptor. In the absence of DA, levels of cAMP are not significantly different between the HEK D1αPan and HEK cell lines (Fig. 3), suggesting D1αPan is not constitutively active when expressed in HEK cells. Similarly, the mammalian D1/1A receptor does not appear to be constitutively active in heterologous expression systems (Missale et al., 1998).
Fig.3.
DA activation of the D1αPan receptor increases [cAMP]. The D1αPan receptor couples positively with cAMP. Changes in cAMP levels in response to increasing [DA] were measured in a stably transfected cell line expressing the D1αPan receptor, HEK D1αPan (filled squares) and in the nontransfected parental cell line, HEK (filled triangles). Data are represented as the mean±S.E.M, n=3.
3.3. D1βPan couples with Gs and Gz in HEK cells, resulting in increased [cAMP]i
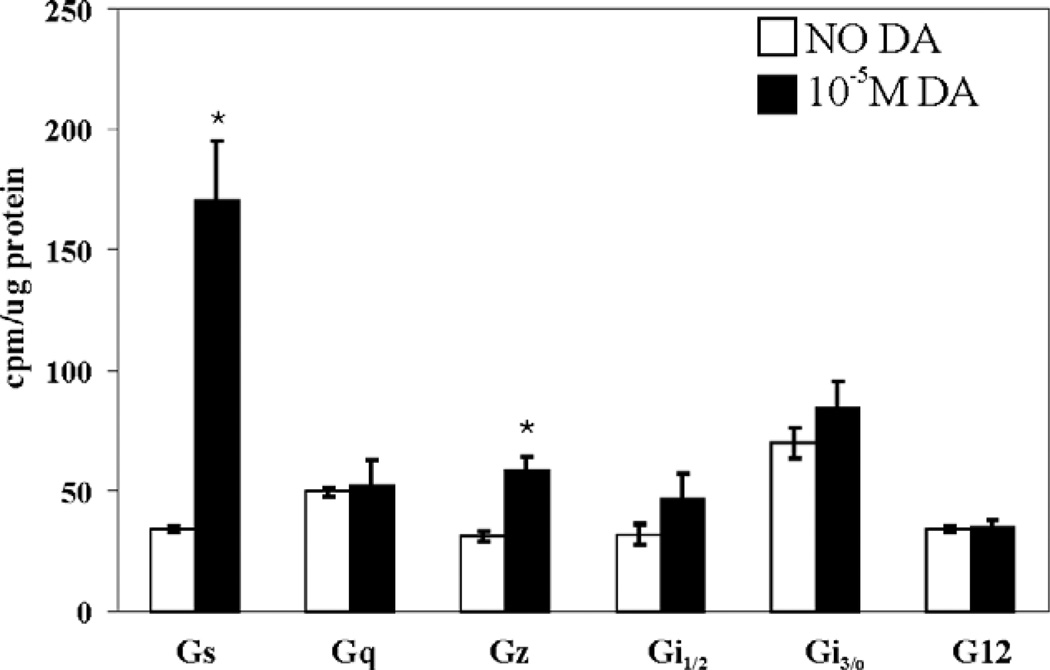
Insect orthologs of the arthropod D1β receptor have previously been shown to positively couple with adenylyl cyclase, suggesting that this receptor couples to Gs (Blenau et al., 1998; Gotzes et al., 1994; Sugamori et al., 1995). We performed the G protein activation assay on the HEKD1βPan cell lines to determine D1βPan receptor-G protein coupling. Fig. 4 indicates that the D1βPan receptor couples with multiple G proteins. A 15 min exposure to 10 µM DA produced a ~5-fold increase in Gαs activity (p<0.002). DA also produced a significant 1.6-fold increase in the activity of Gαz (p<0.004), a PTX insensitive member of the Gi/o family that negatively couples with adenylyl cyclase to reduce cAMP levels (Ho and Wong, 2001). The stimulation of Gs was roughly 3 times larger than that of Gz. The human D1B/D5 receptor has also been shown to couple with Gs and Gz in GH4C1 cells (Sidhu et al., 1998).
Fig. 4.
The D1βPan receptor couples with Gs and Gz. G protein activities in HEK D1βPan membrane preparations were measured in the absence (open bar) vs. the presence (filled bar) of 10−5 M DA for eight G proteins: Gs, Gq, Gz, Gai1, Gai2, Gai3, Gao, Ga12. Data represent the mean±S.E.M., n≥3. Statistically significant differences in the activity of a given G protein are indicated with an asterisk (p<0.05).
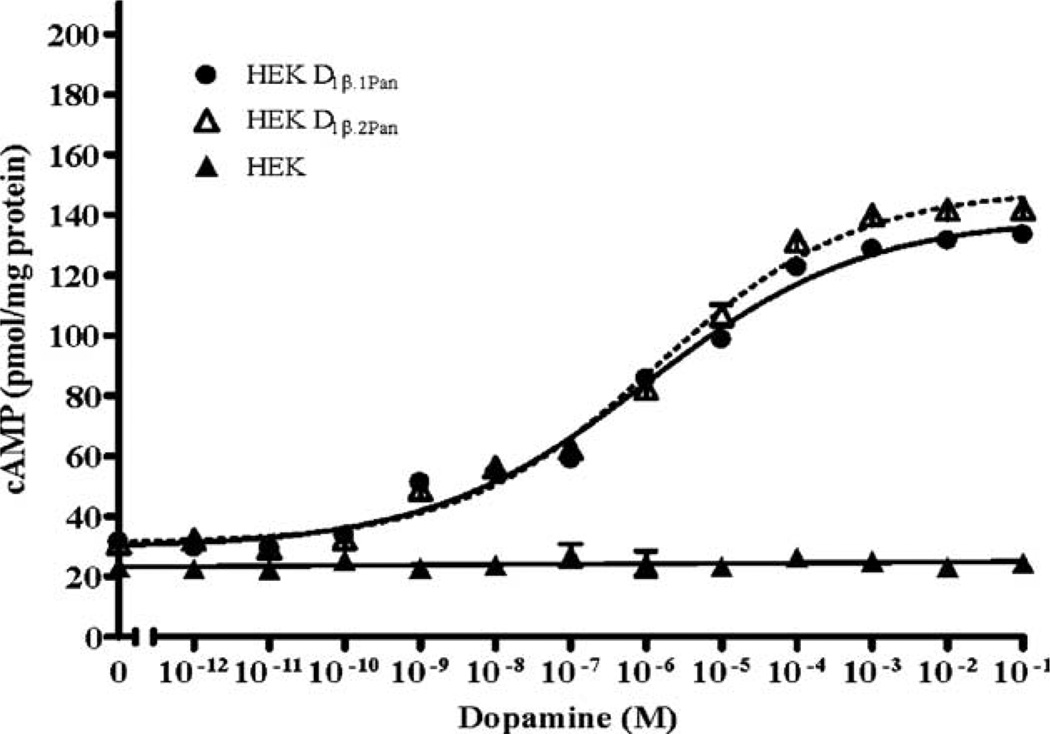
Fig. 3 indicates that the D1βPan receptor couples with G proteins that regulate adenylyl cyclase in opposing directions (i.e., Gs increases adenylyl cyclase activity while Gz decreases adenylyl cyclase activity). Since DA induced Gs activity was three times larger than DA induced Gz activity, we predicted that DA should elicit a net increase in cAMP in HEKD1βPan cell lines. Fig. 5 illustrates that stable cell lines expressing different isoforms of the full-length lobster D1β receptor (HEK D1β.1Pan and HEK D1β.2Pan) show a dose dependent increase in cAMP in response to increasing concentrations of DA, with EC50s between 1 and 1.4 × 10−6. In addition, the D1βPan isoforms appear to be constitutively active. As shown in Fig. 5, in the absence of DA cAMP levels were significantly higher in HEK D1βPan cells relative to parental cells (p<10−5 for both isoforms). Thus, both isoforms of the D1βPan receptor display agonist independent activity like the mammalian D1B/D5 receptor (Demchyshyn et al., 2000). The data do not indicate whether coupling with Gz was also constitutive.
Fig. 5.
DA activation of the D1βPan receptor produces a net increase in cAMP. The D1βPan receptor couples positively with cAMP. Changes in cAMP levels in response to increasing [DA] were measured in the nontransfected parental cell line (HEK, filled triangles) and in two stably transfected cell lines expressing the D1βPan receptor. We identified two isoforms for the D1βPan receptor, and established cell lines for each: HEK D1β.1Pan (circles) and HEK D1β.2Pan (open triangle). Data represent the mean±S.E.M., n=5.
3.4. Dopamine activates lobster type 1 DA-Rs
Monoamines act as circulating neurohormones and neurotransmitters in the STNS. Five endogenous biogenic amines can modulate STNS neurons: dopamine, serotonin (5-HT), tyramine, octopamine and histamine. In some cases, it has been reported that arthropod DARs can respond to multiple monoamines in heterologous expression systems (Hearn et al., 2002). Activation of lobster DARs by multiple monoamines could have important implications for monoaminergic signal transduction in the STNS. We therefore asked which of the endogenous monoamines could activate D1αPan and D1βPan receptors.
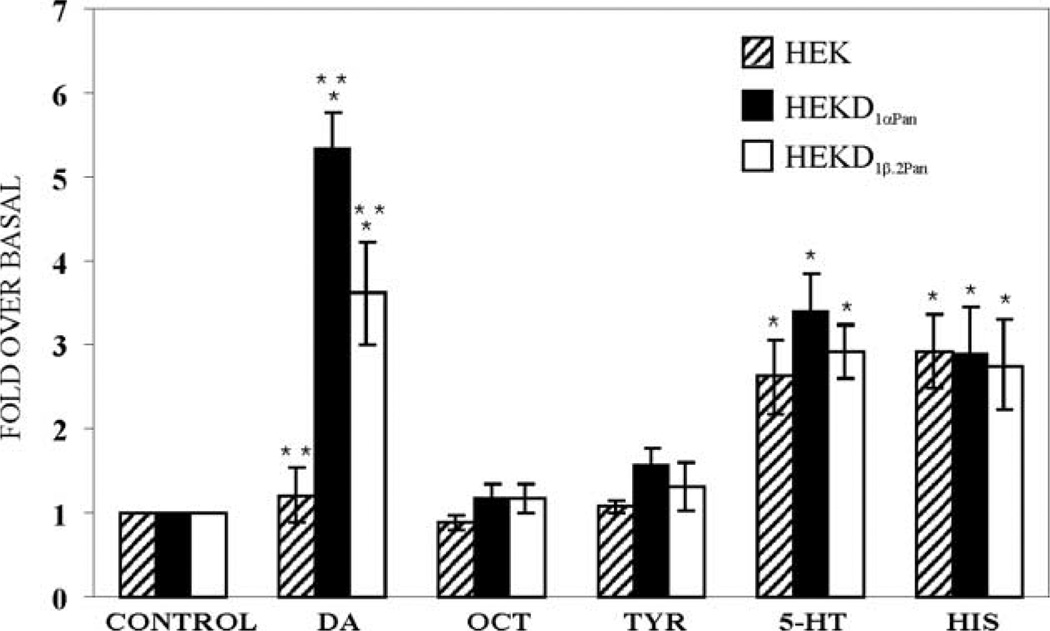
Levels of cAMP were measured in three cell lines (HEK, HEKD1αPan and HEKD1β.2Pan) before and after exposure to one of the five monoamines. Fig. 6 illustrates that DA activation of D1αPan and D1βPan produced significant, approximately 5.3- and 3.6-fold increases in cAMP levels in the HEK D1αPan and HEKD1βPan cell lines, respectively, but had no significant effect on the parental HEK cell line. Thus, the heterologously expressed receptors are responsible for the DA-induced increase in cAMP in HEKD1αPan and HEKD1βPan cell lines.
Fig. 6.
Dopamine is the only endogenous monoamine that activates the D1αPan and D1βPan receptors. Levels of cAMP were measured in HEK (hatched bars), HEKD1αPan (filled bars) and HEKD1βPan.2 (open bars) cell lines under control conditions (no monoamines present) or in the presence of 1 mM of the indicated monoamine. The cAMP levels measured in the presence of the indicated monoamine were normalized by cAMP levels under control conditions. Average fold changes over basal cAMP levels are plotted, error bars indicate the S.E.M, n≥3. * Indicates significant increases in cAMP over basal levels (p<0.05). ** Indicates significant differences (p<0.05) between cell lines within the same condition (e.g., differences between cell lines exposed to DA).
At a concentration of 1 mM, octopamine and tyramine had no significant effect on any of the three cell lines examined. On the other hand, Fig. 6 demonstrates that 1 mM 5-HT produced a significant, roughly 3-fold increase in cAMP in HEK cells, suggesting that the parental cell line contains endogenous 5-HT receptors that are positively coupled to adenylyl cyclase, as has been previously reported (Johnson et al., 2003b). The same increase was observed in the HEKD1αPan and HEKD1βPan cell lines. The 5-HT induced cAMP increases in all three cell lines were not significantly different from one another, suggesting that the responses are due to the endogenous 5-HT receptors and not the heterologously expressed DARs.
Similarly, the parental HEK cell line appears to express endogenous histamine receptors, as 1 mM histamine produced a significant, approximately 3-fold increase in cAMP in HEK cells. This increase was also observed in the HEKD1αPan and HEKD1βPan cell lines. The responses in the three cell lines were not significantly different from one another, suggesting that they were due to the endogenous histamine receptors and not the heterologously expressed DARs. In summary, DA activates D1αPan and D1βPan receptors, but serotonin, histamine, octopamine and tyramine do not.
4. Discussion
The work presented here represents the first step toward defining the molecular underpinnings of dopaminergic neuromodulation in the STNS. We have shown that the structure and function of the spiny lobster DARs, D1αPan and D1βPan, are conserved across class and phyla. D1αPan couples with Gs to increase cAMP while D1βPan couples with Gs and Gz to produce a net increase in cAMP. Moreover, of the 5 biogenic amines tested, only DA activated these receptors.
In all systems, dopaminergic effects are mediated through GPCRs that interact with G proteins. Both G proteins and GPCRs are well conserved across vertebrate/invertebrate lines, especially with regard to interaction domains. Indeed, Table 2 shows that the C-terminal domain of the G protein, which physically interacts with the GPCR, is identical for homologous G proteins in lobsters and humans! There are 6 Gα proteins in arthropods: Gαs, Gαf (Gs-like at the DNA level), Gαq, Gαi, Gαo, Gα12 (http://flybase.net/). Three of the G proteins have been cloned from lobster (McClintock et al., 1992, 1997; Xu et al., 1997). As shown in Table 2, the C-termini of lobster Gαs, Gαq and Gαi are completely conserved with their human homologs. Thus, it is reasonable to probe the coupling specificity of spiny lobster GPCRs in human cell lines.
Table 2.
Comparison of G protein C-termini across species
| Gαs | H: RMHLRQYELL L: RMHLRQYELL |
| Gαq | H: QLNLKEYNLV L: QLNLKEYNLV |
| Gαi1 / 2 | H: KNNLKDCGLF L: KNNLKDCGLF |
| Gαo | H: AKNLRGCGLY F: ANNLRGCGLY |
| Gα12 | H: QENLKDIMLQ F: QRNLNALMLQ |
| Gαf | F: SENVSSMGLF |
H = human, L = lobster, F = fly.
Traditionally, DARs were thought to couple only with Gs to increase [cAMP]i and Gi/o to decrease [cAMP]i; however, recent studies in heterologous expression systems, including the one presented here, suggest that DAR-G protein coupling can be much more diverse (Sidhu and Niznik, 2000). For example, D1/D1A can couple with Gs and Go in reconstitution experiments or in over expression experiments with rat pituitary GH4C1 cells (Kimura et al., 1995a,b). D1B/D5 can couple with Gs and Gz in GH4C1 cells (Sidhu et al., 1998), or with Gs and G12 in immortalized rat renal proximal tubule cells (Zheng et al., 2003). D2 receptors couple with multiple members of the Gi/o family (Banihashemi and Albert, 2002; Ghahremani et al., 1999; Obadiah et al., 1999). When expressed in CHO cells, human D3 receptors may couple with the Gi/o and Gq families (Newman-Tancredi et al., 1999). Studies in native systems also suggest non-classical coupling such that D1 receptors may couple with Gs and Gq in the mammalian and C. elegans CNS (Chase et al., 2004; O'Sullivan et al., 2004; Undie et al., 2000; Wersinger et al., 2003; Zhen et al., 2004).
It is interesting that the lobster D1βPan receptor, like the human D1B/D5 receptor, can couple with both Gs and Gz. We do not know the proteins that interact with the lobster DARs to facilitate multiple couplings. Both post-translational modifications and receptor-interacting proteins can cause a receptor to switch G protein coupling. When phosphorylated by protein kinase A, the β2-adrenergic receptor switches its coupling from Gs to Gi (Baillie et al., 2003; Daaka et al., 1997). Receptor coupling can also be extended by receptor-interacting proteins, like calcyon, which regulates receptor cross-talk and allows D1 receptors to switch coupling between Gs and Gq (Lezcano et al., 2000). It is not obvious why this receptor would couple to multiple cascades that have opposing effects on cAMP. It is possible that the population of cells is heterogeneous so that there is only one type of coupling per cell. On the other hand, when simultaneously activated, opposing cascades in a single cell may be highly localized so that microdomains of cAMP gradients are created (Zaccolo and Pozzan, 2002). Alternatively the cascades may function with different kinetics and interact to generate feedback loops and/or multiphasic responses.
Acknowledgements
We thank Nadja Spitzer and Elizabeth Prince for useful comments on the manuscript. This work was supported, in part, by NIH NS38770 and the STC Program of the National Science Foundation, IBN-9876754. MCC is an MBD fellow.
References
- Baillie GS, Sood A, McPhee I, Gall I, Perry SJ, Lefkowitz RJ, Houslay MD. beta-Arrestin-mediated PDE4 cAMP phosphodiesterase recruitment regulates beta-adrenoceptor switching from Gs to Gi. Proc. Natl. Acad. Sci. U. S. A. 2003;100:940–945. doi: 10.1073/pnas.262787199. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Banihashemi B, Albert PR. Dopamine-D2S receptor inhibition of calcium influx, adenylyl cyclase, and mitogen-activated protein kinase in pituitary cells: distinct Galpha and Gbetagamma requirements. Mol. Endocrinol. 2002;16:2393–2404. doi: 10.1210/me.2001-0220. [DOI] [PubMed] [Google Scholar]
- Beltz BS. Distribution and functional anatomy of amine-containing neurons in decapod crustaceans. Microsc. Res. Tech. 1999;44:105–120. doi: 10.1002/(SICI)1097-0029(19990115/01)44:2/3<105::AID-JEMT5>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Blenau W, Erber J, Baumann A. Characterization of a dopamine D1 receptor from Apis mellifera: cloning, functional expression, pharmacology, and mRNA localization in the brain. J. Neurochem. 1998;70:15–23. doi: 10.1046/j.1471-4159.1998.70010015.x. [DOI] [PubMed] [Google Scholar]
- Bucher D, Thirumalai V, Marder E. Axonal dopamine receptors activate peripheral spike initiation in a stomatogastric motor neuron. J. Neurosci. 2003;23:6866–6875. doi: 10.1523/JNEUROSCI.23-17-06866.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabrera-Vera TM, Vanhauwe J, Thomas TO, Medkova M, Preininger A, Mazzoni MR, Hamm HE. Insights into G protein structure, function, and regulation. Endocr. Rev. 2003;24:765–781. doi: 10.1210/er.2000-0026. [DOI] [PubMed] [Google Scholar]
- Callier S, Snapyan M, Le Crom S, Prou D, Vincent JD, Vernier P. Evolution and cell biology of dopamine receptors in vertebrates. Biol. Cell. 2003;95:489–502. doi: 10.1016/s0248-4900(03)00089-3. [DOI] [PubMed] [Google Scholar]
- Chase DL, Pepper JS, Koelle MR. Mechanism of extrasynaptic dopamine signaling in Caenorhabditis elegans. Nat. Neurosci. 2004;7:1096–1103. doi: 10.1038/nn1316. [DOI] [PubMed] [Google Scholar]
- Clark MC, Dever TE, Dever JJ, Xu P, Rehder V, Sosa MA, Baro DJ. Arthropod 5-HT2 receptors: a neurohormonal receptor in decapod crustaceans that displays agonist independent activity resulting from an evolutionary alteration to the DRY motif. J. Neurosci. 2004;24:3421–3435. doi: 10.1523/JNEUROSCI.0062-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleland TA, Selverston AI. Dopaminergic modulation of inhibitory glutamate receptors in the lobster stomatogastric ganglion. J. Neurophysiol. 1997;78:3450–3452. doi: 10.1152/jn.1997.78.6.3450. [DOI] [PubMed] [Google Scholar]
- Cooper RL, Neckameyer WS. Dopaminergic modulation of motor neuron activity and neuromuscular function in Drosophila melanogaster. Comp. Biochem. Physiol. Part B. 1999 Feb;122(2):199–210. doi: 10.1016/s0305-0491(98)10160-8. [DOI] [PubMed] [Google Scholar]
- Daaka Y, Luttrell LM, Lefkowitz RJ. Switching of the coupling of the beta2-adrenergic receptor to different G proteins by protein kinase A. Nature. 1997;390:88–91. doi: 10.1038/36362. [DOI] [PubMed] [Google Scholar]
- Demchyshyn LL, McConkey F, Niznik HB. Dopamine D5 receptor agonist high affinity and constitutive activity profile conferred by carboxyl-terminal tail sequence. J. Biol. Chem. 2000;275:23446–23455. doi: 10.1074/jbc.M000157200. [DOI] [PubMed] [Google Scholar]
- Feng G, Hannan F, Reale V, Hon YY, Kousky CT, Evans PD, Hall LM. Cloning and functional characterization of a novel dopamine receptor from Drosophila melanogaster. J. Neurosci. 1996;16:3925–3933. doi: 10.1523/JNEUROSCI.16-12-03925.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghahremani MH, Cheng P, Lembo PM, Albert PR. Distinct roles for Galphai2, Galphai3, and Gbeta gamma in modulation of forskolin- or Gs-mediated cAMP accumulation and calcium mobilization by dopamine D2S receptors. J. Biol. Chem. 1999;274:9238–9245. doi: 10.1074/jbc.274.14.9238. [DOI] [PubMed] [Google Scholar]
- Gotzes F, Balfanz S, Baumann A. Primary structure and functional characterization of a Drosophila dopamine receptor with high homology to human D1/5 receptors. Recept. Channels. 1994;2:131–141. [PubMed] [Google Scholar]
- Han KA, Millar NS, Grotewiel MS, Davis RL. DAMB, a novel dopamine receptor expressed specifically in Drosophila mushroom bodies. Neuron. 1996;16:1127–1135. doi: 10.1016/s0896-6273(00)80139-7. [DOI] [PubMed] [Google Scholar]
- Harris-Warrick RM, Nagy F, Nusbaum MP. Neuromodulation of stomatogastric networks by identified neurons and transmitters. In: Harris-Warrick RM, Marder E, Selverston AI, Moulins M, editors. Dynamic Biological Networks: The Stomatogastric Nervous System. Cambridge: MIT Press; 1992. pp. 87–137. [Google Scholar]
- Harris-Warrick RM, Johnson BR, Peck JH, Kloppenburg P, Ayali A, Skarbinski J. Distributed effects of dopamine modulation in the crustacean pyloric network. Ann. N. Y. Acad. Sci. 1998;860:155–167. doi: 10.1111/j.1749-6632.1998.tb09046.x. [DOI] [PubMed] [Google Scholar]
- Hearn MG, Ren Y, McBride EW, Reveillaud I, Beinborn M, Kopin AS. A Drosophila dopamine 2-like receptor: molecular characterization and identification of multiple alternatively spliced variants. Proc. Natl. Acad. Sci. U. S. A. 2002;99:14554–14559. doi: 10.1073/pnas.202498299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho MK, Wong YH. G(z) signaling: emerging divergence from G(i) signaling. Oncogene. 2001;20:1615–1625. doi: 10.1038/sj.onc.1204190. [DOI] [PubMed] [Google Scholar]
- Hooper SL, DiCaprio RA. Crustacean motor pattern generator networks. Neurosignals. 2004;13:50–69. doi: 10.1159/000076158. [DOI] [PubMed] [Google Scholar]
- Johnson BR, Kloppenburg P, Harris-Warrick RM. Dopamine modulation of calcium currents in pyloric neurons of the lobster stomatogastric ganglion. J. Neurophysiol. 2003a;90:631–643. doi: 10.1152/jn.00037.2003. [DOI] [PubMed] [Google Scholar]
- Johnson MS, Lutz EM, Firbank S, Holland PJ, Mitchell R. Functional interactions between native Gs-coupled 5-HT receptors in HEK-293 cells and the heterologously expressed serotonin transporter. Cell. Signal. 2003b;15:803–811. doi: 10.1016/s0898-6568(03)00013-5. [DOI] [PubMed] [Google Scholar]
- Kapsimali M, Le Crom S, Vernier P. A natural history of vertebrate dopamine receptors. In: A S, M L, P V, editors. Dopamine Receptors and Transporters: Function, Imaging and Clinical Implication. second edn. New York: Marcel Dekker, Inc.; 2003. pp. 1–43. [Google Scholar]
- Kimura K, Sela S, Bouvier C, Grandy DK, Sidhu A. Differential coupling of D1 and D5 dopamine receptors to guanine nucleotide binding proteins in transfected GH4C1 rat somatomammotrophic cells. J. Neurochem. 1995a;64:2118–2124. doi: 10.1046/j.1471-4159.1995.64052118.x. [DOI] [PubMed] [Google Scholar]
- Kimura K, White BH, Sidhu A. Coupling of human D-1 dopamine receptors to different guanine nucleotide binding proteins. Evidence that D-1 dopamine receptors can couple to both Gs and G(o) J. Biol. Chem. 1995b;270:14672–14678. doi: 10.1074/jbc.270.24.14672. [DOI] [PubMed] [Google Scholar]
- Kloppenburg P, Levini RM, Harris-Warrick RM. Dopamine modulates two potassium currents and inhibits the intrinsic firing properties of an identified motor neuron in a central pattern generator network. J. Neurophysiol. 1999;81:29–38. doi: 10.1152/jn.1999.81.1.29. [DOI] [PubMed] [Google Scholar]
- Le Crom S, Kapsimali M, Barome PO, Vernier P. Dopamine receptors for every species: gene duplications and functional diversification in craniates. J. Struct. Funct. Genomics. 2003;3:161–176. [PubMed] [Google Scholar]
- Lezcano N, Mrzljak L, Eubanks S, Levenson R, Goldman-Rakic P, Bergson C. Dual signaling regulated by calcyon, a D1 dopamine receptor interacting protein. Science. 2000;287:1660–1664. doi: 10.1126/science.287.5458.1660. [DOI] [PubMed] [Google Scholar]
- McClintock TS, Byrnes AP, Lerner MR. Molecular cloning of a G-protein alpha i subunit from the lobster olfactory organ. Brain Res. Mol. Brain Res. 1992;14:273–276. doi: 10.1016/0169-328x(92)90183-c. [DOI] [PubMed] [Google Scholar]
- McClintock TS, Xu F, Quintero J, Gress AM, Landers TM. Molecular cloning of a lobster G alpha(q) protein expressed in neurons of olfactory organ and brain. J. Neurochem. 1997;68:2248–2254. doi: 10.1046/j.1471-4159.1997.68062248.x. [DOI] [PubMed] [Google Scholar]
- Missale C, Nash SR, Robinson SW, Jaber M, Caron MG. Dopamine receptors: from structure to function. Physiol. Rev. 1998;78:189–225. doi: 10.1152/physrev.1998.78.1.189. [DOI] [PubMed] [Google Scholar]
- Neve KA, Seamans JK, Trantham-Davidson H. Dopamine receptor signaling. J. Recept. Signal Transduct. Res. 2004;24:165–205. doi: 10.1081/rrs-200029981. [DOI] [PubMed] [Google Scholar]
- Newman-Tancredi A, Cussac D, Audinot V, Pasteau V, Gavaudan S, Millan MJ. G protein activation by human dopamine D3 receptors in high-expressing Chinese hamster ovary cells: a guanosine-5′-O-(3-[35S] thio)-triphosphate binding and antibody study. Mol. Pharmacol. 1999;55:564–574. [PubMed] [Google Scholar]
- Nusbaum MP. Regulating peptidergic modulation of rhythmically active neural circuits. Brain Behav. Evol. 2002;60:378–387. doi: 10.1159/000067791. [DOI] [PubMed] [Google Scholar]
- Nusbaum MP, Beenhakker MP. A small-systems approach to motor pattern generation. Nature. 2002;417:343–350. doi: 10.1038/417343a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Sullivan GJ, Roth BL, Kinsella A, Waddington JL. SK and F 83822 distinguishes adenylyl cyclase from phospholipase C-coupled dopamine D1-like receptors: behavioural topography. Eur. J. Pharmacol. 2004;486:273–280. doi: 10.1016/j.ejphar.2004.01.004. [DOI] [PubMed] [Google Scholar]
- Obadiah J, Avidor-Reiss T, Fishburn CS, Carmon S, Bayewitch M, Vogel Z, Fuchs S, Levavi-Sivan B. Adenylyl cyclase interaction with the D2 dopamine receptor family; differential coupling to Gi, Gz, and Gs. Cell. Mol. Neurobiol. 1999;19:653–664. doi: 10.1023/A:1006988603199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peck JH, Nakanishi ST, Yaple R, Harris-Warrick RM. Amine modulation of the transient potassium current in identified cells of the lobster stomatogastric ganglion. J. Neurophysiol. 2001;86:2957–2965. doi: 10.1152/jn.2001.86.6.2957. [DOI] [PubMed] [Google Scholar]
- Seifert R, Wenzel-Seifert K. Constitutive activity of G-protein-coupled receptors: cause of disease and common property of wild-type receptors. Naunyn Schmiedebergs Arch. Pharmacol. 2002;366:381–416. doi: 10.1007/s00210-002-0588-0. [DOI] [PubMed] [Google Scholar]
- Sidhu A, Niznik HB. Coupling of dopamine receptor subtypes to multiple and diverse G proteins. Int. J. Dev. Neurosci. 2000;18:669–677. doi: 10.1016/s0736-5748(00)00033-2. [DOI] [PubMed] [Google Scholar]
- Sidhu A, Kimura K, Uh M, White BH, Patel S. Multiple coupling of human D5 dopamine receptors to guanine nucleotide binding proteins Gs and Gz. J. Neurochem. 1998;70:2459–2467. doi: 10.1046/j.1471-4159.1998.70062459.x. [DOI] [PubMed] [Google Scholar]
- Sosa MA, Spitzer N, Edwards DH, Baro DJ. A crustacean serotonin receptor: cloning and distribution in the thoracic ganglia of crayfish and freshwater prawn. J. Comp. Neurol. 2004;473:526–537. doi: 10.1002/cne.20092. [DOI] [PubMed] [Google Scholar]
- Srivastava DP, Yu EJ, Kennedy K, Chatwin H, Reale V, Hamon M, Smith T, Evans PD. Rapid, nongenomic responses to ecdysteroids and catecholamines mediated by a novel Drosophila G-protein-coupled receptor. J. Neurosci. 2005;25:6145–6155. doi: 10.1523/JNEUROSCI.1005-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strawn JR, Neckameyer WS, Cooper RL. The effects of 5-HT on sensory, central and motor neurons driving the abdominal superficial flexor muscles in the crayfish. Comp. Biochem. Physiol Part B. 2000 Dec;127(4):533–550. doi: 10.1016/s0305-0491(00)00287-x. [DOI] [PubMed] [Google Scholar]
- Sugamori KS, Demchyshyn LL, McConkey F, Forte MA, Niznik HB. A primordial dopamine D1-like adenylyl cyclase-linked receptor from Drosophila melanogaster displaying poor affinity for benzazepines. FEBS Lett. 1995;362:131–138. doi: 10.1016/0014-5793(95)00224-w. [DOI] [PubMed] [Google Scholar]
- Tierney AJ, Greenlaw MA, Dams-O’Connor K, Aig SD, Perna AM. Behavioral effects of serotonin and serotonin agonists in two crayfish species, Procambarus clarkii and Orconectes rusticus. Comp. Biochem. Physiol Part A. 2004 Dec;139(4):495–502. doi: 10.1016/j.cbpb.2004.10.010. [DOI] [PubMed] [Google Scholar]
- Undie AS, Berki AC, Beardsley K. Dopaminergic behaviors and signal transduction mediated through adenylate cyclase and phospholipase C pathways. Neuropharmacology. 2000;39:75–87. doi: 10.1016/s0028-3908(99)00106-9. [DOI] [PubMed] [Google Scholar]
- Wersinger C, White BH, Sidhu A, Senogles SE. Physiology, pharmacology, and pathophysiology of the D1-like family of dopamine receptors in the central nervous system. In: Sidhu A, Laruelle M, Vernier P, editors. Dopamine Receptors and Transporters: Function, Imaging and Clinical Implication. New York: Marcel Dekker, Inc.; 2003. pp. 145–210. [Google Scholar]
- Xu F, Hollins B, Gress AM, Landers TM, McClintock TS. Molecular cloning and characterization of a lobster G alphaS protein expressed in neurons of olfactory organ and brain. J. Neurochem. 1997;69:1793–1800. doi: 10.1046/j.1471-4159.1997.69051793.x. [DOI] [PubMed] [Google Scholar]
- Zaccolo M, Pozzan T. Discrete microdomains with high concentration of cAMP in stimulated rat neonatal cardiac myocytes. Science. 2002;295:1711–1715. doi: 10.1126/science.1069982. [DOI] [PubMed] [Google Scholar]
- Zhen X, Goswami S, Abdali SA, Gil M, Bakshi K, Friedman E. Regulation of cyclin-dependent kinase 5 and calcium/calmodulin-dependent protein kinase II by phosphatidylinositol-linked dopamine receptor in rat brain. Mol. Pharmacol. 2004;66:1500–1507. doi: 10.1124/mol.104.002279. [DOI] [PubMed] [Google Scholar]
- Zheng S, Yu P, Zeng C, Wang Z, Yang Z, Andrews PM, Felder RA, Jose PA. Galpha12- and Galpha13-protein subunit linkage of D5 dopamine receptors in the nephron. Hypertension. 2003;41:604–610. doi: 10.1161/01.HYP.0000057422.75590.D7. [DOI] [PubMed] [Google Scholar]
- Zhou H, Murthy KS. Distinctive G protein-dependent signaling in smooth muscle by sphingosine 1-phosphate receptors S1P1 and S1P2. Am. J. Physiol Cell Physiol. 2004;286:C1130–C1138. doi: 10.1152/ajpcell.00429.2003. [DOI] [PubMed] [Google Scholar]