Abstract
The transcription factor GATA2 plays pivotal roles in early renal development, but its distribution and physiological functions in adult kidney are largely unknown. We examined the GATA2 expression pattern in the adult kidney by tracing green fluorescent protein (GFP) fluorescence in Gata2GFP/+ mice that recapitulate endogenous GATA2 expression and found a robust GFP expression specifically in the renal medulla. Upon purification of the GFP-positive cells, we found that collecting duct (CD)-specific markers, including aquaporin 2 (Aqp2), an important channel for water reabsorption from urine, were abundantly expressed. To address the physiological function of GATA2 in the CD cells, we generated renal tubular cell-specific Gata2-deficient mice (Gata2-CKO) by crossing Gata2 floxed mice with inducible Pax8-Cre mice. We found that the Gata2-CKO mice showed a significant decrease in Aqp2 expression. The Gata2-CKO mice exhibited high 24-h urine volume and low urine osmolality, two important signs of diabetes insipidus. We introduced biotin-tagged GATA2 into a mouse CD-derived cell line and conducted chromatin pulldown assays, which revealed direct GATA2 binding to conserved GATA motifs in the Aqp2 promoter region. A luciferase reporter assay using an Aqp2 promoter-reporter showed that GATA2 trans activates Aqp2 through the GATA motifs. These results demonstrate that GATA2 regulates the Aqp2 gene expression in CD cells and contributes to the maintenance of the body water homeostasis.
INTRODUCTION
Body water homeostasis is tightly regulated through the coordinated function of aquaporin (Aqp) that is expressed in the renal tubular cells. Of the various members of the Aqp family, Aqp1 is expressed in the proximal tubules and is responsible for constitutive reabsorption of water from primary urine. In addition, the final adjustment of urinary osmolality and its volume takes place in the renal collecting duct (CD), which comprises principal cells and intercalated cells (1). Three members of the Aqp family, i.e., Aqp2, Aqp3, and Aqp4, are expressed in principal cells of CD (1) and involved in arginine vasopressin (Avp)-mediated water reabsorption for tight control of body water balance. Avp is an antidiuretic hormone secreted from the hypothalamic neurons, and the primary target of the Avp signaling for urine volume regulation is Aqp2 (2, 3, 4). Avp binds to Avpr2 (Avp receptor type 2) in the principal cells of CD and subsequently induces phosphorylation of Aqp2 (via protein kinase A [PKA]) and its translocation to the luminal side of the principal cells. Aqp2 at the luminal surface of CD reabsorbs water from the tubular lumen to reduce urine volume and maintain systemic blood pressure (5). Since Aqp2-deficient mice exhibit severe urinary concentrating defects and polyuria, the importance of Aqp2 for body water balance has been well recognized (6).
GATA transcription factors are characterized by two zinc fingers that serve as DNA binding domains and are conserved among all six members of the GATA family (GATA1 through GATA6) (7). These zinc fingers bind most avidly to the consensus sequence 5′-(A/G)GATA(A/T)-3′ (8, 9). Among the six members of the GATA transcription factor family, GATA2 and GATA3 participate in the genetic program for renal and urinary tract development (10, 11). Of note, while a 271-kb Gata2 yeast artificial chromosome (YAC) transgene (Gata2 d16B YAC; spanning kb −198 to +73 of the mouse Gata2 locus) rescues Gata2-null mutant embryos from lethal hematopoietic deficiency, most of the YAC-rescued mutant neonates die of defects in urogenital development (12). We have identified two urogenital primordium-specific Gata2 distal enhancers, UG2 and UG4, between 75 kb and 113 kb 3′ to the Gata2 structural gene (13). Furthermore, we have demonstrated that Gata2 knockdown mice show similar urogenital anomalies, reminiscent of human congenital anomalies of the kidney and urinary tract (CAKUT) (14). These anomalies can be rescued by a GATA2 transgene driven by the UG4 distal urogenital enhancer of Gata2 gene (10, 14), indicating that GATA2 is indispensable for proper development of the urogenital system.
Perturbations of Avp signaling lead to nephrogenic diabetes insipidus (NDI), which is a clinical entity featuring abnormally large amounts of urine (15). The majority of congenital NDI cases are caused by mutation of either the Aqp2 or the Avpr2 gene, and the expression level of Aqp2 has been shown to be a critical determinant of normal water reabsorption in the principal cells (16). Long-term water deprivation induces Aqp2 gene expression through activating the Avpr2 and PKA/CREB (cyclic AMP-responsive element binding protein) pathway in the principal cells (17). Moreover, bilateral ureteral obstruction reduces Aqp2 gene expression levels and leads to large urine volume after release of the obstruction, which is reminiscent of acquired NDI (18). On the other hand, increased Aqp2 levels in pregnancy and congestive heart failure coincide with excessive water uptake in principal cells (19, 20). These lines of evidence indicate that regulation of Aqp2 expression levels is crucial for maintenance of the water reabsorption processes in the principal cells of CD. However, it remains uncertain how Aqp2 expression is regulated under physiological and pathological conditions in the principal cells.
Despite accumulating knowledge of GATA2 function during kidney development, GATA2 function in the adult kidney is still unexplored. In this study, we shed light on GATA2 function in the kidney and find that GATA2 is expressed predominantly in the principal cells of CD in adult mouse kidney. We demonstrate that in renal tubular cell-specific GATA2 knockout mice there is a 10-fold increase in urine production and a decrease in urine osmolality. This marked defect of urinary concentration is associated with reduced Aqp2 expression in the CD cells. Collectively, these results demonstrate, for the first time, the prominent participation of GATA2 in body water balance regulation in the kidney through regulation of Aqp2 gene expression.
MATERIALS AND METHODS
Mutant mice.
Gata2 GFP knock-in (Gata2GFP/+), Gata3 LacZ knock-in (Gata2LacZ/+), and Gata2flox mutant mice are maintained in a C57BL/6J genetic background (11, 21, 22). (tetO)7CMV-Cre transgenic mice were kindly provided by Jeffrey Whitsett (23). Rosa26TdTomato (R26T) reporter transgenic mice and Pax8-rtTA transgenic mice were purchased from Jackson Laboratory (24). Four-week-old mice were fed with 1 mg/ml doxycycline (Dox) in drinking water. After 4 weeks of continuous Dox feeding (at 8 weeks old), mice were subjected to analysis (see Fig. 3A). Genomic quantitative real-time PCR was performed to determine the recombination efficiency of the Gata2flox allele after knockout induction as described earlier (25). Primers used are listed in Table 1.
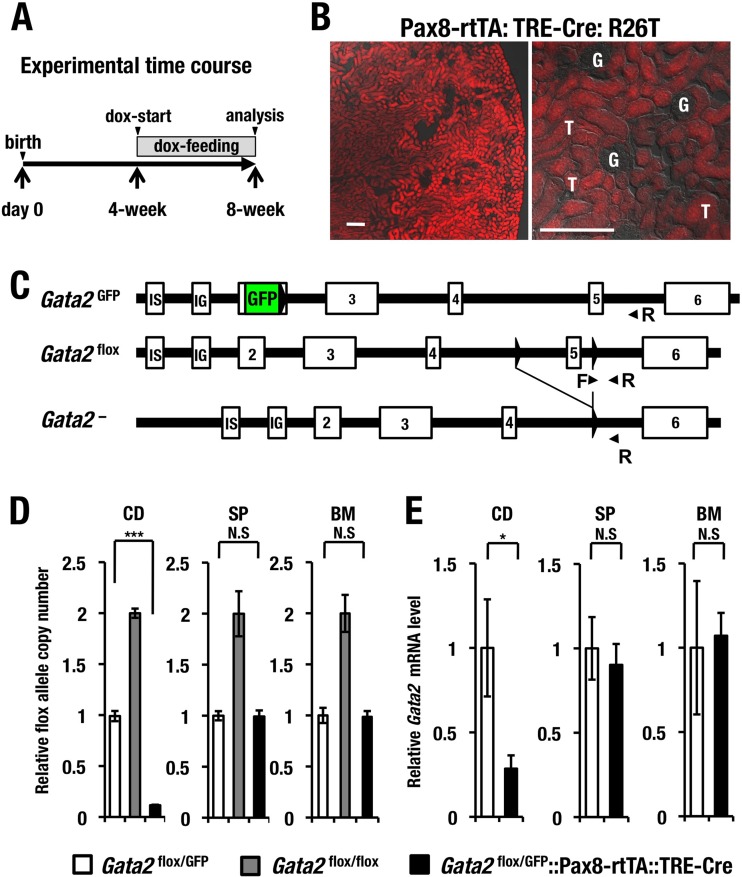
FIG 3.
Renal tubular cell-specific conditional deletion of Gata2 gene (Gata2-CKO). (A) Experimental time course of doxycycline (dox)-induced tubular cell-specific Gata2 deletion. (B) Cre activity indicated by tdTomato are observed exclusively in the tubular cells (T) but not in the glomeruli (G) of the kidney in the Pax8-rtTA::tetO-Cre::Rosa26TdTomato (R26T) reporter transgenic mice. Scale bars, 100 μm. (C) Schematic diagram of Gata2GFP, Gata2flox and recombined Gata2− alleles. Primer pairs used to detect the Gata2flox allele are depicted. (D) Genomic quantitative PCR analysis of the unrecombined Gata2flox allele in the collecting duct (CD), spleen (SP), and bone marrow (BM) cells. Data are shown as means ± SD derived from three independent experiments. (E) Gata2 mRNA expression level in the CD, SP, and BM cells of Gata2flox/GFP control and Gata2-CKO mice. Statistical significance of differences between Gata2flox/GFP (n = 3) and Gata2-CKO (n = 3) mice is depicted (***, P < 0.001; *, P < 0.05; N.S., not significant).
TABLE 1.
Sequence of primers used in quantitative genomic PCR, qRT-PCR, and genotyping
| Gene or genomic element | Sense primer | Antisense primer | Assay |
|---|---|---|---|
| Gata2 | ACCTGTGCAATGCCTGTGGG | TTGCACAACAGGTGCCCGCT | qRT-PCR |
| Gata3 | GGTGGACGTACTTTTTAACAT CGA | CCCTGACGGAGTTTCCGTAG | qRT-PCR |
| Ae1 (Slc4a1) | TATGGGGTCGCCCACATCTAT | AGGCCGAATCTGATCCTCGTA | qRT-PCR |
| Ae4 (Slc4a9) | CCAATTTCCTGGGCATCA | GGGCATCGGGATGAACTT | qRT-PCR |
| Aqp2 | CAGCTCGAAGGAAGGAGACA | GCATTGGCACCCTGGTTCA | qRT-PCR |
| Aqp3 | CTGGGGACCCTCATCCTT | TGGTGAGGAAGCCACCAT | qRT-PCR |
| Aqp4 | TGGAGGATTGGGAGTCACC | TGAACACCAACTGGAAAGTGA | qRT-PCR |
| Creb1 | CCAAACTAGCAGTGGGCAGT | CCCCATCCGTACCATTGTT | qRT-PCR |
| NCC (Slc12a3) | CCTCCATCACCAACTCACCT | CCGCCCACTTGCTGTAGTA | qRT-PCR |
| Nkcc2 (Slc12a1) | ATGCCTCGTATGCCAAATCT | CCCACATGTTGTAAATCCCATA | qRT-PCR |
| Sglt1 (Slc5a1) | CTGGCAGGCCGAAGTATG | TTCCAATGTTACTGGCAAAGAG | qRT-PCR |
| Sglt2 (Slc5a2) | GCTGGATTTGAGTGGAATGC | CGGTCAGATACACTGGCACA | qRT-PCR |
| Umod | CTCAGTGTCCAAGGCTGCTT | GGAAACAACAGCAGCCAGAT | qRT-PCR |
| Avpr2 | GGTCTCGGTCATCCAGTAGC | CTGGTGTCTACCACGTCTGC | qRT-PCR |
| Gapdh | GTCGTGGAGTCTACTGGTGTCTT | GAGATGATGACCCTTTTGGC | qRT-PCR |
| Gata2flox | AGGGACCGGGTACCATAACT | CCTCTAACCCTTCCCTGTCC | Genomic qPCR |
| Aqp2 promoter | CGAGGAAAACAGAGACGTCAA | AAGGCCTATCACCCCATCTT | Genomic qPCR |
| G1-14.4 kb | CAGGGCACAGCGAGTTTAGAG | CCTGTCCTTGGAGCTTGTGAA | Genomic qPCR |
| G1-3.8 kb | CCCTTATCTATGCCTTCCCA | GTGCAAGGCCCAGAAGTC | Genomic qPCR |
| G2-2.8 kb | GCCCTGTACAACCCCATTCTC | TTGTTCCCGGCGAAGATAAT | Genomic qPCR |
| GATA2 flox | TCCGTGGGACCTGTTTCCTTAC | GCCTGCGTCCTCCAACACCTCTAA | Genotyping |
| GATA2 GFP | CTGAAGTTCATCTGCACCACC | GAAGTTGTACTCCAGCTTGTGC | Genotyping |
| Pax8-rtTA | CCATGTCTAGACTGGACAAGA | CTCCAGGCCACATATGATTAG | Genotyping |
| TRE-Cre | ACGTTCACCGGCATCAACGT | CTGCATTACCGGTCGATGCA | Genotyping |
Generation of Gata2 conditional knockout mice (Gata2-CKO).
Gata2-CKO mice were generated by mating Gata2GFP/flox::Pax8-rtTA and Gata2GFP/flox::tetO-Cre mice. Offspring genotyped as Gata2GFP/flox::Pax8-rtTAtetO-Cre or Gata2flox/flox::Pax8-rtTA::tetO-Cre mice were named Gata2-CKO mice, while littermate mice (Gata2flox/flox or Gata2GFP/flox) were used as controls for all experiments. Genotyping primers for Gata2GFP, Gata2flox, Pax8-rtTA, and tetO-Cre are listed in Table 1. Genomic quantitative real-time reverse transcription-PCR (RT-PCR) was performed to determine the recombination efficiency of the mouse Gata2flox allele after knockout induction.
Cell culture and generation of stable transfected cell line.
mIMCD-3, an inner medullary collecting duct (IMCD) cell line, was maintained in Dulbecco's modified Eagle medium (DMEM)—F-12 with 10% fetal bovine serum (FBS), 40 units/ml penicillin, and 40 μg/ml streptomycin. mIMCD cells were transfected with an Escherichia coli BirA-expressing plasmid by the Neon transfection system (Invitrogen), and a stable transfected cell line was selected by 1 mg/ml G418 (Geneticin; Roche). FLBio-GATA2 plasmid was then introduced into the BirA-mIMCD to make a double stable transfected cell line and selected by 1 mg/ml G418 as well as 5 μg/ml puromycin (Sigma).
Plasmid construction, transfection, and luciferase reporter assay.
A 1.5-kb fragment containing the Aqp2 promoter was amplified from mouse kidney genomic DNA by primer STAR DNA polymerase (TaKaRa) with additional MluI and BglII sites (sense, 5′-ACTGACGCGTGACCCGTGTTGTGTATGTGG-3′; antisense, 5′-ACTGAGATCTAGAGGCTAGACTGTGGGCACT-3′). The amplified PCR product was ligated to pGEM-T easy vector (Promega) for generation of substituted mutations in GATA sites (pGEM-T-1.5kb-Aqp2). To mutate the GATA sites, pGEM-T-1.5kb-Aqp2 vector was amplified by primer STAR DNA polymerase with 5′-phosphorylated primers (sense, 5′-GTCAGCTGTGAAAGCTAAGATGGGGTAATAGGCCTTC-3′; antisense, 5′-TAATGTTCTCCTCATTAATGGACTCCAAATAAGGATTGAC-3′). The PCR product was digested with DpnI to eliminate the template plasmid DNA and then self-ligated. A 1.5-kb wild-type (WT)/mutated Aqp2 promoter was cut out and inserted into pGL3-Basic vector by MluI and BglII. pGL3-WT-Aqp2-LUC or pGL3-Mut-Aqp2-LUC was cotransfected with pEF-GATA2 and pRL-null into mIMCD cells by the Neon transfection system (Invitrogen). Forty-eight hours after transfection, luciferase activity was assayed by the dual-luciferase reporter assay system (Promega) and a Lumat LB 9507 luminometer (Berthold Technologies).
To construct the FLBio-GATA2 plasmid, GATA2 cDNA was amplified by PCR with additional BglII and XbaI digestion sites (sense, 5′-CGAGATCTCCATGGAGGTGGCGCCTGAGCAGCC-3′; antisense, 5′-CGTCTAGACTAGCCCATGGCAGTCACCATG-3′). The digested fragment was inserted into the BamHI and XbaI sites of pEF1α/Biotag vector (provided by A. B. Cantor, Children's Hospital and the Dana Farber Cancer Institute, Harvard Medical School).
qRT-PCR.
Total RNA was extracted by Isogen. cDNA was synthesized by SuperScript III (Invitrogen). Quantitative real-time RT-PCR (qRT-PCR) was performed with ABI 7300 and SYBR green master mix (Nippon Gene). The mRNA expression level was normalized to 18S rRNA. At least three Gata2flox/flox control and three Gata2-CKO mice were subjected to the analysis.
The molar ratio of Gata2 transcript against Gata3 transcript was calculated by the following equation (26): molar ratio of mRNA (Gata2/Gata3) = [Lg3 × (1 + Eg3)CTg3]/[Lg2 × (1 + Eg2)CTg2]. Lg2 and Lg3 indicate the lengths of the PCR products for Gata2 and Gata3 transcripts, respectively. Eg2 and Eg3 indicate the amplification efficiency of a primer set for Gata2 and Gata3 transcripts, respectively. CTg2 and CTg3 indicate the numbers of threshold cycles for the Gata2 and Gata3 transcripts, respectively. The primers used in real-time RT-PCR are listed in Table 1.
Renal tubular cell sorting by flow cytometry and biotinylated DBA/streptavidin magnet beads.
Mice were anesthetized and perfused with 10 ml cold phosphate-buffered saline (PBS) from heart to wash blood cells. After removal of kidney membrane, the kidney was immersed with 1 ml fresh-made digestion buffer (0.52 U/ml liberase TM [Roche] and 3 U/ml DNase I [Roche] in Hanks balanced salt solution [HBSS; Invitrogen]). Kidney was minced using scissors and incubated in a 37°C water bath for 2 h by gently pipetting every 30 min. After incubation, the cells were filtrated through a 40-μm cell strainer (BD Bioscience) to remove clots. Fluorescence-activated cell sorter (FACS) Aria (BD Bioscience) was used to separate the GFP-positive population from kidney of Gata2GFP/flox mice. GFP-positive cells were sorted into cold Isogen and stored at −80°C until use. For Dolichos biflorus agglutinin (DBA) magnetic sorting, the kidney cell suspension was incubated with biotinylated DBA (Vector Laboratories) in the cold room for 1 h. DBA-labeled cells were incubated with Dynabeads M-280 streptavidin (Life Technologies) and separated by magnet concentrator 4 times (Life Technologies).
Microarray analyses and data mining.
CD cells separated by biotinylated DBA labeling from Gata2-CKO and wild-type mice were pooled (3 mice/group) and subjected to a whole-mouse genome microarray analysis (4x44K; Agilent Technologies). The expression data were analyzed by Gene Spring software (Agilent Technologies). Heat maps were generated using Cluster 3.0 (http://bonsai.hgc.jp/∼mdehoon/software/cluster/) and JAVA Treeview (http://jtreeview.sourceforge.net/). The classification of the selected genes according to their biological functions was conducted using Ingenuity pathway analysis (IPA) software (Ingenuity system) and the Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway database (http://www.genome.jp/kegg/pathway.html).
Induction of Gata2flox allele recombination and measurement of urine volume and osmolality.
Four-week-old Gata2-CKO mice (Gata2GFP/flox::Pax8-rtTA::tetO-Cre or Gata2flox/flox::Pax8-rtTA::tetO-Cre) and littermate control mice (Gata2flox/flox::Pax8-rtTA or Gata2flox/flox) were fed with doxycycline hyclate solution (1 g/liter Dox [Sigma] and 50 g/liter sucrose [Wako] in double-distilled water) for 4 weeks to delete the Gata2flox allele. After knockout induction, the 24-h urine volume was measured by metabolic cage with a Dox solution supply and osmolality was also measured (SRL, Inc.).
Nuclear extraction, whole-kidney extraction, and Western blotting.
Nuclear extract was prepared from 70% confluent BirA-mIMCD and FLbioG2/BirA-mIMCD cells as described before (10). Equal amounts of nuclear extract were loaded onto 10% SDS-PAGE and transferred to a polyvinylidene difluoride (PVDF) membrane using a semidry device. The same membrane was stained first by anti-GATA2 (RC1.1.1) (12), and antibody was removed and then stained by anti-lamin B (M-20, sc-6217; Santa Cruz). The antibody was removed again and stained by a biotinylation detection kit (Vectastain Elite ABC kit; Vector Laboratories). Whole-kidney extraction was performed after PBS perfusion. One-half volume of buffer A (25 mM HEPES [pH 7.4], 3 mM EDTA, 1 mM dithiothreitol [DTT], 1× protease inhibitor cocktail) was added to one kidney, and the sample was homogenized and added to the same volume of buffer B (25 mM HEPES [pH 7.4], 3 mM EDTA, 1 mM DTT, 1× protease inhibitor cocktail, 10% glycerol). After centrifugation, the supernatant was subjected to SDS-PAGE. The same amount of extract was loaded onto 10% SDS-PAGE. The membrane was first stained by anti-Aqp2 (C-17, sc-9882; Santa Cruz), and then the antibody was removed and stained by antitubulin antibodies (D-10, sc-5274; Santa Cruz). Band intensity was quantified by Image-J software (NIH-image).
Immunostaining.
Kidney was fixed by 4% paraformaldehhyde at 4°C for 3 h and 20% sucrose–PBS at 4°C overnight. The next day, the sample was embedded in O.C.T. compound (Sakura Finetechnical) to make frozen sections. Frozen sections (10 μm) were cut and incubated with 1:100 diluted anti-Aqp2 (sc-9882; Santa Cruz) at 4°C overnight, 1:500 diluted Alexa Fluor 555–donkey anti-goat antibody (Invitrogen) for 1 h, and 1:1,000 diluted 4′,6-diamidino-2-phenylindole (DAPI) for 5 min at room temperature. Fluorescence was observed by an LSM510 confocal imaging system (Carl Zeiss).
ChPD and quantitative PCR (qPCR).
The chromatin pulldown (ChPD) assay was performed as previously described (27). BirA-mIMCD and FLBioGATA2/BirA-mIMCD cells were fixed at room temperature with 1% formaldehyde for 10 min and quenched with 0.125 M glycine. The fixed cells were resuspended in NP-40 Nuclei Swelling buffer (10 mM Tris-HCl [pH 8.0], 10 mM NaCl, 0.5% NP-40) and kept on ice for 10 min. Then, the cells were pelleted by centrifugation and lysed in SDS lysis buffer (50 mM Tris-HCl [pH 8.0], 1% SDS, 10 mM EDTA). The cross-linked chromatin was fragmented by sonication to a chromatin size of 200 to 500 bp. Pulldowns were conducted by incubating the fragmented chromatin with Dynabeads M-280 streptavidin (Invitrogen) in the cold room overnight. After washing the beads using standard protocols (Upstate Biotechnology), reverse cross-linking and DNA elution were done by incubating the beads in elution buffer (20 mM Tris-HCl [pH 7.5], 1% SDS, 5 mM EDTA, 50 mM NaCl) with 1.5 μg/ml proteinase K at 65°C overnight. DNA was recovered by phenol-chloroform extraction and ethanol precipitation. The aliquots of pulldown DNA were subjected to quantitative PCR analysis with normalization to input control. Primers amplifying Aqp2 and Avpr2 loci are listed in Table 1.
Microarray data accession number.
Microarray data are available through the Gene Expression Omnibus database (accession no. GSE52448).
RESULTS
GATA2 is highly expressed in the renal medulla of adult mouse.
To explore the physiological function of GATA2 in adult kidney, we first examined the expression pattern of GATA2 in the kidney, utilizing Gata2 GFP knock-in (Gata2GFP/+) mice, in which GFP expression faithfully represents the tissue-specific distribution of endogenous GATA2 expression (21). In this analysis, we exploited GFP immunohistochemistry for the better resolution of the tissues. We found that GFP was abundantly expressed in renal medulla, while sparse expression of GFP was observed in the cortex (Fig. 1A).
FIG 1.
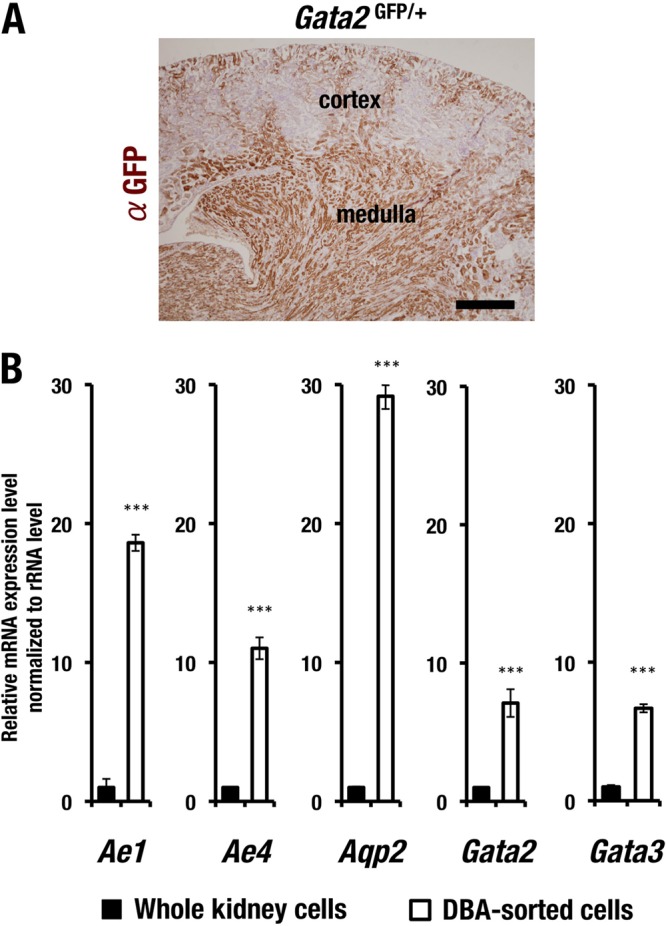
GATA2 expression in adult renal collecting duct (CD) cells. (A) GFP immunoreactive-positive cells are located mainly in the medulla of adult kidney, while sparse GFP expression is observed in the cortex. Scale bar, 0.5 mm. (B) mRNA expression level of the CD cell-affiliated genes. Dolichos biflorus agglutinin (DBA)-labeled CD cells show higher expression levels of Ae1, Ae4, Aqp2, Gata2, and Gata3 than do the whole kidney cells. The experiments were repeated three times. Data are presented as means ± standard deviations (SD). The statistical significance of differences between the whole kidney sample and the DBA-sorted cells are indicated (***, P < 0.01; Student's unpaired t test).
To further clarify the expression of GATA2 in the medulla, we used biotinylated Dolichos biflorus agglutinin (DBA), which specifically labels adult kidney CD cells (28). We purified the biotinylated DBA-labeled mouse CD cells by using streptavidin magnetic beads, and the CD cells were subsequently subjected to the quantification of Gata2 mRNA expression by quantitative RT-PCR. The DBA-labeled CD cells expressed Ae1 and Ae4 mRNAs at a higher level than whole-kidney cells did (Fig. 1B). Both genes encode bicarbonate (HCO3−) transporters preferentially expressed in the CD cells (29).
Aqp2 transcripts were also highly enriched in the DBA-labeled CD cells (Fig. 1B), indicating that the purification methods employing the biotinylated DBA labeling efficiently enriched the CD cells. We also found that Gata2 mRNA was highly expressed in the biotinylated DBA-labeled cells (Fig. 1B). Of note, Gata3 transcripts were also enriched in the CD cells compared to the whole-kidney sample (Fig. 1B). These results thus suggest that Gata2 is highly expressed in CD cells of the renal medulla and that Gata3 expression overlaps that of Gata2.
GFP/GATA2-positive cells are CD cells.
To assess the overlap of biotinylated DBA-labeled cells and GFP-positive cells, we conducted an immunohistochemical double-fluorescence analysis utilizing biotinylated DBA labeling. We found that the vast majority of GFP-positive cells in the kidney of Gata2GFP/+ mice considerably overlapped the DBA-positive CD cells (Fig. 2A, panel c). However, we also found that the GFP signal labeled the contiguous CD cells more thoroughly than the DBA signal did (Fig. 2A, panels a and b). Given these data, we conclude that the GFP-positive cells are CD cells and that the DBA specifically labels CD cells with relatively low efficiency.
FIG 2.
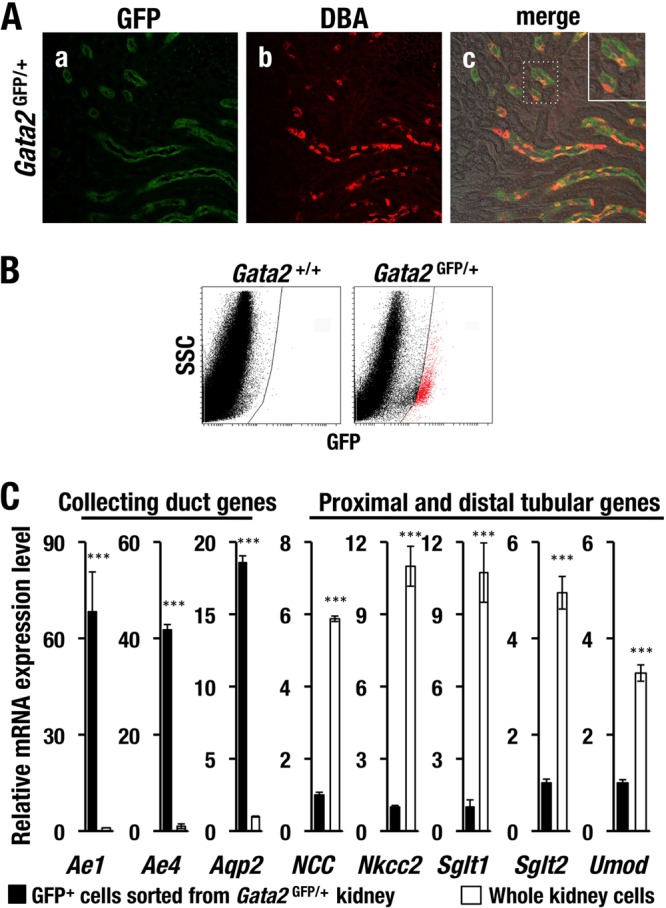
GATA2 is specifically expressed in CD cells of mouse kidney. (A) GFP immunoreactivity (green) in kidney of Gata2GFP/+ mice is colocalized with DBA-lectin-positive (red) CD cells. (B) Flow cytometry analysis of kidney cells. GFP-positive renal tubular cells (red dots) from Gata2GFP/+ are sorted and subjected to the qRT-PCR analysis. SSC, side scatter. (C) Transcripts of CD cell-affiliated genes (Ae1, Ae4, and Aqp2) are highly enriched in the GFP-positive renal cells of Gata2GFP/+ mice in comparison with the whole kidney sample. Expression of proximal or distal tubular markers (NCC, Nkcc2, Sglt1, Sglt2, and Umod) in GFP-positive cells is lower than that in the whole kidney cells. The experiments were repeated three times. Data are shown as the means ± SD. The statistical significance of the differences between the GFP-positive cells and whole kidney cells are indicated (***, P < 0.001; unpaired Student's t test).
To examine the GFP/GATA2-expressing cell-specific gene signature, we conducted flow-cytometric sorting of the GFP-positive cells from Gata2GFP/+ mouse kidney and quantified mRNA expression levels of a series of nephron segment-specific markers. We successfully isolated GFP-positive cells with low side scatter (SSC) from adult Gata2GFP/+ mouse kidney (Fig. 2B). Based on the number of recovered cells and their appearance, we consider that these GFP-positive sorted cells enrich the CD cells (data not shown). Consistent with the double-fluorescence immunohistochemical analysis, GFP-positive cells showed significantly higher levels of CD-affiliated mRNAs (i.e., Ae1, Ae4, and Aqp2) than did the whole kidney cells (Fig. 2C). In contrast, mRNA levels of markers for proximal and distal tubules (NCC, Nkcc2, Sglt1, Sglt2, and Umod) were lower in the GFP-positive cells than in whole-kidney cells (Fig. 2C). These data thus further confirm that GATA2 is predominantly expressed in the CD cells of adult mouse kidney.
Tubular epithelial-cell-specific Gata2 deletion.
To examine the physiological contribution of GATA2 in the CD cells of adult kidney, we utilized the doxycycline-inducible Cre transgenic mouse system and deleted the Gata2 gene's 5th coding exon specifically in the adult renal tubular cells. It has been shown that Pax8-rtTA::tetO-Cre double transgenic mouse expresses Cre recombinase upon doxycycline feeding specifically in the tubular epithelial cells of kidney (24). We generated Gata2GFP/flox::Pax8-rtTA::tetO-Cre (here, referred to as Gata2-CKO) mice by crossing Gata2GFP/flox::Pax8-rtTA and Gata2GFP/flox::tetO-Cre mice. The resulting Gata2-CKO mice were subjected to subsequent analysis, while the littermate mice genotyped as Gata2GFP/flox or Gata2flox/flox were used as controls for experiments.
As mouse kidney development is completed around 14 days after birth (30), we started feeding doxycycline hyclate solution (1 mg/ml) in drinking water to induce recombination of the Gata2flox allele at 4 weeks after birth to circumvent potential toxicity on postnatal nephrogenesis (Fig. 3A). After 4 weeks of Dox feeding (i.e., at 8 weeks after birth), we confirmed the tubular-cell-specific activation of Cre recombinase utilizing separately prepared Pax8-rtTA::tetO-Cre::Rosa26TdTomato (R26T) reporter transgenic mice. Cre activity indicated by tdTomato red fluorescence was observed exclusively in the tubular cells (T in Fig. 3B) but not in the glomeruli (G in Fig. 3B) of the kidney. The structures of Gata2GFP and Gata2flox alleles are depicted in Fig. 3C.
Subsequently, we examined the recombination efficiency of the Gata2flox allele by means of genomic quantitative PCR (gQPCR) analysis using the DBA-sorted CD cells prepared either from the Gata2-CKO or control mouse (Fig. 3D). A primer pair specifically amplifying a fragment containing nucleotide sequences of the 3′ loxP site in the 5th intron was used to quantify the unrecombined Gata2flox allele (Fig. 3C). The threshold cycle (CT) value was normalized with that of the control locus at the kb −2.8 Gata2 IS-promoter upstream region (31). gQPCR analysis demonstrated that the control mice (Gata2flox/GFP) (white bars in Fig. 3D) harbored a single copy of Gata2flox allele in the multiple tissues (i.e., collecting ducts [CD], spleen [SP], and bone marrow [BM]) (Fig. 3D), whereas the Gata2flox/flox littermates (gray bars in Fig. 3D) carried two copies of Gata2flox alleles, confirming the quantitative accuracy of the gQPCR analysis. As anticipated, 90% of the Gata2flox allele was excised in the CD cells of the Gata2-CKO mice, while the Gata2flox allele remained largely intact in hematopoietic tissue, including spleen and bone marrow (black bars in Fig. 3D). Consequently, the Gata2 mRNA level in the DBA-sorted CD cells was decreased to 28.5% in the Gata2-CKO mice compared to that in the Gata2flox/GFP control mice, while Gata2 expression in hematopoietic tissues was largely unchanged in the Gata2-CKO mice (Fig. 3E). These data demonstrate that the 5th coding exon of the Gata2flox allele was deleted specifically and efficiently in the renal CD cells of Gata2-CKO mice.
Gata2 deletion leads to increased urine volume and decreased urine osmolality.
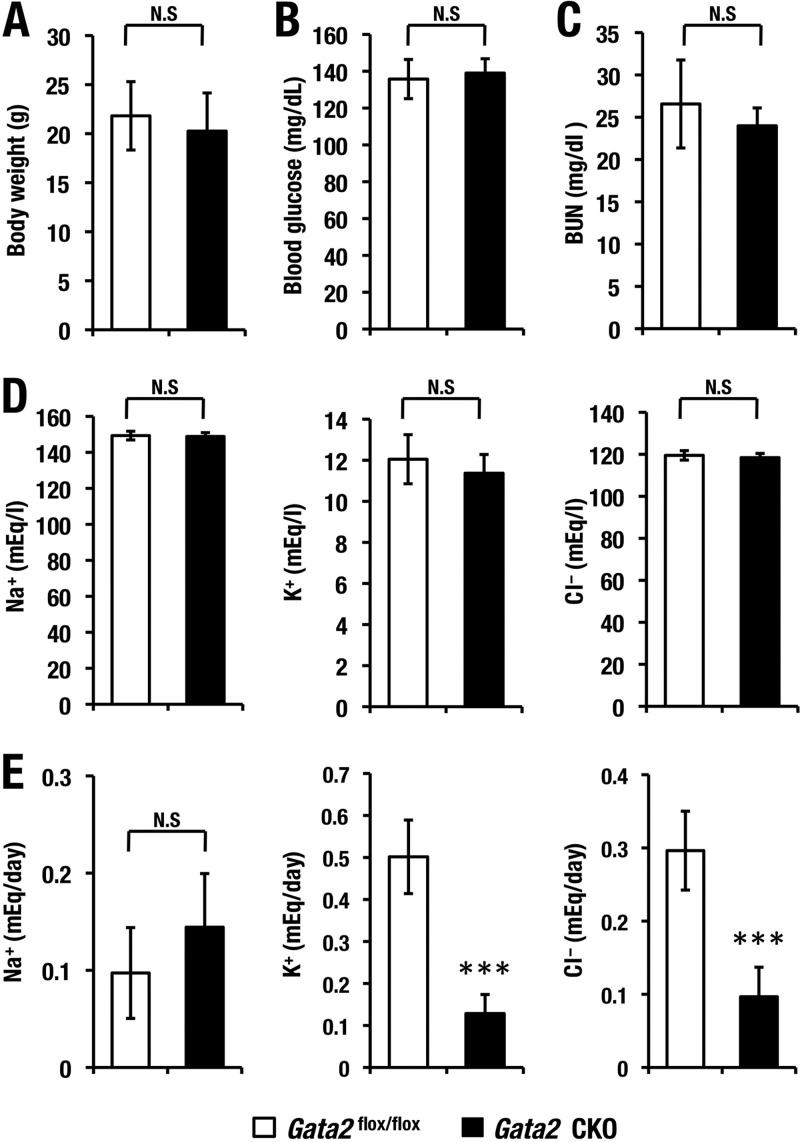
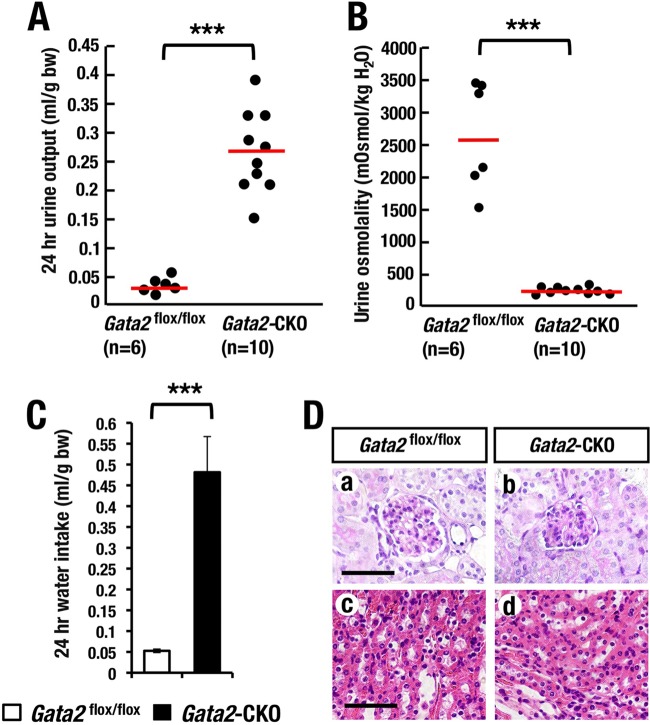
Assuming that the GATA2 deficiency in kidney might cause impairment in renal function in the Gata2-CKO mice, we examined several renal functional and biochemical parameters. We found that the levels of BUN (blood urea nitrogen), electrolytes (sodium, potassium, and chloride) (Fig. 4C and D), and blood glucose (Fig. 4B) in serum were comparable for Gata2-CKO and Gata2flox/flox control mice. We next measured total daily urine volume and daily water intake by collecting 24-h urine from Gata2-CKO mice and Gata2flox/flox littermate control mice using metabolic cages. Interestingly, Gata2-CKO mice showed a 10-fold-more-abundant urine volume than control mice did (Fig. 5A). Consistent with this increase in urine volume, urinary osmolality of Gata2 CKO mice was much lower than that of control mice (Fig. 5B). Concomitantly, Gata2-CKO mice exhibited 10-fold-more-increased daily water intake (Fig. 5C). The total volume of daily urinary sodium excretion was not changed, while that of potassium and that of chloride were decreased in the Gata2-CKO mice (Fig. 4E). Despite a significant increase in the urine volume, Gata2-CKO mice showed neither gross abnormality nor body weight changes after Dox feeding (Fig. 4A and data not shown). Histological examinations of kidney in the Gata2 CKO mice exhibited practically no microscopic abnormality in glomeruli and CDs (Fig. 5D). Collectively, these observations demonstrate that GATA2 deficiency in the renal CD cells results in increased urinary volume without any signs of diabetes mellitus or renal failure. These signs are reminiscent of human nephrogenic diabetes insipidus.
FIG 4.
(A to E) Body weight, blood glucose, serum, and urnie biochemical indexes. Gata2flox/flox (n = 6) and Gata2-CKO (n = 10) mice showed comparable level of body weight (A), blood glucose level (B), serum level of BUN (blood urea nitrogen) (C), Na+, K+, and Cl− (D), and total daily urinary excretion of Na+, K+, and Cl− (E). (N.S., not significant; ***, P < 0.001; Student's unpaired t test).
FIG 5.
Renal tubular cell-specific Gata2 deletion leads to high urine volume and low urine osmolality. (A and B) Volume and osmolality of 24-hour urine in Gata2-CKO (n = 10) and littermate control (Gata2flox/flox; n = 6) mice. Statistical significance of the differences between Gata2-CKO and the control littermates is indicated (***, P < 0.001; Student's unpaired t test). (C) Twenty-four-hour solution intake of control and Gata2-CKO mice (***, P < 0.001; Student's unpaired t test). (D) No obvious histological abnormality in glomeruli (a and b) and CD (c and d) in the Gata2-CKO in comparison with the Gata2flox/GFP littermate control mice. Scale bars, 50 μm.
Gata2 deletion alters expression of multiple water reabsorption-related genes in CD cells.
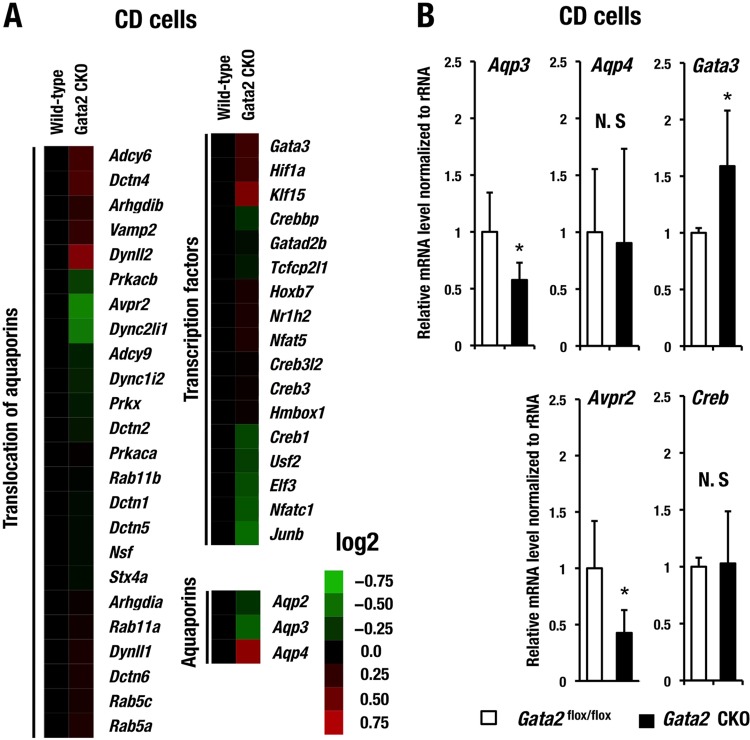
To explore the potential cause of polyuria and urinary concentration defects in the Gata2-CKO mice, we conducted expression microarray analysis using CD cells and searched for potential GATA2 target genes. We purified the CD cells by biotinylated DBA labeling from 3 Gata2-CKO and 3 wild-type mice. RNA samples prepared from the pooled CD cells of either genotype were subjected to expression microarray analysis. We selected a series of genes that are highly expressed in the CD cells and potentially participate in water reabsorption mechanism using Ingenuity pathway analysis (IPA) software and the Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway database (http://www.genome.jp/kegg/pathway.html). We compared the expression profiles and found that expression levels of multiple genes in this category were changed, albeit subtly, in the Gata2-CKO mice (Fig. 6A and Table 2).
FIG 6.
Gene expression profile in CD cells. (A) Heat map generated from DNA microarray data of water reabsorption-related genes changed in CD cells of Gata2 CKO mice. Heat map colors indicate normalized expression level (log2). Genes are categorized into three groups by their function using Ingenuity pathway analysis (IPA) software and the Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway database (http://www.genome.jp/kegg/pathway.html). (B) mRNA expression of Aqp3, Aqp4, Gata3, Creb, and Avpr2 in the CD cells of Gata2flox/flox and Gata2-CKO mice examined by qRT-PCR analysis. The statistical significance of the difference between the Gata2flox/flox and Gata2 CKO mice is indicated (*, P < 0.05; Student's unpaired t test, N.S; not significant).
TABLE 2.
Microarray analysis of water balance-related genes in CD cells of Gata2 CKO mice
| Category and gene name | Description | Fold change (Gata2-CKO/WT) |
|---|---|---|
| Translocation of aquaporins | ||
| Adcy6 | Adenylate cyclase 6 | 1.251 |
| Dctn4 | Dynactin 4 | 1.289 |
| Arhgdib | Rho, GDP dissociation inhibitor (GDI) beta | 1.154 |
| Vamp2 | Vesicle-associated membrane protein 2 | 1.199 |
| Dynll2 | Dynein light chain LC8-type 2 | 1.49 |
| Prkacb | Protein kinase, cAMP dependent, catalytic, beta | 0.811 |
| Avpr2 | Arginine vasopressin receptor 2 | 0.671 |
| Dync2li1 | Dynein cytoplasmic 2 light intermediate chain 1 | 0.689 |
| Adcy9 | Adenylate cyclase 9 | 0.901 |
| Dync1i2 | Dynein cytoplasmic 1 intermediate chain 2 | 0.892 |
| Prkx | Protein kinase, X-linked | 0.919 |
| Dctn2 | Dynactin 2 | 0.931 |
| Prkaca | Protein kinase, cAMP dependent, catalytic, alpha | 1.011 |
| Rab11b | RAB11B, member of RAS oncogene family | 0.995 |
| Dctn1 | Dynactin 1 | 0.975 |
| Dctn5 | Dynactin 5 | 0.973 |
| Nsf | N-Ethylmaleimide-sensitive fusion protein | 0.976 |
| Stx4a | Syntaxin 4A | 0.97 |
| Arhgdia | Rho GDP dissociation inhibitor (GDI) alpha | 1.03 |
| Rab11a | RAB11a, member RAS oncogene family | 1.066 |
| Dynll1 | Dynein light chain LC8-type 1 | 1.094 |
| Dctn6 | Dynactin 6 | 1.094 |
| Rab5c | RAB5C, member RAS oncogene family | 1.083 |
| Rab5a | RAB5A, member RAS oncogene family | 1.111 |
| Transcription factors | ||
| Gata3 | GATA binding protein 3 | 1.23 |
| Hif1a | Hypoxia inducible factor 1, alpha subunit | 1.226 |
| Klf15 | Kruppel-like factor 15 | 1.466 |
| Crebbp | CREB binding protein | 0.856 |
| Gatad2b | GATA zinc finger domain containing 2B | 0.96 |
| Tcfcp2l1 | Transcription factor CP2-like 1 | 0.92 |
| Hoxb7 | Homeobox B7 | 1.089 |
| Nr1h2 | Nuclear receptor subfamily 1, group H, member 2 | 1.098 |
| Nfat5 | Nuclear factor of activated T cells 5 | 1.106 |
| Creb3l2 | cAMP-responsive element binding protein 3-like 2 Gene | 1.013 |
| Creb3 | cAMP-responsive element binding protein 3 | 1.025 |
| Hmbox1 | Homeobox containing 1 | 1.031 |
| Creb1 | cAMP-responsive element binding protein 1 | 0.794 |
| Usf2 | Upstream transcription factor 2 | 0.809 |
| Elf3 | E74-like factor 3 | 0.778 |
| Nfatc1 | Nuclear factor of activated T cells, cytoplasmic, calcineurin-dependent 1 | 0.761 |
| Junb | Jun-B oncogene | 0.715 |
| Water channels | ||
| Aqp2 | Aquaporin 2 | 0.843 |
| Aqp3 | Aquaporin 3 | 0.741 |
| Aqp4 | Aquaporin 4 | 1.533 |
We further selected several candidate genes that were changed in Gata2-CKO mice and confirmed their mRNA expression levels by qRT-PCR. Among these genes, we found that Aqp2 gene expression was most profoundly diminished in the CD cells of Gata2-CKO mice in comparison with the control mice; the decrease reached approximately 62% in qRT-PCR analysis (Fig. 7A). Immunoblotting analysis using anti-Aqp2 antibody showed a decreased level of Aqp2 protein in the whole-kidney cell extract of Gata2-CKO mice (Fig. 7B), and the decrease was approximately 50% (Fig. 7C).
FIG 7.
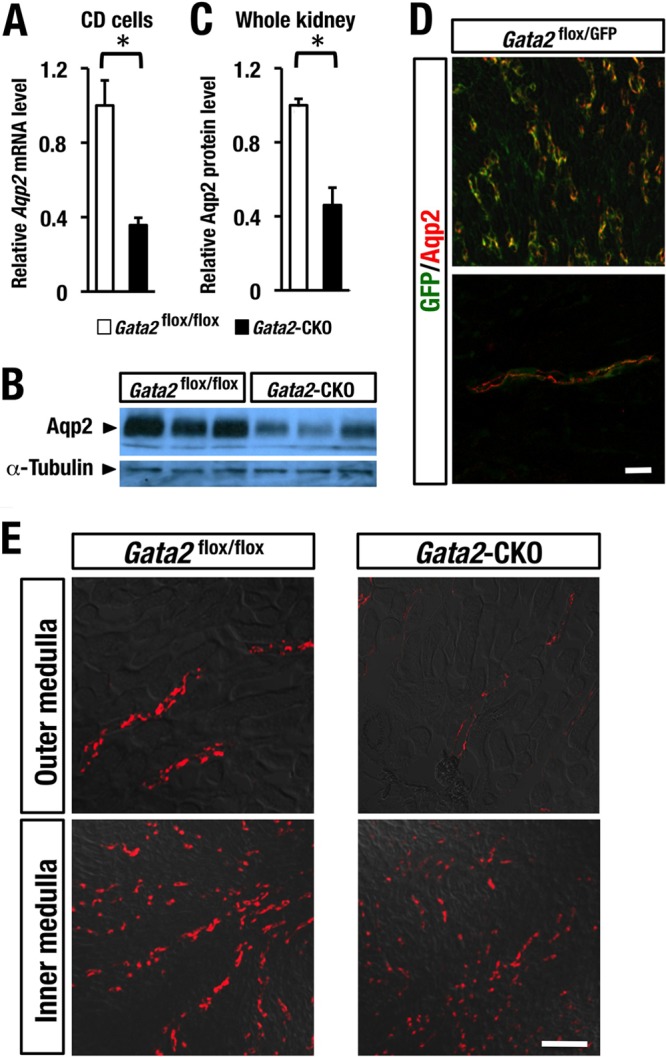
GATA2 is important for maintenance of Aqp2 expression. (A) Aqp2 mRNA level is decreased in the DBA-sorted CD cells of Gata2-CKO mice (n = 3) compared to that of the Gata2flox/GFP control mice (n = 3). (B) Representative immunoblot analysis of Aqp2 using whole-cell extract of kidney in Gata2flox/flox control (n = 3) and Gata2-CKO (n = 3) mice. (C) Aqp2 protein level is quantified and normalized to the α-tubulin level. Data are presented as the means ± SD. The statistical significance of the differences between Gata2 CKO and the Gata2flox/flox control littermates is indicated (*, P < 0.05; Student's unpaired t test). (D) Colocalization of GFP and Aqp2 immunoreactivities in renal tubules of the Gata2flox/GFP mouse. (E) Immunofluorescence analysis shows the reduced Aqp2 expression in the outer and inner medulla of kidney in the Gata2-CKO mouse. Scale bar, 50 μm.
We found that in the kidney of the Gata2GFP/flox control mice, the GFP fluorescence signal nicely overlapped the Aqp2 immunostaining signal (Fig. 7D). This result supports the notion that GATA2 is coexpressed in the Aqp2-expressing principal cells of CD. Immunofluorescence analysis with the Aqp2 antibody showed the reduction of Aqp2 staining signal significantly in the outer medulla and modestly in the inner medulla of Gata2-CKO mouse kidney (Fig. 7E). These results indicate that GATA2 regulates the Aqp2 gene and contributes to the maintenance of the Aqp2 expression level in principal cells of CD (see Fig. 10C).
FIG 10.
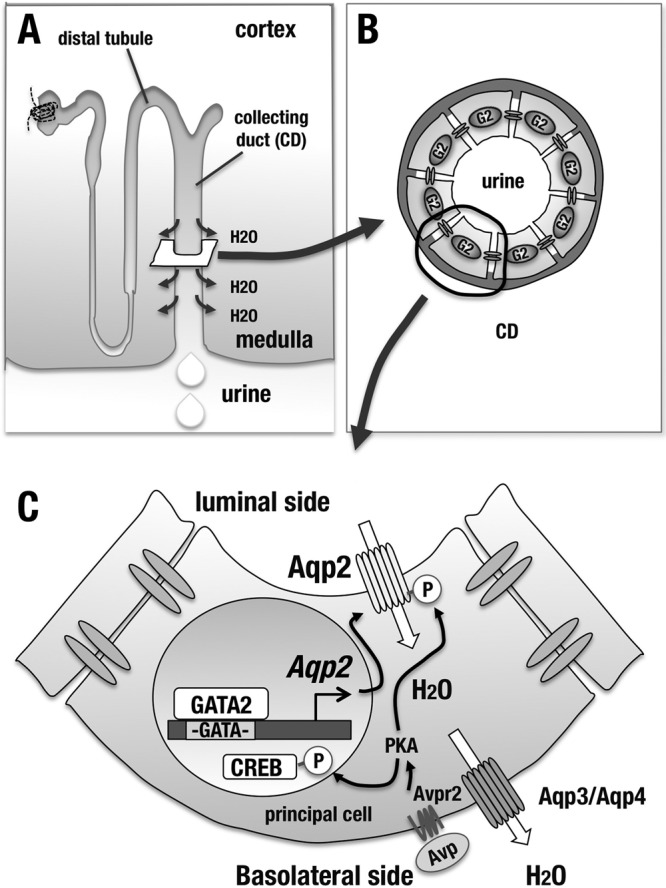
GATA2 regulates the Aqp2 gene expression in the CD and contributes to maintenance of the body water homeostasis. (A) Schematic diagram of nephron. Aqp2-mediated water absorption takes place in the CD. (B) GATA2 is expressed in all the CD cells, including principal cells. (C) In the principal cells of CD, GATA2 directly activates Aqp2 gene expression and maintains the normal water reabsorption mechanism from urine. Upon Avp stimulus, Avpr2 signaling induces PKA (protein kinase A)-mediated phosphorylation of Aqp2 and CREB. Phosphorylated Aqp2 translocates to the luminal side of principal cells. Phosphorylated and activated CREB participates in transactivation of Aqp2 gene.
We observed a 43% reduction of Aqp3 mRNA expression level in the GATA2-deficient CD cells by means of qRT-PCR, while Aqp4 expression levels were comparable for the two genotypes of CD cells (Fig. 6B). Both Aqp3 and Aqp4 are located at the basolateral cell membrane of the principal cells and provide a pathway for water exit from the principal cells (see Fig. 10C) (1). Avpr2 expression was also significantly decreased to 42% in the Gata2-CKO mice (n = 12) compared to the control (n = 9) (Fig. 6B). Transcript levels of Creb, encoding CREB (cyclic AMP-responsive element binding protein), which mediates Avp-mediated transcriptional regulation of Aqp2 (32), were barely changed in the CD cells of Gata2-CKO mice (Fig. 6B). Collectively, these data indicate that GATA2 maintains multiple gene expressions associated with water reabsorption mechanism, particularly including Avpr2, Aqp2, and Aqp3 in the CD cells.
Differential functions of Gata2 and Gata3 in the CD cells.
CD cells of the Gata2 CKO mice showed an increase in mRNA expression of Gata3 (Fig. 6B), another member of the GATA family expressed in the CD cells. Given this observation, we addressed whether total mRNA abundance of the two renal GATA factors (i.e., GATA2 plus GATA3) was changed in the CD cells of the Gata2-CKO mice. To this end, we quantified the molar ratio of Gata2 and Gata3 mRNAs in the CD cells of the Gata2f/f control mice. We found that the Gata2 mRNA level was 2.4-fold higher than that of Gata3 mRNA in the CD cells of the control mice (Fig. 8A). In the CD cells of the Gata2-CKO mice, Gata2 mRNA was decreased to 28.5% of the control, while Gata3 mRNA was increased to 158.9% of the control (Fig. 8A). The total abundance of Gata2 and Gata3 transcripts in the CD cells of the Gata2-CKO mice was still lower than that of control mice (Fig. 8A), suggesting that the increase in GATA3 mRNA is not sufficient to fully compensate the GATA2 loss in the Gata2 CKO mice.
FIG 8.
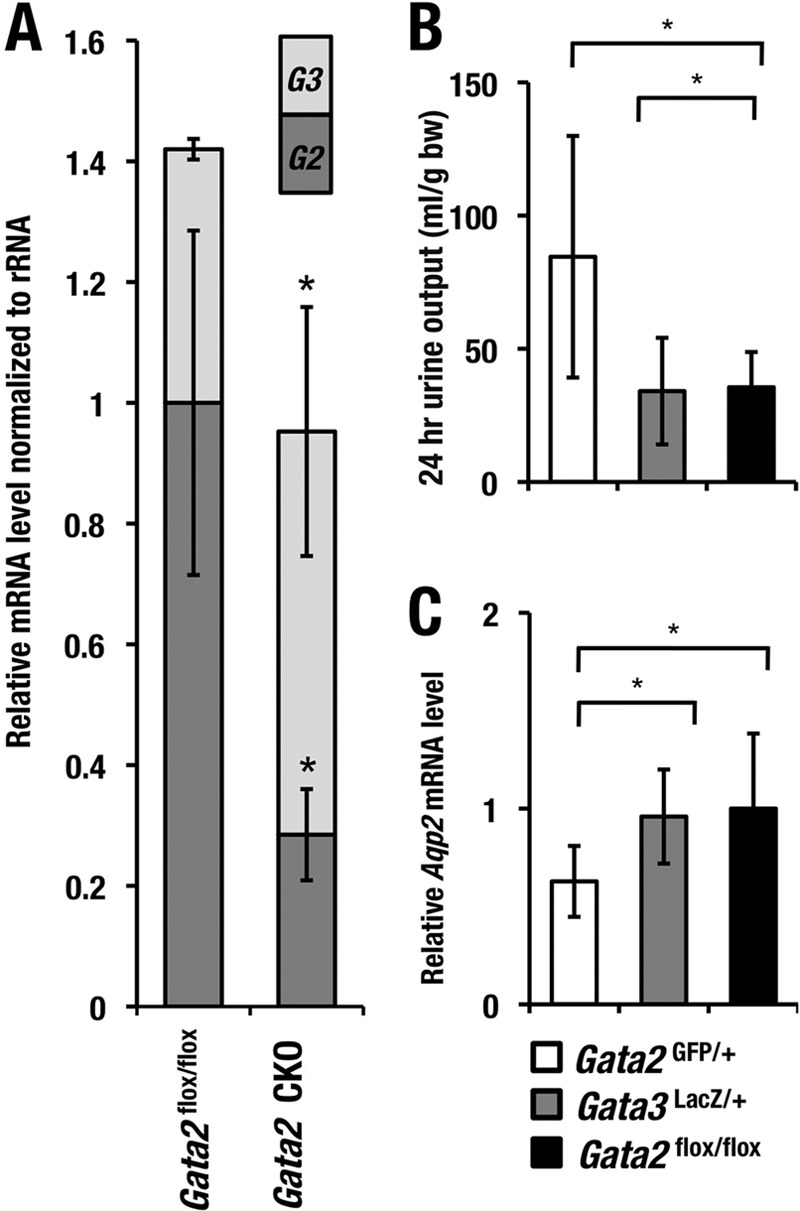
GATA2 is more crucial than GATA3 for Aqp2 trans activation in vivo. (A) The stacked bar graph shows the relative abundances of Gata2 and Gata3 transcripts in the Gata2flox/flox (n = 3) and Gata2 CKO (n = 4) mice. The statistically significant changes in the Gata2 CKO mice compared with the Gata2flox/flox are indicated (*, P < 0.05; Student's unpaired t test). Gata2GFP/+ but not Gata3LacZ/+ mice show increase in urine volume (B) and decrease in Aqp2 mRNA level (C). Statistical significance of differences is indicated (*, P < 0.05; Student's unpaired t test).
To further explore functional differences between Gata2 and Gata3 genes in the urinary concentration processes, we compared both Aqp2 mRNA levels and urine volume between heterozygous deficient mice for Gata2 (Gata2GFP/+) and Gata3 (Gata3LacZ/+) and the control Gata2f/f mice. We found that the Gata2GFP/+ mice showed a decrease in Aqp2 mRNA levels and a resultant increase in urine volume, as is the case for the Gata2-CKO mice (Fig. 8B and C). However, the Gata3LacZ/+ mice showed no significant differences in these parameters. These observations thus indicate that GATA3 does not functionally compensate for GATA2 in the CD cells.
GATA2 directly transactivates Aqp2 gene expression.
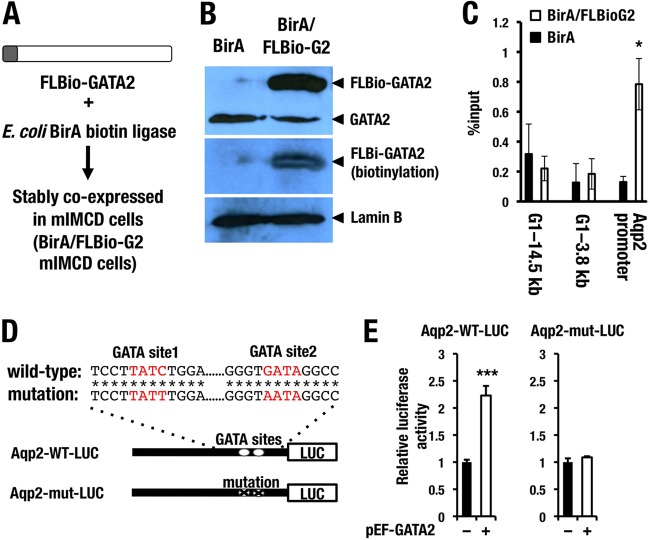
Assuming a major contribution of reduced Aqp2 expression to the urinary concentration defect in the Gata2-CKO mice, we next clarified whether GATA2 directly transactivates Aqp2 gene expression. While no solid evidence for the GATA2-mediated activation of the Aqp2 promoter has been reported, two phylogenetically conserved GATA binding sites are located in the kb −0.3 promoter region of the Aqp2 gene (33, 34). To examine whether GATA2 directly binds to the Aqp2 promoter region, we performed chromatin pulldown (ChPD) experiments using a mouse inner medullary collecting duct cell line. An mIMCD cell line derived from inner medullary cells of mouse kidney is known to retain many differentiated characteristics of CD cells (35). A Flag-biotin tag harboring a sequence motif that can be biotinylated by E. coli BirA biotin ligase was ligated at the N terminus of mouse GATA2 (FLBio-GATA2) (Fig. 9A). We stably transfected E. coli BirA biotin ligase into mIMCD cells to establish BirA-mIMCD cells. Subsequently, the BirA-mIMCD cells were introduced with the FLBio-GATA2 expression plasmid to generate an mIMCD cell line stably coexpressing BirA and FL Bio-GATA2. We refer to this cell line as BirA/FLBio-G2 mIMCD (Fig. 9A).
FIG 9.
GATA2 directly activates Aqp2 expression through the evolutionary conserved GATA sites in the promoter region. (A) BirA/FLBio system for chromatin pulldown assay. Flag and biotinylation tags are fused to the N terminus of GATA2 protein. FLBio-GATA2 and E. coli BirA biotin ligase are stably cotransfected into mIMCD cells. (B) Confirmation of FLBio-GATA2 expression in the BirA/FLBio GATA2 mIMCD cells by immunoblotting analysis. The mIMCD cell line transfected only with BirA is used for control. Immunoblotting using GATA2 antibody detected the FLBio-GATA2 and endogenous GATA2 proteins in the FLBio-GATA2-transfected cells, while the BirA-only transfected cells express only the endogenous GATA2 protein (top panel). Biotinylated GATA2 is detected by streptavidin-HRP and is absent from the BirA-only transfected cells (middle panel). Lamin B antibody is used as loading control (bottom panel). (C) Chromatin pulldown experiments show that FLBio-GATA2 binds to the Aqp2 promoter in the mIMCD cell. Two genomic DNA regions around Gata1 locus (G1-3.8 kb and G1-14.4 kb) are used as negative controls. Data are presented as means ± SD from three independent experiments. Statistical significance of differences is indicated (*, P < 0.05; Student's unpaired t test). (D) Construction of 1.5-kb wild-type (Aqp2-WT-LUC) or GATA site-mutated (Aqp2-mut-LUC) Aqp2 promoter-luciferase reporter. Asterisks indicate nucleotides that are conserved between human and mouse Aqp2 genes. (E) Cotransfection of GATA2-expressing plasmid (pEF-GATA2) in the mIMCD cells activates the Aqp2-WT-LUC reporter but not the Aqp2-WT-LUC reporter. Data are presented as means ± SD from three independent experiments. Statistical significance of differences is indicated (***, P < 0.001; Student's unpaired t test).
In our immunoblotting analysis with GATA2 antibody, we found that BirA/FLBio-G2 mIMCD cells showed an additional tagged band at a higher-molecular-weight region than that of the endogenous GATA2 protein (top panel in Fig. 9B; left lane, BirA mIMCD; right lane, BirA/FLBio-G2 mIMCD). We confirmed biotinylation of the FLBio-GATA2 protein by incubating the membrane with avidin-conjugated horseradish peroxidase (HRP) followed by chemiluminescence reaction (Fig. 9B, middle panel). We used this BirA/FLBio-G2 mIMCD cell line for the subsequent ChPD experiments, and the BirA mIMCD cells were utilized as a negative background control. Results of the ChPD experiments revealed high-level accumulation of FLBio-GATA2 protein around the GATA-binding sites in the Aqp2 promoter region (Fig. 9C). We used two genomic regions (G1-14.5 kb and G1-3.8 kb) in the proximity of mouse Gata1 gene as negative controls (Fig. 9C). Binding of FLBio-GATA2 to these negative loci was not observed in the BirA/FLBio-G2 mIMCD cells.
To further address the question as to whether GATA2 directly activates the Aqp2 promoter through these conserved GATA sites, we performed a luciferase reporter assay using the 1.5-kb Aqp2 promoter with or without GATA site substitution with mIMCD cells (Fig. 9D). Cotransfection of GATA2-expressing plasmid (pEF-GATA2) increased the activity of the wild-type Aqp2 promoter reporter (Aqp2-WT-LUC), while the GATA site-mutated Aqp2 reporter (Aqp2-mut-LUC) was barely activated upon cotransfection of GATA2 (Fig. 9E). This result clearly indicates that GATA2 induces the Aqp2 gene promoter activity through the conserved two GATA sites. Collectively, the present data demonstrate that GATA2 is specifically expressed in the CD cells of kidney (Fig. 10A and B) and participates in the renal regulation of urine concentration through directly activating Aqp2 gene expression (Fig. 10C).
DISCUSSION
Principal cells in the CD (collecting duct) are responsible for Avp-induced water reabsorption, and Avp-induced luminal translocation of Aqp2 has been shown to be a crucial step for the process. In contrast, how regulation of the Aqp2 gene transcription contributes to the Avp-induced water reabsorption remains to be clarified. In this study, we found that GATA2 is predominantly expressed in the CD of adult mouse kidney. Renal tubular cell-specific Gata2-deficient mice exhibit significant reduction of Aqp2 gene expression, thereby giving rise to a large volume of urine with low osmolality. We also found that GATA2 directly binds to the two conserved GATA binding sites in the promoter region of the Aqp2 gene. GATA2 trans activates expression of Aqp2-LUC, an Aqp2 gene-based reporter harboring the two GATA motifs. These results demonstrate that GATA2 is responsible for the body water homeostasis through the epistatic regulatory relationship to Aqp2.
Structural alterations of Avpr2 and Aqp2 caused by genetic mutations of Avpr2 and Aqp2 genes have been shown to lead to inherited forms of nephrogenic diabetes insipidus (NDI), which is characterized by polyuria. Importantly, there are increasing lines of evidence implying that downregulation of the Aqp2 gene expression provokes polyuria associated with acquired forms of NDI in many pathological conditions in humans (16). Indeed, Aqp2 expression level is diminished in multiple water balance disorder animal models, such as puromycin-induced nephritic syndrome (36) and 5/6 nephrectomy-induced chronic renal failure (37). Reversible bilateral urinary obstruction of rats, a model for chronic kidney disease, is also known to reduce the Aqp2 level by approximately 25% to 50%. Consistent with this Aqp2 reduction, a urine-concentrating defect associated with a persistent polyuria emerges after the release of obstruction (18). Additionally, it is well known that in the recovery phase of murine kidney from ischemia-reperfusion injury, robust polyuria is induced following downregulation of Aqp2 (38).
In spite of these observations demonstrating the Aqp2 gene downregulation, the mechanisms underlying the Aqp2 gene suppression associated with pathological conditions of kidney have been unclear. In this regard, it is noteworthy that transgenic reporter mice harboring a 9.5-kb sequence 5′ to the Aqp2 gene in front of GFP recapitulate the principal cell-specific expression of Aqp2 gene and dynamic changes of the expression after the pathological stimuli (39). This observation provides compelling evidence that cis-regulatory elements responsible for the dynamic change of Aqp2 expression in pathological conditions reside in this 9.5-kb region. The 9.5-kb sequence contains several transcription factor-binding motifs (as summarized in reference 33). A conventional reporter transfection assay using cell lines suggests that a cAMP-response element (CRE) located 314 bp upstream of the transcription start site may be responsible for the Avp-mediated induction of the Aqp2 gene (32). Similarly, multiple binding motifs for NFAT (nuclear factor of activated T cells) located 600 to 321 bp upstream of mouse Aqp2 gene are crucial for the gene regulation depending on the osmolality of extracellular fluid (40). In contrast to the reporter transfection-transactivation assays, this study has revealed a critical contribution of GATA2 for maintenance of Aqp2 gene expression in vivo. Indeed, we recently found that Gata2 expression is downregulated in the kidney following ischemia-reperfusion injury (L. Yu and T. Moriguchi, unpublished observation). We surmise that the Gata2 reduction may contribute to the attenuation of Aqp2 gene expression and subsequent alteration of water balance after the renal ischemia-reperfusion injury.
Our microarray analysis using CD cells of the Gata2-CKO mouse revealed downregulation of a number of genes related to water reabsorption in addition to Aqp2. Among these genes, we have confirmed the statistically significant decrease in mRNA level of Aqp3 and Avpr2 by qRT-PCR. Although Aqp4 expression is increased in the Gata2-CKO mice in the microarray analysis, qRT-PCR analysis shows that the expression is not different for Gata2-CKO and control mice. Aqp3 knockout mice are reported to exhibit a marked polyuria, while Aqp4-deficient mice show only a mild defect in urine-concentrating ability (41, 42). Therefore, predominant reduction of Aqp3 and Avpr2 may also contribute to the development of polyuria in the Gata2-CKO mice.
Since we observed the reduction of Avpr2 gene expression in the kidney of Gata2-CKO mouse, this suggests an intriguing possibility. Avpr2 is responsible for Avp-induced intracellular signaling within the principal cells. As summarized in Fig. 10C, it has been reported that upon Avp stimulus, Avpr2 induces luminal translocation of Aqp2 and activation of CREB (32). The activated CREB is postulated to trans activate Aqp2 gene expression. Therefore, GATA2 may contribute to the Avpr2-mediated phosphorylation/activation of Aqp2 and activation of CREB, in addition to the direct transactivation of Aqp2 gene expression. Furthermore, we found an evolutionarily conserved GATA motif at 2.5 kb 5′ to the mouse Avpr2 gene transcription start site, although contribution of the motif to Avpr2 gene regulation needs to be clarified experimentally.
Avpr2-mediated regulation of Aqp2 abundance appears to take place predominantly in the outer medulla and cortical collecting ducts, which are largely impermeable to water in the absence of Avp (43). Indeed, Avpr2 antagonist represses the Aqp2 expression level more predominantly in the outer medulla collecting duct cells than in the inner medulla collecting duct cells (44). Assuming that the Avpr2-mediated regulation of Aqp2 abundance is also under the influence of GATA2, the profound reduction of Aqp2 in the outer medulla of Gata2-CKO mice may potentially be due to the diminished Avpr2 expression in the Gata2 CKO mice.
GATA3 is another member of the hematopoietic GATA family and involved in the regulation of renal and urinary tract development (45). We found that GATA3 is also expressed in the CD cells, and its expression level shows a compensatory increase in the GATA2-deficient CD cells. Genetic redundancy of GATA2 and GATA3 has been observed in several developmental processes, including acoustic neurons and trophoblasts (46, 47). In these tissues, GATA2 and GATA3 cooperatively function for their development, and loss of GATA2 or GATA3 is compensated by increased expression of the other factor. Therefore, it is quite likely that both GATA2 and GATA3 cooperatively function in the principal cells of renal tubules. However, we found that Gata3 mRNA abundance was lower than that of Gata2 mRNA in the CD cells of mouse kidney. Therefore, the total abundance of GATA2 and GATA3 transcripts in the CD cells of Gata2 CKO was still lower than that of control mice. We surmise that Gata3 gene does not fully compensate for Gata2 deficiency in the CD cells.
In summary, we revealed a novel GATA2 function in the principal cells of renal collecting ducts. GATA2 directly activates Aqp2 gene expression, which is crucial for body water homeostasis (summarized in Fig. 10). Disruption of the GATA2-Aqp2 axis might result in water imbalance of the acquired form of NDI in human. With the rise in chronic kidney disease becoming a serious worldwide health care issue, associated NDI has been also emerging as a major complaint. In this regard, the present observation may provide insight into a potential treatment for acquired NDI through complementation of Aqp2 levels by enhancing the activity of GATA2.
ACKNOWLEDGMENTS
We thank Sally A. Camper (University of Michigan) for providing Gata2flox mice. We also thank the Biomedical Research Core of Tohoku University Graduate School of Medicine for technical support.
This study was supported through funding from JSPS KAKENHI, grant numbers 24249015 (M.Y.) and 24590371 (T.M.), the Takeda Foundation (to T.M. and M.Y.), the Naito foundation (to M.Y.), and the Core Research for Evolutional Science and Technology from the JST (M.Y.).
Footnotes
Published ahead of print 17 March 2014
REFERENCES
- 1.Verkman AS. 2006. Roles of aquaporins in kidney revealed by transgenic mice. Semin. Nephrol. 26:200–208. 10.1016/j.semnephrol.2006.02.002 [DOI] [PubMed] [Google Scholar]
- 2.Fushimi K, Uchida S, Hara Y, Hirata Y, Marumo F, Sasaki S. 1993. Cloning and expression of apical membrane water channel of rat kidney collecting tubule. Nature 361:549–552. 10.1038/361549a0 [DOI] [PubMed] [Google Scholar]
- 3.Nielsen S, DiGiovanni SR, Christensen EI, Knepper MA, Harris HW. 1993. Cellular and subcellular immunolocalization of vasopressin-regulated water channel in rat kidney. Proc. Natl. Acad. Sci. U. S. A. 90:11663–11667. 10.1073/pnas.90.24.11663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nielsen S, Frøkiaer J, Marples D, Kwon TH, Agre P, Knepper MA. 2002. Aquaporins in the kidney: from molecules to medicine. Physiol. Rev. 82:205–244 [DOI] [PubMed] [Google Scholar]
- 5.Moeller HB, Olesen ET, Fenton RA. 2011. Regulation of the water channel aquaporin-2 by posttranslational modification. Am. J. Physiol. Renal Physiol. 300:1062–1073. 10.1152/ajprenal.00721.2010 [DOI] [PubMed] [Google Scholar]
- 6.Rojek A, Füchtbauer EM, Kwon TH, Frøkiaer J, Nielsen S. 2006. Severe urinary concentrating defect in renal collecting duct-selective AQP2 conditional-knockout mice. Proc. Natl. Acad. Sci. U. S. A. 103:6037–6042. 10.1073/pnas.0511324103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Patient RK, McGhee JD. 2002. The GATA family (vertebrates and invertebrates). Curr. Opin. Genet. Dev. 12:416–422. 10.1016/S0959-437X(02)00319-2 [DOI] [PubMed] [Google Scholar]
- 8.Ko LJ, Engel JD. 1993. DNA-binding specificities of the GATA transcription factor family. Mol. Cell. Biol. 13:4011–4022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Merika M, Orkin SH. 1993. DNA-binding specificity of GATA family transcription factors. Mol. Cell. Biol. 13:3999–4010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ainoya K, Moriguchi T, Ohmori S, Souma T, Takai J, Morita M, Chandler KJ, Mortlock DP, Shimizi R, Engel JD, Lim KC, Yamamoto M. 2012. UG4 enhancer-driven GATA-2 and bone morphogenetic protein 4 complementation remedies the CAKUT phenotype in Gata2 hypomorphic mutant mice. Mol. Cell. Biol. 32:2312–2322. 10.1128/MCB.06699-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hasegawa SL, Moriguchi T, Rao A, Kuroha T, Engel JD, Lim KC. 2007. Dosage-dependent rescue of definitive nephrogenesis by a distant Gata3 enhancer. Dev. Biol. 301:568–577. 10.1016/j.ydbio.2006.09.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhou Y, Lim KC, Onodera K, Takahashi S, Ohta J, Minegishi N, Tsai FY, Orkin SH, Yamamoto M, Engel JD. 1998. Rescue of the embryonic lethal hematopoietic defect reveals a critical role for GATA-2 in urogenital development. EMBO J. 17:6689–6700. 10.1093/emboj/17.22.6689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Khandekar M, Suzuki N, Lewton J, Yamamoto M, Engel JD. 2004. Multiple, distant Gata2 enhancers specify temporally and tissue-specific patterning in the developing urogenital system. Mol. Cell. Biol. 24:10263–10276. 10.1128/MCB.24.23.10263-10276.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hoshino T, Shimizi R, Ohmori S, Nagano M, Pan X, Ohneda O, Khandekar M, Yamamoto M, Lim KC, Engel JD. 2008. Reduced BMP4 abundance in Gata2 hypomorphic mutant mice result in uropathies resembling human CAKUT. Genes Cells 13:159–170. 10.1111/j.1365-2443.2007.01158.x [DOI] [PubMed] [Google Scholar]
- 15.Babey M, Kopp P, Robertson GL. 2011. Familial forms of diabetes insipidus: clinical and molecular characteristics. Nat. Rev. Endocrinol. 7:701–714. 10.1038/nrendo.2011.100 [DOI] [PubMed] [Google Scholar]
- 16.Moeller HB, Rittig S, Fenton RA. 2013. Nephrogenic diabetes insipidus: essential insights into the molecular background and potential therapies for treatment. Endocr. Rev. 34:278–301. 10.1210/er.2012-1044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hasler U, Leroy V, Martin PY, Féraille E. 2009. Aquaporin-2 abundance in the renal collecting duct: new insights from cultured cell models. Am. J. Physiol. Renal Physiol. 297:10–18. 10.1152/ajprenal.00053.2009 [DOI] [PubMed] [Google Scholar]
- 18.Frøkiaer J, Marples D, Knepper MA, Nielsen S. 1996. Bilateral ureteral obstruction downregulates expression of vasopressin-sensitive AQP-2 water channel in rat kidney. Am. J. Physiol. 270:657–668 [DOI] [PubMed] [Google Scholar]
- 19.Marples D, Frøkiaer J, Nielsen S. 1999. Long-term regulation of aquaporins in the kidney. Am. J. Physiol. 276:331–339 [DOI] [PubMed] [Google Scholar]
- 20.Nielsen S, Kwon TH, Christensen BM, Promeneur D, Frøkiaer J, Marples D. 1999. Physiology and pathophysiology of renal aquaporins. J. Am. Soc. Nephrol. 10:647–663 [DOI] [PubMed] [Google Scholar]
- 21.Suzuki N, Ohneda O, Minegishi N, Nishikawa M, Ohta T, Takahashi S, Engel JD, Yamamoto M. 2006. Combinatorial Gata2 and Sca1 expression defines hematopoietic stem cells in the bone marrow niche. Proc. Natl. Acad. Sci. U. S. A. 103:2202–2207. 10.1073/pnas.0508928103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lim KC, Hosoya T, Brandt W, Ku CJ, Hosoya-Ohmura S, Camper SA, Yamamoto M, Engel JD. 2012. Conditional Gata2 inactivation results in HSC loss and lymphatic mispatterning. J. Clin. Invest. 122:3705–3717. 10.1172/JCI61619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Perl AK, Kist R, Shan Z, Scherer G, Whitsett JA. 2005. Normal lung development and function after Sox9 inactivation in the respiratory epithelium. Genesis 41:23–32. 10.1002/gene.20093 [DOI] [PubMed] [Google Scholar]
- 24.Traykova-Brauch M, Schönig K, Greiner O, Miloud T, Jauch A, Bode M, Felsher DW, Glick AB, Kwiatkowski DJ, Bujard H, Horst J, von Knebel Doeberitz M, Niggli FK, Kriz W, Gröne HJ, Koesters R. 2008. An efficient and versatile system for acute and chronic modulation of renal tubular function in transgenic mice. Nat. Med. 14:979–984. 10.1038/nm.1865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Takai J, Moriguchi T, Suzuki M, Yu L, Ohneda K, Yamamoto M. 2013. The Gata1 5′ region harbors distinct cis-regulatory modules that direct gene activation in erythroid cells and gene inactivation in HSCs. Blood 122:3450–3460. 10.1182/blood-2013-01-476911 [DOI] [PubMed] [Google Scholar]
- 26.Tanabe O, McPhee D, Kobayashi S, Shen Y, Brandt W, Jiang X, Campbell AD, Chen YT, Chang CS, Yamamoto M, Tanimoto K, Engel JD. 2007. Embryonic and fetal beta-globin gene repression by the orphan nuclear receptors, TR2 and TR4. EMBO J. 26:2295–2306. 10.1038/sj.emboj.7601676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.de Boer E, Rodriguez P, Bonte E, Krijgsveld J, Katsantoni E, Heck A, Grosveld F, Strouboulis J. 2003. Efficient biotinylation and single-step purification of tagged transcription factors in mammalian cells and transgenic mice. Proc. Natl. Acad. Sci. U. S. A. 100:7480–7485. 10.1073/pnas.1332608100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Holthöfer H, Schulte BA, Spicer SS. 1987. Expression of binding sites for Dolichos biflorus agglutinin at the apical aspect of collecting duct cells in rat kidney. Cell Tissue Res. 249:481–485 [DOI] [PubMed] [Google Scholar]
- 29.Stehberger PA, Shmukler BE, Stuart-Tilley AK, Peters LL, Alper SL, Wagner CA. 2007. Distal renal tubular acidosis in mice lacking the AE1 (band3) Cl-/HCO3- exchanger (slc4a1). J. Am. Soc. Nephrol. 18:1408–1418. 10.1681/ASN.2006101072 [DOI] [PubMed] [Google Scholar]
- 30.Dickinson H, Walker DW, Cullen-McEwen L, Wintour EM, Moritz K. 2005. The spiny mouse (Acomys cahirinus) completes nephrogenesis before birth. Am. J. Physiol. Renal Physiol. 289:273–279. 10.1152/ajprenal.00400.2004 [DOI] [PubMed] [Google Scholar]
- 31.Grass JA, Boyer ME, Pal S, Wu J, Weiss MJ, Bresnick EH. 2003. GATA-1-dependent transcriptional repression of GATA-2 via disruption of positive autoregulation and domain-wide chromatin remodeling. Proc. Natl. Acad. Sci. U. S. A. 100:8811–8816. 10.1073/pnas.1432147100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yasui M, Zelenin SM, Celsi G, Aperia A. 1997. Adenylate cyclase-coupled vasopressin receptor activates AQP2 promoter via a dual effect on CRE and AP1 elements. Am. J. Physiol. 272:443–450 [DOI] [PubMed] [Google Scholar]
- 33.Yu MJ, Miller RL, Uawithya P, Rinschen MM, Khositseth S, Braucht DW, Chou CL, Pisitkun T, Nelson RD, Knepper MA. 2009. Systems-level analysis of cell-specific AQP2 gene expression in renal collecting duct. Proc. Natl. Acad. Sci. U. S. A. 106:2441–2446. 10.1073/pnas.0813002106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Uchida S, Matsumura Y, Rai T, Sasaki S, Marumo F. 1997. Regulation of aquaporin-2 gene transcription by GATA-3. Biochem. Biophys. Res. Commun. 232:65–68. 10.1006/bbrc.1997.6236 [DOI] [PubMed] [Google Scholar]
- 35.Rauchman MI, Nigam SK, Delpire E, Gullans SR. 1993. An osmotically tolerant inner medullary collecting duct cell line from an SV40 transgenic mouse. Am. J. Physiol. 265:416–424 [DOI] [PubMed] [Google Scholar]
- 36.Apostol E, Ecelbarger CA, Terris J, Bradford AD, Andrews P, Knepper MA. 1997. Reduced renal medullary water channel expression in puromycin aminonucleoside-induced nephrotic syndrome. J. Am. Soc. Nephrol. 8:15–24 [DOI] [PubMed] [Google Scholar]
- 37.Kwon TH, Frøkiaer J, Knepper MA, Nielsen S. 1998. Reduced AQP1, -2, and -3 levels in kidneys of rats with CRF induced by surgical reduction in renal mass. Am. J. Physiol. 275:724–741 [DOI] [PubMed] [Google Scholar]
- 38.Kim EJ, Lee YJ, Ahn YM, Lee H, Kang DG, Lee HS. 2013. Renoprotective effect of Alpiniae oxyphyllae Fructus on ischemia/reperfusion-induced acute renal failure. Arch. Pharm. Res. 36:1004–1012. 10.1007/s12272-013-0117-3 [DOI] [PubMed] [Google Scholar]
- 39.Zharkikh L, Zhu X, Stricklett PK, Kohan DE, Chipman G, Breton S, Brown D, Nelson RD. 2002. Renal principal cell-specific expression of green fluorescent protein in transgenic mice. Am. J. Physiol. Renal Physiol. 283:1351–1364 [DOI] [PubMed] [Google Scholar]
- 40.Li SZ, McDill BW, Kovach PA, Ding L, Go WY, Ho SN, Chen F. 2007. Calcineurin-NFATc signaling pathway regulates AQP2 expression in response to calcium signals and osmotic stress. Am. J. Physiol. Cell Physiol. 292:1606–1616. 10.1152/ajpcell.00588.2005 [DOI] [PubMed] [Google Scholar]
- 41.Ma T, Yang B, Gillespie A, Carlson EJ, Epstein CJ, Verkman AS. 1997. Generation and phenotype of a transgenic knockout mouse lacking the mercurial-insensitive water channel aquaporin-4. J. Clin. Invest. 100:957–962. 10.1172/JCI231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ma T, Song Y, Yang B, Gillespie A, Carlson EJ, Epstein CJ, Verkman AS. 2000. Nephrogenic diabetes insipidus in mice lacking aquaporin-3 water channels. Proc. Natl. Acad. Sci. U. S. A. 97:4386–4391. 10.1073/pnas.080499597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Brenner BM. 2008. Brenner & Rector's the kidney. 8th ed. Saunders Elsevier, Boston, MA [Google Scholar]
- 44.Hadrup N, Petersen JS, Windfeld S, Risom L, Andersen CB, Nielsen S, Christensen S, Jonassen TE. 2007. Differential down-regulation of aquaporin-2 in rat kidney zones by peripheral nociceptin/orphanin FQ receptor agonism and vasopressin type-2 receptor antagonism. J. Pharmacol. Exp. Ther. 323:516–524. 10.1124/jpet.107.123588 [DOI] [PubMed] [Google Scholar]
- 45.Moriguchi T, Takako N, Hamada M, Maeda A, Fujioka Y, Kuroha T, Huber RE, Hasegawa SL, Rao A, Yamamoto M, Takahashi S, Lim KC, Engel JD. 2006. Gata3 participates in a complex transcriptional feedback network to regulate sympathoadrenal differentiation. Development 133:3871–3881. 10.1242/dev.02553 [DOI] [PubMed] [Google Scholar]
- 46.Nardelli J, Thiesson D, Fujiwara Y, Tsai FY, Orkin SH. 1999. Expression and genetic interaction of transcription factors GATA-2 and GATA-3 during development of the mouse central nervous system. Dev. Biol. 210:305–321. 10.1006/dbio.1999.9278 [DOI] [PubMed] [Google Scholar]
- 47.Ma GT, Roth ME, Groskopf JC, Tsai FY, Orkin SH, Grosveld F, Engel JD, Linzer DI. 1997. GATA-2 and GATA-3 regulate trophoblast-specific gene expression in vivo. Development 124:907–914 [DOI] [PubMed] [Google Scholar]