ABSTRACT
T-cell functional avidity is a crucial determinant for efficient pathogen clearance. Although recombinant DNA priming coupled with a vaccinia-vectored vaccine (VACV) boost has been widely used to mount robust CD8+ T-cell responses, how VACV boost shapes the properties of memory CD8+ T cells remains poorly defined. Here, we characterize the memory CD8+ T cells boosted by VACV and demonstrate that the intrinsic expression of MyD88 is critical for their high functional avidity. Independent of selection of clones with high-affinity T-cell receptor (TCR) or of enhanced proximal TCR signaling, the VACV boost significantly increased T-cell functional avidity through a decrease in the activation threshold. VACV-induced inflammatory milieu is not sufficient for this improvement, as simultaneous administration of the DNA vaccine and mock VACV had no effects on the functional avidity of memory CD8+ T cells. Furthermore, reciprocal adoptive transfer models revealed that the intrinsic MyD88 pathway is required for instructing the functional avidity of CD8+ T cells boosted by VACV. Taking these results together, the intrinsic MyD88 pathway is required for the high functional avidity of VACV-boosted CD8+ T cells independent of TCR selection or the VACV infection-induced MyD88-mediated inflammatory milieu.
IMPORTANCE Functional avidity is one of the crucial determinants of T-cell functionality. Interestingly, although it has been demonstrated that a DNA prime-VACV boost regimen elicits high levels of T-cell functional avidity, how VACV changes the low avidity of CD8+ T cells primed by DNA into higher ones in vivo is less defined. Here, we proved that the enhancement of CD8+ T cell avidity induced by VACV boost is mediated by the intrinsic MyD88 pathway but not the MyD88-mediated inflammatory milieu, which might provide prompts in vaccine design.
INTRODUCTION
A regimen of priming with recombinant DNA and boosting with a viral vector has been shown to elicit strong T-cell immune responses (1–3); thus, it is becoming one of the most prevalent vaccine strategies (4). Several regimens have been widely adopted, including the DNA prime-vaccinia vector vaccine boost and the DNA prime-adenoviral vector vaccine boost (5). These modalities are thought to combine the advantages of DNA vaccines to raise focused immune responses against the encoding immunogens in the absence of interference from vector immunogenicity and the advantages of viral vector vaccines to greatly expand the immune responses due to an increased capacity to efficiently express immunogens in vivo and to induce innate immune responses (6). The viral vectors, however, may not only enhance the immunogenicity of the vaccine but also alter the properties of the T-cell responses (7).
Several attributes of CD8+ T cells contribute to the containment of viral replication in vivo, and these include the magnitude of the response, the diversity of the T-cell receptor (TCR), and the functional avidity. Among those factors, the functional avidity, which is also referred to as antigen sensitivity (8), is one of the most critical properties for the determination of CD8+ T-cell efficacy (9, 10). In principle, the strength of the stimulus required for T-cell activation upon exposure to undefined densities of antigen is determined by the functional avidity. T cells with higher avidity are able to recognize virally infected cells with lower surface antigen densities at earlier stages of infection and to elicit stronger responses at a given antigen density compared to T cells with lower avidity (8, 11). Thus, T cells with high avidities exert rapid effector functions at low cognate antigen concentrations, effectively eliminating the virally infected cells before mass viral propagation and immune escape (12). As a result, the functional avidity is a determinant for CD8+ T cell-mediated virus control during various infections (11, 13–15).
It has been reported that a DNA prime-vaccinia virus (VACV) boost regimen could induce antigen-specific CD8+ T cells with higher functional avidity than DNA vaccination alone (16, 17). It remains to be determined how the VACV boost tunes the avidity of T cells induced by DNA prime. Recent studies indicate that pathogen-specific inflammation provides the third signal that dynamically tunes the functional avidity of CD8+ T cells in combination with TCR ligation and costimulatory signaling stimulation (18, 19). Interestingly, VACV could induce inflammatory cytokines through the MyD88-mediated pathway (20, 21), and as such, MyD88-deficient mice display a diminished antigen-specific immune response to recombinant poxvirus-based vaccines (21). Accordingly, a series of clinical trials showed that the poxvirus-based vaccines are highly immunogenic in the context of vaccination regimen of DNA priming and poxvirus-vectored vaccine boost (7, 22). To further characterize CD8+ T cells elicited by this regimen and to understand how the avidity of CD8+ T cells are tuned, we initiated the current study to define the role of the MyD88-mediated pathway in shaping the functional avidity of antigen-specific memory CD8+ T cells induced by a DNA prime-VACV boost vaccination strategy, which will be likely to enlighten the understanding of the formulation mechanism of the functional avidity in vivo.
MATERIALS AND METHODS
DNA vaccines and recombination vaccinia vaccines.
The vector pDRVI SV1.0 contains the cytomegalovirus (CMV) immediate-early promoter and a 72-bp element of simian virus 40 (SV40) enhancer and was used as the DNA vaccine vector (23). DNA vaccines were purified with Qiagen columns (EndoFree plasmid giga kit) and eluted in pyrogen-free deionized water. VACV used in this study was derived from the replication-competent Tiantan vaccinia virus strain and propagated in Vero cells (24). The HIV-1 B′/C recombinant CN54 gag or full-length chicken ovalbumin (ova) was codon optimized and inserted into vaccine vectors. Mock VACV refers to the vaccinia virus vector in the absence of immunogen gene insertion, whereas Tiantan vaccinia viruses carrying the insertion of HIV-1 Gag- and ovalbumin-encoding genes are designated VACV-gag and VACV-ova, respectively.
Animals and immunization.
This study was approved by the Institutional Animal Care and Use Committee (IACUC) of Shanghai Public Health Clinical Center. Six-week-old female BALB/c (H-2Kd) mice were primed with 3 doses of 100 μg DNA-gag 2 weeks apart and boosted with either 100 μg DNA-gag or 107 PFU VACV-gag at 2 weeks postprime (Fig. 1A). In adoptive transfer experiments, 6-week-old female C57BL/6 mice or MyD88−/− mice received 106 OT-I CD8+ T cells and were inoculated with vaccines expressing OVA as shown in Fig. 3A. All mice were immunized in the quadriceps muscle with a total volume of 100 μl of either DNA or VACV vaccine. Both OT-I and MyD88−/− mice used in this study were derived from the C57BL/6 background.
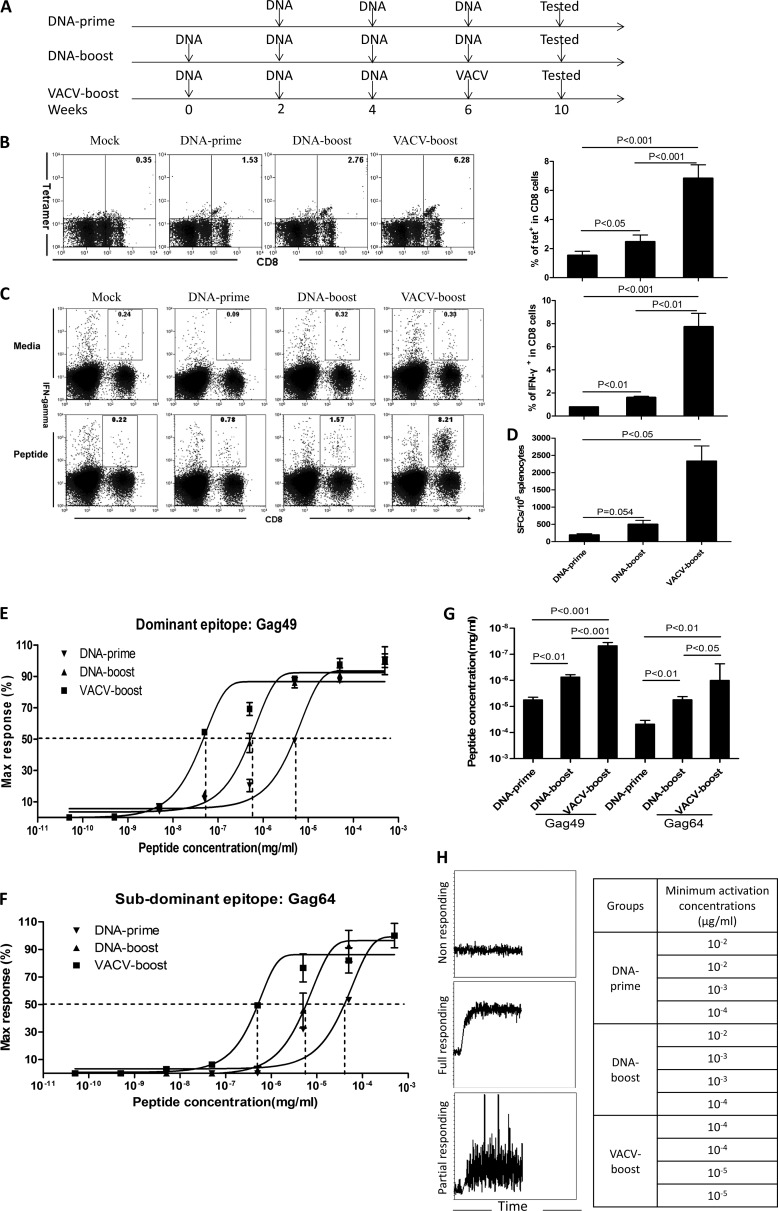
FIG 1.
VACV boosts CD8+ T-cell functional avidity by decreasing the CD8+ T-cell activation threshold. (A) Vaccination schedule. Three vaccination regimens were included in these studies. Vaccine was administered intramuscularly (i.m.) to BALB/c mice at weeks 0, 2, 4, and 6. All assays for characterization of T-cell immunity were carried out 4 weeks after the final inoculation. The vaccines express HIV-1 CN54-Gag. (B to D) Magnitude of Gag-specific CD8+ T-cell responses induced by different regimens. Representative flow-cytometric plots of tetramer (tet) staining (B) and intracellular staining (C) are shown on the left. Summary data are shown on the right. The ELISpot data are shown in panel D. SFCs were counted for 106 cells. (E to G) CD8+ T-cell functional avidity was enhanced by VACV boost. The functional avidity of a dominant epitope (E) and a subdominant epitope (F) are shown. The EC50 data are shown in panel G. (H) The T-cell activation threshold was determined as the sensitivity of CD8+ T cells to anti-CD3ζ antibody stimulation. The immediate responses after stimulation were monitored by Ca2+ influx in antigen-specific CD8+ T cells by flow cytometry for 5 min. Examples of flow-cytometric plots are on the left, and the concentrations of anti-CD3ζ antibodies for activation of Ca2+ influx in tetramer-positive CD8+ T cells from each mouse are displayed on the right. Data are representative of at least three independent experiments with at least 4 mice per group. Statistical analysis was performed by t test using SPSS16.0 software, and the P values of the comparisons between any two groups are labeled.
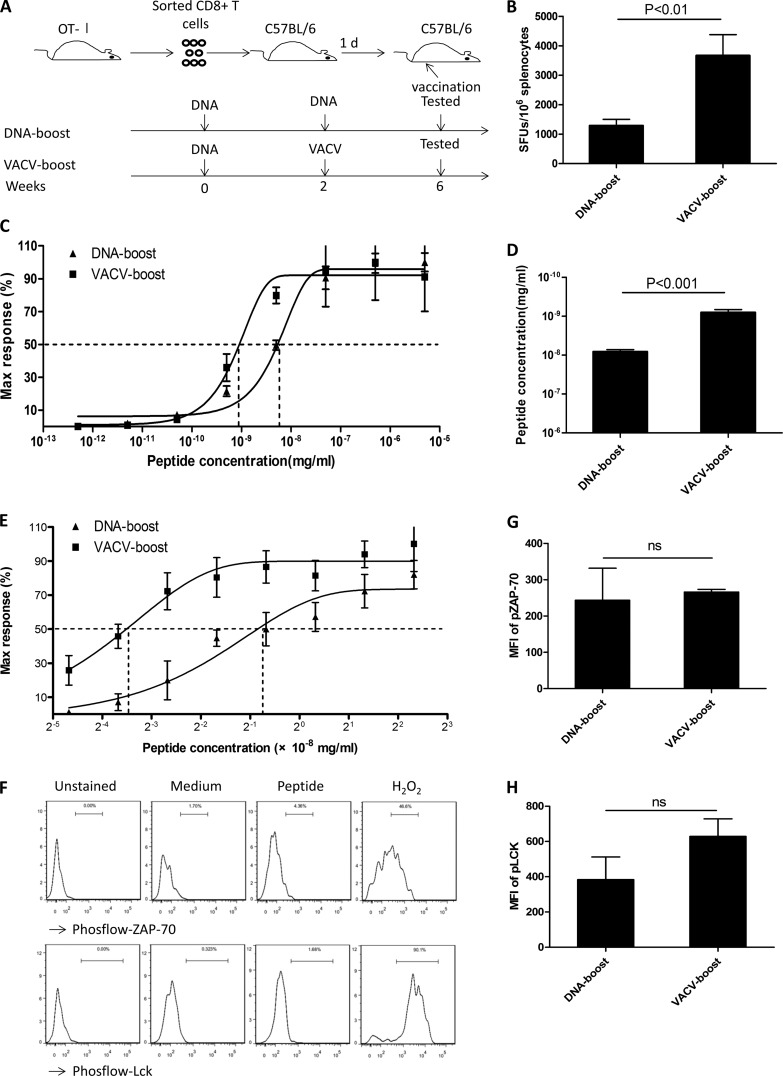
FIG 3.
Enhanced functional avidity induced by VACV boost is independent of TCR selection and enhanced TCR proximal signaling. (A) Wild-type C57BL/6 mice were adoptively transferred with purified monoclonal TCR OT-I CD8+ T cells and immunized with vaccines that expressed OVA. Two vaccination regimens were administered at weeks 0 and 2, and assays were carried out 4 weeks after the last inoculation. (B) Numbers of antigen-specific CD8+ T cells from mice receiving a DNA boost and from mice receiving a VACV boost. (C) Functional avidity as measured by response to decreasing amounts of peptide antigen was assessed in CD8+ T cells from animals receiving either the VACV boost or the DNA boost. (D) EC50 data were calculated for peptide concentrations in cells from animals receiving the VACV boost or the DNA boost. (E) The difference in the functional avidity was confirmed by using 2-fold dilutions of peptides. (F to H) The activation of proximal TCR signaling in the OVA-specific OT-I CD8+ T cells following DNA boost and VACV boost was assessed. (F) Splenocytes were stained for surface markers and stimulated with OVA peptide at 5 μg/ml, and then the levels of phospho-ZAP-70 and phospho-Lck were assessed by intracellular staining. Representative plots of gated CD8+ CD45.1+ cells stained with Phosflow antibodies are shown for the MFI of the activated forms of ZAP-70 (G) and Lck (H). Data are representative of three independent experiments with at least three mice per group. Statistical analysis was performed by t test using SPSS16.0 software, and the P values for the comparisons between any two groups are labeled. ns, not significant.
Preparation of splenocytes and isolation of T cells.
Spleens were mechanically disrupted, and splenocytes were filtered through mesh gauze. Red blood cells (RBCs) were lysed with RBC lysis buffer. CD8+ T cells were positively purified by CD8a (Ly-2) microbeads from Miltenyi. CD8+-depleted splenocytes (CD8− T cells) were enriched from the unlabeled cells using the same kit. The purity of sorted cell populations was greater than 95% as determined by flow cytometry (FACSAria; BD Biosciences).
ELISpot and functional avidity assay.
An enzyme-linked immunosorbent spot (ELISpot) assay was performed according to the gamma interferon (IFN-γ) ELISpot kit instructions (BD Biosciences). A total of 2 × 105 splenocytes were added per well. The spot-forming cells (SFCs) were counted with an immunospot reader (Champspot III; Beijing Sage Creation Science). SFCs at each stimulating concentration of peptide were normalized by maximal value (i.e., the maximum number of SFCs induced by the saturating concentration of peptides), and variable-slope sigmoid regression was used to infer the 50% effective concentration (EC50), which is the peptide concentration required to generate 50% maximal cytokine production. The functional avidity assay in this study is based on IFN-γ production. Peptides were synthesized by GL Biochem (Shanghai, China) with >95% purity. 15mers were used in ELISpot assay for HIV-1 Gag-derived peptide Gag49 (AAMQMLKDTINEEAA) and Gag64 (VPVGDIYKRWIILGL), which were reported as dominant and subdominant epitopes, respectively, in a previous report (25). 8mers used for the dominant epitope (SIINFEKL) were derived from OVA protein (26).
Inflammatory cytokine production using a cytometric bead array.
The mouse inflammatory kit was used by following the manufacturer's instructions (BD Biosciences). For assessment of inflammatory cytokine production, 2 × 105 splenocytes from naive mice were stimulated with mock VACV or UV-inactivated mock VACV at a multiplicity of infection (MOI) of 1 and DNA vaccine vector (5 μg/ml) for 20 h in a 96-well plate at 37°C. Medium and lipopolysaccharide (LPS; 100 ng/ml) were used as controls. The cytometric bead array (CBA) data were generated in graphical and tabular forms using the BD CBA analysis software.
Antibodies, surface staining, and intracellular staining.
For surface staining, cells were incubated on ice with a mixture of antibodies for 30 min. For intracellular cytokine staining (ICS), 1 h after addition of peptide, brefeldin A (eBioscience) and monensin (eBioscience) were added at 1 μg/ml and 1 μM, respectively. Cells were then incubated for an additional 5 h. The stimulated cells were stained with a cocktail of surface antibodies on ice for 30 min and then subjected to fixation and permeation with fix/perm buffer (BD Bioscience). Antibodies against intracellular cytokines were added, and cells were incubated on ice for another 30 min. For intracellular phosphorylated protein analysis, BD Phosflow protocol II for mouse splenocytes or thymocytes was used. The cells were stimulated with OVA peptide (5 μg/ml) at 37°C for 5 min, H2O2 (5 mM) was used as a positive control, and medium was used as a negative control.
The following antibodies were used in this study: CD3-peridinin chlorophyll protein (PerCP) (clone 145-2C11), CD8-Pacific Blue (PB) (clone 53-6.7), CD8-allophycocyanin (APC) (clone 53-6.7), CD44-fluorescein isothiocyanate (FITC) (clone 1M7), CD62L-phycoerythrin (PE)-Cy7 (clone MEL-14), tumor necrosis factor alpha (TNF-α)-PE-Cy7 (clone MP6-XT22), interleukin-2 (IL-2)-allophycocyanin (clone JES6-5H4), IFN-γ-PE (clone XMG1.2), Lck(pY505)-PE (clone 4/LCK-Y505), and ZAP70(pY319)/Syk(Y352)-PE-Cy7 (clone 17A/P-ZAP70), all from BD Biosciences, and CD11c-PE-Cy7 (clone N418), CD14-PerCP-Cy5.5 (clone Sa14-2), CD45.1-FITC (clone A20), CD80-FITC (clone 16-10A1), and CD86-allophycocyanin (clone GL1), all from eBioscience.
Tetramer staining.
PE-conjugated-H2Kd-AMQILKDTI tetramers were provided by the National Institutes of Health Tetramer Facility (Emory University). Splenocytes were coincubated for 30 min at 4°C with dilutions of tetramer at concentrations of 0.005 μg/ml to 2 μg/ml in combination with CD8-allophycocyanin antibody, and then the cells were washed with phosphate-buffered saline (PBS) containing 2% newborn calf serum (NCS) and fixed in 2% paraformaldehyde.
TCR Vβ usage analysis.
Splenocytes were stimulated with 4 μg/ml peptide and then were incubated at 37°C for 18 h. Brefeldin A (10 μg/ml) was added during the final 6 h of incubation. Fixed and permeated cells were stained with IFN-γ-PE, CD3-PerCP, CD8-allophycocyanin, or a panel of TCR Vβ-FITC antibodies (BD Biosciences) for 15 min.
Calcium influx.
CD8+ T cells were loaded with Fluo-3 AM (Biotium) at 37°C, washed with cold PBS, and then incubated on ice with CD8-allophycocyanin antibodies and tetramers for 30 min. The cells were then washed, resuspended into prewarmed medium at 37°C, and analyzed immediately using a FACSCalibur instrument. After establishing a baseline for 30 s, purified anti-CD3 antibody was added at various concentrations (10−5 to 1 μg/ml). Cells were quickly mixed and analyzed by flow cytometry for an additional 5 min. Since the cross-linking of TCR with tetramers could induce a Ca2+ influx, we performed the staining process on ice to minimize this effect (27).
Statistical analysis.
Statistical analysis was performed by t test using SPSS16.0 software.
GEO sequence accession number.
Sequence data determined during this work were deposited in the Gene Expression Omnibus (GEO) database under accession number GSE51849.
RESULTS
Increased antigen-specific memory CD8+ T cells with enhanced functional avidity following VACV boost.
To evaluate the effect of VACV boost on antigen-specific CD8+ T-cell responses that were primed by DNA vaccine, we immunized BALB/c mice with vaccines expressing HIV-Gag (Fig. 1A). Three doses of DNA vaccine were used for priming to induce sufficient responses, since one or two inoculations were unable to reproducibly elicit significant antigen-specific T cells for characterization in our system. A DNA boost group of mice (DNA prime-DNA boost) was included to control for the effect of inoculation time. All subsequent assays were performed with memory-phase cells that were obtained 4 weeks after the final vaccine inoculation. First, we employed tetramer staining to directly enumerate CD8+ T cells that recognize the dominant epitope of HIV-Gag (Gag49). Approximately 6% (6.75% ± 1.23%) of CD8+ T cells from animals that received the VACV boost were tetramer positive, and this percentage was significantly greater than those from animals that received DNA prime alone (1.52% ± 0.32%; P = 0.0002) and animals that received a DNA boost following the DNA priming (2.34% ± 0.53%; P = 0.0006) (Fig. 1B). IFN-γ-based ICS (Fig. 1C) and ELISpot assay (Fig. 1D) confirmed the results of tetramer staining. These data demonstrated that VACV boost mounts higher levels of antigen-specific CD8+ T cells primed by DNA vaccine compared to the levels induced by DNA priming alone or a DNA boost following DNA priming.
We then compared the functional avidity induced by different immunization regimens. We measured the peptide concentration required to generate 50% of the maximum IFN-γ SFUs using ELISpot assay. The VACV boost increased the antigen sensitivity of the responding CD8+ T cells (7.60 × 10−8 ± 1.25 × 10−8 mg/ml) approximately 100-fold compared to that of DNA prime alone (6.39 × 10−6 ± 2.01 × 10−6 mg/ml) and 10-fold compared to that of DNA boost (7.27 × 10−7 ± 9.88 × 10−8 mg/ml), and these findings were consistent with previous reports (16, 17) (Fig. 1E and G). Similar patterns were also observed for a subdominant epitope (Gag64) of HIV-Gag (Fig. 1F and G). This observation was further validated through analysis of CD8+ T-cell responses to epitopes from additional antigens, including HIV-Env and OVA, and in mice with a distinct genetic background (data not shown). Thus, the enhanced functional avidity of antigen-specific CD8+ T cells boosted by VACV is not restricted to a particular model. Collectively, our data demonstrate that antigen-specific CD8+ T cells from mice boosted with VACV were much more sensitive to antigen stimulation than those from mice treated with DNA prime alone or with a DNA boost.
We next determined the activation threshold of antigen-specific T cells by stimulation with serially diluted anti-CD3 antibodies followed immediately by fluorescence-activated cell sorting (FACS) analysis to assess Ca2+ mobilization, which represents an early activation event that could be measured by flow cytometry. Stimulation with anti-CD3 antibodies immediately initiated Ca2+ flux in epitope-specific CD8+ T cells, and the levels of Ca2+ flux did not return to baseline levels during the next ∼4 min (Fig. 1H, left). Interestingly, the epitope-specific CD8+ T cells from animals that received the VACV boost could respond to anti-CD3 antibodies at lower concentrations, from 10−5 μg/ml to 10−4 μg/ml, whereas those from animals that received DNA prime alone or a DNA boost responded to 10−4 μg/ml to 10−2 μg/ml anti-CD3 antibodies (Fig. 1H, right). Thus, epitope-specific CD8+ T cells induced by VACV boost were 10 to 100 times more sensitive than those induced by DNA prime alone or DNA boost (P < 0.05). These data support the in vitro findings that T cells with higher functional avidity require a lesser amount of anti-CD3 antibodies to initiate intracellular signal transduction than low-avidity T cells (28). Altogether, these data demonstrate that VACV boost elicits greater numbers of antigen-specific CD8+ T cells primed by DNA vaccine and increases functional avidity through markedly decreasing the activation threshold.
Characterization of VACV boost-induced antigen-specific CD8+ T cells with high functional avidity.
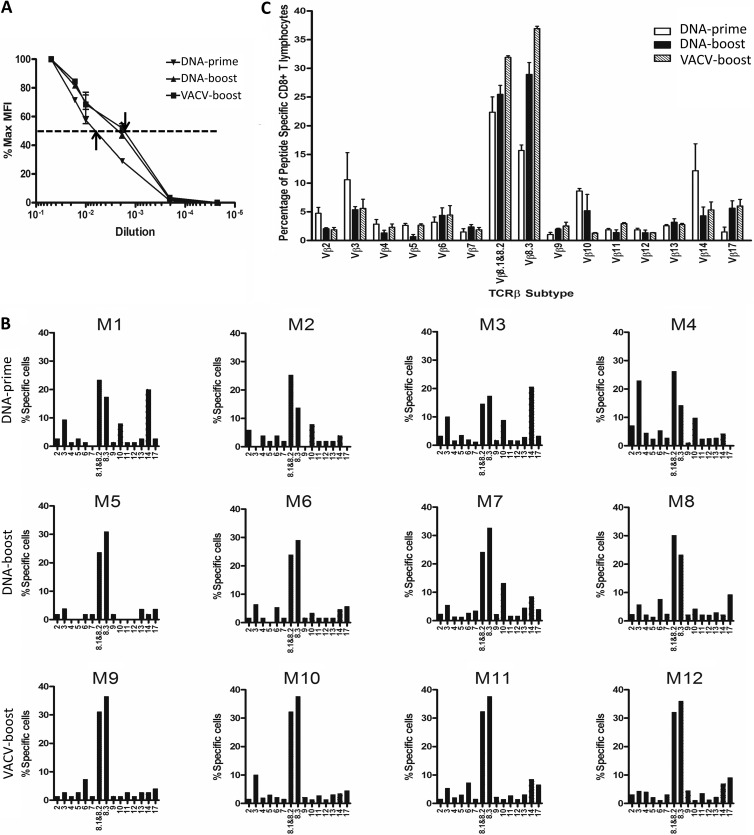
We then evaluated the TCR affinity, as defined by the binding of TCR to the peptide-major histocompatibility (MHC) complex, which is the physical determinant of functional avidity of antigen-specific CD8+ T cells, using the tetramer decay assay (9, 29). As shown in Fig. 2A, the mean fluorescence intensity (MFI) of tetramer on antigen-specific CD8+ T cells from mice that received DNA prime alone decreased much faster than from those that received a boost for all dilutions of the tetramer, whereas no significant difference was observed between cells from DNA-boosted and VACV-boosted mice. These data suggest that enhanced TCR affinity is related to the time of inoculation rather than the vaccination regimen. To determine whether the enhanced TCR affinity during the increased time of inoculation was related to clonal selection, we examined TCR Vβ utilization of the epitope-specific CD8+ T cells. Although Vβ profiles varied among individual mice even within the same group (Fig. 2B), several observations were made. First, a substantial proportion of epitope-specific CD8+ T cells bearing Vβ2, Vβ3, Vβ8.1/8.2, Vβ8.3, Vβ10, Vβ14, and Vβ17 were detected in the majority of mice, whereas other Vβs were used only occasionally (Fig. 2C). After boost with VACV or DNA, the TCR Vβ repertoire became more restricted to Vβ8.1/8.2, Vβ8.3, and Vβ17 with a concomitant profound decrease in the use of Vβ2, Vβ10, and Vβ14, suggesting that both the VACV boost and the DNA boost increase the functional avidity of CD8+ T cells via the selection of the TCR Vβ repertoire (Fig. 2C). Taken together, these data demonstrate that increased time of antigen exposure drives the selection of the TCR repertoire to a high-affinity TCR but that TCR affinity and clonal selection were not major contributors to the difference in functional avidity observed between cells from animals receiving the DNA boost and those receiving the VACV boost.
FIG 2.
Both VACV boost and DNA boost increase the functional avidity of CD8+ T cells by selecting a higher-affinity TCR Vβ repertoire. (A) T-cell affinity was determined with the tetramer dissociation assay. The percentages of MFI of T cells stained with diluted tetramer in the maximal MFI were calculated and graphed. (B and C) TCR Vβ preferential usages were determined with a panel of 15 anti-Vβ antibodies and are displayed for each mouse (B) and as grouped data (C). These experiments were performed as described in the legend to Fig. 1.
To further confirm that the high avidity of the CD8+ T cells boosted by VACV was not due to the selection of high-affinity T cells, we adoptively transferred the purified CD8+ CD45.1+ T cells from OVA-specific monoclonal TCR transgenic OT-I mice into background-compatible CD45.2+ C57BL/6 mice and compared the functional avidity between the OVA-specific CD8+ T cells from animals receiving the DNA boost and those receiving the VACV boost (Fig. 3A). The differences in both the magnitude (Fig. 3B) and the functional avidity (Fig. 3C, D, and E) of the antigen-specific CD8+ T cells remained apparent between these two vaccination regimens, indicating that the increased avidity induced by the VACV boost is independent of TCR clonal selection, as the TCRs in this experiment were monoclonal and, therefore, identical.
The recipients of congenic CD45.1+ OT-I CD8+ T cells provided sufficient cells for further characterization of antigen-responding CD8+ T cells. Proximal TCR signal transduction is mediated by multiple molecules, including CD3ζ, Lck, and ZAP-70 (30, 31). Recent reports suggested that higher functional avidity of CD8+ T cells is associated with increased amounts of activated forms of ZAP-70 and Lck (28, 32). To test whether the increased avidity of CD8+ T cells boosted by VACV was mediated by enhanced TCR signal transduction capabilities, we assessed the levels of phosphorylation of ZAP-70 and Lck in CD45.1+ OT-I CD8+ T cells 4 weeks after the final inoculation. The levels of phosphor-ZAP-70 and phosphor-Lck following OVA peptide stimulation were similar between CD45.1+ OT-I CD8+ T cells from mice that received a DNA boost and those that received a VACV boost (Fig. 3E, G, and H). We further determined the global gene expression profiles of CD45.1+ OT-I CD8+ T cells using cDNA microarray analysis. The CD45.1+ OT-I CD8+ T cells from each vaccination regimen exhibited a unique signature. Specifically, cells from animals receiving the VACV boost selectively activated pathways that are involved in cell proliferation and Wnt signaling, while no significant changes were found in mRNA expression of proximal TCR pathway-related genes (GEO no. GSE51849 and data not shown). Taken together, these data suggest that the VACV boost does not enhance the signal transduction capacity of key TCR proximal molecules compared to that of the DNA boost; instead, the VACV boost may have an impact on other signals for T-cell activation, such as inflammatory cytokine pathways.
The high avidity of antigen-specific memory CD8+ T cells induced by the VACV boost is a cell-intrinsic attribute.
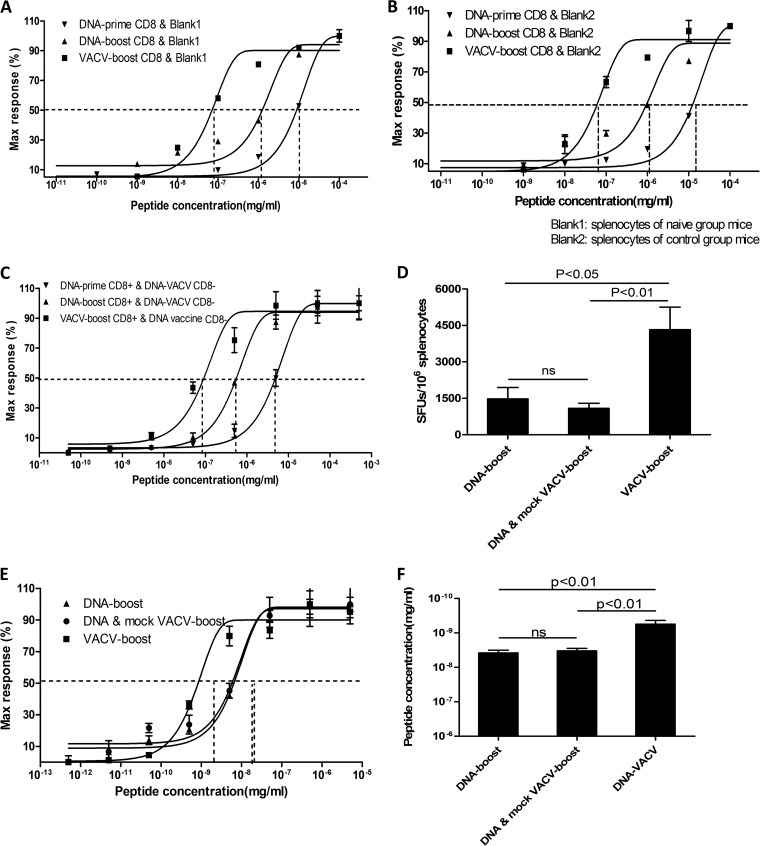
The increased avidity of VACV-boosted CD8+ T cells may stem from the improved antigen presentation by residual VACV replication in autologous splenocytes during ex vivo stimulation in the ELISpot assay, since the replication-competent Tiantan strain persists in vivo for a limited time (∼1 week) (33). Thus, we employed several strategies to exclude this possibility. First, purified CD8+ T cells from each group were mixed with the same number of splenocytes from naive mice, and the order of the avidities among the groups was not altered, although immunoaffinity magnet separation modestly reduced the overall avidity (Fig. 4A). Second, we mixed purified CD8+ T cells with splenocytes derived from mock VACV-infected mice to determine whether the introduction of VACV-sensitized antigen-presenting cells (APCs) enhanced the avidity of CD8+ T cells. The order of the functional avidities among the groups remained unchanged, suggesting that VACV-sensitized APCs do not increase the avidity of CD8+ T cells (Fig. 4B). Third, we mixed isolated CD8+ cells from mice in the DNA-prime and DNA-boost group with the CD8− cells from animals in the VACV-boost group and vice versa. The interchange of CD8− cells did not significantly influence the functional avidity of CD8+ T cells (Fig. 4C). Thus, based on these findings, the functional avidity of CD8+ T cells is unlikely to be affected by the cytokine milieu provided by VACV-sensitized APCs in the ELISpot assay.
FIG 4.
Boost in avidity in CD8+ T cells following VACV vaccination is independent of the inflammatory milieu. The bulk of CD8+ T cells from each immunized mouse group was mixed with equivalent amounts of splenocytes from naive mice (A) or mock VACV-infected mice (B). The functional avidity of CD8+ T cells was measured in response to decreasing peptide concentration. (C) CD8+ and CD8− T cells from DNA vaccine (DNA-prime and DNA-boost) and VACV boost groups were cross-mixed before assessment of functional avidity of the CD8+ T cells. (D to F) Mixed mock VACV and DNA vaccines were administered to concurrently induce the inflammatory milieu with a DNA boost. The magnitude (D) and functional avidity (E and F) were measured. These experiments were performed as described in the legend to Fig. 1, and the dominant epitope, Gag49, was employed for stimulation. Data represent three independent experiments with at least three mice per group. Statistical analysis was performed by t test using SPSS16.0 software, and the P values of comparisons between any two groups are labeled.
We also determined whether other parameters, such as the cytokines produced by CD4+ T helper cells (34), memory phenotypes, or polyfunctionality of antigen-specific CD8+ T cells (11), were related to the functional avidity of the CD8+ T cells. No significant differences in these parameters, however, were observed between the different regimens (data not shown). Taken together, we demonstrated that the enhanced functional avidity following VACV boost is an intrinsic attribute of memory CD8+ T cells.
The inflammatory milieu induced by mock VACV does not enhance the functional avidity of antigen-specific memory CD8+ T cells elicited by DNA boost.
Recent reports indicated that administration of mature dendritic cells (DCs) loaded with peptide concurrently with infection increased the functional avidity of CD8+ T cells as a result of the pathogen-induced inflammatory milieu (18). To determine whether VACV-induced inflammation tunes the functional avidity of CD8+ T cells, we boosted mice with a mixture of DNA vaccine and mock VACV. Unexpectedly, the additional introduction of mock VACV into the DNA boost failed to increase the magnitude (Fig. 4D) or the functional avidity (Fig. 4E and F) of the antigen-specific CD8+ T cells, indicating that the VACV-induced inflammatory milieu is insufficient for generating high-avidity antigen-specific memory CD8+ T cells and that the cis expression of antigen from VACV is required for the enhanced CD8+ T-cell response induced by the VACV boost.
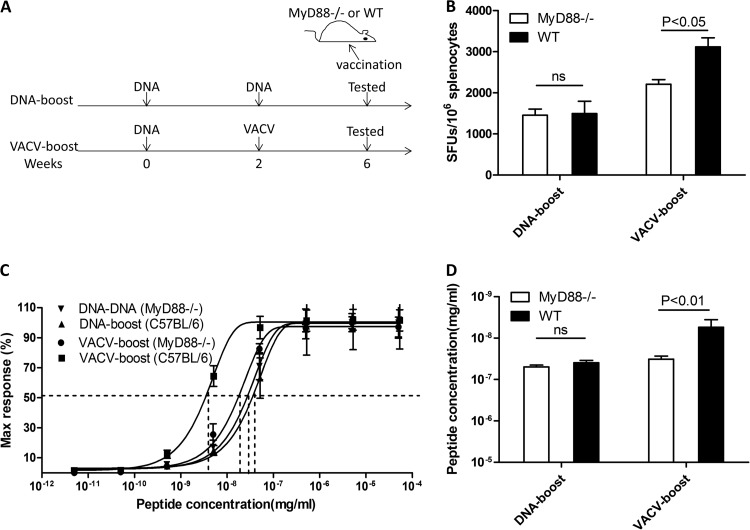
T cell-intrinsic MyD88 is essential for eliciting high-avidity memory CD8+ T cells.
The MyD88 pathway is vital for innate immunity to recognize VACV and to subsequently initiate adaptive immune responses (6, 20, 21). To test whether the MyD88 pathway is engaged in shaping the functional avidity of antigen-specific memory CD8+ cells, we boosted MyD88−/− mice with DNA vaccine or VACV vaccine and then determined the functional avidity of CD8+ T cells (Fig. 5A). The MyD88−/− mice displayed a significant reduction in both the magnitude and the functional avidity of antigen-specific CD8+ T cells following VACV boost, whereas no obvious impact on the immune responses boosted by the DNA vaccine were observed (Fig. 5B, C, and D). As a consequence, the difference in the functional avidities of the cells from animals receiving the DNA boost and those receiving the VACV boost was abrogated in MyD88−/− mice (Fig. 5C and D), demonstrating that the MyD88 pathway is essential for inducing high-avidity memory CD8+ cells with the VACV boost.
FIG 5.
MyD88 is essential for eliciting high-avidity CD8+ T cells. (A) MyD88−/− mice were immunized i.m. with vaccines that express OVA. Two vaccination regimens were performed. The magnitude (B) and the functional avidity (C and D) of OVA-specific CD8+ T cells from MyD88−/− and wild-type mice that were immunized were quantified by ELISpot assay. Statistical analysis was performed by t test using SPSS16.0 software, and the P values of comparisons between any two groups are labeled.
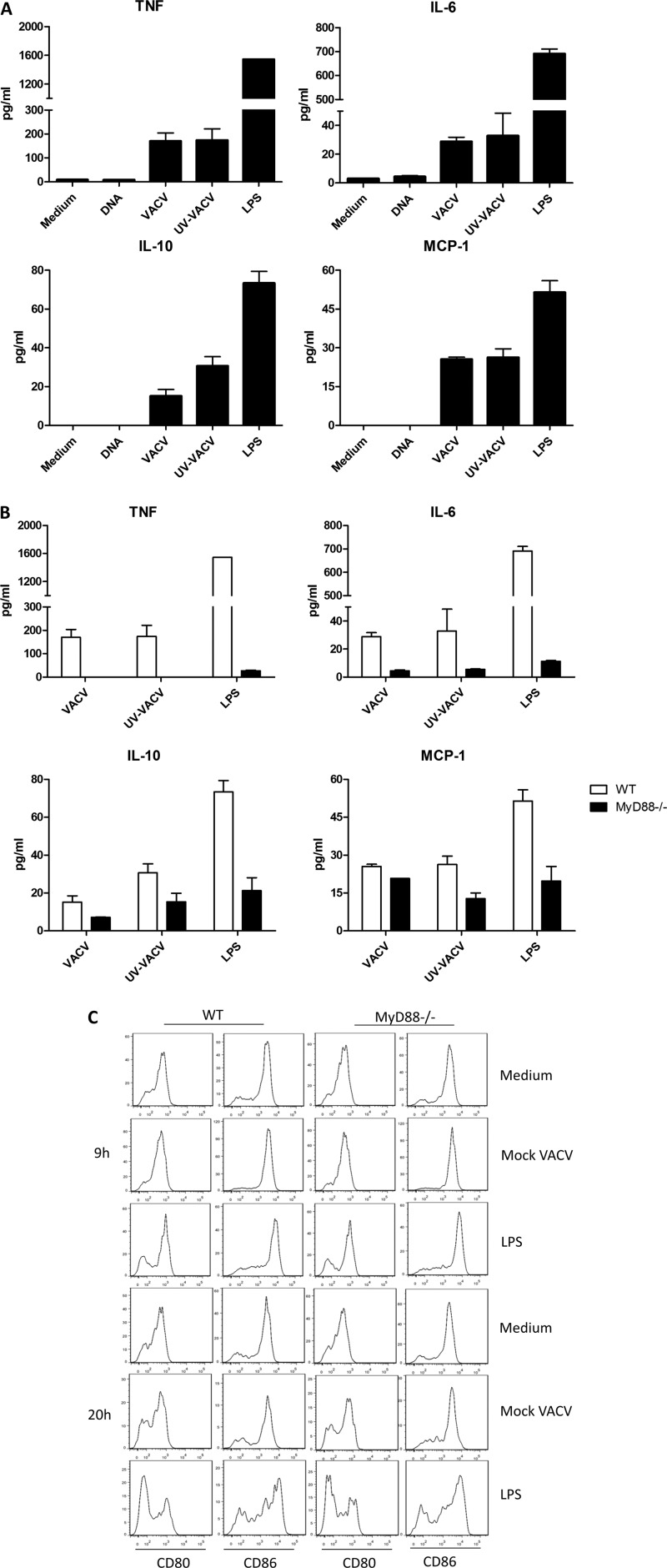
The fact that VACV did not boost CD8+ T cells with high functional avidity in MyD88−/− mice suggests that loss of MyD88 affects APC response to VACV infection. Therefore, we then examined inflammatory cytokine production and DC maturation after MyD88−/− splenocytes were exposed to VACV infection. VACV induced high levels of inflammatory cytokines in splenocytes from WT mice (Fig. 6A). In contrast, the production of cytokines was significantly reduced in MyD88−/− cells (Fig. 6B), whereas MyD88−/− DCs matured normally following in vitro exposure to VACV (Fig. 6C). In agreement with previous reports (20, 21, 35), these data demonstrate that the inflammatory cytokines induced by VACV infection are decreased and that the ability to induce a CD8+ T-cell response is retained in MyD88−/− mice.
FIG 6.
Attenuated cytokine production and normal maturation of MyD88−/− DCs in response to VACV infection. (A) Secretion of inflammatory cytokines by splenocytes from WT mice or MyD88−/− mice stimulated with DNA, mock VACV, and UV-inactivated mock VACV (UV-VACV) for 20 h in 96-well plates at 37°C. Medium was included as a negative control, and LPS was used as a positive control. (B) Comparison of the production of cytokines following treatment with mock VACV and UV-VACV in cells from MyD88−/− and WT mice. LPS was used as a positive control. (C) Splenocytes from MyD88−/− or C57BL/6 mice were harvested and incubated with mock VACV (MOI of 1), LPS (100 ng/ml), or medium in 96-well plates for 9 h and 20 h and then stained with the indicated antibodies. DCs were gated on CD11c+ CD14− cells. The levels of CD80 and CD86 are presented as histograms. Data are representative of three independent experiments with at least three mice per group.
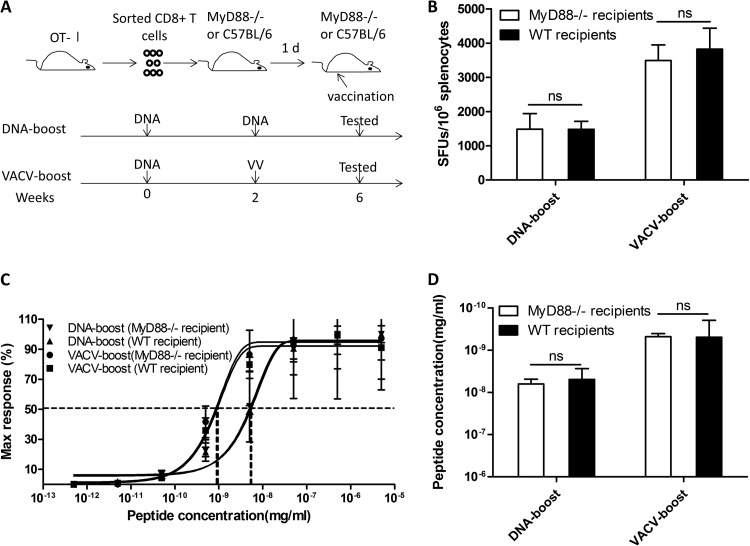
To determine whether the reduction in inflammatory cytokine production in MyD88−/− mice accounts for the decrease in the observed functional avidity of the CD8+ T cells, we adoptively transferred OT-I CD8+ T cells, which express MyD88 and recognize the OVA epitope, into MyD88−/− recipients (Fig. 7A) and then immunized the mice with OVA-expressing DNA, followed by either a DNA or a VACV boost. Interestingly, the VACV boost restored the ability of these cells to boost high-avidity OVA-specific CD8+ T cells, indicating that intrinsic MyD88 signaling in CD8+ T cells dictates the avidity-shaping process following VACV boost (Fig. 7B, C, and D).
FIG 7.
Transferring of WT CD8+ T cells into MyD88−/− mice rescues the enhanced functional avidity of OVA-specific CD8+ T cells boosted by VACV. Purified OT-I CD8+ T cells were adoptively transferred into MyD88−/− mice. The recipients were then immunized according to the schedule shown in panel A. The magnitude (B) and the functional avidity (C and D) of OVA-specific CD8+ T cells were quantified by ELISpot assay. Statistical analysis was performed by t test using SPSS16.0 software, and the P values of comparisons between any two groups are labeled.
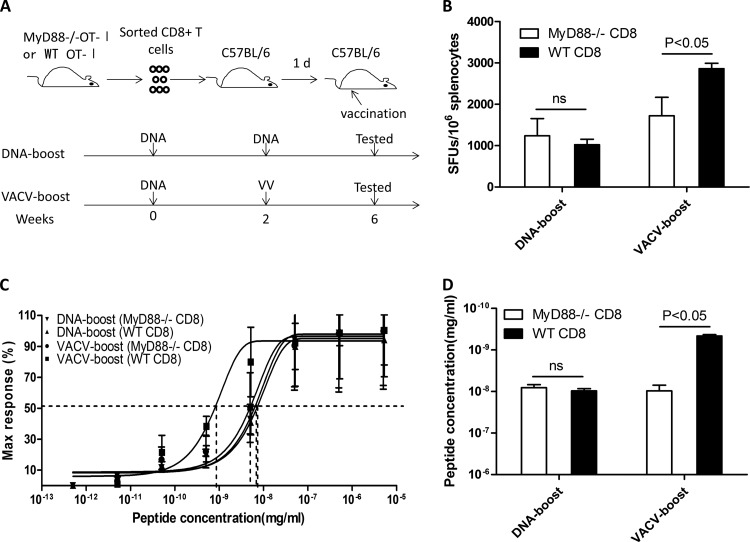
To verify that the functional avidity of CD8+ T cells is determined by the intrinsic MyD88 pathway in CD8+ T cells, we crossed the MyD88−/− mice with OT-I mice to generate MyD88-deficient CD8+ T cells carrying a monoclonal TCR that recognizes OVA (MyD88−/− OT-I). When MyD88−/− OT-I CD8+ T cells were transferred to WT mice, which were then boosted by DNA vaccine or VACV vaccine (Fig. 8A), both the magnitude (Fig. 8B) and the functional avidity (Fig. 8C and D) of the OVA-specific CD8+ T cells induced by VACV boost were significantly impaired, whereas no significant impact was observed on these parameters following DNA boost, demonstrating that the functional avidity of CD8+ T cells is largely determined by the intrinsic MyD88 pathway in CD8+ T cells.
FIG 8.
T-cell-intrinsic MyD88 pathway is required for eliciting high-avidity CD8+ T cells following VACV boost. The OT-I mice were crossed with MyD88−/− mice to generate MyD88-deficient T cells carrying TCR recognizing OVA (MyD88−/− OT-I). Purified CD8+ T cells from MyD88−/− OT-I mice were adoptively transferred into WT mice, which were immunized as depicted in panel A. The magnitude (B) and the functional avidity (C and D) of OVA-specific CD8+ T cells induced by VACV boost or DNA boost in both MyD88−/− and WT transferred cells was analyzed. Statistical analysis was performed by t test using SPSS16.0 software, and the P values of comparisons between any two groups are labeled.
Overall, these data demonstrate that the high functional avidity of memory CD8+ T cells boosted by VACV is independent of the inflammatory milieu, and that the intrinsic MyD88-mediated pathway in CD8+ T cells is essential in shaping the functional avidity in response to VACV boost.
DISCUSSION
Understanding how the components of a vaccine regimen shape the properties of T-cell responses is crucial for optimizing strategies for a better T cell-based vaccine. Previously, multiple properties of antigen-specific T cells have been characterized, including the magnitude of T-cell responses, functionality, and the capacity for proliferation, as well as the functional avidity (9, 10). T cells with high functional avidity not only are able to respond to stimulation with a lower antigen density, thereby attacking virus-infected cells at an earlier stage during infection (9, 10), but also are associated with greatly improved recognition of mutant epitopes (36, 37). Based on these earlier reports, this study aimed at gaining a better understanding of how VACV tunes the functional avidity of CD8+ T cells primed with a DNA vaccine. These findings have important implications for T-cell vaccine development.
A recent study demonstrated that the functional avidity of CD8+ T cells is determined by the regulated control of TCR signaling initiation (28). Here, we showed that the VACV boost in vivo enhanced the functional avidity of CD8+ T cells primed by a DNA vaccine by decreasing the activation threshold. Using congenically marked OT-I transgenic mice and MyD88 knockout mice, we provided evidence that increased functional avidity was not determined by selection of high-affinity TCR or by enhancement of proximal TCR signaling transduction. Furthermore, we demonstrated that intrinsic MyD88 expression is essential in shaping functional avidity in response to the VACV boost. Thus, our data indicate that the increased avidity of CD8+ T cells following DNA vaccine priming and VACV boost does not depend mainly on VACV-induced and MyD88-mediated inflammation, and intrinsic MyD88 in CD8+ T cells is indeed indispensable for this increase. However, the inflammatory cytokines mediated by other pathways beyond MyD88 may also play a role in shaping the antigen-specific immunity induced by VACV immunization.
Our data demonstrate that the functional avidity of CD8+ T cells is orchestrated by at least two signaling pathways, the pMHC-TCR pathway and the MyD88-mediated pathway, and the simultaneous engagement of both pathways was required for generation of high-avidity CD8+ T cells in this system. As shown in previous reports, innate immunity against vaccinia virus is mediated by MyD88 (20), and the MyD88-mediated pathway in CD8+ T cells represents a readily available pathway for responding to vaccinia virus infection and shapes the development of T-cell functionality and the formation of memory T cells (38, 39). Thus, we speculated that this pathway also played a critical role in tuning the functional avidity following VACV boost. As MyD88 functions in multiple innate receptor signaling pathways in CD8+ T cells (40), identification of the innate receptors that are involved in shaping the functional avidity during VACV boosting is a priority. Indeed, MyD88−/− CD8+ T cells were less efficient in pathogen clearance than TLR2−/− CD8+ T cells (39), indicating that MyD88−/− CD8+ T cells are more severely functionally damaged than TLR2−/− CD8+ T cells; thus, MyD88 is involved in more pathways of the innate immune response than TLR2. The MyD88 pathway appears to be triggered by products derived from VACV or VACV-infected host cells. However, further experiments to test this hypothesis are needed.
Importantly, our data reveal that the functional avidity of memory CD8+ T cells may be sequentially tuned by a DNA vaccine followed by a VACV vaccine; thus, these studies provide evidence that this critical property of CD8+ T cells can be modulated by immunization. In this regard, the composition of vaccines in a given protocol will be pivotal for shaping the functional avidity of antigen-specific CD8+ T cells, and these findings have important implications for vaccine development. In particular, different vaccine formulations, such as a DNA-, protein-, or peptide-based vaccines, different viral or bacterial vectors, and different vaccine combinations may generate varied functional avidities of antigen-specific CD8+ T cells, resulting in very different protective efficacies (41, 42).
It remains unknown whether the functional avidity can be both positively and negatively regulated and whether a ceiling capacity for the enhancement of functional avidity exists as well. Interestingly, the addition of mock VACV to the DNA vaccine fails to increase the functional avidity of CD8+ T cells, suggesting that the cis expression of the immunogen from VACV is necessary for this effect and that the inflammatory milieu induced by VACV infection is unlikely to be sufficient for boosting functional avidity. One possible explanation is that VACV infection results in upregulation of costimulatory molecules during APC activation and thereby concomitantly promotes T cell-APC interactions. DNA vaccine mainly enters muscle cells, whereas the VACV vaccine more efficiently infects the epithelial or professional APCs (43–45). Therefore, concurrent inoculation of DNA vaccine and VACV vaccine may not result in ligation of the pMHC-TCR and upregulation of costimulatory molecules into the same synapse. As a result, this vaccination strategy may not boost the functional avidity. In addition, our data demonstrate that the functional avidity of antigen-specific CD8+ T cells is an intrinsic feature of CD8+ T cells and is likely to be forged in vivo during activation and differentiation, as the avidity was not enhanced by coculturing with splenocytes in vitro.
In summary, our data demonstrate that VACV boosts the functional avidity of CD8+ T cells primed by DNA vaccine through the intrinsic MyD88 pathway and that cis expression of immunogen from VACV is required for this enhancement. Therefore, sequential tuning of functional avidity is possible through a structured vaccination protocol. These data shed light on vaccine design and stress the importance of the composition of vaccine strategies.
ACKNOWLEDGMENTS
This work was supported by the National Natural Science Foundation of China (81072496H1014 and U1202228), the National Grand Program on Key Infectious Disease Control (2012ZX10001-006 and 2013ZX10001-002) from the China Ministry of Health, the 973 National Key Basic Research Project (2012CB519005) from the Ministry of Science and Technology of PRC, the Shanghai Science and Technology Committee (11XD1404200), the Mingdao Award for Medical Post-Graduate Students (MDJH2012025) from Fudan University, and the Chinese Scholarship Council (2011610530).
We are grateful to the NIH Tetramer Core Facility for generously providing peptide-MHC tetramers.
We have no conflicting financial interests to report.
Footnotes
Published ahead of print 19 February 2014
REFERENCES
- 1.Harari A, Bart PA, Stohr W, Tapia G, Garcia M, Medjitna-Rais E, Burnet S, Cellerai C, Erlwein O, Barber T, Moog C, Liljestrom P, Wagner R, Wolf H, Kraehenbuhl JP, Esteban M, Heeney J, Frachette MJ, Tartaglia J, McCormack S, Babiker A, Weber J, Pantaleo G. 2008. An HIV-1 clade C DNA prime, NYVAC boost vaccine regimen induces reliable, polyfunctional, and long-lasting T cell responses. J. Exp. Med. 205:63–77. 10.1084/jem.20071331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sandstrom E, Nilsson C, Hejdeman B, Brave A, Bratt G, Robb M, Cox J, Vancott T, Marovich M, Stout R, Aboud S, Bakari M, Pallangyo K, Ljungberg K, Moss B, Earl P, Michael N, Birx D, Mhalu F, Wahren B, Biberfeld G. 2008. Broad immunogenicity of a multigene, multiclade HIV-1 DNA vaccine boosted with heterologous HIV-1 recombinant modified vaccinia virus Ankara. J. Infect. Dis. 198:1482–1490. 10.1086/592507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kibuuka H, Kimutai R, Maboko L, Sawe F, Schunk MS, Kroidl A, Shaffer D, Eller LA, Kibaya R, Eller MA, Schindler KB, Schuetz A, Millard M, Kroll J, Dally L, Hoelscher M, Bailer R, Cox JH, Marovich M, Birx DL, Graham BS, Michael NL, de Souza MS, Robb ML. 2010. A phase 1/2 study of a multiclade HIV-1 DNA plasmid prime and recombinant adenovirus serotype 5 boost vaccine in HIV-uninfected east Africans (RV 172). J. Infect. Dis. 201:600–607. 10.1086/650299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ranasinghe C, Ramshaw IA. 2009. Genetic heterologous prime-boost vaccination strategies for improved systemic and mucosal immunity. Expert Rev. Vaccines 8:1171–1181. 10.1586/erv.09.86 [DOI] [PubMed] [Google Scholar]
- 5.Paris RM, Kim JH, Robb ML, Michael NL. 2010. Prime-boost immunization with poxvirus or adenovirus vectors as a strategy to develop a protective vaccine for HIV-1. Expert Rev. Vaccines 9:1055–1069. 10.1586/erv.10.106 [DOI] [PubMed] [Google Scholar]
- 6.Lousberg EL, Diener KR, Brown MP, Hayball JD. 2011. Innate immune recognition of poxviral vaccine vectors. Expert Rev. Vaccines 10:1435–1449. 10.1586/erv.11.121 [DOI] [PubMed] [Google Scholar]
- 7.Pantaleo G, Esteban M, Jacobs B, Tartaglia J. 2010. Poxvirus vector-based HIV vaccines. Curr. Opin. HIV AIDS 5:391–396. 10.1097/COH.0b013e32833d1e87 [DOI] [PubMed] [Google Scholar]
- 8.Appay V, Douek DC, Price DA. 2008. CD8+ T cell efficacy in vaccination and disease. Nat. Med. 14:623–628. 10.1038/nm.f.1774 [DOI] [PubMed] [Google Scholar]
- 9.Alexander-Miller MA. 2005. High-avidity CD8+ T cells: optimal soldiers in the war against viruses and tumors. Immunol. Res. 31:13–24. 10.1385/IR:31:1:13 [DOI] [PubMed] [Google Scholar]
- 10.Vigano S, Utzschneider DT, Perreau M, Pantaleo G, Zehn D, Harari A. 2012. Functional avidity: a measure to predict the efficacy of effector T cells? Clin. Dev. Immunol. 2012:153863. 10.1155/2012/153863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Almeida JR, Sauce D, Price DA, Papagno L, Shin SY, Moris A, Larsen M, Pancino G, Douek DC, Autran B, Saez-Cirion A, Appay V. 2009. Antigen sensitivity is a major determinant of CD8+ T-cell polyfunctionality and HIV-suppressive activity. Blood 113:6351–6360. 10.1182/blood-2009-02-206557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Derby M, Alexander-Miller M, Tse R, Berzofsky J. 2001. High-avidity CTL exploit two complementary mechanisms to provide better protection against viral infection than low-avidity CTL. J. Immunol. 166:1690–1697 http://www.jimmunol.org/content/166/3/1690.long [DOI] [PubMed] [Google Scholar]
- 13.Mothe B, Llano A, Ibarrondo J, Zamarreno J, Schiaulini M, Miranda C, Ruiz-Riol M, Berger CT, Herrero MJ, Palou E, Plana M, Rolland M, Khatri A, Heckerman D, Pereyra F, Walker BD, Weiner D, Paredes R, Clotet B, Felber BK, Pavlakis GN, Mullins JI, Brander C. 2012. CTL responses of high functional avidity and broad variant cross-reactivity are associated with HIV control. PLoS One 7:e29717. 10.1371/journal.pone.0029717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Appay V, Iglesias MC. 2011. Antigen sensitivity and T-cell receptor avidity as critical determinants of HIV control. Curr. Opin. HIV AIDS 6:157–162. 10.1097/COH.0b013e3283453dfd [DOI] [PubMed] [Google Scholar]
- 15.Almeida JR, Price DA, Papagno L, Arkoub ZA, Sauce D, Bornstein E, Asher TE, Samri A, Schnuriger A, Theodorou I, Costagliola D, Rouzioux C, Agut H, Marcelin AG, Douek D, Autran B, Appay V. 2007. Superior control of HIV-1 replication by CD8+ T cells is reflected by their avidity, polyfunctionality, and clonal turnover. J. Exp. Med. 204:2473–2485. 10.1084/jem.20070784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Estcourt MJ, Ramsay AJ, Brooks A, Thomson SA, Medveckzy CJ, Ramshaw IA. 2002. Prime-boost immunization generates a high frequency, high-avidity CD8(+) cytotoxic T lymphocyte population. Int. Immunol. 14:31–37. 10.1093/intimm/14.1.31 [DOI] [PubMed] [Google Scholar]
- 17.Ranasinghe C, Turner SJ, McArthur C, Sutherland DB, Kim JH, Doherty PC, Ramshaw IA. 2007. Mucosal HIV-1 pox virus prime-boost immunization induces high-avidity CD8+ T cells with regime-dependent cytokine/granzyme B profiles. J. Immunol. 178:2370–2379 http://www.jimmunol.org/content/178/4/2370.long [DOI] [PubMed] [Google Scholar]
- 18.Richer MJ, Nolz JC, Harty JT. 2013. Pathogen-specific inflammatory milieu tune the antigen sensitivity of CD8(+) T cells by enhancing T cell receptor signaling. Immunity 38:140–152. 10.1016/j.immuni.2012.09.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.von Essen MR, Kongsbak M, Geisler C. 2012. Mechanisms behind functional avidity maturation in T cells. Clin. Dev. Immunol. 2012:163453. 10.1155/2012/163453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhu J, Martinez J, Huang X, Yang Y. 2007. Innate immunity against vaccinia virus is mediated by TLR2 and requires TLR-independent production of IFN-beta. Blood 109:619–625. 10.1182/blood-2006-06-027136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lousberg EL, Diener KR, Fraser CK, Phipps S, Foster PS, Chen W, Uematsu S, Akira S, Robertson SA, Brown MP, Hayball JD. 2011. Antigen-specific T-cell responses to a recombinant fowlpox virus are dependent on MyD88 and interleukin-18 and independent of Toll-like receptor 7 (TLR7)- and TLR9-mediated innate immune recognition. J. Virol. 85:3385–3396. 10.1128/JVI.02000-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Elena GC, Perdiguero B, Garcia-Arriaza J, Esteban M. 2012. Poxvirus vectors as HIV/AIDS vaccines in humans. Hum. Vaccin. Immunother. 8:1192–1207. 10.4161/hv.20778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wan Y, Wu L, Liu L, Xu J, Liu Y, Liu Y, Shao Y. 2007. Comparison of immunogenicity between codon optimized HIV-1 Thailand subtype B gp140 and gp145 vaccines. Vaccine 25:4949–4959. 10.1016/j.vaccine.2007.01.118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li HS, Liu Y, Li DF, Zhang RR, Tang HL, Zhang YW, Huang W, Liu Y, Peng H, Xu JQ, Hong KX, Shao YM. 2007. Enhancement of DNA vaccine-induced immune responses by a 72-bp element from SV40 enhancer. Chin Med. J. 120:496–502 http://www.cmj.org/ch/reader/view_abstract.aspx?volume=120&issue=6&start_page=496 [PubMed] [Google Scholar]
- 25.Liu Y, Li F, Liu Y, Hong K, Meng X, Chen J, Zhang Z, Huo Z, Sun M, Self SG, Shao Y. 2011. HIV fragment gag vaccine induces broader T cell response in mice. Vaccine 29:2582–2589. 10.1016/j.vaccine.2011.01.049 [DOI] [PubMed] [Google Scholar]
- 26.van Stipdonk MJ, Hardenberg G, Bijker MS, Lemmens EE, Droin NM, Green DR, Schoenberger SP. 2003. Dynamic programming of CD8+ T lymphocyte responses. Nat. Immunol. 4:361–365. 10.1038/ni912 [DOI] [PubMed] [Google Scholar]
- 27.Reddy J, Bettelli E, Nicholson L, Waldner H, Jang MH, Wucherpfennig KW, Kuchroo VK. 2003. Detection of autoreactive myelin proteolipid protein 139-151-specific T cells by using MHC II (IAs) tetramers. J. Immunol. 170:870–877 http://www.jimmunol.org/content/170/2/870.long [DOI] [PubMed] [Google Scholar]
- 28.Sharma SK, Alexander-Miller MA. 2011. Increased sensitivity to antigen in high avidity CD8(+) T cells results from augmented membrane proximal T-cell receptor signal transduction. Immunology 133:307–317. 10.1111/j.1365-2567.2011.03440.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Busch DH, Pamer EG. 1999. T cell affinity maturation by selective expansion during infection. J. Exp. Med. 189:701–710. 10.1084/jem.189.4.701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Davis MM. 2002. A new trigger for T cells. Cell 110:285–287. 10.1016/S0092-8674(02)00865-6 [DOI] [PubMed] [Google Scholar]
- 31.Luik RM, Lewis RS. 2007. New insights into the molecular mechanisms of store-operated Ca2+ signaling in T cells. Trends Mol. Med. 13:103–107. 10.1016/j.molmed.2007.01.004 [DOI] [PubMed] [Google Scholar]
- 32.Amoah S, Yammani RD, Grayson JM, Alexander-Miller MA. 2012. Changes in functional but not structural avidity during differentiation of CD8+ effector cells in vivo after virus infection. J. Immunol. 189:638–645. 10.4049/jimmunol.1102579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Huang Y, Qiu C, Liu LX, Feng YM, Zhu T, Xu JQ. 2009. A mouse model based on replication-competent Tiantan vaccinia expressing luciferase/HIV-1 Gag fusion protein for the evaluation of protective efficacy of HIV vaccine. Chin Med. J. 122:1655–1659 http://www.cmj.org/ch/reader/view_abstract.aspx?volume=122&issue=14&start_page=1655 [PubMed] [Google Scholar]
- 34.Wilde S, Sommermeyer D, Leisegang M, Frankenberger B, Mosetter B, Uckert W, Schendel DJ. 2012. Human antitumor CD8+ T cells producing Th1 polycytokines show superior antigen sensitivity and tumor recognition. J. Immunol. 189:598–605. 10.4049/jimmunol.1102165 [DOI] [PubMed] [Google Scholar]
- 35.Zhao Y, De Trez C, Flynn R, Ware CF, Croft M, Salek-Ardakani S. 2009. The adaptor molecule MyD88 directly promotes CD8 T cell responses to vaccinia virus. J. Immunol. 182:6278–6286. 10.4049/jimmunol.0803682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Valkenburg SA, Gras S, Guillonneau C, La Gruta NL, Thomas PG, Purcell AW, Rossjohn J, Doherty PC, Turner SJ, Kedzierska K. 2010. Protective efficacy of cross-reactive CD8+ T cells recognising mutant viral epitopes depends on peptide-MHC-I structural interactions and T cell activation threshold. PLoS Pathog. 6:e1001039. 10.1371/journal.ppat.1001039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Varela-Rohena A, Molloy PE, Dunn SM, Li Y, Suhoski MM, Carroll RG, Milicic A, Mahon T, Sutton DH, Laugel B, Moysey R, Cameron BJ, Vuidepot A, Purbhoo MA, Cole DK, Phillips RE, June CH, Jakobsen BK, Sewell AK, Riley JL. 2008. Control of HIV-1 immune escape by CD8 T cells expressing enhanced T-cell receptor. Nat. Med. 14:1390–1395. 10.1038/nm.1779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Quigley M, Martinez J, Huang X, Yang Y. 2009. A critical role for direct TLR2-MyD88 signaling in CD8 T-cell clonal expansion and memory formation following vaccinia viral infection. Blood 113:2256–2264. 10.1182/blood-2008-03-148809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Geng D, Zheng L, Srivastava R, Asprodites N, Velasco-Gonzalez C, Davila E. 2010. When Toll-like receptor and T-cell receptor signals collide: a mechanism for enhanced CD8 T-cell effector function. Blood 116:3494–3504. 10.1182/blood-2010-02-268169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tabiasco J, Devevre E, Rufer N, Salaun B, Cerottini JC, Speiser D, Romero P. 2006. Human effector CD8+ T lymphocytes express TLR3 as a functional coreceptor. J. Immunol. 177:8708–8713 http://www.jimmunol.org/content/177/12/8708.full [DOI] [PubMed] [Google Scholar]
- 41.Reyes-Sandoval A, Rollier CS, Milicic A, Bauza K, Cottingham MG, Tang CK, Dicks MD, Wang D, Longley RJ, Wyllie DH, Hill AV. 2012. Mixed vector immunization with recombinant adenovirus and MVA can improve vaccine efficacy while decreasing antivector immunity. Mol. Ther. 20:1633–1647. 10.1038/mt.2012.25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Park SH, Yang SH, Lee CG, Youn JW, Chang J, Sung YC. 2003. Efficient induction of T helper 1 CD4+ T-cell responses to hepatitis C virus core and E2 by a DNA prime-adenovirus boost. Vaccine 21:4555–4564. 10.1016/S0264-410X(03)00499-7 [DOI] [PubMed] [Google Scholar]
- 43.Wolff JA, Ludtke JJ, Acsadi G, Williams P, Jani A. 1992. Long-term persistence of plasmid DNA and foreign gene expression in mouse muscle. Hum. Mol. Genet. 1:363–369. 10.1093/hmg/1.6.363 [DOI] [PubMed] [Google Scholar]
- 44.Larsson M, Fonteneau JF, Somersan S, Sanders C, Bickham K, Thomas EK, Mahnke K, Bhardwaj N. 2001. Efficiency of cross presentation of vaccinia virus-derived antigens by human dendritic cells. Eur. J. Immunol. 31:3432–3442. [DOI] [PubMed] [Google Scholar]
- 45.Fischer MA, Norbury CC. 2007. Initiation of primary anti-vaccinia virus immunity in vivo. Immunol. Res. 37:113–133. 10.1007/BF02685894 [DOI] [PubMed] [Google Scholar]