Abstract
The continuing spread of methamphetamine (METH) abuse has stimulated research aimed at understanding consequences of its prolonged exposure. Alterations in nigrostriatal dopamine (DA) system parameters have been characterized in experimental studies after discontinuation of long term METH but fewer studies have included similar assessments during METH exposure. Here, we report METH plasma pharmacokinetics and striatal DA system alterations in rat after noncontingent and contingent METH administration for 7.5 weeks. Escalating METH exposure was delivered by dynamic infusion (DI) that incorporated a ‘humanized’ plasma METH half life, or by intravenous self-administration (IVSA) that included binge intakes. Kinetic modeling of DI and IVSA for 24 h periods during the final week of METH exposure showed that plasma METH levels remained between 0.7–1.5 μM. Animals were sacrificed during their last METH administration for autoradiography assessment using [3H]ligands and D2 agonist-induced [35S]GTPγS binding. DA transporter binding was decreased (DI, 34%; IVSA, 15%) while vesicular monoamine transporter binding and substantia nigra DA cell numbers were unchanged. Decreases were measured for D2 receptor (DI and IVSA, 15–20%) and [35S]GTPγS binding (DI, 35%; IVSA, 18%). These similar patterns of DI and IVSA associated decreases in striatal DA markers reflect consequences of cumulative METH exposure and not the drug delivery method. For METH IVSA, individual differences were observed, yet each animal’s total intake was similar within and across three 24 h binges. IVSA rodent models may be useful for identifying molecular mechanisms that are associated with METH binges in humans.
Keywords: Dopamine transporter, Self-administration, Striatum, Tolerance, Binges
INTRODUCTION
Methamphetamine (METH) is a highly abused drug that can produce long term behavioral and cognitive impairments which are particularly manifested during METH exposures continuing over periods of one or more days, i.e., a METH binge. Since METH has profound effects on the brain dopamine system, it can be hypothesized that the expression of those behaviors is associated with concurrent METH-induced alterations in dopamine system neurochemistry. In chronic METH users, decreases in striatal dopamine system integrity have been identified by PET imaging during METH abstinence (Johanson et al., 2006; McCann et al., 1998; Sekine et al., 2001; Volkow et al., 2001) but assessment of further alterations occurring acutely during time periods when METH is being abused cannot be conducted, in vivo. Here, the use of relevant animal studies may be informative, particularly since pharmacokinetic factors of METH binge administration in humans can be modeled and animal brain tissues can be obtained concurrently for analysis.
Accordingly, the design of METH administration protocols in experimental studies needs to be well-justified; otherwise, extrapolation of those results to furthering our understanding of the human condition is limited. For example, the method of drug delivery remains an issue of debate, particularly regarding the relative advantages of contingent and noncontingent administration (Steketee and Kalivas, 2012). The majority of experimental METH studies have been conducted using experimenter-delivered drug with the implicit assumption that the resultant METH concentration in the brain is the critical determinant of behavioral and neurochemical effects. Some comparative studies of noncontingent and contingent METH administration, however, have shown qualitative and quantitative differences in brain neurochemistry profiles (Frankel et al., 2011; Stefanski et al., 2002). In contrast, other studies irrespective of the drug delivery method have elicited similar patterns of locomotor and stereotypy behaviors (Hadamitzky et al., 2012; Segal et al., 2005). Thus, it appears that while the use of either administration protocol can be justified, contingent approaches, i.e., METH intravenous self-administration (IVSA), are increasingly being used with the rationale that they provide greater face validity with respect to patterns of human METH abuse (Cadet et al., 2009; Cho et al., 2001; Danaceau et al., 2007; Davidson et al., 2001; Krasnova et al., 2010; Krasnova et al.; McFadden et al., 2012; Schwendt et al., 2009).
Additionally, the METH dose, frequency of administration, dose escalation, and total duration of exposure need to parallel aspects of human METH abuse conditions. In these studies, we incorporated prolonged METH exposures that are tolerated in the rat without morbidity, and that also include progressively greater doses prior to binge treatments, as is typical in most stimulant-abusing populations (Davidson et al., 2001; Simon et al., 2002). To explore neurochemical consequences of this cumulative METH exposure that may be drug delivery-dependent, we used both an intravenous noncontingent (Dynamic Infusion (DI)) and contingent IVSA drug delivery in rats, in conjunction with assessment of striatal dopamine system integrity during binge-like METH exposures (Hadamitzky et al., 2011; Segal and Kuczenski, 2006). The DI procedure allows for investigator-delivered drug administration that precisely reproduces in rats a plasma METH profile that occurs in humans. This method can provide extended METH exposures in long term studies with plasma concentrations that have been measured in some human METH abuse conditions (Jones et al., 2008; Logan et al., 1998; Melega et al., 2007). For comparison, we developed an IVSA model that included extended periods of daily METH access ranging from 1–12 h/d and that allowed the animal to increase its own drug intake over approximately 7.5 weeks of METH availability. The study design culminated in IVSA continuous access to METH for multiple 24 h periods, i.e., binge-like METH intakes. Although it was not possible to match exactly the total METH exposures for the DI- and IVSA METH protocols, we hypothesized that similar trends in striatal dopamine (DA) system characterizations obtained during DI exposures and IVSA METH binges would suggest that the dominant factor underlying those alterations was the METH exposure, per se, rather than the drug delivery method. Conversely, qualitatively different brain profiles may identify alterations unique to IVSA which, in turn, may be more associated with aspects of drug reinforcement as observed in humans (Angrist, 1983; Curran et al., 2004).
MATERIALS AND METHODS
Subjects
Male Sprague-Dawley rats (275–300g) were obtained from Harlan Labs (Gilroy, CA), and for at least 2 weeks prior to surgery were housed in groups of 2 or 3 animals in wire mesh cages with ad libitum access to food and water. The room was temperature (21.5°C) and humidity-controlled (55 ± 5%), and maintained on a reversed 12 h dark (8:00 am to 8:00 pm), 12 h light cycle to allow for the start of treatment during the normal active phase of the awake/sleep cycle of the rat (Sleipness et al., 2008). During the dark period, all facilities were illuminated with red light to facilitate observation of the animals.
Surgery
All surgical and experimental procedures were conducted in accordance with the National Institutes of Health and Association for the Assessment and Accreditation of Laboratory Animal Care guidelines and were approved by the Institutional Animal Care and Use Committee. The surgical procedures for the DI animals were performed as previously described (Hadamitzky et al., 2011; Segal and Kuczenski, 2006). Briefly, after 2 weeks of acclimation, animals were implanted with i.v. catheters under halothane anesthesia. Catheters were constructed by fitting a 13 cm length of silastic tubing to a guide cannula which was embedded in dental cement and attached to a 2.5 cm circle of marlex mesh and mounted on the back of the animal. Silastic tubing was then passed subcutaneously from the back of the rat to the right external jugular vein. A Tygon cap was inserted over the guide cannula to maintain a closed system. Animals were singly-housed after surgery and on a daily basis between surgery and experimental testing, the catheter was flushed with sterile saline (0.1 ml) containing 3.0 USP units heparin and 3.8 mg timentin.
Drugs
Methamphetamine hydrochloride (Sigma Chemical Co., St. Louis, MO) was dissolved in 0.9% saline and administered intravenously; doses were formulated as METH base (mw 135). Control animals received a comparable administration of vehicle.
Study 1: Escalating Dose – Dynamic Infusion (DI) METH Treatment Protocol
About 2 weeks after surgery, DI animals were placed in individual custom-designed activity chambers (Segal and Kuczenski, 1987) where they remained for the duration of the study. Tubing from a PHM-100 syringe pump (Med Associates Inc., St. Albans, Vt.) was attached to the animal’s catheter via a liquid swivel and a commercially available cannula connector (Plastics One, Roanoke, VA). Each morning throughout the study just prior to the transition from light to dark phase (7:00 am–8:00 am), the behavioral chambers were serviced and the animals were weighed and examined for health. Following a 3 day acclimation period, during which animals received daily dynamic infusions of saline (see below), drug administration was initiated.
Drug Administration
Following acclimation to the activity chambers, animals received either 1 or 2 daily dynamic infusions of METH or comparable volumes of saline, with the METH dose being progressively increased (0.125, 0.187, 0.25, and 0.375 mg/kg) according to the injection schedule until each injection delivered 0.5 mg/kg METH (Table 1). On days when 2 injections were delivered, the inter-injection interval was 4 h. Remote drug or saline administration was initiated at 9:00 a.m. according to the following pattern: drug delivery involved an initial intravenous infusion of METH (e.g., 0.125 mg/kg, administered in 0.105 ml) over a 10 s interval. An 80 dB, 2.2 KHz tone-cue was presented to each animal for 5 s prior to, and during the 10 s drug injection. The audible tone was presented to provide rats with a drug-predictive cue, shown in recent studies to be a potentially significant factor in stimulant-induced neuronal alterations (Ghitza et al., 2003). Since rat plasma METH concentrations decline much more rapidly than in humans, additional METH administration following each dose was required to simulate human METH pharmacokinetics. To model those kinetics, METH was delivered according to a computer-controlled program subsequent to each bolus infusion in the form of short-duration injections (mini-pulses), e.g., 0.28 μl per mini-pulse (1.3 μg/kg for a bolus 0.5 mg/kg dose) over a 16 ms duration, in order to generate a plasma profile of the drug corresponding to a 11 h plasma half-life (for detailed discussion, see (Segal and Kuczenski, 2006). The temporal profile of mini-pulses administered after the initial 6-sec drug injection is summarized in Figure 1. Each animal received a cumulative dose of 4.39 mg/kg over a 24-hr interval to achieve plasma and brain drug exposure in a temporal pattern that is similar to the 11-hr half-life for human METH pharmacokinetics. These minipulses were not anticipated to produce significant increases in extracellular DA insofar as we previously showed that measurable effects on extracellular DA required 20 mini-pulses, equivalent to 26 μg/kg METH/ 24 h (Kuczenski and Segal, 2006).
TABLE 1.
Administration Schedule for Dynamic Infusion (DI) METH with Escalating Dosage.
| Monday | Tuesday | Wednesday | Thursday | Friday | |
|---|---|---|---|---|---|
| Week 1 | 0.125 | 0.125 | 0.125 | 2 × 0.125 | 2 × 0.125 |
| Week 2 | 0.125 | 0.1875 | 0.1875 | 2 × 0.1875 | 2 × 0.1875 |
| Week 3 | 0.1875 | 0.25 | 0.25 | 2 × 0.25 | 2 × 0.25 |
| Week 4 | 0.25 | 0.375 | 0.375 | 2 × 0.375 | 2 × 0.375 |
| Week 5 | 0.375 | 0.5 | 0.5 | 2 × 0.5 | 2 × 0.5 |
| Week 6 | 0.5 | 2 × 0.5 | 2 × 0.5 | 2 × 0.5 | 2 × 0.5 |
| Week 7 | 0.5 | 2 × 0.5 | 2 × 0.5 | 2 × 0.5 | 2 × 0.5 |
| Week 8 | 0.5 | 2 × 0.5 | 2 × 0.5 |
A single DI METH injection/day is indicated by the dosage (mg/kg); Two DI injections/day are indicated 2 × dosage. On days when two DI METH injections were delivered, the inter-injection interval was 4 h.
Fig. 1.
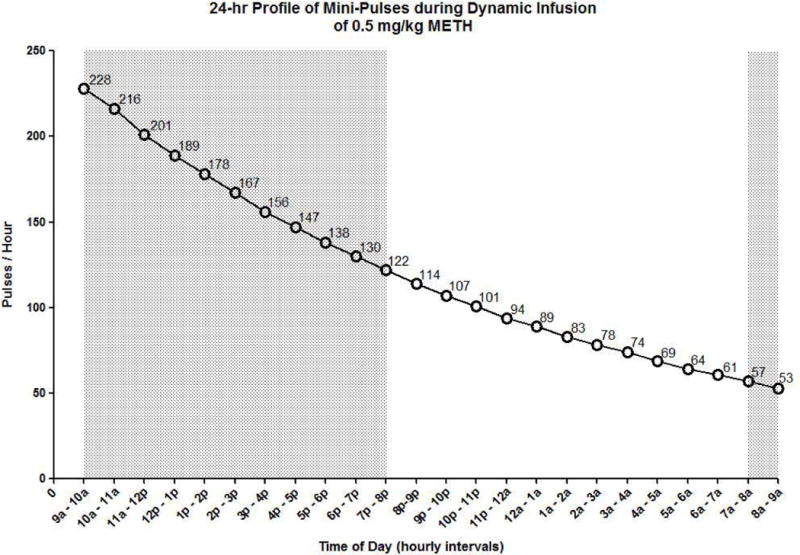
Temporal pattern of mini-pulses over a 24-hr period during a 0.5 mg/kg dynamic infusion of METH. Following a 6-sec injection of drug at 9:00 a.m., METH was delivered according to a computer controlled program in the form of short duration injections or mini-pulses (each 0.28 μl over a 16 msec duration delivering 1.3 μg/kg for a 0.5 mg/kg initial dose) as described in Methods. The values on the curve represent the number of pulses administered during the subsequent hour in order to achieve an 11-hr half-life. Shading represents the dark/active phase of the light-dark cycle.
On the 3rd d of Week 8, DI METH animals received an acute challenge of either saline or DI METH (2 × 0.5 mg/kg), and saline (control) animals received an acute challenge of either saline or DI METH (2 × 0.5 mg/kg). All animals were euthanized at 1 h after their 2nd DI METH- or saline-injection (Table 2). The total duration of METH exposure for all DI groups of animals was ~ 7.5 weeks.
TABLE II.
Administration Schedule for Dynamic Infusion (DI) METH Exposures for Long Term METH and METH Challenge.
| Group | Long Term | METH Challenge |
|---|---|---|
| 1 | saline | saline |
| 2 | saline | DI METH 2 × 0.5 mg/kg |
| 3 | DI METH | Saline |
| 4 | DI METH | DI METH 2 × 0.5 mg/kg |
On the 3rd day of Week 8, all groups were administered the METH Challenge and euthanized at 1 h after their 2nd DI METH – or saline injection.
Study 2: Intravenous Self-Administration (IVSA) METH Treatment Protocol
After a 5 d recovery period from surgery, IVSA METH was initiated in the activity chambers identical to the chambers used in Study 1, with the exception that one wall of the chamber was equipped with two retractable levers (active and inactive, 6 cm apart) located 6 cm above the metal grid floor of the chamber. Active lever presses resulted in an infusion of METH at a dose of 0.05 mg/kg in a volume of 0.05 ml over a period of 1.5 s (fixed-ratio: FR1). Pressing on the inactive lever had no consequences. METH delivery was paired with the activation of the cue light located above the levers, which remained illuminated throughout a 20 s timeout period, while the lever was inactive. Levers were retracted immediately at the end of the session. Rat performance was considered stable when the subject pressed the active lever at least 10 times over 3 consecutive days (achieved by ~ 2 weeks).
IVSA animals were maintained as 3 separate groups (n = 8–10 per group): saline (controls): limited access (LMT) METH, 1h/day, and extended access (EXT) METH, ranging from 1–12 h/d and including 24h/d binges (Table 3). After acquisition of IVSA METH and 6 additional days of 1 h daily exposure sessions (~ 1 week) for all animals, one group remained in this restricted IVSA (~ 4.5 weeks), while the other group, EXT, was started on an IVSA escalating dose protocol (see (Hadamitzky et al., 2011; Hadamitzky et al., 2012). Briefly, drug access time was extended over successive 6 day periods (Table 3). For each period, the daily access time progressed from 3, 6, to 12 h, with 2 drug abstinence days between changes in the duration of IVSA periods (~ 3 weeks). Subsequently, EXT animals were given access to METH for three sessions of 24 h duration (binges), with each binge followed by 2 drug abstinence days. After 6 h of their 4th 24 h binge, EXT animals were euthanized (binge time ~ 1.5 weeks). LMT animals were euthanized immediately at the end of their last 1 h IVSA session. With regard to the euthanasia times for the LMT and EXT groups, we sought to terminate the IVSA animals while they were undergoing stable METH intake. For comparisons, the 6-h time point was selected for the EXT group to approximate the euthanasia time for the DI group. Since the LMT group only had access to drug for 1 hour, they could not be euthanized at an equivalent time point.
TABLE III.
Intravenous Self-Administration (IVSA) METH
| IVSA Access Time | #of IVSA Days | # of Abstinence Days |
|---|---|---|
| IVSA Acquisition/Training for All Animals (2 weeks) | ||
| Limited Access (5.5 weeks) | ||
| 1 h | 6 | 2 |
| Extended Progressive Access (5.5 weeks) | ||
| 1 h | 6 | 2 |
| 3 h | 6 | 2 |
| 6 h | 6 | 2 |
| 12 h | 6 | 2 |
| Binge Sessions | ||
| 24 h | 1 | 2 |
| 24 h | 1 | 2 |
| 24 h | 1 | 2 |
| 6 h | 1 | |
IVSA METH access was provided for 7.5 weeks to the limited- and extended progressive-assess animals. In the final week of IVSA, animals were euthanized after 1 and 6 h of IVSA METH, respectively.
The total duration of METH exposure for all IVSA groups of animals was ~ 7.5 weeks. As previously noted (Hadamitzky et al., 2011; Kuczenski et al., 2009) neither DI nor IVSA animals exhibited evidence of elevated core body temperatures (i.e., hyperthermia) over this dose range using similar treatment schedules. Likewise, in prior studies, we analyzed the effects of temperature on METH – brain effects using comparable long term escalating METH doses and binge treatments in which temperature increases were not observed (Kuczenski et al., 2009; O’Neil et al., 2006; Segal et al., 2003).
Pharmacokinetic Modeling
Estimates of plasma METH levels were calculated for DI and IVSA METH using a one compartment open model with METH pharmacokinetic parameters we had obtained from our prior studies (Vd = 3.9 L/kg; human t1/2 = 11h for DI METH, and rat t1/2 = 70 min for IVSA METH (Cho et al., 2001).
For DI METH, 2 injections of 0.5mg/kg with a 4 h interval were entered into the model with a ‘humanized half-life of 11h. The calculated plasma METH levels were then plotted for a 24 h period.
For IVSA METH, the cumulative IVSA METH intake per each 20 min interval was obtained for each animal (n = 8) using the number of lever presses for each interval × 0.05mg/kg per lever press. A subset of those animals (n = 6), was selected for further analysis of their uptake per 20 min intervals for the 24 h duration of METH access, based on their similar total METH intake. Two outliers (1 high (#1) and 1 low (#3) total METH intake); see Figure 2) were excluded because the graphical analysis was designed to show the mean plasma METH concentrations vs time for animals with similar total METH intake. METH intake values per interval (0–20, 20–40, 40–60 min, etc) from the remaining 6 animals were entered into the model. Their mean plasma METH levels per interval were plotted at mid-interval time points (10, 30, 50 min, etc) for the 24 h access period.
Fig. 2.
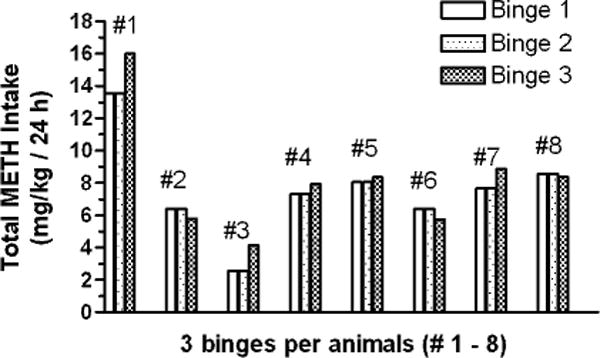
Total intravenous self-administration (IVSA) METH intake per 24 h of continuous access (METH binge). The IVSA METH intake for 8 individual animals is shown across 3 consecutive METH binges, with each binge separated by 2 abstinence days.
The area under the plasma METH concentration vs. time curves (Figure 1) were calculated for DI and IVSA METH 24 h exposures using the trapezoid rule (Graphpad Software, La Jolla, CA).
Post-Mortem Blood and Brain Tissue Preparation
The animals were sacrificed by decapitation; the brains were removed, placed into a rat mold and then singly-sliced coronally to obtain an anterior block that contained the entire striatum and frontal cortex and a posterior block that contained the entire substantia nigra. The anterior block was then hemisected; one half was slowly immersed into isopentane (maintained at −35°C on dry ice) for ~ 30 s, and then stored at −80°C. The remaining half of the anterior block and the entire posterior block were fixed for ~ 24 h in 4% paraformaldehyde, 0.1 M phosphate buffered saline, pH7.4, and cryoprotected through sequential transfers in graded solutions containing 10, 20, 30% sucrose in 0.1M phosphate, pH 7.4; 1% NaN3; blocks were stored in the 30 % solution at −15°C until used.
At the time of sacrifice, trunk blood from the DI-METH animals was collected in EDTA tubes for measuring plasma concentrations of METH and its metabolite, amphetamine (AMPH). Plasma was isolated by centrifugation and immediately frozen on dry ice. The concentrations of METH and AMPH were quantified by NMS Laboratories (Willow Grove, PA).
Ligand-Binding Assays
Hemisected anterior blocks of brain were used to generate sections for multiple ligand-binding studies, starting from mid-anterior striatum/nucleus accumbens to caudal striatum/globus pallidus; 20 μm cryostat sections were obtained (Leica CM-3050 S (Leica Microsystems, Bannockburn, IL) at −20°C and thaw-mounted onto gelatin-subbed slides, and then desiccated under vacuum overnight at 4°C, placed in plastic mailer boxes with desiccant and stored at −80°C until use. Nissl-stained sections containing the striatum and corresponding to AP 1–1.6 mm from a rat brain atlas (Paxinos and Watson, 2005) were used to identify matching sections for radioligand binding. The slides for the different binding assays were selected in the following order (anterior to posterior): D2R-[35S]GTPγS, D2R, DAT, VMAT.
The following studies were conducted according to our previously published protocols for ligand-binding autoradiography (Segal et al., 2005) with minor modifications as stated below. The dorsal striatum was used as the region of interest (ROI), based on its association with compulsive drug seeking and stimulus-response habits (Everitt et al., 2008; Hyman et al., 2006). ROIs were drawn on computerized images of the exposed film using MCID (InterFocus Imaging Ltd., Cambridge, UK) and specific binding was quantified as total – nonspecific binding. The results for each assay were calculated as dpm/mg protein and then expressed as percent of control values.
-
-
DAT binding: 5 nM [3H]WIN 35,428, ([3H]WIN), (86 Ci/mmol; PerkinElmer NEN Radiochemicals; Waltham, MA), nonspecific binding with 30 μM cocaine (Coulter et al., 1995). This ligand has been previously well-characterized for its specific, high affinity binding to the DAT (Madras et al., 1989a; Madras et al., 1989b; Stanwood et al., 2000b).
-
-
VMAT binding: 10 nM [3H]Dihydrotetrabenazine, ([3H]TBzOH), (20 Ci/mmol; American Radiolabeled Chemicals; St. Louis, MO), nonspecific binding with 10 μM tetrabenazine (Darchen et al., 1989)
-
-
D2 Receptor (D2R) Binding: 2 nM [3H]raclopride, ([3H]RAC), (71.2 Ci/mmol; PerkinElmer Life Sciences, Boston, MA), nonspecific binding with 1.0μM (+)butaclamol (Nader et al., 2002). Raclopride D2R binding has also been characterized as D2-like, i.e., D2, D3, D4, (Sibley and Monsma, 1992) and as D2/D3 receptor availability (Volkow et al., 2012). We use the descriptor ‘D2R binding’ here insofar as we analyzed only the dorsal striatum in which the D2 receptor density is predominant over that of the D3 subtype (Stanwood et al., 2000a).
-
-
D2 Receptor Agonist-Induced [35S]GTPγS binding (1250 Ci/mmol, PerkinElmer Life Sciences, Boston, MA). For each assay condition, triplicate sections of rat striatum from at least four animals are used. Slides were incubated at 25°C for 15 min in assay buffer (50 mM Tris-HCl containing 3mM MgCl2, 0.2 mM EGTA, 100 mM NaCl, pH7.4 at 25°C) containing 1 mM GDP and 1μM 8-cyclopentyl-1,3-dipropylxantine, DPCPX, a selective A1 adenosine receptor antagonist. Slides were then transferred to incubation media that contained 0.04 nM [35S]GTPγS (12.5 μCi/nmol), 1 mM GDP (Sim et al., 1995) and 10 μM (R(-)-propylnorapomorphine hydrochloride, (NPA) a highly potent and selective D2R agonist; (NPA activation has been shown to be essentially totally blocked by 10nM spiperone) (O’Connor et al., 2005a; O’Connor et al., 2005b; Rinken et al., 1999).
Basal binding was measured by incubation in the absence of agonist, i.e., with GDP and [35S]GTPγS. Nonspecific binding was assessed in the presence of 10 μM GTPγS. All slides were incubated for 90 min at 30°C and then rinsed twice for 2 min each in ice-cold Tris-washing buffer (50 mM Tris-HCl, pH7.4 at 25°C) and once for 30 s in ice-cold deionized water. Slides were dried for 2 h under a cool stream of air, and then overnight in a desiccator at room temperature. Slides were apposed to Kodak BioMax MR film together with 14C standards for 24 h. Film was scanned on a ScanMaker i900 (Microtek Lab Inc., Carson, CA) and analyzed with MCID. For each dorsal striatum region examined, agonist-stimulated activity for each brain section was calculated by subtracting the optical density in basal sections (GDP only) from the optical density of agonist-stimulated sections; the resultant value was then expressed as nC/mg protein, based on the 14C-standards curve generated for that film.
Histology and Stereological Analysis of Tyrosine Hydroxylase-Positive Immunoreactive (TH+) Cells in the Substantia Nigra Pars Compacta (SNpc)
Midbrains from the DI METH/saline challenge group were selected to determine potential loss of SNpc cells following long term METH exposure. This group received 7.5 weeks of escalating METH, with the last METH exposure ~ 24 h prior to sacrifice; the Saline/saline challenge group was used for comparison.
Methods for immunostaining and quantitation of TH+ neuron cell bodies in the SNpc followed similar protocols that we (Harvey et al., 2000) and others have previously described (McCormack et al., 2002; Zhu et al., 2004). Coronal sections (40 μm) of midbrain were obtained and every 10th serial section was stained in 0.5% cresyl violet for anatomical determination of the SNpc region. Sections for immunohistochemistry first were rinsed in PBS and then endogenous peroxidases were blocked by incubation in 10% methanol/3% H2O2/PBS. Free floating sections were pre-incubated at room temperature for 1 h in 0.1M PBST-Azide (0.3% Triton X100 -PBST, 0.1% sodium azide) with 3% normal rabbit serum (Jackson ImmunoResearch Laboratories, West Grove, PA) and then overnight (in 0.1M PBST-Azide with 1.5% normal rabbit serum and the primary antibody – polyclonal sheep anti-tyrosine hydroxylase (1:1000) (PelFreez, Rogers, AR). The following day, sections were washed in PBS and then incubated at room temperature for 90 min in 0.1 M PBST with the secondary antibody – biotinylated rabbit anti-sheep IgG (1:200) (Vector Labs, Burlingame, CA) and 1.5% normal rabbit serum. Following PBS rinses, sections were incubated at room temperature for 90 min in PBS/avidin-biotin complex (ABC method) (1:200) (Vector Labs) and visualized following 5–10 min incubation in 0.02% 3,3′-diaminobenzidine tetrachloride (Sigma) and 0.0015% H2O2 in 50 mM Tris buffer pH7.5; counterstaining with 0.06% Pyronin Y for 20 min. For control sections, removal of the primary antibody step was followed by all remaining steps in the immunostaining protocol.
For stereological analysis of the TH+ neurons in the SNpc, coronal sections were selected that anatomically corresponded to the midbrain region between coordinates bregma −4.3 to −6.7. The number of TH+ neurons in the entire SNpc (Paxinos and Watson, 2005) was determined using unbiased stereological methods (West, 2002; West et al., 1991) with StereoInvestigator software (MicroBrightfield, Colchester, VT) and an Olympus BX-60 microscope equipped with a motorized stage (Ludl Electronic Products Ltd., Hawthorne, NY). Briefly, the SNpc was viewed and delineated on each section at 4 X objective; a scan grid of 250 × 250 μm was placed onto its contour to define the counting area. A 100 × 100 μm counting frame was randomly placed on the counting area which was then systematically sampled. The counting of TH+ cell bodies was performed with a 40 X objective. Following sampling of the SNpc midbrain region (12–15 sections), the optical fractionator formula was used to estimate the total number of TH+ neurons in the analyzed brain volume. This protocol allowed for coefficient of error (CE) for the estimated cell number as <0.09 (usually, 0.04–0.07).
Data Analysis
[3H] and [35S]Ligand-binding data were statistically analyzed using ANOVA with Bonferroni corrections for specific group comparisons; a t-test was used for the analysis of TH+ cell counts in the SNpc.
RESULTS
Behavior
The behavioral profiles of the DI and IVSA animals are described in detail elsewhere (Hadamitzky et al., 2011; Hadamitzky et al., 2012; Segal and Kuczenski, 2006). Of particular relevance to the present manuscript, at the time of sacrifice, both groups of animals were engaged in intense stereotyped behaviors, consisting predominantly of oral stereotypies.
Plasma METH levels in DI METH and METH IVSA Animals
In the DI METH administration study, plasma METH levels were measured at 1 h after the start of the second DI METH injection. METH levels (mean ± SEM) were 124 ± 21 ng/ml (0.8 μM) for the saline/METH Challenge group and 136 ± 12 ng/ml (0.9 μM) for the DI METH/METH Challenge animals. The AMPH levels were approximately 20% those of METH; 22 ± 4 ng/ml and 25 ± 2 ng/ml, respectively. Thus, prior DI METH exposure did not alter plasma METH and AMPH levels as obtained for the METH challenge dose.
Plasma METH levels for the extended access – IVSA METH administration study had been previously reported (Hadamitzky et al., 2012); for the EXT-METH group – after 6 h IVSA: 99 ± 11 ng/ml (0.66 μM) and for the LMT-METH group – at the end of their 1 h IVSA session: 61 ± 10 ng/ml (0.4 μM). AMPH levels were approximately 20–30% of METH levels: 31 ± 7 ng/ml and 12 ± 2 ng/ml, respectively.
Patterns of IVSA METH Intake
For each animal (n = 8), the total IVSA METH intake per 24 h of continuous access (METH binge) was calculated for each of its 3 consecutive METH binges (Figure 2). Each individual animal’s total METH intake per 24 h binge was similar across the 3 binges. Further, the corresponding mean values of total METH intake for the 3 binges were similar for the majority of animals (6.1 ± 0.4 mg/kg (mean ± SEM); n = 6). Relative to that group’s mean value, 2 animals had mean values of IVSA METH for their three binges of markedly different magnitudes – Animal #1 being higher (12.2 ± 0.7 mg/kg) and Animal #3 being lower (2.5 ± 0.3 mg/kg); these 2 animals’ IVSA METH intake were not included in the graphical analysis (Figure 3).
Fig. 3.
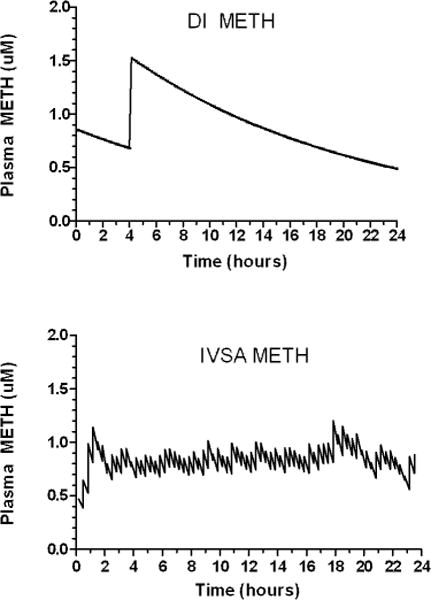
Kinetic modeling of 24 hour – dynamic infusion (DI) and intravenous self-administration (IVSA) METH. A one compartment open model was used to estimate plasma METH levels following DI and IVSA METH delivery in the rat. Upper: DI METH consisted of 2 METH injections (0.5 mg/kg) at a 4 h interval with micro-METH injections to model using a human plasma METH half-life (11 h); Lower: IVSA METH (0.05 mg/kg per lever press-injection) was continuously accessible for 24 h. Mean total METH intake (n = 6 animals) was summated per 20 min time bins and plotted at mid-interval time points throughout a 24 h METH access period, with plasma decay based on model kinetics using a rat plasma half-life of 70 min.
Pharmacokinetics and Kinetic Modeling of Plasma METH Levels for DI and IVSA METH Injections
A one compartment open model was used to model changes in plasma METH levels following DI and IVSA METH delivery. For DI METH, the 2 METH intravenous bolus injections of 0.5 mg/kg at an interval of 4 h, with subsequent microinjections (modeling a humanized plasma METH half-life of 11 h) resulted in an estimated Cmax of 1.5 μM; and Cmin of 0.6 μM across an entire 24 h period.
The IVSA METH modeling showed that for the first 20 min of each access period, an apparent ramping up of IVSA METH consistently occurred (data not shown). For the 24 h access periods, plasma METH levels ramped up to ~ 0.7–0.9 μM by the first hour of IVSA. That range of METH levels was then maintained by IVSA throughout the access period (Figure 3).
Comparison of the AUCs (plasma METH levels vs time) for the DI and IVSA over the 24 h period showed similar cumulative METH exposure: DI 21.2 vs. IVSA 19.5 μM × h.
Substantia Nigra Tyrosine Hydroxylase-Positive Cell Counts
Optical fractionator sampling methods were used to quantify TH+ cell numbers in the SNpc. (Figure 4). The mean ± SEM values for DI METH/Saline challenge (n = 8) vs. Saline/Saline challenge groups (n = 6) counts: 10,716 ± 1880 vs 10,652 ± 1320 were not significantly different.
Fig. 4.
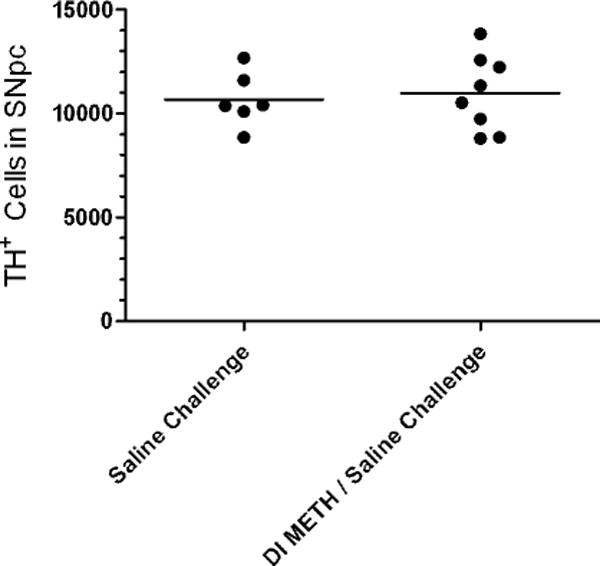
Tyrosine hydroxylase positive (TH+) cell numbers in the SNpc. Significant differences in the mean number of TH+ cells between Saline Challenge, and Dynamic Infusion (DI) METH / Saline Challenge (n = 6–8 per group) were not detected by stereologically-based cell counting methods.
Striatal DAT and VMAT Ligand Binding
[3H]WIN and [3H]TBzOH binding in dorsal striatum sections were measured using autoradiography methods. In the DI METH study, [3H]WIN binding was decreased by 8% in the Saline/METH Challenge group relative to controls but the effect was not statistically significant. For both the DI METH/METH Challenge and DI METH/Saline Challenge groups, [3H]WIN binding was decreased by 34% relative to the Saline/Saline Challenge (p < 0.001) (Figure 5A). In the IVSA METH study, [3H]WIN binding was decreased by 15% in the EXT-24h group (p < 0.05) while values for the LMT-1h group were similar to controls (Figure 6A).
Fig. 5.
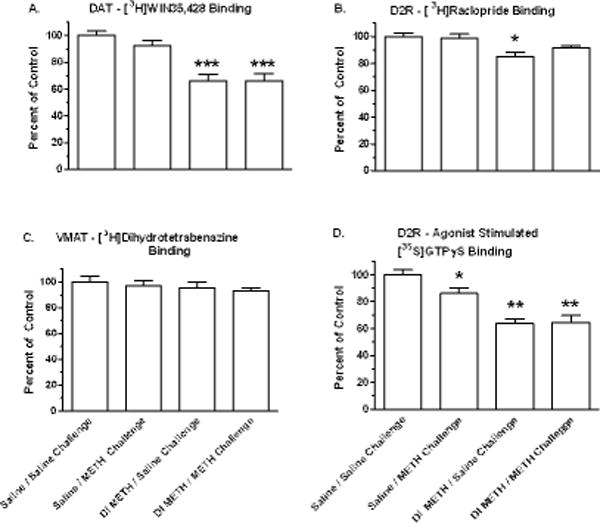
Dopaminergic alterations in dorsal striatum after long term dynamic infusion (DI) METH exposure and acute METH challenge. Drug-naïve animals and animals that had received 7.5 weeks exposure to DI METH were administered either a Saline Challenge or an acute METH Challenge of 2 doses of 0.5 mg/kg METH, i.v., at a 4 h interval, followed by euthanasia at 1 h after the 2nd injection. Ligand-binding autoradiography studies were conducted on contiguous sections containing the dorsal striatum. Each value represents the mean ± SEM for 6–8 animals, and are presented as percent of Saline Challenge (*p < 0.01, *** p < 0.001).
Fig. 6.
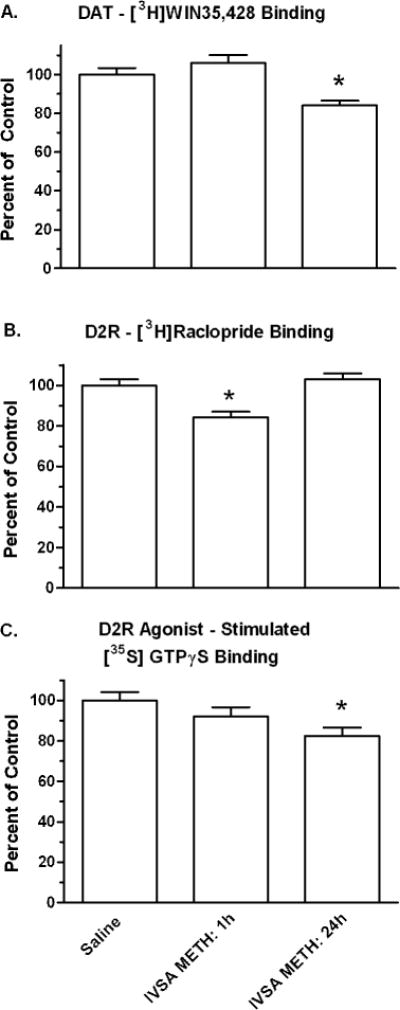
Dopaminergic alterations in dorsal striatum after intravenous self-administration (IVSA) METH. IVSA METH – 1h/d: Animals were provided limited access (1h/d) for 7.5 weeks and then euthanized immediately after the final 1 h/d METH access. IVSA METH – 24 h/d: Animals were provided progressive access for 7.5 weeks that culminated in three 24 h access- binges separated by 2 abstinence days (see Table 3), and then euthanized after 6 h further access to IVSA METH the animals. Ligand-binding autoradiography studies were conducted on contiguous sections containing the dorsal striatum. Each value is presented as the percent (mean ± SEM) of Saline controls for 6–8 subjects, (*p < 0.05).
[3H]TBzOH binding was measured in the DI METH study only. Relative to the Saline/Saline Challenge group, [3H]TBzOH binding was decreased by 3% in the saline/METH challenge group, by 5 % for the DI METH/METH Challenge, and by 7% for the DI METH/Saline Challenge groups but the effects were not statistically significant (Figure 5C).
Striatal D2Receptor and D2Receptor Agonist – Stimulated [35S]GTPγS Binding
In the DI METH study, [3H]RAC binding was not significantly decreased for the Saline/METH Challenge and DI METH/METH Challenge groups (2 and 7 % respectively). [3H]RAC binding was decreased by 15% for the DI METH/Saline Challenge group (p < 0.01), (Figure 5B). In IVSA METH animals, [3H]RAC binding was decreased by 16% for the LMT-1h METH group relative to controls (p < 0.01); statistically significant decreases were not obtained for the EXT-24h METH group (Figure 6B).
In the DI METH study, [35S]GTPγS binding was decreased by 14% in saline/METH Challenge group, and by 35 % for both the DI METH/Saline and DI METH/METH Challenge groups (p < 0.001) (Figure 5D). In the IVSA METH animals, [35S]GTPγS binding was decreased by 18% for the EXT-24 h METH group relative to controls (p < 0.05); an 8% decrease observed for the LMT-1h METH group was not statistically significant (Figure 6C).
DISCUSSION
In these studies, we extended our prior research on modeling aspects of human METH pharmacokinetics in rats. Using contingent and noncontingent administration, we attempted to identify alterations in striatal DA system markers that uniquely emerge during METH exposure. Our DI METH results showed that the decreases in striatal DA profiles we measured after long term DI METH were not further changed during a subsequent acute METH challenge, suggesting that a major determinant of those decreases was the cumulative METH exposure. Further, the profile of DI METH changes was qualitatively similar to that obtained during IVSA METH, suggesting that contingent METH delivery was not a necessary model component to effect those changes.
METH Effects on Nigrostriatal Dopamine System Markers
Both DI METH and EXT (escalating) IVSA METH promoted moderate decreases in striatal DAT ligand binding but had no effect on VMAT-ligand binding. In contrast, limited access METH (1h/d for 7.5 weeks) had no effect on either DAT or VMAT ligand binding suggesting that a threshold METH exposure, either in duration of exposure or in dose is necessary to effect those decreases. Apparently, chronicity of exposure is not itself a critical component since all chronic groups were subjected to 7.5 wks of treatment. Likewise, a relatively high dose is not sufficient since the saline/acute METH group also did not exhibit a decrease in DAT ligand binding.
Overall, the range of decreases in DAT-ligand binding in DI and IVSA exposed animals was in accord with the 10–40% decreases in DAT ligand binding or DAT protein as reported in recent studies that incorporated IVSA METH delivery (Krasnova et al., 2010; McFadden et al., 2012; Schwendt et al., 2009). Further validation for the use of our METH delivery methods is provided by the correspondence of decreases we measured and the 10–30% decreases in striatal DAT-ligand binding without significant decreases in VMAT ligand binding as observed in human PET imaging studies of METH-dependent individuals (Johanson et al., 2006; Sekine et al., 2001). Likewise, in a post-mortem study in humans with documented histories of chronic METH intake, reduced levels of DA, DAT, and tyrosine hydroxylase but not VMAT were reported. Although SNpc TH+ numbers were not assessed in that study, the striatum profile of METH-associated alterations was not indicative of SNpc degeneration (Wilson et al., 1996). Likewise, our striatum characterization for DI METH similarly implied an absence of SNpc cell loss for which confirmation was obtained by stereologic counts of SNpc TH+ cells. These results are in accord with prior escalating METH dose studies in which extensive nigrostriatal DA deficits likewise occurred without TH+ SNpc cell loss (Krasnova et al., 2010; Melega et al., 2008). Thus, the experimental studies provide substantial evidence that significant loss of TH+ SNpc cells is not associated with METH intake and, by analogy, not likely in the human condition as based on striatal profiles from post-mortem and in vivo imaging data showing only minor striatal DA deficits in chronic METH users during abstinence.
METH Effects on Striatal Dopamine Receptors
Our results on the effects of long term METH exposure on D2R-ligand binding are in accord with prior studies, namely, minor decreases (Segal et al., 2005; Stefanski et al., 2002) or no changes (Melega et al., 2008). Likewise, PET imaging studies of striatal D2R binding of chronic METH-dependent subjects have also not shown marked decreases in receptor binding availability. For example, in one study, [11C]raclopride binding was reduced in putamen by 10% and in caudate by 16% (Volkow et al., 2001) while another study showed that [11C[-N-methylspiperone binding was not decreased (Iyo et al., 1993). Additionally, a post-mortem analysis from a cohort of chronic METH users did not detect significant decreases in D2R protein (Worsley et al., 2000).
Although a lack of METH effect on D2R receptor functional activity could be inferred by the small decreases in [3H]RAC binding that we observed with DI and IVSA METH, the greater magnitude of decreases in [35S]GTPγS binding suggested that significant desensitization had occurred in D2R receptor/G-protein coupling. Those decreases were more likely a function of prior cumulative exposure than of acute changes during METH exposure insofar as [35S]GTPγS binding was not further changed during a subsequent acute METH challenge in the DI METH study. This D2R receptor/G-protein effect may be consequent to the long term METH exposure and associated persistent increases in extracellular DA. This proposed mechanism would be consistent with the results of a prior study in which prolonged (21 days) and continuous exposure to the long-acting DA uptake blocker, WF-23, resulted in a profound reduction (>70%) in striatal D2 stimulated [35S]GTPγS binding. Particularly noteworthy in that study was that the reduction in [35S]GTPγS binding occurred in the absence of receptor downregulation (O’Connor et al., 2005a; O’Connor et al., 2005b). Those studies and our DI and IVSA METH results suggest that a continuous elevation in extracellular DA may be a critical variable contributing to the magnitude of decreases in [35S]GTPγS binding. Such decreases in D2R signal transduction could represent a critical component of pharmacodynamic tolerance (O’Connor et al., 2005b) distinct from METH’s effects on well-characterized reductions on DA uptake and DA release mechanisms (Johnson-Davis et al., 2004; O’Neil et al., 2006).
Collectively, our results suggest that contingent vs noncontingent METH drug delivery was not a relevant factor underlying the patterns of METH-associated alterations but rather a function of prior cumulative METH exposure. However, conclusions on whether those decreases reflect neuroadaptations or neurotoxicity cannot be established from our ligand binding-based brain characterizations. Nonetheless, assessments of these dopaminergic markers in dorsal striatum in experimental and clinical studies are not likely to provide insights unique to molecular adaptations underlying METH abuse. Provisional support for that conclusion is suggested by PET imaging studies in human METH users in which METH-associated decreases in DAT and D2R-ligand-binding appear to be associated with only marginal clinical dysfunction (Hart et al., 2012).
DI and IVSA METH Pharmacokinetics
We have consistently argued (O’Neil et al., 2006; Segal and Kuczenski, 1997; Segal and Kuczenski, 2006) that in experimental studies it is the prolonged METH pretreatment that extends from weeks to months with progressively greater doses of the drug prior to a binge treatment that most closely parallels the majority of human abuse patterns. It remains a significant challenge, however, to model appropriate METH human dosage conditions in rats particularly since there are marked species differences in METH elimination half-life. In humans, high-dose METH intake results in being continuously exposed to the drug as a consequence of its 10–12 h half-life. In contrast, using traditional METH delivery approaches in rats of repeated administration at 2h intervals results in dramatic fluctuations in drug plasma levels, including prolonged periods in the absence of drug due to its 60–70 min half-life (Cho et al., 2001). Both our DI and IVSA METH methods were effectively used to resolve this interspecies difference in METH pharmacokinetics. With DI METH, the METH exposure was titrated to achieve plasma METH levels that were calculated to remain continuously between 0.7–1.5 μM, a concentration range that has been measured in some human METH abuse conditions (Gustavsen et al., 2006; Jones et al., 2008; Melega et al., 2007). Under IVSA METH conditions, the issue of METH dosage was titrated by the animal’s self-administration and motivational aspects for lever pressing, thus providing an ‘internal’ validation for the extent of its METH intake. Within the context of animal models, it may be notable that plasma levels maintained by the rat are similar to levels documented in some human abusers (Hadamitzky et al, 2012). However, the correspondence between the low μM METH levels obtained with our DI and IVSA delivery and documented human METH exposures that were without significant psychosis suggests that greater METH intake and corresponding plasma levels are necessary for expression of paranoid syndromes that are frequently associated with METH-associated violent, impulsive, and destructive behaviors. Thus, our METH pharmacokinetics and associated brain characterizations model an intermediate level of METH intake, wherein significant dose escalation and duration of exposure have occurred that are sufficient to effect decreases in markers of striatal DA system integrity.
The pharmacokinetic modeling of that IVSA for a 24 h access period revealed two consistent patterns. Firstly, the METH profile showed that when the animal was given access to IVSA, it apparently adjusted its intake such that the resultant plasma METH concentrations remained within a relatively narrow and low uM range. Secondly, for individual animals, there was remarkable consistency in total IVSA METH intake both within each binge and across all three binges. These consistent patterns of METH intake suggest that neither acute pharmacodynamic tolerance nor sensitization occurred during the METH binges insofar as increases or decreases in METH intake as each binge progressed or across successive binges were not observed.
In conclusion, the long term, escalating IVSA METH delivery designs we have developed in rats approximate relevant aspects of human METH intake, particularly, the pharmacokinetics of METH binges in which the animals self-administered for 24 h. Having now established that consistent IVSA METH intake can be obtained during binges, that time period can be used to investigate molecular mechanisms underlying adaptive responsivity to binge METH. Also, the consistent METH intake/binge within animals may also allow for identification of alterations associated with individual differences in IVSA. The results of such experimental approaches may suggest novel strategies for treatment of METH dependence.
Acknowledgments
This research was supported by the National Institutes of Health grant DA01568-30 from the National Institute on Drug Abuse to Ronald Kuczenski.
References
- Angrist B. In: Stimulants: neurochemical, behavioral and clinical perspectives. C I, editor. New York: Raven Press; 1983. [Google Scholar]
- Cadet JL, McCoy MT, Cai NS, Krasnova IN, Ladenheim B, Beauvais G, Wilson N, Wood W, Becker KG, Hodges AB. Methamphetamine preconditioning alters midbrain transcriptional responses to methamphetamine-induced injury in the rat striatum. PLoS One. 2009;4(11):e7812. doi: 10.1371/journal.pone.0007812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho AK, Melega WP, Kuczenski R, Segal DS. Relevance of pharmacokinetic parameters in animal models of methamphetamine abuse. Synapse. 2001;39(2):161–166. doi: 10.1002/1098-2396(200102)39:2<161::AID-SYN7>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Coulter CL, Happe HK, Bergman DA, Murrin LC. Localization and quantification of the dopamine transporter: comparison of [3H]WIN 35,428 and [125I]RTI-55. Brain Res. 1995;690(2):217–224. doi: 10.1016/0006-8993(95)00614-v. [DOI] [PubMed] [Google Scholar]
- Curran C, Byrappa N, McBride A. Stimulant psychosis: systematic review. Br J Psychiatry. 2004;185:196–204. doi: 10.1192/bjp.185.3.196. [DOI] [PubMed] [Google Scholar]
- Danaceau JP, Deering CE, Day JE, Smeal SJ, Johnson-Davis KL, Fleckenstein AE, Wilkins DG. Persistence of tolerance to methamphetamine-induced monoamine deficits. Eur J Pharmacol. 2007;559(1):46–54. doi: 10.1016/j.ejphar.2006.11.045. [DOI] [PubMed] [Google Scholar]
- Darchen F, Masuo Y, Vial M, Rostene W, Scherman D. Quantitative autoradiography of the rat brain vesicular monoamine transporter using the binding of [3H]dihydrotetrabenazine and 7-amino-8-[125I]iodoketanserin. Neuroscience. 1989;33(2):341–349. doi: 10.1016/0306-4522(89)90214-5. [DOI] [PubMed] [Google Scholar]
- Davidson C, Gow AJ, Lee TH, Ellinwood EH. Methamphetamine neurotoxicity: necrotic and apoptotic mechanisms and relevance to human abuse and treatment. Brain Res Brain Res Rev. 2001;36(1):1–22. doi: 10.1016/s0165-0173(01)00054-6. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Belin D, Economidou D, Pelloux Y, Dalley JW, Robbins TW. Review. Neural mechanisms underlying the vulnerability to develop compulsive drug-seeking habits and addiction. Philos Trans R Soc Lond B Biol Sci. 2008;363(1507):3125–3135. doi: 10.1098/rstb.2008.0089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankel PS, Hoonakker AJ, Alburges ME, McDougall JW, McFadden LM, Fleckenstein AE, Hanson GR. Effect of methamphetamine self-administration on neurotensin systems of the basal ganglia. J Pharmacol Exp Ther. 2011;336(3):809–815. doi: 10.1124/jpet.110.176610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghitza UE, Fabbricatore AT, Prokopenko V, Pawlak AP, West MO. Persistent cue-evoked activity of accumbens neurons after prolonged abstinence from self-administered cocaine. J Neurosci. 2003;23(19):7239–7245. doi: 10.1523/JNEUROSCI.23-19-07239.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustavsen I, Morland J, Bramness JG. Impairment related to blood amphetamine and/or methamphetamine concentrations in suspected drugged drivers. Accid Anal Prev. 2006;38(3):490–495. doi: 10.1016/j.aap.2005.11.005. [DOI] [PubMed] [Google Scholar]
- Hadamitzky M, Markou A, Kuczenski R. Extended access to methamphetamine self-administration affects sensorimotor gating in rats. Behav Brain Res. 2011;217(2):386–390. doi: 10.1016/j.bbr.2010.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadamitzky M, McCunney S, Markou A, Kuczenski R. Development of stereotyped behaviors during prolonged escalation of methamphetamine self-administration in rats. Psychopharmacology (Berl) 2012 doi: 10.1007/s00213-012-2713-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart CL, Marvin CB, Silver R, Smith EE. Is cognitive functioning impaired in methamphetamine users? A critical review. Neuropsychopharmacology. 2012;37(3):586–608. doi: 10.1038/npp.2011.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey DC, Lacan G, Tanious SP, Melega WP. Recovery from methamphetamine induced long-term nigrostriatal dopaminergic deficits without substantia nigra cell loss. Brain Res. 2000;871(2):259–270. doi: 10.1016/s0006-8993(00)02439-2. [DOI] [PubMed] [Google Scholar]
- Hyman SE, Malenka RC, Nestler EJ. Neural mechanisms of addiction: the role of reward-related learning and memory. Annu Rev Neurosci. 2006;29:565–598. doi: 10.1146/annurev.neuro.29.051605.113009. [DOI] [PubMed] [Google Scholar]
- Iyo M, Nishio M, Itoh T, Fukuda H, Suzuki K, Yamasaki T, Fukui S, Tateno Y. Dopamine D2 and serotonin S2 receptors in susceptibility to methamphetamine psychosis detected by positron emission tomography. Psychiatry Res. 1993;50(4):217–231. doi: 10.1016/0925-4927(93)90002-y. [DOI] [PubMed] [Google Scholar]
- Johanson CE, Frey KA, Lundahl LH, Keenan P, Lockhart N, Roll J, Galloway GP, Koeppe RA, Kilbourn MR, Robbins T, Schuster CR. Cognitive function and nigrostriatal markers in abstinent methamphetamine abusers. Psychopharmacology (Berl) 2006;185(3):327–338. doi: 10.1007/s00213-006-0330-6. [DOI] [PubMed] [Google Scholar]
- Johnson-Davis KL, Truong JG, Fleckenstein AE, Wilkins DG. Alterations in vesicular dopamine uptake contribute to tolerance to the neurotoxic effects of methamphetamine. J Pharmacol Exp Ther. 2004;309(2):578–586. doi: 10.1124/jpet.103.062695. [DOI] [PubMed] [Google Scholar]
- Jones AW, Holmgren A, Kugelberg FC. Driving under the influence of central stimulant amines: age and gender differences in concentrations of amphetamine, methamphetamine, and ecstasy in blood. J Stud Alcohol Drugs. 2008;69(2):202–208. doi: 10.15288/jsad.2008.69.202. [DOI] [PubMed] [Google Scholar]
- Krasnova IN, Justinova Z, Ladenheim B, Jayanthi S, McCoy MT, Barnes C, Warner JE, Goldberg SR, Cadet JL. Methamphetamine self-administration is associated with persistent biochemical alterations in striatal and cortical dopaminergic terminals in the rat. PLoS One. 2010;5(1):e8790. doi: 10.1371/journal.pone.0008790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krasnova IN, Ladenheim B, Hodges AB, Volkow ND, Cadet JL. Chronic methamphetamine administration causes differential regulation of transcription factors in the rat midbrain. PLoS One. 2011;6(4):e19179. doi: 10.1371/journal.pone.0019179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuczenski R, Segal DS, Melega WP, Lacan G, McCunney SJ. Human methamphetamine pharmacokinetics simulated in the rat: behavioral and neurochemical effects of a 72-h binge. Neuropsychopharmacology. 2009;34(11):2430–2441. doi: 10.1038/npp.2009.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan BK, Fligner CL, Haddix T. Cause and manner of death in fatalities involving methamphetamine. J Forensic Sci. 1998;43(1):28–34. [PubMed] [Google Scholar]
- Madras BK, Fahey MA, Bergman J, Canfield DR, Spealman RD. Effects of cocaine and related drugs in nonhuman primates. I. [3H]cocaine binding sites in caudate-putamen. J Pharmacol Exp Ther. 1989a;251(1):131–141. [PubMed] [Google Scholar]
- Madras BK, Spealman RD, Fahey MA, Neumeyer JL, Saha JK, Milius RA. Cocaine receptors labeled by [3H]2 beta-carbomethoxy-3 beta-(4-fluorophenyl)tropane. Mol Pharmacol. 1989b;36(4):518–524. [PubMed] [Google Scholar]
- McCann UD, Wong DF, Yokoi F, Villemagne V, Dannals RF, Ricaurte GA. Reduced striatal dopamine transporter density in abstinent methamphetamine and methcathinone users: evidence from positron emission tomography studies with [11C]WIN-35,428. J Neurosci. 1998;18(20):8417–8422. doi: 10.1523/JNEUROSCI.18-20-08417.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormack AL, Thiruchelvam M, Manning-Bog AB, Thiffault C, Langston JW, Cory-Slechta DA, Di Monte DA. Environmental risk factors and Parkinson’s disease: selective degeneration of nigral dopaminergic neurons caused by the herbicide paraquat. Neurobiol Dis. 2002;10(2):119–127. doi: 10.1006/nbdi.2002.0507. [DOI] [PubMed] [Google Scholar]
- McFadden LM, Hadlock GC, Allen SC, Vieira-Brock PL, Stout KA, Ellis JD, Hoonakker AJ, Andrenyak DM, Nielsen SM, Wilkins DG, Hanson GR, Fleckenstein AE. Methamphetamine self-administration causes persistent striatal dopaminergic alterations and mitigates the deficits caused by a subsequent methamphetamine exposure. J Pharmacol Exp Ther. 2012;340(2):295–303. doi: 10.1124/jpet.111.188433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melega WP, Cho AK, Harvey D, Lacan G. Methamphetamine blood concentrations in human abusers: application to pharmacokinetic modeling. Synapse. 2007;61(4):216–220. doi: 10.1002/syn.20365. [DOI] [PubMed] [Google Scholar]
- Melega WP, Jorgensen MJ, Lacan G, Way BM, Pham J, Morton G, Cho AK, Fairbanks LA. Long-term methamphetamine administration in the vervet monkey models aspects of a human exposure: brain neurotoxicity and behavioral profiles. Neuropsychopharmacology. 2008;33(6):1441–1452. doi: 10.1038/sj.npp.1301502. [DOI] [PubMed] [Google Scholar]
- Nader MA, Daunais JB, Moore T, Nader SH, Moore RJ, Smith HR, Friedman DP, Porrino LJ. Effects of cocaine self-administration on striatal dopamine systems in rhesus monkeys: initial and chronic exposure. Neuropsychopharmacology. 2002;27(1):35–46. doi: 10.1016/S0893-133X(01)00427-4. [DOI] [PubMed] [Google Scholar]
- O’Connor KA, Gregg TC, Davies HM, Childers SR. Effects of long-term biogenic amine transporter blockade on receptor/G-protein coupling in rat brain. Neuropharmacology. 2005a;48(1):62–71. doi: 10.1016/j.neuropharm.2004.08.014. [DOI] [PubMed] [Google Scholar]
- O’Connor KA, Porrino LJ, Davies HM, Childers SR. Time-dependent changes in receptor/G-protein coupling in rat brain following chronic monoamine transporter blockade. J Pharmacol Exp Ther. 2005b;313(2):510–517. doi: 10.1124/jpet.104.078451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Neil ML, Kuczenski R, Segal DS, Cho AK, Lacan G, Melega WP. Escalating dose pretreatment induces pharmacodynamic and not pharmacokinetic tolerance to a subsequent high-dose methamphetamine binge. Synapse. 2006;60(6):465–473. doi: 10.1002/syn.20320. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. San Diego: Elsevier Academic Press; 2005. [Google Scholar]
- Rinken A, Finnman UB, Fuxe K. Pharmacological characterization of dopamine-stimulated [35S]-guanosine 5′(gamma-thiotriphosphate) ([35S]GTPgammaS) binding in rat striatal membranes. Biochem Pharmacol. 1999;57(2):155–162. doi: 10.1016/s0006-2952(98)00287-1. [DOI] [PubMed] [Google Scholar]
- Schwendt M, Rocha A, See RE, Pacchioni AM, McGinty JF, Kalivas PW. Extended methamphetamine self-administration in rats results in a selective reduction of dopamine transporter levels in the prefrontal cortex and dorsal striatum not accompanied by marked monoaminergic depletion. J Pharmacol Exp Ther. 2009;331(2):555–562. doi: 10.1124/jpet.109.155770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segal DS, Kuczenski R. Individual differences in responsiveness to single and repeated amphetamine administration: behavioral characteristics and neurochemical correlates. J Pharmacol Exp Ther. 1987;242(3):917–926. [PubMed] [Google Scholar]
- Segal DS, Kuczenski R. Repeated binge exposures to amphetamine and methamphetamine: behavioral and neurochemical characterization. J Pharmacol Exp Ther. 1997;282(2):561–573. [PubMed] [Google Scholar]
- Segal DS, Kuczenski R. Human methamphetamine pharmacokinetics simulated in the rat: single daily intravenous administration reveals elements of sensitization and tolerance. Neuropsychopharmacology. 2006;31(5):941–955. doi: 10.1038/sj.npp.1300865. [DOI] [PubMed] [Google Scholar]
- Segal DS, Kuczenski R, O’Neil ML, Melega WP, Cho AK. Escalating dose methamphetamine pretreatment alters the behavioral and neurochemical profiles associated with exposure to a high-dose methamphetamine binge. Neuropsychopharmacology. 2003;28(10):1730–1740. doi: 10.1038/sj.npp.1300247. [DOI] [PubMed] [Google Scholar]
- Segal DS, Kuczenski R, O’Neil ML, Melega WP, Cho AK. Prolonged exposure of rats to intravenous methamphetamine: behavioral and neurochemical characterization. Psychopharmacology (Berl) 2005;180(3):501–512. doi: 10.1007/s00213-005-2188-4. [DOI] [PubMed] [Google Scholar]
- Sekine Y, Iyo M, Ouchi Y, Matsunaga T, Tsukada H, Okada H, Yoshikawa E, Futatsubashi M, Takei N, Mori N. Methamphetamine-related psychiatric symptoms and reduced brain dopamine transporters studied with PET. Am J Psychiatry. 2001;158(8):1206–1214. doi: 10.1176/appi.ajp.158.8.1206. [DOI] [PubMed] [Google Scholar]
- Sibley DR, Monsma FJ., Jr Molecular biology of dopamine receptors. Trends Pharmacol Sci. 1992;13(2):61–69. doi: 10.1016/0165-6147(92)90025-2. [DOI] [PubMed] [Google Scholar]
- Sim LJ, Selley DE, Childers SR. In vitro autoradiography of receptor-activated G proteins in rat brain by agonist-stimulated guanylyl 5′-[gamma-[35S]thio]-triphosphate binding. Proc Natl Acad Sci U S A. 1995;92(16):7242–7246. doi: 10.1073/pnas.92.16.7242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon SL, Richardson K, Dacey J, Glynn S, Domier CP, Rawson RA, Ling W. A comparison of patterns of methamphetamine and cocaine use. J Addict Dis. 2002;21(1):35–44. doi: 10.1300/j069v21n01_04. [DOI] [PubMed] [Google Scholar]
- Sleipness EP, Jansen HT, Schenk JO, Sorg BA. Time-of-day differences in dopamine clearance in the rat medial prefrontal cortex and nucleus accumbens. Synapse. 2008;62(12):877–885. doi: 10.1002/syn.20552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanwood GD, Artymyshyn RP, Kung MP, Kung HF, Lucki I, McGonigle P. Quantitative autoradiographic mapping of rat brain dopamine D3 binding with [(125)I]7-OH-PIPAT: evidence for the presence of D3 receptors on dopaminergic and nondopaminergic cell bodies and terminals. J Pharmacol Exp Ther. 2000a;295(3):1223–1231. [PubMed] [Google Scholar]
- Stanwood GD, Lucki I, McGonigle P. Differential regulation of dopamine D2 and D3 receptors by chronic drug treatments. J Pharmacol Exp Ther. 2000b;295(3):1232–1240. [PubMed] [Google Scholar]
- Stefanski R, Lee SH, Yasar S, Cadet JL, Goldberg SR. Lack of persistent changes in the dopaminergic system of rats withdrawn from methamphetamine self-administration. Eur J Pharmacol. 2002;439(1–3):59–68. doi: 10.1016/s0014-2999(02)01301-8. [DOI] [PubMed] [Google Scholar]
- Steketee JD, Kalivas PW. Drug wanting: behavioral sensitization and relapse to drug-seeking behavior. Pharmacol Rev. 2012;63(2):348–365. doi: 10.1124/pr.109.001933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Chang L, Wang GJ, Fowler JS, Ding YS, Sedler M, Logan J, Franceschi D, Gatley J, Hitzemann R, Gifford A, Wong C, Pappas N. Low level of brain dopamine D2 receptors in methamphetamine abusers: association with metabolism in the orbitofrontal cortex. Am J Psychiatry. 2001;158(12):2015–2021. doi: 10.1176/appi.ajp.158.12.2015. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Tomasi D, Wang GJ, Telang F, Fowler JS, Logan J, Benveniste H, Kim R, Thanos PK, Ferre S. Evidence that sleep deprivation downregulates dopamine D2R in ventral striatum in the human brain. J Neurosci. 2012;32(19):6711–6717. doi: 10.1523/JNEUROSCI.0045-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West MJ. Design-based stereological methods for counting neurons. Prog Brain Res. 2002;135:43–51. doi: 10.1016/S0079-6123(02)35006-4. [DOI] [PubMed] [Google Scholar]
- West MJ, Slomianka L, Gundersen HJ. Unbiased stereological estimation of the total number of neurons in thesubdivisions of the rat hippocampus using the optical fractionator. Anat Rec. 1991;231(4):482–497. doi: 10.1002/ar.1092310411. [DOI] [PubMed] [Google Scholar]
- Wilson JM, Kalasinsky KS, Levey AI, Bergeron C, Reiber G, Anthony RM, Schmunk GA, Shannak K, Haycock JW, Kish SJ. Striatal dopamine nerve terminal markers in human, chronic methamphetamine users. Nat Med. 1996;2(6):699–703. doi: 10.1038/nm0696-699. [DOI] [PubMed] [Google Scholar]
- Worsley JN, Moszczynska A, Falardeau P, Kalasinsky KS, Schmunk G, Guttman M, Furukawa Y, Ang L, Adams V, Reiber G, Anthony RA, Wickham D, Kish SJ. Dopamine D1 receptor protein is elevated in nucleus accumbens of human, chronic methamphetamine users. Mol Psychiatry. 2000;5(6):664–672. doi: 10.1038/sj.mp.4000760. [DOI] [PubMed] [Google Scholar]
- Zhu C, Vourc’h P, Fernagut PO, Fleming SM, Lacan S, Dicarlo CD, Seaman RL, Chesselet MF. Variable effects of chronic subcutaneous administration of rotenone on striatal histology. J Comp Neurol. 2004;478(4):418–426. doi: 10.1002/cne.20305. [DOI] [PubMed] [Google Scholar]


