ABSTRACT
The mammalian antiviral membrane protein tetherin (BST2/CD317) can be expressed as two isoforms derived from differential translational initiation. The shorter isoform of the human protein (S-tetherin) lacks the first 12 amino acids of the longer (L-tetherin) cytoplasmic tail, which includes a tyrosine motif that acts as both an endocytic recycling signal and a determinant of virus-induced NF-κB activation. S-tetherin is also reported to be less sensitive to the prototypic viral antagonist human immunodeficiency virus type 1 (HIV-1) Vpu. Here we analyzed the relative sensitivities of L- and S-tetherins to primate lentiviral countermeasures. We show that the reduced sensitivity of S-tetherin to HIV-1 Vpu is a feature of all group M proteins, including those of transmitted founder viruses, primarily because it cannot be targeted for endosomal degradation owing to the truncation of its cytoplasmic tail. In contrast, both isoforms of the human and rhesus macaque tetherins display the same sensitivity to nondegradative lentiviral countermeasures of HIV-2 and SIVmac, respectively. Surprisingly, however, the Vpu proteins encoded by simian immunodeficiency viruses (SIVs) of African guenons, as well as that from recently isolated highly pathogenic HIV-1 group N, do not discriminate between tetherin isoforms. Together, these data suggest that the group M HIV-1 Vpu primarily adapted to target L-tetherin upon zoonotic transmission from chimpanzees, and further, we speculate that functions specifically associated with this isoform, such as proinflammatory signaling, play key roles in human tetherin's antiviral function in vivo.
IMPORTANCE The ability of HIV-1 and related viruses to counteract a host antiviral protein, tetherin, is strictly maintained. The adaptation of the HIV-1 Vpu protein to counteract human tetherin is thought to have been one of the key events in the establishment of the HIV/AIDS pandemic. Recent evidence shows that tetherin is expressed as two isoforms and that Vpu preferentially targets the longer form. Here we show that unlike other virus-encoded countermeasures, such as those from primate viruses related to HIV-1, the enhanced ability to counteract the long tetherin isoform is conserved among HIV-1 strains that make up the majority of the human pandemic. This correlates with the ability of Vpu to induce long tetherin degradation. We speculate that functions associated with the human version of this isoform, such as an inflammatory signaling capacity, selected for Vpu's enhanced targeting of long tetherin during its adaptation to humans.
INTRODUCTION
The interferon (IFN)-induced transmembrane (TM) protein tetherin (BST2/CD317/HM1.24) displays antiviral activity in vitro and in vivo (reviewed in reference 1). It has been shown to potently restrict the release of diverse enveloped viruses, including the members of the families Retroviridae (2, 3) and Herpesviridae (4–7) and negative-strand RNA viruses (8), from infected cells by cross-linking them to the plasma membrane (PM). Tetherin is a dimeric glycoprotein that consists of an N-terminal cytoplasmic tail, a conventional TM helix, a coiled-coil extracellular domain, and a C-terminal glycosylphosphatidylinositol (GPI) anchor (1). This structural arrangement is essential for tetherin's antiviral activity (9); the dual-anchor conformation allows predominantly the GPI linkage to partition into budding virions, resulting in stable cross-links of parallel tetherin dimers when viral and cellular membranes separate (10). Thus, tetherin does not need to interact with any virus-encoded structure, which accounts for its wide activity against enveloped viruses. Virions retained by tetherin cross-links can subsequently be endocytosed and trafficked to late endosomes (2). Recent data also demonstrate that human tetherin can mediate signal transduction upon virion retention that activates NF-κB and promotes proinflammatory gene expression, thereby acting as an innate sensor of viral release (11–13).
There are now several examples of virus-encoded proteins that have evolved to counteract tetherin, often in a species-specific manner, highlighting an important selective pressure imposed by tetherin on viral evolution (1). These viral proteins include the primate lentiviral accessory proteins human immunodeficiency virus type 1 (HIV-1) Vpu (2, 14) and simian immunodeficiency virus (SIV) Nef (15, 16); the envelope glycoproteins encoded by HIV-2 and SIVtan (Env) (17, 18), Ebola virus (EBOV-G) (19), and herpes simplex virus 1 (gM) (7); and K5, a membrane-bound ubiquitin ligase encoded by Kaposi's sarcoma-associated herpesvirus (KSHV) (5, 6). Among the primate lentiviruses, genetic evidence strongly suggests that the ability to counteract tetherin is an essential attribute for viral spread in vivo (20). Furthermore, adaptation to target human tetherin efficiently by the Vpu proteins of the HIV-1 group M is thought to have been a key event in determining its spread to become the major agent of the HIV/AIDS pandemic (21).
Recently it was shown that tetherin is expressed as a long (L-tetherin) or short (S-tetherin) isoform because of leaky ribosomal scanning of its mRNA that results in translational initiations at two AUG codons (11). S-tetherin lacks the first 12 amino acids of its cytoplasmic tail (11). Within these 12 residues lies a conserved tyrosine-based motif that acts both as an endocytic recycling sequence (22) and as the determinant of signal transduction in the human protein (11–13). HIV-1 Vpu, a small membrane phosphoprotein, interacts with human and chimpanzee tetherins via direct TM domain interactions (23–26) and blocks the transit of newly synthesized and recycling tetherin to the PM (27, 28). This is then coupled to tetherin's endosomal degradation by a clathrin-dependent transport event that requires both an acidic/dileucine motif in the Vpu cytoplasmic tail and the tyrosine-based sequence in L-tetherin (29). Endosomal degradation of tetherin is ubiquitin and ESCRT dependent (30, 31) and is determined by the recruitment the SCFβTRCP1/2 E3 ligase to a conserved phosphoserine motif (DSGNES) in the Vpu cytoplasmic tail (32, 33). Tetherin can be ubiquitinated on multiple residues in its cytoplasmic tail, but the exact requirements for counteraction and/or degradation are unclear (34, 35). However, a serine-threonine motif found only in L-tetherin has been highlighted as important (35). In contrast, other lentiviral tetherin antagonists do not mediate its degradation but rather promote its endocytosis from the PM for intracellular sequestration (15, 18, 36–38).
Human S-tetherin has been reported to be less sensitive to Vpu of the prototypic HIV-1 molecular clone NL4.3 (11). However, it is not yet known whether such differences in Vpu sensitivity are observable at physiological expression levels. Also, recent findings by our group indicate that the majority of primary Vpu isolate alleles from patients infected with clade B HIV-1 display activity superior to that of NL4.3 Vpu both for counteraction of tetherin's antiviral activity and for suppression of its signaling activity, indicating that it may not be fully representative of wild-type (WT) Vpu function (39). We therefore characterized the sensitivities of the L- and S-tetherins to a diverse panel of Vpu proteins, as well as countermeasures from HIV-2, SIVmac, and KSHV. We found that differential sensitivity of L- and S-tetherins was a conserved feature of all of the group M Vpu proteins tested but not SIVmac Nef, HIV-2 Env, KSHV K5, or the Vpu proteins of SIVs from African guenons, which represent descendants of the viruses from which the 5′ half of the SIVcpz genome is derived (40).
MATERIALS AND METHODS
Cell lines and plasmids.
WT HIV-1 NL4.3 and HIV-1 NL4.3ΔVpu have been described previously (41). The HIV-2 molecular clone pRod10 was obtained from the Centre for AIDS Research (National Institute for Biological Standards and Control, Potters Bar, United Kingdom), and envelope mutants and Env-internal ribosome entry site-green fluorescent protein (Env-IRES-GFP) were described previously (18). SIVmac239 and SIVmacΔNef proviral plasmids were kindly provided by Theodora Hatziioannou, Aaron Diamond AIDS Research Center, New York, NY (16). Tetherin isoforms and species orthologues were made in pCR3.1, pLHCX (Clontech), and pCMS28 by standard molecular biological methods. Vpu proteins from different clades of HIV-1 or clade B transmitted/founder viruses (42) were cloned into a rev-dependent expression vector, pCRV1, as described previously (39). SIVgsn Vpu, SIVmon Vpu, and group N Vpu YBF30 or N1FR2011 (43) were synthesized and cloned into pCRV1. Mona and greater spot-nosed monkey tetherins (21) were similarly synthesized and cloned into pCR3.1.
HEK293T, HEK293, HT1080, and Jurkat cells were obtained from the American Type Culture Collection; the HIV reporter TZM-bl HeLa cell line was kindly provided by John Kappes through the NIH AIDS Reagents Repository Program (ARRP). Jurkat-TAg cells were kindly provided by Marie-Jose Bijlmakers, King's College London. All adherent cells were maintained in Dulbecco's modified Eagle medium (Invitrogen) supplemented with 10% fetal calf serum and gentamicin. Derivatives of cell lines stably expressing human tetherin or mutant forms thereof were produced by transducing the cells with murine leukemia virus-based retroviral vectors packaging a pLHCX or pCMS28 vector genome encoding the tetherin construct and selecting the cells in hygromycin (Invitrogen) or puromycin, respectively. Jurkat and Jurkat-TAg cells were maintained in Roswell Park Memorial Institute medium supplemented with 10% fetal calf serum and gentamicin.
Production of vector and virus stocks.
For full-length vesicular stomatitis virus G protein (VSV-G)-pseudotyped HIV-1 stocks, 293T cells were transfected with 2 μg proviral plasmid and 200 ng pCMV-VSV-G by using polyethylenimine (PEI; 1 mg/ml; Polysciences Europe GmbH). At 48 h posttransfection, supernatants were harvested and endpoint titers were determined in TZM-bl HeLa cells as described previously (18). For KSHV K5 and HIV-1 Vpu vectors, cells were transfected with a 3:2:1 ratio of the pCMS28 K5/Vpu vector plasmid, pMLV-Gag-Pol, and pCMV-VSV-G and supernatants were harvested 48 h posttransfection and used to transduce HT1080 or 293 cells expressing tetherin isoforms.
Virus release assays.
Subconfluent 293T cells were plated on 24-well plates and transfected with 500 ng HIV-1 NL4.3 WT or ΔVpu mutant provirus and various amounts of tetherin expression vector or a constant amount (50 ng) of tetherin and various inputs of Vpu plasmid by using 1 μg/ml PEI. Similarly, 500 ng SIVmac or SIVmacΔNef provirus was cotransfected with various amounts of rhesus macaque tetherin expression vectors. The medium was replaced 6 or 16 h posttransfection, and the supernatants were harvested after 48 h. The infectivity of viral supernatants was determined by infecting TZM-bl HeLa cells, which were assayed 48 h later for β-galactosidase activity with the Tropix GalactoStar chemiluminescence kit (Applied Biosystems). For the analysis of physical virion release, supernatants were filtered (0.22 mm) and pelleted through a 20% sucrose–phosphate-buffered saline (PBS) cushion at 20,000 × g for 90 min at 4°C, and pellets were lysed in SDS-PAGE loading buffer. All HIV-1, HIV-2, and SIVmac virion and cell lysates were then subjected to SDS-PAGE and Western blotted for HIV-1 p24 CA (monoclonal antibody 183-H12-5C; kindly provided by B. Chesebro through the NIH ARRP), rabbit anti-Hsp90 (Santa Cruz Biotechnologies), or polyclonal rabbit antitetherin (kindly provided by K. Strebel through the NIH ARRP) (44) and visualized with a LiCor apparatus by using fluorophore-conjugated secondary antibodies (IRDye 800 goat anti-rabbit, IRDye 680 goat anti-mouse).
Flow cytometry.
Jurkat-TAg cells expressing tetherin isoforms were infected with VSV-G-pseudotyped HIV-1 NL4.3 WT or ΔVpu virus at a multiplicity of infection (MOI) of 2. Cells were washed at 6 or 16 h postinfection. At 48 h postinfection, cells were stained for surface tetherin with a specific anti-BST2 IgG2a monoclonal antibody (Abnova) and a goat anti-mouse IgG2a Alexa 633-conjugated secondary antibody (Molecular Probes, Invitrogen), fixed and permeabilized for 20 min (Cytofix/Cytoperm fixation/permeabilization kit; BD Biosciences), and then stained for intracellular HIV-1 Gag with the KC57 antibody conjugated to phycoerythrin (PE) (Beckman-Coulter). Cells were then analyzed with a FACSCalibur flow cytometer (Becton Dickinson) and FlowJo software. 293 cells expressing tetherin isoforms were plated in 24-well plates and transfected with 500 ng pCRV1-HIV-2-IRES-GFP vector with PEI. At 48 h posttransfection, the cells were harvested in PBS–5 mM EDTA, stained for surface tetherin, and analyzed as described above. HT1080 or 293 cells expressing tetherin isoforms were transduced with K5/K5NTR or different Vpu proteins, respectively. After puromycin selection, cells were stained for surface tetherin with an anti-human CD317-PE antibody (eBioscience) and analyzed as described above.
Immunoprecipitation.
Jurkat cells treated with IFN (1,000 U/ml) or left untreated, Jurkat-TAg L-tetherin, Jurkat-TAg S-tetherin, and Jurkat-TAg empty-vector control cells were lysed on ice for 20 min in buffer containing 50 mM Tris (pH 7.4), 150 mM NaCl, 200 μM sodium orthovanadate, 1% NP-40, 0.5% sodium deoxycholate, 5 mM N-ethylmaleimide, and complete protease inhibitors (Roche). Lysates were cleared by centrifugation, and supernatants were incubated with 5 μg/ml mouse anti-BST2 monoclonal antibody (eBioscience) for 1.5 h at 4°C. Sepharose-protein G beads were washed in lysis buffer before they were added to the samples and incubated for another 3 h. Beads were washed five times in lysis buffer before the peptide–N-glycosidase mixture for deglycosylation was added (New England BioLabs). Samples were incubated at 37°C overnight and resuspended in SDS-PAGE loading buffer. Cell lysates and immunoprecipitates were subjected to SDS-PAGE and Western blotting for tetherin and Hsp90. Blots were visualized with a LiCor apparatus. The secondary antibodies used are described above.
RESULTS
The L- and S-tetherin isoforms are differentially sensitive to HIV-1 Vpu.
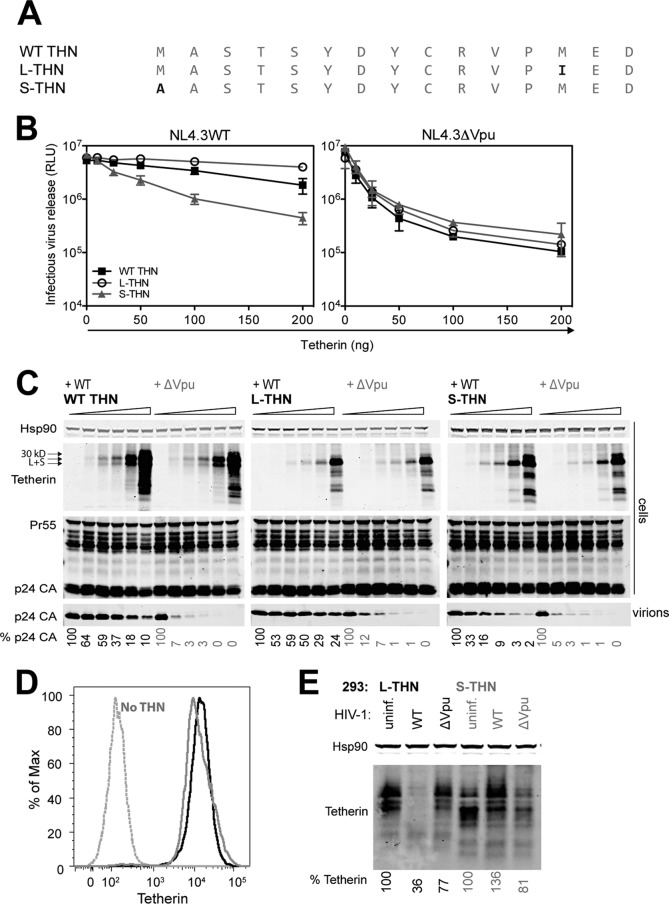
To characterize the roles of the recently identified L- and S-tetherin isoforms (11), we generated constructs encoding human tetherin with its authentic Kozak consensus (WT tetherin), the +1 ATG mutated (S-tetherin), or a semiconservative M13I mutation (L-tetherin) (Fig. 1A). We first sought to confirm the data of Cocka and Bates that S-tetherin displayed reduced sensitivity to HIV-1 Vpu in transient-transfection assays (11). 293T cells were transfected with WT HIV-1 (NL4.3) or a Vpu-defective proviral plasmid in combination with increasing doses of each tetherin expression vector. As expected, increasing the expression of all of the tetherin constructs resulted in an equivalent dose-dependent inhibition of Vpu-defective HIV-1 release, as measured both by infectious titer and by physical virus yield (Fig. 1B and C). All tetherin constructs were well expressed, with the WT tetherin construct apparently expressing similar amounts of both isoforms. The WT virus was essentially resistant to L-tetherin at all expression levels. In contrast, above moderate expression levels, S-tetherin partially restricted the release of WT virus, indicating that Vpu incompletely antagonized it. For the WT tetherin construct, a milder phenotype was observed, with appreciable restriction of WT viral release seen at the highest tetherin input. Therefore, consistent with the previous report (11), S-tetherin homodimers are less sensitive to counteraction by Vpu. Furthermore, since L- and S-tetherins should be able to independently assort to give heterodimers, the approximately 4-fold increased input of WT tetherin required for restriction of WT HIV-1 release implies that L-S heterodimers are as sensitive to Vpu as L-tetherin homodimers assuming a 1:2:1 ratio because of independent assortment (compare 50- and 200-ng inputs in Fig. 1B). Finally, we asked whether S-tetherin displays any evidence of Vpu-induced degradation. Stable 293–L-tetherin or 239–S-tetherin cells that express equivalent levels of the respective isoforms (Fig. 1D) were infected with VSV-G-pseudotyped WT or Vpu-defective virus at an MOI of 2, and cell lysates were analyzed by Western blotting 48 h later (Fig. 1E). As expected, L-tetherin levels were degraded in the presence of the WT virus. However, S-tetherin was not degraded under the same conditions. Interestingly, in cells infected with the WT virus, the apparent molecular mass of S-tetherin increased. This may suggest that a Vpu-induced modification, potentially ubiquitination, may underlie the residual antagonistic effect of Vpu.
FIG 1.
The L- and S-tetherin isoforms are differentially sensitive to HIV-1 Vpu. (A) Schematic representation of the amino acid sequences of WT tetherin (WT THN), L-tetherin (L-THN), and S-tetherin (S-THN). (B and C) 293T cells were transfected with increasing amounts of WT tetherin, L-tetherin, or S-tetherin and NL4.3 WT or ΔVpu proviral plasmid. (B) At 48 h posttransfection, infectious virus release was determined with TZM-bl HeLa reporter cells. Error bars represent standard deviations of the means of three independent experiments. RLU, relative light units. (C) Cell lysates and pelleted viral supernatants from panel B were subjected to SDS-PAGE and analyzed by Western blotting for Hsp90, tetherin, and p24 CA. The percentages of p24 release into the supernatant indicated at the bottom are relative to the release of WT NL4.3 or NL4.3ΔVpu in the absence of tetherin. (D) 293 cells stably expressing the tetherin isoforms were analyzed for surface tetherin expression (L-tetherin, solid black line; S-tetherin, solid gray line; empty-vector 293 cells, dashed gray line [No THN]). (E) 293 cells stably expressing tetherin isoforms were infected with VSV-G-pseudotyped WT NL4.3 or NL4.3ΔVpu virus at an MOI of 2. At 48 h postinfection, cells were harvested and subjected to SDS-PAGE, analyzed by Western blotting for tetherin and Hsp90, and analyzed with a LiCor quantitative imager. The percentages of tetherin levels indicated below the lanes are relative to the tetherin levels in the corresponding uninfected cell lines. Uninf., uninfected.
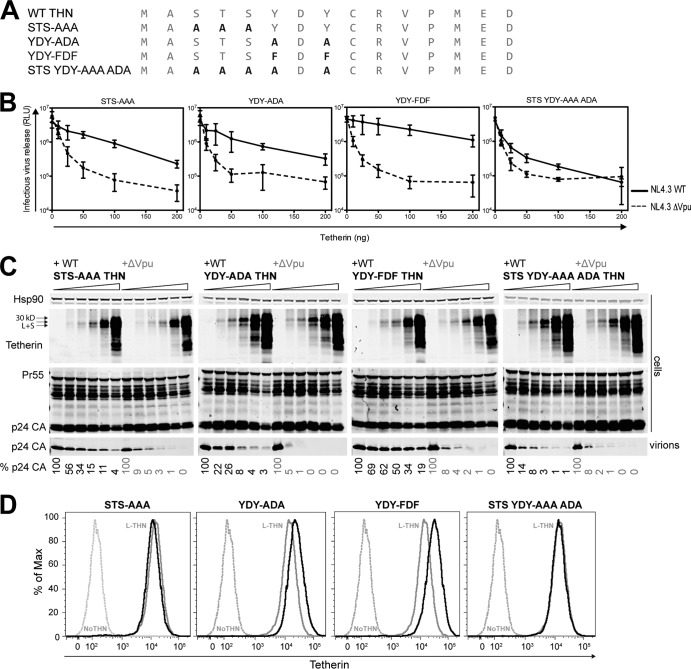
Determinants of Vpu antagonism in the human tetherin cytoplasmic tail have suggested both a role for the tyrosine-based sorting (YDYCRV) motif (29) and a serine-threonine (STS) motif that may be ubiquitinated (35), both of which are absent from S-tetherin. Mutation of either of these motifs to alanines (STS→AAA or YDY→ADA) (Fig. 2A) reduced the sensitivity of WT human tetherin to Vpu (Fig. 2B), and simultaneous disruption had an additive effect with the caveat that this protein is more highly expressed, probably because it lacks sequences important for its natural turnover. Interestingly, mutation of the tyrosine residues to phenylalanines (YDY→FDF) retained sensitivity to Vpu, likely because Phe residues can, to a certain extent, substitute for tyrosines in endocytic sorting motifs (Fig. 2B and C). This site is known to be required for maximal counteraction by Vpu (29). Of note, the reduced sensitivity of these mutants to Vpu did not correlate with overall surface tetherin expression (Fig. 2D).
FIG 2.
Tetherin's sensitivity to Vpu is dependent on serines, a threonine, and tyrosines. (A) Cytoplasmic tail amino acid sequences of mutant tetherins (THNs) used to determine the residues required for sensitivity to HIV-1 NL4.3 Vpu. (B and C) 293T cells were transfected with increasing amounts of different mutant tetherins and a HIV-1 NL4.3 WT or ΔVpu proviral plasmid. (B) At 48 h posttransfection, infectious virus release was determined with TZM-bl HeLa reporter cells. Error bars represent standard deviations of the means of three independent experiments. Solid lines, NL4.3 WT provirus; dashed lines, NL4.3 ΔVpu provirus. RLU, relative light units. (C) Cell lysates and pelleted viral supernatants from panel B were subjected to SDS-PAGE and analyzed by Western blotting for Hsp90, tetherin, and p24 CA. The percentages of p24 release into the supernatant indicated at the bottom are relative to the release of NL4.3 WT or ΔVpu provirus in the absence of tetherin. (D) 293 cells stably expressing the mutant tetherins were analyzed for surface tetherin expression and are shown in comparison to 293 cells expressing L-tetherin (solid gray line) and empty-vector 293 cells (dashed gray line).
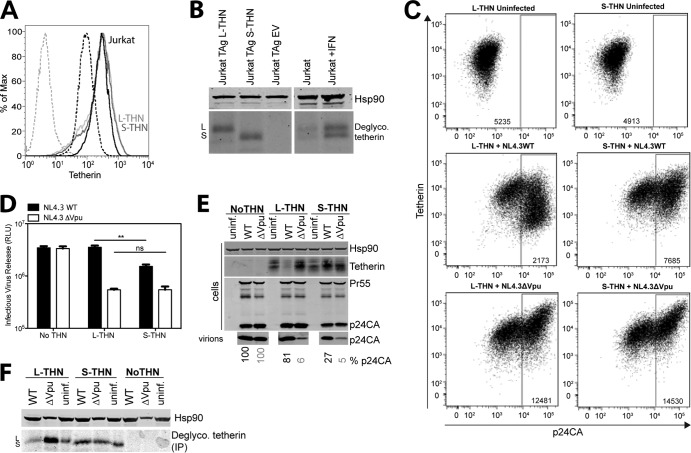
To determine whether tetherin isoforms affect WT virus release in a relevant cell type, we took advantage of a derivative of CD4+ Jurkat cells, Jurkat-TAg cells, that has been shown to have no detectable tetherin on its surface (Fig. 3A) (45). Expression of L- or S-tetherin in these cells resulted in bulk populations whose surface tetherin expression levels were equivalent to those of parental Jurkat cells after type 1 IFN treatment (Fig. 3A). Immunoprecipitation of the tetherin from these cell lines revealed that while Jurkat cells express equivalent levels of both tetherin isoforms and IFN treatment upregulated both equivalently, J-TAg–L-tetherin and J-TAg–S-tetherin cells expressed only the desired isoform (Fig. 3B). Thus, we could use these cells to compare the isoforms in relevant target cells at approximately physiological expression levels. J-TAg–L-tetherin and J-TAg–S-tetherin cells were infected with WT and Vpu-defective HIV-1 and analyzed for surface tetherin and HIV-1 Gag expression 48 h later. As expected, neither tetherin was downregulated in cells infected with Vpu-defective virus, with surface levels enhanced in the p24-high cells probably because of tethered virion accumulation on the surface (Fig. 3C). While L-tetherin was efficiently downmodulated from the surface of cells infected with the WT virus, S-tetherin was not, in keeping with Vpu-mediated targeting for endosomal degradation. Infectious viral release and physical virion yield from the same cultures revealed a significant 3- to 4-fold defect in WT virion release from J-TAg-S-tetherin, demonstrating that at relevant expression levels, the S-tetherin isoform is poorly antagonized (Fig. 3D and E). Since L-tetherin is both induced by pattern recognition (46) and itself capable of proinflammatory signaling (11), a potential confounding issue here is whether WT expression (i.e., expression of both isoforms) is induced in infected cells. While this is a possibility in primary cells, we saw no evidence of tetherin upregulation in infected J-TAg cells (Fig. 3E). Neither did we see any expression of S-tetherin in 293T–L-tetherin cells transfected with Vpu-defective HIV-1 provirus (Fig. 3F), which under these conditions triggers NF-κB activation (12; data not shown). Thus, because of a lack of the cytoplasmic tail residues required for subcellular trafficking and ubiquitination (29, 35), the S-tetherin isoform cannot be degraded or downregulated in CD4+ T cells and this accounts for its reduced sensitivity to HIV-1 Vpu. Furthermore, the residual activity of Vpu against S-tetherin in the absence of surface removal is in keeping with the ability of Vpu to exclude tetherin from virions at the surface to some extent (26, 44).
FIG 3.
Differential sensitivity of tetherin (THN) isoforms to Vpu in CD4+ T cell lines. (A) Jurkat-TAg cells stably expressing L-tetherin (solid light gray line) or S-tetherin (solid dark gray line) were analyzed for surface tetherin expression compared to Jurkat cells treated with IFN for 24 h (solid black line) or left untreated (dashed black line) and to Jurkat-TAg cells (dashed gray line) not expressing tetherin. (B) Jurkat-TAg cells expressing tetherin isoforms and Jurkat cells treated with IFN or left untreated were lysed and immunoprecipitated with antitetherin antibody and deglycosylated (Deglyco.). Lysates and precipitates were subjected to SDS-PAGE and analyzed by Western blotting for Hsp90 and tetherin. (C and D) Jurkat-TAg cells expressing L- or S-tetherin were infected with VSV-G-pseudotyped HIV-1 NL4.3 WT or ΔVpu at an MOI of 2 for 48 h. (C) Cells were analyzed by flow cytometry for intracellular p24 and surface tetherin expression. Median fluorescence intensities (MFI) are indicated. (D) Titers of infectious virus in supernatants from infected cells were determined on TZM-bl cells. Data are from four different experiments, and error bars show the standard errors of the means. **, P < 0.01 (determined by two-tailed t test); ns, no statistically significant difference. RLU, relative light units. (E) Cell lysates and pelleted viral supernatants from panel C were subjected to SDS-PAGE and analyzed by Western blotting for HIV-1 Hsp90, tetherin, and p24 CA. Percent p24 release relative to WT NL4.3 or NL4.3ΔVpu release in the absence of tetherin is indicated at the bottom. uninf., uninfected. (F) 293 cells expressing no tetherin, L-tetherin, or S-tetherin were infected at an MOI of 2. At 48 h postinfection, cells were lysed and immunoprecipitated (IP) with antitetherin antibody and deglycosylated. Lysates and precipitates were subjected to SDS-PAGE and analyzed by Western blotting for Hsp90 and tetherin.
Differential sensitivity of human tetherin isoforms is a feature of HIV-1 group M Vpu proteins.
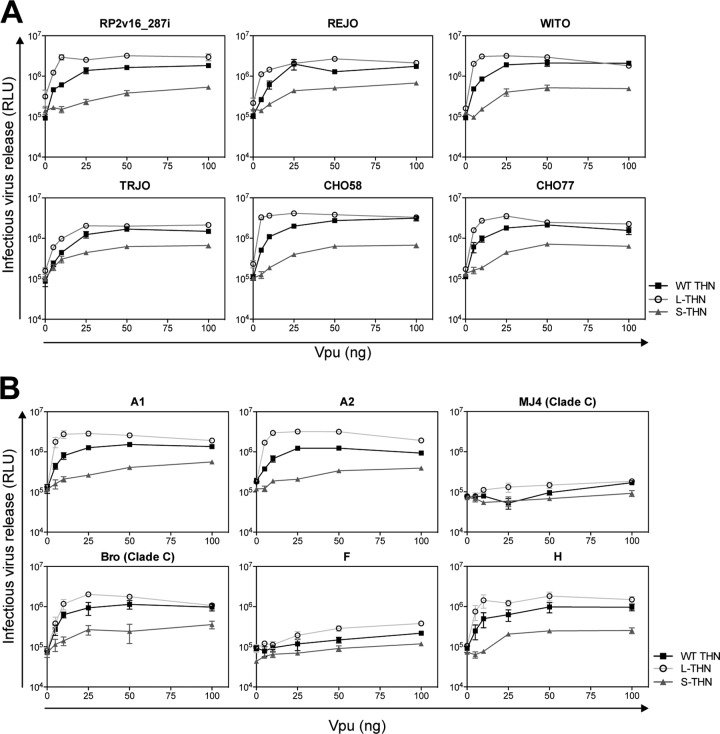
We have recently found that most Vpu alleles from primary isolates display tetherin counteraction activity superior to that encoded by NL4.3, suggesting that accessory gene alleles from lab-adapted strains of HIV-1 may not be representative because of functional drift after prolonged ex vivo passage (39). We therefore examined the sensitivities of human tetherin isoforms to a panel of Vpu alleles from transmitted/founder viruses (42) of HIV-1 subtype B (Fig. 4A) and additional examples from clades A1, A2, C, F, and H (Fig. 4B). In this case, 293T cells were transfected with a Vpu-defective HIV-1 proviral plasmid in the presence of a fixed dose (50 ng) of tetherin and increasing Vpu-encoding plasmid inputs in trans. In all cases where the Vpu displayed antitetherin activity (C MJ4 and F Vpu appeared defective against all tetherin isoforms), L-tetherin was robustly counteracted at low Vpu expression levels. However, as with NL4.3 Vpu, S-tetherin sensitivity to counteraction was much reduced in comparison. In particular, clade A1 and A2 Vpu proteins displayed only marginal activity against S-tetherin, and the weak antagonism of L-tetherin by a clade F Vpu suggests that it may be defective for promotion of degradation. As expected, constructs expressing WT tetherin gave an intermediate phenotype consistent with mixed-isoform expression. Thus, the differential sensitivity of L- and S-tetherins is a feature of the HIV-1 Vpu proteins from group M.
FIG 4.
Differential sensitivity of human tetherin (THN) isoforms to Vpu is a feature of group M. (A and B) 293T cells were transfected with increasing amounts of Vpu, ΔVpu proviral plasmid, and 50 ng of human WT tetherin, L-tetherin, or S-tetherin. At 48 h posttransfection, infectious virus release was determined with TZM-bl HeLa reporter cells. Error bars represent standard deviations of the means of three independent experiments. RLU, relative light units.
Targeting of both tetherin isoforms by an HIV-1 group N Vpu from Togo and Vpu proteins from SIVgsn and SIVmon.
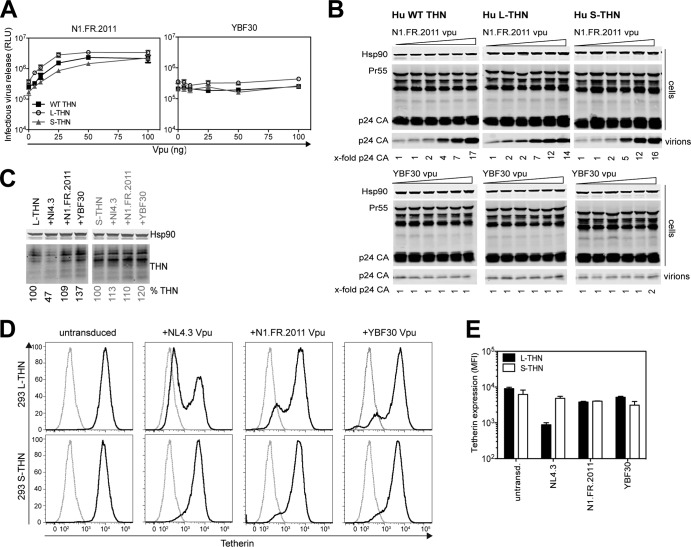
The Vpu proteins of HIV-1 group N display variably weak tetherin counteractivity and are unable to degrade CD4 (21). However, Vpu from a strain isolated from a French national returning from Togo with acute HIV infection revealed evidence of further adaptation to human tetherin in HIV-1 group N (43). In particular, acquisition of a trafficking motif (ExxxLV) found in M-Vpu proteins that is required for promotion of tetherin degradation was associated with tetherin counteraction. While this Vpu (N1.FR.2011) potently targeted L-tetherin in comparison to that from another group N strain, YBF30 (Fig. 5A and B), at higher Vpu expression levels, it could also completely counteract S-tetherin, in contrast to HIV-1 group M Vpu proteins. This enhanced ability to counteract S-tetherin prompted us to examine whether N1.FR.2011 Vpu could downmodulate and degrade S-tetherin. However, expression of N1.FR.2011 Vpu was unable to induce the degradation of S-tetherin (Fig. 5C) and neither did it lead to more than the 2-fold reduction of cell surface S-tetherin that was seen with NL4.3 Vpu (Fig. 5D and E). Thus, N1.FR.2011 Vpu's enhanced activity against S-tetherin may indicate that it is more efficient than group M Vpu proteins at excluding it from budding virions at the cell surface.
FIG 5.
Targeting of both tetherin (THN) isoforms by group N HIV-1 Vpu from Togo. (A) 293T cells were transiently transfected with increasing amounts of Vpu; ΔVpu proviral plasmid; and 50 ng of human WT tetherin, L-tetherin, or S-tetherin. At 48 h posttransfection, infectious virus release was determined with TZM-bl HeLa reporter cells. Error bars represent standard deviations of the means of three independent experiments. RLU, relative light units. (B) Cell lysates and pelleted viral supernatants from panel A were subjected to SDS-PAGE and analyzed by Western blotting for Hsp90 and p24 CA. The fold increases in p24 release into the supernatant indicated at the bottom are relative to the release of NL4.3ΔVpu in the absence of Vpu. (C) 293 cells stably expressing L- or S-tetherin were transduced with retroviral vectors encoding different Vpu proteins. Cell lysates were analyzed for tetherin expression by SDS-PAGE and Western blotting. (D) Vpu-transduced cells from panel C were stained for surface tetherin and analyzed by flow cytometry. Empty-vector 293 cells (not expressing tetherin), dashed gray line. (E) Median fluorescence intensity (MFI) of tetherin in cells from panel D.
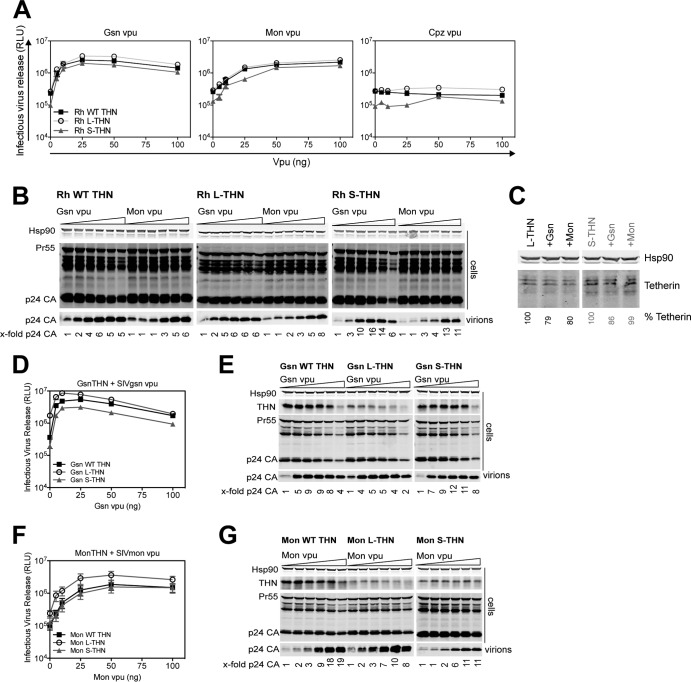
A similar result was obtained with Vpu proteins from SIVgsn and SIVmon that target tetherin in their host species, as well as other primate tetherins, including that from rhesus macaques (21). These are descendants of the ancestral vpu alleles from which SIVcpz and HIV-1 Vpu is derived (40). Again, unlike HIV-1 M Vpu, these proteins equally counteracted the rhesus L- and S-tetherin isoforms, whereas the Vpu from SIVcpzUS, which completely lacks tetherin counteractivity (47), had no effect (Fig. 6A and B). Again, neither SIVgsn nor SIVmon Vpu displayed any degradative activity against either isoform of rhesus tetherin (Fig. 6C). Furthermore, to rule out species-specific effects on tetherin counteraction, we tested these Vpu proteins against their cognate species tetherin isoforms. Although the expression levels of the L isoforms of the greater spot-nosed and mona monkey tetherins were lower than those of the WT and short isoforms, the level of counteraction across Vpu expression levels was equivalent (Fig. 6D to G), again demonstrating equal isoform sensitivity. Thus, the proteins in the guenon SIV lineage that contributed Vpu to HIV-1 are capable of counteracting both isoforms of tetherin equally. This suggests that the readaptation of Vpu to target human tetherin during the zoonotic spread of SIVcpz to become HIV-1 group M resulted in a countermeasure that targeted only one isoform for degradation. The apparently higher potency of the guenon SIV Vpu proteins against S-tetherin suggests that there may be cell biological differences in the mechanisms of action of these proteins that account for this.
FIG 6.
Targeting of both tetherin (THN) isoforms by ancestral Vpu proteins from SIVgsn and SIVmon. (A) 293T cells were transiently transfected with increasing amounts of Vpu; ΔVpu proviral plasmid; and 50 ng of rhesus macaque WT tetherin, L-tetherin, or S-tetherin. At 48 h posttransfection, infectious virus release was determined with TZM-bl HeLa reporter cells. Error bars represent standard deviations of the means of three independent experiments. RLU, relative light units. Rh, rhesus. (B) Cell lysates and pelleted viral supernatants from panel A were subjected to SDS-PAGE and analyzed by Western blotting for Hsp90 and p24 CA. The fold increases in p24 release into the supernatant indicated at the bottom are relative to the release of NL4.3ΔVpu in the absence of Vpu. (C) 293 cells stably expressing L- or S-tetherin were transduced with retroviral vectors encoding different Vpu proteins. Cell lysates were analyzed for tetherin expression by SDS-PAGE and Western blotting. (D and F) 293T cells were transiently transfected with increasing amounts of greater spot-nosed monkey (Gsn) Vpu (D) or mona monkey (Mon) Vpu (F); ΔVpu proviral plasmid; and 50 ng of Gsn (D) or Mon (F) WT tetherin, L-tetherin, or S-tetherin. At 48 h posttransfection, infectious virus release was determined with TZM-bl HeLa reporter cells. Error bars represent standard deviations of the means of three independent experiments. (E and G) Cell lysates and pelleted viral supernatants from panels D and F were subjected to SDS-PAGE and analyzed by Western blotting for Hsp90, tetherin, and p24 CA. The fold increases in p24 release into the supernatant indicated at the bottom are relative to the release of NL4.3ΔVpu in the absence of Vpu.
HIV-2 envelope, SIVmac Nef, and KSHV K5 target tetherin isoforms with equal efficiency.
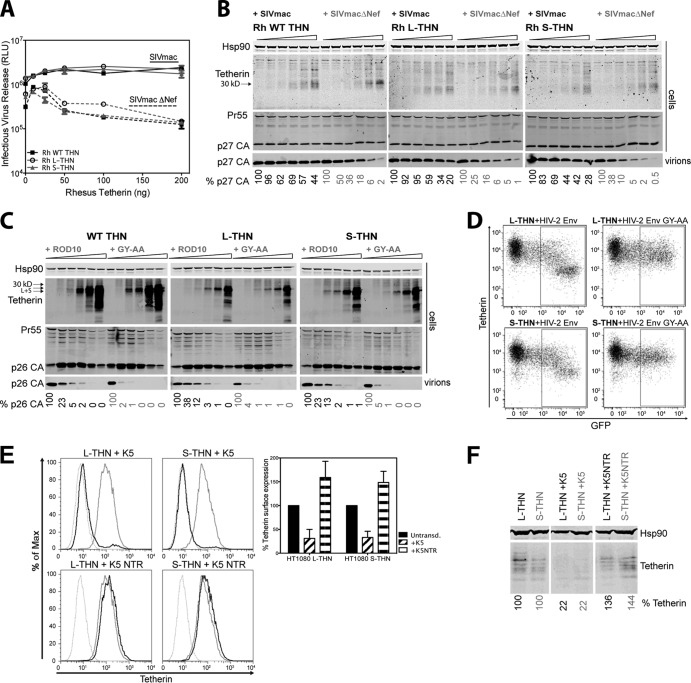
We then examined the sensitivities of human or rhesus L- and S-tetherins to other virus-encoded countermeasures. Most SIVs that lack a vpu gene antagonize their species' tetherins with Nef (15, 16). SIVmac Nef binds to the cytoplasmic tail of macaque tetherin and likely forms a ternary complex with clathrin adaptor protein complex 2 (AP2) (36, 37) to stimulate tetherin endocytosis and intracellular sequestration. Nef's specificity for primate tetherins depends on a (G/D)DIWK motif that is absent from human tetherin. Therefore, rhesus macaque L-tetherin (rhL-tetherin) and rhS-tetherin were tested for the ability to restrict the release of WT or Nef-defective SIVmac239 from transiently transfected 293T cells. To overcome the reduced infectivity of Nef-defective viruses, we cotransfected VSV-G. As with Vpu-defective HIV-1, the release of Nef-defective SIVmac239 was equally sensitive to restriction by either isoform of rh-tetherin (Fig. 7A and B). However, in contrast, the WT virus was resistant to all rh-tetherin isoforms, indicating that the absence of the first 12 amino acids of the tetherin cytoplasmic tail has no effect on its sensitivity to Nef-mediated antagonism. This was also the case for HIV-2, which uses its envelope to target tetherin, again in an AP2-dependent manner (18, 38). Despite being a relatively weak tetherin antagonist, the release of WT HIV-2 Rod10 particles from cells transfected with L-tetherin and S-tetherin was equally superior to a viral Env mutant lacking the major AP2 binding site GYXXV (HIV-2 Rod10 GY-AA) in its cytoplasmic tail (Fig. 7C). Furthermore, expression of the HIV-2 Rod10 envelope protein in 293–L- or 293–S-tetherin cells could reduce cell surface tetherin levels dependent on the AP2-binding site (Fig. 7D). Thus, tetherin counteraction and surface downregulation by both SIVmac Nef and HIV-2 Env are independent of trafficking and ubiquitination motifs that are required for Vpu to target human tetherin for degradation.
FIG 7.
HIV-2 envelope, SIVmac Nef, and KSHV K5 target tetherin (THN) isoforms with equal efficiency. (A and B) 293T cells were transfected with increasing amounts of rhesus (Rh) macaque WT, L-, or S-tetherin and SIVmac or SIVmacΔNef proviral plasmid. (A) Titers of infectious virus released from transfected cells were determined on TZM-bl HeLa reporter cells. Solid lines, SIVmac; dashed lines, SIVmacΔNef. RLU, relative light units. (B) Cell lysates and pelleted supernatant virions were subjected to SDS-PAGE and analyzed by Western blotting for Hsp90, rhesus tetherin, and p27 CA expression. The percentages of p27 CA in the supernatant, relative to virus release in the absence of tetherin, are indicated at the bottom. (C) 293T cells were transfected with increasing amounts of human WT, L-, or S-tetherin and HIV-2 ROD10 or the ROD10 GY-AA mutant proviral plasmid. Cell lysates and pelleted supernatant virions were subjected to SDS-PAGE and analyzed by Western blotting for Hsp90, tetherin, and p26 CA expression. The percentages of p26 CA in the supernatant, relative to virus release in the absence of tetherin, are indicated at the bottom. (D) 293 cells stably expressing L- or S-tetherin were transfected with HIV-2 ROD10 Env-IRES-GFP or HIV-2 ROD10 GY-AA mutant Env-IRES-GFP. At 48 h posttransfection, cells were analyzed by flow cytometry for surface GFP and tetherin expression. (E) HT1080 cells stably expressing tetherin isoforms were transduced with K5 or mutant K5 (K5NTR) defective for tetherin antagonism and selected. Cells were then analyzed by flow cytometry for surface tetherin expression. Dotted gray lines, tetherin-negative HT1080 cells; gray lines, nontransduced tetherin-expressing HT1080 cells; black lines, tetherin-expressing cells transduced with WT K5 (top) or a mutant form (K5NTR; bottom). The graph indicates the surface tetherin expression on transduced cell lines, shown as percentages of the fluorescence of the corresponding nontransduced (Untransd.) tetherin-expressing cell line. (F) Lysates of transduced and nontransduced cells were subjected to SDS-PAGE and analyzed by Western blotting for Hsp90 and tetherin expression. Tetherin expression in lysates as a percentage of that in nontransduced cells is shown at the bottom.
We finally asked whether the K5 ubiquitin ligase encoded by KSHV could also target both tetherin isoforms. K5 targets a single lysine residue (K18) in human tetherin to promote ESCRT-dependent endosomal degradation (5). However, because K5 cannot target rhesus tetherin, it is possible that cytoplasmic tail residues play a role in this specificity. Since 293T cells do not support efficient K5 function (5), HT1080 cells encoding human tetherin isoforms were transduced with puromycin-selective retroviral vectors encoding K5 or a mutant form, K5NTR, bearing a lesion in its C-terminal cytoplasmic tail that abolishes its activity (48). Puromycin-resistant cells expressing K5, but not K5NTR, displayed a marked reduction in the cell surface expression levels of both tetherin isoforms, indicating equal sensitivity to K5 (Fig. 7E). Total tetherin levels in the lysates of the same cells revealed evidence that both tetherin isoforms are sensitive to K5-mediated degradation (Fig. 7F). Together, these data indicate that, unlike Vpu, the first 12 amino acids of the tetherin cytoplasmic tail are dispensable for counteraction by other viral antagonists, thus rendering both isoforms equally sensitive, and further suggest that trafficking determinants in these countermeasures act independently of tetherin's normal recycling mechanism.
DISCUSSION
In this study, we have examined the sensitivities of the L- and S-tetherin isoforms to lentiviral antagonists and found that, uniquely among those studied, the Vpu proteins of HIV-1 group M differ in the potency with which they target isoforms of human tetherin. For the most part, this parallels the ability of M-Vpu to reduce cell surface levels of tetherin and target it for ESCRT-dependent degradation (1). Similarly to a recent study (11), this is determined by the lack of both a clathrin-dependent recycling sequence (YDYCRV) and a putative serine-threonine motif previously reported to act as a ubiquitin acceptor site (35). These data are consistent with the notion that for maximal counteraction of tetherin, Vpu blocks its transit from the trans-Golgi network to the PM and routes it to late endosomes, which requires trafficking motifs in the cytoplasmic tails of both proteins (1). In contrast, SIV Nef and HIV-2 Env do not mediate tetherin degradation but rather lead to tetherin's intracellular sequestration following enhanced internalization from the surface. This is dependent on AP2 binding sites in both viral proteins (18, 36–38, 49). For Nef (36) and also for Env, as shown here, this does not require the presence of the endocytic signal in tetherin itself.
Most of the mammalian tetherins so far sequenced have a second ATG in their first exon that can act as a translation initiation site in the mRNA, leading to the potential to express the L- and S-tetherin isoforms (11). The data so far indicate that both isoforms are expressed equally, and since tetherin is a dimer, independent assembly could give rise to a distribution of homodimers and heterodimers in a 1:2:1 ratio (11). This is borne out in our data showing that approximately 4-fold more expression of WT tetherin than of the short isoform is required to restrict WT HIV-1 release. Polymorphisms in the +1 ATG site have been observed three times so far in different mammalian species. Both the domestic cat (50, 51) and horse (52) tetherin genes lack the +1 ATG, and a similar defect exists in at least one inbred mouse strain, NZW (53). In the latter case, this has been shown to lead to higher tetherin expression levels on lymphocytes and correlates with a better control of murine leukemia virus viremia (53). The facts that both isoforms are equally potent at restricting virion release and that none of the other lentiviral tetherin antagonists tested here differentiate between the isoforms therefore raise the question of why tetherin counteraction and the determinants of L-tetherin degradation are so strictly conserved in group M Vpu.
Key issues are whether the expression of L- and S-tetherin can be differentially regulated and the importance of additional functions associated with the long isoform. The lack of the YDYCRV motif reduces tetherin endocytosis and recycling (22), and in keeping with this, HIV-1 virions do not accumulate in late endosomes in cells expressing either tyrosine mutant or human S-tetherin (12; data not shown). The delivery of retained virions to endosomes for degradation may have immunological consequences in addition to the physical removal of virions from the cell surface. Virion components may be targeted for enhanced antigen presentation in some cell types, or liberation of virion associated pathogen-associated molecular patterns in endosomes may further stimulate cognate pattern recognition receptors in cis (1, 12). In contrast, surface accumulation of virions mediated by S-tetherin may enhance the opsonization of infected cells and debris for clearance by phagocytes (1). In support of this notion, very recent data indicate that tetherin-mediated virion retention sensitizes infected cells to antibody-dependent cellular cytotoxicity (54). Furthermore, tetherin itself may directly regulate pattern recognition receptor function in plasmacytoid dendritic cells through the inhibitory leukocyte receptor ILT-7 (55), although this has been recently challenged (56). Thus, other physiological roles of S-tetherin and L-tetherin bear further investigation. Finally, the ability of tetherin to physically restrict cell-to-cell transmission at virological synapses (VS) of HIV-1 has been controversial (57–59). Given the differential sensitivity of the L and S isoforms to Vpu, it will be important to know whether they partition differently to the VS in a manner that may account for these discrepant observations.
Since most of these attributes should be features of all mammalian tetherins, why only the HIV-1 tetherin countermeasure differentiates between the isoforms so markedly still requires explanation. One further function of tetherin that is limited to the long isoform is the ability to mediate an NF-κB-dependent signal upon virion retention (12, 13). This again requires the YDYCRV motif, and the current data suggest that virion aggregation of tetherin at the cell surface upon restriction of release leads to the recruitment of a TRAF2/TRAF6/TAK1 complex that activates NF-κB (11–13). In keeping with this, human CD4+ T cells infected with Vpu-defective HIV-1 express enhanced levels of proinflammatory cytokines in a tetherin-dependent manner (12). This activity is so far limited to the human and chimpanzee proteins (12). Species-specific changes in the cytoplasmic tails of great ape and hominid tetherins are the determinants of its signaling capacity, with the human protein showing considerably more potent activity (12). Importantly, evidence that S-tetherin dominantly interferes with human tetherin signaling suggests that only homodimers of L-tetherin can signal (11). Since the threshold for such a signaling event may be less than efficient virion retention, we suggest that this function fits well with a further selective pressure exerted specifically by human L-tetherin.
The ability of HIV-1 Vpu to counteract tetherin is a reacquisition of an ancestral guenon SIV function that was lost in SIVcpz Vpu proteins (40). This was probably due to redundancy with Nef, which in SIVcpz acts as the tetherin antagonist (21). SIVcpz derives from a recombination between two lineages of SIV with Vpu- and Nef-mediated tetherin counteraction, respectively (40). However, because Nef associates directly with tetherin, dependent in part on a G/DDIWK motif in the cytoplasmic tail (37) that has been deleted from hominids (60), SIVcpz was without a tetherin antagonist upon zoonotic transfer to humans. Thus, Vpu, which still retained CD4 targeting, adapted to target human tetherin (21). Sequence divergence in SIVcpz Vpu proteins is associated with whether the individual zoonotic transfers that gave rise to groups M, N, O, and P adapted to target human tetherin (40, 43). In particular, (i) the development of an AxxxAxxxAxxxW binding interface in the Vpu TM domain (24, 25), (ii) acidic-dileucine trafficking motifs (29), and (iii) a well-characterized ubiquitin ligase binding site (DSGNES) in the cytoplasmic tail (33, 43) are the key features of the M-Vpu protein that endowed it with tetherin counteractivity. The latter two are essential for L-tetherin degradation by M-Vpu (29, 32, 33). In contrast, O-Vpu lacks tetherin binding in the TM domain and fails to target human tetherin at all, whereas all N-Vpu proteins (save one) have readapted to bind tetherin but fail to counteract it efficiently or degrade it (21, 61, 62). Thus, it has been proposed that efficient tetherin counteraction by Vpu was a key feature in the establishment of the human HIV/AIDS pandemic by group M HIV-1 (40). Coincidentally, the deletion that abolished the Nef sensitivity of human tetherin also gave rise to the enhanced signaling capacity of the long isoform (12), the form that all of the M-Vpu proteins tested herein preferentially target. Thus, we speculate that enhanced targeting of L-tetherin owing to its increased signaling capacity, in addition to its direct antiviral activity, may have contributed to the successful spread of HIV-1 group M in humans, whereas groups N and O remain geographically restricted. In this regard, the increased potency of Vpu from a highly pathogenic group N HIV-1 from Togo is interesting. While it has acquired two cytoplasmic tail attributes important for counteraction (43), in our studies, it did not degrade tetherin. Also, although it is still better at counteracting L-tetherin, it appears to be superior to many M-Vpu proteins at counteracting S-tetherin. Therefore, there may be cell biological differences in its mode of action that account for this. In some experimental systems, Vpu can counteract tetherin to a certain extent at the cell surface by physically excluding it from budding virions without removal from the PM (26). This requires the ExxxLV trafficking motif in Vpu but not the YDYCRV motif in tetherin. Since N1.FR.2011 Vpu has acquired this motif (43), future work will determine if this surface exclusion activity is more potent in this Vpu. Moreover, this may also account for the activity of the SIVgsn and SIVmon Vpu proteins against their cognate species' tetherins, although these do not have equivalent acidic dileucine motifs in their cytoplasmic tails (21). However, given that surface tetherin levels are not reduced by N1.FR.2011, it will also be important to examine the capacity of this Vpu, and other nondegradative tetherin countermeasures such as HIV-2 Env, for the ability to block tetherin-induced signaling.
ACKNOWLEDGMENTS
We thank Theodora Hatziioannou for the kind gift of reagents, Claire Jolly for helpful advice, and Suzanne Pickering and other members of the Neil lab for reagents and support. We are also continually indebted to the NIH AIDS Research Reagents Program and the investigators who make their valuable material available through it.
J.W. performed all the experiments and analyzed the data. J.W. and S.J.D.N. planned the work and wrote the paper.
This work was supported by European Research Council starter grant 281598 and Wellcome Trust senior fellowship WT098049AIA to S.J.D.N.
Footnotes
Published ahead of print 12 March 2014
REFERENCES
- 1.Neil SJ. 2013. The antiviral activities of tetherin. Curr. Top. Microbiol. Immunol. 371:67–104. 10.1007/978-3-642-37765-5_3 [DOI] [PubMed] [Google Scholar]
- 2.Neil SJ, Zang T, Bieniasz PD. 2008. Tetherin inhibits retrovirus release and is antagonized by HIV-1 Vpu. Nature 451:425–430. 10.1038/nature06553 [DOI] [PubMed] [Google Scholar]
- 3.Jouvenet N, Neil SJ, Zhadina M, Zang T, Kratovac Z, Lee Y, McNatt M, Hatziioannou T, Bieniasz PD. 2009. Broad-spectrum inhibition of retroviral and filoviral particle release by tetherin. J. Virol. 83:1837–1844. 10.1128/JVI.02211-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zenner HL, Mauricio R, Banting G, Crump CM. 2013. Herpes simplex virus 1 counteracts tetherin restriction via its virion host shutoff activity. J. Virol. 87:13115–13123. 10.1128/JVI.02167-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pardieu C, Vigan R, Wilson SJ, Calvi A, Zang T, Bieniasz P, Kellam P, Towers GJ, Neil SJ. 2010. The RING-CH ligase K5 antagonizes restriction of KSHV and HIV-1 particle release by mediating ubiquitin-dependent endosomal degradation of tetherin. PLoS Pathog. 6:e1000843. 10.1371/journal.ppat.1000843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mansouri M, Viswanathan K, Douglas JL, Hines J, Gustin J, Moses AV, Fruh K. 2009. Molecular mechanism of BST2/tetherin downregulation by K5/MIR2 of Kaposi's sarcoma-associated herpesvirus. J. Virol. 83:9672–9681. 10.1128/JVI.00597-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blondeau C, Pelchen-Matthews A, Mlcochova P, Marsh M, Milne RS, Towers GJ. 2013. Tetherin restricts herpes simplex virus 1 and is antagonized by glycoprotein m. J. Virol. 87:13124–13133. 10.1128/JVI.02250-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sakuma T, Noda T, Urata S, Kawaoka Y, Yasuda J. 2009. Inhibition of Lassa and Marburg virus production by tetherin. J. Virol. 83:2382–2385. 10.1128/JVI.01607-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Perez-Caballero D, Zang T, Ebrahimi A, McNatt MW, Gregory DA, Johnson MC, Bieniasz PD. 2009. Tetherin inhibits HIV-1 release by directly tethering virions to cells. Cell 139:499–511. 10.1016/j.cell.2009.08.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Venkatesh S, Bieniasz PD. 2013. Mechanism of HIV-1 virion entrapment by tetherin. PLoS Pathog. 9:e1003483. 10.1371/journal.ppat.1003483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cocka LJ, Bates P. 2012. Identification of alternatively translated tetherin isoforms with differing antiviral and signaling activities. PLoS Pathog. 8:e1002931. 10.1371/journal.ppat.1002931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Galão RP, Le Tortorec A, Pickering S, Kueck T, Neil SJ. 2012. Innate sensing of HIV-1 assembly by tetherin induces NFkappaB-dependent proinflammatory responses. Cell Host Microbe 12:633–644. 10.1016/j.chom.2012.10.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tokarev A, Suarez M, Kwan W, Fitzpatrick K, Singh R, Guatelli J. 2013. Stimulation of NF-kappaB activity by the HIV restriction factor BST2. J. Virol. 87:2046–2057. 10.1128/JVI.02272-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Van Damme N, Goff D, Katsura C, Jorgenson RL, Mitchell R, Johnson MC, Stephens EB, Guatelli J. 2008. The interferon-induced protein BST-2 restricts HIV-1 release and is downregulated from the cell surface by the viral Vpu protein. Cell Host Microbe 3:245–252. 10.1016/j.chom.2008.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jia B, Serra-Moreno R, Neidermyer W, Rahmberg A, Mackey J, Fofana IB, Johnson WE, Westmoreland S, Evans DT. 2009. Species-specific activity of SIV Nef and HIV-1 Vpu in overcoming restriction by tetherin/BST2. PLoS Pathog. 5:e1000429. 10.1371/journal.ppat.1000429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang F, Wilson SJ, Landford WC, Virgen B, Gregory D, Johnson MC, Munch J, Kirchhoff F, Bieniasz PD, Hatziioannou T. 2009. Nef proteins from simian immunodeficiency viruses are tetherin antagonists. Cell Host Microbe 6:54–67. 10.1016/j.chom.2009.05.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gupta RK, Mlcochova P, Pelchen-Matthews A, Petit SJ, Mattiuzzo G, Pillay D, Takeuchi Y, Marsh M, Towers GJ. 2009. Simian immunodeficiency virus envelope glycoprotein counteracts tetherin/BST-2/CD317 by intracellular sequestration. Proc. Natl. Acad. Sci. U. S. A. 106:20889–20894. 10.1073/pnas.0907075106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Le Tortorec A, Neil SJ. 2009. Antagonism to and intracellular sequestration of human tetherin by the human immunodeficiency virus type 2 envelope glycoprotein. J. Virol. 83:11966–11978. 10.1128/JVI.01515-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kaletsky RL, Francica JR, Agrawal-Gamse C, Bates P. 2009. Tetherin-mediated restriction of filovirus budding is antagonized by the Ebola glycoprotein. Proc. Natl. Acad. Sci. U. S. A. 106:2886–2891. 10.1073/pnas.0811014106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sauter D, Specht A, Kirchhoff F. 2010. Tetherin: holding on and letting go. Cell 141:392–398. 10.1016/j.cell.2010.04.022 [DOI] [PubMed] [Google Scholar]
- 21.Sauter D, Schindler M, Specht A, Landford WN, Munch J, Kim KA, Votteler J, Schubert U, Bibollet-Ruche F, Keele BF, Takehisa J, Ogando Y, Ochsenbauer C, Kappes JC, Ayouba A, Peeters M, Learn GH, Shaw G, Sharp PM, Bieniasz P, Hahn BH, Hatziioannou T, Kirchhoff F. 2009. Tetherin-driven adaptation of Vpu and Nef function and the evolution of pandemic and nonpandemic HIV-1 strains. Cell Host Microbe 6:409–421. 10.1016/j.chom.2009.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rollason R, Korolchuk V, Hamilton C, Schu P, Banting G. 2007. Clathrin-mediated endocytosis of a lipid-raft-associated protein is mediated through a dual tyrosine motif. J. Cell Sci. 120:3850–3858. 10.1242/jcs.003343 [DOI] [PubMed] [Google Scholar]
- 23.Dubé M, Roy BB, Guiot-Guillain P, Binette J, Mercier J, Chiasson A, Cohen EA. 2010. Antagonism of tetherin restriction of HIV-1 release by Vpu involves binding and sequestration of the restriction factor in a perinuclear compartment. PLoS Pathog. 6:e1000856. 10.1371/journal.ppat.1000856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Skasko M, Wang Y, Tian Y, Tokarev A, Munguia J, Ruiz A, Stephens EB, Opella SJ, Guatelli J. 2012. HIV-1 Vpu protein antagonizes innate restriction factor BST-2 via lipid-embedded helix-helix interactions. J. Biol. Chem. 287:58–67. 10.1074/jbc.M111.296772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vigan R, Neil SJ. 2010. Determinants of tetherin antagonism in the transmembrane domain of the human immunodeficiency virus type 1 Vpu protein. J. Virol. 84:12958–12970. 10.1128/JVI.01699-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McNatt MW, Zang T, Bieniasz PD. 2013. Vpu binds directly to tetherin and displaces it from nascent virions. PLoS Pathog. 9:e1003299. 10.1371/journal.ppat.1003299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dubé M, Paquay C, Roy BB, Bego MG, Mercier J, Cohen EA. 2011. HIV-1 Vpu antagonizes BST-2 by interfering mainly with the trafficking of newly synthesized BST-2 to the cell surface. Traffic 12:1714–1729. 10.1111/j.1600-0854.2011.01277.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schmidt S, Fritz JV, Bitzegeio J, Fackler OT, Keppler OT. 2011. HIV-1 Vpu blocks recycling and biosynthetic transport of the intrinsic immunity factor CD317/tetherin to overcome the virion release restriction. mBio 2:e00036–00011. 10.1128/mBio.00036-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kueck T, Neil SJ. 2012. A cytoplasmic tail determinant in HIV-1 Vpu mediates targeting of tetherin for endosomal degradation and counteracts interferon-induced restriction. PLoS Pathog. 8:e1002609. 10.1371/journal.ppat.1002609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Agromayor M, Soler N, Caballe A, Kueck T, Freund SM, Allen MD, Bycroft M, Perisic O, Ye Y, McDonald B, Scheel H, Hofmann K, Neil SJ, Martin-Serrano J, Williams RL. 2012. The UBAP1 subunit of ESCRT-I interacts with ubiquitin via a SOUBA domain. Structure 20:414–428. 10.1016/j.str.2011.12.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Janvier K, Pelchen-Matthews A, Renaud JB, Caillet M, Marsh M, Berlioz-Torrent C. 2011. The ESCRT-0 component HRS is required for HIV-1 Vpu-mediated BST-2/tetherin down-regulation. PLoS Pathog. 7:e1001265. 10.1371/journal.ppat.1001265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Douglas JL, Viswanathan K, McCarroll MN, Gustin JK, Fruh K, Moses AV. 2009. Vpu directs the degradation of the human immunodeficiency virus restriction factor BST-2/tetherin via a {beta}TrCP-dependent mechanism. J. Virol. 83:7931–7947. 10.1128/JVI.00242-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mitchell RS, Katsura C, Skasko MA, Fitzpatrick K, Lau D, Ruiz A, Stephens EB, Margottin-Goguet F, Benarous R, Guatelli JC. 2009. Vpu antagonizes BST-2-mediated restriction of HIV-1 release via beta-TrCP and endo-lysosomal trafficking. PLoS Pathog. 5:e1000450. 10.1371/journal.ppat.1000450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gustin JK, Douglas JL, Bai Y, Moses AV. 2012. Ubiquitination of BST-2 protein by HIV-1 Vpu protein does not require lysine, serine, or threonine residues within the BST-2 cytoplasmic domain. J. Biol. Chem. 287:14837–14850. 10.1074/jbc.M112.349928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tokarev AA, Munguia J, Guatelli JC. 2011. Serine-threonine ubiquitination mediates downregulation of BST-2/tetherin and relief of restricted virion release by HIV-1 Vpu. J. Virol. 85:51–63. 10.1128/JVI.01795-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang F, Landford WN, Ng M, McNatt MW, Bieniasz PD, Hatziioannou T. 2011. SIV Nef proteins recruit the AP-2 complex to antagonize tetherin and facilitate virion release. PLoS Pathog. 7:e1002039. 10.1371/journal.ppat.1002039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Serra-Moreno R, Zimmermann K, Stern LJ, Evans DT. 2013. Tetherin/BST-2 antagonism by Nef depends on a direct physical interaction between Nef and tetherin, and on clathrin-mediated endocytosis. PLoS Pathog. 9:e1003487. 10.1371/journal.ppat.1003487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lau D, Kwan W, Guatelli J. 2011. Role of the endocytic pathway in the counteraction of BST-2 by human lentiviral pathogens. J. Virol. 85:9834–9846. 10.1128/JVI.02633-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pickering S, Hue S, Kim EY, Reddy S, Wolinsky SM, Neil SJ. 2014. Preservation of tetherin and CD4 counter-activities in circulating Vpu alleles despite extensive sequence variation within HIV-1 infected individuals. PLoS Pathog. 10:e1003895. 10.1371/journal.ppat.1003895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sharp PM, Hahn BH. 2011. Origins of HIV and the AIDS pandemic. Cold Spring Harb. Perspect. Med. 1:a006841. 10.1101/cshperspect.a006841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Neil SJ, Eastman SW, Jouvenet N, Bieniasz PD. 2006. HIV-1 Vpu promotes release and prevents endocytosis of nascent retrovirus particles from the plasma membrane. PLoS Pathog. 2:e39. 10.1371/journal.ppat.0020039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Salazar-Gonzalez JF, Salazar MG, Keele BF, Learn GH, Giorgi EE, Li H, Decker JM, Wang S, Baalwa J, Kraus MH, Parrish NF, Shaw KS, Guffey MB, Bar KJ, Davis KL, Ochsenbauer-Jambor C, Kappes JC, Saag MS, Cohen MS, Mulenga J, Derdeyn CA, Allen S, Hunter E, Markowitz M, Hraber P, Perelson AS, Bhattacharya T, Haynes BF, Korber BT, Hahn BH, Shaw GM. 2009. Genetic identity, biological phenotype, and evolutionary pathways of transmitted/founder viruses in acute and early HIV-1 infection. J. Exp. Med. 206:1273–1289. 10.1084/jem.20090378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sauter D, Unterweger D, Vogl M, Usmani SM, Heigele A, Kluge SF, Hermkes E, Moll M, Barker E, Peeters M, Learn GH, Bibollet-Ruche F, Fritz JV, Fackler OT, Hahn BH, Kirchhoff F. 2012. Human tetherin exerts strong selection pressure on the HIV-1 group N Vpu protein. PLoS Pathog. 8:e1003093. 10.1371/journal.ppat.1003093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Miyagi E, Andrew AJ, Kao S, Strebel K. 2009. Vpu enhances HIV-1 virus release in the absence of Bst-2 cell surface down-modulation and intracellular depletion. Proc. Natl. Acad. Sci. U. S. A. 106:2868–2873. 10.1073/pnas.0813223106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Erikson E, Adam T, Schmidt S, Lehmann-Koch J, Over B, Goffinet C, Harter C, Bekeredjian-Ding I, Sertel S, Lasitschka F, Keppler OT. 2011. In vivo expression profile of the antiviral restriction factor and tumor-targeting antigen CD317/BST-2/HM1.24/tetherin in humans. Proc. Natl. Acad. Sci. U. S. A. 108:13688–13693. 10.1073/pnas.1101684108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bego MG, Mercier J, Cohen EA. 2012. Virus-activated interferon regulatory factor 7 upregulates expression of the interferon-regulated BST2 gene independently of interferon signaling. J. Virol. 86:3513–3527. 10.1128/JVI.06971-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lim ES, Malik HS, Emerman M. 2010. Ancient adaptive evolution of tetherin shaped the functions of Vpu and Nef in human immunodeficiency virus and primate lentiviruses. J. Virol. 84:7124–7134. 10.1128/JVI.00468-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Means RE, Lang SM, Jung JU. 2007. The Kaposi's sarcoma-associated herpesvirus K5 E3 ubiquitin ligase modulates targets by multiple molecular mechanisms. J. Virol. 81:6573–6583. 10.1128/JVI.02751-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Noble B, Abada P, Nunez-Iglesias J, Cannon PM. 2006. Recruitment of the adaptor protein 2 complex by the human immunodeficiency virus type 2 envelope protein is necessary for high levels of virus release. J. Virol. 80:2924–2932. 10.1128/JVI.80.6.2924-2932.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Celestino M, Calistri A, Del Vecchio C, Salata C, Chiuppesi F, Pistello M, Borsetti A, Palu G, Parolin C. 2012. Feline tetherin is characterized by a short N-terminal region and is counteracted by the feline immunodeficiency virus envelope glycoprotein. J. Virol. 86:6688–6700. 10.1128/JVI.07037-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dietrich I, Hosie MJ, Willett BJ. 2011. The role of BST2/tetherin in feline retrovirus infection. Vet. Immunol. Immunopathol. 143:255–264. 10.1016/j.vetimm.2011.06.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yin X, Hu Z, Gu Q, Wu X, Zheng YH, Wei P, Wang X. 2014. Equine tetherin blocks retrovirus release and its activity is antagonized by equine infectious anemia virus envelope protein. J. Virol. 88:1259–1270. 10.1128/JVI.03148-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Barrett BS, Smith DS, Li SX, Guo K, Hasenkrug KJ, Santiago ML. 2012. A single nucleotide polymorphism in tetherin promotes retrovirus restriction in vivo. PLoS Pathog. 8:e1002596. 10.1371/journal.ppat.1002596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pham TN, Lukhele S, Hajjar F, Routy JP, Cohen EA. 2014. HIV Nef and Vpu protect HIV-infected CD4+ T cells from antibody-mediated cell lysis through down-modulation of CD4 and BST2. Retrovirology 11:15. 10.1186/1742-4690-11-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cao W, Bover L, Cho M, Wen X, Hanabuchi S, Bao M, Rosen DB, Wang YH, Shaw JL, Du Q, Li C, Arai N, Yao Z, Lanier LL, Liu YJ. 2009. Regulation of TLR7/9 responses in plasmacytoid dendritic cells by BST2 and ILT7 receptor interaction. J. Exp. Med. 206:1603–1614. 10.1084/jem.20090547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tavano B, Galão RP, Graham DR, Neil SJ, Aquino VN, Fuchs D, Boasso A. 2013. Ig-like transcript 7, but not bone marrow stromal cell antigen 2 (also known as HM1.24, tetherin, or CD317), modulates plasmacytoid dendritic cell function in primary human blood leukocytes. J. Immunol. 190:2622–2630. 10.4049/jimmunol.1202391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Casartelli N, Sourisseau M, Feldmann J, Guivel-Benhassine F, Mallet A, Marcelin AG, Guatelli J, Schwartz O. 2010. Tetherin restricts productive HIV-1 cell-to-cell transmission. PLoS Pathog. 6:e1000955. 10.1371/journal.ppat.1000955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jolly C, Booth NJ, Neil SJ. 2010. Cell-cell spread of human immunodeficiency virus type 1 overcomes tetherin/BST-2-mediated restriction in T cells. J. Virol. 84:12185–12199. 10.1128/JVI.01447-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kuhl BD, Sloan RD, Donahue DA, Bar-Magen T, Liang C, Wainberg MA. 2010. Tetherin restricts direct cell-to-cell infection of HIV-1. Retrovirology 7:115. 10.1186/1742-4690-7-115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sauter D, Vogl M, Kirchhoff F. 2011. Ancient origin of a deletion in human BST2/tetherin that confers protection against viral zoonoses. Hum. Mutat. 32:1243–1245. 10.1002/humu.21571 [DOI] [PubMed] [Google Scholar]
- 61.Vigan R, Neil SJ. 2011. Separable determinants of subcellular localization and interaction account for the inability of group O HIV-1 Vpu to counteract tetherin. J. Virol. 85:9737–9748. 10.1128/JVI.00479-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yang SJ, Lopez LA, Exline CM, Haworth KG, Cannon PM. 2011. Lack of adaptation to human tetherin in HIV-1 group O and P. Retrovirology 8:78. 10.1186/1742-4690-8-78 [DOI] [PMC free article] [PubMed] [Google Scholar]