ABSTRACT
The herpes simplex virus (HSV) tegument protein VP1-2 contains an N-terminal nuclear localization signal (NLS) that is critical for capsid routing to the nuclear pore. Here we analyzed positionally conserved determinants in VP1-2 homologues from each of the alpha, beta, and gamma classes of human herpesviruses. The overall architectures of the VP1-2s were similar, with a conserved N-terminal ubiquitin-specific protease domain separated from an internal region by a linker that was quite poorly conserved in length and sequence. Within this linker region all herpesviruses contained a conserved, highly basic motif which nevertheless exhibited distinct class-specific features. The motif in HSV functioned as a monopartite NLS, while in varicella-zoster virus (VZV) activity required an adjacent basic section defining the motif as a bipartite NLS. Neither the beta- nor gammaherpesvirus VP1-2 motifs were identified by prediction algorithms, but they nevertheless functioned as efficient NLS motifs both in heterologous transfer assays and in HSV VP1-2. Furthermore, though with different efficiencies and with the exception of human herpesvirus 8 (HHV-8), these chimeric variants rescued the replication defect of an HSV mutant lacking its NLS motif. We demonstrate that the lysine at position 428 of HSV is critical for replication, with a single alanine substitution being sufficient to abrogate NLS function and virus growth. We conclude that the basic motifs of each of the VP1-2 proteins are likely to confer a similar function in capsid entry in the homologous setting and that while there is flexibility in the exact type of motif employed, specific individual residues are critical for function.
IMPORTANCE To successfully infect cells, all herpesviruses, along with many other viruses, e.g., HIV, hepatitis B virus, and influenza virus, must navigate through the cytoplasmic environment and dock with nuclear pores for transport of their genomes into the nucleus. However, we still have a limited understanding of the detailed mechanisms involved. Insight into these events is needed and could offer opportunities for therapeutic intervention. This work investigated the role of a specific determinant in the structural protein VP1-2 in herpesvirus entry. We examined this determinant in representative VP1-2s from all herpesvirus subfamilies, demonstrated NLS function, dissected key residues, and showed functional relevance in rescuing replication of the mutant blocked in capsid navigation to the pore. The results are important and strongly support our conclusions of the generality that these motifs are crucial for entry of all herpesviruses. They also facilitate future analysis on selective host interactions and possible routes to disrupt function.
INTRODUCTION
All herpesvirus capsids must be transported across the cytoplasm, targeted to nuclear pores. By unknown mechanisms this promotes capsid structural rearrangements facilitating genome exit and transport across the pore to the nucleus where virus immediate early gene transcription ensues (1). The large tegument protein VP1-2, the product of the UL36 gene, is essential and conserved across the entire family (2–6). It is a complex, multifunctional protein that plays crucial roles at various points in the virus life cycle, including entry, capsid transport, and virion assembly (2–10). This protein is also among a subset of components classed as inner tegument proteins, reflecting tight association with capsids (11–17). Evidence for a key role for VP1-2 early after infection originated from studies of the temperature-sensitive mutant virus tsB7, where at the restrictive temperature, full capsids accumulated at the nuclear pore and virus gene expression was profoundly blocked (3, 18). The defect was mapped specifically to a single amino acid at residue 1453 in VP1-2 (19). Consistent with a role for VP1-2 early in nuclear entry are studies of a full deletion mutant of the UL36 gene wherein mutant capsids lacking VP1-2 were unable to infect nuclei in chemically fused polykaryocytes (7). VP1-2 also promotes capsid motility on microtubules, a role that could be connected to both assembly and entry (6, 9, 13, 15, 17). The protein also encompasses a unique class of ubiquitin-specific protease (USP) embedded within its extreme N terminus (20, 21) which, while potentially dispensable for core functions of virus assembly in tissue culture, may play a role in aspects of replication in vivo, including neuroinvasion or antagonizing innate immune responses (22–24).
The essential nature, the multiplicity of roles, and the very large size of VP1-2 have made difficult the mechanistic dissection of pathways in which it is involved. In particular, deletion of the protein results in failure to assemble virions and prevents further analysis of its role in entry. However, several studies have dissected functional domains of the protein, including essential determinants required for pUL37 and for VP16 binding (4, 25–27), distinct regions involved in pUL25 binding (28, 29), multiple regions involved in binding components of the retrograde motor dynein (17, 30), and the C-terminal region, which has been reported to be dispensable for assembly of virions in infected cells but essential for the retention of VP1-2 on capsids as they enter newly infected cells (31).
We previously demonstrated that VP1-2 contains an efficient nuclear localization signal (NLS) adjacent to the N-terminal USP domain (10, 32). A similar motif in the pseudorabies virus (PRV) VP1-2 homologue has also been shown to be functional in nuclear localization (33). We constructed a herpes simplex virus (HSV) mutant containing a short deletion of this NLS and showed that this determinant is essential for virus spread (10). We showed that the virus mutant lacking the NLS still assembled extracellular virions, that it successfully infected cells, and that the mutant VP1-2 was retained on infecting capsids but that such capsids were profoundly defective for pore docking and unable to initiate gene expression. Our data indicated that the NLS at position 475 in VP1-2 is essential for virus spread and, while dispensable for virion assembly per se, is key to normal entry early in infection via routing to the nuclear pore.
The motif also exhibits positional conservation in the homologues in all herpesviruses (32), and we proposed that it may serve a similar function and be critical for early infection of all herpesviruses. Nevertheless positional conservation does not necessarily correlate with functional conservation, and there are many examples showing lack of functional conservation in overtly similar genes or protein determinants. Moreover, although the putative sequences of certain classical NLS motifs have been defined, there are many examples of sequences that match the consensus but are nonfunctional for nuclear import and of experimentally confirmed NLS motifs that do not match the classical consensus sequences (34). Here we report an analysis of the VP1-2s from representative members of all herpesvirus classes, first to make a more detailed sequence comparison between the different VP1-2s, second to determine whether they each functioned as an NLS, and third to examine whether they would act in a physiologically relevant way and rescue the defect in the HSV VP1-2ΔNLS mutant. We used the last assay as a surrogate measure to evaluate whether each motif functioned in the context of its native setting. A positive result, together with functional data on NLS activity, would be strongly indicative that the corresponding motif performed a similar role in its native setting. This would otherwise require the extremely difficult task of deleting and evaluating virus mutants for each of the representative viruses and classes tested. Our results indicate that the analogous regions from varicella-zoster virus (VZV), human cytomegalovirus (HCMV), Epstein-Barr virus (EBV), and human herpesvirus 8 (HHV-8) functioned as efficient NLS motifs in heterologous protein transfer assays or when transferred into HSV VP1-2 itself. Though with different efficiencies, they also rescued the replication defect of the HSV VP1-2ΔNLS mutant (with the exception of the NLS region from HHV-8 VP1-2). We conclude that the basic motif of each VP1-2 protein is likely to confer a similar function in capsid entry in the homologous setting. Additional analyses nevertheless reveal distinct differences between the motifs which may influence function, identify specific individual residues that are critical for function, and indicate that overlapping determinants with additional functions may reside in the region.
MATERIALS AND METHODS
Cells, virus, infections, and transfection.
RSC (rabbit skin cells) and RSC-HAUL36 cells (7) (a derivative containing the UL36 gene) were grown in Dulbecco's modified minimal essential medium (DMEM) (Gibco) containing 10% fetal bovine serum (FBS), nonessential amino acids (NEAA), and penicillin-streptomycin. Vero cells and HS30 cells, a derivative of Vero cells containing the UL36 gene (2), were grown in DMEM supplemented with 10% newborn calf serum (NCS) and penicillin-streptomycin. RPE-1 cells (immortalized human retinal pigment epithelial cells) were maintained in DMEM F-12 containing 10% FBS, 100 mM l-glutamine, NEAA, and penicillin-streptomycin. HS30 and RSC-HAUL36 media were supplemented with 0.1 and 1 mg/ml Geneticin, respectively. COS cells (African Green monkey kidney cells) or Huh7 cells (human liver carcinoma cells) were used in cotransfection assays for studies of interaction between VP1-2 chimeras and pUL37. These cells were grown in the same medium as RSC cells. Virus strains have been previously described and included HSV-1 (KOS) and KOS.VP1-2ΔNLS, which lacks the core NLS of VP1-2 (10). The parent virus of this mutant is designated KOS.Bac.w/t. We also included the revertant of the mutant, which is designated KOS.VP1-2ΔNLS.R (for revertant).
Plasmid construction. (i) VP1-2 variants containing candidate NLS regions from other herpesviruses.
We created a series of plasmids based on the expression vector for HSV VP1-2.1-1875, plasmid pcDNASV5-NT6 (pMB20) (35). We first constructed a derivative of pMB20, pcDNASV5-NT6v2 (pTH15), by swapping the HindIII/HpaI plasmid backbone for that of pTD3 (32) digested with the same enzymes. This step yields a version of NT6 with a single BamHI site at residue 367 and a single MluI site at residue 588 for use in subsequent cloning strategies, into which we inserted BamHI-MluI fragments with the appropriate sequence. To generate these various BamHI-MluI fragments, we first chemically synthesized an intermediate vector containing the corresponding BamHI-MluI region of HSV-1.VP1-2 (plasmid designated pTH19) (GeneArt; Life Technologies) which contains silent mutations resulting in the creation of unique XhoI and NheI sites at residues 408/409 and 476/477, respectively, flanking the previously described VP1-2 region 1 (R1) (32). We then inserted the 672-bp BamHI-MluI fragment from pTH19 into pcDNASV5-NT6v2. The resulting plasmid, pcDNASV5-NT6v3 (pTH22), encodes VP1-2.1-1875 unchanged (apart from the silent new XhoI and NheI sites) and serves as the parental control for a series of constructs wherein the XhoI-NheI region could now be swapped for sequences from the corresponding regions of VP1-2 homologues of other herpesviruses.
To make these chimeras, we first had to make a series of plasmids (GeneArt) incorporating the chemically synthesized variant R1 sequences with flanking XhoI and NheI sites. These sequences were then inserted as XhoI-NheI fragments into the XhoI-NheI sites of the intermediate vector, pTH19. The larger BamHI-MluI fragments, encompassing these XhoI-NheI sequences, were then isolated and inserted into the unique BamHI-MluI sites of pcDNASV5-NT6v2 to make the final chimeric versions of NT6 termed NT6.R1.VZV, NT6.R1.HCMV, NT6.R1.EBV, and NT6.R1.HHV-8 (see Fig. 6). These encode HSV VP1-2.1-1875 with the R1 sequences derived from VP1-2 homologues of VZV, HCMV, EBV, and HHV-8 inserted in place of the HSV sequence from position 410 to 475. For HHV-8, an additional region adjacent to R1, referred to as region 8 (R8), was constructed by the same strategy. These constructs are summarized in Table 1. In an additional construct, we inserted a completely heterologous monopartite NLS from a host cell protein (myopodin) in place of the core NLS R4 of VP1-2. This was accomplished by the same strategy of chemical synthesis in a vector containing flanking XhoI and NheI sites, in this case incorporating parental sequences from HSV together with the myopodin NLS, resulting in the construct NT6.MyoPNLS.
FIG 6.
Analysis of NLS function in the context of VP1-2. (a) Schematic representation of the expression constructs for the N-terminal half of VP1-2 (NT6), the NT6 variant lacking 7 residues within R4 (NT6ΔNLS), and the candidate NLS motifs or swapped in place of the natural R1 region. (b) Localization was scored into one of three categories (categories 1, 2, and 3). Typical examples are shown at higher magnification. (c) Localization of the parental NT6 and NT6ΔNLS in comparison to versions containing the R1 regions from VZV, HCMV, EBV, HHV-8, and the R8 region from HHV-8 as discussed in the text. (d) Localization of NT6 variants as indicated, where K428 was changed to either an arginine or alanine (NT6.K428R and NT6.K428A), R5 was deleted NT6ΔR5, each basic residue was mutated to alanine (NT6.MutR5), and the core R4 region was replaced with a cellular NLS in the context of R1 (NT6.MyoNLS). Representative fields are shown for each variant as discussed in the text.
TABLE 1.
Summary of region 1 chimeric constructs
| Construct | Protein sequencea |
|---|---|
| 410___________________475 | |
| NT6 | ..VLEMGVVPVGRHRARYSAGLPKRRRPTWTPPSSVEDLTSGEKTKRSAPPAKTKKKSTPKGKTPVGAAVPASVP...... |
| NT6.R1.VZV | ..VLEPWHAVLETTSTGSGVLDCRRRRRPSWTPPSSEENLACIDDGLVNNTHSTDNLHKPAKKVLKFKPTVDVPASVP... |
| NT6.R1.CMV | ..VLEAPSIPVYDPSSSPKKTPEKRRKDLSGSKHGGKKKPPSTTSKTLATASSSPSAIAAASSSSAVPPSYASVP...... |
| NT6.R1.EBV | ..VLEGAAPQTPKRKKGLGKDSPHKKPTSGRRLPLSSTTDTEDDQLPRTHVPPHRPPSAARLPPPVIPIPHASVP...... |
| NT6.R1.KSHV | ..VLEAPSEAPLRRDSTQSQDETRPRRPRVVIPPYDPTDRPRPPHQDRPPEQAAGYGGNKGRGGNKGRGGKTGRGASVP.. |
| NT6.R8.KSHV | ..VLELLSDLTATRGQKRKFSSLKESYPIDSPPSDDDDVSQPSQQTAPDTEDIWIDDPLTPLYPLTDTPSFASVP.. |
| NT6.MyoPNLS | ..VLEMGVVPVGKKRRRRARK..........PPSSVEDLTSGEKTKRSAPPAKTKKKSTPKGKTPVGAAVPASVP...... |
| NT6.Δ448–475 | ..VLEMGVVPVGRHRARYSAGLPKRRRPTWTPPSSVEDL................................ASVP...... |
| NT6.mut448-475 | ..VLEMGVVPVGRHRARYSAGLPKRRRPTWTPPSSVEDLTSGEATALSAPPAATAAASTPAGATPVGAAVPASVP...... |
| NT6.428K>R | ..VLEMGVVPVGRHRARYSAGLPRRRRPTWTPPSSVEDLTSGEKTKRSAPPAKTKKKSTPKGKTPVGAAVPASVP...... |
| NT6.428K>A | ..VLEMGVVPVGRHRARYSAGLPARRRPTWTPPSSVEDLTSGEKTKRSAPPAKTKKKSTPKGKTPVGAAVPASVP...... |
The region from position 410 to 475 of HSV VP1-2 is given on the first line (NT6), with M410 and P475 underlined. In each construct the underlined residues are the first and last residue from aligned positions of the heterologous VP1-2s, inserted in place of HSV positions 410 to 475. Bold lettering of the M and P for NT6 indicates the beginning and end of the R1 region. Bold lettering on all other lines indicates those residues that are changed from the parental HSV NT6 sequence either in mutant or in chimeric constructs. For the cellular myopodin insertion, the overlined sequence of NT6 was replaced with the shorter core myopodin NLS, resulting in a net deletion of 10 residues. The deletion in the R5 region, Δ448-475, is indicated, while the mutation in R5 has all basic residues changed to alanines (indicated in bold italics).
(ii) β-Galactosidase expression vectors.
The series of intermediate plasmids described above which acted as the starting point for ultimate insertion into pcDNASV5-NT6v3 also contained HindIII and BglII sites flanking the XhoI-NheI sites with the addition of an N-terminal hemagglutinin (HA) epitope tag between the HindIII and XhoI sites. This enabled insertion of the same regions used for the NT6 constructs into a second series of constructs to test NLS function in a heterologous setting by insertion into β-galactosidase expression vectors as previously described (32). The plasmids pHA.R1.VZV-β-gal (pTH46), pHA.R1.HCMV-β-gal (pTH47), pHA.R1.EBV-β-gal (pTH67), pR1.HHV-8-β-gal (pTH68), and pR8.HHV-8-β-gal (pTH69) were generated by the digestion of each of the intermediate plasmids in the series with HindIII and BglII and insertion of the HindIII-BglII fragments into pβ-gal (32). This places the R1s from each of the VP1-2 homologues immediately upstream of the reading frame for β-galactosidase. The amino acid residues inserted correspond to the HSV R1 residues used for insertion into NT6 and were as follows (numbered with reference to the start of each respective VP1-2 homologue): VZV, 313 to 381; HCMV, 269 to 333; EBV, 406 to 471; HHV-8.R1, 251 to 320; and HHV-8.R8, 398 to 468. Oligonucleotides for testing the shorter R4s were chemically synthesized (Sigma) with a 5′ HindIII and a 3′ BglII overhang and subsequently inserted into the N terminus of β-galactosidase (Table 1). (For a schematic summarizing these β-galactosidase vectors, see Fig. 2.)
FIG 2.
Sequence analysis and NLS function of the basic regions in VP1-2 in distinct herpesvirus families. (a) Alignment of regions termed R1, encompassing the basic region within the linker region downstream of the USP domain. Basic residues K and R are indicated with a red background. Other color shading is related to chemical property to best highlight conserved and nonconserved residues. Actual numbering of the R1 regions in relation to the parent VP1-2s is listed in full in Materials and Methods. In the alphaherpesvirus alignment, the extents of regions R4 and R5 are indicated by the wavy lines. The minimal regions tested for the beta- and gammaherpesvirus groups to examine sufficiency for NLS activity are indicated by the filled circles. (b) Schematic indicating the test for NLS function, wherein R1 or R4 was inserted in frame into the N terminus of β-galactosidase. (c) Higher-magnification images of typical fields of the parental β-galactosidase and a variant containing the complete R1 from HSV. (d) Lower-magnification images of the various test constructs as indicated.
Purification of viral DNA from K.VP1-2ΔNLS.
For purification of K.VP1-2ΔNLS DNA, RSC-HAUL36 cells (10 175-cm2 flasks) were infected (multiplicity of infection [MOI] of 0.001) and virus isolated from the medium approximately 3 days later after advanced cytopathic effect (CPE) was observed. The medium was clarified by centrifugation (2,000 rpm for 20 min) and virus pelleted from the supernatant (19,000 rpm for 90 min). The pelleted virus was resuspended gently in 5 ml of buffer (10 mM Tris-HCl [pH 7.6], 1 mM EDTA, 1% SDS) containing 50 μg/ml proteinase K and incubated at 50°C for 4 h, and DNA was extracted by successive rounds of phenol and phenol-chloroform extraction. The final aqueous phase was precipitated by addition of 0.1 volume 3 M sodium acetate (pH 5.3) and 3.75 volumes of 100% ethanol (EtOH), gentle mixing, and overnight incubation at −20°C. Finally, the DNA was pelleted (3,500 rpm for 30 min) at 4°C, washed with 70% EtOH, air dried, and then resuspended in 100 to 200 μl of H2O.
Rescue of K.VP1-2ΔNLS by VP1-2s containing heterologous NLS motifs.
RSC cells were transfected with 250 ng of K.VP1-2ΔNLS DNA together with 250 ng of each of the NT6 plasmids. Parental NT6 and NT6ΔNLS were used as positive and negative controls, respectively. Monolayers were monitored for plaque formation and after 3 days incubation at 37°C were harvested and freeze-thawed three times; samples were stored at −80°C. Rescued virus stocks were generated from multiply plaque-purified isolates in RSC cells (under 0.5% agarose), amplified after infection (0.001 PFU/cell), and harvested after 3 days. The presence of the recombined sequences from the VP1-2 homologues in HSV VP1-2 was confirmed by PCR amplification using a combination of HSV-1 and chimera-specific primers and also by sequencing the appropriate region from purified DNA stocks of recombinant or parental virus isolates. The mutant viruses were designated K.VP1-2.NLS.R1.VZV, K.VP1-2.NLS.R1.HCMV, and K.VP1-2.NLS.R1.EBV. Further mutations that could be rescued comprised the myopodin NLS and K428R mutation as described above. Examination of plaque formation and growth curves were performed by standard methods as previously described (10, 35).
Virus characterization.
Viruses expressing chimeric VP1-2 or the K.VP1-2ΔNLS revertant were used to infect monolayers of RSC, RSC-HAUL36, RPE-1, or Vero cells (0.005 PFU/cell in 24-well plates), and total virus yields were harvested at 0, 24, 48, and 72 h after infection. Virus yields were then titrated on RSCUL36 cells. For measuring plaque sizes, infected cultures were incubated for 3 days in the presence of 1 to 2% pooled human serum (Sera Lab) for 3 days and then fixed with 4% formaldehyde in phosphate-buffered saline (PBS) for 20 min. After fixation, samples were permeabilized, blocked in PBS containing 10% goat serum and 0.2% Triton X-100 for 1 h, and stained with rabbit anti-UL37 (1:2,500) followed by goat anti-rabbit–horseradish peroxidase (HRP) (1:1,000) with 3,3′-diaminobenzidine (DAB) (Sigma) as the substrate. The revertant K.VP1-2ΔNLS.R was used as control. Plaque size was measured with the Image Pro Plus 7.0 contour tool and expressed as μm2.
Assay of UL37 interaction with VP1-2.
COS cells (African green monkey kidney cells) or Huh7 cells (human liver carcinoma cells) were grown to 70% confluence in 100-mm dishes in standard medium and transfected with 5 μg of NT6 DNA (or variants thereof) and 5 μg HA-UL37 DNA using GeneJammer as per the manufacturer's protocol (Agilent Technologies). Cells were washed and pelleted in ice-cold PBS and lysed in 500 μl of radioimmunoprecipitation assay (RIPA) buffer containing 150 mM NaCl, 50 mM Tris-HCl, 1% NP-40 or 0.5% 3-[(3-cholamidopropyl)-dimethylammonio]-1-propanesulfonate (CHAPS), 1 mM dithiothreitol (DTT), 2 mM EDTA, 1 μM phenylmethylsulfonyl fluoride (PMSF), and complete protease inhibitor cocktail with gentle agitation at 4°C for 30 min. Extracts were clarified at 4°C at 13,000 rpm for 15 min. Lysates were subsequently precleared with 60 μl of a slurry of protein A beads for 1 h before incubation with primary mouse anti-V5 antibody (1:200; Invitrogen) at 4°C for 3 h. NT6 was precipitated with 60 μl on protein A beads for 1 h at 4°C, washed five times in RIPA buffer, and eluted in SDS sample buffer by boiling for 5 min. SDS-PAGE and Western blotting were performed as per standard protocols using primary mouse anti-V5 (1:10,000) (for VP1-2) and secondary goat anti-mouse–HRP or primary mouse anti-HA (Covance; 1:1,000) with secondary goat anti-mouse–HRP antibody combinations (for pUL37).
Immunofluorescence studies.
Immunofluorescence analysis was performed exactly as described previously (36). Samples were collected at the times indicated, washed with PBS, and fixed with either paraformaldehyde (4%) (followed by permeabilization with 0.1% Triton X-100) or methanol (5 min at −20°C). Samples were blocked with PBS containing 5% goat serum, 5% NCS, and 2% bovine serum albumin (BSA) for 1 h at room temperature. Primary antibodies were mouse anti-V5 (Invitrogen; 1:1,000) and mouse anti-β-galactosidase (Promega; 1:200). DNA was stained with DAPI (4′,6′-diamidino-2-phenylindole) (Sigma) and coverslips mounted in Mowiol supplemented with 2.5% 1,4-diazabicyclo[2.2.2]octane (DABCO). Images were taken using an Axiovert 135 TV with a Zeiss ×10, ×40 LD, or ×63 lens (Plan-Apochromat; 1.4 numerical aperture), captured using a Retiga 2000R camera with Image Pro Plus software or with a Zeiss laser scanning confocal microscope (Zeiss Pascal). Efficiency was measured as cells scoring positive for nuclear import (enumerating cells showing only nuclear or nuclear greater than cytoplasmic localization) as a percentage of total positive cells. For the transfer analyses, the parental large β-galactosidase (130 kDa) requires a specific import signal for nuclear accumulation, and <5% of cells scored as positive. The HSV NLS inserted into β-galactosidase scored greater than 90% positive, and this was classed as efficient for comparison with other variants (see Fig. 2). Analysis of nuclear localization in the context of VP1-2 was as described in Results. Localization was enumerated in at least 30 cells for each construct. Composite illustrations were prepared using Adobe software. Example images shown are representative of numerous images gathered for each sample and condition.
SDS-PAGE and Western blotting.
Mock-infected or infected cell monolayers were harvested in standard SDS sample buffer. Samples were lysed either using a sonicator water bath or by needle shearing using a 25-gauge needle and boiled for 5 min prior to electrophoresis. Equal cell samples were analyzed on 3 to 8% gradient Tris-acetate gels or 10% Tris-glycine gels and transferred to nitrocellulose membranes. Membranes were blocked in PBS containing either 5% dry milk or 0.5× blocking solution (Li-Cor Biosciences) when detection was by fluorescent immunodetection. Primary and secondary antibodies were diluted in PBS plus 0.1% Tween 20 containing 5% dry milk (PBST). Target proteins were visualized using either HRP-conjugated secondary antibodies or DyLight-conjugated secondary antibodies (1/10,000; Pierce). HRP-conjugated species were detected by chemiluminescence, while the DyLight-conjugated species were detected using a Li-Cor Bioscience Odyssey infrared imaging system. Odyssey v3.0 software was used for quantification, with linearity of measurement being confirmed using a standardization bioassay with serial dilutions of sample inputs. Values of specific viral protein intensities were normalized against actin values. Primary antibodies were anti-HA and anti-SV5 (1/10,000; Invitrogen).
RESULTS
Organization of the N-terminal regions of herpesvirus VP1-2 homologues.
We undertook a more detailed analysis of the organization and sequence of the N-terminal region of VP1-2 and its conservation in VP1-2 homologues in other herpesvirus families. Alignment of VP1-2 sequences using the multiple-alignment tool Clustal (http://www.clustal.org/clustal2/) was first performed separately on sets of sequences from alpha-, beta-, or gammaherpesviruses, and a subset of these is illustrated in Fig. 1 to highlight certain broad features, while the NLS region sequences themselves are expanded and analyzed in more detail in Fig. 2a.
FIG 1.
Summary of broad features of organization of VP1-2s from representatives of the alpha-, beta-, and gammaherpesvirus groups. VZV, varicella-zoster virus; PRV, pseudorabies virus; BHV, bovine herpesvirus 1; HCMV, human cytomegalovirus; HHV-6, human herpesvirus 6; EBV, Epstein-Barr virus; CalHV3, callitrichine herpesvirus 3; HHV-8, human herpesvirus 8; HVS, herpesvirus saimiri. Organization and shading are as discussed in the text. Regions involved in pUL37, VP16, or pUL25 interaction are indicated based on studies in either HSV or PRV (4, 5, 12, 26–29).
Alignment of the alphaherpesvirus VP1-2s revealed a clear organization wherein the conserved N-terminal ubiquitin-specific protease domain (USP) is linked to a downstream region (which has a quite well-defined N-terminal boundary beginning around residue 578 in HSV [gray shading]) by a very poorly conserved linker region of variable length and sequence. Within this linker region, the exception was a very highly conserved basic motif encompassing a core NLS and surrounding residues (Fig. 1 and 2a). Separate alignment of the beta- and gammaherpesvirus VP1-2s and subsequent realignment with the alphaherpesvirus family revealed a similar overall organization and the presence of a basic motif (Fig. 2a).
While all VP1-2s contained a region with very high basic content, each family had its own variation on this theme, with this variation being very well conserved within the family and less so between families. For ease of reference and labeling, the broader region encompassing the basic motif(s) is termed region 1 (R1), with boundaries as indicated in Fig. 2a (arrows). Within R1, the alphaherpesvirus VP1-2s all contained two distinct basic clusters separated by a section of 16 to 30 residues rich in prolines (P) and serines (S) or threonines (T). The first of the basic clusters (Fig. 2a, R4) invariably contained a consecutive run of at least 4 basic residues flanked by a very highly conserved P/S/T section. The second C-terminal basic region (Fig. 2a, R5) was more variable and without a consecutive run of four or more residues. We have previously shown for HSV VP1-2 that R4 was sufficient to function as an NLS while R5, though equally basic in overall content, showed no activity as an independent NLS (32).
For the betaherpesvirus VP1-2s, again there was an N-terminal basic motif, in this case with consecutive runs of three or four basic residues (Fig. 2a). This was linked to a C-terminal basic cluster by a shorter region of 9 to 11 residues with no homology to the linker region in the alphaherpesvirus VP1-2s. This overall scheme was well conserved in the betaherpesvirus VP1-2s. For the gammaherpesvirus VP1-2s, these showed somewhat more similarity to the betaherpesvirus configuration. A linker and C-terminal basic cluster were present, though these were less distinct and not conserved in primary sequence. It was particularly noticeable that in the gamma-2-herpesviruses available, while the positionally equivalent area just downstream of the USP domain again showed enrichment in basic residues, there was considerably less sequence conservation not only with other families but within the gamma-2-herpesvirus members themselves. This lack of overall homology made it difficult to align the area with other VP1-2s (apart from alignment of the related herpesvirus saimiri [HVS] and herpesvirus ateles [HVA] sequences with each other [see also Fig. 4]).
FIG 4.

Comparison of the basic motif in gamma-2-herpesviruses. (a) Schematic representation of the HHV-8 VP1-2, with each vertical red line indicating a basic arginine or lysine in the region downstream of the HHV-8 USP domain. This is expanded below, where the boundaries of the various regions tested are indicated. The only positive region for NLS function, termed R8, is further expanded, showing the sequence comparison with other gamma-2-herpesvirus VP1-2s. (b) Comparison of HHV-8 R1, R5, R8, and R9 for NLS function in the context of β-galactosidase.
Examination of transferable NLS function in VP1-2 homologues.
We first examined the ability of the broader R1s to confer nuclear localization upon a heterologous protein. To examine this we inserted the R1s (approximately 75 residues in total [Fig. 2a, bracketed by arrows, top]) into the open reading frame for the large protein β-galactosidase. We initially examined NLS transfer in the context of green fluorescent protein (GFP), but, consistent with previous results of others, we have found that GFP can readily diffuse into the nucleus and localization analyses can be complicated by signals conferring retention in the nucleus rather than nuclear import per se. Generally, while results in both systems were consistent, more interpretable results were obtained by examining localization with β-galactosidase. Thus, while the parent β-galactosidase showed virtually no nuclear accumulation, the R1 motif from HSV exhibited almost uniform nuclear accumulation in all cells positive for expression, an example of which is shown in Fig. 2c. Similarly the R1s from VZV, HCMV, and EBV imparted efficient nuclear localization to the test protein, with little difference discernible in relative efficiency compared to that of the HSV region (Fig. 2d). Measurement of comparative efficiency was performed as described in Materials and Methods. The exception to this was the basic region from HHV-8, which did not impart any significant nuclear localization above background (Fig. 2d).
Since we previously showed that R4 from HSV was sufficient to promote nuclear import, we anticipated that this would be the case with other alphaherpesvirus homologues and tested this with the R4 from the VZV VP1-2. Surprisingly, while as previously shown, HSV R4 acted as an efficient NLS, the precisely equivalent region from VZV (Fig. 2a, overline) was unable to act as an independent NLS (Fig. 2d). We also tested the core basic regions from HCMV, EBV, and HHV-8 (Fig. 2a, bracketed by closed circles). Consistent with the results on the broader R1s, the central R4s from HCMV and EBV also functioned as efficient NLSs, while the R4 from HHV-8 showed no activity.
The lack of activity of the R4 from VZV was unexpected considering the high degree of homology with the HSV R4. However, differences were present (Fig. 3a), and two alterations in particular were noteworthy, i.e., P427→R and K428→R (HSV→VZV). Although this change actually increased the overall basic character of VZV R4, it was possible that either or both changes resulted in failure of the VZV region to act as a monopartite NLS. We tested each residue individually and observed that while HSV P427→R had no detrimental effect on activity, K428→R essentially abolished HSV NLS function. Consistent with this, we found in reciprocal experiments that reverting the VZV residues to those of HSV, either doubly in VZV RR331/2→PK or singly in VZV.R332→K, restored effective NLS function to this first basic section in VZV (Fig. 3b). These results explained the failure of VZV R4 to function. Moreover, considering that the larger VZV R1 was functional, the results indicate that residues outside R4 were required. In VZV there are no upstream basic residues, and the simplest interpretation is that the second downstream basic area (Fig. 2a, R5) was necessary for activity.
FIG 3.

Comparison of the HSV and VZV NLS motifs. (a) Detailed sequence comparison of the alphaherpesvirus R4 regions, highlighting the strong conservation of the region but also indicating differences at proline 427 and lysine 428 (asterisks), which are changed to arginines in VZV. (b) NLS function of variants of the HSV or VZV R4 after insertion into β-galactosidase as discussed in the text. (c) Conversion of R332 of VZV to the K of HSV is sufficient to convert the motif into a strong NLS.
This interpretation is also consistent with additional results where the K428R mutation, which essentially abolished HSV NLS function when assayed in the shorter R4, had only a minor effect when tested in the setting of the broader R1 (data not shown; see also Fig. 6). Taken together, the results indicate that within R1, both basic determinants contribute to overall function. In HSV the upstream R4 is capable of functioning as an independent monopartite NLS, but activity can be contributed to by the second basic patch in R5, while in VZV, due to the single K→R change, this is not the case, and it has an obligatory bipartite NLS involving both regions.
Finally we wished to resolve the failure of the basic R1 in HHV-8 to act as an NLS. Our initial general proposition, considering the overall organizational similarity and enrichment of basic residues in an otherwise variable linker region, had been that all VP1-2s would contain an NLS in this area (Fig. 4a, basic enrichment is indicated by red bars). The inactivity of the HHV-8 region argued against this general proposition. However, further inspection of the HHV-8 sequence revealed a second area with a patch of basic residues (Fig. 4a. R8, with the sequence expanded below). Although this was not predicted to be an NLS, we tested the region in transfer assays similar to those described above. While neither R1 nor, as expected, subregion R4 or R5 (Fig. 2 and 4b) exhibited any NLS activity, in contrast R8 exhibited very potent activity, and consistent with this, the subregion R9 exhibited good activity, though it was slightly weaker (Fig. 4b). This result was somewhat surprising since R9 was no more basic in character than, e.g., R4 or R5, reflecting some specificity in requirement for NLS function. Nevertheless the data taken together indicate that all the linker regions tested indeed contained NLS activity.
We undertook two final experiments to examine the nature of the NLS within the HSV-1 R1 region. First, we substituted the N-terminal basic cluster for a heterologous cellular monopartite NLS from the protein myopodin, which has been shown to function in a heterologous context to import test proteins (37). Second, we examined the involvement of the downstream basic region R5. Although R5 does not function as an independent NLS itself, the results showing function of R1 of VZV but not R4 together with those on substitution of HSV K428 each indicated a role for the R5 region. We examined this by deletion of R5 or by substituting the basic residues within it. First, the results demonstrated that the R1 with insertion of the cellular myopodin NLS functioned as an efficient NLS in the context of β-galactosidase (Fig. 5). Second, deletion of R5 or substitution the basic residues within it (data not shown) had no significant effect on NLS function. We could discern no significant difference in efficiency between the complete HSV R1 and either of these variants (Fig. 5). However, as shown below, we obtained somewhat different results when assaying NLS function in the context of VP1-2 itself.
FIG 5.
Insertion of the cellular NLS from myopodin in place of R4 and analysis of R5 in HSV. (a) Schematic representation of the test regions wherein a confirmed cellular monopartite NLS from the protein myopodin was inserted in place of the core R4 NLS from HSV. This variant R1 region was termed R1.HSV.Myo. Arrows indicate the extent of the residues swapped out, which included part of the P/T/S-rich linker, with the resulting overall R1 10 residues shorter. In another test construct, the whole of R5 from residue 448 to 475 was deleted in R1.HSV.Δ448. (b) Test of NLS activity in the context of β-galactosidase, showing efficient function of the myopodin chimeric region and that the entire R5 could be deleted without significant impact on NLS activity. The latter result is consistent with data shown here and previously (32) that in HSV, R4 is sufficient for NLS activity.
Function of NLS determinants in the context of HSV VP1-2.
Prior to analysis of the ability of NLS variants and the regions from VP1-2 homologues to rescue the defect of K.VP1-2ΔNLS in virus replication assays, we first wished to expand our analysis of the NLS by examining activity in the context of VP1-2 itself, in addition to analysis in the heterologous β-galactosidase setting.
Full-length VP1-2 exhibits a complex localization profile, with the majority of the population present in the cytoplasm but with distinct subpopulations in the nucleus (30, 31, 33, 36, 38). The extreme C terminus is partly responsible for this and promotes retention of the protein in the cytoplasm (30, 31). To simplify study of VP1-2 NLS function, we tested variants in the context of the large N-terminal segment (approximately 1,900 residues) (Fig. 6a, NT6). This large peptide is imported into the nucleus, dependent upon a functional NLS within R1 (35, 36). For the current studies, localization was scored in one of three categories as shown in Fig. 6b: 1, exclusively or highly enriched nuclear; 2, approximately equal nuclear and cytoplasmic; and 3, exclusively or enriched cytoplasmic. NT6 typically scored >70% in category 1 or 2 and was deemed efficient. NT6ΔNLS typically scored none in category 1 and >70% in category 2 or 3 and was deemed to show no nuclear enrichment. NT6 variants could then be classed as efficient as the parent HSV (i.e., >70% category 1 or 2) or less efficient, (i.e., above background of NT6ΔNLS but <70% category 1 or 2). The results showing typical fields for each construct are shown in Fig. 6c. While deletion of 7 amino acids of the core NLS essentially abolished selective nuclear localization (Fig. 6c), each of the chimeras containing the corresponding regions from VZV, HCMV, EBV, and HHV-8 restored nuclear localization (Fig. 6c). As with the assays in the setting of β-galactosidase, the positionally corresponding region of HHV-8, R1, exhibited no activity above background, while the adjacent R8 functioned as efficiently as the homologous HSV R1. Consistent with the results in the β-galactosidase setting, each of the regions from HCMV, EBV, and HHV-8 (R8) functioned as efficiently as the homologous region from HSV, typically with >70% of cells showing exclusive or enriched nuclear localization. While VZV R1 restored nuclear localization, it was not as efficient as the parental NT6 and frequently showed significant cytosolic as well as nuclear localization (Fig. 6c). That this lower efficiency was likely explained by the single K428→R change between HSV and VZV was confirmed by testing this single substitution now in the context of NT6. Mutation K428R resulted in an overall reduction in efficiency with significant levels of the protein still being detected in the cytoplasm, though clearly the mutant exhibited activity above the background level of the ΔNLS (Fig. 6d). The critical importance of K428 was also further highlighted since replacement of this residue by a nonbasic amino acid, alanine, reduced nuclear import to essentially background levels notwithstanding the presence of the remainder of the region and adjacent basic R5 (Fig. 6d).
On the other hand, R5 also contributed to activity, with deletion of the region (NT6ΔR5 448 to 475) or replacement of each basic residue by alanine (NT6MutR5 448 to 475) having a detrimental effect on import efficiency (Fig. 6d). Note that each of these variants retained activity above the background seen for the ΔNLS mutant. Taken together, these results are consistent with those obtained above and indicate that the core NLS within R4 is essential for activity and that within this region the K428 is critical. While NLS activity will tolerate a K→R substitution, it will not tolerate a K→A change, even when the remainder of the overall basic region, including R5, is present. Equally, however, R4 does not account for total activity, with R5, although not showing independent function, nevertheless augmenting overall activity. A detailed mutational analysis of the EBV, HCMV, and HHV-8 regions is beyond the scope of this work. However, these regions appear to function as efficiently as the entire HSV R1, implying that while organizationally distinct, the combined activity of R4-linker-R5 is encompassed within the more compact motif from these viruses.
Interaction of VP1-2 variants with pUL37.
Prior to examining the ability of the various VP1-2 variants and mutants to rescue the defect in the virus K.VP1-2ΔNLS, we first examined one other function which has been reported to involve determinants close to the NLS region. Both in PRV and in HSV, VP1-2 interacts with pUL37 (4, 12, 26, 27), and in HSV a minimal region has been refined to within residues 512 to 567 (27). Although this is not within the region swapped or mutated, it could be that the variants within R1 (positions 400 to 475) may have affected pUL37 interaction. To examine this, we expressed the epitope-tagged NT6 variants without or with HA-tagged pUL37, immunoprecipitated the NT6 species (anti-V5 tag), and assayed by Western blotting for coprecipitation of pUL37 (Fig. 7). In control experiments, pUL37 was not precipitated in the absence of NT6 (Fig. 7a, lane 2) but was readily detected in the presence of NT6 (lane 4). Also, when the two species were coexpressed but precipitation assays were performed without primary antibody, neither NT6 nor pUL37 was detected (lane 5), confirming that any detection of pUL37 was not due to nonspecific precipitation or retention on the beads. All of the variants containing the chimeric NLS regions interacted with pUL37 with equal efficiency (Fig. 7a, lanes 4 to 10). In similar experiments none of the mutations or variations in the HSV R1 affected pUL37 interaction (Fig. 7b). These results are consistent with data indicating that the core region of pUL37 interactions is outside the VP1-2 NLS R1 (27) and indicate that neither the changes due to swapping R1s from the homologues nor the mutations within HSV R1 affected pUL37 binding.
FIG 7.

The NLS region is not required for VP1-2 interaction with pUL37. (a) NT6 variants as indicated were transfected with HA-tagged pUL37 and immunoprecipitated by virtue of the epitope tag (V5) on the NT6 construct. Controls in lanes 1, 2, and 5 are as discussed in the text. Each of the variants interacted with pUL37, with no discernible difference in efficiency (lanes 6 to 10). (b) As for panel a, with results indicating that none of the substitution or deletion variants affected VP1-2-pUL37 interaction (c.f. lane 4 with lanes 6 to 11).
VP1-2 NLSs rescue the entry defect of K.VP1-2ΔNLS.
To examine physiological function of the VP1-2 chimera and variants, we cotransfected DNA from the K.VP1-2ΔNLS mutant virus together with DNA from the series of NT6 variants described above to examine rescue of viable virus able to replicate in noncomplementing RSC cells (Fig. 8a).
FIG 8.
Rescue of viable virus using VP1-2 with heterologous NLS regions. (a) Schematic showing the experimental protocol wherein purified genomic DNA from the mutant virus KOS.VP1-2ΔNLS was cotransfected with plasmid NT6 DNA containing the N-terminal region of VP1-2 encompassing the R1 regions as indicated. (b) RSC cells were transfected as described in Materials and Methods and plates stained 3 days later for CPE and plaque formation. Representative results from several independent analyses are shown. (c) Quantitative analysis by titration on RSC cells of the yield of viable virus from transfection assays, with results discussed in the text. The asterisk over NT6.Mut5R indicates that recovered viruses were wt revertants as discussed in the text.
In control experiments, transfection of K.VP1-2ΔNLS DNA without any rescuing VP1-2 DNA yielded little if any viable virus. This reflects the tight phenotype of the mutant and very low levels of background wild-type (wt) virus that might result from recombination during mutant stock propagation in complementing lines (Fig. 8b, plate 1). Conversely, when the mutant virus DNA was cotransfected with the wt NT6 N-terminal fragment, abundant virus replication was observed (Fig. 8b, plate 2), while cotransfection with the same fragment lacking the 7 residues around the core NLS of R4 effectively abolished rescue and reduced the production of replication-competent virus to the background level (Fig. 8b, plate 3).
For the chimeric NT6 fragments encompassing the R1s from VZV, HCMV, and EBV, successful rescue was observed in all cases, though with lower efficiencies than with the wt HSV fragment (Fig. 8b, plates 4 to 6). Yields of virus capable of replicating on noncomplementing cells are quantitated in Fig. 8c. Perhaps not surprisingly, the variant containing the VZV R1, i.e., the nearest in sequence homology to HSV, initially gave the better yields, though these were 50- to 80-fold lower than those from the parental fragment, and yields from the EBV and HCMV chimeras were a further 5- to 10-fold decreased. Individual virus plaques from these experiments were isolated and sequenced across the VP1-2 N terminus, demonstrating them to be recombinant viruses containing the expected chimeric VP1-2s incorporating the R1 from the appropriate homologue (see below). However, we did not obtain viable virus from several attempts using the NT6 containing the HHV-8 NLS region, despite the fact that the corresponding fusion protein exhibited good nuclear localization (Fig. 8b, plate 7, and c).
We also tested the mutations in the key residue K428. While K428R resulted in reasonably efficient virus production, with a modest reduction from the parental virus (but better than the chimera with the VZV region), in contrast the single K428A change virtually eliminated production of viable virus (Fig. 8c). The latter result is consistent with the assay for nuclear import in the context of VP1-2, where K428A abolished VP1-2 nuclear accumulation (Fig. 6c).
Finally, we also analyzed the effect of mutation of the second basic cluster in R5. Deletion of R5 essentially abolished virus replication. The same deletion in the context of VP1-2 nuclear import had a detrimental effect, though the overall efficiency, while reduced, was approximately equivalent to that of the VZV chimera, which did yield replication-competent virus (see Discussion). Mutation of the basic residues in R5 to alanines yielded a very low level of virus (Fig. 8c), but upon isolation and sequencing of several independent purified isolates, these were all found to be wt recombinant virus. No viable virus with the R5 region mutated was detected, and we interpret this result to indicate that not only would deletion of R5 abolish replicative capacity, replacement of the basic residues essentially has the same effect. Taken together, these data are consistent and indicate the likely essential nature of R5 for replication, even though in protein nuclear import assays, diminished but distinct activity was observed without it. (The reason for obtaining a small number of revertants with the R5 mutation is presumably because with both R4 and R5 essential, recombination between mutant virus genome [R4 negative, R5 positive] and mutant fragment [R4 positive, R5 negative] in the very short region between R4 and R5 would be easier with the R5 sequence still present than with R5 deleted.)
Growth characteristics of viruses with heterologous NLS motifs.
To complete the analysis, individual isolates of rescued viruses were plaque purified and grown on RSC cells. The genomic regions of purified stocks were also sequenced, confirming replacement of the HSV R1 from K.VP1-2ΔNLS with the corresponding R1 from each of VZV, HCMV, and EBV. The resultant viruses were named K.VP1-2ΔNLS.R1VZV, etc., to indicate the parent virus source and the replacement of the 75-amino-acid R1 but for ease of reference here are referred to as R1.VZV, etc. Growth characteristics were then examined under multiround or single-step conditions in several different cell types (Fig. 9 and data not shown). Overall, in RSC cells (used for routine propagation and titration), each of the viruses grew with modestly reduced overall efficiency. Final yields were reduced by 5- to 10-fold, and the kinetics of virus production were slightly delayed, compared to those for either the parental KOS virus or the revertant of the mutant virus containing the homologous HSV sequence, K.VP1-2ΔNLS.R (Fig. 9a). This delay in RSC cells was not significantly different in the complementing line, RSCUL36 (Fig. 9b). Moreover, no significant delay was observed in another cell type, retinal pigment epithelium (RPE) cells, with each virus producing similar overall yields with similar kinetics (Fig. 9c). This notwithstanding, we did observe a highly significant difference in replication in Vero cells. Each of the three viruses, i.e., R1.CMV, R1.EBV, and R1.VZV exhibited substantially reduced kinetics of infection and reduced yields, with the most debilitated virus being the R1.VZV variant, which replicated slowly and poorly with overall yields reduced by over 3,000-fold (Fig. 9d). Similarly, in single-cycle growth analysis (data not shown), R1.VZV yields were significantly reduced compared to those of the revertant virus, though less so (10- to 20-fold) than in multistep analysis.
FIG 9.
Rescue and replication of viruses containing heterologous NLS motifs. Purified virus isolates with sequence-confirmed insertion of variant NLS motifs (as indicated in the key) were inoculated at low MOI (0.002 PFU/cell) into each of several lines (RSC, RSCUL36, RPE, and Vero), and total yields on infectious progeny were assayed at various times thereafter on RSCUL36 cells. While in RPE and RSC cells each of the viruses grew normally (RPE cells) or with at most a modest reduction in yield (RSC cells), in Vero cells growth of the chimeric viruses, in particular that containing the VZV R1 NLS region, was significantly reduced.
To analyze this further, we examined the most debilitated virus, R1.VZV, and the revertant virus containing the homologous HSV sequence in plaque formation assays in both RSC and Vero cells, using immunoperoxidase to examine infection and spread. Typical results are shown in Fig. 10. For the revertant virus, plaque formation in RSC cells was robust, with typical sizes somewhat larger than those in Vero cells, though frequently for RSC cells exhibiting more turbid interiors with less overt detachment from the culture dish (Fig. 10, panel i). For R1.VZV in RSC cells, plaques were reduced in size (30 to 50% of the diameter of the control) but were readily detected (Fig. 10, panel ii). The data are consistent with replication in the multistep growth curve showing delayed and modestly reduced yields. However, in Vero cells, in contrast to the readily observed plaques observed for the revertant virus (Fig. 10, panel iii), R1.VZV formed extremely small clusters involving limited cell numbers (Fig. 10, panel iv, arrowheads). Indeed, the contrast was such that these tiny foci could be observed only by immunoperoxidase staining and were not easily detected by phase observation or general cell staining such as with crystal violet. This pronounced defect was complemented in the HS30 cell line, a derivative of Vero cells containing the wt VP1-2 gene, wherein no significant difference could be observed between the revertant and the R1.VZV mutant (Fig. 10, panel vi). Considering that R1.VZV infects and propagates in RPE and RSC cells with at most a modest deficiency, this result indicates that some cell type-specific restriction may operate, which reveals differences in the efficiency or function of the NLS region (see below).
FIG 10.
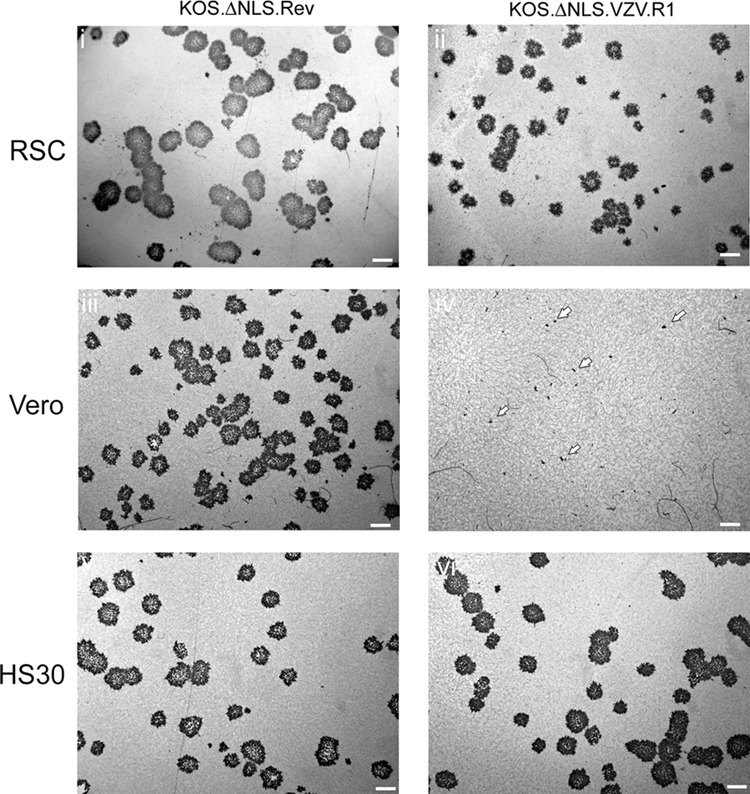
Pronounced defect in spread of viruses with a variant NLS motif in Vero cells. A comparison of plaque formation by K.VP1-2ΔNLS.R1VZV and the revertant virus containing the homologous HSV sequence is shown. Plaques were fixed and examined by immunoperoxidase staining as described in Materials and Methods. Extremely small clusters of infection in Vero cells for K.VP1-2ΔNLS.R1VZV are indicated by outlined arrowheads (panel iv). Scale bars equal 1 mm.
DISCUSSION
We previously showed that the HSV inner tegument protein VP1-2 contains an N-terminal basic motif that functions as an efficient nuclear localization signal and is critical for capsid routing to the nuclear pore during entry (10, 32). The motif is not critical for the known assembly functions of VP1-2, indicating that if VP1-2 is recruited onto capsids or is otherwise important in the nucleus, the NLS is unlikely to play a critical role in that function. Here we undertook a comparative evaluation of the corresponding regions of VP1-2 homologues from human herpesviruses from each major family, which reveals similarities and differences between them.
Comparative organization of NLS determinants.
In the alphaherpesviruses, the NLS is contained within a linker region which itself shows extremely little conservation in sequence or length (apart from the central basic determinant within it). This basic determinant extends for approximately 40 residues (the longest of the three classes of VP1-2s) and is divided into an N-terminal highly conserved motif (R4 for reference), a C-terminal basic region with somewhat less conservation (R5), and a linker region between the two that is rich in P, S, and T amino acids. As discussed in detail in Results, the betaherpesvirus and gammaherpesvirus homologues show the same overall organizational theme of a basic region between the N-terminal USP domain and the body of the protein, but they also exhibit distinct family-specific differences. These differences in organization within an overall conserved framework between the USP and body of the protein may reflect functional virus specific variations on a conserved theme (see below). Nonetheless, each of the motifs from HSV, VZV, HCMV, EBV, and HHV-8 acted as a potent NLS when analyzed in the large test protein β-galactosidase and when inserted in place of the corresponding region in HSV VP1-2.
Requirements for NLS function and its relationship to virus replication.
The direct comparison of the HSV and VZV motifs revealed aspects involved in NLS function and virus replication which we had not previously appreciated. Thus, having shown the HSV core R4 region was sufficient for NLS function in transfer assays, we anticipated that this would be the case for the highly conserved VZV R4 motif. This was not the case. The explanation was the presence of a lysine in HSV at the start of the R4 basic cluster versus an arginine in the VZV cluster. Although the definition of NLS motifs has now expanded, with more flexibility than first appreciated (34, 39), nonetheless classical monopartite NLS motifs have the consensus K[K/R]x[K/R], and a very strong requirement for the first position (bold, P1) to be a lysine. Our results are entirely consistent with this requirement. A single change at this equivalent lysine was sufficient to abrogate NLS function in HSV R4, and likewise, restoring the arginine to a lysine was sufficient to convert VZV R4 to an efficient NLS. However, since we also show that the extended region R1 did function in VZV, the simplest conclusion is that the downstream region R5 plays an important role in NLS function in VZV. A detailed analysis of which individual residues are important in R5 is beyond the scope of the current work and is not critical for current conclusions. However, it is intriguing that the R5 basic cluster in VZV is located some 30 residues away from the upstream R4, a spacing which is exceptional in NLS motifs and distinct from the organization in beta- and gammaherpesviruses (see also below). The implications of this analysis are that at least in VZV the motif obligatorily consists of a bipartite NLS, while in HSV the motif could function as a monopartite or a bipartite NLS. In relation to this point, we find that while HSV R4 is sufficient for NLS activity in the protein transfer assays in the context of VP1-2, R5 deletion or mutation did diminish the efficiency of nuclear localization. A reasonable conclusion consistent with all the data is that in HSV too, efficient nuclear localization of VP1-2 involves the activity of a bipartite NLS with functional requirements in both R4 and R5. It is noteworthy that replacement of the single lysine 428 by alanine (instead of arginine) was sufficient to almost completely abrogate VP1-2 nuclear import and to abolish virus replication, notwithstanding the presence of numerous basic residues in the surrounding R4 and R5 regions. We note also that this result is consistent with recent data indicating the essential requirement for lysine 287, the corresponding lysine in HCMV VP1-2, both for NLS function and for HCMV replication (40). Although speculative, this pronounced sensitivity of K428 may be open to exploitation in the design of classes of inhibitors which might interfere with early infection, whether experimentally to study infection or as a therapeutic agent.
While there was generally good agreement between the two different assays for nuclear import, i.e., in the context of a transferable NLS linking to β-galactosidase or in VP1-2, and in turn between these assays and virus replication, there were exceptions to this general consistency which warrant discussion. The NLS transfer assay, even though in the context of the large β-galactosidase target protein, exhibited less stringent requirements than the assay in the context of VP1-2. The involvement of R5 in NLS activity in the context of VP1-2 is also consistent with our results on mutant virus rescue, where R5 deletion or mutation completely abrogated production of viable virus. We note that while our conclusions are consistent with recent data on HCMV (40), confirming our prediction that the region would function as an NLS and be required for replication (10), there are differences for which our current results now provide a satisfactory explanation. While the HCMV motif was shown to be a bipartite NLS, involving both the N-terminal and C-terminal basic clusters, the HSV motif could not be readily swapped into HCMV VP1-2. Thus, substitution of the HCMV motif with the HSV motif resulted in a virus that had a major growth defect, with on the order of a 1,000-fold reduction in yield (40). This is likely due to the fact that the HSV motif used in that study encompassed only R4, which as we now show is not sufficient in VP1-2 itself. We also show directly that this motif is required for HSV replication. Moreover, we now also have performed the reciprocal experiment showing that the HCMV motif can be placed in the HSV VP1-2, resulting in viable virus with at most a modest growth defect despite containing 75 HCMV residues encompassing the NLS and surrounding nonconserved residues.
The requirement for R5 in the native context versus its dispensability in nuclear transport of β-galactosidase is not unusual, and determinants involved in activity in the native context frequently extend beyond or differ from those required in heterologous transfer assays during analysis of nuclear import (34, 41, 42). There could be several distinct but not mutually exclusive explanations. It could be envisioned that presentation of a core NLS (e.g., R4) in this internal region in VP1-2 could require R5, without the latter being an integral part of the NLS. However, since even in the context of transfer to the N terminus of β-galactosidase the VZV R4 did not function independently, we favor an explanation where R5 is an integral part of the NLS in VP1-2, required for efficient interaction with the nuclear import machinery.
The general conclusion, therefore, is that the VP1-2s can be classed into three general types. The first class, within the alphaherpesviruses, encompasses a particular variant of the bipartite motif with an exceptionally long linker. The second class, in the beta- and gamma-1-herpesvirus VP1-2s, appear to be more classical bipartite NLS motifs. The third class is the gamma-2-herpesvirus motifs, which fit into neither of the above schemes. Considering that the motifs are all functional for nuclear import and, with the exception of HHV-8, supported HSV replication, we currently do not have an explanation for what appears to be a distinct subgrouping. However, several features warrant speculation, and the cell type-specific differences discussed below may reflect such functional subgrouping.
For the alphaherpesvirus class, the N-terminal basic cluster has a clear consecutive run, which could for some species (HSV, PRV, and equine herpesvirus 1 [EHV-1]) allow it to act as a monopartite motif. The linker regions of classical bipartite motifs are generally limited to approximately 10 to 12 residues. Exceptionally long linkers of 20 of more residues have been found and sometimes experimentally verified (e.g., that from the yeast Ty1 integrase [43] or SMAD4 [44]), but these exceptions prove the rule that generally the linkers of bipartite NLS motifs are 8 to 12 residues. Indeed, systematically increasing the length to 15 or 20 residues can have a significant impact on classical bipartite motif activity (39). However, all the alphaherpesvirus VP1-2 NLS motifs retain this long linker structure, as well as a defined P/S/T-rich element. It is possible that this structure and long linker region may contribute to NLS function at least under certain circumstances. Bipartite NLS motifs are thought to generally interact with importin α, which has two binding sites for NLS motifs, a major binding pocket and a minor binding pocket (45). Considering that the linker region between the upstream and downstream clusters can make contacts with importin α and contribute to binding, it could be that differences in the linkers could contribute to function. For example, since it is known that neurons can express distinct repertoires of nuclear import machinery, it is possible that the distinct organization of the NLS in alphaherpesviruses, while not being critical for replication in culture, is important for some aspect of transport, import, and replication in vivo (46, 47).
NLS function in protein import does not always correlate with virus replication.
We currently do not understand why insertion of the HHV-8 NLS into VP1-2 did not restore virus replication despite the HHV-8 NLS acting as an efficient NLS either in the context of transfer to β-galactosidase or in VP1-2 itself. However, the sequence of the HHV-8 motif and surrounding residues was the most distant and indeed, to keep parity with other inserts and spacing, included a region highly enriched in acidic residues (Fig. 4). It could be that, despite restoring NLS function in the context of soluble protein import, this motif interfered with function in the context of the HSV capsid, whether in preventing virus assembly in the first place or in entry functions. Thus, perhaps the HHV-8 motif acted as a dominant negative domain in the context of the virus. However, trimming the HHV-8 insertion, e.g., to just the core R9 is unlikely to work, since we already have shown that, e.g., HSV R4 alone, a single upstream basic motif, does not function in virus replication. We favor an explanation of interference between the more distant related regions and in future work plan to test this proposal with additional variants.
An additional unexpected observation in this work was the cell type difference in replication of chimeras with the heterologous NLS motifs. While all the chimeras replicated and with at most a modest deficiency in RSC cells or RPE cells, in Vero cells each of the variants was debilitated to a significant extent, with the greatest decrease in the virus containing the motif from VZV, the most closely related in overall sequence. It should be noted, however, that although it is the most closely related, the VZV motif was in fact the least efficient and specifically lacked the critical K in P1 that confers monopartite NLS activity to a basic consecutive run (Fig. 2 and 3). Therefore, this cell type-specific deficiency in replication correlated at least to some degree with NLS efficiency. There could be several not mutually exclusive explanations to account for a more pronounced deficiency in replication in one cell type versus another, related to the efficiency of the VP1-2 NLS. For example, it could be that in certain cells relevant import components are in more limited supply than in other cell types, and this reveals lower overall efficiency of one NLS compared to another in that particular cell type. It could also be that there are restrictions or host responses to infection that operate in one cell type versus another. In this case, it is possible that, e.g., lower efficiency or kinetics of entry then allows a competing host response to restrict infection in a cell type-specific manner. Yet other possibilities include the proposal that in certain cells there is redundancy in entry pathways, with some routes less dependent on NLS efficiency while other cells have restricted entry routes completely dependent upon the VP1-2 NLS. An example here could be an auxiliary pathway wherein cell division or nuclear envelope breakdown facilitated entry. While the precise explanation requires further analysis and could involve one or more of such possibilities, the chimeric viruses we have constructed will facilitate additional investigation into the role of the NLS and the prospect for complex early virus-host interactions where entry pathways are integrated with host structure or innate immune responses.
Future work will also examine the specificity and affinity of the different NLS motifs for different components of the now considerably expanded nuclear import machinery both in the context of the protein itself and in the context of purified capsids from each of the variant viruses we have constructed. One previous report indicated that detergent-extracted HSV virions could bind to nuclear pores in vitro (48), and this could be partially inhibited by antibody to either nucleoporins or importin β. Importin β was reported to promote capsid binding to isolated nuclei (48), but no specific virus receptors were identified for this. While it could be that these studies reflect the involvement of the NLS of VP1-2, other capsid proteins are plausible candidates. For example the abundant capsid protein VP19c also localizes to the nucleus, also possesses an NLS, and indeed is responsible for import of soluble capsid components during assembly (49–51). Moreover, it was reported that importin α, which directly interacts with bipartite NLS motifs of the type in VP1-2, did not support capsid binding and that an NLS-peptide conjugate could not compete for capsid-pore docking (52, 53). On the other hand, the VP19c NLS has been reported to interact with importin β and could be involved in the previously reported recruitment of capsid to pores. However, the VP19C NLS can be deleted and still allow virus replication and spread (51), so if it did account for importin β binding, its relevance to virus replication may be modest. In contrast the VP1-2 NLS is critical for replication (10, 40).
In conclusion, therefore, our results together with those we have previously reported showing (i) conserved organizational variations within an overall similar structure, (ii) nuclear import function of defined NLS motifs, and (iii) the ability of such motifs to restore replication to an alphaherpesvirus debilitated in nuclear import taken together indicate that the motifs from the various virus VP1-2s are likely to play similar functions in their native contexts. Equally, the lack of function of the HHV-8 motif in virus rescue despite its function in nuclear import and the profound cell type-specific defect in replication of viruses with the heterologous motifs remain to be explained. The latter results indicate the possibilities of additional potentially independent determinants within the region and also of cell type-specific restrictions either at the level of specific infectivity or due to host cell responses which may restrict infection of viruses with suboptimal early entry pathways or kinetics. Without construction of recombinant viruses with mutant or variant determinants which are demonstrably involved in replication, studies of the protein in isolation cannot be augmented with studies of the protein in the physiological context, i.e., the capsid during entry. With the construction of such variant viruses, we are now in a position to expand our work and undertake a more detailed mechanistic investigation both at the protein level and with isolated capsids and virions of replication and restriction in various settings, including in vivo pathogenesis studies.
ACKNOWLEDGMENTS
We thank Fraser Rixon and Valerie Preston for antibodies and cells and Prashant Desai for the complementing cell line HS30.
T.H. was in receipt of a Ph.D. scholarship from the Wellcome Trust.
Footnotes
Published ahead of print 26 February 2014
REFERENCES
- 1.Flint SJ, Enquist LW, Krug RM, Racaniello VR, Skalka AM. 2009. Principles of virology. ASM Press, Washington, DC [Google Scholar]
- 2.Desai PJ. 2000. A null mutation in the UL36 gene of herpes simplex virus type 1 results in accumulation of unenveloped DNA-filled capsids in the cytoplasm of infected cells. J. Virol. 74:11608–116018. 10.1128/JVI.74.24.11608-11618.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Knipe DM, Batterson W, Nosal C, Roizman B, Buchan A. 1981. Molecular genetics of herpes simplex virus. VI. Characterization of a temperature-sensitive mutant defective in the expression of all early viral gene products. J. Virol. 38:539–547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fuchs W, Klupp BG, Granzow H, Mettenleiter TC. 2004. Essential function of the pseudorabies virus UL36 gene product is independent of its interaction with the UL37 protein. J. Virol. 78:11879–11889. 10.1128/JVI.78.21.11879-11889.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee JI, Luxton GW, Smith GA. 2006. Identification of an essential domain in the herpesvirus VP1/2 tegument protein: the carboxy terminus directs incorporation into capsid assemblons. J. Virol. 80:12086–12094. 10.1128/JVI.01184-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Luxton GW, Lee JI, Haverlock-Moyns S, Schober JM, Smith GA. 2006. The pseudorabies virus VP1/2 tegument protein is required for intracellular capsid transport. J. Virol. 80:201–209. 10.1128/JVI.80.1.201-209.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Roberts AP, Abaitua F, O'Hare P, McNab D, Rixon FJ, Pasdeloup D. 2009. Differing roles of inner tegument proteins pUL36 and pUL37 during entry of herpes simplex virus type 1 (HSV-1). J. Virol. 83:105–116. 10.1128/JVI.01032-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bottcher S, Granzow H, Maresch C, Mohl B, Klupp BG, Mettenleiter TC. 2007. Identification of functional domains within the essential large tegument protein pUL36 of pseudorabies virus. J. Virol. 81:13403–13411. 10.1128/JVI.01643-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shanda SK, Wilson DW. 2008. UL36p is required for efficient transport of membrane-associated herpes simplex virus type 1 along microtubules. J. Virol. 82:7388–7394. 10.1128/JVI.00225-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Abaitua F, Hollinshead M, Bolstad M, Crump CM, O'Hare P. 2012. A nuclear localization signal in herpesvirus protein VP1-2 is essential for infection via capsid routing to the nuclear pore. J. Virol. 86:8998–9014. 10.1128/JVI.01209-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Granzow H, Klupp BG, Mettenleiter TC. 2005. Entry of pseudorabies virus: an immunogold-labeling study. J. Virol. 79:3200–3205. 10.1128/JVI.79.5.3200-3205.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Klupp BG, Fuchs W, Granzow H, Nixdorf R, Mettenleiter TC. 2002. Pseudorabies virus UL36 tegument protein physically interacts with the UL37 protein. J. Virol. 76:3065–3071. 10.1128/JVI.76.6.3065-3071.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Luxton GW, Haverlock S, Coller KE, Antinone SE, Pincetic A, Smith GA. 2005. Targeting of herpesvirus capsid transport in axons is coupled to association with specific sets of tegument proteins. Proc. Natl. Acad. Sci. U. S. A. 102:5832–5837. 10.1073/pnas.0500803102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Michael K, Klupp BG, Mettenleiter TC, Karger A. 2006. Composition of pseudorabies virus particles lacking tegument protein US3, UL47, or UL49 or envelope glycoprotein E. J. Virol. 80:1332–1339. 10.1128/JVI.80.3.1332-1339.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wolfstein A, Nagel CH, Radtke K, Dohner K, Allan VJ, Sodeik B. 2006. The inner tegument promotes herpes simplex virus capsid motility along microtubules in vitro. Traffic 7:227–237. 10.1111/j.1600-0854.2005.00379.x [DOI] [PubMed] [Google Scholar]
- 16.Newcomb WW, Brown JC. 2010. Structure and capsid association of the herpesvirus large tegument protein UL36. J. Virol. 84:9408–9414. 10.1128/JVI.00361-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Radtke K, Kieneke D, Wolfstein A, Michael K, Steffen W, Scholz T, Karger A, Sodeik B. 2010. Plus- and minus-end directed microtubule motors bind simultaneously to herpes simplex virus capsids using different inner tegument structures. PLoS pathogens 6:e1000991. 10.1371/journal.ppat.1000991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Batterson W, Roizman B. 1983. Characterization of the herpes simplex virion-associated factor responsible for the induction of alpha genes. J. Virol. 46:371–377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Abaitua F, Daikoku T, Crump CM, Bolstad M, O'Hare P. 2011. A single mutation responsible for temperature sensitive entry and assembly defects in the VP1-2 protein of HSV. J. Virol. 85:2024–2036. 10.1128/JVI.01895-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kattenhorn LM, Korbel GA, Kessler BM, Spooner E, Ploegh HL. 2005. A deubiquitinating enzyme encoded by HSV-1 belongs to a family of cysteine proteases that is conserved across the family Herpesviridae. Mol. Cell 19:547–557. 10.1016/j.molcel.2005.07.003 [DOI] [PubMed] [Google Scholar]
- 21.Schlieker C, Weihofen WA, Frijns E, Kattenhorn LM, Gaudet R, Ploegh HL. 2007. Structure of a herpesvirus-encoded cysteine protease reveals a unique class of deubiquitinating enzymes. Mol. Cell 25:677–687. 10.1016/j.molcel.2007.01.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee JI, Sollars PJ, Baver SB, Pickard GE, Leelawong M, Smith GA. 2009. A herpesvirus encoded deubiquitinase is a novel neuroinvasive determinant. PLoS Pathog. 5:e1000387. 10.1371/journal.ppat.1000387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jarosinski K, Kattenhorn L, Kaufer B, Ploegh H, Osterrieder N. 2007. A herpesvirus ubiquitin-specific protease is critical for efficient T cell lymphoma formation. Proc. Natl. Acad. Sci. U. S. A. 104:20025–20030. 10.1073/pnas.0706295104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang S, Wang K, Li J, Zheng C. 2013. Herpes simplex virus 1 ubiquitin-specific protease UL36 inhibits beta interferon production by deubiquitinating TRAF3. J. Virol. 87:11851–11860. 10.1128/JVI.01211-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ko DH, Cunningham AL, Diefenbach RJ. 2010. The major determinant for addition of tegument protein pUL48 (VP16) to capsids in herpes simplex virus type 1 is the presence of the major tegument protein pUL36 (VP1/2). J. Virol. 84:1397–1405. 10.1128/JVI.01721-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mijatov B, Cunningham AL, Diefenbach RJ. 2007. Residues F593 and E596 of HSV-1 tegument protein pUL36 (VP1/2) mediate binding of tegument protein pUL37. Virology 368:26–31. 10.1016/j.virol.2007.07.005 [DOI] [PubMed] [Google Scholar]
- 27.Vittone V, Diefenbach E, Triffett D, Douglas MW, Cunningham AL, Diefenbach RJ. 2005. Determination of interactions between tegument proteins of herpes simplex virus type 1. J. Virol. 79:9566–9571. 10.1128/JVI.79.15.9566-9571.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pasdeloup D, Blondel D, Isidro AL, Rixon FJ. 2009. Herpesvirus capsid association with the nuclear pore complex and viral DNA release involve the nucleoporin CAN/Nup214 and the capsid protein pUL25. J. Virol. 83:6610–6623. 10.1128/JVI.02655-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Coller KE, Lee JI, Ueda A, Smith GA. 2007. The capsid and tegument of the alpha herpesviruses are linked by an interaction between the UL25 and VP1-2 proteins. J. Virol. 81:11790–11797. 10.1128/JVI.01113-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zaichick SV, Bohannon KP, Hughes A, Sollars PJ, Pickard GE, Smith GA. 2013. The herpesvirus VP1/2 protein is an effector of dynein-mediated capsid transport and neuroinvasion. Cell Host Microbe 13:193–203. 10.1016/j.chom.2013.01.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schipke J, Pohlmann A, Diestel R, Binz A, Rudolph K, Nagel CH, Bauerfeind R, Sodeik B. 2012. The C terminus of the large tegument protein pUL36 contains multiple capsid binding sites that function differently during assembly and cell entry of herpes simplex virus. J. Virol. 86:3682–3700. 10.1128/JVI.06432-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Abaitua F, O'Hare P. 2008. Identification of a highly conserved, functional nuclear localization signal within the N-terminal region of herpes simplex virus type 1 VP1-2 tegument protein. J. Virol. 82:5234–5244. 10.1128/JVI.02497-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mohl BS, Bottcher S, Granzow H, Kuhn J, Klupp BG, Mettenleiter TC. 2009. Intracellular localization of the pseudorabies virus large tegument protein pUL36. J. Virol. 83:9641–9651. 10.1128/JVI.01045-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kosugi S, Hasebe M, Matsumura N, Takashima H, Miyamoto-Sato E, Tomita M, Yanagawa H. 2009. Six classes of nuclear localization signals specific to different binding grooves of importin alpha. J. Biol. Chem. 284:478–485. 10.1074/jbc.M807017200 [DOI] [PubMed] [Google Scholar]
- 35.Bolstad M, Abaitua F, Crump CM, O'Hare P. 2011. Autocatalytic activity of the ubiquitin-specific protease domain of HSV-1 VP1-2. J. Virol. 85:8738–8751. 10.1128/JVI.00798-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Abaitua F, Souto RN, Browne H, Daikoku T, O'Hare P. 2009. Characterization of the herpes simplex virus (HSV)-1 tegument protein VP1-2 during infection with the HSV temperature-sensitive mutant tsB7. J. Gen. Virol. 90:2353–2363. 10.1099/vir.0.012492-0 [DOI] [PubMed] [Google Scholar]
- 37.De Ganck A, Hubert T, Van Impe K, Geelen D, Vandekerckhove J, De Corte V, Gettemans J. 2005. A monopartite nuclear localization sequence regulates nuclear targeting of the actin binding protein myopodin. FEBS Lett. 579:6673–6680. 10.1016/j.febslet.2005.10.054 [DOI] [PubMed] [Google Scholar]
- 38.Bucks MA, O'Regan KJ, Murphy MA, Wills JW, Courtney RJ. 2007. Herpes simplex virus type 1 tegument proteins VP1/2 and UL37 are associated with intranuclear capsids. Virology 361:316–324. 10.1016/j.virol.2006.11.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lange A, McLane LM, Mills RE, Devine SE, Corbett AH. 2010. Expanding the definition of the classical bipartite nuclear localization signal. Traffic 11:311–323. 10.1111/j.1600-0854.2009.01028.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brock I, Kruger M, Mertens T, von Einem J. 2013. Nuclear targeting of human cytomegalovirus large tegument protein pUL48 is essential for viral growth. J. Virol. 87:6005–6019. 10.1128/JVI.03558-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xu D, Farmer A, Chook YM. 2010. Recognition of nuclear targeting signals by Karyopherin-beta proteins. Curr. Opin. Struct. Biol. 20:782–790. 10.1016/j.sbi.2010.09.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dingwall C, Laskey RA. 1991. Nuclear targetting sequences—a consensus? Trends Biochem. Sci. 16:585–587 [DOI] [PubMed] [Google Scholar]
- 43.Kenna MA, Brachmann CB, Devine SE, Boeke JD. 1998. Invading the yeast nucleus: a nuclear localization signal at the C terminus of Ty1 integrase is required for transposition in vivo. Mol. Cell. Biol. 18:1115–1124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Xiao Z, Latek R, Lodish HF. 2003. An extended bipartite nuclear localization signal in Smad4 is required for its nuclear import and transcriptional activity. Oncogene 22:1057–1069. 10.1038/sj.onc.1206212 [DOI] [PubMed] [Google Scholar]
- 45.Chang CW, Counago RM, Williams SJ, Boden M, Kobe B. 2013. Distinctive conformation of minor site-specific nuclear localization signals bound to importin-alpha. Traffic 14:1144–1154. 10.1111/tra.12098 [DOI] [PubMed] [Google Scholar]
- 46.Hanz S, Perlson E, Willis D, Zheng JQ, Massarwa R, Huerta JJ, Koltzenburg M, Kohler M, van Minnen J, Twiss JL, Fainzilber M. 2003. Axoplasmic importins enable retrograde injury signaling in lesioned nerve. Neuron 40:1095–1104. 10.1016/S0896-6273(03)00770-0 [DOI] [PubMed] [Google Scholar]
- 47.Perry RB, Fainzilber M. 2009. Nuclear transport factors in neuronal function. Semin. Cell Dev. Biol. 20:600–606. 10.1016/j.semcdb.2009.04.014 [DOI] [PubMed] [Google Scholar]
- 48.Ojala PM, Sodeik B, Ebersold MW, Kutay U, Helenius A. 2000. Herpes simplex virus type 1 entry into host cells: reconstitution of capsid binding and uncoating at the nuclear pore complex in vitro. Mol. Cell. Biol. 20:4922–4931. 10.1128/MCB.20.13.4922-4931.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rixon FJ, Addison C, McGregor A, Macnab SJ, Nicholson P, Preston VG, Tatman JD. 1996. Multiple interactions control the intracellular localization of the herpes simplex virus type 1 capsid proteins. J. Gen. Virol. 77:2251–2260. 10.1099/0022-1317-77-9-2251 [DOI] [PubMed] [Google Scholar]
- 50.Nicholson P, Addison C, Cross AM, Kennard J, Preston VG, Rixon FJ. 1994. Localization of the herpes simplex virus type 1 major capsid protein VP5 to the cell nucleus requires the abundant scaffolding protein VP22a. J. Gen. Virol. 75:1091–1099. 10.1099/0022-1317-75-5-1091 [DOI] [PubMed] [Google Scholar]
- 51.Li Y, Zhao L, Wang S, Xing J, Zheng C. 2012. Identification of a novel NLS of herpes simplex virus type 1 (HSV-1) VP19C and its nuclear localization is required for efficient production of HSV-1. J. Gen. Virol. 93:1869–1875. 10.1099/vir.0.042697-0 [DOI] [PubMed] [Google Scholar]
- 52.Goldfarb DS, Corbett AH, Mason DA, Harreman MT, Adam SA. 2004. Importin alpha: a multipurpose nuclear-transport receptor. Trends Cell Biol. 14:505–514. 10.1016/j.tcb.2004.07.016 [DOI] [PubMed] [Google Scholar]
- 53.Gorlich D, Kutay U. 1999. Transport between the cell nucleus and the cytoplasm. Annu. Rev. Cell Dev. Biol. 15:607–660. 10.1146/annurev.cellbio.15.1.607 [DOI] [PubMed] [Google Scholar]








