Abstract
Background
Previous studies of air pollution and birth outcomes have not evaluated whether complicated pregnancies might be susceptible to the adverse effects of air pollution. We hypothesized that trimester mean pollutant concentrations would be associated with fetal growth restriction, with larger risks among complicated pregnancies.
Methods
We used a multiyear linked birth certificate and maternal/newborn hospital discharge dataset of singleton, term births to mothers residing in New Jersey at the time of birth, who were White (non-Hispanic), African American (non-Hispanic), or Hispanic. We defined very small for gestational age (VSGA) as a fetal growth ratio <0.75, small for gestational age (SGA) as ≥0.75 and <0.85, and ‘reference’ births as ≥0.85. Using polytomous logistic regression, we examined associations between mean pollutant concentrations during the 1st, 2nd, and 3rd trimesters and the risks of SGA/VSGA, as well as effect modification of these associations by several pregnancy complications.
Results
We found significantly increased risk of SGA associated with 1st and 3rd trimester PM2.5, and increased risk of VSGA associated with 1st, 2nd, and 3rd trimester NO2 concentrations. Pregnancies complicated by placental abruption and premature rupture of the membrane had ~2-5 fold greater excess risks of SGA/VSGA than pregnancies not complicated by these conditions, although these estimates were not statistically significant.
Conclusions
These findings suggest that ambient air pollution, perhaps specifically traffic emissions during early and late pregnancy and/or factors associated with residence near a roadway during pregnancy, may affect fetal growth. Further, pregnancy complications may increase susceptibility to these effects in late pregnancy.
Keywords: parturition, pregnancy complications, fetal development, epidemiology, air pollution, abruption placentae
INTRODUCTION
A body of evidence is emerging from several countries on the adverse consequences of ambient air pollution on fetal/birth outcomes, including preterm birth and fetal growth restriction.1-20 However, the biological mechanism(s) by which ambient air pollution may impact adverse birth outcomes, which may be different in complicated and uncomplicated pregnancies, is/are not clearly established.
Pathophysiologic changes that have been proposed as plausible mechanisms for fetal growth restriction (i.e. decreased oxygen saturation, endothelial dysfunction, increased blood viscosity, thrombosis, etc.), also have been associated with air pollution in studies of acute pollution/cardio-respiratory responses.21-24 Further, these mechanisms also may play an important role in the occurrence of pregnancy complications including preeclampsia, placental abruption and placenta previa.25-29 Thus, air pollution related fetal growth restriction, some pregnancy complications (e.g., placental abruption) and cardio-respiratory disease may share common mechanisms. Therefore, we hypothesized that elevated levels of air pollution affect fetal growth in uncomplicated pregnancies, and that pregnancy complications adversely modify the pollution/fetal growth association making the risk of impaired fetal growth more pronounced among complicated pregnancies.
Using a multi-year, New Jersey (NJ) statewide, linked birth certificate and maternal hospital discharge dataset, and PM2.5 (particulate matter <2.5 μm in aerodynamic diameter), nitrogen dioxide (NO2), sulfur dioxide (SO2), and carbon monoxide (CO) measurements made at monitoring locations across NJ, we examined the effect(s) of ambient air pollutant concentrations during early, middle, and late pregnancy on fetal growth restriction among term births. These linked data provide more complete recording of pregnancy complications than birth certificates alone, and provide an opportunity to examine if the effect of air pollution on fetal growth differs between uncomplicated and complicated pregnancies.
METHODS
Study population
Using linked birth certificate and maternal/newborn hospital discharge summaries maintained by the Division of Family Health Services, NJ Department of Health and Senior Services (NJDHSS), we selected all singleton births in NJ from 1999-2003 to White (non-Hispanic), African American (non-Hispanic), or Hispanic mothers who were residents of NJ at the time of birth, with a gestational age of 37-42 completed weeks and a birth weight ≥ 500g. The study was approved by both UMDNJ and NJDHSS Institutional Review Boards.
From the birth certificate, we extracted data on maternal characteristics (i.e. age, race/ethnicity, marital status, education level, and cigarette smoking, drug use, and alcohol use during pregnancy), maternal place of residence at the time of birth, trimester of 1st prenatal care visit, infant birth weight, and gender. Also from the birth certificate, we retained data on the start day, month, and year of the last menstrual period (LMP), and the clinical estimate of gestational age. If either the birth certificate or maternal discharge data indicated a specific pregnancy complications (gestational hypertension, preeclampsia, eclampsia, gestational diabetes, placenta previa, placental abruption, or premature rupture of the membranes), we coded that subject as having that complication. This approach provides a higher sensitivity and specificity than use of birth certificates or maternal hospital discharge data alone.30-33
Outcome definition
We estimated gestational age based on LMP using the algorithm proposed by the National Center for Health Statistics.34 Gestational age information reported on the basis of women’s menstrual history has been shown to be reasonably reliable.33,35,36 For each birth, we calculated a fetal growth ratio as a measure of newborn size.37,38 For each gestational age/gender/race specific stratum (e.g. white males with gestational age of 38 weeks), we calculated the median birth weight. Each newborn’s/birth’s fetal growth ratio was then calculated as the newborn’s birth weight divided by the median birth weight of the corresponding stratum. We then defined VSGA as a fetal growth ratio <0.75, SGA as ≥0.75 and <0.85, with all fetal growth ratios ≥ 0.85 comprising the reference group. The cutoff values for defining VSGA and SGA have been validated by other investigators.37,38 This method of measuring fetal growth has been used previously by our group39 and others.37,38
Air pollution
All pollutant measurements by the NJ Department of Environmental Protection were retrieved from the United States Environmental Protection Agency website.40 PM2.5 measurements (24 hour period) were made every third day at 20 monitoring sites in NJ from September 1999 through December 2003. NO2 was measured continuously at 11 stations, SO2 continuously at 16 stations, and CO continuously at 16 stations for the study period.
To each subject/birth, we assigned measurements from the PM2.5 monitor closest to the maternal residence at birth. However, we excluded all births whose maternal residence was >10km from the closest monitoring station. Using the estimated date of conception, we calculated the mean 1st trimester (1st 93 days from estimated date of conception) and mean 2nd trimester PM2.5 concentrations (2nd 93 days from estimated date of conception). The mean 3rd trimester PM2.5 concentration was calculated as the mean PM2.5 concentration during the remaining pregnancy time (3rd trimester ranged from 73 to 108 days). We calculated a mean concentration for only those trimesters with <30% of the scheduled PM2.5 measurements missing. If ≥30% were missing, we set that trimester specific mean PM2.5 concentration to missing. We then calculated trimester specific NO2, SO2, and CO concentrations in the same manner, and used these concentrations in all subsequent analyses.
Neighborhood level socio-economic status (SES)
To control for neighborhood characteristics of the maternal residence that may both be associated with birth outcomes and correlated with air pollution concentrations, we abstracted the following variables from the 2000 US Census, by census tract:41 percentage of persons aged 25 and older with less than a high school education, percentage of persons aged 25 and older with at least 4 years of college education, and percentage of persons below the federally defined poverty line. These area-based variables have been shown to be reasonable measures of neighborhood level SES,42 which may predict health risks associated with neighborhood characteristics independent of individual level SES measures.43 The latitude and longitude of the maternal residence at birth were used to identify the census tract in which each mother resided, using ArcGIS v.9.2 (©ESRI, Redlands, CA). We then assigned each birth/mother values of these three area-based US census variables.
Statistical analysis
Main analysis
We used a cohort study design and polytomous logistic regression (SAS Proc Catmod, ©SAS Inc, Cary, NC) to estimate the risk of SGA and VSGA, compared to the reference group, associated with incremental increases in mean PM2.5 concentration in the 1st trimester. In this model we included those covariates that were not thought to be on the causal pathway from PM2.5 to SGA/VSGA, which changed the pollutant effect estimate by 10% and/or were predictors of SGA/VSGA. These included maternal age, education, and race, trimester of prenatal care initiation, maternal smoking, drug use, and alcohol use during pregnancy, marital status, percentage of the maternal residence census tract’s population 25 years and older with < 12 years of education, percentage with ≥ 4 years of college education, and the percentage of the census tract’s population living below the poverty line. We then re-ran this same model without the 1st trimester PM2.5 to separately examine effects associated with 2nd and then 3rd trimester mean PM2.5 concentrations, as well as 1st, 2nd, and 3rd trimester mean NO2, SO2, and CO concentrations. From each model, we report the excess risk and its 95% confidence interval.
Sensitivity analyses
To evaluate our assumption of a linear concentration response, we replaced the continuous pollutant concentration (e.g. 1st trimester PM2.5) with indicator variables based on quintiles and re-ran the same one pollutant model described above. We then used an ordinal variable to replace these quintiles to perform a test for trend. To assess the stability of our single pollutant model risk estimates (e.g. 1st trimester PM2.5) after adjustment for other pollutant concentrations, we ran the same models including two pollutant concentrations from the same trimester (e.g. 1st trimester PM2.5 and 1st trimester NO2). To determine if our findings were sensitive to the definitions of SGA/VSGA used (i.e. fetal growth ratio vs. <10%tile), we redefined VSGA as a birth weight less than the 3rd percentile of the corresponding gestational age, gender, and race specific distribution of birth weights, SGA as greater than or equal to the 3rd percentile and less than the 10th percentile, and our reference birth group as greater than or equal to the 10th percentile. We then re-ran the same model described above. To evaluate if our findings were restricted to one racial/ethnic group, we evaluated effect modification by maternal race. To evaluate whether our findings were sensitive to control for long term trends, season, and temperature, we included indicator variables for the month and calendar year of birth, and linear and quadratic terms of 1st trimester mean apparent temperature.44 For each subject we used temperature and dew point measurements made at the closest airport to the maternal residence, and from these calculated apparent temperature as a measure of the subject’s perceived air temperature given the humidity.
Effect modification by pregnancy complications
We investigated whether the association between fetal growth restriction and PM2.5 differed in those women with and without pregnancy complications. We created an indicator variable for the presence of each pregnancy complication (i.e. gestational hypertension, gestational diabetes, pre-eclampsia, eclampsia, placenta previa, placental abruption, and premature rupture of the membrane), and then included an interaction term (PM2.5 * Pregnancy Complication) in the model. All statistical analyses were done using SAS v.9.1 (©SAS, Inc. Cary, NC).
RESULTS
There were 492,678 singleton births to White (non-Hispanic), African American (non-Hispanic), and Hispanic mothers who were residents of NJ from 1999 to 2003. After retaining only those births with gestational ages 37 to 42 weeks, and excluding all observations with missing data on birth weight, date of birth, LMP, and other covariates, 350,107 births remained (n=27,943 SGA births [8%] and n=7,773 [2%] VSGA births). Births with a maternal residence >10 km from a monitoring station, or those missing trimester specific mean pollutant concentrations were then excluded, leaving n=88,678 births for analyses involving PM2.5, n=132,888 for SO2, n=114,411 for NO2, and n=134,798 births for analyses involving CO. There were n=199,221 births included in at least 1 pollutant specific analysis.
Mothers of SGA and VSGA infants were more likely to be less than 25 years old and less likely to have completed high school, compared to mothers of appropriate size births (Table 1). They were also more likely to be single, African American and have smoked during pregnancy. The frequencies of gestational hypertension, preeclampsia, fetal distress, placental abruption and premature rupture of membranes were highest for mothers of VSGA infants, intermediate for mothers of SGA infants, and lowest for mothers of appropriate size infants. Mothers of VSGA and SGA infants lived in census tracts where greater proportions of residents had less than a high school education and lived in poverty, compared to mothers of births in the referent group (Table 1).
Table 1.
Characteristics of Study Population (Births in at Least One Pollutant Specific Analysis), by Birth Category. New Jersey Air Pollution and Adverse Birth Outcomes Study. 1999-2003 (N=199,221 Term Births).
| REFERENCE BIRTHS |
SGA* BIRTHS |
VSGA† BIRTHS |
||||||
|---|---|---|---|---|---|---|---|---|
| CHARACTERISTIC | N | % | N | % | p-value χ2 test (SGA vs. Reference) |
N | % | p-value χ2 test (VSGA vs. Reference) |
| Sample Size | 178,198 | 90 | 16,340 | 8 | 4,683 | 2 | ||
|
| ||||||||
| Maternal Age (years) | <0.01 | <0.01 | ||||||
| <20 | 14,223 | 8 | 2,007 | 13 | 597 | 13 | ||
| 20-24 | 34,420 | 19 | 3,769 | 23 | 1,070 | 23 | ||
| 25-29 | 45,010 | 25 | 3,947 | 24 | 1,103 | 24 | ||
| 30-34 | 51,615 | 29 | 3,960 | 24 | 1,096 | 23 | ||
| ≥35 | 32,930 | 19 | 2,657 | 16 | 817 | 17 | ||
|
| ||||||||
| Prenatal Care Initiation | <0.01 | <0.01 | ||||||
| 1st Trimester | 141,153 | 79 | 12,208 | 75 | 3,387 | 72 | ||
| 2nd Trimester | 29,605 | 17 | 3,218 | 20 | 992 | 21 | ||
| 3rd Trimester | 7,440 | 4 | 914 | 5 | 304 | 7 | ||
|
| ||||||||
| Maternal Smoking during pregnancy | 14,350 | 8 | 2,384 | 15 | <0.01 | 893 | 19 | <0.01 |
|
| ||||||||
|
Maternal Alcohol Use during
pregnancy |
1,867 | 1 | 297 | 2 | <0.01 | 121 | 3 | <0.01 |
|
| ||||||||
| Maternal Drug Use during pregnancy | 2,419 | 1 | 553 | 3 | <0.01 | 305 | 7 | <0.01 |
|
| ||||||||
| Maternal Marital Status | <0.01 | <0.01 | ||||||
| Single | 63,534 | 36 | 7,348 | 45 | 2,361 | 51 | ||
| Married | 112,997 | 63 | 8,817 | 54 | 2,265 | 48 | ||
| Separated, Divorced, Widowed | 1,667 | 1 | 175 | 1 | 57 | 1 | ||
|
| ||||||||
| Maternal Education | <0.01 | <0.01 | ||||||
| < High School | 30,160 | 17 | 3,589 | 22 | 1,140 | 24 | ||
| High School Graduate | 57,743 | 32 | 5,548 | 34 | 1,741 | 37 | ||
| Some College or More | 90,295 | 51 | 7,203 | 44 | 1,802 | 39 | ||
| Maternal Race | <0.01 | <0.01 | ||||||
| White (non-Hispanic) | 84,747 | 47 | 7,478 | 46 | 1,930 | 41 | ||
| African American (non-Hispanic) | 38,978 | 22 | 3,952 | 24 | 1,363 | 29 | ||
| Hispanic | 54,473 | 31 | 4,910 | 30 | 1,390 | 30 | ||
|
| ||||||||
| Pregnancy Complications | ||||||||
| Pre-pregnancy Hypertension | 1,990 | 1 | 256 | 2 | <0.01 | 139 | 3 | <0.01 |
| Gestational Hypertension | 10,028 | 6 | 1,452 | 9 | <0.01 | 788 | 17 | <0.01 |
| Pre-eclampsia | 3,742 | 2 | 688 | 4 | <0.01 | 491 | 10 | <0.01 |
| Eclampsia | 142 | 0 | 15 | 0 | 0.60 | 21 | 0 | <0.01 |
| Diabetes Mellitus (Type I and II) | 1,491 | 1 | 109 | 1 | 0.02 | 47 | 1 | 0.22 |
| Gestational Diabetes | 8,416 | 5 | 574 | 4 | <0.01 | 198 | 4 | 0.11 |
| Placenta Previa | 766 | 0 | 92 | 1 | 0.01 | 53 | 1 | <0.01 |
| Fetal Distress | 9,740 | 5 | 1,336 | 8 | <0.01 | 600 | 13 | <0.01 |
| Placental Abruption | 839 | 0 | 177 | 1 | <0.01 | 136 | 3 | <0.01 |
| Premature Rupture of Membrane | 5,757 | 3 | 797 | 5 | <0.01 | 369 | 8 | <0.01 |
|
| ||||||||
|
Percentage of population(≥ 25 years)
in maternal residence census tract with < 12 years of education |
<0.01 | <0.01 | ||||||
| <15 | 68,143 | 38 | 5,847 | 36 | 1,564 | 33 | ||
| 15 to <25 | 39,325 | 22 | 3,686 | 23 | 1,015 | 22 | ||
| 25 to <40 | 41,354 | 23 | 3,935 | 24 | 1,236 | 26 | ||
| ≥40 | 29,376 | 17 | 2,872 | 17 | 868 | 19 | ||
|
| ||||||||
|
Percentage of population(≥ 25 years)
in maternal residence census tract with at least 4 years of college |
<0.01 | <0.01 | ||||||
| <15 | 62,346 | 35 | 6,021 | 37 | 1,836 | 39 | ||
| 15 to <25 | 41,095 | 23 | 3,846 | 24 | 1,111 | 24 | ||
| 25 to <40 | 35,765 | 20 | 3,123 | 19 | 871 | 19 | ||
| ≥40 | 38,992 | 22 | 3,350 | 20 | 865 | 18 | ||
|
Percentage of population in maternal
residence census tract below federally defined poverty line |
<0.01 | <0.01 | ||||||
| <5 | 67,854 | 38 | 5,762 | 35 | 1,519 | 32 | ||
| 5 to <10 | 35,328 | 20 | 3,334 | 21 | 929 | 20 | ||
| 10 to <20 | 40,086 | 22 | 3,759 | 23 | 1,110 | 24 | ||
| ≥20 | 34,930 | 20 | 3,485 | 21 | 1,125 | 24 | ||
SGA = “Small for gestational age” defined as a fetal growth ratio (i.e. newborn’s birth weight divided by median birth weight of corresponding gestational age/gender/race specific stratum) ≥0.75 and <0.85.
VSGA = “Very small for gestational age” defined as fetal growth ratio <0.75.
Mothers of infants excluded from the analysis (i.e. no pollutant monitoring station <10 km from the maternal residence) were generally older (23% ≥ 35 years), had earlier prenatal care (86% in the 1st trimester), and were more likely to be white (77%), married (77%), and have had some college education (63%), than the mothers of reference births included in the analysis (Table 1). The frequencies of specific pregnancy complications, however, were similar (e.g. preeclampsia 2%; gestational diabetes 4%; placental abruption 1%; premature rupture of the membrane 4%) to the reference group.
Subject specific 1st trimester mean PM2.5 concentrations ranged from 2 to 29 μg/m3, NO2 from 5 to 47 ppb, SO2 from 1 to 14 ppb, and CO from 0.137 to 2.195 ppm. The mean and standard deviation for subject’s trimester specific mean pollutant concentrations are shown in Table 2. Subject specific 1st, 2nd, and 3rd trimester NO2 and CO concentrations were each highly correlated (e.g. 1st trimester CO and 2nd trimester CO: r=0.88), but subject specific 1st, 2nd, and 3rd trimester SO2 and PM2.5 concentrations were not (Table 3). Trimester specific NO2 and CO concentrations were moderately correlated (e.g. 1st trimester NO2 and 1st trimester CO: r=0.51), and all other pollutant/trimester pairs uncorrelated.
Table 2.
Mean and standard deviation (SD) pollutant concentration by trimester and fetal growth category. New Jersey Air Pollution and Adverse Birth Outcomes Study. 1999-2003 (N=199,221 Term Births).
| REFERENCE BIRTHS |
SGA* BIRTHS |
VSGA† BIRTHS |
|||
|---|---|---|---|---|---|
| Mean (SD) | Mean (SD) | p-value t-test (SGA Vs. Reference) |
Mean (SD) | p-value t-test (VSGA Vs. Reference) |
|
| PM2.5 concentration (μg/m3) | |||||
| 1st Trimester | 13.8 (2.5) | 13.9 (2.5) | <0.01 | 13.9 (2.4) | 0.01 |
| 2nd Trimester | 13.8 (2.5) | 13.8 (2.5) | 0.32 | 13.9 (2.4) | 0.09 |
| 3rd Trimester | 13.7 (2.7) | 13.8 (2.7) | <0.01 | 13.8 (2.7) | <0.01 |
| SO2 concentration (ppb) | |||||
| 1st Trimester | 5.7 (2.3) | 5.8 (2.3) | 0.06 | 5.8 (2.3) | 0.33 |
| 2nd Trimester | 5.6 (2.3) | 5.6 (2.3) | 0.20 | 5.7 (2.3) | 0.01 |
| 3rd Trimester | 5.4(2.2) | 5.5 (2.2) | 0.23 | 5.5 (2.2) | 0.01 |
| NO2 concentration (ppb) | |||||
| 1st Trimester | 25.8 (8.0) | 25.9 (7.8) | 0.35 | 26.3 (7.6) | <0.01 |
| 2nd Trimester | 25.8 (8.2) | 25.9 (8.0) | 0.29 | 26.4 (7.7) | <0.01 |
| 3rd Trimester | 25.9 (8.4) | 25.9 (8.2) | 0.39 | 26.4 (7.9) | <0.01 |
| CO concentration (ppm) | |||||
| 1st Trimester | 0.925 (0.320) | 0.925 (0.322) | 0.94 | 0.918 (0.316) | 0.21 |
| 2nd Trimester | 0.935 (0.329) | 0.933 (0.331) | 0.51 | 0.933 (0.327) | 0.71 |
| 3rd Trimester | 0.946 (0.338) | 0.944 (0.341) | 0.59 | 0.950 (0.336) | 0.48 |
SGA = “Small for gestational age” defined as a fetal growth ratio (i.e. newborn’s birth weight divided by median birth weight of corresponding gestational age/gender/race specific stratum) ≥0.75 and <0.85.
VSGA = “Very small for gestational age” defined as fetal growth ratio <0.75.
Table 3.
Pearson correlation coefficients (r) for subjects’ trimester specific pollutant concentrations.
| Trimester specific pollutant |
PM2.5 | NO2 | SO2 | CO | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1st | 2nd | 3rd | 1st | 2nd | 3rd | 1st | 2nd | 3rd | 1st | 2nd | 3rd | ||
| PM2.5 | 1st | --- | −0.09 | 0.30 | 0.01 | 0.06 | 0.14 | 0.17 | −0.11 | 0.33 | 0.25 | 0.21 | 0.31 |
| 2nd | −0.09 | --- | −0.17 | 0.05 | −0.02 | 0.04 | 0.04 | 0.17 | −0.02 | 0.31 | 0.22 | 0.21 | |
| 3rd | 0.30 | −0.17 | --- | 0.16 | 0.08 | −0.00 | 0.37 | 0.04 | 0.19 | 0.26 | 0.30 | 0.22 | |
| NO2 | 1st | 0.01 | 0.05 | 0.16 | --- | 0.85 | 0.75 | 0.16 | 0.06 | −0.03 | 0.51 | 0.52 | 0.44 |
| 2nd | 0.06 | −0.02 | 0.08 | 0.85 | --- | 0.87 | −0.05 | 0.12 | 0.16 | 0.47 | 0.54 | 0.54 | |
| 3rd | 0.14 | 0.04 | −0.00 | 0.75 | 0.87 | --- | −0.08 | −0.06 | 0.17 | 0.48 | 0.48 | 0.53 | |
| SO2 | 1st | 0.17 | 0.04 | 0.37 | 0.16 | −0.05 | −0.08 | --- | 0.37 | 0.14 | 0.22 | 0.18 | 0.02 |
| 2nd | −0.11 | 0.17 | 0.04 | 0.06 | 0.12 | −0.06 | 0.37 | --- | 0.47 | 0.12 | 0.25 | 0.21 | |
| 3rd | 0.33 | −0.02 | 0.19 | −0.03 | 0.16 | 0.17 | 0.14 | 0.47 | --- | 0.21 | 0.24 | 0.38 | |
| CO | 1st | 0.25 | 0.31 | 0.26 | 0.51 | 0.47 | 0.48 | 0.22 | 0.12 | 0.21 | --- | 0.88 | 0.83 |
| 2nd | 0.21 | 0.22 | 0.30 | 0.52 | 0.54 | 0.48 | 0.18 | 0.25 | 0.24 | 0.88 | --- | 0.89 | |
| 3rd | 0.31 | 0.21 | 0.22 | 0.44 | 0.54 | 0.53 | 0.02 | 0.21 | 0.38 | 0.83 | 0.89 | --- | |
When we evaluated each trimester specific pollutant concentration separately, each 4 μg/m3 increase in both the 1st and 3rd trimester mean PM2.5 concentration was associated with significantly increased risk of SGA (Table 4). The 1st and 3rd trimester VSGA excess risk estimates were also greater than 0, but not statistically significant. Each 10 ppb increase in each of the 1st, 2nd, and 3rd trimester mean NO2 concentrations was associated with significantly increased risk of VSGA, but not SGA. No trimester specific mean SO2 or CO concentration was associated with increased risk of SGA or VSGA (Table 4).
Table 4.
Percent Change in Risk (and 95% Confidence Intervals) of SGA and VSGA Associated with Each Incremental (Interquartile Range) Increase in Mean Trimester Specific Pollutant Concentration. New Jersey Air Pollution and Adverse Birth Outcomes Study 1999-2003.
| Pollutant (n) |
Interquartile range |
Trimester of mean concentration |
Small for gestational age |
Very small for gestational age |
|---|---|---|---|---|
| Fine particles - PM2.5 (n=88,678) |
4 μg/m3 | 1st Trimester | 4.5 (0.5, 8.7) |
2.6 (−4.4, 10.0) |
| 2nd Trimester | −1.8 (−5.6, 2.2) |
0.2 (−6.7, 7.5) |
||
| 3rd Trimester | 4.1 (0.3, 8.0) |
4.2 (−2.4, 11.2) |
||
|
| ||||
| Sulfur Dioxide (n=132,888) |
3 ppb | 1st Trimester | 1.7 (−0.9, 4.3) |
0.0 (−4.6, 4.8) |
| 2nd Trimester | 0.2 (−2.4, 2.9) |
2.5 (−2.2, 7.4) |
||
| 3rd Trimester | −0.1 (−2.8, 2.6) |
3.1 (−1.8, 8.3) |
||
|
| ||||
| Nitrogen Dioxide (n=114,411) |
10 ppb | 1st Trimester | 1.2 (−1.6, 4.0) |
7.0 (1.8, 12.4) |
| 2nd Trimester | 1.1 (−1.6, 3.9) |
7.7 (2.6, 13.0) |
||
| 3rd Trimester | 1.0 (−1.7, 3.7) |
7.4 (2.5, 12.5) |
||
|
| ||||
| Carbon Monoxide (n=134,798) |
0.5 ppm | 1st Trimester | 1.1 (−2.0, 4.3) |
−4.1 (−9.4, 1.4) |
| 2nd Trimester | 0.0 (−3.0, 3.1) |
−1.9 (−7.1, 3.6) |
||
| 3rd Trimester | 0.1 (−2.8, 3.1) |
1.1 (−4.1, 6.5) |
||
NOTE: Each trimester specific pollutant concentration was modeled separately. All risk estimates adjusted for maternal race/ethnicity, maternal education, maternal age, marital status, trimester prenatal care began; maternal alcohol use, maternal smoking, maternal drug use, percentage of population (≥ 25 years) in maternal residence census tract with < 12 years of education, percentage of population (≥ 25 years) in maternal residence census tract with at least 4 years of college, and percentage of population in maternal residence census tract below federally defined poverty line.
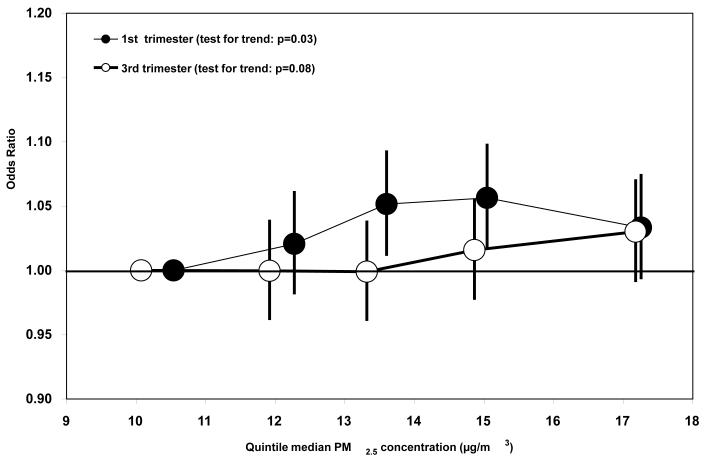
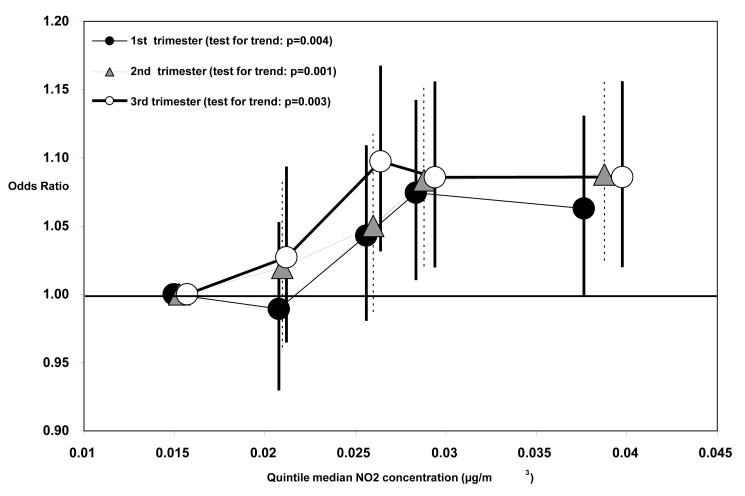
When including 1st trimester PM2.5 and NO2 concentrations in a model simultaneously (n=59,955 births with both PM2.5 and NO2 trimester mean concentrations), the PM2.5/SGA and NO2/VSGA risk estimates were not substantially different than the risk estimates from single pollutant models on those same n=59,955 subjects (Table 5). This was also true for the 2nd and 3rd trimester risk estimates. Risk of SGA or VSGA generally increased with increasing quintiles of 1st and 3rd trimester PM2.5 concentration (Figure 1) and 1st, 2nd, and 3rd trimester NO2 concentrations, although not always (Figure 2).
Table 5.
Percent Change in Risk (And 95% Confidence Intervals) of SGA and VSGA Associated with Each Incremental (Interquartile Range) Increase in Mean Trimester Specific PM2.5 and NO2 Concentrations (Single pollutant and Two pollutant models). New Jersey Air Pollution and Adverse Birth Outcomes Study 1999-2003 (n=59,955).
| Trimester of mean concentration |
Model Type (Single or Two pollutant) |
Pollutant | Interquartile range |
Small for gestational age |
Very small for gestational age |
|---|---|---|---|---|---|
| 1st Trimester | Single* | PM2.5 | 4 μg/m3 | 4.6 (−0.3, 9.8) |
4.5 (−4.0, 13.7) |
| Single* | Nitrogen Dioxide |
10 ppb | 1.0 (−2.9, 5.0) |
9.2 (2.0, 17.0) |
|
|
| |||||
| Two | PM2.5 | 4 μg/m3 | 4.5 (−0.4, 9.7) |
3.2 (−5.2, 12.4) |
|
| Nitrogen Dioxide |
10 ppb | 0.6 (−3.3, 4.6) |
8.9 (1.6, 16.7) |
||
|
| |||||
| 2nd Trimester | Single* | PM2.5 | 4 μg/m3 | −3.2 (−7.8, 1.6) |
−0.6 (−8.7, 8.3) |
| Single* | Nitrogen Dioxide |
10 ppb | 0.3 (−3.2, 4.0) |
9.5 (2.8, 16.8) |
|
|
| |||||
| Two | PM2.5 | 4 μg/m3 | −3.3 (−8.0, 1.5) |
−2.0 (−10.1, 6.9) |
|
| Nitrogen Dioxide |
10 ppb | 0.6 (−3.0, 4.4) |
9.7 (2.9, 17.0) |
||
|
| |||||
| 3rd Trimester | Single* | PM2.5 | 4 μg/m3 | 8.2 (3.4, 13.2) |
6.4 (−1.7, 15.2) |
| Single* | Nitrogen Dioxide |
10 ppb | 0.3 (−3.2, 3.8) |
9.1 (2.5, 16.0) |
|
|
| |||||
| Two | PM2.5 | 4 μg/m3 | 8.2 (3.5, 13.2) |
5.3 (−2.8, 14.1) |
|
| Nitrogen Dioxide |
10 ppb | −0.4 (−3.8, 3.2) |
8.6 (2.1, 15.5) |
||
NOTE: All risk estimates adjusted for maternal race/ethnicity, maternal education, maternal age, marital status, trimester prenatal care began; maternal alcohol use, maternal smoking, maternal drug use, percentage of population (≥ 25 years) in maternal residence census tract with < 12 years of education, percentage of population (≥ 25 years) in maternal residence census tract with at least 4 years of college, and percentage of population in maternal residence census tract below federally defined poverty line.
Note, these single pollutant models differ from those in Table 4 only by the number of births used in the analysis.
Figure 1.
Relative Odds and 95% Confidence Intervals of SGA Associated with Each Quintile of 1st and 3rd Trimester Mean PM2.5 Concentration, by Median PM2.5 Concentration (μg/m3) of Each Quintile.
Figure 2.
Relative Odds and 95% Confidence Intervals of SGA Associated with Each Quintile of 1st, 2nd, and 3rd Trimester Mean PM2.5 Concentration, by Median PM2.5 Concentration (μg/m3) of Each Quintile.
When we redefined SGA and VSGA as less than the 10th and 3rd percentiles, respectively, the excess risk estimates were generally consistent with our previous PM2.5/SGA estimate (1st trimester: 4.5%, 95% CI = -0.5%, 8.7%; 3rd trimester: 4.1%, 95% CI = 0.3%, 8.0%), and our NO2/VSGA estimates (1st trimester: 7.0%, 95% CI = 1.8%, 12.4%; 2nd trimester: 7.7%, 95% CI= 2.6%, 13.0%; 3rd trimester: 7.4%, 95% CI = 2.5%, 12.5%). When we included apparent temperature, calendar month and year of birth in our models, our excess risk estimates were consistent with our previous PM2.5/SGA estimates (1st trimester: 5.5%, 95% CI: 0.3%, 11.0%; 3rd trimester: 3.3%, 95% CI = -1.7%, 8.6%) and our NO2/VSGA estimates (1st trimester: 7.5%, 95% CI = 1.9%, 13.4%; 2nd trimester: 7.3%, 95% CI = 1.8%, 13.0%; 3rd trimester: 8.0%, 95% CI = 2.7%, 13.7%).
When we evaluated effect modification by maternal race, the 3rd trimester NO2/VSGA excess risk estimate was greatest for Hispanic mothers (9.5%; 95% CI = 0.5%, 19.2%), and smaller but similar for White (non-Hispanic) (5.2%, 95% CI = -2.3%, 13.3%) and African American (non-Hispanic) mothers (5.0%, 95% CI = -3.9%, 14.8%). However, the 3rd trimester PM2.5/SGA risk estimate was greatest for African American mothers (7.9%, 95% CU = 0.1%, 16.2%), smaller for White mothers (4.2%, 95% CI = -1.4%, 10.1%), but there was no apparent effect in Hispanic mothers (-0.1%, 95% CI = -6.4%, 6.7%).
Last, we evaluated whether the association between late pregnancy (i.e. 3rd trimester) mean PM2.5 concentration and the risk of SGA/VSGA was modified by several pregnancy complications. Among those pregnancies with at least one pregnancy complication, each 4 μg/m3 increase in 3rd trimester mean PM2.5 concentration was associated with a 12.6% greater risk of VSGA. Among uncomplicated pregnancies, this excess risk estimate was ~5 times smaller (1.5%; Table 6). We did not observe a similar pattern when estimating the risk of SGA associated with the same incremental PM2.5 increase, and neither of these interaction terms were statistically significant. We then evaluated each pregnancy complication separately in the same manner. Although none of the complication-specific interaction terms were statistically significant, we did observe ~2 to 5 fold larger SGA/VSGA excess risk estimates in those pregnancies complicated by placental abruption compared to those without placental abruption, and premature rupture of the membrane compared to those without this condition. For the other pregnancy complications, we did not observe larger excess risks of both SGA and VSGA associated with incremental PM2.5 concentration increases for complicated pregnancies compared to uncomplicated pregnancies (Table 6).
Table 6.
Percent Increase in Risk (and 95% Confidence Intervals) of SGA/VSGA Associated with Each 4 μg/m3 Increase in Mean 3rd Trimester PM2.5 Concentration, by Pregnancy Complication. New Jersey Air Pollution and Adverse Birth Outcomes Study 1999-2003 (n=88,678)
| SMALL FOR GESTATIONAL AGE | VERY SMALL FOR GESTATIONAL AGE |
|||
|---|---|---|---|---|
| Percent increase in risk |
95% confidence interval |
Percent increase in risk |
95% confidence interval |
|
| Any Complication | ||||
| No | 4.7 | (0.6, 9.0) | 1.5 | (−6.1, 9.7) |
| Yes | 2.2 | (−6.1, 11.3) | 12.6 | (0.1, 26.7) |
| Placental Abruption | ||||
| No | 4.0 | (0.3, 7.9) | 4.1 | (−2.6, 11.2) |
| Yes | 11.7 | (−21.7, 59.5) | 7.6 | (−29.8, 64.9) |
| Placenta Previa | ||||
| No | 3.9 | (0.2, 7.8) | 4.1 | (−2.5, 11.2) |
| Yes | 23.2 | (−20.9, 91.9) | 3.2 | (−43.0, 86.9) |
| Pre-clampsia | ||||
| No | 4.2 | (0.4, 8.2) | 4.4 | (−2.6, 11.9) |
| Yes | 2.7 | (−13.8, 22.3) | 3.9 | (−15.7, 28.1) |
| Gestational Hypertension | ||||
| No | 4.3 | (0.4, 8.4) | 3.2 | (−4.0, 10.9) |
| Yes | 3.9 | (−7.8, 17.1) | 12.9 | (−3.3, 31.9) |
| Premature Rupture of the Membrane | ||||
| No | 3.7 | (−0.1, 7.7) | 3.3 | (−3.5, 10.5) |
| Yes | 14.6 | (−3.3, 35.9) | 21.9 | (−3.6, 54.2) |
| Gestational Diabetes | ||||
| No | 4.6 | (0.8, 8.6) | 4.3 | (−2.5, 11.5) |
| Yes | −9.3 | (−24.7, 9.3) | 1.4 | (−27.0, 40.9) |
NOTE: All risk estimates adjusted for maternal race/ethnicity, maternal education, maternal age, marital status, trimester prenatal care began; maternal alcohol use, maternal smoking, maternal drug use, percentage of population (≥ 25 years) in maternal residence census tract with < 12 years of education, percentage of population (≥ 25 years) in maternal residence census tract with at least 4 years of college, and percentage of population in maternal residence census tract below federally defined poverty line.
DISCUSSION
In this large, multiyear, statewide cohort study of ambient air pollution and risk of fetal growth restriction, we found significantly increased risk of SGA associated with each 4 μg/m3 increase in mean PM2.5 concentration in the 1st and 3rd trimesters, and significantly increased risk of VSGA associated with each 10 ppb increase in 1st, 2nd, and 3rd trimester mean NO2 concentrations, after controlling for known risk factors. These estimates were not attenuated when both PM2.5 and NO2 were included in the same model, and each pollutant effect was generally consistent with an increasing concentration-response relationship. However, there were differences in the magnitude of the 3rd trimester risk estimates by race/ethnicity, but the pattern of effect modification was not the same for PM2.5 (highest for African American mothers) and NO2 (highest for Hispanic mothers). Last, we found evidence of effect modification by several pregnancy complications including placental abruption and premature rupture of membranes, although these effects were not statistically significant, likely because of the rarity of these complications.
Our findings are consistent with previous studies reporting greater risk of fetal growth restriction or low birth weight associated with 1st trimester pollutant concentration1,5,8,9,12,16,20 and 3rd trimester pollutant concentrations,1,7,9-13,16-18 although the specific pollutants responsible for those increased risks may be different. Associations with NO2 suggest local traffic pollution and/or residence near a source of traffic pollution during the pregnancy may be important risk factors. Future analyses will estimate risks associated with pregnancy exposures to specific PM2.5 components (i.e. sulfates, elemental carbon, organic carbon, etc) or other traffic related pollutants (e.g. specific polycyclic aromatic hydrocarbons) to explore these PM2.5 and traffic pollution findings further.
The biological mechanism(s) by which ambient air pollution affect(s) fetal growth is/are largely unknown and may differ between early and late-onset fetal growth restriction, as well as between uncomplicated and complicated pregnancies. Mechanisms may include a defective trophoblast invasion,45,46 decreased vascular reactivity,47 decreased oxygen and nutrient delivery,48 and increased trophoblast apoptosis,49 which may act independently or jointly. Mechanisms may also include the direct transfer of pollutants across the maternal blood-placenta barrier and direct binding to the fetal DNA regulating its transcription. Polycyclic aromatic hydrocarbons (PAH) previously have been associated with DNA adducts, which have been reported to adversely affect fetal growth and development,50 especially during the period of rapid fetal growth. PAH exposure during pregnancy also has been associated with increased risk of fetal growth restriction.51,52
We observed approximately two to five fold larger SGA/VSGA risk estimates in those pregnancies complicated by placental abruption and premature rupture of membranes compared to those without these complications. Although fetal growth restriction and placental abruption share a common mechanism of defective placental implantation early during embryogenesis,53 elevated levels of pollution late in pregnancy may exaggerate decidual necrosis, microinfarcts and atheromatous/fibrinoid changes in the placenta of pregnancies that are prone to abruption,29 accentuating their effect on fetal growth restriction. The reason(s) for the synergy between elevated air pollution and premature rupture of membranes is not clear. Premature rupture of membranes may serve as an indicator of chronic infection as it is associated with chorioamnionitis.54 Thus, mothers developing certain pregnancy complications, such as placental abruption and premature rupture of the membranes, may represent a parturient group particularly susceptible to the adverse health effects of elevated air pollution. However, our results need confirmation.
Although our study had several strengths, including the large number of subjects and the use of statewide, multiyear linked data from birth certificates and maternal hospital discharges, there were some limitations that should be considered. First, we had a limited number of VSGA births and pregnancy complications, and therefore less precision in these risk estimates. Second, it is likely that smoking, illicit drug use, and alcohol use are underreported on birth certificates and hospital discharge data. Nonetheless, because these data are recorded during prenatal visits, it is unlikely that this misclassification is differential with respect to normal versus restricted fetal growth. However, residual confounding cannot be ruled out. Third, there is likely non-differential exposure misclassification, and therefore underestimation of risk, as we assigned pollutant concentrations based on residential proximity to fixed pollutant monitoring sites. Although we still found increased risks associated with PM2.5 and NO2, this non-differential misclassification may explain the lack of association with CO, a more spatially heterogeneous pollutant.
Fourth, although we assumed the maternal residence at birth was the same throughout the pregnancy, previous studies have shown that between 25% and 33% of pregnant women move during pregnancy,55 with 62% moving within the same municipality,56 and 70% moving within the same county.57 Since we matched air pollution concentrations from the monitor closest to the maternal residence at birth, we may have mismatched some pollution monitors if the mother changed residences during pregnancy. Assuming this mismatching/exposure error was non-differential with respect to fetal growth category, this misclassification may have resulted in a bias towards the null and underestimation of risk. However, the magnitude of this bias may be minimal, as movement within a municipality or to a neighboring municipality may not have resulted in a change in the air pollution monitor.
Last, only 25% of births with complete covariate data (88,678 of 350,107), had a maternal residence ≤10 km from a PM2.5 monitoring station and were thus retained for PM2.5 analyses. Since many of these monitors were located in urban areas, there were clear differences in the sociodemographic characteristics between those included (births to mothers from mostly urban areas) and excluded from analyses (births to mothers from urban, suburban, and rural areas). Although this is not an issue of internal validity, these differences in subject characteristics between those included and excluded from this analysis may limit the generalizability of these findings.
Future work to examine associations between pregnancy exposure to specific PM components/sources and adverse birth outcomes, and/or to examine more powerfully the role of pregnancy complications as effect modifiers of this association or as outcomes themselves, are needed.
WHAT IS ALREADY KNOWN ON THIS SUBJECT?
1 – Although the relationship between ambient air pollution and adverse birth outcomes is an active area of investigation, more data is needed to establish the time(s) during pregnancy when mothers are most at risk.
2 – Also, whether the presence of pregnancy complications late in pregnancy infer greater susceptibility to the adverse effects of ambient air pollution on birth outcomes is not known.
WHAT THIS STUDY ADDS.
1 – Our findings suggest that ambient air pollution, perhaps specifically traffic emissions during early and late pregnancy and/or factors associated with residence near a roadway during pregnancy, may affect fetal growth.
2 - Using more comprehensive data encompassing birth certificates and hospital discharge abstracts at the time of delivery, pregnancies complicated by placental abruption and premature rupture of the membrane had greater excess risks of SGA/VSGA than pregnancies not complicated by these conditions.
Acknowledgements
The authors would like to thank Dr. Lakota Kruse and Neetu Jain of NJDHSS for their assistance in constructing the health dataset, and Dr.’s Junfeng Zhang and Barbara Turpin for their assistance with interpretation of air pollution analyses.
Funding: This work was funded by a grant from the Foundation of the University of Medicine and Dentistry of New Jersey, and the NIEHS sponsored UMDNJ Center for Environmental Exposures and Disease (CEED), Grant # NIEHS P30ES005022.
REFERENCES
- 1.Bell ML, Ebisu K, Belanger K. Ambient air pollution and low birth weight in Connecticut and Massachusetts. Environ Health Perspect. 2007;115(7):1118–24. doi: 10.1289/ehp.9759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bobak M. Outdoor air pollution, low birth weight, and prematurity. Environ Health Perspect. 2000;108(2):173–6. doi: 10.1289/ehp.00108173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gouveia N, Bremner SA, Novaes HM. Association between ambient air pollution and birth weight in Sao Paulo, Brazil. J Epidemiol Community Health. 2004;58(1):11–7. doi: 10.1136/jech.58.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ha EH, Hong YC, Lee BE, Woo BH, Schwartz J, Christiani DC. Is air pollution a risk factor for low birth weight in Seoul? Epidemiology. 2001;12:643–648. doi: 10.1097/00001648-200111000-00011. [DOI] [PubMed] [Google Scholar]
- 5.Huynh M, Woodruff TJ, Parker JD, Schoendorf KC. Relationships between air pollution and preterm birth in California. Paediatr Perinat Epidemiol. 2006;20(6):454–61. doi: 10.1111/j.1365-3016.2006.00759.x. [DOI] [PubMed] [Google Scholar]
- 6.Lee BE, Ha EH, Park HS, Kim YJ, Hong YC, Kim H, Lee JT. Exposure to air pollution during different gestational phases contributes to risks of low birth weight. Human Reproduction. 2003;18(3):638–643. doi: 10.1093/humrep/deg102. [DOI] [PubMed] [Google Scholar]
- 7.Lin CM, Li CY, Yang GY, Mao IF. Association between maternal exposure to elevated ambient sulfur dioxide during pregnancy and term low birth weight. Environ Res. 2004;96(1):41–50. doi: 10.1016/j.envres.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 8.Liu S, Krewski D, Shi Y, Chen Y, Burnett RT. Association between gaseous ambient air pollutants and adverse pregnancy outcomes in Vancouver, Canada. Environ Health Perspect. 2003;111(14):1773–8. doi: 10.1289/ehp.6251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu S, Krewski D, SHi Y, Chen Y, Burnett RT. Association between maternal exposure to ambient air pollutants during pregnancy and fetal growth restriction. Journal of Exposure Science and Environmental Epidemiology. 2007;17:426–432. doi: 10.1038/sj.jes.7500503. [DOI] [PubMed] [Google Scholar]
- 10.Maisonet M, Bush TJ, Correa A, Jaakkola JJ. Relation between ambient air pollution and low birth weight in the Northeastern United States. Environ Health Perspect. 2001;109(Suppl 3):351–6. doi: 10.1289/ehp.01109s3351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mannes T, Jalaludin B, Morgan G, Lincoln D, Sheppeard V, Corbett S. Impact of ambient air pollution on birth weight in Sydney, Australia. Occup Environ Med. 2005;62(8):524–30. doi: 10.1136/oem.2004.014282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Parker JD, Woodruff TJ, Basu R, Schoendorf KC. Air pollution and birth weight among term infants in California. Pediatrics. 2005;115(1):121–8. doi: 10.1542/peds.2004-0889. [DOI] [PubMed] [Google Scholar]
- 13.Ritz B, Yu F. The effect of ambient carbon monoxide on low birth weight among children born in Southern California between 1989 and 1993. Environ Health Perspect. 1999;107:17–25. doi: 10.1289/ehp.9910717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ritz B, Yu F, Chapa G, Fruin S. Effect of air pollution on preterm birth among children born in Southern California between 1989 and 1993. Epidemiology. 2000;11(5):502–11. doi: 10.1097/00001648-200009000-00004. [DOI] [PubMed] [Google Scholar]
- 15.Sagiv SK, Mendola P, Loomis D, Herring AH, Neas LM, Savitz DA, Poole C. A time-series analysis of air pollution and preterm birth in Pennsylvania, 1997-2001. Environ Health Perspect. 2005;113(5):602–6. doi: 10.1289/ehp.7646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Salam MT, Millstein J, Li CY, Lurmann FW, Margolis HG, Gilliland FD. Birth outcomes and prenatal exposures to ozone, carbon monoxide, and particulate matter: results from the Children’s Health Study. Environ Health Perspect. 2005;113:1638–1644. doi: 10.1289/ehp.8111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang X, Ding H, Ryan L, Xu X. Association between air pollution and low birth weight: a community based study. Environ Health Perspect. 1997;105:514–520. doi: 10.1289/ehp.97105514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wilhelm M, Ritz B. Local variations in CO and particulate air pollution and adverse birth outcomes in Los Angeles County, California, USA. Environ Health Perspect. 2005;113(9):1212–21. doi: 10.1289/ehp.7751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xu X, Ding H, Wang X. Acute effects of total suspended particles and sulfur dioxides on preterm delivery: a community-based cohort study. Arch Environ Health. 1995;50(6):407–15. doi: 10.1080/00039896.1995.9935976. [DOI] [PubMed] [Google Scholar]
- 20.Yang CY, Tseng YT, Chang CC. Effects of air pollution on birth weight among children born between 1995 and 1997 in Kaohsiung, Taiwan. J Toxicol Environ Health A. 2003;66(9):807–16. doi: 10.1080/15287390306385. [DOI] [PubMed] [Google Scholar]
- 21.Brook RD, Franklin B, Cascio W, Hong Y, Howard G, Lipsett M, Luepker R, Mittleman M, Samet J, Smith SC, Jr., Tager I. Air pollution and cardiovascular disease: a statement for healthcare professionals from the Expert Panel on Population and Prevention Science of the American Heart Association. Circulation. 2004;109(21):2655–71. doi: 10.1161/01.CIR.0000128587.30041.C8. [DOI] [PubMed] [Google Scholar]
- 22.DeMeo DL, Zanobetti A, Litonjua AA, Coull BA, Schwartz J, Gold DR. Ambient air pollution and oxygen saturation. Am J Respir Crit Care Med. 2004;170(4):383–7. doi: 10.1164/rccm.200402-244OC. [DOI] [PubMed] [Google Scholar]
- 23.Mills NL, Tornqvist H, Robinson SD, Gonzalez M, Darnley K, MacNee W, Boon NA, Donaldson K, Blomberg A, Sandstrom T, Newby DE. Diesel exhaust inhalation causes vascular dysfunction and impaired endogenous fibrinolysis. Circulation. 2005;112(25):3930–6. doi: 10.1161/CIRCULATIONAHA.105.588962. [DOI] [PubMed] [Google Scholar]
- 24.O’Neill MS, Veves A, Zanobetti A, Sarnat JA, Gold DR, Economides PA, Horton ES, Schwartz J. Diabetes enhances vulnerability to particulate air pollution-associated impairment in vascular reactivity and endothelial function. Circulation. 2005;111(22):2913–20. doi: 10.1161/CIRCULATIONAHA.104.517110. [DOI] [PubMed] [Google Scholar]
- 25.Andres RL, Day MC. Perinatal complications associated with maternal tobacco use. Semin Neonatol. 2000;5(3):231–41. doi: 10.1053/siny.2000.0025. [DOI] [PubMed] [Google Scholar]
- 26.Crispi F, Dominguez C, Llurba E, Martin-Gallan P, Cabero L, Gratacos E. Placental angiogenic growth factors and uterine artery Doppler findings for characterization of different subsets in preeclampsia and in isolated intrauterine growth restriction. Am J Obstet Gynecol. 2006;195(1):201–7. doi: 10.1016/j.ajog.2006.01.014. [DOI] [PubMed] [Google Scholar]
- 27.Goswami D, Tannetta DS, Magee LA, Fuchisawa A, Redman CW, Sargent IL, von Dadelszen P. Excess syncytiotrophoblast microparticle shedding is a feature of early-onset pre-eclampsia, but not normotensive intrauterine growth restriction. Placenta. 2006;27(1):56–61. doi: 10.1016/j.placenta.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 28.Naeye RL. Placenta previa. Predisposing factors and effects on the fetus and surviving infants. Obstet Gynecol. 1978;52(5):521–5. [PubMed] [Google Scholar]
- 29.Naeye RL. Abruptio placentae and placenta previa: frequency, perinatal mortality, and cigarette smoking. Obstet Gynecol. 1980;55(6):701–4. [PubMed] [Google Scholar]
- 30.Demissie K, Rhoads GG, Smulian JC, Balasubramanian BA, Gandhi K, Joseph KS, Kramer M. Operative vaginal delivery and neonatal and infant adverse outcomes: population based retrospective analysis. BMJ. 2004;329(7456):24–9. doi: 10.1136/bmj.329.7456.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Green DC, Moore JM, Adams MM, Berg CJ, Wilcox LS, McCarthy BJ. Are we underestimating rates of vaginal birth after previous cesarean birth? The validity of delivery methods from birth certificates. Am J Epidemiol. 1998;147(6):581–6. doi: 10.1093/oxfordjournals.aje.a009490. [DOI] [PubMed] [Google Scholar]
- 32.Parrish KM, Holt VL, Connell FA, Williams B, LoGerfo JP. Variations in the accuracy of obstetric procedures and diagnoses on birth records in Washington State, 1989. Am J Epidemiol. 1993;138(2):119–27. doi: 10.1093/oxfordjournals.aje.a116834. [DOI] [PubMed] [Google Scholar]
- 33.Piper JM, Mitchel EF, Jr., Snowden M, Hall C, Adams M, Taylor P. Validation of 1989 Tennessee birth certificates using maternal and newborn hospital records. Am J Epidemiol. 1993;137(7):758–68. doi: 10.1093/oxfordjournals.aje.a116736. [DOI] [PubMed] [Google Scholar]
- 34.NCHS . Computer Edits for Natality Data, Effective 1993. National Center for Health Statistics. Vital Statistics Data Preparation; 1995. Instruction Manual Part 12. [Google Scholar]
- 35.Emery ES, 3rd, Eaton A, Grether JK, Nelson KB. Assessment of gestational age using birth certificate data compared with medical record data. Paediatr Perinat Epidemiol. 1997;11(3):313–21. doi: 10.1111/j.1365-3016.1997.tb00009.x. [DOI] [PubMed] [Google Scholar]
- 36.Kline J, Stein Z, Susser M. Conception to birth: epidemiology of prenatal development. Oxford University Press; New York, NY: 1989. [Google Scholar]
- 37.Kramer MS, McLean FH, Olivier M, Willis DM, Usher RH. Body proportionality and head and length ‘sparing’ in growth-retarded neonates: a critical reappraisal. Pediatrics. 1989;84(4):717–23. [PubMed] [Google Scholar]
- 38.Wen SW, Kramer MS, Usher RH. Comparison of birth weight distributions between Chinese and Caucasian infants. Am J Epidemiol. 1995;141(12):1177–87. doi: 10.1093/oxfordjournals.aje.a117391. [DOI] [PubMed] [Google Scholar]
- 39.Demissie K, Breckenridge MB, Rhoads GG. Infant and maternal outcomes in the pregnancies of asthmatic women. Am J Respir Crit Care Med. 1998;158(4):1091–5. doi: 10.1164/ajrccm.158.4.9802053. [DOI] [PubMed] [Google Scholar]
- 40.US EPA Technology transfer network - Air quality system. Download AQS data. United States Environmental Protection Agency. 2007 [Google Scholar]
- 41.US Census “Variables extracted from detailed tables of Census 2000 consisting of social, economic and housing characteristics. 2000.
- 42.Subramanian SV, Chen JT, Rehkopf DH, Waterman PD, Krieger N. Comparing individual- and area-based socioeconomic measures for the surveillance of health disparities: A multilevel analysis of Massachusetts births, 1989-1991. Am J Epidemiol. 2006;164(9):823–34. doi: 10.1093/aje/kwj313. [DOI] [PubMed] [Google Scholar]
- 43.Krieger N, Chen JT, Waterman PD, Soobader MJ, Subramanian SV, Carson R. Choosing area based socioeconomic measures to monitor social inequalities in low birth weight and childhood lead poisoning: The Public Health Disparities Geocoding Project (US) J Epidemiol Community Health. 2003;57(3):186–99. doi: 10.1136/jech.57.3.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kalkstein LS, Valimont KM. An evaluation of summer discomfort in the United States using a relative climatological index. B Am Meteorol Soc. 1986;1986(67) [Google Scholar]
- 45.Duvekot JJ, Cheriex EC, Pieters FA, Peeters LL. Severely impaired fetal growth is preceded by maternal hemodynamic maladaptation in very early pregnancy. Acta Obstet Gynecol Scand. 1995;74(9):693–7. doi: 10.3109/00016349509021176. [DOI] [PubMed] [Google Scholar]
- 46.Khong TY, De Wolf F, Robertson WB, Brosens I. Inadequate maternal vascular response to placentation in pregnancies complicated by pre-eclampsia and by small-for-gestational age infants. Br J Obstet Gynaecol. 1986;93(10):1049–59. doi: 10.1111/j.1471-0528.1986.tb07830.x. [DOI] [PubMed] [Google Scholar]
- 47.Peters A, Doring A, Wichmann HE, Koenig W. Increased plasma viscosity during an air pollution episode: a link to mortality? Lancet. 1997;349(9065):1582–7. doi: 10.1016/S0140-6736(97)01211-7. [DOI] [PubMed] [Google Scholar]
- 48.Vorherr H. Factors influencing fetal growth. Am J Obstet Gynecol. 1982;142(5):577–88. doi: 10.1016/0002-9378(82)90765-7. [DOI] [PubMed] [Google Scholar]
- 49.Kadyrov M, Kingdom JC, Huppertz B. Divergent trophoblast invasion and apoptosis in placental bed spiral arteries from pregnancies complicated by maternal anemia and early-onset preeclampsia/intrauterine growth restriction. Am J Obstet Gynecol. 2006;194(2):557–63. doi: 10.1016/j.ajog.2005.07.035. [DOI] [PubMed] [Google Scholar]
- 50.Perera FP, Rauh V, Whyatt RM, Tsai WY, Bernert JT, Tu YH, Andrews H, Ramirez J, Qu L, Tang D. Molecular evidence of an interaction between prenatal environmental exposures and birth outcomes in a multiethnic population. Environ Health Perspect. 2004;112:626–630. doi: 10.1289/ehp.6617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dejmek J, Solansky I, Benes I, Lenickek J, Sram RJ. The impact of polycyclic aromatric hydrocarbons and fine particles on pregnancy outcomes. Environ Health Perspect. 2000;118:1159–1164. doi: 10.1289/ehp.001081159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Perera FP, Rauh V, Tsai WY, Kinney P, Camann D, Barr D, Bernert T, Garfinkel R, Tu YH, Diaz D, Dietrich J, Whyatt RM. Effects of transplacental exposure to environmental pollutants on birth outcomes in a multiethnic population. Environ Health Perspect. 2003;111:201–205. doi: 10.1289/ehp.5742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dommisse J, Tiltman AJ. Placental bed biopsies in placental abruption. Br J Obstet Gynaecol. 1992;99(8):651–4. doi: 10.1111/j.1471-0528.1992.tb13848.x. [DOI] [PubMed] [Google Scholar]
- 54.Hannah ME, Ohlsson A, Farine D, Hewson SA, Hodnett ED, Myhr TL, Wang EE, Weston JA, Willan AR. Induction of labor compared with expectant management for prelabor rupture of the membranes at term. TERMPROM Study Group. N Engl J Med. 1996;334(16):1005–10. doi: 10.1056/NEJM199604183341601. [DOI] [PubMed] [Google Scholar]
- 55.Canfield MA, Ramadhani TA, Langlois PH, Waller DK. Residential mobility patterns and exposure misclassification in epidemiologic studies of birth defects. J Expo Sci Environ Epidemiol. 2006 doi: 10.1038/sj.jes.7500501. [DOI] [PubMed] [Google Scholar]
- 56.Fell DB, Dodds L, King WD. Residential mobility during pregnancy. Paediatr Perinat Epidemiol. 2004;18(6):408–14. doi: 10.1111/j.1365-3016.2004.00580.x. [DOI] [PubMed] [Google Scholar]
- 57.Shaw GM, Schulman J, Frisch JD, Cummins SK, Harris JA. Congenital malformations and birth weight in areas with potential environmental contamination. Arch Environ Health. 1992;47(2):147–54. doi: 10.1080/00039896.1992.10118769. [DOI] [PubMed] [Google Scholar]